Abstract
Purpose of the study
We used a rabbit model infected with high phenotypic reactivators (HPR) as well as recombinant HSV-1 with deletions to study their effect on corneal innervations after latency was established.
Materials and methods
Corneas from non-infected New Zealand white rabbits were used to obtain the entire map of corneal innervation. Others were inoculated with the HSV-1 strains McKrae, 17 Syn+ or recombinant mutants with glycoprotein K (gk) deletion or with infected early protein 0 (ICP0) deletion. The animals were euthanized at 124 to 125 days post-infection and the corneas were immunostained with a mouse monoclonal anti-βIII tubulin antibody. Images were acquired with a fluorescence microscope and corneal sub-basal nerve density was calculated on the basis of the whole mount images. Differences between the HSV-infected eyes, and comparison with normal control, were analyzed.
Results
In the non-infected rabbit, the stroma was densely innervated in the central area and as a consequence the subbasal epithelial nerve bundles were shorter, and no vortex was found. The HSV-infected corneas showed nerve damage in both epithelial and stromal nerves. Corneas infected with ICP0 and gk deletion mutants showed mild to moderate damage, while those infected with 17Syn+ and McKrae strains were seriously damaged. In the eyes infected with ICP0 and gk deletion, there were reduced numbers of subbasal nerve bundles, but most of the corneas retained a normal stromal network. Corneas infected with 17 Syn+ and McKrae displayed destroyed nerve structures and formation of a scar tissue in the central cornea, in which only a few nerve fibers could be detected.
Conclusion
HSV-1 primary corneal infection seriously damages the corneal nerves, persisting for more than 4 months. Reduction of axonal transport (by gk deletion) or virus replication (by ICP0 deletion) significantly attenuated the nerve damage induced by the virus.
Keywords: HSV-1, herpetic stromal keratitis, corneal nerve, corneal sensitivity, immunofluorescence
Introduction
The cornea is predominantly innervated by the ophthalmic division of the trigeminal ganglion (TG). These nerves innervate both the corneal epithelium and the stroma, and they serve an important role in maintaining a healthy corneal surface. Interruption of corneal innervation may result in altered epithelial morphology and function, poor tear film, and delayed wound healing.1–3 Our laboratory previously unveiled the entire human corneal nerve architecture and studied the changes that occur during diabetes and corneal dystrophies.4–6 Using the rabbit as a model, we also studied the nerve regeneration that occurs after lamellar keratectomy in corneas treated with pigment epithelium derived factor (PEDF) plus docosahexaenoic acid (DHA) or the DHA-derived lipid mediator, neuroprotectin D1 (NPD1).7–10
Herpetic stromal keratitis (HSK) is one of the most damaging ocular infections. It is the leading cause of infectious blindness in the developed world, with a reported prevalence of ocular herpetic manifestations in 11.8 out of 100,000 individuals in the United States.11–12 Once HSV-1 infects the corneal epithelium, the virus then penetrates the nerve terminals and, by retrograde axonal transport, reaches the TG, where it establishes a latent infection. It is estimated that 33% of the global population has this virus in latency.11 Reactivation can be induced through multiple factors, and it occurs when the virus is carried by anterograde transport to the initial sites of infection or any site innervated by the latently-infected ganglia. Resulting complications include severe dry eye and loss of sensitivity, susceptibility to injury, damage to the epithelium and, in the most advanced stages, HSK that can lead to stromal scarring, thinning, opacity, and neovascularization.13,14
The rabbit model is useful for studying the mechanisms of HSV-1 latency, reactivation, and recurrence.15–17 An important component of the structure of the virus is glycoprotein K (gK), and deletion of the gK gene inhibits efficient replication and spread of the virus in the corneal epithelium and TG.18,19 Another important protein encoded by the virus is the infected cell polypeptide 0 (ICP0). The protein is produced at the beginning of the infection and transported to the nucleus of the infected cells, where it stimulates transcription of viral genes.20
Alterations of corneal innervation have been observed in patients with HSV-1 infection,21–23 but experimental evidence supporting this observation remains limited24. Here we describe, for the first time, the entire corneal map in the normal rabbit, and we investigate corneal nerve density in animals infected with HSV-1 and the gk- or ICP0-deficient HSV-1 virus after an extended period of latency.
Material and methods
Animals
New Zealand white rabbits of both sexes and weighing between 2.5 and 3.5 kg were housed in the animal care facility at Lousiana State University Health, New Orleans. Animals were treated in compliance with guidelines of the ARVO Resolution on the Use of Animals in Ophthalmic Research. Experimental protocols were approved by the Institutional Animal Care and Use Committee, Louisiana State University Health, New Orleans.
Rabbit inoculation
Forty rabbits were anesthetized with an intramuscular injection of xylazine (10mg/kg) and ketamine hydrochloride (50mg/Kg). Corneas were topically anesthetized with proparacaine hydrochloride, and a light scarification was made. Rabbits were inoculated with 200,000–300,000 PFU of wild type virus strains: McKrae (10 rabbits) or 17Syn+ (10 rabbits). In addition, 10 rabbits were inoculated with recombinant mutants of the McKrae wild type virus with gk deletion19 (a gift from Dr. Gus Kousoulas), and another 10 rabbits were inoculated with a recombinant mutant with ICPO deletion (constructed by Dr. Bill Halford).25 Rabbit corneal infection was monitored by slit lamp examination from days 3 to 14 for herpes-specific dendritic lesions. On days 124–125 post-infection, the rabbits were euthanized with an overdose of sodium pentobarbital via ear vain injection.
Prior to sacrificing the rabbits, corneal swabs were performed as previously described,26 and the corneas negative for the infectious virus were used in this study.
To study the normal corneal nerve architecture, five non-infected rabbits of the same age were used.
Immunofluorescence and Imaging
Corneas were fixed in freshly-prepared 4% paraformaldehyde in 0.01M phosphate buffer (pH 7.4) for 2 hours at room temperature. After gradient dehydration in 10%, 15%, and 20% sucrose in 0.01M phosphate-buffered saline (PBS) for 2 hours each, the whole corneas were kept in a 24-well plate (one cornea per well) and incubated with 10% normal goat serum plus 0.3% Triton X-100 solution in PBS for 1 hour at room temperature to block non-specific binding. This was followed by incubation with the mouse monoclonal anti-βIII-tubulin (Tuj1, MMS-435p, Covance Antibody Services Inc., Berkeley, CA; 1:1000) in 0.1M PBS containing 1.5% normal goat serum plus 0.1% Triton X-100 for 72 hours at 4°C. After thorough washing with PBS-bovine serum albumin (BSA, 4 × 15 minutes), the corneas were incubated with the secondary antibodies Alexa fluor® 488 goat anti-mouse Ig G (1: 1500) for 24 hours at 4°C and washed again with PBS-BSA. To exclude non-specific labeling, the primary antibody was replaced by serum IgG of the same host species as the primary antibody. In controls without primary antibodies, there were no stains (data not shown). Images were acquired with a Nikon Eclipse TE200 fluorescent microscope equipped with a digital camera using the Meta Vue imaging software in time-lapse mode, as previously described.4–6 The images were recorded on the same plane at adjoining points from each cornea and merged together to build a whole view of the rabbit corneal nerve architecture.
Data Analysis
To calculate the sub-basal epithelial nerve density, six to eight images of different areas in the central cornea were analyzed for each rabbit. Then the fluorescent images were changed to grayscale mode and placed against a white background to provide better contrast. The nerve fibers of each image were carefully drawn with 4-pixel lines following the course of each fiber by using the brush tool in Photoshop imaging software (Adobe Systems, Inc. San Jose, CA). The percentage of the nerve area obtained from each image was quantified by comparison to the total area, and calculations were performed in a blind fashion. Differences in nerve density between the different groups and normal control corneas were analyzed by ANOVA.
Results
Nerve architecture of rabbit cornea
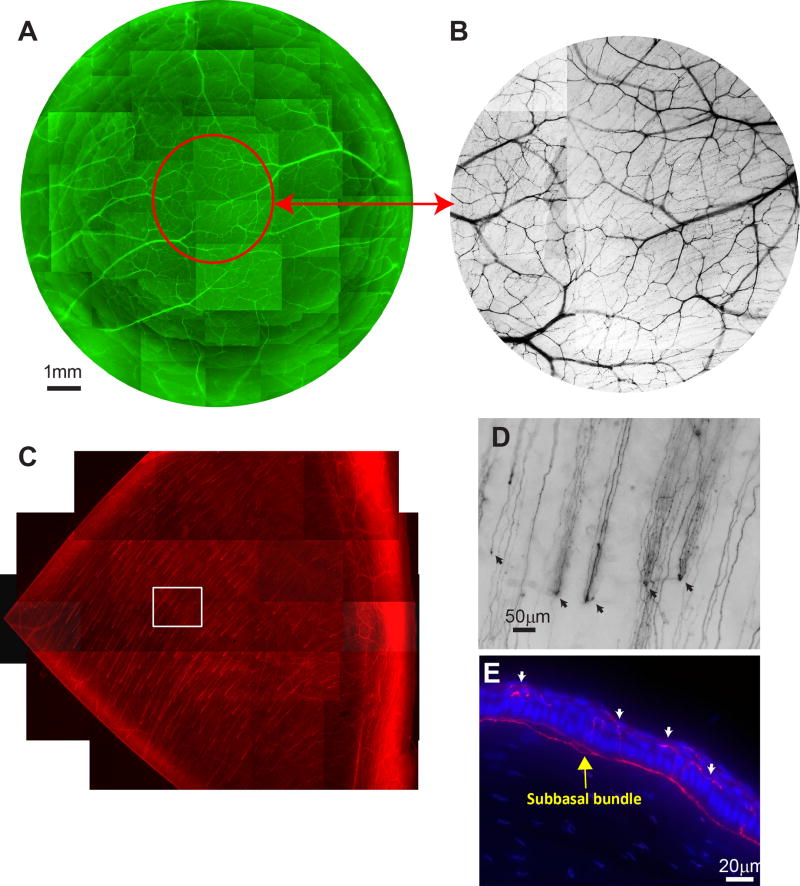
In the healthy cornea, thick stromal nerve trunks entered the cornea around the limbus and advanced toward the center, dividing into branches that ran toward the anterior stroma (Figure 1A). In the adult rabbit, many of these branches connected with each other to form a network in the central area (Figure 1B), subsequently dividing into smaller branches that penetrated upwards into the epithelium to give origin to the sub-basal nerve bundles (Figure 1C). Each penetrating point provided an origin for several bundles (Figure 1D). Along the course, fine terminals budded from the bundles to innervate the epithelial cells (Figure 1E). Unlike those seen on the human cornea,4–6 the epithelial bundles in the rabbit were shorter and did not connect with each other to form a network-like structure that converged in the apex of cornea.
FIGURE 1.
Rabbit corneal nerve architecture. A) Whole mount view of corneal stromal nerves. Images were acquired in a time lapse mode with a Nikon Eclipse TE200 and a 5× lens in compliance with the shape of the cornea. About 350 stacks were used to build the complete view. B) Highlighted image of the center of the corneal stromal nerves. C) Subbasal epithelial bundles in a quarter of the cornea. D) Highlighted image as framed in (C) shows the tips of the stromal nerves penetrating the epithelium and forming the subbasal nerve bundles. A video recorded from the same area shows the detailed nerve architectures at the planes of superficial, subbasal, and mid-stromal layers (see Supplementary Video 1). E) A cross section counter-stained with DAPI shows the locations of subbasal nerve bundles and terminals (dashed arrows) innervating the epithelia.
Nerve density in HSV-1 latently infected corneas
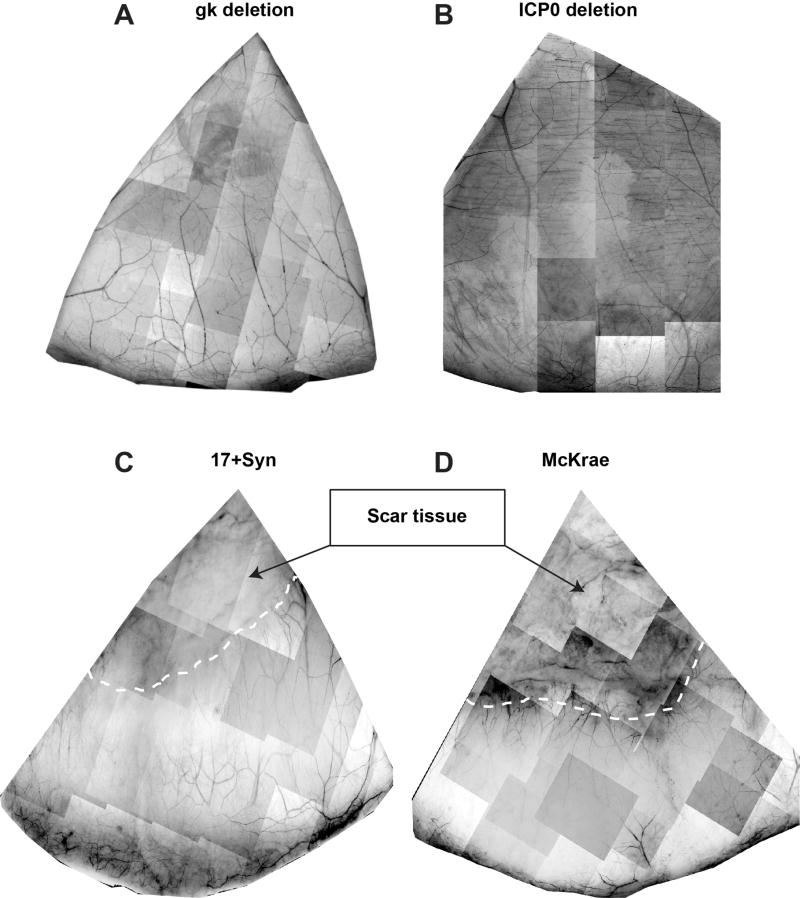
Twenty-two rabbits of the 40 inoculated with the virus had scores for epithelial keratitis from 0–0.5 (normal to non-specific superficial lesions) when measured with the slit lamp,27 and they had negative swabs. There were three rabbits infected with the McKrae strain, four rabbits with the 17Syn+, eight rabbits with the ICP0 deletion, and seven rabbits with the gk deletion. Corneas of these rabbits were fixed and immune-labeled with βIII-tubulin. Images were captured, and the subbasal nerve density was calculated as explained in the Methods. Compared to the normal corneas, all the HSV-1-infected rabbits showed a significant decrease in corneal nerve density after more than four months of infection (Figure 2). Corneas infected with the 17Syn+ and McKrae viruses showed intense epithelial and stromal nerve damage as well as formation of scar tissue in the central cornea, where very few nerve fibers could be detected.
FIGURE 2.
Corneal nerve topography after HSV-1 infection with different phenotypes and mutants. Representative whole mount images showing the nerve architecture in one quarter of rabbit corneas in each infected condition stained with βIII tubulin antibody. Corneas infected with 17Syn+ and McKrae virus show scar tissue in the central cornea, in which a few nerve fibers could be detected.
In the ICP0-infected rabbits, the damage appeared as a decrease in the number of subbasal nerve bundles, but most of the corneas remained within the normal stromal network. In the corneas infected with the gk deletion, there was (in addition to a subbasal nerve decrease) some stromal nerve damage.
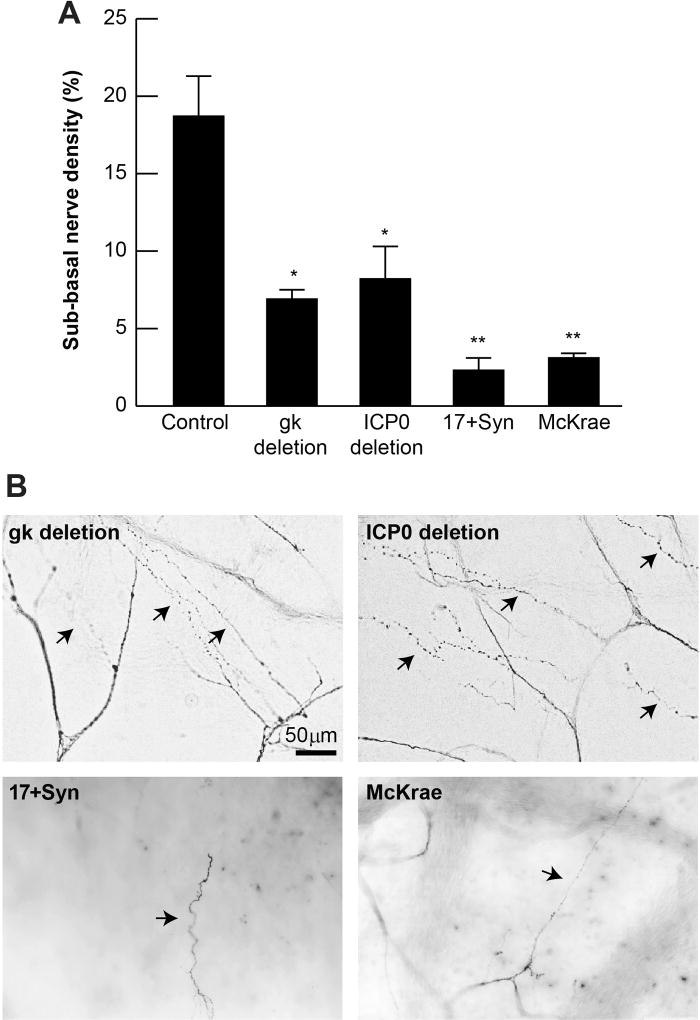
Figure 3A compares the sub-epithelial nerve density in healthy rabbits with rabbits infected with the different viruses after a relatively long period of infection and without reactivation. There was an 88% decrease of nerve density, with respect to the control, in the corneas infected with the 17Syn+ virus, and there was an 84% decrease of nerve density compared to normal corneas in the animals inoculated with the McKrae phenotype (p<0.00005 and 0.0001, respectively). Corneas infected with the gk and ICP0 deletion mutants showed a 63% and a 56% decrease in nerve density with respect to control corneas (p<0.001 and 0.0005, respectively). This means that corneas inoculated with the gk mutant had between 2–3 times less nerve degeneration than corneas infected with the McKrae or 17Syn+ viruses, while corneas inoculated with the ICP0 mutant had between 2.6–3.6 times less nerve degeneration than the corneas infected with the wild phenotypes. Figure 3B shows the difference in regeneration of subbasal nerve bundles (arrows) in corneas inoculated with the different strains and mutants.
FIGURE 3.
Sub-epithelial nerve density in HSV-1 latently-infected rabbit corneas. A) An average of six to eight images of sub-epithelial nerves/rabbit cornea were taken. *Statistically significant lower nerve areas were found in all the conditions with respect to control. Control, n=5 rabbits; gk deletion, n=7 rabbits; ICP0 deletion, n=8 rabbits, 17Syn+, n=4 rabbits; McKrae, n=3 rabbits. B) Representative images of infected corneas showing regenerating sub-basal nerve bundles (arrows).
Discussion
For the first time, we describe here the entire nerve map of the rabbit cornea, an animal model commonly used in research, including studies of HSV infection.15 As we previously described for human and mouse corneas,4–6,28 the nerves reach the cornea through the limbal area of the stroma. However, in contrast to humans and mice, rabbit corneal innervation shows some different features. One difference is that distribution of the central stromal nerves is much denser than what is seen in humans and adult mice. As a result, there are more penetrating sites from the stromal nerves to the subbasal epithelium in the center than in the periphery. Another feature is that the subbasal bundles are shorter and have fewer connections between them, so that they do not form a network or a typical whorl-like or vortex structure in the central zone.
HSV-1 is a neurotrophic virus, and following corneal primary infection the virus establishes latency in the TG. Also, the virus can undergo periodic reactivation in response to certain stimuli, leading to recurrent disease and HSK. Patients with HSK have low corneal sensitivity and a decrease in corneal subbasal nerve density associated with development of neurotrophic keratitis; however, the causes and pathogenesis of corneal nerve damage are poorly explored.21–23. In the current study, we investigated the changes in rabbit corneal innervation after four months of primary HSV-1 infection. We found that the infected corneas displayed serious damage to both epithelial and stromal nerves. Formation of scar tissue and the significant decrease in nerve density after a relatively long post-infection period suggest that corneal infection with HSV-1 results in inflammation that can persist long after clearance of the infectious virus. In fact, it was reported that, in an HSV-1 mouse model, there was an inflammatory reaction that persisted and was associated with loss of corneal sensation.24 Recent studies in our laboratory have shown that rabbit corneas infected with the 17Syn+ strain induced an inflammatory response, which started at three to four days post-infection and persisted for more than two weeks. Loss of sensitivity occurred in the first week and was not recovered during the experimental eight-week period.29
The severity of nerve damage was related to the virus strains. The corneas infected with the McKrae or 17Syn+ strains showed the most serious nerve damage, while those infected with the mutant strains had less damage. McKrae and 17Syn+ are known as high phenotypic reactivators (HPRs). Early studies have revealed that inoculation of rabbits with HPR strains results in high mortality, and corneal inoculation with HPR strains results in stromal opacity in 5–10% of corneas.14, 15 In our study, of the 20 rabbits infected with HPR strains, only seven did not show signs of reactivation 125 days post-inoculation.
HSV-1 is an enveloped, double-stranded DNA virus that is covered by glycoproteins, one of which (gk) plays an important role in the entry of the virus into the epithelial cells, membrane fusion, intracellular transport of virions, and late morphogenetic events in infectious virion formation.29 In mice, deletion of gk from the virus inhibits efficient replication and spread of the virus in the corneal epithelium, as well as axonal transport to the TG.18,19 In the current study, the inefficient replication and spread of the virus may attenuate acute inflammatory reaction and lead to less corneal nerve destruction. This is supported by our observation that the corneas infected with gk deletion showed no central scar formation.
ICP0 is a multi-functional phosphoprotein with an important role in the initiation of the productive cycle of infection.20, 31,32 It has been reported that ICP0 enhances viral gene expression by inactivating a number of components related to host cell antiviral defense and stress response pathways, attenuating the innate immune response.33, 34 Therefore, it is conceivable that deletion of ICP0 will reduce viral replication, accelerate virus clearance, and ameliorate inflammation after primary infection. As a result, there is less damage on corneal nerves, as revealed in the current study.
In summary, we used a modified method of immunofluorescence and imaging to investigate the changes of the corneal nerve architecture after four months of latent infection with two HPR strains and two deletion mutants. Rabbits infected with McKrae or 17Syn+ strains had serious corneal nerve damage and scar tissue, while those infected with the gk or ICP0 mutant strains showed significant increase in nerve regeneration, reaffirming the importance of these components not only in the infection and replication of the virus, but in the serious consequences of decreased corneal innervation and sensitivity.
Supplementary Material
Acknowledgments
This work was supported by National Institute of General Medical Sciences grant P30 GM103340 and the Research to Prevent Blindness.
Footnotes
Disclosure: J. He, None; R. Cosby, None; JM. Hill, None; HEP. Bazan, None.
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. The authors declared that no conflicts of interest or commercial relationship existed in the form of financial support or personal financial interest.
References
- 1.Epstein DL, Paton D. Keratitis from misuse of corneal anesthetics. N Engl J Med. 1968;278:396–399. doi: 10.1056/NEJM196808222790802. [DOI] [PubMed] [Google Scholar]
- 2.Araki K, Ohashi Y, Kinoshita S, Hayashi K, Kuwayama Y, Tano Y. Epithelial wound healing in the denervated cornea. Curr Eye Res. 1994;13:203–211. doi: 10.3109/02713689408995778. [DOI] [PubMed] [Google Scholar]
- 3.Murphy CJ, Marfurt CF, McDermott A, Bentley E, Abrams GA, Reid TW, et al. Spontaneous chronic corneal epithelial defects (SCCED) in dogs: clinical features, innervation, and effect of topical SP, with or without IGF-1. Invest Ophthalmol Vis Sci. 2001;42:2252–2261. [PubMed] [Google Scholar]
- 4.He J, Bazan NG, Bazan HEP. Mapping the entire human corneal nerve architecture. Exp Eye Res. 2010;91:513–523. doi: 10.1016/j.exer.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He J, Bazan HEP. Mapping the nerve architecture of diabetic human corneas. Ophthalmology. 2012;119:956–964. doi: 10.1016/j.ophtha.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He J, Bazan HEP. Corneal nerve architecture in a donor with unilateral corneal epithelial basement membrane dystrophy. Ophthalmic Res. 2013;49:185–191. doi: 10.1159/000345766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortina MS, He J, Li N, Bazan NG, Bazan HEP. Neuroprotectin D1 synthesis and corneal nerve regeneration after experimental surgery and treatment with PEDF plus DHA. Invest Ophthalmol Vis Sci. 2010;51:804–810. doi: 10.1167/iovs.09-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortina MS, He J, Li N, Bazan NG, Bazan HEP. Recovery of corneal sensitivity, calcitonin gene-related peptide-positive nerves, and increased wound healing induced by pigment epithelial-derived factor plus docosahexaenoic acid after experimental surgery. Arch Ophthalmol. 2012;130:76–83. doi: 10.1001/archophthalmol.2011.287. [DOI] [PubMed] [Google Scholar]
- 9.Cortina MS, He J, Russ T, Bazan NG, Bazan HEP. Neuroprotectin D1 restores corneal nerve integrity and function after damage from experimental surgery. Invest Ophthalmol Vis Sci. 2013;54:4109–4116. doi: 10.1167/iovs.13-12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He J, Cortina MS, Kakazu A, Bazan HEP. The PEDF neuroprotective domain plus DHA selectively induces corneal nerve regeneration after experimental surgery. Invest Ophthalmol Vis Sci. 2015;56:3505–3513. doi: 10.1167/iovs.15-16755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1–13. doi: 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Young RC, Hodge DO, Liesegang TJ, Baratz KH. Incidence, recurrence, and outcomes of herpes simplex virus eye disease in Olmsted County, Minnesota, 1976–2007: the effect of oral antiviral prophylaxis. Arch Ophthalmol. 2010;128:1178–1183. doi: 10.1001/archophthalmol.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Dujaili LJ, Clerkin PP, Clement C, McFerrin HE, Bhattacharjee PS, Varnell ED, et al. Ocular herpes simplex virus: how are latency, reactivation, recurrent disease and therapy interrelated? Future Microbiology. 2011;6:877–907. doi: 10.2217/fmb.11.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toma HS, Murina AT, Areaux RG, Jr, Neumann DM, Bhattacharjee PS, Foster TP, et al. Ocular HSV-1 latency, reactivation and recurrent disease. Semin Ophthalmology. 2008;23:249–273. doi: 10.1080/08820530802111085. [DOI] [PubMed] [Google Scholar]
- 15.Webre JM, Hill JM, Nolan NM, Clement C, McFerrin HE, Bhattacharjee PS, et al. Rabbit and mouse models of HSV-1 latency, reactivation, and recurrent eye diseases. J Biomed Biotechnol. 2012;2012:612316. doi: 10.1155/2012/612316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bloom DC, Stevens JG, Hill JM, Tran RK. Mutagenesis of a cAMP response element within the latency-associated transcript promoter of HSV-1 reduces adrenergic reactivation. Virology. 1997;236:202–207. doi: 10.1006/viro.1997.8723. [DOI] [PubMed] [Google Scholar]
- 17.Perng GC, Slanina SM, Yukht A, Ghiasi H, Nesburn AB, Wechsler SL. The latency-associated transcript gene enhances establishment of herpes simplex virus type 1 latency in rabbits. J Virol. 2000;74:1885–1891. doi: 10.1128/jvi.74.4.1885-1891.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.David AT, Baghian A, Foster TP, Chouljenko VN, Kousoulas KG. The herpes simplex virus type 1 (HSV-1) glycoprotein K (gK) is essential for viral corneal spread and neuroinvasiveness. Curr Eye Res. 2008;33:455–467. doi: 10.1080/02713680802130362. [DOI] [PubMed] [Google Scholar]
- 19.David AT, Saied A, Charles A, Subramanian R, Chouljenko VN, Kousoulas KG. A herpes simplex virus 1 (McKrae) mutant lacking the glycoprotein K gene is unable to Infect via neuronal axons and egress from neuronal cell bodies. MBio. 2012;3:e00144–12. doi: 10.1128/mBio.00144-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai W, Schaffer PA. Herpes simplex virus type 1 ICP0 regulates expression of immediate-early, early, and late genes in productively infected cells. J Virol. 1992;66:2904–2915. doi: 10.1128/jvi.66.5.2904-2915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberg ME, Tervo TM, Müller LJ, Moilanen JA, Vesaluoma MH. In vivo confocal microscopy after herpes keratitis. Cornea. 2002;21:265–269. doi: 10.1097/00003226-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Hamrah P, Cruzat A, Dastjerdi MH, Zheng L, Shahatit BM, Bayhan HA, et al. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2010;117:1930–1936. doi: 10.1016/j.ophtha.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chucair-Elliott AJ, Zheng M, Carr DJ. Degeneration and regeneration of corneal nerves in response to HSV-1 infection. Invest Ophthalmol Vis Sci. 2015;56:1097–1107. doi: 10.1167/iovs.14-15596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yun H, Rowe AM, Lathrop KL, Harvey SAK, Hendricks RL. Reversible nerve damage and corneal pathology in murine herpes simplex stromal keratitis. J Virol. 2014;88:7870–7880. doi: 10.1128/JVI.01146-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halford WP, Weisend C, Grace J, Sobeliski M, Carr DJJ, Balliet JW, et al. ICP0 antagonizes Stat 1-dependent repression of herpes simplex virus: implication for the regulation of viral latency. Virol J. 2006;3:44. doi: 10.1186/1743-422X-3-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harura Y, Roorman DS, Xie L, Kiritoshi A, Hill JM. Recurrent HSV-1 corneal lesions in rabbits induced by cyclophosphamide and dexamethasone. Invest Ophthalmol Vis Sci. 1989;30:371–376. [PubMed] [Google Scholar]
- 27.Anand BS, Hill JM, Dey S, Maruyama K, Bhattacharjee PS, Myles ME, et al. In vivo antiviral efficacy of a dipeptide acyclovir prodrug, val-val-acyclovir, against HSV-1 epithelial and stromal keratitis in the rabbit eye model. Invest Ophthalmol Vis Sci. 2003;44:2529–2534. doi: 10.1167/iovs.02-1251. [DOI] [PubMed] [Google Scholar]
- 28.He J, Bazan HEP. Neuroanatomy and neurochemistry of mouse cornea. Invest Ophthalmol Vis Sci. 2016;57:664–674. doi: 10.1167/iovs.15-18019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cortina MS, He J, Kakazu AH, Neumann D, Bazan NG, Bazan HEP. PEDF plus DHA induce a dual response of the immune system and increase nerve regeneration in HSV-1 infected corneas. ARVO. 2015:3075–D0271. (E-abstract) [Google Scholar]
- 30.Johnson DC, Baines JD. Herpes virus remodel host membranes for virus egress. Nat Rev Microbiol. 2011;9:382–394. doi: 10.1038/nrmicro2559. [DOI] [PubMed] [Google Scholar]
- 31.Everett RD. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays. 2000;22:761–770. doi: 10.1002/1521-1878(200008)22:8<761::AID-BIES10>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 32.Hagglund R, Roizman B. Role of ICP0 in the strategy of conquest of the host cell by herpes simplex virus 1. J Virol. 2004;78:2169–2178. doi: 10.1128/JVI.78.5.2169-2178.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edison KM, Hobbs WE, Manning BJ, Carlson P, DeLuca NA. Expression of herpes simplex virus ICP0 inhibits induction of interferon-stimulated genes by viral infection. J Virol. 2002;76:2180–2191. doi: 10.1128/jvi.76.5.2180-2191.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melroe G, DeLuca N, Knipe DM. Herpes simplex virus 1 has multiple mechanism for blocking virus induced interferon production. J Virol. 2004;78:8411–8420. doi: 10.1128/JVI.78.16.8411-8420.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.