Abstract
Purpose
Trial**** was a phase III randomized trial designed to determine the optimal duration of androgen deprivation therapy (ADT) when combined with definitive radiation therapy (RT) in the treatment of locally advanced non-metastatic adenocarcinoma of the prostate. Long-term follow-up results of this study now available are relevant to the management of this disease.
Materials and Methods
Men (N=1,554) with adenocarcinoma of the prostate (cT2c-T4, N0- Nx) with a prostate specific antigen (PSA) <150ng/ml and no evidence of distant metastasis were randomized (June 1992 to April 1995) to short term ADT (STAD: 4 months of flutamide 250mg three times per day and goserelin 3.6mg per month) and definitive RT verses long term ADT (LTAD: STAD with definitive RT plus an additional 24 months of monthly goserelin).
Results
Among 1,520 protocol eligible and evaluable patients, median follow up for this analysis was 19.6 years. In analysis adjusted for prognostic covariates, LTAD improved disease free survival (29% relative reduction in failure rate, p<0.0001), local progression (46% relative reduction, p=0.02), distant metastases (36% relative reduction, p<0.0001), disease specific survival (30% relative reduction, p=0.003), and overall survival (12% relative reduction, p=0.03). Other cause (non-prostate cancer) mortality did not differ (5% relative reduction, p=0.48).
Conclusions
LTAD and RT is superior to STAD and RT for the treatment of locally advanced non-metastatic adenocarcinoma of the prostate and should be considered the standard of care.
Introduction
The benefit of androgen deprivation therapy (ADT) in addition to radiation therapy for locally advanced adenocarcinoma of the prostate has been well established since the results of several phase III randomized trials were reported in the late 1990s and early 2000s.1–3 These trials randomized patients between radiation therapy (RT) alone versus RT and ADT. The ADT was given for varying lengths of time from 4 months2 to three years3 to indefinitely.1 Since all of these trials showed a benefit to the ADT plus RT arms in terms of prostate cancer control, the next obvious question was what was the optimal duration of ADT. Both the European Organisation for Research and the Treatment of Cancer (EORTC)4 and the ****5 (****) addressed this question with a phase III randomized trial of short course versus longer course ADT in addition to pelvic lymph node and prostate RT for locally advanced disease patients.
Results from the EORTC trial showed a benefit to the long term (36 months) arm over the 6 month arm in terms of clinical progression free survival, the primary endpoint of the study.4 Trial**** investigated the addition of 24 months of adjuvant ADT versus no adjuvant ADT following four month duration neoadjuvant and concurrent ADT and standard RT to the prostate and pelvic lymph nodes.6 This report represents the final update of that trial with respect to treatment efficacy outcomes and toxicities.
Patients and Methods
Patient Population
Men with histologically confirmed adenocarcinoma of the prostate (clinical T2c -T4, N0-NX, based on 1992 American Joint Committee on Cancer Staging Manual7 and meeting the following criteria were eligible for Trial****. Pretreatment prostate specific antigen (PSA) <150 ng/mL, Karnofsky performance status > 70% or greater, no evidence of distant metastasis, and no prior ADT, RT, or chemotherapy. Institutional Review Board approval was required at each participating center before any patient enrollment or data transfer could occur. Informed consent was obtained for each patient before enrollment, random assignment, and treatment. The details of pretreatment patient evaluations have been summarized in a previous report.5 Follow up as previously reported occurred after the RT was complete with PSA occurring every 3–6 months during the first 5 years and then annually.
Enrollment and Treatment
Trial**** opened for accrual June 26, 1992 and closed April1 5, 1995 and enrolled 1554 patients.
After registration and consent, patients were randomized within strata defined by stage (T2c v T3 v T4), pretreatment PSA (≤ 30 v > 30 mg/mL), grade (2–5, 6, 7, 8–10), and nodal status (NX-N2) using a permuted block method.8 Patients were randomly assigned to short term androgen deprivation (STAD) or long term androgen deprivation (LTAD), as defined below.
External beam radiation therapy was performed on all patients utilizing conventional pelvic fields using a “4-field technique” with megavoltage x-rays of ≥ 4MV. This treatment was delivered at 1.8–2.0 Gy once daily to a dose of 44–46 Gy and was followed by reduced fields to the prostate for a total of 65–70 Gy for T2c tumors and 67.5–70 Gy for T3 and T4 tumors. The prescribed dose was recorded as an isocenter dose at the center of the prostate target volume.
All patients began ADT two months before the start of RT, and received flutamide (250 mg three times per day) with goserelin (3.6mg subcutaneously monthly) until the RT was completed (four months total duration), and then continued to no further treatment (STAD) or an additional 24 months of monthly goserelin (LTAD), depending on their randomly assigned treatment arm.
Statistical Considerations
Study Design and Endpoints
The primary trial endpoint was disease-free survival (DFS), defined as time until local progression, distant metastasis, biochemical failure, or death prior to these events. This study was designed to provide at least 90% power at (one-sided) alpha= 0.05 to detect an absolute 10% improvement in disease free survival (DFS) from 40% to 50% at 5 years. Additional endpoints include local progression (LP), distant metastasis (DM), biochemical failure (BF), disease-specific survival (DSS), and overall survival (OS). Local progression was defined as clinical evidence of local recurrence by any method or persistent disease. Distant metastasis was defined as clinical evidence of distant disease by any method. BF was originally defined as the earliest of the following: 3 consecutive rises after a post treatment PSA nadir (the 1997 American Society for Therapeutic Radiology Oncology “ASTRO definition”), any point where the patients received additional ADT or an absolute PSA >4ng/mL. In this report we use the more commonly applied Phoenix definition of nadir plus 2.0ng/mL.9 DSS was defined as death resulting from prostate cancer, treatment toxicity, or unknown cause with distant metastasis. All event times were measured from the date of randomization. Acute RT toxicities were defined as those occurring within 90 days from the start of RT. Any toxicity continuing or developing after 90 days was considered a late RT toxicity. These were summarized as frequencies of greatest toxicity grade per type, and for selected adverse events, cumulative probability of occurrence of grade 3 or greater toxicities.
Analysis Methods
The Kaplan-Meier method was used to estimate the OS and DFS distributions.10 The cumulative incidence approach was used to estimate the cumulative probability for LP, DM, BF, and DS deaths in the presence of competing risks.11 In graphical displays, the complement (i.e., 1 minus) the probability was plotted against time from randomization to represent the event-free probability over time. The logrank test was used to test for differences in DFS and OS between treatment arms, and was also used to compare cause-specific hazards for LP, DM, BF, and DSS.12 For each endpoint, hazard ratios with 95% confidence intervals were computed from the Cox proportional hazards model for hazards (DFS and OS) or cause-specific hazards (LP, DM, BF, DSS).13 For endpoints where competing risks are present, analyses using Gray’s test and the associated competing risks hazard regression model were also conducted,14, 15 as both cause-specific hazards and cumulative incidence methods can be relevant to interpretation, particularly in long-term follow-up.16 To explore the potential for larger treatment benefit in patients at particularly high risk, an analysis of treatment outcomes in the subset of patients with a Gleason score of 8–10 was performed in earlier analyses and those findings are updated for this report.
Results
Table 1 shows the pretreatment characteristics of the 1554 patients enrolled (1520 analyzable). There were no statistically significant differences between the two treatment arms with regards to the stratification variables and other characteristics. As previously reported RT as assigned was completed in 96% of the cases in the STAD arm and 95% in the LTAD arm with 4% and 3% of the reviewed cases judged unacceptable major deviations in the STAD and LTAD arms respectively. The median follow up for all living patients was 19.6 years.
Table 1.
Pre-treatment Characteristics for Patients Included in Analysis
| Characteristics | STAD+RT (n=762) | LTAD+RT (n=758) | p | |||
|---|---|---|---|---|---|---|
| Age, years | 0.39 | |||||
| Mean | 69.4 | 69.7 | ||||
| Median (range) | 70 (43–87) | 70 (43–88) | ||||
| No. | % | No. | % | |||
| Race | 0.41 | |||||
| White | 642 | 84.3 | 637 | 84.0 | ||
| Hispanic | 17 | 2.2 | 10 | 1.3 | ||
| African American | 92 | 12.1 | 105 | 13.9 | ||
| Native Hawaiian, other Pacific Islanders, American Indian, or Alaska Native | 6 | 0.8 | 4 | 0.5 | ||
| Unknown | 5 | 0.7 | 2 | 0.3 | ||
| PSA, ng/mL | 0.88 | |||||
| <=30 | 510 | 66.9 | 510 | 67.3 | ||
| >30 | 252 | 33.1 | 248 | 32.7 | ||
| Karnofsky Status, % | 0.04 | |||||
| 70 | 11 | 1.4 | 2 | 0.3 | ||
| 80 | 54 | 7.1 | 48 | 6.3 | ||
| 90 | 376 | 49.3 | 358 | 47.2 | ||
| 100 | 321 | 42.1 | 350 | 46.2 | ||
| Intercurrent Disease | 0.89 | |||||
| No | 253 | 33.2 | 246 | 32.5 | ||
| Yes | 503 | 66.0 | 508 | 67.0 | ||
| Unknown | 6 | 0.8 | 4 | 0.5 | ||
| Clinical Stage | 0.07 | |||||
| T2 | 347 | 45.5 | 344 | 45.4 | ||
| T3 | 394 | 51.7 | 376 | 49.6 | ||
| T4 | 21 | 2.8 | 38 | 5.0 | ||
| Pathological Nodal Stage | 0.63 | |||||
| NX | 657 | 86.2 | 648 | 85.5 | ||
| N0 | 70 | 9.2 | 81 | 10.7 | ||
| N1 | 24 | 3.2 | 18 | 2.4 | ||
| N2 | 11 | 1.4 | 11 | 1.5 | ||
| Institutional Gleason Score | 0.34 | |||||
| 2 – 5 | 142 | 18.6 | 137 | 18.1 | ||
| 6 | 149 | 19.6 | 154 | 20.3 | ||
| 7 | 226 | 29.7 | 251 | 33.1 | ||
| 8 – 10 | 187 | 24.5 | 174 | 23.0 | ||
| Unknown | 58 | 7.6 | 42 | 5.5 | ||
Treatment Outcomes
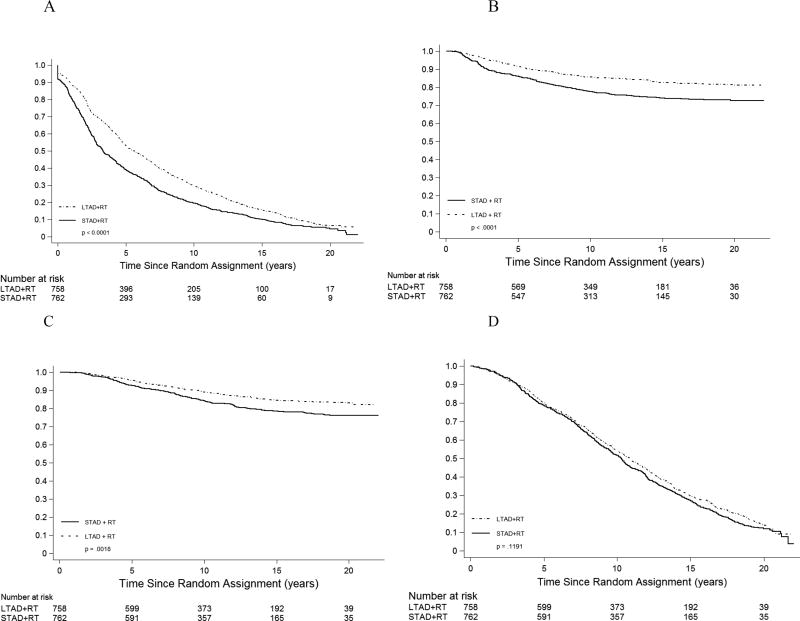
The primary endpoint disease-free survival was improved with long-term androgen deprivation therapy (Figure 1, Table 2). At 15 years, DFS estimates were 10% in the STAD arm versus 16% in the LTAD arm. Overall, there was a 29% reduction in risk of failure for LTAD relative to STAD (p<0.0001, Table 2). Disease events comprising DFS were also reduced with LTAD (Table 2), with a 46% relative risk reduction in LP and 15-year failure-free estimates of 77% (STAD) vs. 87% (LTAD), a 36% relative risk reduction in DM (74% vs. 83% DM-free at 15 years), and a 42% relative risk reduction in BF (Phoenix definition, 39% v 55% BF-free at 15 years).
Figure 1.
Disease-free survival (A), distant metastasis (B), death from prostate cancer (C), and overall survival (death from any cause) (D) by treatment group. Plots in panels A and D are Kaplan-Meier curves; plots in panels B and C are (1 – cumulative incidence estimator). P-values are from unadjusted logrank tests. See Table 2 for adjusted hazard ratios and p-values.
Table 2.
Analysis of LTAD Effects on Trial Endpoints
| Total Events | Unadjusted 15-year Estimates (% Event-free) (95% confidence interval) |
Adjusted Hazard Ratio* | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Outcome | STAD | LTAD | STAD | LTAD | LTAD/STAD Hazard Ratio |
95% CI | p |
|
| |||||||
| Disease progression endpoints | |||||||
|
| |||||||
| Disease-free survival | 700 | 670 | 10.0 | 15.7 | 0.71 | 0.64 – 0.79 | < 0.0001 |
| 6.8 – 12.4 | 10.1 – 18.5 | ||||||
|
| |||||||
| Local progression | 176 | 100 | 76.8 | 87.1 | 0.54 | 0.42 – 0.69 | < 0.0001 |
| 73.8 – 79.8 | 84.5 – 89.4 | ||||||
|
| |||||||
| Distant metastasis | 198 | 134 | 74.0 | 82.6 | 0.64 | 0.51 – 0.80 | < 0.0001 |
| 70.8 – 77.1 | 79.8 – 85.3 | ||||||
|
| |||||||
| Biochemical failure | 461 | 341 | 38.8 | 54.6 | 0.58 | 0.50 – 0.66 | < 0.0001 |
| 35.4 – 42.4 | 51.0 – 58.2 | ||||||
|
| |||||||
| Mortality endpoints | |||||||
|
| |||||||
| Disease-specific deaths | 168 | 121 | 78.4 | 84.4 | 0.70 | 0.55 – 0.89 | 0.003 |
| 75.3 – 81.4 | 81.7 – 87.0 | ||||||
|
| |||||||
| Non-cancer deaths | 443 | 477 | 48.7 | 45.3 | 0.95 | 0.84 – 1.09 | 0.48 |
| 45.0 – 52.5 | 41.7 – 49.1 | ||||||
|
| |||||||
| Overall survival | 611 | 598 | 27.1 | 29.8 | 0.88 | 0.79 – 0.98 | 0.03 |
| 23.8 – 30.0 | 26.4 – 33.2 | ||||||
Adjusted HR from Cox proportional hazard models including the following covariates: PSA (≤30, >30 ng/mL), T stage (T2, T3, T4), N stage (N0/NX, N1/N2), Gleason score (8–10, < 8), age, and treatment (STAD+RT,LTAD+RT)
Prostate cancer-specific survival at 15 years was 78% for patients receiving STAD and 84% for those receiving LTAD (Figure 1). Overall, there was a 30% risk reduction in death due to prostate cancer with the use of LTAD (Table 2, p =0.003). Death due to other causes did not differ significantly by treatment arm; 49% for STAD versus 45% for LTAD at 15 years (Table 2). The relative risk of other-cause mortality was not significantly influenced by treatment arm (HR=0.95, Table 2). Overall survival at 15 years was 27% for STAD versus 30% for LTAD (Table 2, Figure 1). The risk of death from any cause was reduced approximately 12% by LTAD (p=0.03, Table 2).
The influence of patient and disease characteristics on outcomes were largely as expected, with characteristics related to more aggressive or advanced disease (higher Gleason score, greater baseline PSA, higher stage) associated with greater risk of failure for all disease outcomes. Increasing age was associated with greater failure for disease-free and overall survival (Supplemental Table 1). An additional analysis of endpoints with competing risks (local progression, distant metastasis, biochemical failure, disease-specific survival) with an alternative model to the cause-specific hazard did not produce materially different estimates or inference for treatment effects described in Table 2.
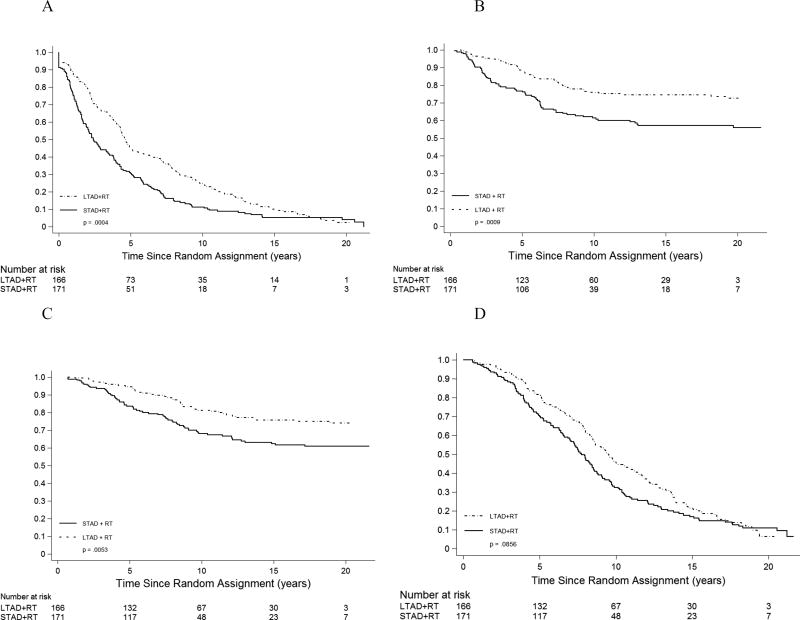
Specific patient subsets were identified based on expected prognosis, and the benefit of long term AD examined within these. As noted in earlier reports from this trial, for patients with high Gleason Scores (GS) the impact of LTAD was greater (Table 3). For the 337 patients with GS 8–10 and N0/NX node status there was a relative risk reduction of 33% in DFS, 48% in DM, and 50% in BF in favor of the LTAD arm. Disease-specific death risk was reduced by 45% while overall mortality was reduced by 25% (Table 3, Figure 2).
Table 3.
Analysis of LTAD Effects on Trial Endpoints among Patients with Gleason Score 8–10 and N0/NX
| Total Events | Unadjusted 15-year Estimate (% Event-free) (95% Confidence Interval) |
Adjusted Hazard Ratio* | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Outcome | STAD | LDAT | STAD | LDAT | LDAT/STA D HR |
95% CI | p |
|
| |||||||
| Disease progression endpoints | |||||||
|
| |||||||
| Disease-free Survival | 162 | 152 | 5.2 | 9.9 | 0.64 | 0.51 – 0.81 | < 0.0001 |
| 2.4 – 9.6 | 5.8 – 15.4 | ||||||
|
| |||||||
| Local Progression | 47 | 29 | 72.9 | 82.3 | 0.57 | 0.35 – 0.91 | 0.02 |
| 66.0 – 79.4 | 76.1 – 87.7 | ||||||
|
| |||||||
| Distant metastasis | 72 | 43 | 57.0 | 74.3 | 0.53 | 0.36 – 0.77 | <0.0001 |
| 49.5 – 64.7 | 67.3 – 80.8 | ||||||
|
| |||||||
| Biochemical failure | 117 | 84 | 30.9 | 48.3 | 0.52 | 0.39 – 0.69 | <0.0001 |
| 24.4 – 38.6 | 40.8 – 56.5 | ||||||
|
| |||||||
| Mortality endpoints | |||||||
|
| |||||||
| Disease-specific survival | 63 | 40 | 62.2 | 75.6 | 0.54 | 0.36 – 0.81 | 0.003 |
| 54.6 – 69.7 | 68.5 – 82.1 | ||||||
|
| |||||||
| Other cause of death | 80 | 99 | 54.8 | 45.8 | 0.87 | 0.64 – 1.19 | 0.39 |
| 47.1 – 62.7 | 38.2 -- 54.1 | ||||||
|
| |||||||
| Overall survival | 143 | 139 | 16.9 | 21.4 | 0.75 | 0.59 – 0.95 | 0.02 |
| 11.4 – 23.4 | 15.1 – 28.3 | ||||||
Adjusted HR from Cox proportional hazard models including the following covariates: PSA (≤30, >30 ng/mL), T stage (T2, T3, T4), N stage (N0/NX, N1/N2), Gleason score (8–10, < 8), age, and treatment (STAD+RT,LTAD+RT)
Figure 2.
Disease-free survival (A), distant metastasis (B), death from prostate cancer (C), and overall survival (death from any cause) (D) by treatment group for patients with Gleason score 8- 10 and N0/NX nodal status. Plots in panels A and D are Kaplan-Meier curves; plots in panels B and C are (1 – cumulative incidence estimator). P-values are from unadjusted logrank tests. See Table 3 for adjusted hazard ratios and p-values.
Further explorations into combinations of GS and age at diagnosis were undertaken to investigate how disease-specific risk and other-cause death risk influence the relative benefit of LTAD. These did not reveal clearly differential treatment benefits according to subset examined (data not shown).
Toxicities
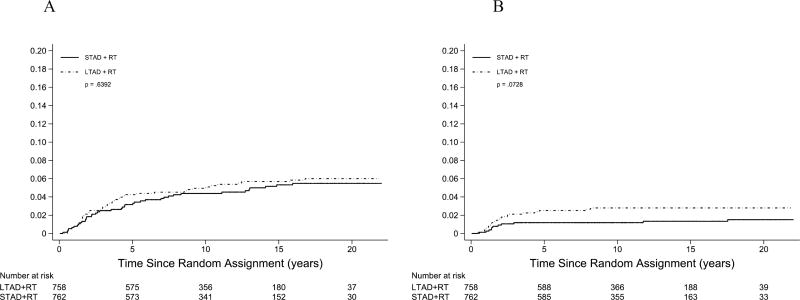
Toxicity from treatment was scored using the previously reported RTOG criteria. 17 Acute toxicity has been previously reported.6 There was no statistical difference in acute toxicity by treatment arm with a maximum acute toxicity of > grade 3 in 10% on the STAD arm and 8% on the LTAD arm. Late toxicity was defined as toxicity developing after 90 days from the start of radiation is shown in Supplemental Table 2. There was no statistical difference in grade > 3 late genitourinary (GU) toxicity between the two arms. However, there was a statistically significant difference in late grade > 3 gastrointestinal (GI) toxicity with a frequency of 1.5% (n=11) in the STAD arm and 3.0% (n=23) in the LTAD arm p=0.04. Frequency of other > grade 3 toxicity (not GI or GU) was not different between the two arms, 0.8% for STAD compared to 1.3% for LTAD. Analysis of the distribution of time to occurrence for late grade > 3 GU and GI toxicity (Figure 3) showed that the cumulative probability over time of late GI toxicity was somewhat greater for men in the LTAD arm.
Figure 3.
Cumulative incidence of grade 3 or greater genitourinary (GU) late toxicity (A) and gastrointestinal (GI) late toxicity (B). P-value is from Gray’s test.
With respect to long-term consequences of LTAD, of particular note is the fact that there was no significant difference in risk of all other-cause death combined between the two treatment arms (Table 2).
Discussion
The benefit of the addition of ADT to RT for locally advanced and/or high risk prostate cancer patients has been well studied.1–3 Each of these randomized trials has shown a clear benefit to the use of ADT in addition to RT for these patients. The challenge amongst these trials is that although a benefit was seen, the duration of ADT in each of the trials was different ranging from 4 months to indefinite. Thus the need for a trial looking at duration of ADT was obvious. Both the EORTC and the **** trials addressed this need.4, 5
This analysis reflects the long-term update of treatment benefits of LTAD in Trial****, with a median follow-up of 20 years. The addition of two years of ADT after neoadjuvant and concurrent ADT with RT resulted in significant improvement in DFS, LP, DM, BF, and DSS that have persisted with additional patient follow up. A modest overall mortality risk reduction of about 8–12% and absolute advantage of 2–3% for LTAD has been consistently observed since the first report5 and only nominally reaches conventional statistical significance in this update. Benefits of LTAD were greater for patients with higher Gleason Score (GS 8–10), including a statistically significant OS advantage in the first report and subsequent update.6 In this update, the observed 25% mortality risk reduction and absolute advantage of 4% (Table 3) is slightly smaller than in the earlier report, likely owing in part to most prostate cancer deaths having occurred earlier in follow-up (and more frequently with STAD), whereas at 15 years post- diagnosis and beyond, most deaths are due to other causes, resulting in no further separation of the disease-specific survival curves and convergence of overall survival curves (Figure 1). This phenomenon causes the hazard ratio to diminish and corresponding p-value to increase. However, no causal relationship between treatment group and non-cancer deaths is necessary or implied by such an observation.16 It is reasonable to conclude that LTAD may be associated with a small survival advantage that is difficult to reliably distinguish from chance variation, and for higher risk patients, OS continues to be reliably improved via reduction in prostate cancer deaths.
It is imperative to put this data into the pool of data regarding this question. Our results mirror similar findings in the EORTC trial of 6 months versus 36 months of ADT in addition to RT for locally advanced prostate cancer patients.4 This data also suggests that longer duration of ADT is clearly better for these patients. Yet the Canadian Prostate Cancer Study IV (NCT 0023145), which18 evaluated 18 months ADT versus 36 months in addition to RT showed in a preliminary report no difference in OS or DSS. This does point to a question we all should ask; just how long does the longer course ADT need to be?
Finally the question of dose of RT has to be addressed. The doses used in Trial**** of 65–70 Gy (isocenter doses) are clearly too low by today standards. One must ask if the benefits seen in the LTAD arm could be off set with more appropriate RT doses to the prostate such as 75–80 Gy? The DART 01–05 GICOR trial19 addressed this question with a phase III randomized trial four months versus 28 months of ADT combined with 76 Gy to the prostate. At a median follow up 63 months, results of this trial showed a benefit to the LTAD arm in terms of OS and biochemical control, especially for high risk patients. Thus the answer seems quite clear for locally advanced/high risk prostate cancer patients the addition of LTAD improves their chance of cancer control significantly and therefore, needs to be viewed as the standard of care for these patients relative to short duration AD.
Supplementary Material
Summary.
Several clinical trials have shown a benefit to adding androgen deprivation therapy (ADT) to definitive radiation therapy (RT) to treat locally advanced adenocarcinoma of the prostate. The length of time on ADT varied resulting in a question of the optimal timing. Trial**** addressed this question in a phase III randomized trial of RT and 4 months of ADT versus 28 months. Longer ADT was superior to shorter ADT.
Acknowledgments
This project was supported by grants U10CA180822 (NRG Oncology SDMC), U10CA180868 (NRG Oncology Operations) from the National Cancer Institute (NCI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: Dr. Sandler reports grants from ACR-RTOG, during the conduct of the study; consulting fees from Sanofi, Medivation, Clovis Oncology, Janssen, Ferring, and Blue Earth Diagnostics, speaking fees from Varian outside the submitted work.
References
- 1.****
- 2.Pilepich MV, Winter K, John MJ, et al. Phase III radiation therapy oncology group (RTOG) trial 8610 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Rad Onc Biol & Phys. 2001;50:1243–1252. doi: 10.1016/s0360-3016(01)01579-6. [DOI] [PubMed] [Google Scholar]
- 3.Bolla M, Collette L, Blank L, et al. Long term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): A phase III randomized trial. Lancet. 2002;360:103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 4.Bolla M, de Reijke T, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. New Eng Jour of Med. 2009;360(24):2516–2027. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 5.****
- 6.****
- 7.Beahrs OH, Hanson DE, Hunter RVP, et al., editors. Manual for the Staging of Cancer. 4. Philadelphia, PA: JB Lippincott; 1992. American Joint Commission on Cancer: Prostate; pp. 181–186. [Google Scholar]
- 8.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27:365–375. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 9.****
- 10.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 11.Korn EL, Dorey FJ. Applications of crude incidence curves. Stat Med. 1992;11:813–29. doi: 10.1002/sim.4780110611. [DOI] [PubMed] [Google Scholar]
- 12.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 13.Prentice RL, Kalbfleisch JD, Peterson AV, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–54. [PubMed] [Google Scholar]
- 14.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a comparing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 15.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 16.****
- 17.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiation Oncology Biology Physics. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 18.Nabid A, Carrier N, Martin A-G, Bahary J-P, Souhami L, Duclos M, Vincent F, Vass S, Bahoric B, Archambault R, Lemaire C. Duration of androgen deprivation therapy in highrisk prostate cancer: A randomized trial. J Clin Oncol (Meeting Abstracts) 2013 May;31 no. 15_suppl LBA4510. [Google Scholar]
- 19.Zapatero A, Gurerrero A, Maldonado X, et al. High-dose radiotherapy with short-term or long-term androgen deprivation in localized prostate cancer (DART 01/05 GICOR): a randomised, controlled phase 3 trial. The Lancet Oncology. 16(3):320–327. doi: 10.1016/S1470-2045(15)70045-8. 3/15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.