Abstract
The aim of the present study was to evaluate the effect of addition of 5% of apple peel powder (APP) and sugars (1, 5% of glucose, fructose, sucrose or trehalose) in apple purée on its phenolic composition and antioxidant activity (AOA). Apple purée samples were processed by freezing and freeze-drying. Apple purées containing APP showed higher phenolics, and AOA than the purées without APP. In freeze–dried samples the highest retention of individual polyphenols was achieved with addition of 1% of fructose or trehalose. However, in frozen samples the best impact on polyphenol retention had 5% of sucrose or trehalose. The results from this study showed the antioxidant potential of apple peel as a naturally-sourced antioxidants as well as a new insight into phenol–sugar interaction. These new findings could be of interest for the development of new food products rich in antioxidants which can improve human health.
Keywords: Apple purée, Apple peel powder, Polyphenols, Antioxidant activity, Trehalose
Introduction
To successfully address the new challenges faced by the food industry interests in the research on utilization of less invasive processing and utilization of food waste on fruit and vegetable produce have been promoted. One of the lees invasive processing is freezing. Freezing is one of the oldest and most widely used methods of food preservation, which allows preservation of taste, texture, and nutritional value at a great measure (Kozłowicz and Kluza 2006). On the other hand freeze-drying is considered as one of the best method of water removal with final products of highest quality compared to other methods of food drying. Biological and sensory qualities of the freeze-dried material mainly retained in the product (Desai and Park 2005). The other approach in improving the quality of the food product is the use of additives. Some additives that can be used, for that purpose, are simple sugars. Certain mechanisms have been put forward to explain the mechanism of the action of sugars such as: locking-in compounds, mimic the hydrogen bonding character of water, glass transformation, increasing the surface tension of the bulk solvent, preventing thermotropic phase separations in lipid bilayers, preventing the fusion of membranes water replacement and chemical stability (Komes et al. 2007).
But when it comes to food antioxidants there has been an increasing interest by the food industry and a growing trend in consumer preferences for natural antioxidants (Siripatrawan et al. 2013). Lot of studies revealed that fruit and vegetable peel have higher phenol content and antioxidant activity than fruit and vegetable flesh, and usually peel is treated as a waste. Apple skin which is generated during apple sauce, and canned apple manufacturing, has higher content of phenols and antioxidants than apple flesh. The nature and distribution of these phytochemicals between the flesh and the peel of the apple is also different. For example, while the fleshy part of the apple contains catechins, procyanidins, phloridzin, phloretin glycosides, caffeic acid and chlorogenic acid, the apple peel possesses all of these compounds and has additional flavonoids not found in the flesh, such as quercetin glycosides and cyanidin glycosides (Denis et al. 2013). Wolf et al. (2003) established that apple skin extracts inhibited the human hepatocellular liver carcinoma cell proliferation in higher extent than whole apple extract. Due to high contents of phenolics and dietary fibers, investigation of apple skin powder addition to muffins on their quality was conducted. Investigation showed that it is possible to replace wheat with apple skin powder (16% weight basis) and still receive favorable sensory properties, as well as increase of phenolics and antioxidants (Rupasinghe et al. 2009). Investigated apple skin extracts for their ability to inhibit lipid oxidation in aqueous eicosapentaenoic acid emulsions and bulk fish oil due to their antioxidant activity showed that extracts were effective in reducing the oxidation induced by heat, UV light and peroxyl radical (Rupasinghe et al. 2010). Sekhon-Loodu et al. (2013) investigated influence of fractionated apple peel phenolics on inhibition of fish oil oxidation. The authors found out that the fractionated polyphenols from dried and frozen apple peel showed higher inhibition of lipid oxidation compared to alpha-tocopherol, butylated hydroxytoluene and crude apple peel extracts. However, there has been only a limited study on this matter, such as addition of by-product to food products.
Apple purée is the product as itself but it can be used for the production of apple sauce, nectars, cloudy juices, baby food and other products. The aim of this study is to investigate the effect of freeze-dried apple peel (Gold Rush—GR and Granny Smith—GS) for polyphenol enrichment of processed (frozen and freeze-dried) apple purée (Gold Rush—GR and Granny Smith—GS), and sugars (1, 5% of glucose, fructose, sucrose or trehalose) for which is known that can enhance nutritional and sensorial properties of fruit products (Kopjar et al. 2013).
Materials and methods
Materials
Apple cultivars, Granny Smith (GS) and Gold Rush (GR), were obtained from Agricultural Institute (Osijek, Croatia). The cultivar GS was chosen because it is availabe throughout the year and cultivar GR was chosen due to its high content of polyphenols.
Folin–Ciocalteu reagent was purchased from Kemika (Zagreb, Croatia), caffeic and chlorogenic acid, phloretin, catechin, epicatechin, rutin and quercetin from Sigma Chemical Co. (St. Louis, USA), 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonate), procyanidin B2 from Fluka (St. Louis, USA) phlorizin from Aldricht (St. Louis, USA), methanol (HPLC gradient grade), and o-phosphoric acid (85%) from Panareac (Barcelona, Spain). Glucose was obtained from Kemika (Zagreb, Croatia), fructose from Merick (Damstadt, Germany), sucrose was purchased from Gram-mol (Zagreb, Croatia), and trehalose Hayashibara Co. (Japan).
Purée preparation
Before apples were disintegrated (Braun Multiquick Professional 600 Watt Turbo), for the analysis, apples were washed and the core was removed. After disintegration apple peel powder (5%) and/or sugars, 1 or 5% glucose (G), fructose (F), sucrose (S) or trehalose (T) were added. Apple purée samples used for freezing were placed in the plastic bags and frozen in a laboratory freezer. Samples were maintained at −18 °C. Before freeze-drying apple purée was frozen at −18 °C for 24 h and then freeze-dried in laboratory freeze-dryer (Christ Freeze Dryer, Aplha 1-4 LSC, Germany). Conditions for drying process were as follows: freezing temperature −55 °C; the temperature of sublimation −35–0 °C; and the vacuum level 0.220 mbar. The temperature of isothermal desorption varied from 0 °C to 22 °C under the vacuum of 0.060 mbar. Freeze-drying lasted about 48 h until the total solids content was 94–98%. Apple peel was removed by peeler (Adamo lo sbuccia, Italy) and frozen at −18 °C before freeze-drying, under the conditions mentioned above. Freeze-dried apple peel was powdered using a grinder (Končar 130 Watt) in order to obtained apple peel powder (APP).
Extraction of polyphenols
The extraction of polyphenols from prepared apple purée was carried in accordance to Loncaric et al. (2014). Polyphenols were extracted with acidified methanol (1 g apple purée in 10 mL acidified methanol). The samples were held for 1 h at the ambient temperature. After 1 h mixture was filtered through pleated filter paper. The extracts were used for the determination of total phenol content (TPC) and antioxidant activity (AOA). For HPLC identification of polyphenols extraction was performed as follows: phenolics were extracted from 250 mg freeze-dried samples using 80% aqueous methanol (5 mL). The mixture was sonicated for 15 min and centrifuged at the room temperature for 15 min. 0.45 µm poly (tetrafluoro-ethylene) syringe-tip filter was used for samples extraction.
Total phenol content
The total phenol content in the apple samples were measured by using Folin–Ciocalteu method; 0.2 mL of apple extract and 1.8 mL of deionizer water were mixed with 10 mL (1:10) of Folin–Ciocalteu reagent and 8 mL of 7.5% solution of sodium carbonate. The color was developed in 120 min, and the absorbance was read at 765 nm by spectrophotometer (Jenway 6300, Bibby Scientific, UK) (Singleton and Rossi 1965). For each sample, the measurements were performed in triplicates and the average value was interpolated on a gallic acid calibration curve and expressed as g of gallic acid equivalents per kg of sample (g GAE/kg).
Antioxidant activity
Spectrophotometric method of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) assay was used for determination of antioxidant activity. The ABTS assay followed the method of Arnao et al. (2001) with some modifications. The results were expressed using Trolox as standard in mmol TE/100 g of sample. Additional dilution was needed if the ABTS value measured was over the linear range of the standard curve. The measurements were performed in triplicates.
Identification of phenolics
The analytical HPLC system employed consisted of a Varian LC system (USA) equipped with a ProStar 230 solvent delivery module, and ProStar 330 PDA Detector. Phenolic compounds separation was done in an OmniSpher C18 column (250 × 4.6 mm inner diameter, 5 μm, Varian, USA) protected with guard column (ChromSep 1 cm × 3 mm, Varian, USA). In determination of phenolic acids and flavonols the same solvents and gradient elution program were used. Solvent A was 0.1% phosphoric acid and solvent B was 100% HPLC grade methanol. The elution conditions were as follows: 0-30 min from 5% B to 80% B; 30–33 min 80% B; 33–35 min from 80%, B to 5% B; with flow rate = 0.8 mL/min (Loncaric et al. 2014). For each sample, three replicated HPLC analyses were performed, and results were given in mg/100 g of fresh weight (FW).
Statistical analysis
All measurements were done in triplicate and data were expressed as mean ± standard deviation. The experimental data were subjected to a one-way analysis of variance (ANOVA) and Fisher’s LSD was calculated to detect significant difference (p ≤ 0.05) between the mean values. MS Excel (Microsoft Office 2007 Professional) statistical program was used for statistical analysis. Pearson’s correlation coefficient was calculated using Microsoft Excel 2007.
Results and discussion
Table 1 presents the levels of TPC in the apple purées (fresh, frozen and freeze-dried). Lower levels of TPC in frozen and freeze-dried purées were result of the chemical and enzymatic oxidation reactions which occurred during preparation and processing. AOA of frozen purées, shown in Table 1, was higher than AOA of freeze-dried purées. The higher levels of AOA can be explained by the presence of polyphenols with an intermediate oxidation state. Partially oxidized polyphenols exhibited higher radical scavenging activity than the non-oxidized ones due to their increased ability to donate a hydrogen atom from the aromatic hydroxyl group to a free radical, and/or to the capacity of their aromatic structures to support the unpaired electron through delocalization around the π-electron system (Nicoli et al. 1999). Tables 2, 3, 4 and 5 present the impact of processing and addition of sugars and APP on polyphenol groups in apple purée. Polyphenol groups were calculated from individual polyphenols which were determined by HPLC. Flavan-3-ols were calculated from (+) epicatechin and procyanidin B2, dihydrochalcones from phloretin and phloridzin, and flavonols from rutin and quercetin glycosides (RT = 24.181, 24.440, 24.997, 25.213 min). From the results presented in Table 2 it could be seen that freeze-drying of GR purée had the highest effect on loss of flavan-3-ols and flavonols 100 and 99%, respectively. In purées with sugar addition the highest impact on retention of these polyphenolic groups had the addition of 1% T. With the addition of 1% T this loss was reduced to 47% for flavan-3-ols and to 25% for flavonols. Addition of APP into GR purée greatly affects the polyphenol profile of the purée and the combination of APP with 1% T had the best results. With the addition of APP and 1% T the loss of flavan-3-ols was reduced on 17%, and the content of flavonols was 18% higher compared to fresh GR purée. This combination had also the highest impact on the levels of dihydrochalcones reducing the loss from 70% to only 3% (Table 2). Addition of APP had the lowest impact on phenolic acids which could be explained by the fact that the polyphenol profile of GR peel was mainly consisted of flavan-3-ols and flavonols (Loncaric and Pilizota 2014). Regarding frozen GR purées (Table 3), it could be seen that freezing affects the loss of flavan-3-ols and dihydrochalcones: 100, 58%, respectively. In purées with addition of 5% T had the best effect and reduced the loss of flavan-3-ols and dihydrochalcones on 81 and 44%, respectively. The content of phenolic acids was higher in fresh GR purée compared to purées with sugar addition. The content of flavonols in frozen GR purée was higher than the content in fresh GR purée. As in the freeze-dried GR purées the addition of APP had high impact on polyphenol profile in frozen GR purée. Addition of APP in combination with 1% S reduced the loss of flavan-3-ols to 43%, and increased the content of dihydrochalcones for 17% compared to fresh GR purée. Combination of APP and 5% T reduced the loss of phenolic acids upto 82% and increased the content of flavonols upto 110% compared to fresh GR purée. In the case of GS purée, freeze-drying significantly caused the loss of flavan-3-ols, dihydrochalcones and phenolic acids. Addition of sugar reduced the loss of flavan-3-ols and dihydrochalcones. Addition of 1% F reduced the loss of falavan-3-ols from 100 to 62% and dihydrochalcones from 100 to 49%. The impact of 1% F on dihydrochalcones was no different from addition of 5% F (p ≤ 0.05). Addition of 1% T reduced the loss of flavonols from 73 to 21%. Furthermore, the addition of 1% F in combination with APP was the best in reducing the loss of flavan-3-ols and dihydrochalcones, the loss was reduced to 51 and 16%, respectively. This combination also increased flavonols for 39% in comparison to fresh apple purée. In frozen GR purée addition of 5% T reduced the loss of flavan-3-ols and dihydrochalcones from 100 to 83 and 32%, respectively. Addition of 5% F increased the content of flavonols for 23% compared to fresh GS purée.
Table 1.
Total phenol content (TPC) and antioxidant activity (AOA) of apple purées
| Samples | TPC (g GAE/kg) | AOA (mmol TE/100 g) |
|---|---|---|
| Gold Rush | ||
| Fresh | 1.14 ± 0.007a | 12.78 ± 0.880a |
| Frozen | 0.61 ± 0.027b | 11.69 ± 0.246a |
| Freeze-dried | 0.64 ± 0.013b | 8.00 ± 0.348b |
| Granny Smith | ||
| Fresh | 1.29 ± 0.068a | 7.35 ± 1.068a |
| Frozen | 0.40 ± 0.019b | 6.00 ± 0.480ab |
| Freeze-dried | 0.45 ± 0.022b | 5.60 ± 0.393b |
Each value is expressed as mean ± SD (n = 3). Within the same column and samples with the same additives, means followed by different letters are significantly different at p ≤ 0.05, (ANOVA, Fisher’s LSD)
Table 2.
Content of polyphenol groups (mg /100 g edible FW)a in freeze–dried apple purée made from apple cultivar Gold Rush (GR) without and with addition of sugars and apple peel powder (APP) analyzed by HPLC
| Sample | Flavan-3-ols | Dihydrochalcones | Fenolic acids | Flavonols |
|---|---|---|---|---|
| GR purée | 3.63 ± 0.218 | 0.62 ± 0.112 | 1.20 ± 0.047 | 1.43 ± 0.073 |
| Freeze-dried | ||||
| GR purée | 0.00e | 0.19 ± 0.162b | 0.16 ± 0.156d | 0.21 ± 0.000f |
| +5% S | 1.25 ± 0.088c | 0.50 ± 0.097a | 0.31 ± 0.019abc | 0.94 ± 0.065b |
| +1% S | 1.19 ± 0.042c | 0.43 ± 0.076a | 0.39 ± 0.037a | 0.95 ± 0.109b |
| +5% G | 1.17 ± 0.061c | 0.40 ± 0.107a | 0.36 ± 0.066ab | 0.48 ± 0.049e |
| +1% G | 0.61 ± 0.143d | 0.51 ± 0.088a | 0.23 ± 0.054cd | 0.78 ± 0.068c |
| +5% F | 1.78 ± 0.083a | 0.42 ± 0.094a | 0.18 ± 0.039d | 0.66 ± 0.060d |
| +1% F | 1.42 ± 0.137b | 0.49 ± 0.043a | 0.33 ± 0.035abc | 0.94 ± 0.086b |
| +5% T | 1.82 ± 0.117a | 0.45 ± 0.041a | 0.26 ± 0.051bcd | 0.65 ± 0.050d |
| +1% T | 1.92 ± 0.082a | 0.45 ± 0.077a | 0.40 ± 0.050a | 1.07 ± 0.068a |
| Freeze-dried + APP | ||||
| GR purée | 0.51 ± 0.012f | 0.53 ± 0.029ab | 0.34 ± 0.003bc | 0.67 ± 0.100de |
| +5% S | 1.52 ± 0.107de | 0.51 ± 0.079ab | 0.39 ± 0.060b | 1.25 ± 0.100b |
| +1% S | 1.75 ± 0.157c | 0.52 ± 0.107ab | 0.39 ± 0.060b | 1.25 ± 0.101b |
| +5% G | 1.37 ± 0.103e | 0.43 ± 0.057b | 0.44 ± 0.064ab | 0.43 ± 0.089f |
| +1% G | 1.61 ± 0.078cd | 0.41 ± 0.082b | 0.25 ± 0.018c | 0.58 ± 0.109e |
| +5% F | 1.52 ± 0.081de | 0.51 ± 0.116ab | 0.25 ± 0.026c | 0.48 ± 0.068ef |
| +1% F | 2.40 ± 0.055b | 0.60 ± 0.099a | 0.49 ± 0.086a | 1.10 ± 0.068c |
| +5% T | 1.63 ± 0.150cd | 0.52 ± 0.081ab | 0.38 ± 0.070b | 0.76 ± 0.081d |
| +1% T | 3.03 ± 0.064a | 0.61 ± 0.095a | 0.43 ± 0.017ab | 1.69 ± 0.058a |
Each value is expressed as mean ± SD (n = 3). Within the same column and samples with the same additives, means followed by different letters are significantly different at p ≤ 0.05, (ANOVA, Fisher’s LSD)
S sucrose, G glucose, F fructose, T trehalose
Table 3.
Content of polyphenol groups (mg /100 g edible FW)a in frozen apple purée made from apple cultivar Gold Rush (GR) without and with addition of sugars and apple peel powder (APP) analyzed by HPLC
| Flavan-3-ols | Dihydrochalcones | Fenolic acids | Flavonols | |
|---|---|---|---|---|
| Frozen | ||||
| GR purée | n.d. | 0.26 ± 0.065c | 0.13 ± 0.011a | 1.54 ± 0.009cd |
| +5% S | n.d. | n.d. | n.d. | 1.10 ± 0.006f |
| +1% S | n.d. | n.d. | n.d. | 1.53 ± 0.007d |
| +5% G | n.d. | n.d. | n.d. | 1.06 ± 0.008g |
| +1% G | n.d. | n.d. | n.d. | 1.55 ± 0.004c |
| +5% F | n.d. | n.d. | n.d. | 1.65 ± 0.020b |
| +1% F | n.d. | n.d. | n.d. | 1.40 ± 0.009e |
| +5% T | 0.67 ± 0.002a | 0.35 ± 0.001a | 0.01 ± 0.000b | 1.96 ± 0.004a |
| +1% T | 0.35 ± 0.000b | 0.29 ± 0.000b | 0.01 ± 0.000b | 1.54 ± 0.005c |
| Frozen + APP | ||||
| GR purée | 1.49 ± 0.026d | 0.15 ± 0.001d | 0.27 ± 0.002d | 2.33 ± 0.031e |
| +5% S | 2.07 ± 0.084a | 0.73 ± 0.059a | 0.21 ± 0.077d | 3.01 ± 0.084a |
| +1% S | 1.88 ± 0.105b | 0.56 ± 0.085b | 0.66 ± 0.023ab | 2.66 ± 0.097bc |
| +5% G | 1.71 ± 0.076c | 0.54 ± 0.129bc | 0.67 ± 0.029ab | 2.79 ± 0.076b |
| +1% G | 0.79 ± 0.151f | 0.44 ± 0.018bc | 0.65 ± 0.035ab | 2.51 ± 0.072d |
| +5% F | 0.98 ± 0.104e | 0.39 ± 0.084c | 0.61 ± 0.022b | 2.62 ± 0.065cd |
| +1% F | 1.57 ± 0.092cd | 0.43 ± 0.090bc | 0.70 ± 0.033ab | 2.60 ± 0.107cd |
| +5% T | 1.68 ± 0.051c | 0.57 ± 0.079b | 0.71 ± 0.094a | 3.12 ± 0.080a |
| +1% T | 0.83 ± 0.025ef | 0.41 ± 0.126c | 0.46 ± 0.071 | 2.53 ± 0.085cd |
Each value is expressed as mean ± SD (n = 3). Within the same column and samples with the same additives, means followed by different letters are significantly different at p ≤ 0.05, (ANOVA, Fisher’s LSD)
S sucrose, G glucose, F fructose, T trehalose
Table 4.
Content of polyphenol groups (mg /100 g edible FW)a in freeze–dried apple purée made from apple cultivar Granny Smith (GS) without and with addition of sugars and apple peel powder (APP) analyzed by HPLC
| Flavan-3-ols | Dihydrochalcones | Fenolic acids | Flavonols | |
|---|---|---|---|---|
| GS purée | 3.15 ± 0.033 | 0.73 ± 0.023 | 0.38 ± 0.028 | 1.49 ± 0.066 |
| Freeze-dried | ||||
| GS purée | n.d. | n.d. | n.d. | 0.40 ± 0.101f |
| +5% S | 0.64 ± 0.059d | 0.21 ± 0.027e | n.d. | 0.50 ± 0.013f |
| +1% S | 0.91 ± 0.045bc | 0.28 ± 0.031d | n.d. | 0.75 ± 0.037de |
| +5% G | 1.05 ± 0.378ab | 0.28 ± 0.013cd | n.d. | 0.75 ± 0.107de |
| +1% G | 0.56 ± 0.078d | 0.32 ± 0.017bc | n.d. | 0.71 ± 0.033e |
| +5% F | 0.75 ± 0.025cd | 0.40 ± 0.029a | n.d. | 1.01 ± 0.099bc |
| +1% F | 1.20 ± 0.016a | 0.37 ± 0.021a | n.d. | 1.08 ± 0.191ab |
| +5% T | 1.08 ± 0.010ab | 0.31 ± 0.009bc | 0.08 ± 0.007a | 0.91 ± 0.003cd |
| +1% T | 0.76 ± 0.000cd | 0.33 ± 0.005b | 0.04 ± 0.009b | 1.18 ± 0.034a |
| Freeze-dried +APP | ||||
| GS purée | 0.40 ± 0.226g | 0.62 ± 0.027ab | n.d. | 0.66 ± 0.379e |
| +5% S | 1.02 ± 0.062c | 0.44 ± 0.077e | n.d. | 1.27 ± 0.059d |
| +1% S | 0.74 ± 0.015e | 0.50 ± 0.009cde | n.d. | 1.22 ± 0.021d |
| +5% G | 0.66 ± 0.013f | 0.48 ± 0.002de | n.d. | 1.92 ± 0.016b |
| +1% G | 0.45 ± 0.040g | 0.44 ± 0.015e | n.d. | 1.73 ± 0.021c |
| +5% F | 1.03 ± 0.034c | 0.54 ± 0.028bcd | n.d. | 1.98 ± 0.039ab |
| +1% F | 1.55 ± 0.085a | 0.61 ± 0.028a | n.d. | 2.07 ± 0.029a |
| +5% T | 1.21 ± 0.023b | 0.56 ± 0.048abc | n.d. | 1.91 ± 0.045b |
| +1% T | 0.94 ± 0.031d | 0.46 ± 0.050e | n.d. | 1.70 ± 0.053c |
Each value is expressed as mean ± SD (n = 3). Within the same column and samples with the same additives, means followed by different letters are significantly different at p ≤ 0.05, (ANOVA, Fisher’s LSD)
S sucrose, G glucose, F fructose, T trehalose
Table 5.
Content of polyphenol groups (mg/100 g edible FW)a in frozen apple purée made from apple cultivar Granny Smith (GS) without and with addition of sugars and apple peel powder (APP) analyzed by HPLC
| Flavan-3-ols | Dihydrochalcones | Fenolic acids | Flavonols | |
|---|---|---|---|---|
| Frozen | ||||
| GS purée | n.d. | n.d. | n.d. | 0.52 ± 0.123h |
| +5% S | n.d. | n.d. | n.d. | 1.22 ± 0.016d |
| +1% S | n.d. | n.d. | n.d. | 1.32 ± 0.014c |
| +5% G | n.d. | n.d. | n.d. | 1.30 ± 0.056c |
| +1% G | n.d. | n.d. | n.d. | 1.41 ± 0.008b |
| +5% F | n.d. | n.d. | n.d. | 1.83 ± 0.012a |
| +1% F | n.d. | n.d. | n.d. | 0.95 ± 0.014f |
| +5% T | 0.51 ± 0.005a | 0.50 ± 0.001a | n.d. | 0.84 ± 0.017g |
| +1% T | 0.38 ± 0.009b | 0.49 ± 0.006a | n.d. | 1.07 ± 0.012e |
| Frozen + APP | ||||
| GS purée | 0.34 ± 0.021d | 0.20 ± 0.045b | n.d. | 2.58 ± 0.131ab |
| +5% S | 0.75 ± 0.038c | 0.74 ± 0.141a | n.d. | 2.17 ± 0.083cd |
| +1% S | 0.74 ± 0.057c | 0.68 ± 0.063a | n.d. | 1.72 ± 0.134e |
| +5% G | 0.84 ± 0.119bc | 0.76 ± 0.117a | n.d. | 2.32 ± 0.065bcd |
| +1% G | 0.72 ± 0.098c | 0.72 ± 0.101a | n.d. | 2.35 ± 0.064bc |
| +5% F | 0.83 ± 0.039bc | 0.68 ± 0.115a | n.d. | 2.09 ± 0.090d |
| +1% F | 0.88 ± 0.032b | 0.78 ± 0.139a | n.d. | 2.72 ± 0.086a |
| +5% T | 0.89 ± 0.073b | 0.77 ± 0.119a | n.d. | 2.47 ± 0.074ab |
| +1% T | 1.20 ± 0.092a | 0.83 ± 0.164a | n.d. | 2.39 ± 0.072bc |
Each value is expressed as mean ± SD (n = 3). Within the same column and samples with the same additives, means followed by different letters are significantly different at p ≤ 0.05, (ANOVA, Fisher’s LSD)
S sucrose, G glucose, F fructose, T trehalose
Addition of APP in combination with 1% T had the best impact on reduction of flavan-3-ols loss. The loss was reduced from 89 to 62%. The same combination increased of dihydrochalcones level to 13% compared to fresh GS purée while combination of APP and 1% F increased flavonols level to 83% compared to control sample (fresh prepared GS purée).
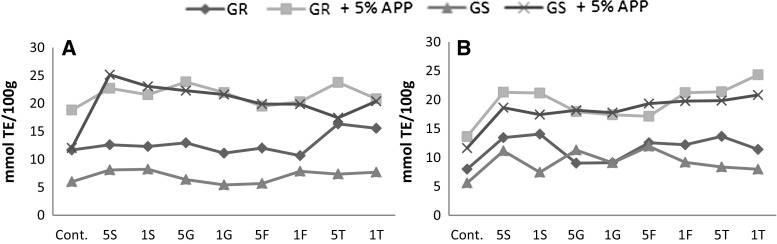
Influence of sugar and apple peel powder addition on AOA is showed in Fig. 1. In samples obtained by freezing, the highest AOA (25.15 mmol TE/100 g) had GS with the addition of apple peel powder and 5% S. Considering only sugar influence, the highest AOA (16.36 mmol TE/100 g) had GR with addition of 5% T. The highest AOA (24.32 mmol TE/100 g) in freeze-dried samples had GR with addition of apple peel powder and 1% T. Considering only sugar influence, the highest AOA (14.05 mmol TE/100 g) had GR purée with addition of 1% S. A correlation analysis was done among the sum of individual polyphenols determined by HLPC and the antioxidant activity measured with ABTS. Antioxidant activity exhibited a significant correlation (p < 0.05) with polyphenols in frozen samples GR (r = 0.964) and GS (r = 0.896). In freeze-dried samples correlation was lower but still significant for GR (r = 0.693) and GS (r = 0.773).
Fig. 1.
Antioxidant activity of frozen (a) and freeze-dried (b) Gold Rush (GR) and Granny Smith (GS) apple purées without and with addition of sugars and apple peel powder (APP). S sucrose; G glucose; F fructose; T trehalose (1 and 5%)
Conclusion
Addition of freeze-dried apple peels in apple purées increases the levels of flavan-3-ols and flavonols of apple purées. The highest impact on retention of studied polyphenols in freeze-dried samples had addition of 1% of F and T. In frozen purées the highest retention was achieved with addition of 5% for S and T, while only in frozen GS purée the highest polyphenol levels was measured in samples with 1% T. Antioxidant activity exhibited a significant correlation with polyphenols in frozen samples and freeze-dried samples. Presented results indicate that APP could be considered as an alternative polyphenol source or specialty food ingredient for food products or selected functional foods and nutraceuticals. Better use of the by-product, such as apple peel, could provide benefits to the food industry as well as solutions for environment concerns associated with the waste disposal.
Acknowledgements
This research was supported by Croatian Ministry of Science, Education and Sport, Grant 113-1130473-0340. We would like to thank Agricultural Institute (Osijek, Croatia) for providing apples required for experiments and we would like, also, to thank Hayashibara Co. (Japan) for providing us a trehalose.
References
- Arnao MB, Cano A, Acosta M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001;73:239–244. doi: 10.1016/S0308-8146(00)00324-1. [DOI] [Google Scholar]
- Denis MC, Furtos A, Dudonne S, Montoudis A, Garofalo C, Desjardins Y, Delvin E, Levy E. Apple peel polyphenols and their beneficial actions on oxidative stress and inflammation. PLoS ONE. 2013;8:e53725. doi: 10.1371/journal.pone.0053725. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Desai KGH, Park HJ. Recent developments in microencapsulation of food ingredients. Dry Technol. 2005;23:1361–1394. doi: 10.1081/DRT-200063478. [DOI] [Google Scholar]
- Komes D, Lovric T, Ganic KK. Aroma of dehydrated pear products. LWT. 2007;40:1578–1586. doi: 10.1016/j.lwt.2006.12.011. [DOI] [Google Scholar]
- Kopjar M, Hribar J, Simcic M, Zlatic E, Pozrl T, Pilizota V. Effect of trehalose addition on volatiles responsible for strawberry aroma. Nat Prod Commun. 2013;8:1767–1770. [PubMed] [Google Scholar]
- Kozłowicz K, Kluza F. Experimental characteristics of freezing of apple, pear purée with sweetening substances addition. Acta Agrophys. 2006;7:105–112. [Google Scholar]
- Loncaric A, Pilizota V. Effect of variety, growing season and storage on polyphenol profile and antioxidant activity of apple peels. Food Health Dis. 2014;3(2):96–105. [Google Scholar]
- Loncaric A, Dugalic K, Mihaljevic I, Jakobek L, Pilizota V. Effects of sugar addition on total polyphenol content and antioxidant activity of frozen and freeze-dried apple purée. J Agric Food Chem. 2014;62:1674–1682. doi: 10.1021/jf405003u. [DOI] [PubMed] [Google Scholar]
- Nicoli MC, Anese M, Parpinel M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci Technol. 1999;10:94–100. doi: 10.1016/S0924-2244(99)00023-0. [DOI] [Google Scholar]
- Rupasinghe HPV, Wang LX, Pitts NL, Astatkie T. Baking and sensory characteristics of muffins incorporated with apple skin powder. J Food Qual. 2009;32:685–694. doi: 10.1111/j.1745-4557.2009.00275.x. [DOI] [Google Scholar]
- Rupasinghe HPV, Erkan N, Yasmin A. Antioxidant protection of eicosapentaenoic acid and fish oil oxidation by polyphenolic, enriched apple skin extract. J Agric Food Chem. 2010;58:1233–1239. doi: 10.1021/jf903162k. [DOI] [PubMed] [Google Scholar]
- Sekhon-Loodu S, Warnakulasuriya SN, Rupasinghe HPV, Shahidi F. Antioxidant ability of fractionated apple peel phenolics to inhibit fish oil oxidation. Food Chem. 2013;140:189–196. doi: 10.1016/j.foodchem.2013.02.040. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Siripatrawan U, Vitchayakitti W, Sanguandeekul R. Antioxidant and antimicrobial properties of Thai propolis extracted using ethanol aqueous solution. Int J Food Sci Technol. 2013;48:22–27. doi: 10.1111/j.1365-2621.2012.03152.x. [DOI] [Google Scholar]
- Wolf K, Wu XZ, Liu RH. Antioxidant activity of apple peels. J Agri Food Chem. 2003;51:609–614. doi: 10.1021/jf020782a. [DOI] [PubMed] [Google Scholar]