Abstract
Background
Recently, we have shown that an immunosuppression regimen including costimulation blockade via anti-CD154 antibody significantly prolongs the cardiac xenograft survival in a GTKO.hCD46Tg pig-to-baboon heterotopic xenotransplantation model. Unfortunately, many coagulation disorders were observed with the use of anti-CD154 antibody, and recipient survival was markedly reduced by these complications.
Material and Methods
In this experiment, we replaced anti-CD154 antibody with a more clinically acceptable anti-CD40 antibody while keeping the rest of the immunosuppressive regimen and the donor pig genetics the same. This was carried out to evaluate the antibody's role in xenograft survival and prevention of coagulopathies. Two available clones of anti-CD40 antibody were tested. One mouse anti-human CD40 antibody, (clone 3A8), activated B lymphocytes in vitro and only modestly suppressed antibody production in vivo. Whereas a recombinant mouse non-human primate chimeric raised against macaque CD40, (clone 2C10R4), blocked B-cell activation in vitro and completely blocked antibody production in vivo.
Results
The thrombotic complications seen with anti-CD154 antibody were effectively avoided but the graft survival, although extended, was not as prolonged as observed with anti-CD154 antibody treatment. The longest survival for the 3A8 antibody group was 27 days, and the longest graft survival in the 2C10R4 antibody group was 146 days. All of the grafts except two rejected and were explanted. Only two recipient baboons had to be euthanized due to unrelated complications, and the rest of the baboons remained healthy throughout the graft survival period or after graft explantation. In contrast to our anti-CD 154 antibody-treated baboons, the non-Gal antibody levels started to rise after B cells made their appearance around 8 weeks post-transplantation.
Conclusions
Anti-CD40 antibody at the current dose does not induce any coagulopathies but while effective, had reduced efficacy to induce similar long-term graft survival as with anti-CD154 antibody perhaps due to ineffective control of B-cell function and antibody production at the present dose. More experiments are required to determine antibody affinity and effective dose for inducing long-term cardiac xenograft survival.
Keywords: 2C10R4, Anti-CD40 antibody, cardiac xenograft, CD46, Gal KO pigs, non-Gal antibodies, xenotransplantation
Introduction
Costimulation blockade is an essential component of an effective immunosuppressive regimen to suppress allo- and xenograft rejection [1–4]. Unfortunately, coagulopathy, due to rapid activation and consumption of platelets in baboons, is frequently observed with anti-CD154 antibody [5–7]. Platelets express CD154 on their surface, and binding of anti-CD154 antibody to this molecule causes platelet activation, aggregation, and depletion, leading to consumptive coagulopathy (CC). This condition is further aggravated by the incompatibility of pig thromboregulatory molecules with baboon coagulation factors. Simultaneous use of ketorolac, a platelet aggregation inhibitor, prevented anti-CD154 antibody-mediated CC in most animals [8– 10], but due to its potential for thrombogenic complication, the anti-CD154 antibody has not been recommended for clinical use [11]. Recently, anti-CD40 antibodies have been produced that target the same CD40-CD40L costimulation pathway as anti-CD154 antibody [3,12,13]. The anti-CD40 antibody has not been shown to activate platelets; therefore, it is not expected to induce platelet aggregation/depletion and cause CC. Also just preventing CC may not be enough for graft survival, and further modifications in donor pig genetics may be required to achieve clinically relevant graft survival [14]. To test the potential of this anti-CD40 antibody and the role of human complement regulatory protein CD46, in this study, we used donor pigs that were α1-3 galactosidase knockout (GTKO) and also transgenic for the human CD46 (hCD46Tg). We also examined the role of anti-CD40 antibody from two different clones in a pig-to-baboon heterotopic cardiac xenotransplantation model.
Material and methods
Animal
Specific pathogen-free (SPF) baboons weighing 7– 15 kg from University of Oklahoma (Norman, OK, USA) were housed in a clean pathogen-free facility. GTKO.hCD46Tg pigs (Revivicor Inc., Blacksburg, VA, USA) were used at 3–8 weeks old with a maximum weight of 12 kg. The origin of the CD46 transgene was the same as described before [15]. This line of CD46 pigs was made using a CD46 minigene that demonstrated a high expression of human CD46 in all tissues of these pigs, including in heart [15]. This line of CD46 pigs was cross-bred with Revivicor GTKO pigs [16] over more than four generations. The transgene is stable at the genomic level, and expression of hCD46 protein is consistent with high level across all GTKO/ hCD46 pigs, as in the hCD46Tg-only lines. All animals were used in compliance with guidelines provided by the National Heart, Lung and Blood Institute (NHLBI) Animal Care and Use Committee (ACUC).
Surgical procedures
All transplant procedures were performed at a NHLBI core surgical facility. The heterotopic xenotransplantation procedure has previously been described [17]. Briefly, the recipient baboon's infrarenal aorta and inferior vena cava are exposed through a midline abdominal incision. Side-biting clamps are applied; an aortotomy and venotomy performed, and the end-to-side anastomosis are performed between donor and recipient aorta and donor pulmonary artery with recipient inferior vena cava.
Immunosuppressive regimen
As described in Table 1, all animals received induction treatment with anti-thymocyte globulin (ATG), anti-CD20 antibody, anti-CD40 antibody, and cobra venom factor (CVF). The maintenance treatment regimen included mycophenolate mofetil (MMF) and corticosteroids along with costimulation blockade with anti-CD40 antibody. Our preliminary experiments indicate that protection by CD46 molecule was not complete and CVF is still required to prevent complement-mediated rejection. Based on the clone of anti-CD40 antibody used, animals were divided into two groups: Group A (n = 3) received mouse IgG2b antibody from clone 3A8 [18,19]; Group B (n = 6) received recombinant mouse-rhesus chimeric IgG4 antibody from clone 2C10R4 [20]. The antibody dose was tapered over 60-day period (20 mg/kg on days − 1, 0, & 5; 10 mg/kg on days 9, 14, & 21; and 5 mg/kg q weekly until day 60). As these antibodies were used for the first time in a pig-to-baboon cardiac xenotransplantation model, the tapering dose of antibody was selected after consulting with the manufacturer and was based on the experience of islet xenotransplantation experiments, utilizing the same antibodies for different period of times [19,20].
Table 1. Treatment regimen.
| Drugs | Dose | Timing |
|---|---|---|
| Induction | ||
| Anti-CD20 antibody | 19 mg/kg | Preoperative days −7, 0, 7 & 14 |
| ATG | 40–50 mg/kg | Preoperative days −2 & −1 |
| Anti-CD40 (3A8) or (2C10) antibody | 20 mg/kg | Preoperative days −1 & 0 |
| CVF | 50–100 U/kg | Preoperative days −1, 0 & 1 |
| Maintenance | ||
| Anti-CD40 (3A8) or (2C10 antibody) | 5–20 mg/kg | 20 mg/kg on postoperative day 5, 10 mg/kg on day 9,14, h & 21, 5 mg/kg q weekly until day 60. |
| MMF | 20 mg/kg/2 h IV infusion | BID daily |
| Steroids | 2 mg/kg | BID, tapered off in 7 weeks |
| Aspirin | 81 mg | Daily |
| Heparin | Maintain ACT h 2× baseline | Continuous infusion |
| Supportive | ||
| Ganciclovir | 5 mg/kg/day | Daily |
| Cefazolin | 250 mg | BID for 7 days |
| Epogen | 200 U/kg | Daily from −7 to 7 then weekly |
ACT, activated clotting time; ATG, anti-thymocyte globulin; CVF, cobra venom factor; MMF, mycophenolate mofetil.
Treatment of rejection
Upon detecting rejection by telemetry, rescue therapy was initiated with intravenous methyl prednisolone (10–15 mg/kg) for 6 days. Heparin dose was also increased to prevent thrombus formation, whenever a drop in activated clotting time (ACT) below the target value (2× the baseline value) was observed.
Cross-reactivity of anti-CD40 antibody with baboon
Anti-CD40 monoclonal antibody clones 3A8 and 2C10 were conjugated to R-phycoerythrin (PE). Peripheral blood mononuclear cells (PBMCs) were isolated from human and baboon whole blood and then stained with anti-CD20-FITC and 2C10-PE or 3A8-PE. Samples were washed, fixed in PBS/ 1% formaldehyde, and analyzed on a flow cytometer using light scatter to gate lymphocytes and CD20 fluorescence to gate B cells.
Telemetry implantation as a method of evaluating graft function
A telemetry device (Konigsberg Instruments, Inc, Pasadena, CA, USA) was implanted into the recipient to monitor graft left ventricular pressure (LVP) and electrocardiogram (EKG), as well as, the recipient's temperature. The telemetry device data are transmitted wirelessly to a receiver attached to the animal's cage (RMISS, Wilmington, DE, USA). The parameters recorded include peak systolic pressure (PEAK), end diastolic pressure (END), left ventricular pressure (PEAK-END; LVP), heart rate based on LVP (LVPHR) by counting the PEAK LVP tracings in a minute, EKG, heart rate based on EKG (EKGHR) by counting the QRS complexes in a minute and recipient's body temperature (TEMP). Heart function was continuously evaluated by telemetry, and a drop in LVP below 60 mmHg indicated the time point at which the rejection process starts to affect the graft contractility, and a pressure below 10 mmHg was an indication of complete cessation of graft contractility.
Measurement of graft survival
Graft survival was measured by multiple methods. LVP measurements were used as a main indicator of strength of contractions, and heart rate based on both LVP and EKG was recorded. Besides telemetry, periodic measurements by palpation and determination of heart function using echocar-diography (Echo) were also conducted. Manual palpation was scored from ++++ (strong contractility) to 0 (no palpable contractions). Echo was used to visualize the contractility, measure wall thickening, blood flow, and intracavity thrombus formation. Final determination of graft rejection was made on histological examination.
Measurement of B cell and non-Gal antibody levels
Flow cytometry was used to measure the numbers of B cells in the peripheral blood as described before [4]. Porcine aortic endothelial cells (PAEC) were isolated and cultured from GTKO.hCD46 Tg pigs. Serum samples were collected from the transplanted baboons every 2–3 days for a month, then biweekly throughout the study period. The method of staining using PAEC has been described previously [17]. Mean fluorescence intensity (MFI) was used to determine the non-Gal antibody titers in the serum.
Laboratory tests
Complete blood count (CBC), liver enzymes, electrolytes, BUN, creatinine, ACT, and troponin levels were measured weekly for the first 2 months and then biweekly until the graft was rejected or explanted.
Histology
Paraffin sections from biopsies and explanted xenografts were stained with hematoxylin and eosin for light microscopy. Sections were analyzed semi-quantitatively for the presence of hemorrhage, necrosis, thrombosis, and cellular infiltrates. Rejection was scored from 0 to 4 based on percentage of graft affected (0 = none, 1 = ≤20%, 2 = ≤50%, 3 = ≤75%, 4 = ≥75%).
Statistical test
Anti-non-Gal antibody levels and platelet numbers are presented as mean ± SEM. The differences between the groups were determined by Student's t-test. P values of <0.05 were considered significant.
Results
Measurement of cross-reactivity of anti-CD40 antibody with baboon
To confirm that anti-CD40 antibodies 3A8 and 2C10 recognized baboon CD40, we compared the binding of PE conjugates of both antibodies on human and baboon blood B cells. As shown in Fig. 1, fluorescence intensity of each antibody was similar for human and baboon B cells. The lower fluorescence intensity seen for 2C10 is likely due to a lower fluorophore conjugation efficiency in the preparation used because 2C10 has a higher affinity for CD40 than 3A8 (R. Wang, K.A. Reimann, unpublished data).
Fig. 1.
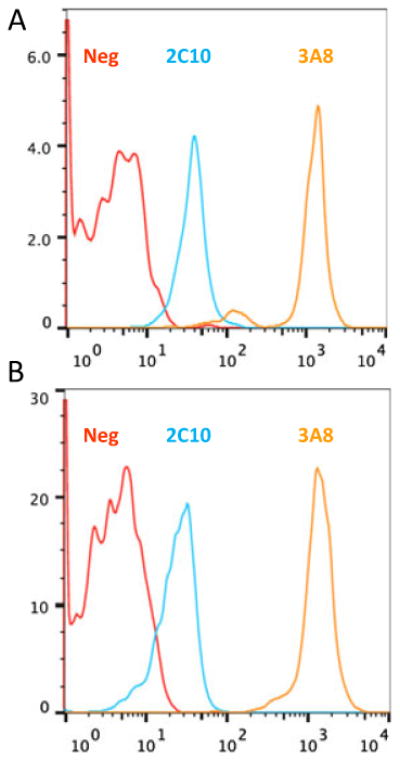
Anti-CD40 antibodies from clone 3A8 and 2C10 bind similarly to human and baboon B cells: (A) Human or (B) baboon PBMCs were stained with anti-CD20-FITC and anti-CD40-PE (clone 3A8 or 2C10). PE fluorescence on CD20+ B cells confirms both antibodies bind similarly to human and baboon CD40.
Xenograft survival
As shown in Table 2, all grafts in Group A receiving 3A8 antibody treatment rejected within 3-4 weeks. Whereas grafts in Group B receiving 2C10 antibody had the longest survival of 149 days. The median survival time of all grafts in this group was 84 ± 48 days (range 60–149 days for the grafts that were ultimately rejected). Two animals had to be euthanized, while the transplanted heart was still viable, due to gastrointestinal bleeding or pulmonary hemorrhage that was discovered at necropsy. The etiology of bleeding could not be determined. These baboons died within 40 days of transplantation and were receiving low dose (50 U/h) heparin infusion. All the other baboons remained healthy throughout the study period and completely rejected the xenograft. On rejection, the xenograft was explanted for histological analysis, and the recipient baboons were allowed to recover. These baboons remained healthy and complication free after the explanation. Graft rejection correlated with drop in LVP and decrease in manual palpation scores as shown in Table 2. All the rejected grafts had LVP below 10 and palpation scores of 0. The rescue therapy with steroids had no obvious benefit in preventing or delaying rejection. Increase in heparin dosage delayed the progression of thrombus formation in the ventricular cavities.
Table 2. Graft survival.
| No | Baboon ID | Baboon weight (Kg) | Pig ID | Pig weight (Kg) | Treatment | Graft survival (days) | Graft score | LVP mmHg | Complications | Graft/recipient status |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10308 | 8.4 | 452-01 | 10 | A | 21 | 0 | <10 | None | EXP/Alive |
| 2 | 7807 | 9.0 | 444-07 | 7.5 | A | 21 | 0 | <10 | None | EXP/Alive |
| 3 | 11008 | 7.7 | 452-05 | 12 | A | 28 | 0 | <10 | None | EXP/Alive |
| 4 | 9708 | 8.8 | 483-01 | 8.0 | B | 146 | 0 | <10 | None | EXP/Alive |
| 5 | 6907 | 12 | 483-02 | 9.0 | B | 149 | 0 | <10 | None | EXP/Alive |
| 6 | 2207 | 13.1 | 485-03 | 8.2 | B | 107 | 0 | <10 | None | EXP/Alive |
| 7 | 13908 | 9.5 | 488-01 | 8.4 | B | >30 | +++ | >50 | GI bleeding | Euthanized |
| 8 | 9908 | 8.3 | 498-05 | 8.0 | B | 60 | 0 | <10 | None | EXP/Alive |
| 9 | 509 | 10.2 | 505-07 | 10 | B | >40 | ++ | >60 | Pulmonary hemorrhage | Euthanized |
A = 3A8 antibody.
B = 2C10 antibody.
EXP, explant; LVP, left ventricular pressure.
Measurement of B-lymphocyte numbers and non-Gal antibody levels in the recipient baboons
The B lymphocytes were completely depleted by the pre-treatment of four doses of anti-CD20 antibody. On FACS analyses performed weekly, no B lymphocytes were detected for 60 days post-transplantation (Fig. 2A). The B lymphocytes slowly made their appearance after 60 days in three Group B baboons surviving for more than 60 days and regained their pre-transplant numbers shortly thereafter. One baboon demonstrated a rebound with much higher B-lymphocyte numbers than its pre-transplant level (Fig. 2A). The SPF baboons used had low baseline non-Gal antibody titers compared with conventional baboons [4]. Both IgG and IgM non-Gal antibodies were increased in all the animals treated with antibody from clone 3A8 (Group A). Whereas, these antibodies were suppressed in baboons treated with antibody from 2C10 clone (except in two of six animals where the titers were moderately increased; Group B). A marked rise in antibody titer was observed in Group B after the termination of anti-CD40 treatment at day 60, when the B lymphocytes also start to reappear. Suppression of non-Gal antibody production was not as prominent in Group A, indicating that the 3A8 clone was less effective than the 2C10 clone at a similar dose (Fig. 2B). The comparison of average values of antibody titer before and after transplantation (both IgM and IgG) in both treatment groups at 0–60 days time points (Fig. 2C) indicates that a statistically higher amount of antibody was produced in 3A8-treated animals than the 2C10-treated group. However, a much higher amount of non-Gal IgM and IgG antibody was detected in Group B during the 61–150 day period, indicating that long-term treatment with 2C10 antibody is required.
Fig. 2.
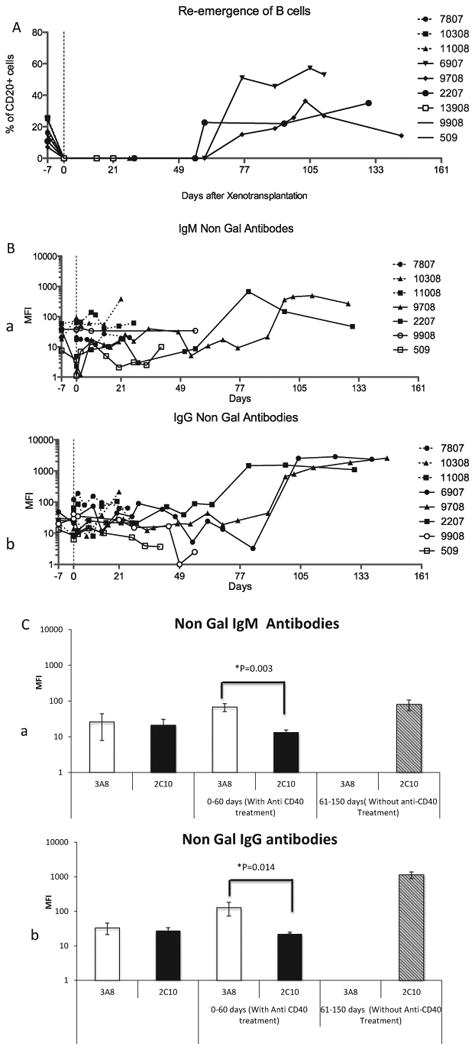
B lymphocytes and non-Gal antibody titers: (A) Percentage of CD20+ B lymphocytes present in the peripheral blood throughout the graft survival period, (B) non-Gal IgM (a) and IgG (b) antibodies before and after transplantation, and (C) comparison of average mean fluorescence intensity of non-Gal IgM (a) and IgG (b) antibody in two treatment groups before transplantation, after transplantation up to day 60 and after discontinuing the antibody after day 60 until the graft rejection.
CBC, chemistry, and troponin levels
Baboons in all groups maintained their normal hemoglobin (11–14 gm/dl), hematocrit (33–43%), and RBC counts (4.3–5.6 million/mm3; Fig. 3). All of the baboons had a moderate increase in liver enzymes. Except for two baboons, which had GI and intrapulmonary bleeding and required blood transfusions, no other baboons developed anemia. The platelet numbers, after an initial drop (≤100 thousand/mm3) immediately after the surgery, were maintained at a healthy level (≥150 thousand/mm3) throughout the graft survival period (Fig. 4A,B). The initial platelet drop was more significant in the 3A8 group than 2C10 group between days 0–7 (P = 0.018) and on days 8–30 (P = 0.0014). A very low dose of heparin (50– 100 U/h) was required to maintain the intravenous lines and to keep the ACT to a 29 normal level (≥200 s; Fig. 4C). We have used the heparin therapy in all baboons receiving pig cardiac xenograft in our transplantation program (n ≥ 50). Heparin is found to help reduce the incidence of thrombus formation in the ventricular cavities. The troponin levels were increased (≥1) immediately after the surgery but dropped to zero within a week. Levels were maintained at this mark until the graft started showing signs of rejection (gradual decrease in LVP and graft contractility), at which time the troponin levels gradually increased to its maximum levels (>8) and then start to decrease after the necrosis and fibrosis sets in following complete rejection (no evidence of contractility on palpation and echo, and LVP below 10 mmHg; Fig. 5). The decline in troponin levels at this stage is most likely due to degradation of troponin by proteolytic enzymes released by the necrotic tissue [21]. Hearts were explanted at this time point, and baboons were survived.
Fig. 3.
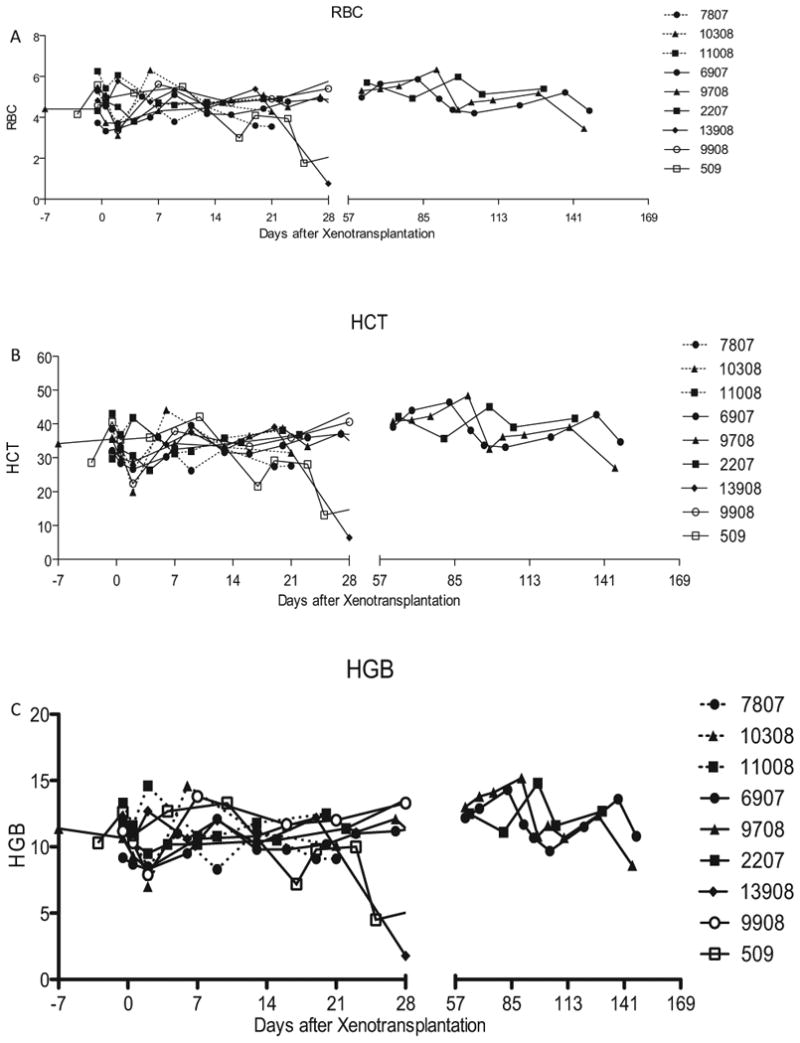
Laboratory tests: Results for both Groups A and B showing the values for (A) red blood cell numbers (B) hematocrit and (C) hemoglobin.
Fig. 4.
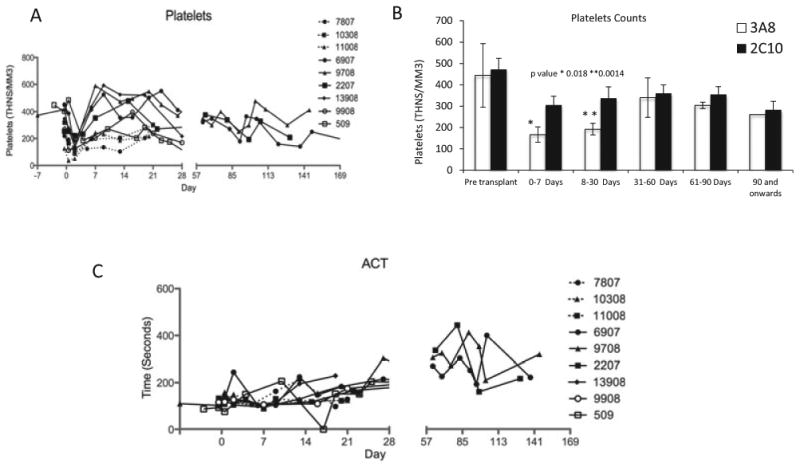
Platelet counts and activated clotting time (ACT) values for Groups A and B: (A) Platelet numbers throughout the graft survival, (B) platelet levels in two groups at different time points, and (C) ACT values for the baboons in two groups for the entire survival period. Animals were survived after explantation of rejected hearts, and platelet counts were measured even after the graft explantation.
Fig. 5.
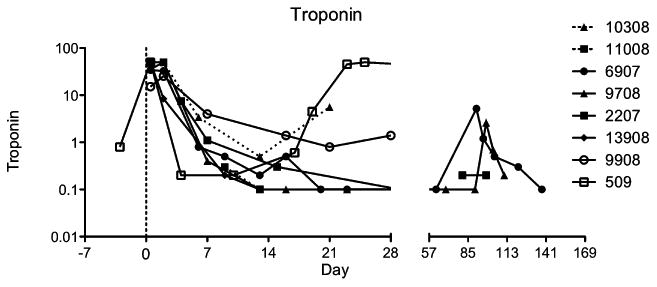
Troponin values for both Groups A and B: Grafts were harvested when troponin levels have reached their peaks and started to decline.
Histology
The hearts from the two groups had a rejection score of four and as shown in Fig. 6 gave somewhat similar pictures of microvascular thrombosis (A & C), hemorrhage (D), and focal areas of myocyte necrosis and fibrosis (A & D). Lymphocytic infiltration was also seen in one 3A8-treated baboon (Fig. 6B). Although the histological picture was similar, the rejection was delayed in Group B compared with Group A grafts. Perhaps the rejection process was slowed down by the treatment in Group B, but not completely prevented resulting in similar histological appearance in the end. We found the immunohistochemistry comparison of rejected grafts to be very similar as both IgG and IgM antibody deposition were present in all groups including the long-term surviving grafts reported earlier irrespective of the time of rejection.
Fig. 6.
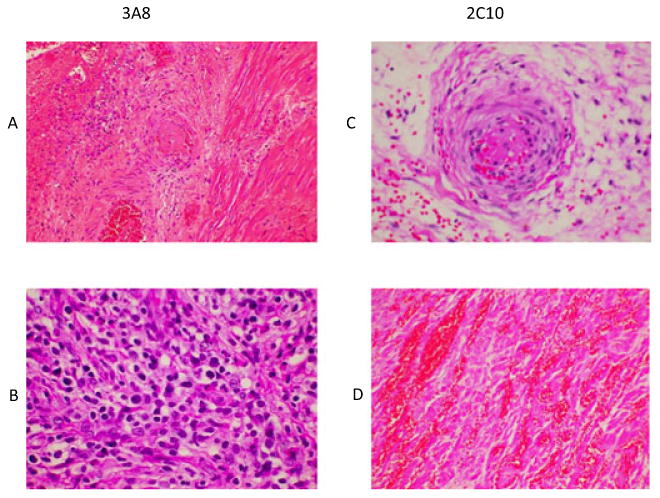
Histology: Findings from both groups were similar showing interstitial micro vascular thrombosis (A and C), interstitial hemorrhage (D), myocyte necrosis (A and D), and rare lymphocyte infiltration (B).
Discussion
The mechanism of rejection of cardiac xenograft in a pig-to-baboon xenotransplantation model is very complex [22]. There are layers of defense mechanisms present, which are usually discovered after the preceding rejection mechanism is avoided. Overcoming the anti-Gal antibody barrier was a major milestone, but the remaining hurdles were not thought to be as significant as naturally occurring Gal antibodies mediated hyperacute rejection (HAR). It soon became evident that even after avoiding the HAR, the non-Gal antibodies continue to cause delayed graft rejection [23,24]. Several improvements in pig genetics have been tried even before the GTKO animals became available. These modifications included expression of human complement regulatory proteins such as DAF and CD46 [25,26]. Variable graft survival results with these genetic modifications are reported [27]. Some recent modifications to GTKO pigs include expression of human thromboregulatory proteins such as CD39 and thrombomodulin [28,29] but the graft survival data for organs from these pigs are not yet available. Besides genetic modifications of the donor, the immunosuppressive regimen is continuously being modified with more emphasis on reducing the lethal side effects by replacing generalized immunosuppression with target-specific immunosuppression or immunomodulation. The B lymphocytes-mediated rejection continues to be a hurdle [30]. We have previously shown that by depleting B cells with anti-CD20 antibody, costimulation blockade by anti-CD154 antibody, and treatment with MMF, we can prolong cardiac xenograft survival in a pig-to-baboon model for up to 236 days [4]. Despite achieving significant graft prolongation in the above group of experiments, we encountered several complications leading to recipient death or need for euthanasia before the transplanted heart fully rejected [31]. In our experience and also by others, anti-CD154 antibody use resulted in complications including TM, CC, and bleeding [8,32]. In a few anti-CD154-treated animals, we also observed drop in platelet numbers, and blood transfusion was required to overcome thrombocytopenia. In most cases, high fever (temperatures over 39 °C) preceded thrombocytopenia, perhaps indicating a role of infection in triggering this effect. Although thrombocytopenia or CC has also been shown to occur in transplant experiments not using anti-CD154 antibody, in our experience, thrombocytopenia was more pronounced with anti-CD154 antibody treatment. Some of the other complications encountered in CD154 antibody-treated baboons included cardiac rupture, bronchopneumonia after aspiration, intestinal obstruction, and rupture [31]. Infections, both bacterial and fungal, were also witnessed but were rare. Perhaps the reason why these infections were a non-factor in recipient survival was due to our ability to detect the onset of fever early with the help of the in vivo telemetry system and the ability to institute broad-spectrum antibiotic treatment at an early stage of infection.
The importance of the CD40–CD40L pathway in transplantation is now very well established. To further modify the immunosuppression regimen and overcome the thrombotic complications of anti-CD154, we explored the use of the recently available anti-CD40 antibodies that block the interaction between CD40L and CD40 molecules. The first antibody made available to us was mouse IgG2b (clone 3A8). This clone activated B cells in vitro but was able to extend graft survival in experimental allotransplant models [19]. The other antibody was mouse/non-human primate chimeric IgG4 (clone 2C10), and this antibody functioned similar to anti-CD154 antibody in vitro. The primatized (mouse–rhesus chimeric) 2C10 antibody had many favorable characteristics including: suppression of CD154-mediated B-cell activation without depleting them, binding to a different epitope of CD40 than the other anti-CD40 antibodies and prevention of antigen-specific antibody formation [19,20]. The 2C10 antibody has also shown to successfully suppress the allo response and has prolonged the survival of islet allografts in non-human primates [20].
Our laboratory was the first one to use both anti-CD40 antibodies from clone 3A8 and clone 2C10 in a pig-to-baboon cardiac xenotransplantation model. The dosages of both anti-CD40 clones used in this experiment were chosen after consultation with the manufacturer and considering their prior use in islet transplantation studies [19] [20]. The reason why the mouse antibody was relatively ineffective in our model could be due to the specificity of this antibody, elicitation of anti-mouse Ig antibody responses, and also due to its ability to activate B lymphocytes. Although the graft survival was prolonged significantly with 2C10 antibody treatment, the survival period was much less than the grafts in anti-CD154-treated baboons. This difference could also be attributed to the tapering dose of antibody, generation of anti-Ig antibodies, and also termination of antibody use after 2 months. In comparison with non-Gal antibody production between the two treatment groups for the first 60 days (Group A grafts lasted for less than a month), it was revealed that on average, a higher amount of anti-non-Gal antibody (both IgG and IgM) was produced in our 3A8-treated group vs. 2C10 group, indicating better control of antibody production by 2C10 antibody even with a tapering dose. It was also noted that after stopping the antibody treatment, the non-Gal antibody titers started to rise, which may be a contributing factor for graft rejection. Our results suggest that a longer duration and higher dose of species-matched recombinant antibodies are required to better control the antibody production and achieve consistent long-term graft survival.
One of the promising finding in this study was the relative absence of persistent thrombocytopenia and CC after treatment with anti-CD40 antibody. Transient thrombocytopenia (<100 000) was observed immediately after the transplantation in both groups (more in 3A8 group than 2C10 group), but adequate platelet numbers (>150 000) were regained within a week. As CD154 is expressed on platelets, anti-CD154 antibody directly binds to it on the platelets leading to platelet activation and CC. In both anti-CD40 antibody treatment groups, CC was not observed, indicating that anti-CD40 antibody can be safely used without the complication of platelet activation. In this study, there was also no reduction in baboon's hematocrit, hemoglobin, and red blood cell numbers. Only one of the two baboons with bleeding complications required blood transfusion, and in the second baboon, pulmonary hemorrhage was discovered on necropsy. Infections were also not observed in any of these transplanted baboons.
In conclusion, the primatized (mouse–rhesus chimeric) anti-CD40 antibody (2C10R4) has an encouraging clinical potential, as its binding site constituting first 61 amino acids is well conserved between human and baboons. The retention of 2C10 affinity for human CD40 in in vitro experiments [20] indicates that this antibody is likely to be as effective in humans as it is in our NHP model. Our experiments indicate that the antibody dosage selected for this study is not sufficient to prolong the cardiac xenograft survival beyond 140 days. Perhaps a higher maintenance dose of 2C10 or better species matching of recombinant anti-CD40 antibody is required throughout the period of graft survival. The 3A8 antibody, at the current dose, was relatively ineffective in our large animal xenotransplantation model. The newly available pigs with modified genetics also have a greater potential to help in reducing the number of targets requiring suppression.
Acknowledgments
Authors would like to acknowledge the following: Ms. Patricia Jackson for the administrative support. Mr. Caleb Seavey for designing Microsoft excel-based software to analyze hematologic parameters. Ms. Caitlin Mayhew and Mr. Matt Holzner for their support in data collection. NHLBI core facility staff at the animal program for their support in surgical procedures and Flow cytometry core for their help with FACS analyses, and Division of Veterinary resource staff for the animal care.
Footnotes
Disclosure: Dr. David Ayares is the Executive Vice President and Chief Scientific Officer of Revivicor. Inc. Dr. Keith Reimann holds equity in Primatope Therapeutics who has licensed the 2C10 antibody.
References
- 1.Larsen CP, Elwood ET, Alexander DZ, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 2.Cardona K, Korbutt GS, Milas Z, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12:304–306. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 3.Thompson P, Cardona K, Russell M, et al. CD40-specific costimulation blockade enhances neonatal porcine islet survival in nonhuman primates. Am J Transplant. 2011;11:947–957. doi: 10.1111/j.1600-6143.2011.03509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohiuddin MM, Corcoran PC, Singh AK, et al. B-cell depletion extends the survival of GTKO.hCD46Tg pig heart xenografts in baboons for up to 8 months. Am J Transplant. 2012;12:763–771. doi: 10.1111/j.1600-6143.2011.03846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dor FJ, Kuwaki K, Tseng YL, et al. Potential of aspirin to inhibit thrombotic microangiopathy in alpha1,3-galactosyltransferase gene-knockout pig hearts after transplantation in baboons. Transplant Proc. 2005;37:489–490. doi: 10.1016/j.transproceed.2004.12.235. [DOI] [PubMed] [Google Scholar]
- 6.Larsen CP, Elwood ET, Alexander DZ, et al. Pillars article: long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;1996(381):434–438. J Immunol 2011; 2693–2697. [PubMed] [Google Scholar]
- 7.Haanstra KG, Ringers J, Sick EA, et al. Prevention of kidney allograft rejection using anti-CD40 and anti-CD86 in primates. Transplantation. 2003;75:637–643. doi: 10.1097/01.TP.0000054835.58014.C2. [DOI] [PubMed] [Google Scholar]
- 8.Koyama I, Kawai T, Andrews D, et al. Thrombophilia associated with anti-CD154 monoclonal antibody treatment and its prophylaxis in nonhuman primates. Transplantation. 2004;77:460–462. doi: 10.1097/01.TP.0000110291.29370.C0. [DOI] [PubMed] [Google Scholar]
- 9.Buhler L, Yamada K, Kitamura H, et al. Pig kidney transplantation in baboons: anti-Gal(alpha)1-3Gal IgM alone is associated with acute humoral xenograft rejection and disseminated intravascular coagulation. Transplantation. 2001;72:1743–1752. doi: 10.1097/00007890-200112150-00007. [DOI] [PubMed] [Google Scholar]
- 10.Lin CC, Ezzelarab M, Shapiro R, et al. Recipient tissue factor expression is associated with consumptive coagulopathy in pig-to-primate kidney xenotransplantation. Am J Transplant. 2010;10:1556–1568. doi: 10.1111/j.1600-6143.2010.03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6:114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 12.Aoyagi T, Yamashita K, Suzuki T, et al. A human anti-CD40 monoclonal antibody, 4D11, for kidney transplantation in cynomolgus monkeys: induction and maintenance therapy. Am J Transplant. 2009;9:1732–1741. doi: 10.1111/j.1600-6143.2009.02693.x. [DOI] [PubMed] [Google Scholar]
- 13.Ossevoort MA, Ringers J, Boon L, et al. Blocking of costimulation prevents kidney graft rejection in rhesus monkeys. Transplant Proc. 1998;30:2165–2166. doi: 10.1016/s0041-1345(98)00576-4. [DOI] [PubMed] [Google Scholar]
- 14.Ekser B, Rigotti P, Gridelli B, Cooper DK. Xenotrans-plantation of solid organs in the pig-to-primate model. Transpl Immunol. 2009;21:87–92. doi: 10.1016/j.trim.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Loveland BE, Milland J, Kyriakou P, et al. Characterization of a CD46 transgenic pig and protection of trans-genic kidneys against hyperacute rejection in non-immunosuppressed baboons. Xenotransplantation. 2004;11:171–183. doi: 10.1046/j.1399-3089.2003.00103.x. [DOI] [PubMed] [Google Scholar]
- 16.Phelps CJ, Koike C, Vaught TD, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu G, Pfeiffer S, Schroder C, et al. Co-stimulation blockade targeting CD154 and CD28/B7 modulates the induced antibody response after a pig-to-baboon cardiac xenograft. Xenotransplantation. 2005;12:197–208. doi: 10.1111/j.1399-3089.2005.00221.x. [DOI] [PubMed] [Google Scholar]
- 18.Kwekkeboom J, De Boer M, Tager JM, De Groot C. CD40 plays an essential role in the activation of human B cells by murine EL4B5 cells. Immunology. 1993;79:439–444. [PMC free article] [PubMed] [Google Scholar]
- 19.Badell IR, ThompsonPW, Turner AP, et al. Nondepleting Anti-CD40-Based Therapy Prolongs Allograft Survival in Nonhuman Primates. Am J Transplant. 2012;12:126–135. doi: 10.1111/j.1600-6143.2011.03736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowe M, Badell IR, Thompson P, et al. A novel monoclonal antibody to CD40 prolongs islet allograft survival. Am J Transplant. 2012;12:2079–2087. doi: 10.1111/j.1600-6143.2012.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katrukha AG, Bereznikova AV, Filatov VL, et al. Degradation of cardiac troponin I: implication for reliable immunodetection. Clin Chem. 1998;44:2433–2440. [PubMed] [Google Scholar]
- 22.Mohiuddin MM. Clinical xenotransplantation of organs: why aren't we there yet? PLoS Med. 2007;4:e75. doi: 10.1371/journal.pmed.0040075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tazelaar HD, Byrne GW, McGregor CG. Comparison of Gal and non-Gal-mediated cardiac xenograft rejection. Transplantation. 2011;91:968–975. doi: 10.1097/TP.0b013e318212c7fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen G, Qian H, Starzl T, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med. 2005;11:1295–1298. doi: 10.1038/nm1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams DH, Kadner A, Chen RH, Farivar RS. Human membrane cofactor protein (MCP, CD 46) protects transgenic pig hearts from hyperacute rejection in primates. Xenotransplantation. 2001;8:36–40. doi: 10.1046/j.0908-665x.2000.00085.x. [DOI] [PubMed] [Google Scholar]
- 26.Buhler L, Yamada K, Alwayn I, et al. Miniature swine and hDAF pig kidney transplantation in baboons treated with a nonmyeloablative regimen and CD154 blockade. Transplant Proc. 2001;33:716. doi: 10.1016/s0041-1345(00)02220-x. [DOI] [PubMed] [Google Scholar]
- 27.Ekser B, Cooper DK. Overcoming the barriers to xeno-transplantation: prospects for the future. Expert Rev Clin Immunol. 2010;6:219–230. doi: 10.1586/eci.09.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yazaki S, Iwamoto M, Onishi A, et al. Production of cloned pigs expressing human thrombomodulin in endo-thelial cells. Xenotransplantation. 2012;19:82–91. doi: 10.1111/j.1399-3089.2012.00696.x. [DOI] [PubMed] [Google Scholar]
- 29.Wheeler DG, Joseph ME, Mahamud SD, et al. Transgenic swine: expression of human CD39 protects against myocardial injury. J Mol Cell Cardiol. 2012;52:958–961. doi: 10.1016/j.yjmcc.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierson RN., III Antibody-mediated xenograft injury: mechanisms and protective strategies. Transpl Immunol. 2009;21:65–69. doi: 10.1016/j.trim.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corcoran PC, Horvath KA, Singh AK, et al. Surgical and nonsurgical complications of a pig to baboon hetero-topic heart transplantation model. Transplant Proc. 2010;42:2149–2151. doi: 10.1016/j.transproceed.2010.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buhler L, Alwayn IP, Appel JZ, III, Robson SC, Cooper DK. Anti-CD154 monoclonal antibody and thromboembolism. Transplantation. 2001;71:491. doi: 10.1097/00007890-200102150-00028. [DOI] [PubMed] [Google Scholar]


