Abstract
OBJECTIVE
To determine the association between H. pylori infection and risk of incident diabetes in adults at high risk for diabetes who participated in the Diabetes Prevention Program (DPP) study.
METHODS
In a nested case-control study conducted among 421 adults with newly diagnosed diabetes and 421 matched controls, we examined the association between serological status of H. pylori at baseline and risk of incident diabetes over a mean follow-up period of 2.6 years. Using data from the baseline visit of the DPP, we also examined the cross-sectional association between presence of H. pylori antibodies and insulin sensitivity, insulin secretion and the disposition index-like measure after a 75-gram oral glucose tolerance test (OGTT).
RESULTS
At baseline, H. pylori antibodies were present in 40% of participants who developed diabetes and 39% of controls. After adjusting for matching factors, there was no association between exposure to H. pylori and incident diabetes (odds ratio [OR] of 1.04 (95% CI, 0.77 to 1.40). In cross-sectional analyses, H. pylori status was not significantly associated with insulin sensitivity and disposition index-like measure from OGTT.
CONCLUSIONS
In adults at high risk for diabetes, H. pylori seropositivity was not associated with risk of developing diabetes.
Keywords: pylori, case-control, Diabetes Prevention Program, Diabetes mellitus, metabolic syndrome, observational study
1. INTRODUCTION
Diabetes affects approximately 8 percent of the adult population in the U.S. 1. Although overall incidence of diabetes appears to have plateaued, diabetes continues to grow in Hispanic and non-Hispanic blacks and in people attaining a high school education or less 1. Nearly 9 out of 10 new cases are due to type 2 diabetes. Lifestyle (e.g., diet, physical activity), genetic, and socioeconomic factors are well-established risk factors for type 2 diabetes 2,3 but do not fully account for the total diabetes risk. In clinical trials, lifestyle changes aiming at weight loss have been successful at lowering risk of type 2 disease 4. However, long-term maintenance of weight loss has proved elusive outside of clinical trials. Moreover, even after successful weight loss, there is still significant residual risk. Therefore, searching for additional risk factors for type 2 diabetes is important.
Helicobacter pylori (H. pylori) colonizes the human stomach and has been implicated in the development of several gastric conditions 5. Whereas H. pylori was initially thought only to cause disease in the upper gut, this microorganism has been implicated in several extra-digestive conditions 6 Recently, infection with H. pylori has emerged as a potential risk factor for type 2 diabetes 7,8. In some observational studies, H. pylori infection has been associated with glucose intolerance or diabetes, but this has not been a consistent finding 9–20. Except for one study 20, the available observational studies have been cross-sectional designs. Since they cannot determine the temporal sequence of the association, and the observed associations may be confounded by a variety of factors (e.g. age, race/ethnicity), the hypothesis that H. pylori may be a risk factor for t2DM remains unresolved.
The purpose of the present study was to (1) evaluate whether infection with H. pylori is associated with development of type 2 diabetes and (2) identify pathophysiologic mechanisms that may mediate the association among participants at high risk for diabetes in the Diabetes Prevention Program (DPP), a controlled trial comparing different treatment modalities to prevent diabetes.
2. RESEARCH DESIGN AND METHODS
2.1 Study Participants
The DPP was a randomized controlled clinical trial conducted between 1996 and 2001 at 27 sites in the U.S., comparing the effects of intensive lifestyle intervention, metformin, or placebo on the development of diabetes in adults at high risk for the disease 4. The eligibility criteria, design, and methods of the DPP have been described in detail elsewhere 4,21. Inclusion criteria included age ≥25 years, body mass index (BMI) ≥24 kg/m2 (≥22 kg/m2 in Asian Americans), fasting plasma glucose between 5.3 to 6.9 mmol/L (95 to 125 mg/dL) (≤6.9 mmol/L for American Indian sites) and plasma glucose between 7.8 to 11 mmol/L (140 to 199 mg/dL) after a 75-gram oral glucose tolerance test. Persons taking any medicine known to alter glucose tolerance were excluded. All DPP participants were given standard advice on healthy diet and physical activity before randomization to one of 3 arms: intensive program of lifestyle modification (aiming to achieve a weight reduction of at least 7 percent of initial body weight), standard lifestyle recommendations plus metformin or standard lifestyle recommendations plus placebo. The Institutional Review Board at each site approved the DPP protocol and all participants gave written informed consent. The Tufts University Institutional Review Board approved the present ancillary observational study.
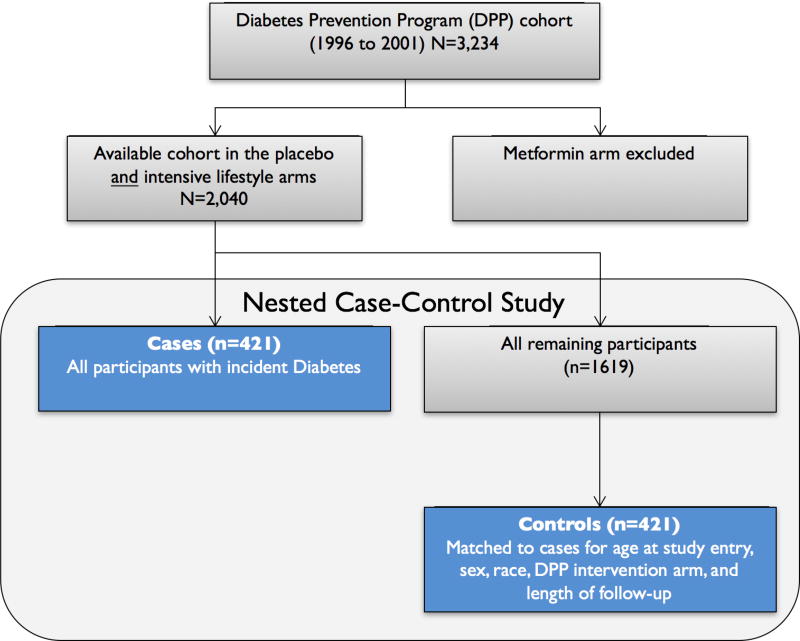
2.2 Nested Case-Control Study Design
We employed a case-control study nested among DPP participants randomized to the intensive lifestyle or placebo groups. We excluded the metformin arm to avoid potential confounding of results by a hypoglycemic medication. Cases were all participants (n=421) who developed diabetes during follow-up. Controls were selected from the remaining participants, matched 1:1 with cases using the “greedy” algorithm 22. Matching criteria were age at study entry (+/− 5 years), sex (male or female), race/ethnicity (Non-Hispanic White vs. other), DPP intervention (lifestyle vs. placebo), and length of follow-up time in the DPP study.
2.3 Assessment of H. pylori infection
Testing for H. pylori infection was done in stored samples from the baseline visit, when all participants were free of diabetes, by measuring H. pylori immunoglobulin G (IgG) antibody in serum using H. pylori IgG enzyme-linked immunosorbent assay (ELISA, Diasorin Diagnostics Srl, manufactured by Hycor Biomedical GmbH). Presence of antibodies indicates past or current infection with H. pylori. The clinical laboratory at Tufts Medical Center performed the testing and all quality control procedures were followed. Samples from matched case-control pairs were assayed in the same analytical run by personnel blinded to the case-control status of the samples. For each specimen, an immune status ratio (ISR) was calculated based on Optical density (OD) values. A value of ISR less than 0.9 was considered to be negative, ISR between 0.91–1.09 was considered indeterminate and ISR greater than 1.1 was considered positive. In sensitivity analysis, we did not find a significant change when indeterminate samples (27 samples) were grouped with either negative or positive samples, so we considered all samples with ISR of 0.91 or greater as positive, as done in prior studies 18.
2.4 Ascertainment of Incident Diabetes
The primary DPP outcome, incident diabetes, was assessed following strict laboratory criteria, based on a 75-gram oral glucose tolerance testing performed annually and fasting plasma glucose performed semiannually or when symptoms consistent with hyperglycemia occurred 4,21. The diagnosis required confirmation by repeat testing.
2.5 Ascertainment of Glucose-Insulin Dynamic Measures
A composite beta-cell function (disposition index-like measure from OGTT) was estimated by the product of the corrected insulin response (CIR) and insulin-sensitivity index (ISI), as previously used in the DPP 23. Corrected insulin response was calculated as follows: (100 × insulin at 30 minutes) ÷ (glucose at 30 minutes × [glucose at 30 minutes – 70 mg/dL]). Insulin-sensitivity index, which is the reciprocal of insulin resistance according to the homeostasis model assessment, was calculated as follows: 22.5 ÷ (fasting insulin × [fasting glucose ÷ 18.01]). Glucose and insulin are expressed as mg/dL and µU/mL, respectively, unless otherwise specified.
2.6 Assessment of Potential Confounders and Laboratory Assessment
Self-reported race and ethnicity were classified according to the 1990 U.S. Census questionnaire. Self-reported level of leisure physical activity was assessed annually with the Modifiable Activity Questionnaire and expressed as the average metabolic equivalent (MET-hours) per week for the previous year. Standardized interviewer-administered questionnaires were used annually to obtain self-reported data on personal medical history, smoking, medications, alcohol use, and family medical history. Weight was measured using a standard calibrated scale and height was measured with a standard stadiometer and body mass index was calculated (Kg/m2). Fasting blood was obtained and processed following standardized procedures. Measurement methods for hemoglobin A1c, glucose and insulin, and C-reactive protein have been published 4,21.
2.7 Statistical Analyses
We describe the differences in baseline characteristics between cases and controls using means for continuous measures and percentages for categorical measures. Statistical tests of the mean differences between cases and controls were performed with t-tests and chi-square tests for proportional differences. On the basis of H. pylori immunoassay results, as described above, participants were classified as H. pylori positive or negative. Odds ratios (OR) and their 95% confidence intervals were used to estimate the association between H. pylori status and incident diabetes using conditional logistic regression analysis for matched pairs data. The first model adjusted for matching factors only. Two additional regression models were built adjusting for clinically important baseline characteristics. The first additional model included baseline body mass index. The second additional model was built adjusting further for smoking status (never, past, or currently smoking), alcohol consumption (g/day) and physical activity (MET-hours per week).
In subgroup analyses, we tested the association between H. pylori and incident diabetes by race/ethnicity (Non-Hispanic White vs. Other), BMI (non-obese vs. obese [<30 and >=30 kg/m2]), age (two groups based on median [<57 and >=57 years]) and DPP intervention (lifestyle and placebo). We also tested for statistical interaction between race, baseline BMI, age, and DPP intervention × H. pylori status on diabetes incidence by including an interaction term in the conditional logistic regression model.
In cross-sectional analyses, we assessed the association between H. pylori status and glucose-insulin dynamic measures. Average differences of insulin sensitivity index, insulin secretion and disposition index-like measure from OGTT at baseline between H. pylori groups was calculated from multivariable linear regression models adjusting for the same co-variates above.
For all analyses a two-sided alpha of 0.05 was used as the threshold for determining statistical significance. Analyses were performed using SAS (version 9.3).
3. RESULTS
3.1 Participant characteristics
At baseline, the mean age of the cases and controls was 49.6 years and 66% were women (Table 1). Participants who developed diabetes were more likely to be smokers and reported lower alcohol consumption. As expected, cases had a higher baseline BMI (35.6 vs. 33.2 kg/m2), hemoglobin A1c (6.1 vs. 5.8%), fasting plasma glucose (112.1 vs. 104.9 mg/dL) and C-reactive protein (6.8 vs. 5.5 mg/L) as compared to controls (Table 1).
Table 1.
Baseline characteristics of participants
| Characteristic | Diabetes cases (n=421) |
Controls (n=421) |
P-value1 |
|---|---|---|---|
| Age, mean (SD), y | 49.6 (10.0) | 49.6 (9.9) | 0.96 |
| Sex, No. (%) women | 278 (66.0) | 278 (66.0) | 0.99 |
| Race/ethnicity, No. (%) | |||
| Non-Hispanic White | 228 (54.2) | 228 (54.2) | 0.22 |
| African-American | 95 (22.6) | 78 (18.5) | |
| Other (Hispanic, American Indian, Asian) | 98 (23.3) | 115 (27.3) | |
| Weight, mean (SD), kg | 99.8 (22.8) | 91.9 (18.9) | <0.01 |
| DPP lifestyle intervention arm, No (%) | 139 (33.0) | 139 (33.0) | 0.99 |
| Body mass index, mean (SD), kg/m2 | 35.6 (7.5) | 33.2 (6.1) | <0.01 |
| Waist circumference, mean (SD), cm | 109.4 (16.1) | 103.2 (13.8) | <0.01 |
| Family history of diabetes, No. (%) | 281 (66.9) | 300 (71.3) | 0.17 |
| Physical Activity, mean (SD), MET-hours 3 | 16.6 (23.2) | 15.1 (19.7) | 0.33 |
| Smoking status, No. (%) | |||
| Never | 230 (54.6) | 257 (61.0) | 0.01 |
| Past | 150 (35.6) | 144 (34.2) | |
| Current | 41 (9.7) | 20 (4.8) | |
| Alcohol consumption, mean (SD), g/day | 1.8 (4.6) | 2.3 (5.8) | 0.19 |
| Fasting plasma glucose, mean (SD), mg/dL | 112.1 (9.4) | 104.9 (6.3) | <0.01 |
| Hemoglobin A1c, mean (SD), % | 6.1 (0.6) | 5.8 (0.5) | <0.01 |
| C-reactive protein, mean (SD), mg/L | 6.8 (9.3) | 5.5 (6.2) | 0.02 |
| Insulin sensitivity index (ISI), mean (SD), [(µU/mL)*( mg/dL)]−1 | 0.16 (0.12) | 0.21 (0.14) | <0.01 |
| Corrected insulin response (CIR), mean (SD), [(µU/mL)/(mg/dL)2] | 0.53 (0.34) | 0.66 (0.41) | <0.01 |
| Disposition index-like measure from OGTT (LN(CIR) * LN (Insulin sensitivity index)) | 0.07 (0.04) | 0.12 (0.08) | <0.01 |
| H. pylori positive, N (%) | 169 (40%) | 166 (39%) | |
SD, standard deviation
Characteristics of diabetes cases and controls were compared using the t test (for means) or chi-square test (for percentages). Age (+/− 5 years), sex (men or women), race/ethnicity (Non-Hispanic White vs. African-American vs. Other), DPP intervention arm (lifestyle or placebo) and length of follow up were matching variables.
MET denotes metabolic equivalent. MET-hours represent the average amount of time engaged in specified physical activities multiplied by the MET value of each activity.
3.2 H. pylori infection and incident diabetes
Participants were followed for an average of 2.6 years. After adjusting for matching factors, H. pylori seropositivity was not significantly associated with increased risk of type 2 diabetes (odds ratio [OR] of 1.04 (95% CI, 0.77 to 1.40) (Table 2). After further adjustment for BMI at baseline, there was no change in the association (OR 1.04; 95% CI, 0.76 to1.42). Further multivariable adjustment for other covariates at baseline, including smoking status, alcohol consumption and physical activity also did not change results.
Table 2.
Odds ratio for incident diabetes, according to H. pylori status in participants at risk for diabetes in the Diabetes and Prevention Program study.
| H. pylori status* | P-value | ||
|---|---|---|---|
| Negative (N=507) |
Positive (N=335) |
||
| Cases / controls, No. | 252 / 255 | 169 / 166 | |
| Odds ratio (95% confidence interval) | |||
| Model 1 1 | 1.00 (reference) | 1.04 (0.77, 1.40) | 0.82 |
| Model 2 2 | 1.00 (reference) | 1.04 (0.76, 1.42) | 0.79 |
| Model 3 3 | 1.00 (reference) | 1.03 (0.74, 1.42) | 0.88 |
Model 1 adjusted for matching factors (age [years], sex [male or female], race/ethnicity [White vs. Non-White], intervention arm [lifestyle or placebo] and length of follow up) through conditional logistic regression.
Model 2 adjusted for everything in model 1 plus body mass index (kg/m2) at baseline.
Model 3 adjusted for everything in model 2 plus smoking status (never, past, or currently smoking), alcohol consumption (g/day), self-reported physical activity (MET-hours per week).
3.3 Subgroup Analyses
We conducted subgroup analyses, to explore whether race/ethnicity, baseline BMI, age or DPP intervention (intensive or placebo) would modify the association between H. pylori seropositivity and incident diabetes (Table 3). The test for interaction was nearly statistically significant for H. pylori × BMI (p= 0.08), but the association between H. pylori seropositivity and incident diabetes was not significant within the two BMI subgroups. The tests for interactions were not statistically significant in any of the other subgroups analyzed.
Table 3.
Odds ratio for incident diabetes, according to H. pylori status in participants at risk for diabetes in the Diabetes and Prevention Program study by race/ethnicity, baseline body mass index, age and DPP intervention arm.
| H. pylori status | P-value1 | P for interaction6 | ||
|---|---|---|---|---|
| Negative | Positive | |||
| Race/ethnicity 2 | ||||
| Non-Hispanic White | 1.00 (reference) | 1.22 (0.81, 1.86) | 0.35 | 0.25 |
| Other | 1.00 (reference) | 0.88 (0.59, 1.32) | 0.53 | |
| Body Mass Index (kg/m2) 3 | ||||
| BMI < 30 | 1.00 (reference) | 1.10 (0.66, 1.83) | 0.73 | 0.08 |
| BMI ≥ 30 | 1.00 (reference) | 1.03 (0.72, 1.47) | 0.87 | |
| Age (median, years) 4 | ||||
| < 57 | 1.00 (reference) | 1.00 (0.71, 1.41) | 0.99 | 0.84 |
| ≥ 57 | 1.00 (reference) | 1.14 (0.64, 2.02) | 0.65 | |
| DPP Arm | ||||
| Lifestyle | 1.00 (reference) | 1.00 (0.59, 1.68) | 0.99 | 0.87 |
| Placebo | 1.00 (reference) | 1.05 (0.74, 1.49) | 0.79 | |
P-values for subgroup analyses are based on logistic regression adjusted for matching factors (age [years], sex [male or female], race/ethnicity [Non-Hispanic White vs. Other], DPP intervention arm [lifestyle or placebo]) and length of follow up.
Model for White includes 228 cases and controls; model for non-White includes 193 cases and controls.
Model for BMI<30 kg/m2 includes 110 cases and 149 controls; model for BMI≥30 kg/m2 includes 311 cases and 272 controls
Model for age<57 years includes 318 cases and 316 controls; model for age≥57 years includes 103 cases and 105 controls
Model for DPP lifestyle arm includes 139 cases and controls; model for placebo arm includes 282 cases and controls.
P-values for interaction effect are adjusted for matched variables (age [years], sex [male or female], race/ethnicity [Non-Hispanic White vs. Other], intervention arm [lifestyle or placebo]) and length of follow up through conditional logistic regression.
3.4 H. pylori seropositivity and Glucose-Insulin Dynamic Measures
We also examined the cross-sectional association between H. pylori seropositivity and insulin sensitivity index, corrected insulin response and disposition index-like measure from OGTT at baseline adjusting for matching factors and other covariates including smoking status, alcohol consumption and self-reported physical activity. H. pylori seropositivity was negatively associated with insulin sensitivity index and disposition index-like measure from OGTT and positively associated with corrected insulin response, but these associations were not statistically significant (Table 4).
Table 4.
Adjusted average difference of insulin secretion and insulin sensitivity according to H. pylori status at baseline in participants at risk for diabetes in the Diabetes and Prevention Program (DPP) study.
| H. pylori status * | P-value | ||
|---|---|---|---|
| Negative | Positive | ||
| Insulin sensitivity index, [(µU/mL)*( mg/dL)]-1 † | (reference) | −0.02 (−0.10, 0.05) | 0.53 |
| Corrected insulin response, CIR (µU/mL)/(mg/dL)2 † | (reference) | 0.08 (−0.01, 0.17) | 0.08 |
| Disposition index-like measure from OGTT (LN(CIR) * LN (Insulin sensitivity index)) | (reference) | −0.12 (−0.29, 0.05) | 0.17 |
Results are presented by H. pylori status; average difference between groups was calculated from a multivariate linear regression model; all models are adjusted for matching factors (age [years], sex [male or female], race/ethnicity [Non-Hispanic White vs. Other], DPP intervention arm [lifestyle or placebo] and length of follow up), BMI at baseline, smoking status (never, past, or currently smoking), alcohol consumption (g/day), self-reported physical activity (MET-hours per week).
Dependent variable log transformed
4. DISCUSSION
In this observational longitudinal study nested within the DPP cohort, there was no association between the presence of H. pylori antibodies (representing past or current infection) and incident diabetes, even after adjustment for potential confounders and risk factors. No associations were observed in subgroups defined by race/ethnicity, baseline BMI, age or DPP intervention arm. Also, there was no statistically significant cross-sectional association between H. pylori antibodies and measures of glucose-insulin dynamics at baseline.
Cross-sectional studies have reported a higher prevalence (~38–85%) of H. pylori seropositivity or positive gastric histology among patients with glucose intolerance/diabetes compared to controls (18–65%) with normal glucose tolerance 9,10,12,13,18. H. pylori seropositivity 15,16,24 or positive gastric histology 25,26 have also been associated with insulin resistance (assessed in the fasting state) among individuals without known diabetes. Potential biological mechanisms that might explain these observations include H. pylori having a role in energy homeostasis by affecting the production of ghrelin and leptin, which are important hormones in the regulation of appetite and energy expenditure 27–29 or through an effect on secretion of pro-inflammatory cytokines 30. However, other studies have reported no differences in H. pylori between patients with diabetes and without diabetes, or even a lower rate of infection in patients with diabetes 14,17,19,31.
The results of the present case-control study nested within a well-conducted randomized trial do not support the hypothesis that infection with H. pylori is associated with risk of type 2 diabetes and are consistent with a recent meta-analysis 32. A potential explanation for the lack of association may be that the effect of H. pylori on diabetes pathophysiology may have occurred before the onset of pre-diabetes. Furthermore, H. pylori strains exhibit a significant degree of diversity and some strains contain pathogenic genes, such as cytokine-associated gene A (CagA), which have been associated with more severe clinical manifestations and may also be relevant to diabetes 33,34. We did not measure for the presence of the CagA protein and could not assess this hypothesis. Although the overall prevalence of H. pylori in the US is declining, seroprevalence is overall still high, especially in populations (older, minorities, overweight) who are at greatest risk for diabetes 35–37 and there is evidence that obesity may modify the association between H. pylori and glucose tolerance 18. However, subgroup analyses by race/ethnicity and weight status did not reveal any significant associations. In contrast, the only other longitudinal study available reported a positive association between H. pylori infection and incident diabetes in Latino elderly 20.
Several studies have reported on the association between H. pylori infection and insulin resistance with mixed results 15,16,21,38 but none have reported the association between H. pylori and composite beta-cell function as measured by disposition index-like measure from OGTT, which is a measure of pancreatic beta cell function that captures both insulin secretion and insulin sensitivity 23. A low disposition index-like measure from OGTT indicates an impaired pancreatic beta cell function and is a validated predictor of diabetes risk 39,40. The inverse association between the presence of H. pylori antibodies and disposition index-like measure from OGTT we observed was not statistically significant.
The present study has several advantages over prior studies. Nearly all prior studies reporting associations between H. pylori and diabetes are from non-US populations while our study focuses on a cohort representative of the U.S. population at risk for t2DM (older, minorities). Cases were defined based on a new diagnosis of diabetes (incident cases only) using strict biochemical glycemic criteria, which avoids risk of misclassification and bias towards null associated with self-reporting of diabetes status. The assessment of diabetes risk factors and other potential confounders was carried out prospectively before diabetes development, and was considerably more detailed than prior studies. There are some limitations: We did not have data on the use of antibiotics during the follow up period, which could have altered the course of H. pylori infection. Conceivably some subjects may have been treated specifically to eradicate H. pylori. Antibiotic monotherapy given for other infections is rarely effective at eradicating H. pylori but it could have resulted in under-estimation of the risk if more frequently taken by the cohort with diabetes than the controls. In addition, the follow-up period may not have been long enough to detect a significant association.
In conclusion, we did not find an association between the presence of H. pylori antibodies and diabetes incidence in adults at high risk for diabetes. Insulin sensitivity, insulin secretion and oral disposition index-like measure from OGTT did not significantly differ between participants with or without serological evidence of H. pylori infection. Further studies comparing the effects of H. pylori strains carrying the cag pathogenicity island with cag-negative strains may provide additional insight.
Figure 1.
Nested case-control design
Acknowledgments
This work was supported by grants R01-DK-76092 and R01-DK-79003 (to A.G.P.) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institutes of Health, and the National Institutes of Health Office of Dietary Supplements; Grant UL1RR025752 (to Tufts University) from the National Center for Research Resources; the U.S. Department of Agriculture Agreement 58-1950-9001 (to B.D-H.); and Grant UO1-DK-48489 from the NIDDK to the DPP clinical centers and the Coordinating Center for the design and conduct of the DPP study. The study was supported in part by the Intramural Research Program of the NIDDK.
S.A. researched data, wrote the manuscript and interpreted the results. A.P. contributed to the discussion, interpreted the results and reviewed/edited the manuscript. J.P. contributed to the discussion and reviewed/edited the manuscript. J.N. conducted analyses, reviewed and edited the manuscript. S.M and W.C.K. contributed to the discussion and reviewed and edited the manuscript. W.C.K. contributed to the design, management, and data collection in the DPP. S.A. and A.G.P. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The investigators gratefully acknowledge the commitment and dedication of the participants of the DPP. We thank Robin Ruthazer, MPH. Associate Director, Research Design Center/Biostatistics Research Center (RDC/BRC), Tufts Clinical and Translational Science Institute, Tufts University, Boston, MA for her valuable input into interpretation of the results.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Financial Conflicts of Interest: None disclosed.
Contributor Information
Saud Alzahrani, Division of Endocrinology, Diabetes and Metabolism, Tufts Medical Center, Boston, MA1.
Jason Nelson, Predictive Analytics and Comparative Effectiveness (PACE) Center, Institute for Clinical Research and Health Policy Studies, Tufts Medical Center, Boston, Massachusetts.
Steven F. Moss, Division of Gastroenterology, Brown University, Providence, RI.
Jessica K. Paulus, Predictive Analytics and Comparative Effectiveness (PACE) Center, Institute for Clinical Research and Health Policy Studies, Tufts Medical Center, Boston, Massachusetts.
William C. Knowler, National Institute of Diabetes and Digestive and Kidney Diseases, Phoenix, AZ.
Anastassios G. Pittas, Division of Endocrinology, Diabetes and Metabolism, Tufts Medical Center, Boston, MA1.
References
- 1.Geiss LS, Wang J, Cheng YJ, et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA. 2014;312(12):1218–1226. doi: 10.1001/jama.2014.11494. [DOI] [PubMed] [Google Scholar]
- 2.Qi L, Hu FB, Hu G. Genes, environment, and interactions in prevention of type 2 diabetes: a focus on physical activity and lifestyle changes. Curr Mol Med. 2008;8(6):519–532. doi: 10.2174/156652408785747915. [DOI] [PubMed] [Google Scholar]
- 3.Agardh E, Allebeck P, Hallqvist J, Moradi T, Sidorchuk A. Type 2 diabetes incidence and socio-economic position: a systematic review and meta-analysis. Int J Epidemiol. 2011;40(3):804–818. doi: 10.1093/ije/dyr029. [DOI] [PubMed] [Google Scholar]
- 4.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347(15):1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 6.Goni E, Franceschi F. Helicobacter pylori and extragastric diseases. Helicobacter. 2016;21(Suppl 1):45–48. doi: 10.1111/hel.12340. [DOI] [PubMed] [Google Scholar]
- 7.Polyzos SA, Kountouras J, Zavos C, Deretzi G. The association between Helicobacter pylori infection and insulin resistance: a systematic review. Helicobacter. 2011;16(2):79–88. doi: 10.1111/j.1523-5378.2011.00822.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhou X, Zhang C, Wu J, Zhang G. Association between Helicobacter pylori infection and diabetes mellitus: a meta-analysis of observational studies. Diabetes Res Clin Pract. 2013;99(2):200–208. doi: 10.1016/j.diabres.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Quadri R, Rossi C, Catalfamo E, et al. Helicobacter pylori infection in type 2 diabetic patients. Nutr Metab Cardiovasc Dis. 2000;10(5):263–266. [PubMed] [Google Scholar]
- 10.Marrollo M, Latella G, Melideo D, et al. Increased prevalence of Helicobacter pylori in patients with diabetes mellitus. Dig Liver Dis. 2001;33(1):21–29. doi: 10.1016/s1590-8658(01)80131-6. [DOI] [PubMed] [Google Scholar]
- 11.de Luis DA, de la Calle H, Roy G, et al. Helicobacter pylori infection and insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1998;39(2):143–146. doi: 10.1016/s0168-8227(97)00127-7. [DOI] [PubMed] [Google Scholar]
- 12.Fernandini-Paredes GG, Mezones-Holguin E, Vargas-Gonzales R, Pozo-Briceno E, Rodriguez-Morales AJ. In patients with type 2 diabetes mellitus, are glycosylated hemoglobin levels higher for those with Helicobacter pylori infection than those without infection? Clin Infect Dis. 2008;47(1):144–146. doi: 10.1086/588846. [DOI] [PubMed] [Google Scholar]
- 13.Pocecco M, Buratti E, Tommasini A, Torre G, Not T. High risk of Helicobacter pylori infection associated with cow's milk antibodies in young diabetics. Acta Paediatr. 1997;86(7):700–703. doi: 10.1111/j.1651-2227.1997.tb08571.x. [DOI] [PubMed] [Google Scholar]
- 14.Gillum RF. Infection with Helicobacter pylori, coronary heart disease, cardiovascular risk factors, and systemic inflammation: the Third National Health and Nutrition Examination Survey. J Natl Med Assoc. 2004;96(11):1470–1476. [PMC free article] [PubMed] [Google Scholar]
- 15.Eshraghian A, Hashemi SA, Hamidian Jahromi A, et al. Helicobacter pylori infection as a risk factor for insulin resistance. Dig Dis Sci. 2009;54(9):1966–1970. doi: 10.1007/s10620-008-0557-7. [DOI] [PubMed] [Google Scholar]
- 16.Gunji T, Matsuhashi N, Sato H, et al. Helicobacter pylori infection significantly increases insulin resistance in the asymptomatic Japanese population. Helicobacter. 2009;14(5):144–150. doi: 10.1111/j.1523-5378.2009.00705.x. [DOI] [PubMed] [Google Scholar]
- 17.Lutsey PL, Pankow JS, Bertoni AG, Szklo M, Folsom AR. Serological evidence of infections and Type 2 diabetes: the MultiEthnic Study of Atherosclerosis. Diabet Med. 2009;26(2):149–152. doi: 10.1111/j.1464-5491.2008.02632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Blaser MJ. Association between gastric Helicobacter pylori colonization and glycated hemoglobin levels. The Journal of infectious diseases. 2012;205(8):1195–1202. doi: 10.1093/infdis/jis106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jafarzadeh A, Rezayati MT, Nemati M. Helicobacter pylori Seropositivity in Patients with Type 2 Diabetes Mellitus in South-East of Iran. Acta Med Iran. 2013;51(12):892–896. [PubMed] [Google Scholar]
- 20.Jeon CY, Haan MN, Cheng C, et al. Helicobacter pylori infection is associated with an increased rate of diabetes. Diabetes care. 2012;35(3):520–525. doi: 10.2337/dc11-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes care. 1999;22(4):623–634. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenbaum PR. Optimal Matching for Observational Studies. Journal of the American Statistical Association. 1989;84(408):1024–1032. [Google Scholar]
- 23.Kitabchi AE, Temprosa M, Knowler WC, et al. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes. 2005;54(8):2404–2414. doi: 10.2337/diabetes.54.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ekesbo R, Nilsson PM, Lindholm LH, Persson K, Wadstrom T. Combined seropositivity for H. pylori and C. pneumoniae is associated with age, obesity and social factors. J Cardiovasc Risk. 2000;7(3):191–195. doi: 10.1177/204748730000700305. [DOI] [PubMed] [Google Scholar]
- 25.Aydemir S, Bayraktaroglu T, Sert M, et al. The effect of Helicobacter pylori on insulin resistance. Dig Dis Sci. 2005;50(11):2090–2093. doi: 10.1007/s10620-005-3012-z. [DOI] [PubMed] [Google Scholar]
- 26.Aslan M, Horoz M, Nazligul Y, et al. Insulin resistance in H pylori infection and its association with oxidative stress. World J Gastroenterol. 2006;12(42):6865–6868. doi: 10.3748/wjg.v12.i42.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isomoto H, Nakazato M, Ueno H, et al. Low plasma ghrelin levels in patients with Helicobacter pylori-associated gastritis. Am J Med. 2004;117(6):429–432. doi: 10.1016/j.amjmed.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 28.Isomoto H, Nishi Y, Ohnita K, et al. The Relationship between Plasma and Gastric Ghrelin Levels and Strain Diversity in Helicobacter pylori Virulence. Am J Gastroenterol. 2005;100(6):1425–1427. doi: 10.1111/j.1572-0241.2005.41929_7.x. [DOI] [PubMed] [Google Scholar]
- 29.Pacifico L, Anania C, Osborn JF, et al. Long-term effects of Helicobacter pylori eradication on circulating ghrelin and leptin concentrations and body composition in prepubertal children. Eur J Endocrinol. 2008;158(3):323–332. doi: 10.1530/EJE-07-0438. [DOI] [PubMed] [Google Scholar]
- 30.Gen R, Demir M, Ataseven H. Effect of Helicobacter pylori eradication on insulin resistance, serum lipids and low-grade inflammation. South Med J. 2010;103(3):190–196. doi: 10.1097/SMJ.0b013e3181cf373f. [DOI] [PubMed] [Google Scholar]
- 31.Dore MP, Bilotta M, Malaty HM, et al. Diabetes mellitus and Helicobacter pylori infection. Nutrition. 2000;16(6):407–410. doi: 10.1016/s0899-9007(00)00267-7. [DOI] [PubMed] [Google Scholar]
- 32.Horikawa C, Kodama S, Fujihara K, et al. Association of Helicobacter pylori infection with glycemic control in patients with diabetes: a meta-analysis. Journal of diabetes research. 2014;2014:250620. doi: 10.1155/2014/250620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tohidpour A. CagA-mediated pathogenesis of Helicobacter pylori. Microb Pathog. 2016;93:44–55. doi: 10.1016/j.micpath.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Delitala AP, Pes GM, Malaty HM, Pisanu G, Delitala G, Dore MP. Implication of Cytotoxic Helicobacter pylori Infection in Autoimmune Diabetes. Journal of diabetes research. 2016;2016:7347065. doi: 10.1155/2016/7347065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imai J, Yamada T, Saito T, et al. Eradication of insulin resistance. Lancet. 2009;374(9685):264. doi: 10.1016/S0140-6736(09)60872-2. [DOI] [PubMed] [Google Scholar]
- 36.Everhart JE, Kruszon-Moran D, Perez-Perez GI, Tralka TS, McQuillan G. Seroprevalence and ethnic differences in Helicobacter pylori infection among adults in the United States. The Journal of infectious diseases. 2000;181(4):1359–1363. doi: 10.1086/315384. [DOI] [PubMed] [Google Scholar]
- 37.Grad YH, Lipsitch M, Aiello AE. Secular trends in Helicobacter pylori seroprevalence in adults in the United States: evidence for sustained race/ethnic disparities. American journal of epidemiology. 2012;175(1):54–59. doi: 10.1093/aje/kwr288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vafaeimanesh J, Heidari A, Effatpanah M, Parham M. Serum Adiponectin Level in Diabetic Patients with and without Helicobacter pylori Infection: Is There Any Difference? Scientific World Journal. 2014;2014:402685. doi: 10.1155/2014/402685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Utzschneider KM, Prigeon RL, Faulenbach MV, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes care. 2009;32(2):335–341. doi: 10.2337/dc08-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lorenzo C, Wagenknecht LE, D'Agostino RB, Jr, Rewers MJ, Karter AJ, Haffner SM. Insulin resistance, beta-cell dysfunction, and conversion to type 2 diabetes in a multiethnic population: the Insulin Resistance Atherosclerosis Study. Diabetes care. 2010;33(1):67–72. doi: 10.2337/dc09-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]