Abstract
Abstract
Fruit phenolics are important dietary antioxidant and antidiabetic constituents. The fruit parts (pulp, seed, seed coat, kernel) of underutilized indigenous six black jamun landraces (Syzygium cumini L.), found in Gir forest region of India and differed in their fruit size, shape and weight, are evaluated and correlated with antidiabetic, DPPH radical scavenging and phenolic constituents. The α-amylase inhibitors propose an efficient antidiabetic strategy and the levels of postprandial hyperglycemia were lowered by restraining starch breakdown. The sequential solvent systems with ascending polarity—petroleum ether, ethyl acetate, methanol and water were performed for soxhlet extraction by hot percolation method and extractive yield was found maximum with methanolic fruit part extracts of six landraces. The methanolic extracts of fruit parts also evidenced higher antidiabetic activity and hence utilized for further characterization. Among the six landraces, pulp and kernel of BJLR-6 (very small, oblong fruits) evidenced maximum 53.8 and 98.2% inhibition of α-amylase activity, respectively. The seed attained inhibitory activity mostly contributed by the kernel fraction. The inhibition of DPPH radical scavenging activity was positively correlated with phenol constituents. An HPLC–PDA technique was used to quantify the seven individual phenolics. The seed and kernel of BJLR-6 exhibited higher individual phenolics—gallic, catechin, ellagic, ferulic acids and quercetin, whereas pulp evidenced higher with gallic acid and catechin as α-amylase inhibitors. The IC50 value indicates concentration of fruit extracts exhibiting ≥50% inhibition on porcine pancreatic α-amylase (PPA) activity. The kernel fraction of BJLR6 evidenced lowest (8.3 µg ml−1) IC50 value followed by seed (12.9 µg ml−1), seed coat (50.8 µg ml−1) and pulp (270 µg ml−1). The seed and kernel of BJLR-6 inhibited PPA at much lower concentrations than standard acarbose (24.7 µg ml−1) considering good candidates for antidiabetic herbal formulations.
Graphical Abstract
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2756-8) contains supplementary material, which is available to authorized users.
Keywords: Syzygium cumini L., Fruit parts, α-Amylase inhibition, Antioxidant activity, HPLC profile, Herbal formulation
Introduction
The deficiency of insulin secretion and action causes disorder in endocrine system and it disturbed carbohydrate metabolism known as diabetes mellitus (Alberti and Zimmet 1998). About 800 antidiabetic plants have been reported in the Indian subcontinent. Except for few, the mechanism of antidiabetic action of these plants have been remain yet to study (Grover et al. 2000; Mukherjee et al. 2006). Plant extracts of Barringtonia racemosa, Phyllanthus amarus and others collected from various regions of the world were examined for the occurrence of pancreatic α-amylase inhibitors (Ali et al. 2006; Kotowaroo et al. 2006).
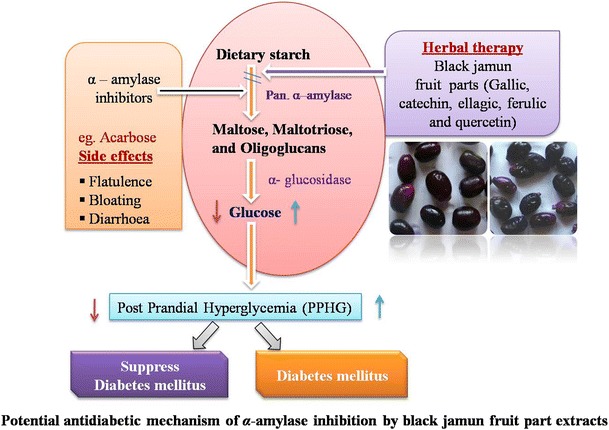
Pancreatic α-amylase is a key digestive enzyme and hydrolyzed starch to maltose and finally to glucose. The dietary starch degraded rapidly by pancreatic α-amylase and elevated post prandial hyperglycemia. The human pancreatic α-amylase (HPA) found in the small intestine correlates positively with the levels of post-prandial glucose, the control of an important aspect in treatment of diabetes (Eichler et al. 1984). Hence, inhibition of α-amylase enzyme would play a key role in the control of diabetes by withdrawing starch digestion.
The black jamun (Syzygium cumini L.) is an important indigenous plant of the family Myrtaceae originally from Indonesia and India. It produced a fruits in various size which is underutilized. The fruit pulp is sweet and seeds are acrid, sour, tonic. The pup and seeds are used for traditional medicine against diabetes, diarrhoea and ringworm (Benherlal and Arumughan 2007). The ripen fruits are purplish black in colour due to the presence of anthocyanins. Fruits are rich in minerals and have high antioxidant property which contributes to many health benefits. Jamun is highly perishable, therefore, very difficult to store and market at distant places. Jamun seeds are used in traditional medicine. The presence of oxalic, tannic, gallic acids and other alkaloids create one to feel such an astringency taste. The secondary metabolites have been reported to be potent free radical scavengers (Ayyanar and Subash-babu 2012). Phenolic and flavonoid compounds present in S. cumini are responsible for antioxidant and anti-inflammatory activities (De Bona et al. 2016; Hossain et al. 2016).
The indigenous jamun tree produced different size of fruits with round and oblong shape. The present study categorized indigenous black jamun landraces of gir forest region of western Gujarat (India), based on fruit size and morphology. Study aimed to (1) observe antidiabetic properties of fruit parts (pulp, seed, seed coat, kernel) of black jamun by in vitro α-amylase inhibition activity, (2) identify phenolic compounds present in best solvent fraction of fruit parts of various black jamun landraces (BJLR) by high performance liquid chromatography (HPLC) analysis; and (3) correlate 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity and phenolics with antidiabetic assay and thereby identify the black jamun landraces with its best fruit part as an antidiabetic value.
Materials and methods
Plant materials
The indigenous jamun trees, found in gir forest region of western Gujarat (India), were produced fruits of different size, shape and weight. Six landraces of black jamun categorized on the basis of fruit weight and size viz., BJLR-1 (big fruit); BJLR-2 (medium to big fruit); BJLR-3 (medium fruit); BJLR-4 (medium to small fruit); BJLR-5 (small fruit) and BJLR-6 (very small fruit) (Table 1). Mature fruits were harvested from the individual land races as three independent replications. Fruits were washed with distilled water followed by drying on filter paper. The fruits were sealed in polythene bags and stored at −20 °C for antidiabetic, antioxidant and phenol profiling.
Table 1.
Black jamun landraces with fruit morphology
| Sr. no. | Code of landraces | Single fruit weight (g) | Classified as | Fruit shape | Photographic image |
|---|---|---|---|---|---|
| 1 | BJLR-1 | >11.00 | Big fruit | Round |

|
| 2 | BJLR-2 | 8.00–11.00 | Medium to big fruit | Round |

|
| 3 | BJLR-3 | 6.00–8.00 | Medium fruit | Round |

|
| 4 | BJLR-4 | 5.00–6.00 | Medium to small fruit | Round and Oblong |

|
| 5 | BJLR-5 | 3.00–5.00 | Small fruit | Oblong |

|
| 6 | BJLR-6 | <3.00 | Very small fruit | Oblong |

|
Preparation of solvent extracts from fruit parts of black jamun
The fruit parts (pulp, seed, seed coat, kernel) of each landraces were separated manually by hand-picking method and dried in hot air oven at 50 °C. Dried coarse powder of the fruit parts (20 g) was placed into the extractor of a Soxhlet apparatus and subjected to extraction by hot percolation method. The extraction was carried out using successive solvent systems with increasing polarity starting from petroleum ether, ethyl acetate, methanol and water. The extraction was carried out with 200 ml of each solvent for a period of 72 h. The extractant in the respective solvent systems were lyophilized under reduced pressure at 40 °C. The extractive yield was calculated by gravimetrical method (Muthumani et al. 2010). The fruit extract of each parts (pulp, seed, seed coat, kernel) were prepared in respective mother solvent (mg ml−1) for further biochemical assay.
Antidiabetic assay by in vitro α-amylase inhibitory activity
The porcine pancreatic α-amylase (PPA) was used for screening of α-amylase inhibitors from the fruit part extracts. The inhibition assay was performed using the chromogenic di-nitro salicylic acid (DNSA) method (Miller 1959). The negative control was also included to eliminate the absorbance produced by fruit extracts. One unit of enzyme activity is defined as the amount of enzyme required to release one micromole of maltose from starch per min under the assay conditions. The per cent α-amylase inhibition was calculated as [100 − (enzyme activity with fruit extract/enzyme activity without fruit extract) × 100]. The IC50 values were defined as the concentration of the fruit extract, containing the α-amylase inhibitor that inhibited 50% of the PPA activity. The assay was performed as above except that the inhibitor/fruit extract concentrations were varied from 0.1 to 300 µg for the determination of the inhibitor concentration at which 50% inhibition of enzyme activity occurs (IC50). Acarbose was used as a positive control at a concentration range of 8–40 µg.
Antioxidant assay by DPPH radical scavenging activity
The antioxidant activity was measured by 1, 1-diphenyl-2-picrylhydrazyl (DPPH) method (McCune and Johns 2002). The intensity of disappearance of violet color was measured at 517 nm in spectrophotometer. Ascorbic acid (10–50 µg) was used as a positive control. The percent of inhibition or scavenging of free radicals was determined by the formula—per cent Inhibition of DPPH = [(Control OD − Sample OD)/Control OD] × 100, where control was prepared as above without fruit extract.
Total phenol assay by folin phenol method
The methanolic extract (mg ml−1) of fruit part samples of black jamun landraces was prepared in their mother solvent. The total phenolic content was determined with suitable aliquot using the folin phenol reagent (Jayasinghe et al. 2003). A standard curve was prepared using 10–50 µg concentrations of gallic acid. The total phenolic content was expressed as mg gallic acid equivalent g−1 extracts.
Phenol profile by HPLC–PDA
High-performance liquid chromatography (HPLC) of the extract samples was performed in triplicate according to Gupta et al. (2012). The quaternary gradient prep HPLC (Waters, Empower2) was equipped with 2996 photodiode array (PDA) detector and 600e multi solvent delivery system; and reverse-phase chromatographic analysis was carried out using a Xterra MSC18 HPLC column (7.8 × 100 mm), particle size 5 µm, C18 (Phenomenex; Torrance, CA, USA) at 25 °C. Samples were filtered through a membrane filter (pore size 0.45 µm; Merck: Mumbai, India) before injection in the sample loop. Phenolic extracts of the sample (20 µl) were separated using a mobile phase based on a linear gradient which included solvents system—solvent-A (2% acetic acid in water) and solvent-B (80% methanol). The gradient programme begun with 100% solvent-A for 5 min followed by solvent-B for 60 min with a flow rate 0.750 ml min−1 and attenuation 0.03. The acquisition wavelength was set in the range of 200–600 nm to identify targeted eight individual phenolics. The standard of phenolics were mixed and run with single injection. It was found that UV detection of mixed phenolics was quite pronounced at wavelength 290 nm.
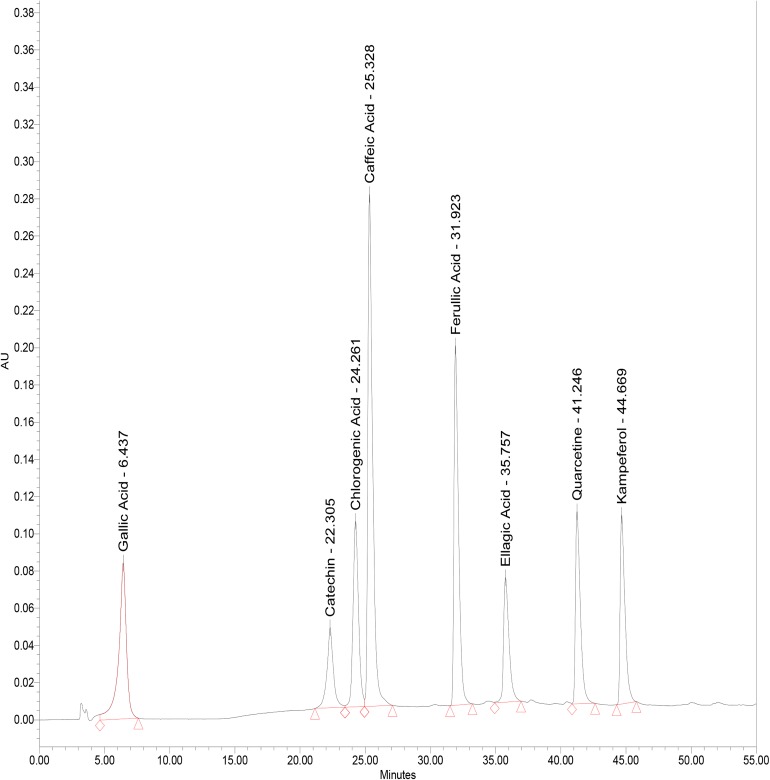
Gallic, catechin, chlorogenic, caffeic, ferulic, ellagic acids, quercetin and Kampeferol were used as standards. Phenolic compounds present in the samples were identified by comparing retention time of standards and by co-injection. The individual phenolics quantified by comparing peak areas of standard phenolic compounds with those in the samples run under the same elution conditions (Fig. 1).
Fig. 1.
Chromatogram of phenol standards separated by HPLC–PDA
Statistical analysis
Fruit samples were collected from three independent trees as three replications for each landraces. Analyses were performed in duplicate for each parameter from bulk samples of five fruits per replication. Statistical analysis was performed by subjecting the data for analysis of variance and analyzing them by two factor complete block randomized design (2FCRD) (F1-Landraces; 2F-Fruit parts) as statistical tools for interpretation of data of antidiabetic and antioxidant activities (Snedecor and Cochran 1967). Individual phenolics were analyzed in HPLC from three independent replications and quantified using a reference standard. The variations in individual phenolics were analyzed as standard deviations. The SPSS software was used for analysis of data including correlation matrix between antodiabetic, antioxidant and phenolic compounds.
Results and discussion
The black jamun landraces were examined for fruit shape and weight (Table 1). The fruit shape of six landraces was found to be round and oblong. The fruits of BJLR-1, BJLR-2, BJLR-3 and BJLR-4 were found round shape while BJLR-5 and BJLR-6 were observed oblong in shape. Roy et al. (2013) reported that the black jamun fruits are found with round to oblong in shape which agreed with present results. Total six landraces of black jamun were categorized on the basis of fruit weight and size viz., BJLR-1 (>11 g—big fruit); BJLR-2 (8–11 g—medium to big fruit); BJLR-3 (6–8 g—medium fruit); BJLR-4 (5–6 g—medium to small fruit); BJLR-5 (3–5 g—mall fruit) and BJLR-6 (<3 g—very small fruit). The extractive yield varied among the four different fruit parts of landraces and also among the solvents used Table S1. Methanol extracts showed higher extractive yield in different fruit parts followed by aqueous water, ethyl acetate and petroleum ether extracts.
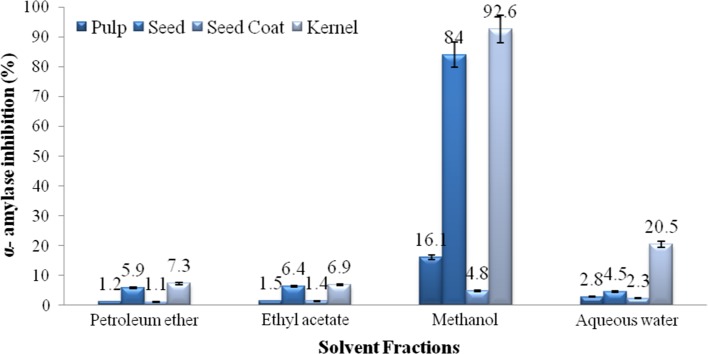
Fruit parts (pulp, seed, seed coat, kernel) of representative landraces (BJLR-4) were sequentially extracted with non-polar to polar solvents and subjected to antidiabetic assay by α-amylase inhibition (Fig. 2). The methanolic extracts of pulp (16.1%), seed (84%), seed coat (4.8%) and kernel (92.6%) were evident higher antidiabetic potential compared with aqueous water, ethyl acetate and petroleum ether. Thus, methanolic fraction of fruit parts of six landraces were utilized for evaluation of antidiabetic and free radical scavenging activities, phenolic constituents by HPLC profile, and identify the black jamun landraces with its best fruit part as an antidiabetic values.
Fig. 2.
Screening of solvent fraction for antidiabetic potential by α-amylase inhibitory activity in fruit parts of black jamun (BJLR-4). Bars indicate standard deviation between replications
Antidiabetic activity from fruit part extracts of black jamun landraces
Antidiabetic activity of different fruit parts (pulp, seed, seed coat, kernel) of six landraces are measured by in vitro α- amylase inhibition. Methanolic extract of landraces showed significant variation in antidiabetic activity (Table 2). Irrespective of fruit parts, mean landraces of antidiabetic activity showed significant differences. The highest activity was recorded in BJLR-6 (62.7%) followed by BJLR-5 (52.0%). The antidiabetic activity was recorded minimum in BJLR-1 (35.7%). Among the fruit parts, kernel contained higher (86.2%) antidiabetic activity followed by seed (79.4%). Interaction effect between landraces and fruit parts showed significant differences. The kernel of BJLR-6 exhibited highest (98.2%) antidiabetic activity followed by BJLR-5 (93.8%). Comparatively, seed contained higher inhibitory activity particularly because of the it’s kernel fraction. However, pulp fraction evident lower activity compared with seed and kernel part in all landraces. BJLR-6 exhibited maximum (53.8%) antidiabetic activity among the fruit pulp of all landraces.
Table 2.
α-amylase inhibitory (antidiabetic) activity from methanolic fraction of fruit parts of black jamun landraces
| Black jamun landraces | Fruit parts (% inhibition of PPA) | Mean (LRx) | |||
|---|---|---|---|---|---|
| Pulp | Seed | Seed coat | Kernel | ||
| BJLR 1 | 8.3 | 59.1 | 2.9 | 72.6 | 35.7 |
| BJLR 2 | 8.5 | 70.5 | 1.3 | 71.2 | 37.9 |
| BJLR 3 | 7.8 | 82.3 | 3.0 | 89.0 | 46.7 |
| BJLR 4 | 16.1 | 84.0 | 4.8 | 92.6 | 49.4 |
| BJLR 5 | 19.3 | 89.7 | 5.2 | 93.8 | 52.0 |
| BJLR 6 | 53.8 | 90.6 | 8.3 | 98.2 | 62.7 |
| Mean (FPx) | 18.9 | 79.4 | 4.3 | 86.2 | |
| S.Em. ± | C.D. at 5% | CV % | |||
| LR | 0.61 | 1.74 | 5.35 | ||
| FP | 0.50 | 1.42 | |||
| LR X FP | 1.22 | 3.48 | |||
Values are mean of three independent replications; PPA porcine pancreatic α- amylase
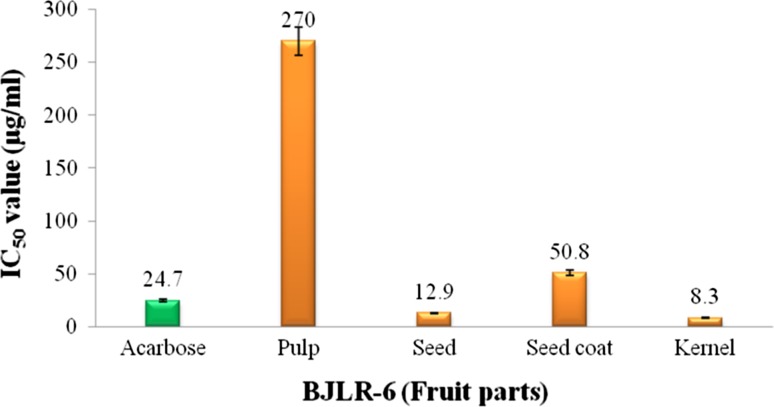
The BJLR-6 landraces was evident for potential α-amylase inhibition activity in the methanolic extract of fruit parts compared with other landraces. Hence, it was examined for IC50 values. The IC50 value indicates concentration of fruit extracts exhibiting ≥50% inhibition on PPA activity. A lower IC50 value indicates a higher α-amylase inhibitory activity. Among the fruit parts of BJLR-6, the methanolic kernel extract evident lowest (8.3 µg ml−1) IC50 value followed by seed (12.9 µg ml−1), seed coat (50.8 µg ml−1) and pulp (270 µg ml−1) (Fig. 3). The crude extracts of seed and kernel could be inhibited PPA at much lower concentrations than even standard acarbose (24.7 µg ml−1) and would, therefore, be good candidates for antidiabetic activity. The synthetic hypoglycemic agent like acarbose have limitations and are known to produce serious side effects. The look for more secure, definite, and efficient hypoglycemic agent was an important area of investigation with natural extracts from readily available traditional medicinal plants offering great potential for discovery of new antidiabetic drugs (Patwardhan et al. 2004).
Fig. 3.
IC50 values of methanol fraction of BJLR-6 fruit parts exhibiting ≥50 inhibition of porcine pancreatic α amylase enzyme activity. Bars indicate standard deviation between replications
Ponnusamy et al. (2010) studied on antidiabetic potentials of medicinal plants inhibiting in vitro human pancreatic amylase activity and found that pancreatic α-amylase lower the levels of post perandial hyperglycemia via control of starch breakdown. The probable mechanism of action of the fractions is due to their inhibitory action on HPA, thereby reducing the rate of starch hydrolysis leading to lowered glucose levels. Phytochemical analysis revealed inhibitory compounds like proteins, alkaloids, glycosides, flavonoids, tannins, saponins and steroids.
Advanced molecular studies showed that methanol extract of black jamun plant modulate the expression of glucose transporter (Glut-4), peroxisome proliferator activator receptor gamma (PPARγ) and phosphatidylinositol-3-kinase (PI3 kinase) comparable with insulin and rosiglitazone (Anandharajan et al. 2006). Evaluation of black jamun containing antidiabetic poly herbal formulation in alloxan induced diabetic rats also showed significant hypoglycemic activity, positive glucose tolerance activity and reduced lipid peroxidation in various organs compared to that of the diabetic control animals (Joshi et al. 2007). Meshram et al. (2011) studied on hypoglycaemic action of black jamun seeds and concluded that the methanol extracts of black jamun seeds may act in a variety of diabetic conditions with or without functioning of pancreatic β-cells.
Antioxidant activity from fruit part extracts of black jamun landraces
The methanolic extract of different fruit parts of six landraces showed significant variation in antioxidant activity (Table 3). Irrespective of fruit parts, the highest mean activity was examined in BJLR 6 (87.6%) followed by BJLR-5 (79.3%) and BJLR-4 (77.8). However, among the fruit parts, kernel contained higher (84.7%) antioxidant activity followed by seed (80.3%). Interaction effect between landraces and fruit parts showed significant differences. The kernel of BJLR-6 exhibited highest (94.8%) DPPH radical scavenging activity followed by BJLR-5 (85.2%) and BJLR-4 (84.6%). Seed contributed higher antioxidant activity particularly because of their kernel fraction compared with seed coat.
Table 3.
DPPH free radical scavenging (antioxidant) activity from methanolic fraction of fruit parts of black jamun landraces
| Landraces | Fruit parts (% inhibition of DPPH) | Mean (LRx) | |||
|---|---|---|---|---|---|
| Pulp | Seed | Seed coat | Kernel | ||
| BJLR 1 | 66.7 | 73.8 | 52.3 | 80.4 | 68.3 |
| BJLR 2 | 74.6 | 75.1 | 54.6 | 82.1 | 71.6 |
| BJLR 3 | 77.8 | 78.6 | 60.8 | 81.1 | 74.6 |
| BJLR 4 | 75.2 | 80.7 | 70.5 | 84.6 | 77.8 |
| BJLR 5 | 75.6 | 81.3 | 75.2 | 85.2 | 79.3 |
| BJLR 6 | 83.7 | 92.4 | 79.5 | 94.8 | 87.6 |
| Mean (FPx) | 75.6 | 80.3 | 65.5 | 84.7 | |
| S.Em.± | C.D. at 5% | CV % | |||
| LR | 0.08 | 0.24 | 1.34 | ||
| FP | 0.07 | 0.20 | |||
| LR X FP | 0.17 | 0.48 | |||
Values are mean of three independent replications
Hasan et al. (2009) studied DPPH radical scavenging activity of black jamun seed extracted in methanol. Our results evident methanolic extract of seeds of BJLR-6 was attained highest free radical scavenging activity followed by kernel and pulp.
The phenolic compounds like flavonoids, polyphenols, tannins, and phenolic terpenes presence in plant products showed antioxidant effect due to their radical scavenging activity (Rahman and Moon 2007). Similar to our results, an increased reducing power potential was observed in aqueous, ethanolic and methanolic extracts in a concentration dependent manner and the strongest reducing power potential was observed in methanolic extract by Atale et al. (2011). Liang and Yi (2009) identified hydrolysable tannins (ellagitannins) extracted from black jamun fruit showed a very good DPPH radical scavenging activity and ferric reducing/antioxidant power. The results are promising and indicating the utilization of the fruit of black jamun as a significant source of natural antioxidants. Antioxidant potency of black jamun is more evident from its capacity to scavenge free radicals and reactive oxygen species produced in consequent to ionizing radiation exposure. The radical scavenging capacity was found to be proportional to phenolic compounds present in the fruit and their skin (Banerjee et al. 2005; Luximon et al. 2003).
Prior et al. (2016) studied multi-radical (ORAC) antioxidant capacity of selected berries and effects of food processing. The antioxidant quenching potential using five different radical/oxidant sources of different berries and fruits varied widely and understanding this variation may be helpful in understanding health benefits of different berries and foods. Blueberry contains multiple antioxidants, which inhibit plasma lipid oxidation induced by diverse oxidants (Morita et al. 2017). The blueberry skin was more effective than fruit and may be utilized as antioxidant for processed foods, beverages, and synthetic compounds. The strawberry is a rich source of several nutritive and non-nutritive bioactive compounds, which are implicated in various health-promoting and disease preventive effects (Afrin et al. 2016; Forbes-Hernandez et al. 2016). Berries are a relevant source of micronutrients and nonessential phytochemicals, such as polyphenol compounds, that play a synergistic and cumulative role in human health promotion (Mazzoni et al. 2016). Edirisinghe and Burton-Freeman (2016) reviewed the anti-diabetic effects of berry polyphenolic compounds with special emphasis on the cellular and molecular mechanisms involved in the beneficial effects of berry polyphenol compounds.
The demand for processed food is increasing rapidly with increasing urbanization. Peanut (Arachis hypogaea L.) is a rich source of heart healthy nutrients such as protein, vitamin E, folic acid, soluble fiber, arginine, plant sterols, and minerals. It may be predicted that the use of peanuts in the form of peanut butter may improve the nutritional value of biscuits as it contributes high quantity and quality of proteins and also lowers the saturated fat content in biscuits. Peanut butter supplemented biscuits contained beneficial MUFA and PUFA in addition to natural antioxidants (Gajera et al. 2008, 2010). These fatty acids have been shown to lower blood cholesterol level and thereby reduced risk of coronary heart diseases.
Phenolic constituents from fruit part extracts of black jamun landraces
Total phenol from methanolic extracts of different fruit part of black jamun landraces were estimated by colorimetric Folin phenol method and HPLC profile performed to quantify individual phenolics. Total phenol content was found to be significant in different landraces of black jamun and their fruit parts (Table 4). The phenol content was found to be highest in kernel of BJLR-6 (508.58 mg g−1) followed by BJLR-5 (503.26 mg g−1). The content represented higher in the kernel compared with seed coat parts of the seed. Pulp contained significantly lower phenols in all landraces compared with seed and its parts. Among fruit pulp of the landraces, pulp of BJLR-6 evident highest (82.28 mg g−1) phenols followed by BJLR-5 (64.60 mg g−1). Research indicates that total phenol content found maximum in methanolic extract of black jamun leaves (Zhi et al. 2008; Atale et al. 2011) and are very important plant constituents because of their radical scavenging ability due to their hydroxyl groups (Banerjee et al. 2005).
Table 4.
Phenolic constituents from methanolic fraction of fruit parts of black jamun landraces
| Landraces (Fruit parts) | Total Phenol (mg g−1) | Individual phenolics (µg g−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| Gallic acid | Catechin | Chloro-genic acid | Caffeic acid | Ferulic acid | Ellagic acid | Quer cetine | ||
| BJLR-1 | ||||||||
| Pulp | 41.40 ± 2.07 | 0.87 ± 0.04 | 0.11 ± 0.01 | 0.07 ± 0.01 | nd | 0.04 ± 0.01 | 0.36 ± 0.02 | nd |
| Seed | 406.15 ± 18.25 | 14.95 ± 0.80 | 7.88 ± 0.39 | 0.89 ± 0.04 | nd | 1.50 ± 0.06 | 18.65 ± 0.94 | nd |
| Seed coat | 164.27 ± 8.20 | 3.44 ± 0.15 | 0.51 ± 0.04 | nd | nd | 0.13 ± 0.02 | 7.14 ± 0.42 | nd |
| Kernel | 401.18 ± 15.03 | 30.49 ± 1.45 | 15.98 ± 0.70 | 1.75 ± 0.09 | nd | 1.79 ± 0.08 | 36.91 ± 1.83 | nd |
| BJLR-2 | ||||||||
| Pulp | 56.30 ± 2.82 | 0.69 ± 0.04 | 0.06 ± 0.012 | 0.04 ± 0.005 | nd | 0.03 ± 0.002 | 0.24 ± 0.006 | nd |
| Seed | 361.16 ± 17.56 | 20.76 ± 1.07 | 5.36 ± 0.28 | nd | 1.16 ± 0.01 | 2.34 ± 0.05 | 23.93 ± 1.25 | nd |
| Seed coat | 160.29 ± 9.25 | 5.85 ± 0.35 | nd | nd | nd | nd | 8.20 ± 0.42 | nd |
| Kernel | 411.17 ± 20.55 | 34.00 ± 1.70 | 16.05 ± 0.80 | nd | 1.35 ± 0.22 | 2.57 ± 0.15 | 37.50 ± 1.85 | nd |
| BJLR-3 | ||||||||
| Pulp | 51.40 ± 2.57 | 0.35 ± 0.02 | 0.03 ± 0.01 | 0.05 ± 0.013 | nd | 0.02 ± 0.001 | 0.13 ± 0.002 | nd |
| Seed | 441.12 ± 21.84 | 18.64 ± 0.98 | 9.00 ± 0.45 | 6.19 ± 0.32 | nd | 2.16 ± 0.05 | 19.79 ± 0.99 | nd |
| Seed coat | 184.35 ± 9.48 | 5.25 ± 0.26 | nd | nd | nd | nd | 7.50 ± 0.39 | nd |
| Kernel | 429.15 ± 22.64 | 30.94 ± 1.55 | 8.10 ± 0.41 | 6.73 ± 0.65 | nd | 2.33 ± 0.12 | 36.00 ± 1.80 | nd |
| BJLR-4 | ||||||||
| Pulp | 59.60 ± 2.98 | 0.67 ± 0.03 | 0.12 ± 0.01 | 0.13 ± 0.01 | 0.01 ± 0.001 | 0.02 ± 0.001 | 0.21 ± 0.01 | nd |
| Seed | 438.10 ± 21.52 | 15.74 ± 0.79 | 5.79 ± 0.29 | 6.32 ± 0.28 | 1.02 ± 0.35 | 2.29 ± 0.07 | 19.90 ± 1.00 | nd |
| Seed coat | 215.27 ± 10.75 | 15.74 ± 0.84 | 3.79 ± 0.18 | nd | nd | 1.07 ± 0.05 | 8.90 ± 0.45 | nd |
| Kernel | 479.24 ± 21.80 | 21.93 ± 1.15 | 17.30 ± 0.87 | 6.95 ± 0.38 | 1.14 ± 0.55 | 3.37 ± 0.19 | 34.60 ± 1.85 | nd |
| BJLR-5 | ||||||||
| Pulp | 64.60 ± 3.23 | 0.62 ± 0.03 | 0.05 ± 0.002 | 0.03 ± 0.001 | 0.01 ± 0.001 | 0.03 ± 0.001 | 0.25 ± 0.01 | nd |
| Seed | 441.26 ± 22.10 | 20.00 ± 1.05 | 9.83 ± 0.52 | 6.18 ± 0.34 | 3.29 ± 0.16 | 5.07 ± 0.05 | 20.47 ± 1.02 | nd |
| Seed coat | 191.26 ± 10.50 | 3.86 ± 0.19 | nd | nd | nd | 0.57 ± 0.03 | 10.50 ± 0.53 | nd |
| Kernel | 503.26 ± 25.45 | 34.40 ± 1.61 | 10.95 ± 0.55 | 6.35 ± 0.34 | 3.95 ± 0.20 | 5.90 ± 0.09 | 40.54 ± 2.05 | nd |
| BJLR-6 | ||||||||
| Pulp | 82.28 ± 4.12 | 1.21 ± 0.06 | 0.45 ± 0.02 | 0.07 ± 0.003 | 0.03 ± 0.001 | 0.04 ± 0.001 | 0.22 ± 0.015 | nd |
| Seed | 496.26 ± 23.57 | 32.40 ± 1.64 | 16.80 ± 0.85 | 6.80 ± 0.49 | 4.73 ± 0.19 | 8.21 ± 0.15 | 32.70 ± 1.16 | 0.04 ± 0.001 |
| Seed coat | 241.34 ± 12.04 | 9.42 ± 0.48 | 6.74 ± 0.35 | nd | nd | 1.45 ± 0.08 | 15.30 ± 0.78 | nd |
| Kernel | 508.58 ± 25.28 | 47.90 ± 1.75 | 22.70 ± 1.14 | 7.06 ± 0.52 | 5.63 ± 0.28 | 9.27 ± 0.23 | 48.37 ± 2.48 | 0.05 ± 0.002 |
Values are mean of three independent replications; value after ± indicates standard deviation between replications; nd not detected
Individual phenolics were detected by HPLC analysis with identified corresponding retention time (RT) of standard phenolic compounds. Out of eight, total seven phenolics (gallic acid, catechin, chlorogenic acid, caffeic acid, ferulic acid, ellagic acid, quarcetin) were identified among landraces and quantified using respective standard concentration. However, kampeferol was not identified in any of the fruit parts of landraces.
Among the fruit parts, seed contained higher gallic acid in all landraces compared with kernel, seed coat and pulp. The highest gallic acid were recorded in kernel of BJLR-6 (47.90 µg g−1) followed by BJLR-5 (34.40 µg g−1). The content was examined relatively higher (1.21 µg g−1) in fruit pulp of BJLR-6 (very small fruit) compared with pulp of other landraces (Table 4). Stanely et al. (2011) examined the defensive action of gallic acid as antioxidant which diminished brain lipid peroxidation in streptozotocin induced type II diabetes mellitus. A diet containing gallic acid may be beneficial to type II diabetic patients.
The kernel contained higher catechin in all landraces compared with seed, seed coat and pulp (Table 4). The catechin was observed to be highest (22.70 µg g−1) in kernel of BJLR-6 followed by BJLR-4 (17.3 µg g−1). The content was examined relatively higher in kernel compared with seed coat fractions of seeds. Catechin was not detected in seed coat fraction of BJLR-2, 3 and 5 landraces. The fruit pulp of landraces examined with low range of catechin content (0.03 to 0.45 µg g−1) among which the content represented higher (0.45 µg g−1) in BJLR-6 landrace.
Meguro et al. (2009) investigated the effects of continuous ingestion of a catechin rich beverage in patients with type 2 diabetes. The significant increase in insulin level was observed to patients fed with green tea containing the catechin. Rizvi et al. (2005) evaluated the effect of tea catechins on markers of oxidative stress in erythrocytes from type 2 diabetics. Higher intake of catechin rich food by diabetic patients may provide some protection against the development of long term complications of diabetes.
Among the fruit parts, seed contained higher chlorogenic acid in black jamun landraces compared with pulp, seed coat and kernel except BJLR-2 where seed and its fraction could not be noticed with chlorogenic acid (Table 4). The highest chlorogenic acid were recorded in kernel of BJLR-6 (7.06 µg g−1) followed by BJLR-4 (6.95 µg g−1). The seed of all landraces contained chlorogenic acid mostly in their kernel fraction and seed coat could not be detected with the same. The content also could not be exhibited in significant level for fruit pulp of black jamun landraces. The chlorogenic acid may delay glucose absorption in the intestine through inhibition of glucose-6-phosphate translocase 1 and reduction of the sodium gradient driven apical glucose transport. In vitro studies and animal studies exhibited decrease in hepatic glucose output through inhibition of glucose-6-phospatase by chlorogenic acid derivates (Van Dam 2006).
The seed particularly in its kernel fraction contained higher caffeic acid in BJLR-5 and 6 landraces compared with other landraces (Table 4). The highest caffeic acid (5.63 µg g−1) was found with kernel of BJLR-6 followed by BJLR-5 (3.95 µg g−1). The content was not detected remarkable in pulp and seed coat of black jamun landraces.
Jung et al. (2006b) examined antioxidant activity of caffeic acid and lowering the effect of blood glucose in mice. A significant decrease in the levels of blood glucose and glycosylated hemoglobin were induced by caffeic acid compared to the control group. The noticeably elevation of mRNA expression of glucokinase, its activity and glycogen content caused by caffeic acid which simultaneously lowered glucose-6-phosphatase and phosphoenol pyruvate carboxykinase activities and their respective mRNA expressions resulted to diminish the glucose transporter 2 expression in the liver.
The ferulic acid were recorded higher (8.21 µg g−1) in kernel of BJLR-6 followed by BJLR-5 (5.07 µg g−1) and contributed largely by their kernel fraction. The content was not detected considerably in fruit pulp of black jamun landraces (Table 4). Zhi et al. (2008) investigated the antioxidant activity of black jamun leaf extracts. The antioxidant activity of leaf extracts was due to major phenlics—ferulic acid and catechin.
Diabetes, when uncontrolled, causes dyslipidemia often followed by atherogenic abnormalities. Balasubashini et al. (2003) examined role of ferulic acid (flavonoid) in diabetes induced dyslipidemia. Study demonstrates that ferulic acid lowers the lipid levels in diabetic rats and hence prevents further complications.
The type 1 and type 2 diabetic in mice was controlled by ferulic acid as it enhanced insulin secretion and lowered blood glucose level (Nomura 2001). Diabetic mice was given rice derived ferulic acid for 17 days and results showed that plasma insulin level increased while blood sugar level decreased significantly compared with control (Jung et al. 2006a). Ferulic acid may be beneficial in Type 2 diabetic and for the management of diabetic complications.
The ellagic acid examined highest (48.4 µg g−1) in kernel of BJLR-6 followed by BJLR-5 (40.5 µg g−1). The content was not found significant with fruit pulp of any landraces. Among the six landraces, only seed and kernel of BJLR-6 detected with quercetin (Table 4).
Hussain et al. (2012) indicated that quercetin can decrease postprandial glucose level after disaccharides loading, which may be mainly attributed to inhibition of α-glucosidase as one of the expected mechanisms for the reduction of plasma glucose. This effect subsequently leads to suppression of postprandial hyperglycemia. Thus, quercetin can be considered as a potential candidate for the management of type 2 diabetes mellitus. Medicines that reduce postprandial hyperglycemia by suppressing the absorption of carbohydrates are shown to be effective for prevention and treatment of non-insulin dependent diabetes mellitus (McCune and Johns 2002). Quercetin inhibited in vitro the intestinal α-glucosidase activity (Jo et al. 2009). It has been also assumed that quercetin activates tyrosine kinase. Phosphorylation of the specific region of the subunit in insulin receptor (including Tyr-1158, Tyr-1161 and Tyr-1162) correlates with receptor tyrosine kinase activation and the propagation of the biological actions of the hormone (Rosen 1987).
De Bona et al. (2016) noticed that S. cumini leaf extract affects the purinergic system and may modulate adenosine levels, indicating that the extract of this plant exhibits immunomodulatory properties. Leaf extract also may potentially prevent the cellular injury induced by oxidative stress, highlighting its cytoprotective effects. HPLC fingerprinting of leaf extract revealed the presence of catechin, epicatechin, rutin, quercitrin, isoquercitrin, quercetin, kaempferol and chlorogenic, caffeic, gallic and ellagic acids. Hossain et al. (2016) also pointed out the phenolic and flavonoid compounds are responsible for acute anti-inflammatory and antioxidant activities of S. jambos.
Correlations between antidiabetic, antiradical and phenolic compounds
Fruit size measured as fruit volume was negatively correlated (P0.001) with antidiabetic and antioxidant activities, and also negatively correlated with phenolic acids (P0.001) or (P0.01) except ellagic acid. Smaller the fruit size evidenced with higher antidiabetic and antioxidant activities with elevation of phenolic acids. The antidiabetic activity of fruit parts of black jamun landraces was positively correlated with free radical scavenging activity and individual phenolic constituents (Table 5). Total phenols and individual phenolics are positively correlated with antidiabetic and antiradical activities but vary with different level of significances. Individual phenolics—gallic, catechin, ellagic and ferulic acids are highly positively correlated (P0.001) with antidiabetic and free radical scavenging activity. The positive correlation (P0.01) was established for caffeic and chlorogenic acids to scavenge free radicals and α amylase inhibitory activity (antidiabetic) for methanolic extract of black jamun fruit parts. The quercetin was found only in seed and its part kernel fraction of BJLR-6 (very small size fruits) and found to be positively correlated (P0.05) with antidiabetic activity. Among the fruit parts of black jamun land races, seed exhibited maximum seven individual phenolics and total phenols, particularly in their kernel parts. Among the individual phenolics, gallic acid was most diverse phenolic constituents which significantly positively correlated (P0.001) with inhibition of α amylase activity and DPPH radical scavengers followed by catechin, ellagic and ferulic acids in different fruit parts of black jamun land races. Similar to present study, Muniappan et al. (2012) reviewed the black jamun as an antidiabetic plant containing ellagic acid, glycosides, anthocynine, kampferol, marcein and isoquarcetine; and halt the diastolic conversation of starch into sugar.
Table 5.
Correlation between antidiabetic, antioxidant and phenolic compounds
| Parameters | Fruit size (Vol. cm3) | Antidia-betic activity | Antioxi-dant activity | Total phenol | Gallic acid | Cate-chin | Chloro-genic acid | Caffeic acid | Ferulic acid | Ellagic acid | Quarce tine |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fruit size (Vol. cm3) | 1.000 | ||||||||||
| Antidiabetic activity | −0.984c | 1.000 | |||||||||
| Antioxidant activity | −0.801c | 0.633c | 1.000 | ||||||||
| Total phenol | −0.795c | 0.807c | 0.880c | 1.000 | |||||||
| Gallic acid | −0.673c | 0.846c | 0.792c | 0.913c | 1.000 | ||||||
| Catechin | −0.506b | 0.793c | 0.715c | 0.839c | 0.850c | 1.000 | |||||
| Chlorogenic acid | −0.568b | 0.590b | 0.501b | 0.637c | 0.518b | 0.739c | 1.000 | ||||
| Caffeic acid | −0.724c | 0.645b | 0.445 | 0.579b | 0.555b | 0.788c | 0.667c | 1.000 | |||
| Ferulic acid | −0.519b | 0.785c | 0.713c | 0.837c | 0.841c | 0.923c | 0.768c | 0.805c | 1.000 | ||
| Ellagic acid | −0.300 | 0.792c | 0.740c | 0.837c | 0.928c | 0.833c | 0.503b | 0.604b | 0.791c | 1.000 | |
| Quercetin | −0.611c | 0.396a | 0.279 | 0.389a | 0.422a | 0.351 | 0.530a | 0.408a | 0.523b | 0.343 | 1.000 |
n = 24; Critical value: a P(0.05) = 0.381; b P(0.01) = 0.487; c P(0.001) = 0.597
Conclusion
The six black jamun landraces produced different size of fruits. Fruit size was negatively correlated with antidiabetic, antioxidant activities, and phenolic acids. Small fruit evidenced with higher antidiabetic and antioxidant activities in their fruit parts (pulp, seed coat and kernel) compared to larger one. The seed part of all six land races contained higher phenolic constituents compared with pulp. The seed evidenced higher antioxidant activity particularly because of their kernel fraction. The methanolic kernel extract of BJLR-6 evidenced lowest IC50 value followed by seed, seed coat and pulp. Methanolic extracts of discarded seed and kernel of BJLR-6 (Small fruits), showing high inhibition of PPA, could be considered the better source for herbal formulations of antidiabetic drugs.
This is to certify that there is no any conflict of interest, all co-authors are agreed for submission to the journal. The research work is original and MS is prepared according to Journal format. The manuscript has not been previously published, is not currently submitted for review to any other journal, and will not be submitted elsewhere before one decision is made.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- DPPH
1,1-diphenyl-2-picrylhydrazyl
- NIDDM
Non-insulin dependent diabetes mellitus
- WHO
World health organization
- BJLR
Black jamun landraces
- HPLC
High-performance liquid chromatography
- DNSA
Di-nitro salicylic acid
- PDA
Photodiode array
- 2FCRD
Two factor complete block randomized design
- PPA
Porcine pancreatic α-amylase
- IC50
50% inhibition of enzyme activity
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2756-8) contains supplementary material, which is available to authorized users.
Contributor Information
H. P. Gajera, Email: harsukhgajera@yahoo.com
Shila N. Gevariya, Email: shila_patel88@yahoo.com
Darshna G. Hirpara, Email: darshnahirapara1@gmail.com
S. V. Patel, Email: svpatel@jau.in
B. A. Golakiya, Email: bag@jau.in
References
- Afrin S, Gasparrini M, Forbes-Hernandez TY, Reboredo-Rodriguez P, Mezzetti B, Varela-López A, Giampieri F, Battino M. Promising health benefits of the strawberry: a focus on clinical studies. J Agric Food Chem. 2016;64(22):4435–4449. doi: 10.1021/acs.jafc.6b00857. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Zimmet PZ. Definition diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Report of a WHO Consultation. Diabet Med. 1998;15:534–539. doi: 10.1002/(SICI)1096-9136(199807)15:7<535::AID-DIA670>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Ali H, Houghton P, Soumyanath A. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J Ethnopharmacol. 2006;107:449–455. doi: 10.1016/j.jep.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Anandharajan R, Jaiganesh S, Shankernarayanan N, Viswakarma R, Balakrishnan A. In vitro glucose uptake activity of Aegles marmelos and Syzygium cumini by activation of Glut-4, PI3 kinase and PPAR. Phytomedicine. 2006;13:434–441. doi: 10.1016/j.phymed.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Atale N, Jaiswal A, Chhabra A, Malhotra U, Kohli S, Mohanty S, Rani V. Phytochemical and antioxidant screening of Syzygium cumini seed extracts: a comparative study. J Pharm Res. 2011;4(12):4530–4532. [Google Scholar]
- Ayyanar M, Subash-babu P. Syzygium cumini (L.) Skeels: a review of its phytochemical constituents and traditional uses. Asian Pac J Trop Biomed. 2012;2:240–246. doi: 10.1016/S2221-1691(12)60050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubashini M, Rukkumani R, Menon V. Protective effects of ferulic acid on hyperlipidemic diabetic rats. Acta Diabetol. 2003;40:118–122. doi: 10.1007/s00592-003-0099-6. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Nabasree D, Bratati D. In vitro study of antioxidant activity of Syzygium cumini fruit. Food Chem. 2005;90:727–733. doi: 10.1016/j.foodchem.2004.04.033. [DOI] [Google Scholar]
- Benherlal P, Arumughan C. Chemical composition and in vitro antioxidant studies on Syzygium cumini fruit. J Sci Food Agric. 2007;87:2560–2569. doi: 10.1002/jsfa.2957. [DOI] [PubMed] [Google Scholar]
- De Bona KS, Bonfanti G, Bitencourt PE, da Silva TP, Borges RM, Boligon A, Pigatto A, Athayde ML, Moretto MB. Protective effect of gallic acid and Syzygium cumini extract against oxidative stress-induced cellular injury in human lymphocytes. Drug Chem Toxicol. 2016;39(3):256–263. doi: 10.3109/01480545.2015.1084631. [DOI] [PubMed] [Google Scholar]
- Edirisinghe I, Burton-Freeman B. Anti-diabetic actions of Berry polyphenols—review on proposed mechanisms of action. J Berry Res. 2016;6:237–250. doi: 10.3233/JBR-160137. [DOI] [Google Scholar]
- Eichler H, Korn A, Gasic S. The effect of a new specific α-amylase inhibitor on post-prandial glucose and insulin excursions in normal and Type 2 (non-insulindependent) diabetic patients. Diabetologia. 1984;26:278–281. doi: 10.1007/BF00283650. [DOI] [PubMed] [Google Scholar]
- Forbes-Hernandez TY, Gasparrini M, Afrin S, Bompadre S, Mezzetti B, Quiles JL, Giampieri F, Battino M. The healthy effects of strawberry polyphenols: Which strategy behind antioxidant capacity? Crit Rev Food Sci Nutr. 2016;56(1):S46–S59. doi: 10.1080/10408398.2015.1051919. [DOI] [PubMed] [Google Scholar]
- Gajera HP, Kapopara MB, Patel VH, Patel MM. Influence of peanut butter on quality characteristics of biscuits. J Food Sci Technol. 2008;45(4):373–375. [Google Scholar]
- Gajera HP, Kapopara MB, Patel VH. Application of peanut butter to improve fatty acid composition of biscuits. J Food Sci Technol. 2010;47(3):285–289. doi: 10.1007/s13197-010-0044-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover J, Vats V, Rathi S. Anti-hyperglycemic effect of Eugenia jambolana and Tinospora cordifolia in experimental diabetes and their effects on key metabolic enzymes involved in carbohydrate metabolism. J Ethnopharmacol. 2000;73:461–470. doi: 10.1016/S0378-8741(00)00319-6. [DOI] [PubMed] [Google Scholar]
- Gupta M, Sasmal S, Majumandar S, Mukherjee A. HPLC profile of standard phenolics compounds present in medicinal plant. Int J Pharmacol. 2012;4:162–167. [Google Scholar]
- Hasan R, Mokarram M, Raushanara A, Mariam J, Ehsanul H, Mazumder N, Shafiqur R. DPPH free radical scavenging activity of some Bangladeshi medicinal plants. J Med Plants Res. 2009;3:875–879. [Google Scholar]
- Hossain H, Rahman SE, Akbar PN, Khan TA, Rahman M, Jahan IR. HPLC profiling, antioxidant and in vivo anti-inflammatory activity of the ethanol extract of Syzygium jambos available in Bangladesh. BMC Res Notes. 2016;9:191–198. doi: 10.1186/s13104-016-2000-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain SA, Ahmed ZA, Mahwi TO, Aziz TA. Quercetin dampens postprandial hyperglycemia in type 2 diabetic patients challenged with carbohydrates load. Int J Diabetes Res. 2012;1:32–35. doi: 10.5923/j.diabetes.20120103.01. [DOI] [Google Scholar]
- Jayasinghe C, Gotoh N, Aoki T, Wada S. Phenolic composition and antioxidant activity of Sweet Basil. J Agric Food Chem. 2003;51:4442–4449. doi: 10.1021/jf034269o. [DOI] [PubMed] [Google Scholar]
- Jo SH, Ka EH, Lee HS. Comparison of antioxidant potential and rat intestinal α-glucosidases inihibitory activities of quercetin, rutin, and isoquercetin. Int J Appl Res Nat Prod. 2009;2:52–60. [Google Scholar]
- Joshi C, Priya E, Venkataraman S. Hypoglycemic and antilipidperoxidative effects of a polyherbal formulation, Diakyur, in experimental animal models. J Health Sci. 2007;53:734–739. doi: 10.1248/jhs.53.734. [DOI] [Google Scholar]
- Jung EH, Kim SR, Hwang IK, Ha TY. Hypoglycemic effects of a phenolic acid fraction of rice bran and ferulic acid in C57BL/KsJ-db/db mice. J Agric Food Chem. 2006;55:980–984. doi: 10.1021/jf0714463. [DOI] [PubMed] [Google Scholar]
- Jung UJ, Lee MK, Park YB, Jeon SM, Choi MS. Antihyperglycemic and antioxidant properties of caffeic acid in db/db mice. J Pharmacol Exp Ther. 2006;318:476–483. doi: 10.1124/jpet.106.105163. [DOI] [PubMed] [Google Scholar]
- Kotowaroo MI, Mahomoodally MF, Gurib-Fakim A, Subratty AH. Screening of traditional antidiabetic medicinal plants of Mauritius for possible α-amylase inhibitory effects in vitro. Phytother Res. 2006;20:228–231. doi: 10.1002/ptr.1839. [DOI] [PubMed] [Google Scholar]
- Liang LZ, Yi ML. Antioxidant tannins from Syzygium cumini fruit. Afr J Biotechnol. 2009;8:2301–2309. [Google Scholar]
- Luximon RA, Bahorun T, Crozier A. Antioxidant actions and phenolic and vitamin C contents of common Mauritian exotic fruits. J Sci Food Agric. 2003;83:496–502. doi: 10.1002/jsfa.1365. [DOI] [Google Scholar]
- Mazzoni L, Perez-Lopez P, Giampieri F, Alvarez-Suarez JM, Gasparrini M, Forbes-Hernandez TY, Quiles JL, Mezzetti B, Battino M. The genetic aspects of berries: from field to health. J Sci Food Agric. 2016;96(2):365–371. doi: 10.1002/jsfa.7216. [DOI] [PubMed] [Google Scholar]
- McCune L, Johns T. Antioxidant activity in medicinal plants associated with the symptoms of diabetes mellitus used by the indigenous peoples of the North American boreal forest. J Ethnopharmacol. 2002;82:197–205. doi: 10.1016/S0378-8741(02)00180-0. [DOI] [PubMed] [Google Scholar]
- Meguro S, Nagao T, Hase T, Otsuka K, Komikado M, Tokimitsu I, Yamamoto T, Yamamoto K. A catechin-rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity (Silver Spring) 2009;17:310–317. doi: 10.1038/oby.2008.505. [DOI] [PubMed] [Google Scholar]
- Meshram G, Yadav S, Dattatraya S, Patil B, Singh D. Antibacterial study and effect of ethanolic extracts of Syzygium cumini seeds powder on glucoamylase in vitro. J Pharm Sci Res. 2011;3:1060–1063. [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Morita M, Naito Y, Yoshikawa T, Niki E. Antioxidant capacity of blueberry extracts: peroxyl radical scavenging and inhibition of plasma lipid oxidation induced by multiple oxidants. J Berry Res. 2017;7(1):1–9. doi: 10.3233/JBR-170152. [DOI] [Google Scholar]
- Mukherjee P, Maiti K, Mukherjee K, Houghton PJ. Leads from Indian medicinal plants with hypoglycaemic potentials. J Ethnopharmacol. 2006;106:1–28. doi: 10.1016/j.jep.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Muniappan A, Pandurangan P, Subash B. Syzygium cumini (L.) Skeels: a review of its phytochemical constituents and traditional uses. Asian Pac J Trop Biomed. 2012;3:240–246. doi: 10.1016/S2221-1691(12)60050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthumani P, Ramseshu KV, Meera R, Devi P. Phytochemical investigation and anti microbial and enzyme inhibition activity of Murraya Koenigii (Linn) spreng. Int J Pharm Biol Sci Arch. 2010;1(4):345–349. [Google Scholar]
- Nomura H (2001) Acceleration of ferulic acid and related compounds on insulin secession. Research report of Wakayama industrial technology center, pp 17–19
- Patwardhan B, Vaidya D, Chorghade M. Ayurveda and natural products drug discovery. Curr Sci. 2004;86:789–799. [Google Scholar]
- Ponnusamy S, Ravindran R, Zinjarde S, Bhargava S, Kumar A. Evaluation of traditional indian antidiabetic medicinal plants for human pancreatic amylase inhibitory effect in vitro. Evid Based Complement Altern Med. 2010;4:401–407. doi: 10.1155/2011/515647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior RL, Sintara M, Chang T. Multi-radical (ORAC) antioxidant capacity of selected berries and effects of food processing MR5. J Berry Res. 2016;6:159–173. doi: 10.3233/JBR-160127. [DOI] [Google Scholar]
- Rahman MA, Moon SS. Antioxidant polyphenol glycosides from the Plant Draba nemorosa. Bull Korean Chem Soc. 2007;28:827–831. doi: 10.5012/bkcs.2007.28.5.827. [DOI] [Google Scholar]
- Rizvi SI, Zaid MA, Anis R, Mishra N. Protective role of tea catechins against oxidation-induced damage of type 2 diabetic erythrocytes. Clin Exp Pharmacol Physiol. 2005;32:70–75. doi: 10.1111/j.1440-1681.2005.04160.x. [DOI] [PubMed] [Google Scholar]
- Rosen OM. After insulin binds. Science. 1987;237:1452–1458. doi: 10.1126/science.2442814. [DOI] [PubMed] [Google Scholar]
- Roy G, Malla S, Chakravarty S. Integrated processing of jamun (Syzygium cumini Skeels) fruit for value addition and assessment of its impact on health and nutrition. Clin Diagn Lab Immunol. 2013;21:65–69. [Google Scholar]
- Snedecor GW, Cochran WG. Statistical methods. 6. Culcatta: Oxford and IBH Publishing Co.; 1967. [Google Scholar]
- Stanely M, Prince M, Kumar C, Selvakumari J. Effects of gallic acid on brain lipid peroxide and lipid metabolism in streptozotocin-induced diabetic Wistar rats. J Biochem Mol Toxicol. 2011;25:101–107. doi: 10.1002/jbt.20365. [DOI] [PubMed] [Google Scholar]
- Van Dam RM. Coffee and type 2 diabetes: from beans to beta-cells. Nutr Metab Cardiovasc Dis. 2006;16:69–77. doi: 10.1016/j.numecd.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Zhi PR, Liang LZ, Yi ML. Evaluation of the antioxidant activity of Syzygium cumini leaves. Molecules. 2008;13:2545–2556. doi: 10.3390/molecules13102545. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.