Abstract
The major priming event in neurodegeneration is loss of neurons. Loss of neurons by apoptotic mechanisms is a theme for studies focused on determining therapeutic strategies. Neurons following an insult, activate a number of signal transduction pathways, of which, kinases are the leading members. Cyclin-dependent kinase 5 (Cdk5) is one of the kinases that have been linked to neurodegeneration. Cdk5 along with its principal activator p35 is involved in multiple cellular functions ranging from neuronal differentiation and migration to synaptic transmission. However, during neurotoxic stress, intracellular rise in Ca2+ activates calpain, which cleaves p35 to generate p25. The long half-life of Cdk5/p25 results in a hyperactive, aberrant Cdk5 that hyperphosphorylates Tau, neurofilament and other cytoskeletal proteins. These hyperphosphorylated cytoskeletal proteins set the groundwork to forming neurofibrillary tangles and aggregates of phosphorylated proteins, hallmarks of neurodegenerative diseases like Alzheimer’s disease, Parkinson’s disease and Amyotropic Lateral Sclerosis. Attempts to selectively target Cdk5/p25 activity without affecting Cdk5/p35 have been largely unsuccessful. A polypeptide inhibitor, CIP (Cdk5 inhibitory peptide), developed in our laboratory, successfully inhibits Cdk5/p25 activity in vitro, in cultured primary neurons, and is currently undergoing validation tests in mouse models of neurodegeneration. Here, we discuss the therapeutic potential of CIP in regenerating neurons that are exposed to neurodegenerative stimuli.
Keywords: Cdk5, Neuron regeneration, Tau, Hyperphosphorylation, Neurodegeneration
Introduction
In neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD) and Amyotropic Lateral Sclerosis (ALS), loss of neurons occurs in particular regions of the nervous system. Due to the loss of neurons, cognitive or motor functions are adversely affected that define the symptoms of these neurodegenerative diseases. Although many proteins and gene mutations have been linked to the neuron loss, the exact mechanisms still remain unknown. A number of studies have focused on designing various therapeutic strategies to prevent neuron loss. One of such strategies is based on the finding that adult brains are capable of generating new neurons (neurogenesis) (Gross 2000). This intrinsic capacity of the adult nervous system can be exploited to replace dying neurons (Zhao et al. 2008). Another strategy is to interfere with the process that induces neuron death. There are evidences that neurogenesis is impaired in neurodegenerative diseases including AD, PD and ALS (Geraerts et al. 2007; Shan et al. 2006; Yoshimi et al. 2005; Zhao et al. 2003). It is also not established whether impaired or failed neurogenesis in the adult brain contributes to neurodegeneration. These two strategies may not be exclusive of each other in terms of preventing or reversing neurodegeneration. However, each strategy independently could have the potential to regenerate the nervous system that begins to go awry.
Regeneration within the nervous system is a complex process involving multiple steps. Major steps include: (1) survival of the injured neurons (prevention of cell death and degeneration), and (2) replacing injured neurons by transplantation of new cells (cell replacement). A major difference between neuronal and most non-neuronal cell death is that the neuron is post-mitotic and therefore there is no turn over, while most non-neuronal cells have quick turn over so as to replace the dead cells. The nervous system obviously is equipped with more complex machinery adapted to the specific function of keeping the neurons alive. But at times, the machinery fails and neuronal death ensues. Moreover, it is not known whether apoptotic mechanisms contribute to neurodegenerative diseases (Yuan and Yankner 2000). If in fact it does, then a large number of options at multiple points of possible interference open up as an umpteen number of anti-apoptotic and apoptotic pathways are involved in neuronal death. For instance, several transcription factors regulating apoptosis, such as p53, Fas, c-Jun, Bax and Bcl-2 may be targets of therapeutic intervention (Yuan and Yankner 2000), while caspase inhibitor peptides also show promise in mouse models of ALS (Li et al. 2000) and protect neurons against MPTP toxicity (Yang et al. 2004).
Cyclin-dependent kinase 5 (Cdk5) belongs to the family of serine/threonine cyclin-dependent kinase (Meyerson et al. 1992). Cdk5 is found in mitotic cells, but its activity is mostly restricted to neuronal cells due to the expression of neuron-specific activators, p35 and p39 (Dhavan and Tsai 2001). Although Cdk5 is ubiquitously expressed in all cells and shares a high degree of homology with other members of the cyclin-dependent kinase family (CDKs), its activity is found specifically in post-mitotic neurons because its activators, p35 and p39, are expressed primarily in neurons (Ko et al. 2001). Cdk5 is a multi-functional S/T protein kinase that is involved in a wide range of neuronal functions from neurite outgrowth and neuronal migration to synaptic activity and cell survival (Cruz and Tsai 2004). Cdk5 knockout mice exhibit defects in organization of the cortex and cerebellum and are embryonically lethal (Ohshima et al. 1996). In addition, regulation and deregulation of Cdk5 activity plays an important role in a range of physiological and pathological processes that include involvement in nervous system development and neurodegeneration (Dhavan and Tsai 2001; Shelton and Johnson 2004), and neuronal differentiation (Cicero and Herrup 2005).
Cyclin-dependent kinase 5 and Neurodegeneration
A major function of Cdk5 is its role in cell survival. In vitro, Cdk5 protects neurons from cell death by direct interaction and activation of the anti-apoptotic protein Bcl-2 (Cheung et al. 2008; Wang et al. 2006) and by inhibiting sustained ERK1/2 activation in PC12 cells (Zheng et al. 2007). Cdk5 and GSK3β have been identified as prime candidates for neurodegenerative pathogenesis (Drewes 2004; Sato et al. 2002). Normally, cytoskeletal proteins are phosphorylated on (S/T-P) residues selectively in the axonal compartment of the neuron. However, in a number of neuropathological conditions, such as amyotrophic lateral sclerosis (ALS), Alzheimer’s disease (AD), Neiman Pick’s Type C disease, the proline-directed S/T-P residues on cytoskeletal proteins are aberrantly hyperphosphorylated within cell bodies, resulting in the accumulation of abnormal cellular aggregates and massive neuronal cell death. Cdk5 is one of such kinases responsible for phosphorylation of neuronal cytoskeletal proteins specifically in their S/T-P residues (Shetty et al. 1993).
During neuronal insults, increase in intracellular calcium and activation of calpains result in the cleavage of p35–p25 thereby inducing deregulation and hyperactivation of Cdk5. In outcome, aberrant hyperphosphorylation of cytoskeletal proteins (e.g., NFs, MAPs, Tau) occurs, forming aggregates of these proteins in the cell body and consequently inducing neuronal death (Fig. 1). This process has been associated with a large number of neurodegenerative diseases (Ko et al. 2001). Cdk5 is not only involved in phosphorylating the NFs, MAPs, and Tau but also involved directly or indirectly in modulating the other kinase activities that phosphorylate the same proteins as well as other proteins. Cross-talk of Cdk5 with many different signal transduction pathways is involved in nervous system development and neurodegeneration (Kesavapany et al. 2003).
Fig. 1.
Normal and aberrant Cdk5 activities in the neurons. Cdk5/p35, by phosphorylating specific substrates, regulates a number of cellular functions, such as the nervous system development, neuron survival, migration, exocytosis and neuronal cytoskeletal protein maintenance and stability. Following various insults (Ca2+ influx leading to calpain activation, Aβ, oxidative stress, cytokines like IL-6 and IL-18), p35 is cleaved to generate p25. P25 lacks the myristoylation site of the N-terminal region that is retained in the cleaved p10 polypeptide. The mislocalized Cdk5/p25 in the cytoplasm is hyperactivated possibly due to its longer half-life than Cdk5/p35. The hyperactivated Cdk5/p25 aberrantly hyperphosphorylates Tau and neurofilament (NF) proteins, which are considered as the primers of neuronal degeneration
Cyclin-dependent kinase 5 inhibits Erk1/2 activity by phosphorylating the upstream kinase MEK1 and intense immunostaining of phosphorylated cytoskeletal proteins is observed in the neuronal cell bodies of Cdk5−/− mice compared to the wild type mice (Sharma et al. 2002). Also in p35−/− mice, there is an inverse relationship between Cdk5 and Erk1/2 activities, while the expression levels of Erk1/2 remains unchanged (Sharma et al. 2002). Such sustained high activity of Erk1/2 in the neuronal cells has been implicated as the cause of cell death (Cheung and Slack 2004). Consistent with this assumption, extensive apoptosis in Cdk−/− brains compared to the wild type brains is observed as evidenced by the upregulation of the apoptosis marker, cleaved-caspase-3 and appearance of TUNEL-positive cells (Zheng et al. 2005). Evidence from mouse model, the PC12 cell line, and rat primary cortical neuron cultures demonstrate that Cdk5 modulates MAPK (MEK/Erk1/2) pathway and is involved in neuronal survival, in which Cdk5/p35 seems to act as a “molecular switch” to modulate the duration of Erk1/2 activation thereby resulting in NF-M/H and Tau phosphorylation (Harada et al. 2001; Sharma et al. 2002). Cdk5-Ras GRF cross talk upstream of the MAPK pathway also regulates neuronal apoptosis through nuclear condensation of neurons (Kesavapany et al. 2004, 2006). Sustained Erk1/2 activation by inhibiting Cdk5 activity using roscovitine induces apoptosis of primary neurons in culture, where Tau and NF are also hyperphosphosphorylated (Zheng et al. 2007).
The other kinase that has been linked to neurodegeneration along with Cdk5 is glycogen synthase kinase 3β (GSK3β). It was observed that low concentrations of olomoucine, which inhibits Cdk5 activity, also inhibits fast axonal transport and GSK3 phosphorylation of kinesin light chains significantly inhibits anterograde but not retrograde fast transport (Morfini et al. 2002). Inhibiting Cdk5 with roscovitine or olomoucine activates PP1 phosphatase, which, in turn, dephosphorylates and activates GSK3β thereby inducing phosphorylation and dissociation of kinesin from the vesicles (Morfini et al. 2004). Both, Cdk5 and GSK3β generate disease-associated phospho-epitopes on Tau, and they co-localize with filamentous Tau aggregates in the brains of patients (Imahori and Uchida 1997; Shelton and Johnson 2004) and in a transgenic mouse model of tauopathy (Imahori and Uchida 1997; Ishizawa et al. 2003). Cdk5 and GSK3 also regulate Aβ production in vivo (Cruz et al. 2006; Phiel et al. 2003). These studies link Cdk5 to neurodegeneration.
Cdk5 Activators and Neurodegeneration
The reported neurotoxic effects of Cdk5 have been linked to p25 production, a proteolytically cleaved product of p35, the major activator of neuronal Cdk5. In primary cortical neuron cultures, p25/Cdk5 complex phosphorylates Tau more efficiently than does the p35/Cdk5 complex, since the half-life of p25/Cdk5 is longer than the p35/Cdk5 complex (Patrick et al. 1999). In vitro, Tau phosphorylation assays have demonstrated that p25 accelerates Cdk5 catalytic activity by ~2.4-fold over p35 (Hashiguchi et al. 2002). Further evidence comes from the preferential increase in Tau phosphorylation in p25 transgenic mice (Ahlijanian et al. 2000; Cruz et al. 2003), while p35 transgenic mice displaying increased Cdk5 catalytic activity, do not show increased Tau phosphorylation (Van den Haute et al. 2001). These findings are complemented by the observation that Cdk5-deficient mice show decreased Tau phosphorylation (Takahashi et al. 2003). Surprisingly, however, a new strain of p35-deficient mice display increased Tau phosphorylation (Hallows et al. 2003). It is possible that in these mice Tau phosphorylation occurs due to the compensatory increases in another Cdk5 activator, p39 level or by Cdk-crosstalk as discussed earlier. The compensatory increase in p39 expression has been reported in p35-deficient mice (Chae et al. 1997). Moreover, p39-mediated Tau phosphorylation is more efficient than p35-mediated Tau phosphorylation (Iijima et al. 2000). P39-derived p29 is also potent in phosphorylating Tau (Patzke and Tsai 2002).
Recent studies now implicate p25 as a “normal” player in modulating synaptic function, LTD, learning and memory in specific brain regions in young animals (Angelo et al. 2006; Fischer et al. 2005, 2003). Transgenic mice expressing low level of p25, or with expression restricted spatiotemporally to specific brain regions, show a Cdk5/p25 positive transient effect on LTD in the hippocampus and water maze learning in these animals. Prolonged expression of p25, in older animals, however, does exert a predictable effect on β-amyloid and Tau phosphorylation and neurodegeneration (Cruz and Tsai 2004; Cruz et al. 2003). The differential phosphorylation potentials of p25 and p35 are also associated with another Cdk5 substrate, APP, which is involved in neurodegeneration. Cdk5 phosphorylates amyloid precursor protein (APP) in its cytoplasmic domain at Thr668 (Iijima et al. 2000). Increased APP Thr668 phosphorylation is observed in p25 transgenic mice in which p35/Cdk5 activity remains unaltered (Cruz et al. 2003). These findings reveal that the role of Cdk5/p25 in neuropathological diseases is complex.
Inducers of Cdk5/p25 Activity
To date, no single hypothesis on AD pathogenesis can sufficiently explain the cause of neurodegeneration (Haass and Selkoe 2007; Maccioni et al. 2001). One of the leading hypotheses for the etiology of Alzheimer’s disease (AD) is the amyloid hypothesis (Hardy and Selkoe 2002). In AD, extracellular accumulation of Aβ42 that readily aggregates into amyloid plaques occurs due to an altered ratio of Aβ generation and clearance. One of the downstream events of this elevated Aβ42 levels is the aberrant activation of kinases and inhibition of phosphatases. The resultant imbalance in the kinase/phosphatase activities causes neurofibrillary tangle formation and neuronal death. Myriads of reports now link Cdk5 to Aβ42 toxicity and Tau pathology leading to neurodegeneration. In primary neurons, Aβ42 induces the cleavage of p35–p25 (Alvarez et al. 1999; Lee et al. 2000; Town et al. 2002; Zheng et al. 2002). P25 accumulation is found in mutant APP transgenic mice that display elevated Aβ42 levels (Otth et al. 2002). P25 accumulation may precede the formation of NFT in the AD brain (Patrick et al. 1999). Inhibition of Cdk5 activity also attenuates Aβ42-induced neuronal death (Alvarez et al. 1999; Lee et al. 2000). These findings indicate that Aβ42 is a potent activator of p25/Cdk5 activity.
Pathogenesis of AD is triggered long before the clinical onset of the disease and the risk factors involved in AD pathophysiology include blood lipid disorders, head injury, oxidative stress, shear stress, mechanical cell damage, K+ efflux and membrane permeabilization (Rojo et al. 2008). Recently, long-term activation of the innate immune system by any risk factor triggering an inflammatory cascade culminating in Tau aggregation and paired helical filament formation has been emphasized (Fernandez et al. 2008). The risk factors inducing such pathophysiological changes range from Aβ oligomers, cytokines, high lipid content and redox iron (Fernandez et al. 2008). IL-1β over-expression in AD brains was reported earlier (Li et al. 2003), and hippocampal neurons treated with a physiological dose of IL-6 has been shown to induce Tau phosphorylation, possibly mediated by the increased levels of intraneuronal levels of Cdk5 and p35 (Quintanilla et al. 2004). A more recent report showed that the pro-inflammatory IL-18 could induce Cdk5/p35 and GSK3β protein expression leading to increased Tau phosphorylation in SH-SY5Y neuroblastoma cells with an increase in Cdk5/p35 or p25 complex after 24–48 h of IL-18 treatment (Ojala et al. 2008).
Targeting Cdk5/p25 to Promote Neuron Regeneration
Degenerating neuronal cells in AD show phenotypic changes characteristic of cells re-entering cell division (McShea et al. 2007). It has been suggested that in neurons, Tau hyperphosphorylation may induce abnormal, incomplete cell cycle re-entry (Andorfer et al. 2005). Subsequently, activation of related signal transduction pathways and cell cycle-dependent kinases along with transcriptional activation could lead to cytoskeletal alterations and DNA replication (McShea et al. 2007; Nagy 2000; Yang et al. 2001). It is hypothesized that some neurons re-enter the cell cycle upon neuronal insult in an effort to recover, since hippocampi of patients with mild cognitive impairment show increased expression of G/S phase regulating cyclin G1 (CG1), Cdk2 and Cdk5 (Sultana and Butterfield 2007). Expression of CG1 has been shown to associate with Cdk5 and possibly nerve regeneration (Morita et al. 1996). The role of CG1 in AD remains unclear, although it may act as a mediator between apoptosis and the cell cycle (Crocker et al. 2003a, b; Okamoto and Beach 1994; Zauberman et al. 1995).
Neurotrophic factor delivery and stem cell transplantation are considered potentially promising in treating neurodegenerative diseases (Apfel 2000; Thomson et al. 1998). Forced expression of calpastatin (an inhibitor of calpains) and dominant-negative Cdk5 by adenoviral infection of the nigrostriatal pathway have been shown to protect against MPTP toxicity and also reveal an alternative cell death pathway (Crocker et al. 2003a, b). NF- and Tau-directed protein kinases are potential targets for modification, when it comes to therapeutic interventions of AD. Pin1, a peptidylprolyl cis/trans-isomerase substantially increases neurofilament phosphorylation by proline-directed kinases, Erk1/2, Cdk5/p35 and JNK3 in a concentration-dependent manner (Kesavapany et al. 2007; Rudrabhatla et al. 2008) and targeting Pin1 for therapeutic intervention is speculated.
Several protein kinases including Cdk5 that phosphorylate Tau and NFs also phosphorylate other substrates that are critical for normal cellular physiology. Both Cdk5 and GSK3β are involved in AD but also play a role in the development of neurons (Hooper and Turner 2008; Johnson and Stoothoff 2004; Naska et al. 2006; Nguyen et al. 2002; Ohshima et al. 1996). Inhibitors of protein kinases, for this reason, prove to be clinically futile in treating neurodegeneration. Also, usually kinase inhibitors target the ATP binding sites thereby losing their selectivity since ATP binding sites are common to many kinases. Complications arise when there is no demarcation between a normal functional kinase and the same kinase gone awry and becoming a pathological factor. Specific inhibitors that do not inhibit normal kinase activity, but specifically inhibit the aberrant, hyperactive kinase activity, would be biologically more acceptable a treatment strategy for neurodegenerative disease.
The polypeptide CIP (Cdk5/p25 inhibitory peptide), a derivative of p35 that inhibits Cdk5/p25 activity in vitro, in HEK cells as well as in rat cortical primary neurons in culture (Zheng et al. 2002), shows the potential for therapeutic intervention since it specifically inhibits the Cdk5/p25 deregulated hyperactivity (Fig. 2). Moreover, proven therapeutic agents may not efficiently reach and stably remain in the target tissues in vivo. There must be strategies to overcome this impediment. Recently, using CIP in nanoparticles, inhibition of Cdk5/p25 activity in order to treat ischemic stroke has been proposed (Slevin and Krupinski 2008). According to the model in this report, CIP in nanoparticles or nanogels (polymer-linked hydrogels) can be labeled with antibodies to cell-specific markers (e.g., S100-beta in neurons) and delivered to the neurons. CIP is a fragment of p35 spanning amino acids 154–279. CIP competes against p25, thus inhibiting the hyperphosphorylating activity of Cdk5/p25. In another report, TAT-CIP has been shown to prevent Cdk5 activation by p25 in vitro (Sun et al. 2008). Although no specific compound was found functional in inhibiting Cdk5/p25, specific inhibition of GSK3β, another kinase involved in NFT formation by a 1-Aza-9-oxafluorenes has been reported (Voigt et al. 2008). Further screening of such modified pharmacological inhibitors may yield novel inhibitors of Cdk5/p25. It is relevant to note that CIP does not inhibit Cdk5/p35 activity, and therefore, does not interfere with the normal Cdk5-regulated phosphorylation needed for cellular functions.
Fig. 2.
CIP specifically inhibits Cdk5/p25 activity. a Schematic representation of the size of CIP, a derivative of p35. Full-length p35 is a 307-amino acid protein. CIP constitutes amino acids spanning from 154–279. b Forced expression of CIP inhibits Cdk5/p25 hyperactivity in cortical neurons. Transduction of cells with lentivirus-delivered p25 exhibits an increase in Cdk5 activity compared with the uninfected and empty lentivirus vector-infected neurons. Co-expression of CIP with p25 reduces p25-mediated activation of endogenous Cdk5. c CIP does not perturb Cdk5/p35 activity in neurons. Co-expression of CIP or absence of CIP with Cdk5/p35 does not affect Cdk5 activity. Basal level of Cdk5 activity is observed in the neurons harboring empty vector
Whether CIP can successfully inhibit Cdk5/p25 in vivo in mouse models of neurodegeneration is in the works in our laboratory. The major questions we are beginning to address are: does CIP have the potential to regenerate a sub-lethally injured neuron? Are the effects of factors like aging, Aβ accumulation, oxidative stress and inflammation, which are contributory factors to eventual death of neurons, be counteracted by CIP? Is the definitive direction of a neuron under a sub-lethal insult toward apoptosis be reversed? Finally, if possible, then at what strategic point CIP would be a best fit for this scenario? When a neuron receives sub-lethal insult, several stress-induced molecules such as JNK, p38 kinase (Gallo and Johnson 2002), transcription factor p53, caspases and calpain turn on their signaling cascades leading to apoptosis. In addition, sub-sets of molecules that are involved in cell cycle re-entry (Cyclin G1, Cdk5, p35/p25) are also induced (McShea et al. 2007) that results in cytoskeletal alterations (McShea et al. 2007; Nagy 2000; Yang et al. 2001). Tau hyperphosphorylation may also induce abnormal, incomplete cell cycle re-entry (Andorfer et al. 2005). An apoptosis-promoting stimulus has been thought to induce a dedifferentiation process in mature neurons (Heintz 1993). However, this reactivation of neuronal cell cycle remains incomplete. This very attempt of the cell’s machinery may be exploited to regenerate a neuron that has received the signal to die. In Fig. 3, the therapeutic intervention point for CIP is shown. By inhibiting the aberrant Cdk5/p25, the direction of a neuron under nascent insult can be diverted so as for the neuron, survival over death is achieved. The injured neuron’s inevitable degeneration may thus be avoided. Under sustained intrinsic and extrinsic insults, the probability of neurons withstanding the death-inducing impact with a constant presence of CIP, although seems hypothetically possible based on our results from studies on in vitro and cultured primary neurons, remains to be experimentally determined.
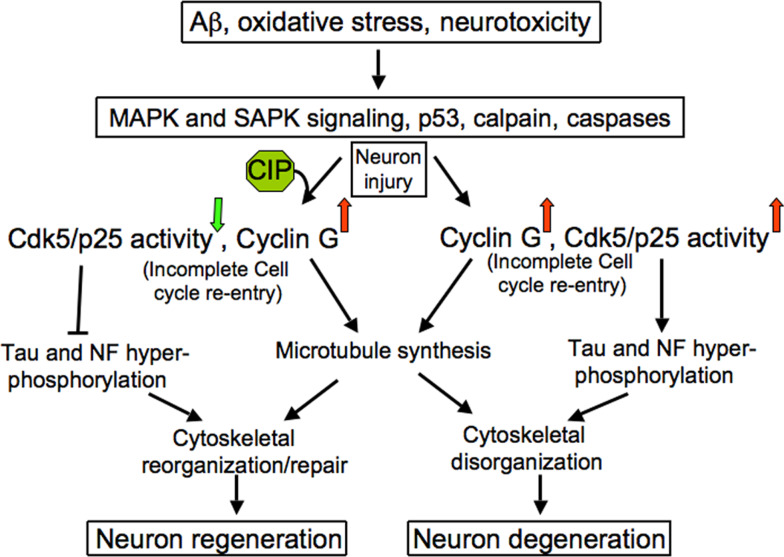
Fig. 3.
Simplified schematic representation of CIP-induced neuronal regeneration under insults from Aβ, oxidative stress and neurotoxicity. Normally, under these conditions, neurons would undergo degeneration following hyperphosphorylation of Tau and neurofilament (NF) proteins, especially by Cdk5/p25. Although, as an intrinsic defense mechanism, the neurons attempt cell cycle re-entry whilst microtubule synthesis and/or repair occur, eventually with an incomplete cell cycle re-entry, the neurons undergo degeneration and death. However, in the presence of CIP, Cdk5/p25 activity is down-regulated and although the post-mitotic neuron may not re-enter cell cycle by this specific inhibition, the cell’s re-course from a degenerating route would occur
Conclusion
Neurons under apoptotic stimuli may be protected from undergoing degeneration by therapeutic agents that prevent various cell death pathways. Among inhibitors of Cdk5, roscovitine reduces ischemia-induced tauopathy (Wen et al. 2007); and olomoucine inhibits ischemia-induced reactive astrogliosis (Zhu et al. 2007). These general inhibitors of Cdk5 are not without their demerits when it comes to an in vivo situation. Cdk5/p35 activity is essential for cell survival. Therefore, inhibiting the aberrantly active Cdk5/p25 without interfering with Cdk5/p35 would be therapeutically beneficial. CIP, a peptide inhibitor derived from p35, has been shown to inhibit Cdk5/p25, but not Cdk5/p35 activity. In addition to having the specificity, CIP also possesses a certain degree of safety factor being a polypeptide. These characteristics, as a therapeutic agent, would be conducive when treatment strategies would require its long residency within the cell and durable biological activities of a molecule that is not as foreign to the cell as the chemical agents.
Acknowledgments
This work was supported by intramural funds from the National Institute of Neurological Disorders and Stroke, National Institutes of Health.
References
- Ahlijanian MK, Barrezueta NX, Williams RD, Jakowski A, Kowsz KP, McCarthy S, Coskran T, Carlo A, Seymour PA, Burkhardt JE et al (2000) Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc Natl Acad Sci USA 97:2910–2915. doi:10.1073/pnas.040577797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez A, Toro R, Caceres A, Maccioni RB (1999) Inhibition of tau phosphorylating protein kinase cdk5 prevents beta-amyloid-induced neuronal death. FEBS Lett 459:421–426. doi:10.1016/S0014-5793(99)01279-X [DOI] [PubMed] [Google Scholar]
- Andorfer C, Acker CM, Kress Y, Hof PR, Duff K, Davies P (2005) Cell-cycle reentry and cell death in transgenic mice expressing nonmutant human tau isoforms. J Neurosci 25:5446–5454. doi:10.1523/JNEUROSCI.4637-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelo M, Plattner F, Giese KP (2006) Cyclin-dependent kinase 5 in synaptic plasticity, learning and memory. J Neurochem 99:353–370. doi:10.1111/j.1471-4159.2006.04040.x [DOI] [PubMed] [Google Scholar]
- Apfel SC (2000) Neurotrophic factors and pain. Clin J Pain 16:S7–S11 [DOI] [PubMed] [Google Scholar]
- Chae T, Kwon YT, Bronson R, Dikkes P, Li E, Tsai LH (1997) Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron 18:29–42. doi:10.1016/S0896-6273(01)80044-1 [DOI] [PubMed] [Google Scholar]
- Cheung EC, Slack RS (2004) Emerging role for ERK as a key regulator of neuronal apoptosis. Sci STKE 2004:PE45. doi:10.1126/stke.2512004pe45 [DOI] [PubMed] [Google Scholar]
- Cheung ZH, Gong K, Ip NY (2008) Cyclin-dependent kinase 5 supports neuronal survival through phosphorylation of Bcl-2. J Neurosci 28:4872–4877. doi:10.1523/JNEUROSCI.0689-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero S, Herrup K (2005) Cyclin-dependent kinase 5 is essential for neuronal cell cycle arrest and differentiation. J Neurosci 25:9658–9668. doi:10.1523/JNEUROSCI.1773-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker SJ, Liston P, Anisman H, Lee CJ, Smith PD, Earl N, Thompson CS, Park DS, Korneluk RG, Robertson GS (2003a) Attenuation of MPTP-induced neurotoxicity and behavioural impairment in NSE-XIAP transgenic mice. Neurobiol Dis 12:150–161. doi:10.1016/S0969-9961(02)00020-7 [DOI] [PubMed] [Google Scholar]
- Crocker SJ, Smith PD, Jackson-Lewis V, Lamba WR, Hayley SP, Grimm E, Callaghan SM, Slack RS, Melloni E, Przedborski S et al (2003b) Inhibition of calpains prevents neuronal and behavioral deficits in an MPTP mouse model of Parkinson’s disease. J Neurosci 23:4081–4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz JC, Tsai LH (2004) A Jekyll and Hyde kinase: roles for Cdk5 in brain development and disease. Curr Opin Neurobiol 14:390–394. doi:10.1016/j.conb.2004.05.002 [DOI] [PubMed] [Google Scholar]
- Cruz JC, Tseng HC, Goldman JA, Shih H, Tsai LH (2003) Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron 40:471–483. doi:10.1016/S0896-6273(03)00627-5 [DOI] [PubMed] [Google Scholar]
- Cruz JC, Kim D, Moy LY, Dobbin MM, Sun X, Bronson RT, Tsai LH (2006) p25/cyclin-dependent kinase 5 induces production and intraneuronal accumulation of amyloid beta in vivo. J Neurosci 26:10536–10541. doi:10.1523/JNEUROSCI.3133-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhavan R, Tsai LH (2001) A decade of CDK5. Nat Rev Mol Cell Biol 2:749–759. doi:10.1038/35096019 [DOI] [PubMed] [Google Scholar]
- Drewes G (2004) MARKing tau for tangles and toxicity. Trends Biochem Sci 29:548–555. doi:10.1016/j.tibs.2004.08.001 [DOI] [PubMed] [Google Scholar]
- Fernandez JA, Rojo L, Kuljis RO, Maccioni RB (2008) The damage signals hypothesis of Alzheimer’s disease pathogenesis. J Alzheimers Dis 14:329–333 [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Spiess J, Radulovic J (2003) Cdk5: a novel role in learning and memory. Neurosignals 12:200–208. doi:10.1159/000074621 [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Pang PT, Lu B, Tsai LH (2005) Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron 48:825–838. doi:10.1016/j.neuron.2005.10.033 [DOI] [PubMed] [Google Scholar]
- Gallo KA, Johnson GL (2002) Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol 3:663–672. doi:10.1038/nrm906 [DOI] [PubMed] [Google Scholar]
- Geraerts M, Krylyshkina O, Debyser Z, Baekelandt V (2007) Concise review: therapeutic strategies for Parkinson disease based on the modulation of adult neurogenesis. Stem Cells 25:263–270. doi:10.1634/stemcells.2006-0364 [DOI] [PubMed] [Google Scholar]
- Gross CG (2000) Neurogenesis in the adult brain: death of a dogma. Nat Rev Neurosci 1:67–73. doi:10.1038/35036235 [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol 8:101–112. doi:10.1038/nrm2101 [DOI] [PubMed] [Google Scholar]
- Hallows JL, Chen K, DePinho RA, Vincent I (2003) Decreased cyclin-dependent kinase 5 (cdk5) activity is accompanied by redistribution of cdk5 and cytoskeletal proteins and increased cytoskeletal protein phosphorylation in p35 null mice. J Neurosci 23:10633–10644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, Morooka T, Ogawa S, Nishida E (2001) ERK induces p35, a neuron-specific activator of Cdk5, through induction of Egr1. Nat Cell Biol 3:453–459. doi:10.1038/35074516 [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356. doi:10.1126/science.1072994 [DOI] [PubMed] [Google Scholar]
- Hashiguchi M, Saito T, Hisanaga S, Hashiguchi T (2002) Truncation of CDK5 activator p35 induces intensive phosphorylation of Ser202/Thr205 of human tau. J Biol Chem 277:44525–44530. doi:10.1074/jbc.M207426200 [DOI] [PubMed] [Google Scholar]
- Heintz N (1993) Cell death and the cell cycle: a relationship between transformation and neurodegeneration? Trends Biochem Sci 18:157–159. doi:10.1016/0968-0004(93)90103-T [DOI] [PubMed] [Google Scholar]
- Hooper NM, Turner AJ (2008) A new take on prions: preventing Alzheimer’s disease. Trends Biochem Sci 33:151–155. doi:10.1016/j.tibs.2008.01.004 [DOI] [PubMed] [Google Scholar]
- Iijima K, Ando K, Takeda S, Satoh Y, Seki T, Itohara S, Greengard P, Kirino Y, Nairn AC, Suzuki T (2000) Neuron-specific phosphorylation of Alzheimer’s beta-amyloid precursor protein by cyclin-dependent kinase 5. J Neurochem 75:1085–1091. doi:10.1046/j.1471-4159.2000.0751085.x [DOI] [PubMed] [Google Scholar]
- Imahori K, Uchida T (1997) Physiology and pathology of tau protein kinases in relation to Alzheimer’s disease. J Biochem 121:179–188 [PubMed] [Google Scholar]
- Ishizawa T, Sahara N, Ishiguro K, Kersh J, McGowan E, Lewis J, Hutton M, Dickson DW, Yen SH (2003) Co-localization of glycogen synthase kinase-3 with neurofibrillary tangles and granulovacuolar degeneration in transgenic mice. Am J Pathol 163:1057–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GV, Stoothoff WH (2004) Tau phosphorylation in neuronal cell function and dysfunction. J Cell Sci 117:5721–5729. doi:10.1242/jcs.01558 [DOI] [PubMed] [Google Scholar]
- Kesavapany S, Lau KF, Ackerley S, Banner SJ, Shemilt SJ, Cooper JD, Leigh PN, Shaw CE, McLoughlin DM, Miller CC (2003) Identification of a novel, membrane-associated neuronal kinase, cyclin-dependent kinase 5/p35-regulated kinase. J Neurosci 23:4975–4983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavapany S, Amin N, Zheng YL, Nijhara R, Jaffe H, Sihag R, Gutkind JS, Takahashi S, Kulkarni A, Grant P, Pant HC (2004) p35/cyclin-dependent kinase 5 phosphorylation of ras guanine nucleotide releasing factor 2 (RasGRF2) mediates Rac-dependent Extracellular Signal-regulated kinase 1/2 activity, altering RasGRF2 and microtubule-associated protein 1b distribution in neurons. J Neurosci 24:4421–4431. doi:10.1523/JNEUROSCI.0690-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavapany S, Pareek TK, Zheng YL, Amin N, Gutkind JS, Ma W, Kulkarni AB, Grant P, Pant HC (2006) Neuronal nuclear organization is controlled by cyclin-dependent kinase 5 phosphorylation of Ras Guanine nucleotide releasing factor-1. Neurosignals 15:157–173. doi:10.1159/000095130 [DOI] [PubMed] [Google Scholar]
- Kesavapany S, Patel V, Zheng YL, Pareek TK, Bjelogrlic M, Albers W, Amin N, Jaffe H, Gutkind JS, Strong MJ et al (2007) Inhibition of Pin1 reduces glutamate-induced perikaryal accumulation of phosphorylated neurofilament-H in neurons. Mol Biol Cell 18:3645–3655. doi:10.1091/mbc.E07-03-0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J, Humbert S, Bronson RT, Takahashi S, Kulkarni AB, Li E, Tsai LH (2001) p35 and p39 are essential for cyclin-dependent kinase 5 function during neurodevelopment. J Neurosci 21:6758–6771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH (2000) Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature 405:360–364. doi:10.1038/35012636 [DOI] [PubMed] [Google Scholar]
- Li M, Ona VO, Guegan C, Chen M, Jackson-Lewis V, Andrews LJ, Olszewski AJ, Stieg PE, Lee JP, Przedborski S, Friedlander RM (2000) Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science 288:335–339. doi:10.1126/science.288.5464.335 [DOI] [PubMed] [Google Scholar]
- Li Y, Liu L, Barger SW, Griffin WS (2003) Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J Neurosci 23:1605–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccioni RB, Munoz JP, Barbeito L (2001) The molecular bases of Alzheimer’s disease and other neurodegenerative disorders. Arch Med Res 32:367–381. doi:10.1016/S0188-4409(01)00316-2 [DOI] [PubMed] [Google Scholar]
- McShea A, Lee HG, Petersen RB, Casadesus G, Vincent I, Linford NJ, Funk JO, Shapiro RA, Smith MA (2007) Neuronal cell cycle re-entry mediates Alzheimer disease-type changes. Biochim Biophys Acta 1772:467–472 [DOI] [PubMed] [Google Scholar]
- Meyerson M, Enders GH, Wu CL, Su LK, Gorka C, Nelson C, Harlow E, Tsai LH (1992) A family of human cdc2-related protein kinases. EMBO J 11:2909–2917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G, Szebenyi G, Elluru R, Ratner N, Brady ST (2002) Glycogen synthase kinase 3 phosphorylates kinesin light chains and negatively regulates kinesin-based motility. EMBO J 21:281–293. doi:10.1093/emboj/21.3.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G, Szebenyi G, Brown H, Pant HC, Pigino G, DeBoer S, Beffert U, Brady ST (2004) A novel CDK5-dependent pathway for regulating GSK3 activity and kinesin-driven motility in neurons. EMBO J 23:2235–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita N, Kiryu S, Kiyama H (1996) p53-independent cyclin G expression in a group of mature neurons and its enhanced expression during nerve regeneration. J Neurosci 16:5961–5966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z (2000) Cell cycle regulatory failure in neurones: causes and consequences. Neurobiol Aging 21:761–769. doi:10.1016/S0197-4580(00)00223-2 [DOI] [PubMed] [Google Scholar]
- Naska S, Park KJ, Hannigan GE, Dedhar S, Miller FD, Kaplan DR (2006) An essential role for the integrin-linked kinase-glycogen synthase kinase-3 beta pathway during dendrite initiation and growth. J Neurosci 26:13344–13356. doi:10.1523/JNEUROSCI.4462-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MD, Mushynski WE, Julien JP (2002) Cycling at the interface between neurodevelopment and neurodegeneration. Cell Death Differ 9:1294–1306. doi:10.1038/sj.cdd.4401108 [DOI] [PubMed] [Google Scholar]
- Ohshima T, Ward JM, Huh CG, Longenecker G, Veeranna, Pant HC, Brady RO, Martin LJ, Kulkarni AB (1996) Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci USA 93:11173–11178. doi:10.1073/pnas.93.20.11173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojala JO, Sutinen EM, Salminen A, Pirttila T (2008) Interleukin-18 increases expression of kinases involved in tau phosphorylation in SH-SY5Y neuroblastoma cells. J Neuroimmunol 205:86–93. doi:10.1016/j.jneuroim.2008.09.012 [DOI] [PubMed] [Google Scholar]
- Okamoto K, Beach D (1994) Cyclin G is a transcriptional target of the p53 tumor suppressor protein. EMBO J 13:4816–4822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otth C, Concha II, Arendt T, Stieler J, Schliebs R, Gonzalez-Billault C, Maccioni RB (2002) AbetaPP induces cdk5-dependent tau hyperphosphorylation in transgenic mice Tg2576. J Alzheimers Dis 4:417–430 [DOI] [PubMed] [Google Scholar]
- Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH (1999) Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 402:615–622. doi:10.1038/45159 [DOI] [PubMed] [Google Scholar]
- Patzke H, Tsai LH (2002) Calpain-mediated cleavage of the cyclin-dependent kinase-5 activator p39 to p29. J Biol Chem 277:8054–8060. doi:10.1074/jbc.M109645200 [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Wilson CA, Lee VM, Klein PS (2003) GSK-3alpha regulates production of Alzheimer’s disease amyloid-beta peptides. Nature 423:435–439. doi:10.1038/nature01640 [DOI] [PubMed] [Google Scholar]
- Quintanilla RA, Orellana DI, Gonzalez-Billault C, Maccioni RB (2004) Interleukin-6 induces Alzheimer-type phosphorylation of tau protein by deregulating the cdk5/p35 pathway. Exp Cell Res 295:245–257. doi:10.1016/j.yexcr.2004.01.002 [DOI] [PubMed] [Google Scholar]
- Rojo LE, Fernandez JA, Maccioni AA, Jimenez JM, Maccioni RB (2008) Neuroinflammation: implications for the pathogenesis and molecular diagnosis of Alzheimer’s disease. Arch Med Res 39:1–16. doi:10.1016/j.arcmed.2007.10.001 [DOI] [PubMed] [Google Scholar]
- Rudrabhatla P, Zheng YL, Amin ND, Kesavapany S, Albers W, Pant HC (2008) Pin1-dependent prolyl isomerization modulates the stress-induced phosphorylation of high molecular weight neurofilament protein. J Biol Chem 283:26737–26747. doi:10.1074/jbc.M801633200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Nishimoto I, Matsuoka M (2002) Ik3–2, a relative to ik3–1/cables, is associated with cdk3, cdk5, and c-abl. Biochim Biophys Acta 1574:157–163 [DOI] [PubMed] [Google Scholar]
- Shan X, Chi L, Bishop M, Luo C, Lien L, Zhang Z, Liu R (2006) Enhanced de novo neurogenesis and dopaminergic neurogenesis in the substantia nigra of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced Parkinson’s disease-like mice. Stem Cells 24:1280–1287. doi:10.1634/stemcells.2005-0487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Veeranna, Sharma M, Amin ND, Sihag RK, Grant P, Ahn N, Kulkarni AB, Pant HC (2002) Phosphorylation of MEK1 by cdk5/p35 down-regulates the mitogen-activated protein kinase pathway. J Biol Chem 277:528–534. doi:10.1074/jbc.M109324200 [DOI] [PubMed] [Google Scholar]
- Shelton SB, Johnson GV (2004) Cyclin-dependent kinase-5 in neurodegeneration. J Neurochem 88:1313–1326 [DOI] [PubMed] [Google Scholar]
- Shetty KT, Link WT, Pant HC (1993) Cdc2-like kinase from rat spinal cord specifically phosphorylates KSPXK motifs in neurofilament proteins: isolation and characterization. Proc Natl Acad Sci USA 90:6844–6848. doi:10.1073/pnas.90.14.6844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slevin M, Krupinski J (2008) Cyclin-dependent kinase-5 targeting for ischaemic stroke. Curr Opin Pharmacol 9:119–124 [DOI] [PubMed] [Google Scholar]
- Sultana R, Butterfield DA (2007) Regional expression of key cell cycle proteins in brain from subjects with amnestic mild cognitive impairment. Neurochem Res 32:655–662. doi:10.1007/s11064-006-9123-x [DOI] [PubMed] [Google Scholar]
- Sun KH, de Pablo Y, Vincent F, Johnson EO, Chavers AK, Shah K (2008) Novel genetic tools reveal Cdk5’s major role in golgi fragmentation in Alzheimer’s disease. Mol Biol Cell 19:3052–3069. doi:10.1091/mbc.E07-11-1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Saito T, Hisanaga S, Pant HC, Kulkarni AB (2003) Tau phosphorylation by cyclin-dependent kinase 5/p39 during brain development reduces its affinity for microtubules. J Biol Chem 278:10506–10515. doi:10.1074/jbc.M211964200 [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147. doi:10.1126/science.282.5391.1145 [DOI] [PubMed] [Google Scholar]
- Town T, Zolton J, Shaffner R, Schnell B, Crescentini R, Wu Y, Zeng J, DelleDonne A, Obregon D, Tan J, Mullan M (2002) p35/Cdk5 pathway mediates soluble amyloid-beta peptide-induced tau phosphorylation in vitro. J Neurosci Res 69:362–372. doi:10.1002/jnr.10299 [DOI] [PubMed] [Google Scholar]
- Van den Haute C, Spittaels K, Van Dorpe J, Lasrado R, Vandezande K, Laenen I, Geerts H, Van Leuven F (2001) Coexpression of human cdk5 and its activator p35 with human protein tau in neurons in brain of triple transgenic mice. Neurobiol Dis 8:32–44. doi:10.1006/nbdi.2000.0333 [DOI] [PubMed] [Google Scholar]
- Voigt B, Krug M, Schachtele C, Totzke F, Hilgeroth A (2008) Probing novel 1-aza-9-oxafluorenes as selective GSK-3beta inhibitors. ChemMedChem 3:120–126. doi:10.1002/cmdc.200700175 [DOI] [PubMed] [Google Scholar]
- Wang CX, Song JH, Song DK, Yong VW, Shuaib A, Hao C (2006) Cyclin-dependent kinase-5 prevents neuronal apoptosis through ERK-mediated upregulation of Bcl-2. Cell Death Differ 13:1203–1212. doi:10.1038/sj.cdd.4401804 [DOI] [PubMed] [Google Scholar]
- Wen Y, Yang SH, Liu R, Perez EJ, Brun-Zinkernagel AM, Koulen P, Simpkins JW (2007) Cdk5 is involved in NFT-like tauopathy induced by transient cerebral ischemia in female rats. Biochim Biophys Acta 1772:473–483 [DOI] [PubMed] [Google Scholar]
- Yang Z, Zhu Q, Luo K, Zhou Q (2001) The 7SK small nuclear RNA inhibits the CDK9/cyclin T1 kinase to control transcription. Nature 414:317–322. doi:10.1038/35104575 [DOI] [PubMed] [Google Scholar]
- Yang L, Sugama S, Mischak RP, Kiaei M, Bizat N, Brouillet E, Joh TH, Beal MF (2004) A novel systemically active caspase inhibitor attenuates the toxicities of MPTP, malonate, and 3NP in vivo. Neurobiol Dis 17:250–259. doi:10.1016/j.nbd.2004.07.021 [DOI] [PubMed] [Google Scholar]
- Yoshimi K, Ren YR, Seki T, Yamada M, Ooizumi H, Onodera M, Saito Y, Murayama S, Okano H, Mizuno Y, Mochizuki H (2005) Possibility for neurogenesis in substantia nigra of parkinsonian brain. Ann Neurol 58:31–40. doi:10.1002/ana.20506 [DOI] [PubMed] [Google Scholar]
- Yuan J, Yankner BA (2000) Apoptosis in the nervous system. Nature 407:802–809. doi:10.1038/35037739 [DOI] [PubMed] [Google Scholar]
- Zauberman A, Lupo A, Oren M (1995) Identification of p53 target genes through immune selection of genomic DNA: the cyclin G gene contains two distinct p53 binding sites. Oncogene 10:2361–2366 [PubMed] [Google Scholar]
- Zhao M, Momma S, Delfani K, Carlen M, Cassidy RM, Johansson CB, Brismar H, Shupliakov O, Frisen J, Janson AM (2003) Evidence for neurogenesis in the adult mammalian substantia nigra. Proc Natl Acad Sci USA 100:7925–7930. doi:10.1073/pnas.1131955100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132:645–660. doi:10.1016/j.cell.2008.01.033 [DOI] [PubMed] [Google Scholar]
- Zheng YL, Li BS, Amin ND, Albers W, Pant HC (2002) A peptide derived from cyclin-dependent kinase activator (p35) specifically inhibits Cdk5 activity and phosphorylation of tau protein in transfected cells. Eur J Biochem 269:4427–4434. doi:10.1046/j.1432-1033.2002.03133.x [DOI] [PubMed] [Google Scholar]
- Zheng YL, Kesavapany S, Gravell M, Hamilton RS, Schubert M, Amin N, Albers W, Grant P, Pant HC (2005) A Cdk5 inhibitory peptide reduces tau hyperphosphorylation and apoptosis in neurons. EMBO J 24:209–220. doi:10.1038/sj.emboj.7600441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YL, Li BS, Kanungo J, Kesavapany S, Amin N, Grant P, Pant HC (2007) Cdk5 Modulation of mitogen-activated protein kinase signaling regulates neuronal survival. Mol Biol Cell 18:404–413. doi:10.1091/mbc.E06-09-0851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Zhang Q, Yu Z, Zhang L, Tian D, Zhu S, Bu B, Xie M, Wang W (2007) Inhibiting cell cycle progression reduces reactive astrogliosis initiated by scratch injury in vitro and by cerebral ischemia in vivo. Glia 55:546–558. doi:10.1002/glia.20476 [DOI] [PubMed] [Google Scholar]