Abstract
Background
Substantial data indicate greater muscle fatigue in individuals with spinal cord injury (SCI) compared with healthy control subjects when tested by using electrical stimulation protocols. Few studies have investigated the extent of volitional fatigue in motor incomplete SCI.
Methods
Repeated, maximal volitional effort (MVE) isometric contractions of the knee extensors (KE) were performed in 14 subjects with a motor incomplete SCI and in 10 intact subjects. Subjects performed 20 repeated, intermittent MVEs (5 seconds contraction/5 seconds rest) with KE torques and thigh electromyographic (EMG) activity recorded.
Results
Peak KE torques declined to 64% of baseline MVEs with repeated efforts in control subjects. Conversely, subjects with SCI increased peak torques during the first 5 contractions by 15%, with little evidence of fatigue after 20 repeated efforts. Increases in peak KE torques and the rate of torque increase during the first 5 contractions were attributed primarily to increases in quadriceps EMG activity, but not to decreased knee flexor co-activation. The observed initial increases in peak torque were dependent on the subject’s volitional activation and were consistent on the same or different days, indicating little contribution of learning or accommodation to the testing conditions. Sustained MVEs did not elicit substantial increases in peak KE torques as compared to repeated intermittent efforts.
Conclusions
These data revealed a marked divergence from expected results of increased fatigability in subjects with SCI, and may be a result of complex interactions between mechanisms underlying spastic motor activity and changes in intrinsic motoneuron properties.
Keywords: Fatigue, Spasticity, Upper motor neuron
Human spinal cord injury (SCI) is associated with the loss of volitional motor control and manifestation of spastic motor behaviors that lead to alterations in neuromuscular integrity. Previous evidence has demonstrated substantial muscle atrophy,1 fiber type conversion,2 decreased oxidative capacity,3 and alterations in transmembrane ionic exchange4 and muscle blood flow5 in muscles below the lesion level (ie, upper motoneuron lesions). These changes often result in substantial neuromuscular fatigue, defined as a decline in maximal force-generating capability with repeated or sustained contractions.6 Assessment of fatigue in chronically paralyzed muscle is typically performed using surface neuromuscular electrical stimulation (NMES), which bypasses injured spinal pathways in the absence of volitional control. Previous studies using this technique have demonstrated greater fatigability of paralyzed muscle as compared to unimpaired subjects,7 with rapidly decreasing fatigue resistance in the year following initial SCI.8
Despite extensive evidence of decreased fatigue resistance in human complete SCI during NMES, surprisingly little research has been performed to quantify mechanisms of altered force production and fatigue during volitional, repeated contractions in individuals with partial preservation of descending pathways after SCI.9,10 Considering the increasing prevalence of motor incomplete SCI,11 the ability to repeatedly generate torques over a sustained duration, as occurs during various tasks (eg, locomotion), is critical for functional independence.
The purpose of the present study was to investigate alterations in peak torque and electromyographic (EMG) activity in individuals with motor incomplete SCI during repeated maximal volitional effort (MVE) contractions of the knee extensors (KE). Toward this aim, we used an experimental paradigm to elicit volitional fatigue, with subjects performing repeated, intermittent, isometric MVEs.12 Consistent with substantial published data from individuals with complete SCI,13,14 the primary expectation was that subjects with incomplete SCI would demonstrate greater fatigue as compared to neurologically intact individuals.
In preliminary testing, control subjects decreased their peak torques substantially with repeated MVEs, while the opposite behavior was observed in subjects with incomplete SCI. Specifically, subjects with incomplete SCI demonstrated an increase in the magnitude and rate of rise of KE torque during repeated, intermittent MVEs, particularly during the first few attempts. After 20 repeated MVEs, there was no significant decline in peak torques with some subjects able to generate greater KE torques at the 20th versus 1st MVE. Such data contrast with the extensive literature suggesting greater fatigue in human SCI.2 In the present study, we characterize the changes in the magnitude and rate of rise of KE torque and quadriceps EMG activity during repeated MVEs. We further demonstrate the repeatability of the observed behaviors both within and between experimental sessions, and differences between sustained versus intermittent MVEs. In general, the data presented provide evidence for consistent facilitation of volitional force with repeated maximal efforts, with little evidence for volitional fatigue in individuals with incomplete SCI.
Methods
Subjects
Subjects with SCI were recruited from the outpatient clinics of the Rehabilitation Institute of Chicago (RIC). Experiments were performed on 14 subjects (12 males) with chronic (> 1 year) motor incomplete lesions above the T10 neurological level (see Table 1). All SCI subjects were classified as either C or D using the American Spinal Injury Association (ASIA) Impairment Scale and demonstrated residual volitional KE strength in at least 1 limb. Exclusion criteria included a medical history of multiple CNS lesions, lower limb peripheral nerve injury, or an orthopedic injury that may limit maximal effort KE contractions as determined through examination of medical records. Tendon-tap reflex responses of knee extensors of all SCI subjects were judged hyper-excitable or within normal range during clinical examination. None of the subjects were using anti-spasticity medication at the time of the study, and all had previous experience using the testing apparatus during single but not repeated MVE contractions of the KE extensors. All subjects were further aware of the study protocol to assess volitional fatigue, but were unaware of the preliminary data or hypothesis. An additional 10 subjects (8 males) of similar age and without neurological or orthopedic injury also participated. All procedures were approved by the Institutional Review Board of Northwestern University.
Table 1.
Subject Demographics
| Subject No. | Age (years) | Duration Post-SCI (months) | Neurological Level | ASIA Classification | LEMS |
|---|---|---|---|---|---|
| 1 | 48 | 282 | C5 | D | 39 |
| 2 | 39 | 46 | C5 | C | 15 |
| 3 | 43 | 58 | C4 | D | 39 |
| 4 | 37 | 38 | C4 | D | 50 |
| 5 | 30 | 48 | T6 | D | 42 |
| 6 | 30 | 105 | C6 | D | 44 |
| 7 | 59 | 44 | T8 | D | 49 |
| 8 | 52 | 50 | T8 | D | 50 |
| 9 | 44 | 88 | C6 | C | 29 |
| 10 | 36 | 115 | C5 | D | 31 |
| 11 | 49 | 74 | C5 | D | 36 |
| 12 | 44 | 133 | C5 | D | 47 |
| 13 | 32 | 80 | C5 | C | 27 |
| 14 | 58 | 396 | T7 | C | 21 |
Abbreviations: SCI, spinal cord injury; ASIA, American Spinal Injury Association; LEMS, lower extremity motor scores.
Experimental Design
Experiments lasted approximately 1 to 1.5 hours. Subjects were seated in the adjustable height chair of the testing apparatus (System 3; Biodex Medical Systems, Shirley, NY) with the hips flexed comfortably to 80 to 90° and the knee positioned at 90°. The distal shank was secured to the dynamometer arm, which was coupled to a 6 degree of freedom load cell (ATI, Apex, NC) used to calculate joint torques. Torque signals were low-pass filtered at 200 Hz and collected at 1000 Hz. Surface EMG was recorded using active bipolar electrodes (Delsys, Boston, MA) applied to the skin over the vastus lateralis (VL), vastus medialis (VM), rectus femoris (RF), and medial hamstring (MH). Signals were amplified (×1000), filtered at 20 to 450 Hz, and sampled at 1000 Hz simultaneously with the torque data.
Experimental Protocol
Torque and EMG data were collected on all subjects with SCI on the more impaired limb as determined during clinical evaluation using the single limb lower extremity motor score (LEMS),15 and later confirmed by differences in central activation ratio (CAR) values between limbs. In 7 subjects, differences in lower extremity strength between limbs were detected during clinical testing, and data were collected on both limbs on separate days, yielding 21 experimental trials. All subjects began each session by performing 3 baseline MVEs, each lasting 3 to 8 seconds, with > 1 minute between attempts. Subjects were instructed to extend the knee as hard and as fast as possible, with vigorous verbal encouragement. No visual feedback was provided to subjects during baseline or repeated MVEs. During each of the baseline MVEs, a supramaximal train of electrical stimulation16 (10 pulses, 600 μs duration, 100 Hz, 135 V; S48 Grass, West Warwick, RI) was delivered to the quadriceps through 3″ × 5″ self-adhesive, stimulating electrodes (ConMed Corp, Utica, NY) placed over the distal VM and the proximal VL. The stimulation was triggered manually by the experimenters when the KE torque peaked and appeared visually to reach a plateau. The electrically elicited torque was used to estimate voluntary KE activation.
Following baseline contractions, subjects performed 20 consecutive MVEs intended to elicit quadriceps fatigue. Each MVE was maintained for 5 seconds with a 5-second recovery period between attempts,12 with verbal encouragement provided during each MVE. Electrical stimulation was superimposed on the 5, 10, 15, and 20th MVE. Subjects rested for 10 minutes after 20 maximal efforts, followed by a single MVE.
The repeatability of the observed phenomena both between sessions and within the same session was tested on a subset of the subjects, using 2 separate protocols performed on separate days. To assess consistency between sessions, 7 individuals who performed the initial experiment returned to the laboratory within 5 days and were retested using the identical protocol (20 repeated MVEs) with the same limb. In a separate set of experiments performed on a different day, we tested the within-session repeatability of the torque augmentation during the first 5 repeated MVEs. After baseline efforts, 7 subjects performed 3 trials of 5 repeated MVEs (5 seconds MVE/5 seconds rest), with > 5 minutes between trials.
In a separate set of experiments, we determined whether sustained MVEs would result in similar increases in KE torques as observed during repeated MVEs. After baseline testing as described above, 7 subjects with SCI (1 subject was tested bilaterally on separate days) and 6 control subjects performed a sustained MVE for ~30 seconds during simultaneous recording of KE torque and quadriceps and MH EMGs. Verbal encouragement was provided throughout the sustained contraction, with no visual feedback and no electrical stimulation applied to the contraction.
Data Collection and Analysis
Data were acquired and analyzed using custom LabView software (National Instruments Austin, TX). Electromyographic signals were first filtered with a zero-phase lag, 4th order band-stop Butterworth filter at 58 to 62 Hz to remove any 60 Hz noise. EMG signals were then rectified and smoothed using a 4th order, 10 Hz low-pass Butterworth filter to create a linear envelope for further analysis. Torque signals were low-pass filtered at 10 Hz using a 4th order Butterworth filter with zero phase delay. Peak torque was identified for each contraction and the period corresponding to ±50 ms was then averaged to represent peak torque. The largest torque elicited during the 3 baseline MVEs was used to normalize the subject’s KE torques during the repeated contractions. EMG activity during the repeated contractions was also normalized to the mean EMG activity present 0 to 100 ms before the peak torque found during baseline efforts. Pooled extensor EMG activity was calculated as the average of the normalized (% increase) VL, VM, and RF activity. MH EMG activity was analyzed to assess alterations in antagonist activity during repeated MVEs. Voluntary activation was quantified as the central activation ratio (CAR = Tvoluntary/Telectrical), where Tvoluntary refers to the voluntary torque produced 100 ms before the electrical stimulation and Telectrical refers to the peak electrically elicited KE torque.17 CAR was determined during baseline and 5th, 10th, 15th and 20th repeated MVEs, with values < 1.00 thought to estimate the extent of activation failure (see, however, works by Kendall et al, Kooistra et al, and Herbert and Gandevia18–20).
Changes in the rate of torque development were calculated as the duration between 20% and 80% of the MVEs.21 Due to the nature of the fatiguing protocol, T20–80 was calculated only for the baseline efforts and the first 3 repeated MVEs (ie, torque declined < 80% in control subjects). Similar estimates of the rate of EMG increase were made for pooled, smoothed quadriceps activity (EMG20–80) with 100% pooled EMG established as described.
Data in text are presented as mean ± standard deviation, while data in figures present standard errors. All statistical analyses were performed using computer software (Statview; SAS Institute, NC) with α = .05. Data were assessed for normality using the Kolmogorov-Smirnov test, with nonparametric tests used for data that were not normally distributed. Difference in baseline KE torques and CAR values between subject groups was analyzed using Mann-Whitney U tests. Comparisons of torque and EMG responses were made using data normalized to peak baseline contractions, with specific comparisons made between baseline, 1st through 5th, 10th, 15th, and 20th MVEs using parametric statistics. Two-way, repeated measures analyses of variance (ANOVAs) were used to assess differences in both normalized (% of peak baseline) and absolute change (Nm) in torque and EMG (% change) activity between subject groups produced during the initial 20 MVEs. Single factor repeated measures ANOVAs or t tests, followed by post hoc Tukey-Kramer analysis were used as appropriate to determine individual differences following significant results. Repeatability of the observed data was assessed using paired comparisons or repeated ANOVAs as necessary. Correlation and regression analyses between key variables were determined using Pearson product moments, and stepwise, multiple linear regression.
Results
Peak KE torques were determined as the highest of the 3 initial (ie, baseline) MVEs, with no significant differences between the 3 attempts in SCI subjects (χ2 = 1.69, P = .43). Significant differences in maximal KE torques were observed between subject groups, with SCI subjects (74 ± 54 Nm) generating substantially lower torques than control subjects (226 ± 91 Nm; Mann-Whitney U test; P < .001). Control subjects demonstrated CAR values of 0.97 ± 0.04 during baseline MVEs, consistent with previous reports of CAR values in young and elderly subjects.22 Conversely, the average CAR for SCI subjects was 0.52 ± 0.27 (Mann-Whitney U test, P < .0001).
Torque and EMG Activity During Repeated MVEs
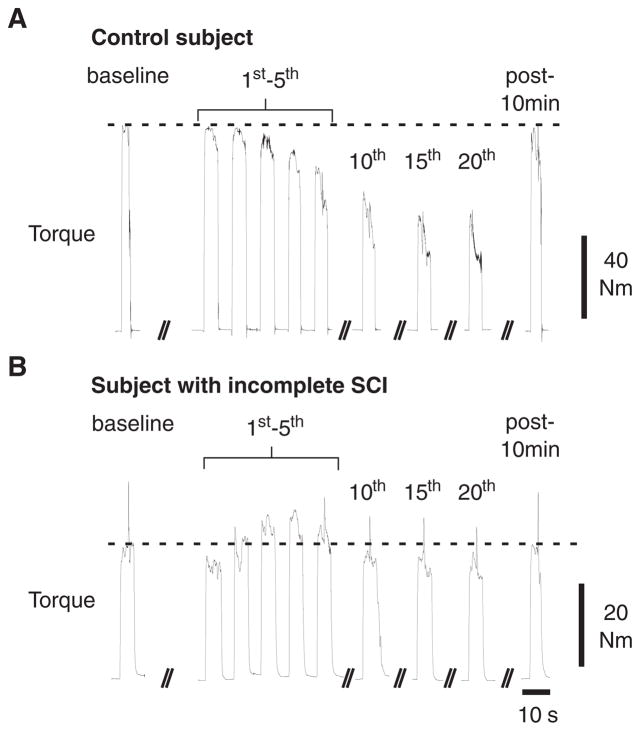
For repeated MVEs, substantially different volitional activation patterns were observed between representative control (Figure 1A) and SCI (Figure 1B) subjects. An immediate and gradual decline in KE torque and quadriceps EMGs from peak baseline MVEs was observed in the control subject with repeated efforts. In contrast, and despite substantially lower peak KE torques, the subject with SCI showed a marked increase in torque and quadriceps EMG above the baseline MVE with repeated contractions, particularly during the first 5 efforts. Furthermore, only a small decline in KE torque was observed by the 20th MVE in the subject with SCI. After 10 minutes, peak KE torque during a single MVE approximated that of the initial baseline or first repeated MVE.
Figure 1. Alterations in Knee Extensors Torque and Electromyographic Activity.
Note: Typical examples of alterations in knee extensors torque and electromyographic activity from the vastus lateralis, vastus medialis, rectus femoris, and medial hamstring during baseline and repeated maximal volitional efforts (MVEs) in a representative control (A) and spinal cord injury (SCI, B) subject, followed by a single MVE 10 minutes after repeated efforts. Central activation ratios were determined during baseline efforts, every 5th repeated MVE, and at the 10 minutes following repeated efforts.
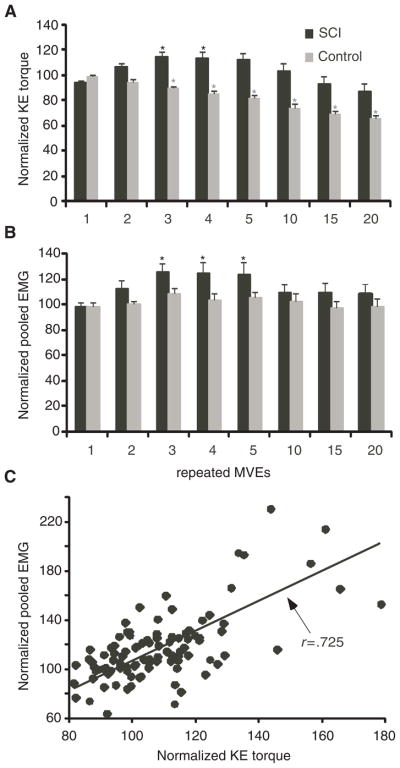
Population data from 21 experimental trials (14 subjects) are presented in Figure 2, with data analyzed following normalization of KE torques and EMG activity to the highest individual baseline MVE. Statistical analysis of KE torques was performed using both normalized values (expressed as a percentage of baseline), and absolute torque changes from baseline, yielding similar results. Significant main effects for normalized torque were observed for subject group (P < .001) and repeated effort (P < .0001), with a significant interaction (P < .0001; Figure 2A). Post hoc repeated measures ANOVA for repeated efforts revealed a significant decline in peak torque in control subjects, with Tukey-Kramer tests revealing differences at the 3rd (89 ± 6.1% of baseline, or 13 ± 20 Nm decline) through the 20th effort (decrease to 65 ± 10% of baseline, or 66 ± 27 Nm decline). In contrast, significant increases in KE torques in SCI subjects were observed at the 3rd and 4th MVEs versus baseline or 1st efforts, with maximal improvements of 15 ± 15% (increase of 11 ± 11 Nm) at the 3rd MVE. Furthermore, KE torques in SCI subjects did not decrease significantly upon the last (20th) MVE (85 ± 26%; 10 ± 22 Nm decline). Significant differences (P < .01) in torque between groups and within the SCI group were also observed when the less impaired limb of subjects tested bilaterally was removed from the analysis.
Figure 2. KE Torques and Quadriceps EMG Activity.
Note: Averaged, normalized peak knee extensors (KE) torques (A) and pooled quadriceps electromyographic (EMG) activity (B) during the 1st through 5th, 10th, 15th, and 20th repeated maximal volitional efforts (MVEs) in spinal cord injury (SCI; black) and control (gray) subjects. Asterisks (*) indicate significant increases in torque or EMG during repeated versus baseline MVEs in SCI, or decreases in torque in control subjects. (C) The significant correlation between peak KE torques and pooled EMG for the first 5 repeated MVEs in SCI subjects is also shown (P < .001).
Changes in CAR values during repeated MVEs indicated small but nonsignificant decreases from baseline values in control subjects (range, 0.93–0.95). In SCI subjects, CAR values during the 5th MVEs (0.54 ± 0.27) were significantly higher than CARs at the 15th (0.48 ± 0.27) and 20th effort (0.47 ± 0.28), but not different when compared with baseline efforts.
Quadriceps EMG was recorded during 20/21 experimental trials (technical difficulty with EMG recordings in 1 session). Muscle activity generally increased during the first few repeated MVEs in the SCI subjects, and remained elevated with continued efforts. Figure 2B demonstrates changes in pooled EMG activity from the VL, VM, and RF during repeated contractions in SCI subjects, with data normalized to the largest baseline effort. Pooled EMG increased by up to 26% at the 3rd and 4th repeated MVEs, and was maintained (10 ± 26% increase) at the 20th effort. In contrast, control subjects demonstrated a smaller, nonsignificant increase in pooled EMG activity (8 ± 13% increase at the 3rd effort), with minimal decline during repeated MVEs (97 ± 20% of baseline at the 20th effort). Significant main effects for repeated effort (P < .01) but not subject group were observed, with no significant interaction. Post hoc analysis indicated that pooled EMG was significantly increased at the 3rd through 5th MVEs compared with baseline EMG in the subjects with SCI, although no significant changes were observed in the control subjects. Normalized, pooled EMG responses of SCI subjects were correlated significantly with peak KE torques during the first 5 repeated MVEs (r = .725; P < .001; Figure 2C).
Analysis of individual quadriceps muscle activity in subjects with SCI revealed the largest changes in VL EMG (143 ± 81% of baseline at 5th MVE), which declined slightly with repeated efforts. Smaller increases were observed in the VM, with maximal values at the 5th effort (19 ± 42% increase at 5th MVE), and in the RF, which increased initially (11 ± 37% increase at 3rd MVE) and subsequently declined. There were no significant differences between muscle groups with repeated efforts (P > .10). Stepwise, multiple linear regression analysis revealed that VL activity was the largest contributor to augmented torques responses (r = .595; P < .001), with nonsignificant contributions from other muscles. In addition, MH activity did not change significantly during the repeated MVEs (range of mean values between 88 and 100% of baseline; P > .05), suggesting little change in antagonist activity during the repeated efforts.
Increased Rate of Rise of Torque and EMG With Repeated MVEs
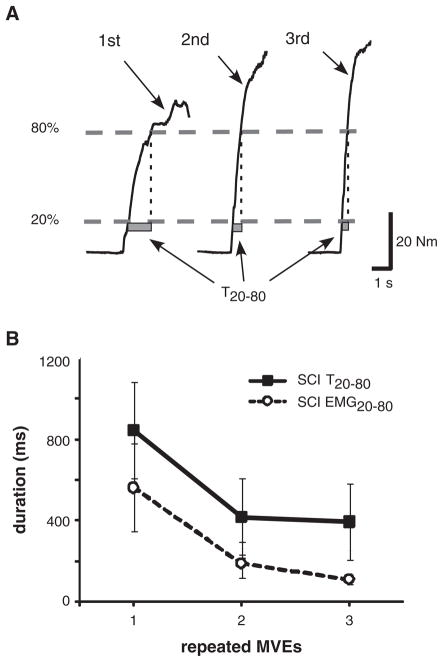
The rate of rise of KE torque (Figure 3A) increased significantly with repeated MVEs in SCI but not the control group. Calculation of T20–80 was performed for the first 3 repeated MVEs only, because some control subjects were unable to generate 80% of their peak baseline torque by the 4th MVE. All SCI subjects were able to generate > 80% of baseline torques for the first 5 efforts and were able to decrease KE torque below 20% of peak baseline values between repeated efforts. T20–80 values for control subjects were 257 ± 99 ms during the initial MVEs, and did not change substantially during the 2nd (257 ± 118 ms) or 3rd contraction (313 ± 181 ms). For SCI subjects, T20–80 values were much longer upon the first MVE (895 ± 879 ms) but decreased significantly upon the 2nd (449 ± 693 ms) and 3rd (535 ± 837 ms) contractions (P < .001).
Figure 3. Representative Data.
Note: (A) One subject with spinal cord injury (SCI) portraying increasing rate of torque increase (indicated by shorter T20–80 duration; gray bars) during the first 3 repeated maximal volitional efforts (MVEs). (B) The mean alterations in T20–80 and EMG20–80 in subjects with SCI. Differences in T20–80 and EMG20–80 from the 1st to 2nd repeated MVEs were significantly correlated (r = 725; P < .001). EMG, electromyographic data.
To determine whether changes in the rate of torque increases were driven by alterations in muscle activation, EMG20–80 was calculated from the linear envelopes for the first 3 MVEs, using data points identified initially by the T20–80 values (please see the Methods section). Such data were only available for 16 experimental trials (14 subjects), as some individuals were unable to reduce pooled EMG activity below 20% of maximal values between consecutive efforts. Figure 3B demonstrates changes in both T20–80 and EMG20–80 during the first 3 MVEs, with similar modulations of the rate of torque and EMG increases (P < .05 for repeated measures ANOVA for EMG20–80). Changes in T20–80 and EMG20–80 from the 1st to 2nd repeated MVEs were significantly correlated (r = .748; P < .01).
Increases in KE Torques Were Dependent on Volitional Activation
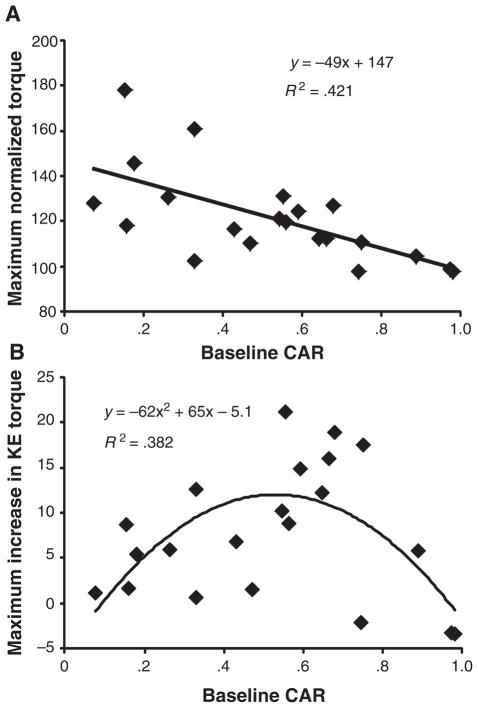
In subjects with SCI, the increase in KE torque during repeated efforts was dependent on volitional quadriceps activation. A significant negative relationship was observed between the highest percentage increase in KE torque (observed during the first 5 repeated MVEs) and CAR values obtained at baseline (r = −.656; P < .01; Figure 4A). When peak increases in KE torques are expressed in absolute values (Nm), a moderate curvilinear relationship (r = .616; P < .05; Figure 4B) is observed, where those with very high or very low CARs demonstrated smaller increases with repeated MVEs.
Figure 4. Correlation Between CAR Values.
Note: A significant negative correlation (P < .001) between central activation ratio (CAR) values determined during baseline maximal volitional efforts and largest relative (percentage) increase in torque during the 1st through 5th repeated effort is demonstrated (A), with a significant curvilinear relationship demonstrated when absolute peak increases in knee extensors (KE) torque are plotted versus CAR (B).
Repeatability of Increased KE Torques During Repeated MVEs
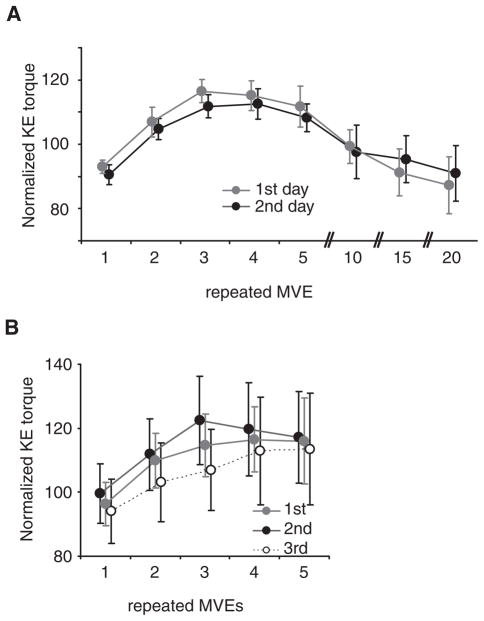
To assess the repeatability of the observed behaviors, 7 subjects with SCI selected by convenience from the pool of subjects returned within 5 days (range, 2–5 days) for retesting (mean CAR = 0.60; range, 0.35–0.84). During reassessment, the identical experimental protocol was performed on the same leg, with the same moment arm. Paired comparisons revealed no significant difference in baseline KE torque between the days (day 1: 74 ± 24 Nm vs day 2: 75 ± 40 Nm; P = .83), and a 2-way, repeated measures ANOVA indicated no significant differences in the relative magnitude or time course of KE torque generation between days (P = .86). Normalized peak KE torques were < 4% different throughout repeated efforts, indicating very little effect of practice and/or learning to the observed phenomenon.
Seven SCI subjects performed a second protocol to determine within-session repeatability of the increased KE torques during repeated MVEs (mean CAR = 0.57; range, 0.31–0.78). On a separate session, subjects performed only 5 repeated MVEs (eg, 5 seconds on, 5 seconds off) at least 3 times, with > 5 minutes between efforts. Normalized KE torques are presented in Figure 5B, with increases in KE torques observed consistently during all attempts. There was a slight increase in normalized KE torques during the 2nd repeated efforts, although the relative increases were similar across the 3 trials (P = .56).
Figure 5. Changes in KE Torques.
Note: (A) Repeatability of the observed changes in knee extensors (KE) torques were tested on 7 subjects with spinal cord injury on different days (<5 days between sessions), using an identical testing paradigm of 20 repeated maximal volitional efforts. (B) Assessment of the repeatability of the increase in KE torque during the first 5 repeated efforts was performed on the same day. There were small, nonsignificant differences with repeated testing. MVE, maximum voluntary effort.
Increased MVE Torques During Intermittent Versus Sustained Efforts
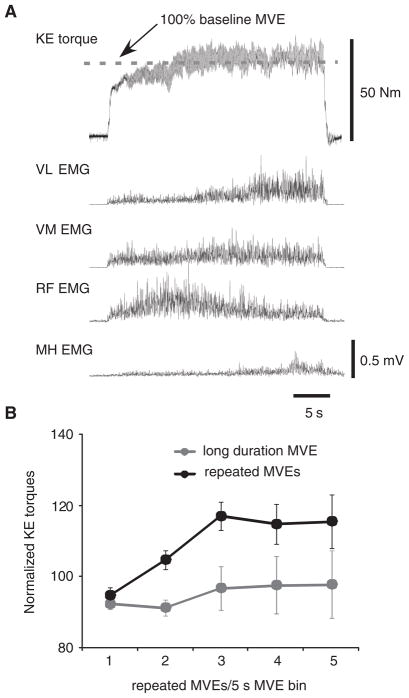
To alleviate the concern that increases in KE torques and quadriceps EMG during repeated MVEs occurred due to insufficient time allowed to reach peak KE torques, additional experiments were performed to investigate whether sustained MVEs could elicit increased efferent output. On separate days and following determination of peak KE torques during baseline MVEs, 7 SCI subjects (1 tested bilaterally) performed a single, sustained MVE for ~30 seconds. Data from a single subject are shown in Figure 6A, where KE torque gradually increased during the first 15 seconds, then reached a plateau. Notably, increased variability in torque signals is observed, revealing clonic motor activity at ~7 Hz.23 Such data were present during both repeated intermittent or sustained contractions in subjects with SCI, but were not evident in control subjects. Individual quadriceps EMGs increased as well, with the largest increase above initial levels observed in the VL, consistent with data collected during repeated MVEs.
Figure 6. Alterations in KE Torque and EMG Activity.
Note: (A) Single subject data of the alterations in knee extensors (KE) torque and electromyographic (EMG) activity during a sustained (~30 seconds) maximal volitional effort (MVE). (B) Changes in peak KE torques during repeated (black circles) versus sustained (gray circles) efforts in spinal cord injury. Significant increases in KE torques were observed only with intermittent MVEs, with peak KE torques consistently higher than those achieved during sustained efforts. VL, vastus lateralis; VM, vastus medialis; RF, rectus femoris; MH, medial hamstring.
Figure 6B depicts mean changes in KE torques during sustained (30 seconds) MVEs versus repeated, intermittent MVEs for the 7 subjects who completed both testing sessions. During sustained MVEs, peak torques were identified every 5 seconds for the first 25 seconds of the contraction (ie, five 5-second data analysis epochs). As described previously, significant increases in peak KE torques were observed during intermittent MVEs performed during these 8 trials (P < .01), although in the same subjects, no significant increase in KE torques was observed during sustained efforts (P = .72). Only 1 subject increased their KE torques substantially (ie, > 50%) during the sustained MVEs, whereas all other subjects demonstrated peak KE torques < 12% above baseline MVEs. In every subject, the peak increase in joint torque during the first 5 repeated MVE contractions was always greater than peak torques generated during sustained MVEs (difference of 17 ± 8.3%; P < .01 using paired comparisons). In contrast, 6 intact subjects who performed the sustained MVE contractions demonstrated decreases in peak torque by the last 5-second epoch (20th–25th sec), with a significant decline to 85 ± 19% of their initial baseline torque output (P < .05), consistent with previously published reports.24 There was no difference in the decline in torques during repeated versus intermittent MVEs in control subjects (P = .66).
Evaluation of pooled EMG activity revealed very little change in efferent drive in nearly all subjects (92 ± 21% of baseline) with the exception of the 1 subject who demonstrated a large increase in KE torque (ie, pooled EMG activity > 200% of baseline MVEs). The CAR of this subject was 0.32, indicating substantial quadriceps weakness secondary to reduced voluntary activation. However, other subjects with lower CARs demonstrated decreases, not increases, in EMG and KE torques during sustained MVEs.
Discussion
The main finding of the present study was that subjects with motor incomplete SCI exhibited no significant decline in peak torque during the performance of an established experimental paradigm to assess volitional fatigue. Rather, performance of repeated intermittent MVEs consistently resulted in brief improvements in KE torque and EMG. The present data represent a substantial departure from a large body of literature indicating enhanced muscular fatigue in patients with SCI using a NMES paradigm as compared to neurologically intact individuals.13
Minimal Evidence of Fatigue With Repeated MVEs
Multiple mechanisms may account for the findings of reduced fatigability in motor incomplete SCI versus intact subjects. For example, reduced volitional activation (ie, low CAR values) may minimize peripheral contractile failure during repeated MVEs and reduce fatigue. This may also account for the delayed fatigue (ie, increased endurance time) observed previously by others during submaximal volitional efforts in incomplete SCI subjects.9 Our findings of reduced volitional activation in SCI subjects could also minimize blood flow occlusion using repeated MVEs and reduce the onset of fatigue,25 although the contributions of these mechanisms to our observations are unclear.
A related explanation for the observed differences between control and SCI subjects could be altered motivation during performance of repeated MVEs. Specifically, both reduced motivation and altered integrity of supraspinal pathways could limit volitional activation in our SCI population, and may have contributed to reduced evidence of fatigue. We are unable to separately quantify the relative contribution of each factor, although all SCI and control subjects were provided similar encouragement and categorically stated they exerted maximal effort despite marked differences in their behaviors. The consistency of baseline peak torques in SCI subjects between testing days, and the repeatability of our observations both within and between sessions argue that our observations are due to physiological not motivational differences between subject populations.
Our data also contrast sharply with published reports of increased fatigue in complete SCI, and may be accounted for by at least 2 specific mechanisms. Greater daily lower limb activity in subjects with motor incomplete SCI, particularly those who are ambulatory, may offset many of the detrimental neuromuscular alterations associated with SCI and disuse. Indeed in subjects with impaired activation of selected motor units, compensatory increased volitional drive to the motoneurons that receive greater descending synaptic input may increase fatigue resistance of the innervated muscle fibers with continued use. Furthermore, the presence of spastic motor behaviors in all subjects tested may have contributed to preservation of Type I muscle fibers after injury,26 thereby potentially minimizing the extent of neuromuscular fatigue and contributing to the present results (see, however, Harris et al27). While we did not assess neuromuscular fatigability in control versus SCI subjects using NMES,7 a recent report indicated little difference in muscle properties between subjects with incomplete SCI and control participants,28 although further investigation of this issue is warranted.
Another likely explanation for the differences between the present versus previous studies in human SCI is the methodology used to assess fatigue. In particular, NMES is required in studies performed on subjects with complete SCI, and recruits motor units in a spatially-fixed and temporally synchronous manner,29 with some evidence for preferential activation of larger, fast fatigable fibers (see, however, Binder-Macleod et al30 and Feiereisen et al31). Use of NMES, therefore, eliminates the flexibility of the nervous system to alter motor unit recruitment and rate coding strategies that allow for maintained volitional force during fatiguing efforts.32 Although NMES-elicited fatigue can be partially offset by altering stimulation frequencies33 (see, however, Fuglevand and Keen34), evidence for greater muscular fatigue during electrical versus volitional contractions in intact subjects is well established.32 Indeed, maintained elevation of EMG patterns during the final repeated effort suggests that central mechanisms play a substantial role in the observed resistance to fatigue. More importantly, the increase in quadriceps EMG was significantly related to increases in KE torque, which is of primary interest in the current study.
Elevated Central Activation During “Fatiguing” Efforts
The findings of increased KE torques were initially unexpected and, to our knowledge, have not been described previously. Recent data have described improvements in volitional torque production (29 ± 8.2%) and rate of torque development (T20–80; 77 ± 103 ms) in incomplete SCI subjects after 12 weeks of resistance/plyometric training.33 In the present study, approximately half of this increase in KE torque was realized during repeated MVEs, with dramatic increases in the rate of force production. While muscle potentiation may partly contribute to the findings,35 the increasing magnitude and rate of increasing KE torques were strongly correlated with increasing quadriceps, primarily VL, muscle activity, indicating a central (ie, spinal or supraspinal) origin for the observed behaviors. The minimal changes in MH EMG further indicate little contribution of decreased antagonist activation to increased KE torques. The observation of augmented torques was also consistent on the same or on different days, suggesting little influence of learning or accommodation to the testing apparatus. Furthermore, peak KE torque generated during repeated MVEs were not a result of insufficient duration to achieve maximal efferent drive, as sustained MVEs consistently resulted in smaller increases in peak torque output.
Potential contributors to increased efferent output include augmented supraspinal or segmental inputs, and/or modulation of intrinsic motoneuronal excitability. For example, the phenomenon of postexercise facilitation of corticomotoneuronal excitability is well established, with increases in motor evoked potentials (MEPs) observed using transcranial magnetic stimulation (TMS) following volitional contractions of varying duration and intensity in intact subjects.36,37 Some have suggested a supraspinal origin for the behaviors, as direct transcranial electrical stimulation (TES) presumed to activate proximal segments of pyramidal cell axons,37 did not elicit increased postcontraction MEPs. More recent data suggest that TES can activate intracortical circuits, however,38 and the contribution of changes in descending drive are unclear.
Synaptic input from afferent pathways could also contribute to altered muscle force during repeated MVEs, although most research has focused on proprioceptive inputs that contribute to the development of fatigue (eg, disfacilitation of excitatory Ia drive39 or increases in group III–IV afferent inhibition40). Alternatively, few studies have discussed the contribution of increased excitatory afferent drive during repeated contractions41 although recent data suggest a spinal locus for increased efferent output during similar conditions.42 In particular, continuous or repeated afferent input, as occurs with tendon vibration43 or low-intensity, wide pulse width (1 ms) NMES,44 can elicit prolonged, elevated motor discharge that outlasts the afferent stimulus. Such behaviors have been attributed to persistent inward (Ca2+ and Na+) currents (PICs) in spinal motoneurons,45 which give rise to elevated and/or sustained depolarization (i.e., plateau potentials). In addition, PIC activation is time dependent, and characterized by a decrease in the depolarization required to activate these currents (ie, reduction of PIC threshold) with repeated stimuli (ie, “warm-up” or “wind-up”), as observed in spastic motor activity in human SCI.46,47 Indeed, the manifestation of spastic motor activity in SCI has been linked to PIC activity as well,45 in which sustained motor output (ie, spasms) may occur in the absence of continuous synaptic input. In our current study, motor output during repeated intermittent or sustained MVEs often demonstrated characteristics of spastic motor behavior (ie, clonic activity; Figure 6A). Few studies have linked PIC activity to altered volitional force in human SCI although motor unit firing patterns consistent with PIC activity in control subjects48 have been observed in SCI subjects during volitional efforts.49 While the relative contributions of spinal and supraspinal alterations underlying increasing efferent drive during repeated MVEs are unclear, the present data and potential explanations provide a framework of testable hypotheses to determine the mechanisms underlying augmented torque and EMG activity with repeated maximal efforts.50
Differences Between Sustained Versus Intermittent MVEs
To determine whether similar increases in efferent output would be observed if SCI subjects were simply asked to sustain their MVEs, we discovered smaller increases in KE torques or quadriceps EMG, particularly when compared with repeated MVEs. Previous studies in intact subjects have attributed differences in fatigability during sustained versus intermittent contractions to reflex inhibition by means of group III–IV afferent discharge activated by decreased removal of metabolites with blood flow occlusion,51 with potentially greater fatigue in extensor versus flexor muscles in the upper limbs.40 The mechanisms underlying facilitation of KE torques may, therefore, be masked during sustained MVEs by the increase in negative feedback from group III–IV afferents. However, the mechanism through which group III–IV feedback inhibits extensor versus flexor motoneuron pools in lower limbs, particularly in SCI subjects, is not well understood and requires further investigation.
Limitations
Limitations of the current study should be addressed. Specifically, both control and SCI subjects were not provided visual feedback during performance of MVEs. This was avoided purposefully to minimize any expectation of the subjects during the maximal “fatiguing” efforts. Whether providing visual feedback of the KE torques may have altered our results in SCI subjects is unclear, and requires investigation.
Second, the increases in KE torques observed across patients were related to estimates of volitional activation (CAR), as assessed using high intensity NMES applied during baseline or repeated efforts. The accuracy of the CAR to determine volitional activation using various stimulation parameters has been questioned,16,19 with other data suggesting that current electrophysiological techniques may result in overestimation of volitional activation.18 In the present study, however, the determination of CAR was performed only to provide an approximation of volitional activation and its contribution to the primary findings. Use of different methods to estimate volitional activation may be of interest in incomplete SCI, although would likely not change the general finding that the augmentation of force with repeated MVEs depends on the ability to volitionally generate KE torques.
Finally, the lack of determination of specific mechanisms underlying the primary results (ie, augmented torque and reduced fatigue) is a limitation in the current article, and is the focus of future work.50 Specifically, the central mechanisms underlying the increase in volitional torque in incomplete SCI are uncertain, as alterations in motor unit recruitment and/or discharge rate may account for the increased EMG activity with repeated maximal efforts, and may not be accurately estimated from surface recordings.52 Such recordings may also provide further insight into the potential contribution of PICs to the present work, as performed previously.42
Clinical Significance
The relationship between the present observations of increased volitional motor output and the generation of spastic motor activity in chronic SCI presents an interesting dichotomy in the management of patients with SCI. More directly, spastic motor activity has traditionally been viewed as a negative consequence of neurological injury and is suppressed through pharmacological interventions. However, a possible mechanism underlying the observed phenomenon, specifically PIC-activity, is associated with manifestation of spastic motor behaviors in human SCI. In addition, many individuals with SCI demonstrated volitional activity indicative of spasticity/spasms during the repeated or sustained MVEs (ie, Figure 6A). This finding could be interpreted as increased motor unit synchronization during moderate to high intensity contractions, which could account for the increased EMG activity and force variability.53 Alternatively, such behavior is consistent with the hypothesis that subjects used their involuntary reflex activity (ie, “spasticity”) to augment KE torques. In both intact and SCI subjects, reflex activation during static54 and dynamic55 behaviors is critical for generating and sustaining volitional motor output. Indeed, professionals in rehabilitation medicine have long acknowledged that subjects with SCI often use their spastic behaviors to perform functional tasks56 (see comment by Dietz57). In addition, nearly all subjects were able to minimize their torques and EMGs between repeated MVEs to less that 20% of peak baseline contractions. The combined data indicate that the augmented efferent activity was largely controlled through volitional initiation and termination, regardless of reflex and intrinsic motoneuronal contributions. Despite the lack of identified mechanisms, the current data represent a “reserve” of efferent drive used in incomplete SCI during repeated MVEs to increase peak torques. Whether this reserve can be harnessed through specific interventions to improve strength is an area of future study.
Acknowledgments
The authors thank Zachary Riley, PhD, Carol Mottram, PT, PhD, and Arun Jayamaran, PT, PhD for their critical review of the manuscript before submission. Funding for the present work was provided by NIH/NICHD R21-HD046876 and the Craig H. Neilsen Foundation (grant # 36830) to TGH.
References
- 1.Castro MJ, Apple DF, Jr, Staron RS, et al. Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J Appl Physiol. 1999;86:350–358. doi: 10.1152/jappl.1999.86.1.350. [DOI] [PubMed] [Google Scholar]
- 2.Shields RK. Fatigability, relaxation properties, and electromyographic responses of the human paralyzed soleus muscle. J Neurophysiol. 1995;73:2195–2206. doi: 10.1152/jn.1995.73.6.2195. [DOI] [PubMed] [Google Scholar]
- 3.Crameri RM, Weston AR, Rutkowski S, et al. Effects of electrical stimulation leg training during the acute phase of spinal cord injury: a pilot study. Eur J Appl Physiol. 2000;83:409–415. doi: 10.1007/s004210000263. [DOI] [PubMed] [Google Scholar]
- 4.Talmadge RJ, Castro MJ, Apple DF, Jr, et al. Phenotypic adaptations in human muscle fibers 6 and 24 wk after spinal cord injury. J Appl Physiol. 2002;92:147–154. doi: 10.1152/japplphysiol.000247.2001. [DOI] [PubMed] [Google Scholar]
- 5.Olive JL, Dudley GA, McCully KK. Vascular remodeling after spinal cord injury. Med Sci Sports Exerc. 2003;35:901–907. doi: 10.1249/01.MSS.0000069755.40046.96. [DOI] [PubMed] [Google Scholar]
- 6.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 7.Gerrits HL, De Haan A, Hopman MT, et al. Contractile properties of the quadriceps muscle in individuals with spinal cord injury. Muscle Nerve. 1999;22:1249–1256. doi: 10.1002/(sici)1097-4598(199909)22:9<1249::aid-mus13>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 8.Shields RK, Dudley-Javoroski S. Musculoskeletal plasticity after acute spinal cord injury: effects of long-term neuromuscular electrical stimulation training. J Neurophysiol. 2006;95:2380–2390. doi: 10.1152/jn.01181.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas CK, del Valle A. The role of motor unit rate modulation versus recruitment in repeated submaximal voluntary contractions performed by control and spinal cord injured subjects. J Electromyogr Kinesiol. 2001;11:217–229. doi: 10.1016/s1050-6411(00)00055-9. [DOI] [PubMed] [Google Scholar]
- 10.Thomas CK, Tucker ME, Bigland-Ritchie B. Voluntary muscle weakness and co-activation after chronic cervical spinal cord injury. J Neurotrauma. 1998;15:149–161. doi: 10.1089/neu.1998.15.149. [DOI] [PubMed] [Google Scholar]
- 11.Calancie B, Molano MR, Broton JG. Epidemiology and demography of acute spinal cord injury in a large urban setting. J Spinal Cord Med. 2005;28:92–96. doi: 10.1080/10790268.2005.11753804. [DOI] [PubMed] [Google Scholar]
- 12.Russ DW, Kent-Braun JA. Sex differences in human skeletal muscle fatigue are eliminated under ischemic conditions. J Appl Physiol. 2003;94:2414–2422. doi: 10.1152/japplphysiol.01145.2002. [DOI] [PubMed] [Google Scholar]
- 13.Shields RK. Muscular, skeletal, and neural adaptations following spinal cord injury. J Orthop Sports Phys Ther. 2002;32:65–74. doi: 10.2519/jospt.2002.32.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas CK, Zijdewind I. Fatigue of muscles weakened by death of motoneurons. Muscle Nerve. 2006;33:21–41. doi: 10.1002/mus.20400. [DOI] [PubMed] [Google Scholar]
- 15.American Spinal Injury Association. International Standards for Neurological and Functional Classification of Spinal Cord Injury. Chicago: ASIA; 1994. [DOI] [PubMed] [Google Scholar]
- 16.Miller M, Downham D, Lexell J. Superimposed single impulse and pulse train electrical stimulation: a quantitative assessment during submaximal isometric knee extension in young, healthy men. Muscle Nerve. 1999;22:1038–1046. doi: 10.1002/(sici)1097-4598(199908)22:8<1038::aid-mus5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 17.Kent-Braun JA, Le Blanc R. Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle Nerve. 1996;19:861–869. doi: 10.1002/(SICI)1097-4598(199607)19:7<861::AID-MUS8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Kendall TL, Black CD, Elder CP, et al. Determining the extent of neural activation during maximal effort. Med Sci Sports Exerc. 2006;38:1470–1475. doi: 10.1249/01.mss.0000228953.52473.ce. [DOI] [PubMed] [Google Scholar]
- 19.Kooistra RD, de Ruiter CJ, de Haan A. Conventionally assessed voluntary activation does not represent relative voluntary torque production. Eur J Appl Physiol. 2007;100:309–320. doi: 10.1007/s00421-007-0425-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herbert RD, Gandevia SC. Twitch interpolation in human muscles: mechanisms and implications for measurement of voluntary activation. J Neurophysiol. 1999;82:2271–2283. doi: 10.1152/jn.1999.82.5.2271. [DOI] [PubMed] [Google Scholar]
- 21.Gregory CM, Dixon W, Bickel CS. Impact of varying pulse frequency and duration on muscle torque production and fatigue. Muscle Nerve. 2007;35:504–509. doi: 10.1002/mus.20710. [DOI] [PubMed] [Google Scholar]
- 22.Stackhouse SK, Stevens JE, Lee SC, et al. Maximum voluntary activation in nonfatigued and fatigued muscle of young and elderly individuals. Phys Ther. 2001;81:1102–1109. [PubMed] [Google Scholar]
- 23.Beres-Jones JA, Johnson TD, Harkema SJ. Clonus after human spinal cord injury cannot be attributed solely to recurrent muscle-tendon stretch. Exp Brain Res. 2003;149:222–236. doi: 10.1007/s00221-002-1349-5. [DOI] [PubMed] [Google Scholar]
- 24.Bigland-Ritchie B, Jones DA, Hosking GP, et al. Central and peripheral fatigue in sustained maximum voluntary contractions of human quadriceps muscle. Clin Sci Mol Med. 1978;54:609–614. doi: 10.1042/cs0540609. [DOI] [PubMed] [Google Scholar]
- 25.de Ruiter CJ, Goudsmit JF, Van Tricht JA, et al. The isometric torque at which knee-extensor muscle reoxygenation stops. Med Sci Sports Exerc. 2007;39:443–453. doi: 10.1249/mss.0b013e31802dd3cc. [DOI] [PubMed] [Google Scholar]
- 26.Hidler JM, Harvey RL, Rymer WZ. Frequency response characteristics of ankle plantar flexors in humans following spinal cord injury: relation to degree of spasticity. Ann Biomed Eng. 2002;30:969–981. doi: 10.1114/1.1500409. [DOI] [PubMed] [Google Scholar]
- 27.Harris RL, Bobet J, Sanelli L, et al. Tail muscles become slow but fatigable in chronic sacral spinal rats with spasticity. J Neurophysiol. 2006;95:1124–1133. doi: 10.1152/jn.00456.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jayaraman A, Gregory CM, Bowden M, et al. Lower extremity skeletal muscle function in persons with incomplete spinal cord injury. Spinal Cord. 2006;44:680–687. doi: 10.1038/sj.sc.3101892. [DOI] [PubMed] [Google Scholar]
- 29.Gregory CM, Bickel CS. Recruitment patterns in human skeletal muscle during electrical stimulation. Phys Ther. 2005;85:358–364. [PubMed] [Google Scholar]
- 30.Binder-Macleod SA, Halden EE, Jungles KA. Effects of stimulation intensity on the physiological responses of human motor units. Med Sci Sports Exerc. 1995;27:556–565. [PubMed] [Google Scholar]
- 31.Feiereisen P, Duchateau J, Hainaut K. Motor unit recruitment order during voluntary and electrically induced contractions in the tibialis anterior. Exp Brain Res. 1997;114:117–123. doi: 10.1007/pl00005610. [DOI] [PubMed] [Google Scholar]
- 32.Dobkin BH. Fatigue versus activity-dependent fatigability in patients with central or peripheral motor impairments. Neurorehabil Neural Repair. 2008;22:105–110. doi: 10.1177/1545968308315046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gregory CM, Bowden MG, Jayaraman A, et al. Resistance training and locomotor recovery after incomplete spinal cord injury: a case series. Spinal Cord. 2007;45:522–530. doi: 10.1038/sj.sc.3102002. [DOI] [PubMed] [Google Scholar]
- 34.Fuglevand AJ, Keen DA. Re-evaluation of muscle wisdom in the human adductor pollicis using physiological rates of stimulation. J Physiol. 2003;549(pt 3):865–875. doi: 10.1113/jphysiol.2003.038836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shields RK, Dudley-Javoroski S, Littmann AE. Postfatigue potentiation of the paralyzed soleus muscle: evidence for adaptation with long-term electrical stimulation training. J Appl Physiol. 2006;101:556–565. doi: 10.1152/japplphysiol.00099.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norgaard P, Nielsen JF, Andersen H. Post-exercise facilitation of compound muscle action potentials evoked by transcranial magnetic stimulation in healthy subjects. Exp Brain Res. 2000;132:517–522. doi: 10.1007/s002219900318. [DOI] [PubMed] [Google Scholar]
- 37.Samii A, Wassermann EM, Ikoma K, et al. Characterization of postexercise facilitation and depression of motor evoked potentials to transcranial magnetic stimulation. Neurology. 1996;46:1376–1382. doi: 10.1212/wnl.46.5.1376. [DOI] [PubMed] [Google Scholar]
- 38.Brocke J, Irlbacher K, Hauptmann B, et al. Transcranial magnetic and electrical stimulation compared: does TES activate intracortical neuronal circuits? Clin Neurophysiol. 2005;116:2748–2756. doi: 10.1016/j.clinph.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 39.Macefield G, Hagbarth KE, Gorman R, et al. Decline in spindle support to alpha-motoneurones during sustained voluntary contractions. J Physiol. 1991;440:497–512. doi: 10.1113/jphysiol.1991.sp018721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin PG, Smith JL, Butler JE, et al. Fatigue-sensitive afferents inhibit extensor but not flexor motoneurons in humans. J Neurosci. 2006;26:4796–4802. doi: 10.1523/JNEUROSCI.5487-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki S, Hayami A, Suzuki M, et al. Reductions in recruitment force thresholds in human single motor units by successive voluntary contractions. Exp Brain Res. 1990;82:227–230. doi: 10.1007/BF00230858. [DOI] [PubMed] [Google Scholar]
- 42.Gorassini M, Yang JF, Siu M, et al. Intrinsic activation of human motoneurons: reduction of motor unit recruitment thresholds by repeated contractions. J Neurophysiol. 2002;87:1859–1866. doi: 10.1152/jn.00025.2001. [DOI] [PubMed] [Google Scholar]
- 43.Kiehn O, Eken T. Prolonged firing in motor units: evidence of plateau potentials in human motoneurons? J Neurophysiol. 1997;78:3061–3068. doi: 10.1152/jn.1997.78.6.3061. [DOI] [PubMed] [Google Scholar]
- 44.Collins DF, Gorassini M, Bennett D, et al. Recent evidence for plateau potentials in human motoneurones. Adv Exp Med Biol. 2002;508:227–235. doi: 10.1007/978-1-4615-0713-0_28. [DOI] [PubMed] [Google Scholar]
- 45.Heckmann CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve. 2005;31:135–156. doi: 10.1002/mus.20261. [DOI] [PubMed] [Google Scholar]
- 46.Hornby TG, Kahn JH, Wu M, et al. Temporal facilitation of spastic stretch reflexes following human spinal cord injury. J Physiol. 2006;571(pt 3):593–604. doi: 10.1113/jphysiol.2005.102046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hornby TG, Rymer WZ, Benz EN, et al. Windup of flexion reflexes in chronic human spinal cord injury: a marker for neuronal plateau potentials? J Neurophysiol. 2003;89:416–426. doi: 10.1152/jn.00979.2001. [DOI] [PubMed] [Google Scholar]
- 48.Gorassini M, Yang JF, Siu M, et al. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol. 2002;87:1850–1858. doi: 10.1152/jn.00024.2001. [DOI] [PubMed] [Google Scholar]
- 49.Zijdewind I, Thomas CK. Motor unit firing during and after voluntary contractions of human thenar muscles weakened by spinal cord injury. J Neurophysiol. 2003;89:2065–2071. doi: 10.1152/jn.00492.2002. [DOI] [PubMed] [Google Scholar]
- 50.Hornby TG, Thompson CK, Lewek MD. Potential neuromuscular mechanisms underlying torque augmentation during “fatiguing” contractions in human incomplete spinal cord injury. Proceedings of the Annual Society for Neuroscience; San Diego, CA. 2007. [Google Scholar]
- 51.Duchateau J, Balestra C, Carpentier A, et al. Reflex regulation during sustained and intermittent submaximal contractions in humans. J Physiol. 2002;541(pt 3):959–967. doi: 10.1113/jphysiol.2002.016790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol. 2004;96:1486–1495. doi: 10.1152/japplphysiol.01070.2003. [DOI] [PubMed] [Google Scholar]
- 53.Yao W, Fuglevand RJ, Enoka RM. Motor-unit synchronization increases EMG amplitude and decreases force steadiness of simulated contractions. J Neurophysiol. 2000;83:441–452. doi: 10.1152/jn.2000.83.1.441. [DOI] [PubMed] [Google Scholar]
- 54.Macefield VG, Gandevia SC, Bigland-Ritchie B, et al. The firing rates of human motoneurones voluntarily activated in the absence of muscle afferent feedback. J Physiol. 1993;471:429–443. doi: 10.1113/jphysiol.1993.sp019908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harkema S, Hurley SL, Patel UK, et al. Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol. 1997;77:797–811. doi: 10.1152/jn.1997.77.2.797. [DOI] [PubMed] [Google Scholar]
- 56.Gittmann L. Proceedings of the Symposium on Spinal Injuries. Edinburgh: R Cole Sor; 1963. Initial treatment of traumatic paraplegia and tetraplegia. [Google Scholar]
- 57.Dietz V. Spasticity-spastic movement disorder. Spinal Cord. 2008;46:588. doi: 10.1038/sc.2008.45. [DOI] [PubMed] [Google Scholar]