Abstract
Preparation of oil-in-water nanoemulsions has emerged as a subject of interest for the encapsulation of lipophilic functional ingredients to increase their stability and activity. In this study, black cumin essential oil nanoemulsions (BCO-NE) using different ratios of essential oil with canola and flax seed oils (ripening inhibitors) were formulated and stabilized with octenyl succinic anhydride (OSA) modified waxy maize starch. The nanoemulsions exhibited monomodal size distributions with mean droplet diameter below 200 nm and zeta potential above −30, indicating a strong electrostatic repulsion between the dispersed oil droplets. Further, during storage (4 weeks at 25 °C ± 2) emulsions showed shear thinning phenomena and stability towards coalescence. Antimicrobial properties of nanoemulsions were determined by minimum inhibitory concentration and time-kill method against two Gram-positive bacterial (GPB) strains (Bacillus cereus and Listeria monocytogenes). Negatively charged BCO-NE showed prolonged bactericidal activities as compared to pure BCO due to better stability, controlled release and self-assembly with GPB cell membrane followed by destruction of cellular constituents. Our results suggest the application of BCO-NE may be exploited in aqueous food systems for extending the shelf life and other functional properties.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2800-8) contains supplementary material, which is available to authorized users.
Keywords: Black cumin essential oil, OSA modified starch, Nanoemulsions, Rheology, Antimicrobial properties
Introduction
Essential oils (EOS) extracted from plants and spices bear a number of biologically active compounds on their credit. In recent years, EOS have emerged as potential candidates for uses in agricultural, pharmaceutical and food applications. In food industry, EOS are being exploited as natural antimicrobial additives to replace the synthetic antimicrobial compounds with an ultimate aim of extending the shelf life of food products (Liang et al. 2012). Black cumin (Nigella sativa L.) is an important spice which has been remained in used for both culinary and medicinal purposes (Butt and Sultan 2010; Fawzy Ramadan 2015). Thymoquinone, p-cymene, α-thujene, longifolene, thymol, carvacrol, dithymoquinone, thymohydroquinone are the main biological active constituents of black cumin essential oil (BCO) which exhibits the antioxidant, antimicrobial, anticarcinogenic and antiulcer activities (Hassanien et al. 2015; Singh 2014; Solati and Badlishah 2015; Venkatachallam et al. 2010).
Antimicrobial constituents of EOs attack on bacterial cell membranes and alter the composition of cell contents followed by their leakage (Burt 2004). Pasqua et al. (2007) observed a significant alteration in unsaturated fatty acids of different bacterial strains treated with thymol, carvacrol, eugenol, limonene and cinnamaldehyde. Furthermore, Deepak et al. (2011) has also confirmed significant inhibitory activity of BCO against many strains of bacteria, including V. cholera, S. aureus and E. coli. Although, Most of the EOs are categorized as “generally recognized as safe” (GRAS) yet their use in different food products and beverages is limited owning to their low water solubility, high volatility, strong odor and less stability against the oxidation (Liang et al. 2012; Zinoviadou et al. 2009). Currently, encapsulation of EOs is gaining a considerable attention among the researchers as a promising strategy which may help in keeping their integral physicochemical stability and also enhance the water solubility and ultimate bioactivity (Sharif et al. 2017).
Among different encapsulations approaches, oil-in-water nanoemulsions are commercially valuable delivery systems due to the characteristics like small size, high surface area, optically clear and low rate of gravitational separation and flocculation. Microfluidization is a widely adopted efficient method to reduce emulsion droplet size (Salvia-Trujillo et al. 2015). However, droplet size of nanoemulsions containing EOs may change due to Ostwald ripening phenomena and ripening inhibitor (long-chain triacylglycerol) is added in dispersed phase to avoid this (Donsi et al. 2012; Majeed et al. 2016). Surfactants lower the interfacial tension between oil and water phase and play a pivotal role in preparation of stable nanoemulsions. Octenyl succinic anhydride (OSA) modified starches are good candidates to stabilize oil-in-water emulsions because of their good emulsifying properties, stability against environmental factors and consumer friendly label (Liang et al. 2012; Qian et al. 2011). Among OSA modified starches Purity Gum Ultra (PGU) is a newly developed succinylated waxy maize starch which provides stability at lower concentrations (1–2%) (Abbas et al. 2015; Majeed et al. 2016; Sharif et al. 2017).
Studies documenting the characterization and pharmacological potential of black cumin essential oil, black cumin seed extracts and thymoquinone (black cumin essential oil most bioactive component) loaded nanoparticles have been reported (Alam et al. 2012; Al-Haj et al. 2010; Deepak et al. 2011; Periasamy et al. 2016; Shaaban et al. 2015; Verma et al. 2013). However, still there exist scarcity of sufficient data reporting the utilization of OSA modified starch for formulation of BCO nanoemulsions and their antimicrobial activity. Hence, in this current study we hypnotized to formulate PGU stabilized nanoemulsions using different ratios of BCO (antimicrobial agent) with ripening inhibitors (canola and flax seed oils) and to observe the stability during storage. Additionally, the antimicrobial activities of nanoemulsions were also assessed for two common food borne Gram-positive pathogenic microorganisms, Bacillus cereus and Listeria monocytogenes.
Materials and methods
Materials
Black cumin essential oil (major compounds: thymoquinone, longifolene, p-cymene, β-pinene, borneol, α-pinene and α-thujene) was purchased from Jishui obedient medicinal spice oil refinery (Jiangxi, China). Canola oil (CO) and flax seed oil (FSO) were purchased from local market and TA Foods Ltd. (Yorkton, Saskatchewan, Canada), respectively. Purity gum ultra (PGU), an octenyl succinic anhydride modified waxy maize starch, was obtained from National Starch (Bridgewater, NJ, USA). The bacterial strains Listeria monocytogenes (ATCC19114) and Bacillus cereus (ATCC14579) were purchased from Haibo Biotechnology Co. Ltd, (Qingdao, China). All other chemicals used in this study were of analytical grade and purchased from Sigma-Aldrich (St. Louis, MO, USA).
Measurements of interfacial tension (IT)
The interfacial tension between oils (CO and FSO) and aqueous phase and mixture of oils (BCO + CO and BCO + FSO) at 6:4 and PGU (2%) aqueous solution was measured using a digital Data Physics® Tensiometer (Model: DCAT21, Germany) and a small Wilhelmy-plate PT 9 made of platinum-iridium (length: 10 mm, width: 9.95 mm, thickness: 0.2 mm) at 20 °C.
Preparation of nanoemulsions
The continuous phase (aqueous phase) was prepared by dispersing 2% (w/v) PGU powder into double distilled water and stirred for overnight at room temperature. Dispersed phase (oil phase) was consisted of 10% (v/v) pure CO or FSO or mixture of BCO with CO and FSO at different ratios (2:8, 4:6, 6:4 and 8:2), respectively. Coarse emulsions were prepared by homogenizing 10% (v/v) dispersed phase into 90% (v/v) continuous phase with a high speed homogenizer (Ultra-Turrax T25 IKA Janke and Kunkle, GmbH and CO KG, Germany) at 18,000 rpm for 3 min at room temperature. Finally, the coarse emulsions were passed through a microfluidizer (Model 101, Microfluidics, Newton, MA) at 100 MPa pressure with 5 processing cycles.
Measurement of emulsion mean droplet diameter (MDD), polydispersity index (PDI) and Zeta potential (ZP)
The MDD, PDI and ZP of emulsions were determined by dynamic light scattering (Zetasizer Nano ZS, Malvern Instruments, Malvern, U.K.). Emulsions were diluted 100-fold with deionized water and agitated well to avoid multiple light scattering effects. Size measurements of fresh and stored emulsions were carried out and expressed in nm, and the PDI was calculated by cumulant analysis. ZP was calculated from the electrophoretic mobility using the Smoluchowski equation and values were expressed in mV. All measurements were made in triplicate at 25 °C.
Observation of emulsions through confocal laser scanning microscopy (CLSM)
Confocal laser scanning microscopy (CLSM) images were taken to illustrate the morphology of emulsions stored for 4 weeks at 25 °C. Emulsion samples were dyed with Nile red (1 mg/ml ethanol), a lipophilic fluorescent dye excited with a 543 nm continuous-wave argon ion laser. A Zeiss LSM 710 confocal microscope (Leica, Heidelberg, Germany) with a 40 × oil immersion objective lens was used to capture the confocal images. Images were taken and processed using the instrument software program (LSM 710 ZEN version 7.2.3, Germany).
Rheological measurements
The rheological behavior of emulsions were determined by using the Discovery Hybrid Rheometer (DHR-2, TA-Instruments, New Castle, DE, USA) with a cone and plate geometry (cone diameter = 40 mm, angle = 2o, gap = 48 µm) and the temperature was maintained at 25 °C. Emulsion sample (1 mL) was loaded between cone and plate fixtures and viscosity was measured by a steady state flow program with the shear rate ranging from 0 s−1 to 500 s−1. Experimental flow curves were fitted to a power law model
| 1 |
where η = viscosity (Pa·s), K = consistency index (Pa·sn), γ = shear rate (s−1) and n = flow behavior index. There exists three value ranges for n: n < 1 for a shear-thinning fluid, n = 1 for a Newtonian fluid, and n > 1 for a shear-thickening fluid.
Evaluation of antimicrobial activity of pure BCO and BCO nanoemulsions (BCO-NE)
Determination of minimum inhibitory concentration (MIC)
Two bacterial strains (B. cereus and L. monocytogenes) were selected for the determination of MIC of BCO and BCO-NE. The agar dilution method was used and a series of dilutions (0.01, 0.02, 0.04, 0.06, 0.08, 0.1, 0.12, 0.14, 0.16, 0.18 and 0.20% v/v) of BCO and BCO-NE were prepared in tryticase soy agar yeast extract (TSAYE) as described in our previous study in our group (Majeed et al. 2016). Solidified plates were inoculated with 10 µl of bacterial containing (~104 CFU/ml) suspensions separately. Media alone and media with FSO/CO nanoemulsions without BCO were used as growth control and nanoemulsion control (NE control), respectively. The MIC was defined as the lowest concentration of samples that had no visible growth of cultures. All experiments were repeated three times.
Time-kill assay
Pure BCO and BCO-NE at their respective MIC were mixed and further tested to investigate the dynamic time-kill plots of B. cereus and L. monocytogenes (104–105 CFU/ml). An aliquot (0.1 ml) was taken from each sample after 0, 2, 4, 6, 8, 10, 12, 24, 36, 48 h and were serially diluted and spread in duplicate on TSAYE. The plates were further incubated at 37 °C for 24 h. Microbial colonies were counted and calculated by dilution times and time kill curves were constructed. All experiments were performed in triplicate.
Results and discussion
Mean droplet diameter (MDD), polydispersity index (PDI) and Zeta potential (ZP) of emulsions
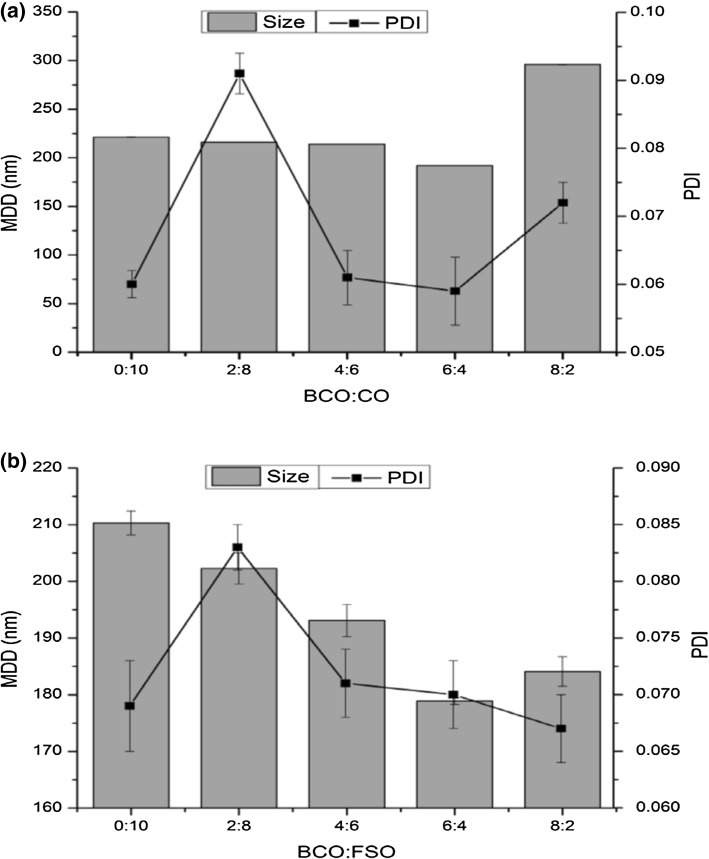
Addition of ripening inhibitor (long-chain triacylglycerol) to the dispersed phase is a common practice to avoid Ostwald ripening phenomena in essential oil emulsion. Therefore, various ratios of BCO with carrier oils (CO and FSO) were taken as organic phase (total 10% v/v) to determine the influence on MDD and PDI of emulsions stabilized by PGU (2% w/v). It has been shown in Fig. 1a that with the increasing ratio of BCO in CO from 0 to 6, there was a continuous reduction in MDD was observed. Maximum reduction was noted at 6:4 (BCO:CO) and further increase in BCO caused to rise in MDD again. This was due to the critical limit of CO to prevent from Ostwald ripening of BCO in emulsion system. We have already observed this phenomenon in our earlier study (Majeed et al. 2016). Results of BCO blending with FSO (at same ratio) also exhibited almost a similar trend for MDD (Fig. 1b). However, BCO-FSO emulsions showed slight lower MDD (178.9 nm) as compared to BCO:CO (191.8 nm) at 6:4.
Fig. 1.
Influence of different ratios of total 10% v/v oil phase a BCO with CO and b BCO with FSO on mean droplet diameter and PDI of PGU stabilized nanoemulsions
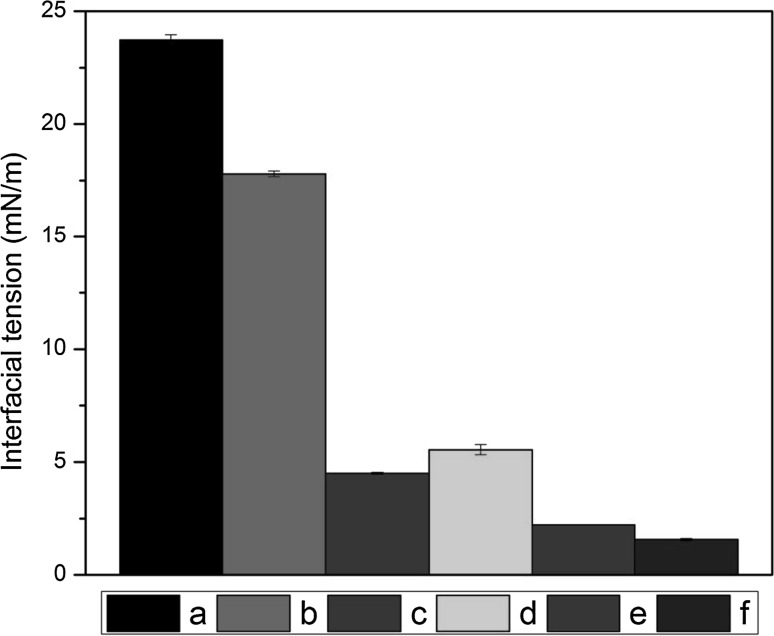
In order to understand the difference in MDD of both emulsions, interfacial tension (IFT) measurements were carried out between distilled water to carrier oils, aqueous dispersion of PGU 2% (w/w) to carrier oils and aqueous dispersion of PGU 2% (w/w) to BCO-carrier oil mixtures (6:4). It has been shown in Fig. 2 that IFT between FSO and distilled water was quite lower as compared to CO. Further, both the addition of emulsifier into aqueous phase and BCO into oil phase caused significant reduction in IFT. Lower IFT values of FSO in all experiments could be the reason for relatively less MDD values as compared to CO. Adamczak et al. (2013) also reported relatively lower IFT values for FSO as compared to soybean oil. The phenomenon of reduction in IFT values with the addition of emulsifier in aqueous phase or essential oil in organic phase has already been observed (Abbas et al. 2014; Majeed et al. 2016).
Fig. 2.
Interfacial tension (mN/m) measurements (n = 3) of (a and d) distilled water with CO and FSO, (b and e) aqueous dispersion of PGU 2% (w/w) with CO and FSO and (c and f) aqueous dispersion of PGU 2% (w/w) with BCO + CO mixture (6:4) and BCO + FSO mixture (6:4)
Poly dispersity index (PDI) is an important parameter which represents the particle size distribution of the droplets. Smaller PDI values indicate narrow particle size distribution and in our case, all emulsion formulations of BCO with both carrier oils stabilized by PGU showed PDI values lower than 0.1. Zeta potential is also an important parameter and higher ZP values (>+30 or −30) are considered to provide better stability to the dispersed particles (Heurtault et al. 2003). Starches modification through succinylation caused appearance of negative charge on surface. Our emulsions stabilized by PGU showed high negative values (> −30) of zeta potential as shown in Fig. S1 (Supplementary information), indicating strong electrostatic repulsion between the dispersed oil droplets in aqueous phase. However, a little change in ZP of both emulsions was might be due to the presence of different charge containing moieties in oil mixtures (Bonilla et al. 2012).
Storage stability of BCO-NE
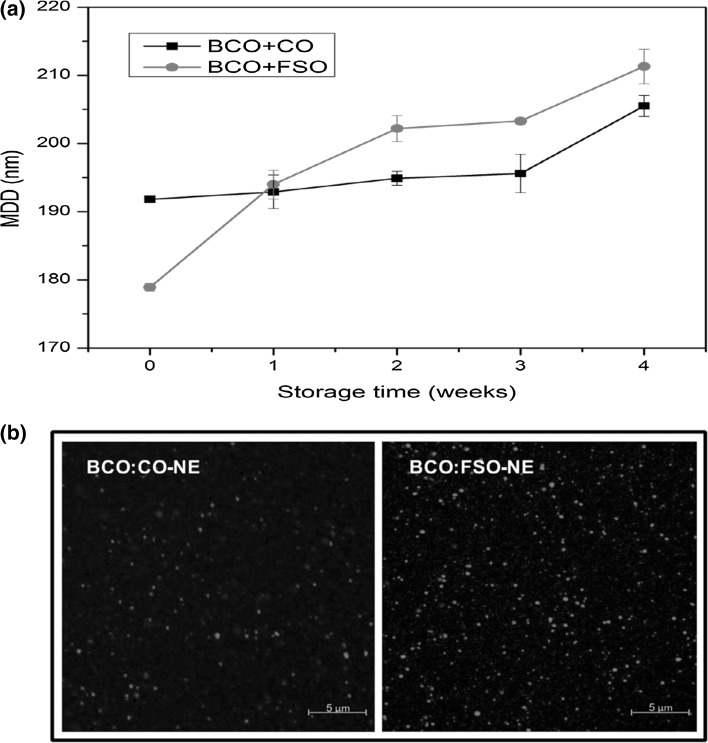
Emulsions of BCO with both carrier oils at ratio 6:4 showed smallest size and stored further for 4 weeks 25C to study the effect on MDD and morphology. Both emulsions showed a gradual increase in MDD over time (Fig. 3a). However, emulsion containing BCO:FSO (oil phase) showed more increase in MDD (~32 nm) as compared to BCO:CO (~15 nm). In earlier studies, 20–30 nm change in droplet diameter of the nanoemulsions was observed during 1 month of storage at room temperature (Abbas et al. 2015; Liang et al. 2012; Majeed et al. 2016). It was not possible to get CLSM images of fresh nanometric emulsions due to limit of microscope, However, both emulsions showed no signs of flocculation and coalescence during storage as seen in Fig. 3b.
Fig. 3.
Influence of storage (4 weeks) at 25 °C ± 2 on (a) mean droplet diameter (n = 3) and b morphology (observed through confocal laser scanning microscope) of PGU stabilized BCO + CO (6:4) and BCO + FSO (6:4) nanoemulsions
Rheological properties
Rheological behavior of emulsions, especially shear thinning (lowering of viscosity under flow during consumption) dictates their application in food and beverages. The viscosity of BCO based fresh and stored nanoemulsions under the influence of shear rate were also determined to evaluate their stability and shown in Fig. S2 (Supplementary information). Both fresh emulsions with different carrier oils showed a non-significant difference, however, during storage a reduction in viscosity was observed. In an earlier study, Liang et al. (2012) also found same trend (reduction in viscosity) which could be due to the increase in size of emulsions during storage. Rheological data given in Table 1 revealed that rheological parameters fitted with a power law model and the square of the correlation index (R 2) was >0.95, directing that the model is suitable for the emulsions studied in this work. The flow behavior index (n) also indicating that all emulsions (fresh and stored) showed shear-thinning behavior (n < 1.0), which varied as a function of oil composition and storage time (Table 1). Overall, our emulsion’s rheological parameters indicate their suitability to incorporate into dairy and beverages.
Table 1.
Rheological parameters obtained from fitting using power law model for BCO-NE (fresh and stored for 4 weeks) stabilized by PGU
| Sample | K (Pa.sn) | N | R2 |
|---|---|---|---|
| BCO + FSO-NE | 0.0853 | 0.658 | 0.96 |
| BCO + CO-NE | 0.0961 | 0.674 | 0.96 |
| BCO + FSO-NE stored | 0.0644 | 0.589 | 0.95 |
| BCO + CO-NE stored | 0.0639 | 0.504 | 0.95 |
Antimicrobial activity of pure BCO and BCO-NE
Pure BCO as well as BCO-NE showed overall higher MIC values for B. cereus over L. monocytogenes as shown in Table 2. However, MIC of BCO:FSO-NE was found slightly lower as compared to pure BCO and BCO:CO-NE for B. cereus. In comparison to B. cereus, MIC of BCO:CO-NE and BCO:FSO-NE were found lower as compared to pure BCO for L. monocytogenes. Our determined MIC values of pure BCO are in between the earlier findings (Harzallah et al. 2011; Piras et al. 2013; Singh et al. 2005). Whereas, Manju et al. (2016) and Shaaban et al. (2015) reported lower MIC values for encapsulated BCO systems than our BCO-NE. In earlier studies Liang et al. (2012) and Majeed et al. (2016) noted slightly lower or equal MIC values for pure antimicrobial component and their encapsulated form.
Table 2.
Minimum inhibitory concentrations (MIC) of BCO and BCO-NE for Bacillus cereus and Listeria monocytogenes (n = 3)
| Bacterial strain | Sample | MIC (% v/v) |
|---|---|---|
| B. cereus | Pure BCO BCO:CO-NE BCO:FSO-NE |
0.08 0.08 0.06 |
| L. monocytogenes | Pure BCO BCO:CO-NE BCO:FSO-NE |
0.12 0.1 0.1 |
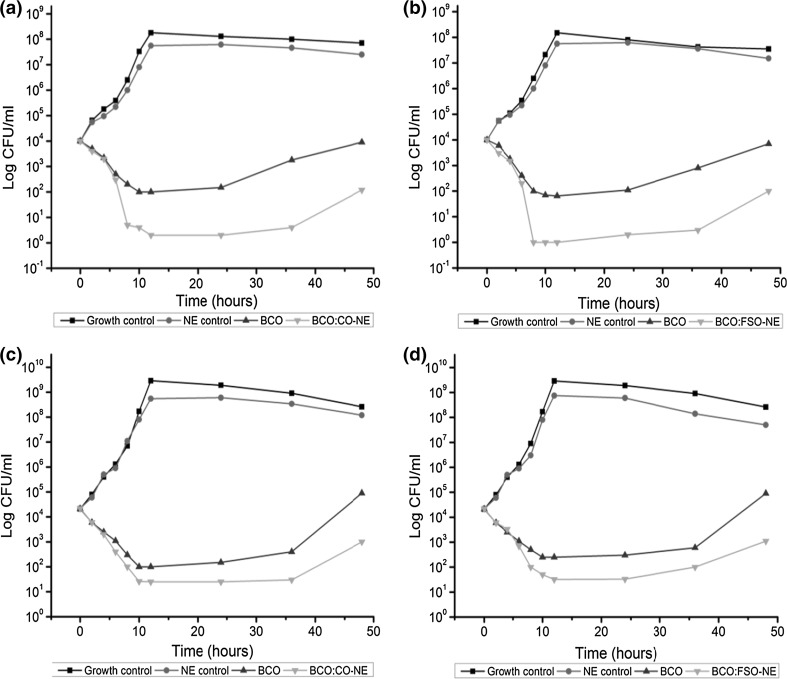
A time-kill dynamic experiment was conducted by using the same concentrations of BCO in either pure or emulsified form as their respective MIC values against B. cereus and L. monocytogenes. Both bacterial strains showed almost the similar trend of high growth over time in growth control (media alone) and NE control (media with FOS/CO nanoemulsion without BCO) as shown in Fig. 4. Further, with the addition of BCO either in pure or emulsified form caused a strong inhibition of bacterial growth. However, growth inhibition was stronger in BCO-NE as compared to pure BCO for both bacterial strains. Pure BCO caused more initial inhibition of B. cereus relative to L. monocytogenes and after 10 h both bacterial strains again started to increase in number, which went up to around 104 and 105 CFU/ml, respectively after 48 h. Similar to pure BCO, BCO-NE also showed stronger inhibition activity against B. cereus as compared to L. monocytogenes for first 10 h and later no significant increase was noted for up to 36 h. Finally, at 48 h again increase in bacterial growth was noted around 102 to 103 CFU/ml for B. cereus and L. monocytogenes, respectively. However, our BCO-NE was found stronger against L. monocytogenes as compared to peppermint oil and peppermint oil nanoemulsions (Liang et al. 2012). Long term inhibition of bacterial growth provided by the encapsulated antimicrobial compound relative to its free form could be due to the better stability and control release, which further extend its activity (Liang et al. 2012).
Fig. 4.
Time kill plots for BCO + CO-NE against (a and c) Bacillus cereus and Listeria monocytogenes and BCO + FSO-NE against (b and d) Bacillus cereus and Listeria monocytogenes (n = 3). Both nanoemulsions contained total 10% v/v oil mixture of BCO + CO (6:4) and BCO + FSO (6:4)
The difference in bacterial growth inhibition by the both nanoemulsions (BCO:CO-NE and BCO:FSO-NE) was non-significant, indicating no effect of carrier oil and L. monocytogenes was found less sensitive to both BCO and BCO-NE as compared to B. cereus. In an earlier study, Shaaban et al. (2015) found ineffectiveness of BCO microemulsions stabilized by Tween–20 (non-ionic surfactant) against L. monocytogenes. Contrarily, our BCO-nanoemulsions stabilized by PGU (anionic surfactant) showed a significant inhibition against L. monocytogenes, indicating the better suitability of PGU as emulsifier to stabilize essential oil-in-water nanoemulsions for their bactericidal action. In our previous study (Majeed et al. 2016) it has been observed that the chemical interaction between PGU stabilized antimicrobial nanoemulsions and Gram-positive bacterial cell membrane caused disruption of cellular and cytoplasmic membranes followed by release of cellular constituents.
Conclusion
Black cumin essential oil nanoemulsions formulated by mixing with CO and FSO showed stability against phase separation and coalescence during 4 weeks storage at room temperature. Antimicrobial experiments showed that L. monocytogenes was less sensitive to both BCO and BCO-NE as compared to B. cereus. However, PGU stabilized BCO-NE showed higher bactericidal activity against two Gram positive pathogenic bacterial strains as compared to pure BCO. The better work of BCO in negatively charged NE droplets form was credited to better stability, controlled release and self-assembly with GPB cell membrane followed by destruction of cellular constituents. Overall, the rheological and antimicrobial properties of BCO-NE suggested their applications in dairy and beverages to formulate new delivery systems with extended shelf life.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors greatly appreciate the support of state key laboratory of food science and technology, school of food science and technology, jiangnan university, China.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2800-8) contains supplementary material, which is available to authorized users.
References
- Abbas S, Mohanad B, Waseem A, Wei WL, Xiaoming Z. Process optimization of ultrasound-assisted curcumin nanoemulsions stabilized by OSA-modified starch. Ultrason Sonochem. 2014;21:1265–1274. doi: 10.1016/j.ultsonch.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Abbas S, Eric K, Mohanad B, Khizar H, Xiao H, Hafiz RS, Zhang X. Fabrication of polymeric nanocapsules from curcumin-loaded nanoemulsion templates by self-assembly. Ultrason Sonochem. 2015;23:81–92. doi: 10.1016/j.ultsonch.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Adamczak M, Para G, Simon C, Warszyński P. Natural oil nanoemulsions as cores for layer-by-layer encapsulation. J Microencapsul. 2013;30(5):479–489. doi: 10.3109/02652048.2012.752536. [DOI] [PubMed] [Google Scholar]
- Alam S, Zeenat IK, Gulam M, Manish K, Fakhrul I, Aseem B, Farhan JA. Development and evaluation of thymoquinone-encapsulated chitosan nanoparticles for nose-to-brain targeting: a pharmacoscintigraphic study. Int J Nanomed. 2012;7:5705–5718. doi: 10.2147/IJN.S35329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Haj NA, Mariana NS, Norfarrah MA, Hana FZ, Ahmad B, Siddig I, Rasedee A. Characterization of Nigella sativa L. essential oil-loaded solid lipid nanoparticles. Am J Pharmacol and Toxicol. 2010;5(1):52–57. doi: 10.3844/ajptsp.2010.52.57. [DOI] [Google Scholar]
- Bonilla J, Atares L, Vargas M, Chiralt A. Effect of essential oils and homogenization conditions on properties of chitosan-based films. Food Hydrocolloid. 2012;26(1):9–16. doi: 10.1016/j.foodhyd.2011.03.015. [DOI] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods–a review. Int J Food Microbiol. 2004;94(3):223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Butt MS, Sultan MT. Nigella sativa: reduces the risk of various maladies. Crit Rev Food Sci. 2010;50:654–665. doi: 10.1080/10408390902768797. [DOI] [PubMed] [Google Scholar]
- Deepak Suruchi S, Mohd S, Veena G, Mohd S. Entrapment of seed extract of Nigella sativa into thermosensitive (NIPAAm–Co–VP) co-polymeric micelles and its antibacterial activity. Int J Pharm Sci Drug Res. 2011;3(3):246–252. [Google Scholar]
- Donsi F, Annunziata M, Vincensi M, Ferrari G. Design of nanoemulsion based delivery systems of natural antimicrobials: effect of the emulsifier. J Biotechnol. 2012;159:324–350. doi: 10.1016/j.jbiotec.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Fawzy Ramadan M. Nutritional value and applications of Nigella sativa essential oil: a mini review. J Essent Oil Res. 2015;27:271–275. doi: 10.1080/10412905.2015.1045564. [DOI] [Google Scholar]
- Harzallah HJ, Bochra K, Guido F, Amina B, Touhami M. Chemical composition, antimicrobial potential against cariogenic bacteria and cytotoxic activity of Tunisian Nigella sativa essential oil and thymoquinone. Food Chem. 2011;129:1469–1474. doi: 10.1016/j.foodchem.2011.05.117. [DOI] [Google Scholar]
- Hassanien MFR, Adel MAA, Ahmed MA, Hesham FO. Health-promoting value and food applications of black cumin essential oil: an overview. J Food Sci Technol. 2015;52(10):6136–6142. doi: 10.1007/s13197-015-1785-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurtault B, Saulnier P, Pech B, Proust J-E, Benoit J-P. Physico-chemical stability of colloidal lipid particles. Biomaterials. 2003;24(23):4283–4300. doi: 10.1016/S0142-9612(03)00331-4. [DOI] [PubMed] [Google Scholar]
- Liang R, Xu S, Shoemaker CF, Li Y, Zhong F, Huang Q. Physical and antimicrobial properties of peppermint oil nanoemulsions. J Agric Food Chem. 2012;60(30):7548–7555. doi: 10.1021/jf301129k. [DOI] [PubMed] [Google Scholar]
- Majeed H, Fei L, Joseph H, Hafiz RS, Jing Q, Barkat A, Yuan-Yuan B, Jianguo M, Wallace Y, Fang Z. Bactericidal action mechanism of negatively charged food grade clove oil nanoemulsions. Food Chem. 2016;197:75–83. doi: 10.1016/j.foodchem.2015.10.015. [DOI] [PubMed] [Google Scholar]
- Manju S, Balasubramanian M, Sekar V, Sathappan S, Ameeramja J, Perumal E, Baskaralingam V. Antibacterial, antibiofilm and cytotoxic effects of Nigella sativa essential oil coated gold nanoparticles. Microb Pathogenesis. 2016;91:129–135. doi: 10.1016/j.micpath.2015.11.021. [DOI] [PubMed] [Google Scholar]
- Pasqua RD, Betts G, Hoskins N, Edwards M, Ercolini D, Mauriello G. Membrane toxicity of antimicrobial compounds from essential oils. J Agric Food Chem. 2007;55:4863–4870. doi: 10.1021/jf0636465. [DOI] [PubMed] [Google Scholar]
- Periasamy VS, Jegan A, Ali AA. Anticancer activity of an ultrasonic nanoemulsion formulation of Nigella sativa L. essential oil on human breast cancer cells. Ultrason Sonochem. 2016;31:449–455. doi: 10.1016/j.ultsonch.2016.01.035. [DOI] [PubMed] [Google Scholar]
- Piras A, Rosa A, Marongiu B, Porcedda S, Falconieri D, Dessì MA, Ozcelik B, Koca U. Chemical composition and in vitro bioactivity of the volatile and fixed oils of Nigella sativa L. extracted by supercritical carbon dioxide. Ind Crop Prod. 2013;46:317–323. doi: 10.1016/j.indcrop.2013.02.013. [DOI] [Google Scholar]
- Qian C, Decker EA, Xiao H, McClements DJ. Comparison of biopolymer emulsifier performance in formation and stabilization of orange oil in water emulsions. J Am Oil Chem Soc. 2011;88:47–55. doi: 10.1007/s11746-010-1658-y. [DOI] [Google Scholar]
- Salvia-Trujillo L, Alejandra R-G, Robert S-F, Olga M-B. Physicochemical characterization and antimicrobial activity of food-grade emulsions and nanoemulsions incorporating essential oils. Food Hydrocolloid. 2015;43:547–556. doi: 10.1016/j.foodhyd.2014.07.012. [DOI] [Google Scholar]
- Shaaban HA, Zainab S, Emr EE, Amal S-H. Analysis and antimicrobial activity of Nigella sativa essential oil formulated in microemulsion system. J Oleo Sci. 2015;64(2):223–232. doi: 10.5650/jos.ess14177. [DOI] [PubMed] [Google Scholar]
- Sharif HR, Williams PA, Sharif MK, Khan MA, Majeed H, Safdar W, Shamoon M, Shoaib M, Haider J, Zhong F. Influence of OSA-starch on the physico chemical characteristics of flax seed oil-eugenol nanoemulsions. Food Hydrocolloid. 2017;66:365–377. doi: 10.1016/j.foodhyd.2016.12.002. [DOI] [Google Scholar]
- Singh S. Composition, in vitro antioxidant and antimicrobial activities of essential oil and oleoresins obtained from black cumin seeds (Nigella sativa L.) Biomed Res. 2014 doi: 10.1155/2014/918209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G, Palanisamy M, Sde-H Carola, Cesar C. Chemical constituents and antimicrobial and antioxidant potentials of essential oil and acetone extract of Nigella sativa seeds. J Sci Food Agr. 2005;85:2297–2306. doi: 10.1002/jsfa.2255. [DOI] [Google Scholar]
- Solati Z, Badlishah SB. Antioxidant effect of supercritical CO2 extracted Nigella sativa L. seed extract on deep fried oil quality parameters. J Food Sci Technol. 2015;52(6):3475–3484. doi: 10.1007/s13197-014-1409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachallam SKT, Hajimalang P, Soundar D, Udaya SK. Chemical composition of Nigella sativa L. seed extracts obtained by supercritical carbon dioxide. J Food Sci Technol. 2010;47(6):598–605. doi: 10.1007/s13197-010-0109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma SK, Shweta R, Kalim J, Mohd A, Indu A, Mohd S. Nanothymoquinone, a novel hepatotargeted delivery system for treating CCl4 mediated hepatotoxicity in rats. J Mater Chem B. 2013;1:2956–2966. doi: 10.1039/c3tb20379d. [DOI] [PubMed] [Google Scholar]
- Zinoviadou KG, Konstantinos PK, Costas GB. Physico-chemical properties of whey protein isolate films containing oregano oil and their antimicrobial action against spoilage flora of fresh beef. Meat Sci. 2009;82:338–345. doi: 10.1016/j.meatsci.2009.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.