Abstract
The use of therapeutic antibodies, delivered by intravenous (IV) instillation, is a rapidly expanding area of biomedical treatment for a variety of conditions. However, little is known about how the antibodies are anatomically distributed following infusion and the underlying mechanism mediating therapeutic antibody distribution to specific anatomical sites remains to be elucidated. Current efforts utilize low resolution and sensitivity methods such as ELISA and indirect labeling imaging techniques, which often leads to high background and difficulty in assessing biodistribution. Here, using the in vivo non-human primate model, we demonstrate that it is possible to utilize the fluorophores Cy5 and Cy3 directly conjugated to antibodies for direct visualization and quantification of passively transferred antibodies in plasma, tissue, and in mucosal secretions. Antibodies were formulated with 1–2 fluorophores per antibody to minimally influence antibody function. Fluorophore conjugated Gamunex-C(pooled human IgG) were tested for binding to protein A, via surface plasmon resonance, and showed similar levels of binding when compared to unlabeled Gamunex-C. In order to assess the effect fluorophore labeling has on turnover and localization, rhesus macaques were IV infused with either labeled or unlabeled Gamunex-C. Plasma, vaginal Weck-Cel fluid, cervicovaginal mucus, and vaginal/rectal tissue biopsies were collected up to 8 weeks. Similar turnover and biodistribution was observed between labeled and unlabeled antibodies, showing that the labeling process did not have an obvious deleterious effect on localization or turnover. Cy5 and Cy3 labeled antibodies were readily detected in the same pattern regardless of fluorophore. Tissue distribution was measured in macaque vaginal and rectal biopsies. The labeled antibody in macaque biopsies was found to have similar biodistribution pattern to endogenous antibodies in macaque and human tissues. In the vaginal and rectal mucosa, endogenous and infused antibodies were found primarily within the lamina propria. In the mucosal squamous epithelium of the vaginal vault, significant antibody was also observed in a striated pattern in the superficial, nonviable, stratum corneum. Endogenous antibody distribution in both human and macaque squamous tissues exhibited a similar pattern as seen with the labeled and unlabeled antibodies. This proof-of-principle study reveals that the labeled antibody is stable and physiologically similar relative to endogenous antibody setting the stage for future work to better understand the mechanisms of how antibodies reach unique anatomical sites. Direct visualization of fluorophore-conjugated antibodies following passive infusion can be utilized to assess the kinetics of biodistribution of infused antibodies and may be a useful approach to monitor and predict efficacy of therapeutic antibodies.
1. Introduction
Monoclonal antibodies are the fastest growing sector of the therapeutic protein market (Ecker, Jones et al. 2015). Understanding how antibodies are metabolized and biodistributed following IV infusion is critical. Likewise, some therapeutic antibodies need to be delivered to specific sites, such as mucosal surfaces, or have unique characteristics such as glycoform, that facilitate optimal effector domain, and in turn therapeutic function(Johansson and Ekman 1999, Hristodorov, Fischer et al. 2013). Although ELISA and indirect immunofluorescence are used routinely to assess antibody localization and turnover, there are many drawbacks to using these methods. For example, false positive results from ELISA and high secondary background in tissue have made assessing IV infused IgG unreliable(Guven, Duus et al. 2014, Ivell, Teerds et al. 2014, Terato, Do et al. 2014). Thus, the development of alternative approaches to accurately measure antibody distribution and turnover is imperative.
Intravenous infusion of antibodies is actively administered in the clinic for a myriad of conditions ranging from states of immunodeficiency, cancer, infection, and autoimmunity. Multiple factors dictate how long antibodies persist in circulation and how effective they are following IV infusion. These include, but are not limited to subclass, Fc glycosylation, the neonatal Fc receptor (FcRn) and antigen binding affinities (Tabrizi, Bornstein et al. 2010, Zheng, Bantog et al. 2011). The rate at which antibodies turn over, as well as their biodistribution, are critical components for the efficacy of protection that these antibodies possess.
Fluorophore conjugation to antibodies is not a new method; in fact Coon et al. first pioneered it back in the 1940’s(Coons, Creech et al. 1941, Coons, Creech et al. 1942). The concept of using fluorophore-conjugated antibodies in vivo has been met with mixed reactions. Direct labeling of antibody is a highly sensitive method for imaging, as it does not rely on secondary techniques to visualize. However, other studies illustrate that conjugation of antibodies can alter the antibody-antigen interaction, leading to non-specific staining, with fluorophore to fluorophore variation depending on the degree of labeling (Pittman, Herbert et al. 1967, Gruber, Hahn et al. 2000, Vira, Mekhedov et al. 2010).
The method described here utilizes conjugation of fluorophores to antibodies for direct fluorescent intensity measurements within plasma, mucosal secretions and tissue following IV infusion in rhesus macaques. We show that the conjugation of the fluorophore to the antibody does not noticeably affect its turnover, distribution kinetics, or localization upon IV infusion. This method has the potential for a multitude of downstream applications. For example, beyond the direct detection of infused antibodies, this method could potentially identify optimal bioavailability of therapeutic proteins by monitoring their behavior in competitive or multiplexed infusion experiments.
2. Materials and Methods
2.1. Ethics statement
All macaques (Macaca mulatta) were housed at the Tulane National Primate Research Center (TNPRC) in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care International standards. All primate studies were reviewed and approved by the Tulane University Institutional Animal Care and Use Committee under protocol number P0240. Rhesus macaques were provided ad libitum with Monkey chow (Lab Fiber Plus Primate diet-DT, PMI Nutrition International, St. Louis, MO) and supplemented with fruits, vitamins and Noyes’ treats (Research Diets, New Brunswick, NJ). All clinical procedures were carried out under the direction of a laboratory animal veterinarian and were performed under anesthesia using ketamine, often in combination with telazol, with all efforts made to minimize stress, improve housing conditions, and to provide enrichment opportunities (e.g., objects to manipulate in cage, varied food supplements, foraging and task-oriented feeding methods, interaction with caregivers and research staff).
2.2. Macaques
Two sets of macaques were intravenously injected with labeled and unlabeled Gamunex-C at either 50mg/kg or 100mg/kg. In the first set of macaques, macaque IL24 received 50mg/kg of unlabeled Gamunex-C and macaque BV98 received 50mg/kg of Cy3 labeled Gamunex-C IgG. In the second set of macaques, macaque HL03 received 100mg/kg of unlabeled Gamunex-C and macaque JM01 received a mixture of 50mg/kg of Cy3 labeled Gamunex-C IgG and 50mg/kg of Cy5 labeled Gamunex-C IgG for a total of 100mg/kg. JM01 was 4.7kg, HL03 was 7.3 kg, BV98 was 10.6kg and IL24 was 7.6kg at time of injection. Vaginal and rectal biopsies were collected weekly for 8 weeks from each animal. Tissue samples were preserved in optimal cutting temperature (OCT) compound and stored at −80°C. Plasma, vaginal Weck-Cel® fluid, and cervicovaginal mucus were collected at 0 hours, 2 hours, 4 hours, 24 hours, 48 hours, 72 hours, 1 week and every week thereafter out to 8 weeks.
2.3. Tissue source and culture
Cervical tissue samples were obtained from consenting donors following surgery at Northwestern Memorial Hospital. All samples were collected in accordance with IRB guidelines and with ethics approval. Tissue was collected and processed within 2 hours of surgery, dissected into 1cm3 ectocervical explants. All donor tissue was then snap-frozen at −80°C, in OCT. Tissue was sectioned (12µm), immunofluorescently stained for endogenous antibodies and imaged by deconvolution microscopy (GE DeltaVision).
2.4. Immunofluorescence
Sectioned tissues were fixed in 3.7% formaldehyde in PIPES buffer and blocked with normal donkey serum prior to staining. For adherens junction identification in macaque tissues, anti-E-cadherin (BD Pharmingen) was used. To identify endogenous antibodies in human samples, tissues were stained with anti-IgG (BD Biosciences). Macaque endogenous antibodies were also detected with macaque specific anti-IgG (BD Biosciences). Likewise, 8E11 (KeraFAST) was used to identify passively transferred unlabeled Gamunex-C antibodies in rhesus macaque tissue. Secondary antibodies labeled with Rhodamine RedX (Jackson ImmunoResearch) or Cy5 (Jackson ImmunoResearch), were also utilized. Antibody specificity was confirmed by negative results with respective isotype and secondary control antibodies. Hoechst DAPI (Invitrogen) was used for staining of nuclear material. After staining, mounting medium (DakoCytomation) and coverslips were applied and sealed with clear nail polish.
2.5. Imaging and Image analysis
Images were obtained by deconvolution microscopy on a DeltaVision RT system collected on a digital camera (CoolSNAP HQ; Photometrics) using a 40× or 100× oil objective. In order to fully visualize antibody localization within tissues, 40× panel images were acquired to include the epithelium and lamina propria. To assess mean fluorescent intensities in tissue, we took ten 100× images of each tissue type per macaque. We measured the mean fluorescent intensity of each image using in-house algorithms written in IDL 7.1 (Harris Geospatial Solutions) and the Bio-Formats library(Linkert, Rueden et al. 2010).
2.6. Fluorophore labeling of antibodies
Gamunex-C (pooled human IgG) was purchased from the Northwestern pharmacy. Prior to labeling, Gamunex-C was diluted to 50mg/mL in 10mL PBS and buffer exchanged into PBS using two 10mL Zeba columns (Thermo Fisher), 5mL for each column. 500mg of Gamunex-C was labeled using 0.5mg of Sulfo-NHS-ester Cy5 or Cy3 (Lumiprobe) in 10mL PBS with 100mM Sodium Bicarbonate. The reaction was covered from light and allowed to rock gently for 1hr at room temperature. Following the labeling reaction, the 10mL reaction was passed through two 10mL Zeba columns (5mL per column) buffer exchanged with PBS to remove free dye. This step was repeated again to ensure free dye removal. Finally the labeled antibody was passed through a 0.45µm filter and stored at 4°C, protected from the light, until infusion.
2.7. Degree of labeling calculation
The degree of labeling (DOL) is the ratio of the number of fluorophores per protein, averaged over all proteins in a solution. We calculate this in the standard way; by applying the Beer-Lambert law to the absorbance spectrum of labeled protein.
Where Mfluorophore and Mprotein are the molarities of the label and IgG, and εprotein and ελmax are the coefficients of linear extinction for the protein at 280nm and for the label at its maximal absorbance wavelength. A correction factor (c280nm) is used to account for the absorbance at 280nm that is caused by the dye and not the protein.
Fluorophore-labeled antibodies were run on a Nanodrop (Thermo Scientific) and measurements for A280nm, A550nm and A650nm were recorded for IgG, Cy3, and Cy5 respectively. The extinction coefficient for IgG was set to Σprotein = 210,000 M−1cm−1. The coefficient of extinction and 280nm correction factor for Cy5 were set to Σmax=250,000 M−1cm−1 and c280nm =0.05. The coefficient of extinction and 280nm correction factor for Cy3 were set to Σmax=150,000 M−1cm−1; c280nm =0.08.
2.8. Fluorescent intensity measurement of antibodies in solution
The fluorescent intensity of labeled antibodies was measured on a FLUOstar OPTIMA (BMG Labtech). 100µL of plasma were placed into wells in triplicate. Mucus samples were prepared by mixing 10µL mucus with 40µL of 6M GuHCl and then neutralized with 5µL of 1M Tris-HCl. Finally, 60µL of PBS were added and sample was aliquoted at 50µL per well in duplicate. Weck-Cel fluid was added at 50µL per well in duplicate. FLUOstar OPTIMA gain was set at 1000 for plasma Cy3, 1500 for plasma Cy5, 2000 for mucus Cy3 and Weck-Cel Cy3, and 3000 for mucus Cy5 and Weck-Cel Cy5.
2.9. Human IgG ELISA
Human IgG ELISA (Immunology Consultants Laboratory, Inc.) was carried out according to manufacturer instructions. Plasma was diluted 1:1000 in assay dilution buffer, plated in duplicate, and incubated for 1hr. The plate was next washed and then incubated with enzyme-antibody conjugate for 20min. The plate was washed and developed with TMB substrate solution for 10min. The reaction was stopped and the plate was read on the FLUOstar OPTIMA (BMG Labtech) at 450nm. Antibody concentrations were determined based on an IgG standard curve using a 4-parameter fit.
2.10. Surface Plasmon Resonance
Protein A was captured onto individual channels of an SPR Biacore CM5 chip (GE). The CM5 chip was activated using EDC/NHS capture protocol (Biacore manual, GE). Briefly, carboxydextran surfaces were activated by injection of a 0.2M solution of 1-Ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC) and 0.05M N-hydroxysuccinimide (NHS-Sigma). The capture protein, protein A, was diluted at to required concentration in acetate buffer pH 6.5 and was injected to the chip to the desired channel at 10 µl/min. Unreacted sites were blocked using 0.1 M ethanolamine (Sigma). Antibodies; Gamunex Unlabeled, Gamunex-Cy5 tagged and Gamunex-Cy3 tagged were serially diluted at 1:4 dilution factor and flown over the CM5 protein A chip at 10 µl/min for 300 seconds. All experiments were conducted in triplicate and using running buffer (filtered and degassed PBS with 0.005% Tween 20 (pH 7.2)) at 25°C. The bound antibody was allowed to dissociated by flowing running buffer at 10 µL/min for 300 seconds. Ten µl of 10 mM HCl was injected for 15 seconds to regenerate the surfaces between each sample injection.
Following double reference subtraction with buffer and control surface, (Req) values for each concentration of the antibody were calculated using separate fit analysis for association and dissociation phase for each concentration using BiaEvaluation 4.0 software (GE) and averaged for the affinity model analysis. Steady state binding level is related to concentration (C) in M and the number of binding sites on the ligand (n, which was 1 in all cases) according to the applied Langmuir equation Req = KA CRmax/(1+ KACn) from which the kon, koff and KD values were calculated.
2.11. Statistics
Statistics were analyzed used GraphPad Prism. A Pearson correlation coefficient was used to compare the concentration of unlabeled Gamunex-C versus fluorophore-labeled Gamunex-C in the plasma of rhesus macaques. This test was also used for comparing between Cy5 and Cy3 fluorescent intensity in plasma, Weck-Cel fluid, cervicovaginal mucus and tissue biopsies. Two-tailed p-values for the Pearson correlation are indicated where statistical significance was achieved.
3. Results
3.1. Labeling of antibodies with 1–2 fluorophores per IgG
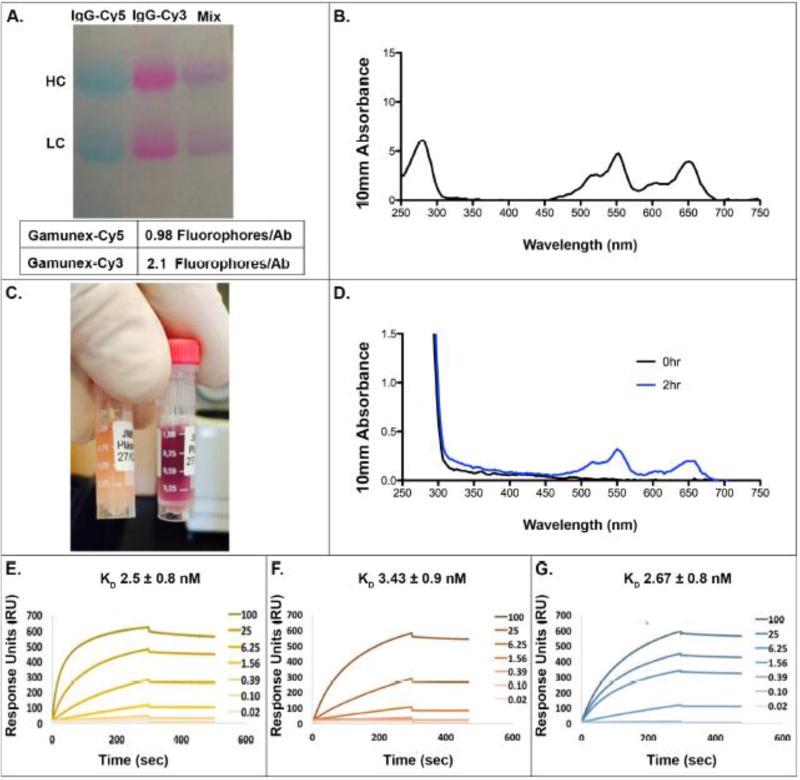
A major concern of using fluorophore-labeled antibodies is that the labeling process may decrease the stability and function of the antibody following IV infusion. To validate that fluorophore-labeled and unlabeled antibodies have similar biodistribution and turnover, we conjugated 1–2 fluorophores per IgG molecule. This low degree of labeling (DOL) was chosen to minimize changes to the natural state of the antibody. We measured the DOL of the antibodies as described above. Utilizing 100µL pilot runs, we optimized the reaction conditions to achieve 1–2 fluorophores per antibody, and then scaled up to 10mL volumes. Following purification, the Cy5- and Cy3- labeled Gamunex-C had 1 and 2 fluorophores per antibody, respectively (Fig 1A–B). The final antibody solutions were run on SDS PAGE to ensure efficient antibody labeling and that there was no free dye remaining (Fig 1A).
Figure 1. Generation of Cy5- and Cy3-labeled Gamunex-C.
Gamunex-C was labeled with Cy5 and Cy3 and run on SDS PAGE (A). The absorption spectrum for the pre-infusion inoculate containing both Cy5 and Cy3 labeled Gamunex-C was measured with a Nanodrop (B). JM01 Plasma samples at 0hrs and 2hrs post-infusion was assessed both by eye (left, 0hr; right, 2hr) (C) and their spectrum by nanodrop (D). Surface plasmon resonance binding to a CM5 protein A chip for unlabeled Gamunex-C (E), Cy5 labeled Gamunex-C (F), and Cy3 labeled Gamunex-C (G). Antibodies were added in a titration series starting at 100ug/ml and going down to 20ng/ml.
3.2. Fluorophore conjugation does not affect plasma antibody turnover
To confirm that fluorescent labeling did not obviously alter stability of the antibody, one rhesus macaque (JM01) was infused with 50mg/kg each of Cy5- and Cy3-labeled human IgG, and a second animal (HL03) with 100mg/kg of the same antibody, but unlabeled. Plasma samples were collected out to 8 weeks. At 2 hours, it was evident that the plasma samples contained labeled antibodies as the plasma had changed from a yellow plasma color prior to injection (Fig 1C left) to a purple color due to the mixing of cy5 and cy3 fluorophores (Fig 1C right) after injection. The absorption spectra of plasma pre- and 2 hours post-infusion are plotted in figure 1D. The appearance of an absorption profile similar to that of the injected antibody solution (Fig 1B) confirms the presence of the two fluorophores at this early time-point. Importantly, the relative abundance of one fluorophore compared to the other is also conserved and we detect no preferential loss of a particular fluorophore labeled antibody over time (Fig1D).
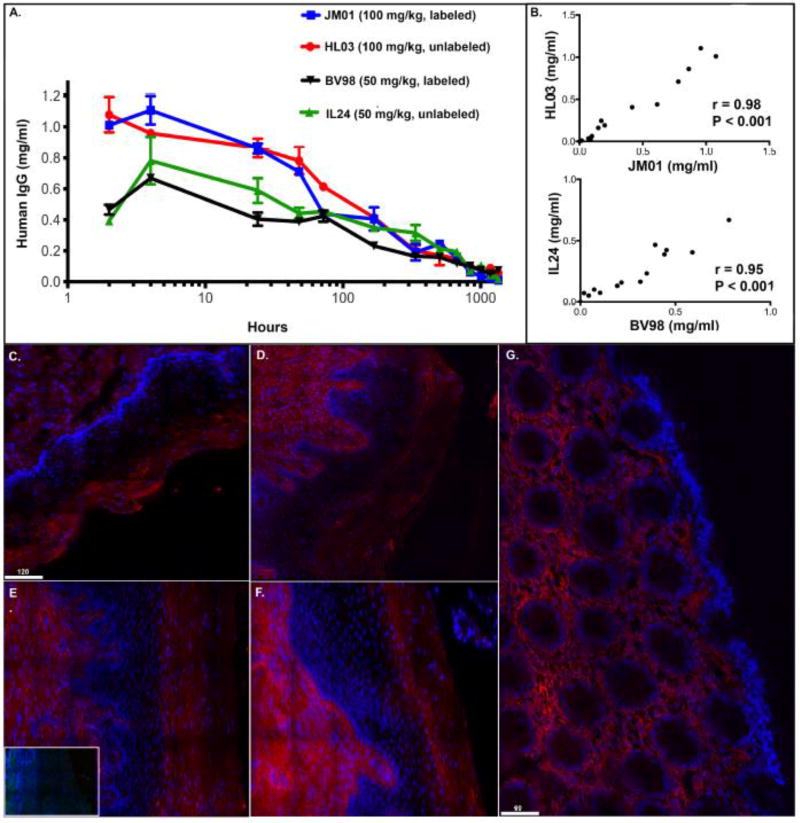
To explore the effect the labeling process had on antibody function, we also measured binding of the labeled and unlabeled Gamunex-C to protein A. Through surface plasmon resonance(SPR) we found no change in binding when comparing unlabeled Gamunex-C(1E), Cy5 labeled Gamunex-C(1.2 fluorophore/ab) (1F) and Cy3 labeled Gamunex-C(0.97 Fluorophore/ab) (1G). The turnover of labeled(JM01) and unlabeled(HL03) human IgG in macaque plasma was measured using human IgG ELISA. In the plasma of macaques, we found that injected human IgG peaked around 2–4 hours and over time, turned over at a similar rate in the presence or absence of label (r = 0.98)(p<.001) (Fig 2A–B). Although the antibody levels varied between macaques the overall trend of antibody turnover was similar and there was not an overall deleterious effect of the label. Similar studies using a single-fluorophore labeled antibody were done to further explore any clear effects that the label had on turnover. One rhesus macaque (BV98) was infused with 50mg/kg of Cy3-labeled human IgG, and a second macaque (IL24) was infused with 50mg/kg of the same unlabeled antibody. Similar to JM01 and HL03, human IgG levels in the plasma peaked around 2 hours and decreased steadily out to 8 weeks(Fig 2A). Human IgG turned over at a similar rate between labeled and unlabeled IgG and the Pearson correlation coefficient between these curves was 0.95 and statistically significant (p<.001) (Fig 2B). Taken together this data shows that the label does not alter antibody binding to protein A and does not have an obvious impact on antibody turnover following IV injection.
Figure 2. Fluorophore conjugation does not have a deleterious effect on plasma antibody turnover or antibody localization.
Human IgG ELISA measuring Cy5 and Cy3 labeled(JM01) and unlabeled(HL03) Gamunex-C from macaque plasma in the 100mg/kg animals and Cy3 labeled(BV98) and unlabeled(IL24) Gamunex-C from macaque plasma in the 50mg/kg animals (A). Spearman correlation correlations for JM01 and HL03(B) and BV98 and IL 24(B). Fluorescent deconvolution images (40×) of IgG in mucosal tissues. IgG (red) and DAPI (blue). Endogenous IgG in human cervical tissue (ectocervix) (C). Endogenous IgG in macaque vaginal tissue (D). IL24 vaginal tissue stained for anti-human IgG. Inset image: Secondary control (E). Gamunex-C Cy5 in JM01 vaginal biopsy tissue 1-week post-infusion (F). Gamunex-C Cy5 in JM01 rectal biopsy tissue 1-week post-infusion(G) (Size bars are 60 and 120 µm.)
3.3 Fluorophore conjugation does not affect tissue distribution of infused antibodies
Prior to evaluating the biodistribution of infused fluorophore-labeled antibodies, we examined the epithelial distribution of endogenous IgG in humans and macaques. Human ectocervical tissues were stained for endogenous human IgG and macaque vaginal tissue was stained for primate IgG (Fig 2C–D). In both human and macaque tissues, endogenous IgG antibodies were found primarily within the lamina propria and the superficial, non-viable stratum corneum of the squamous epithelium. In contrast, the viable stratum malpighii did not exhibit strong IgG fluorescent signals. Next, rhesus macaque vaginal and rectal biopsies were analyzed for labeled and unlabeled antibodies post infusion for up to 8 weeks post injection. In macaque JM01, the animal that received both Cy3 and Cy5 tagged, IgG was, once again, located in the lamina propria and the non-viable stratum corneum in vaginal biopsies (Fig 2E), phenotypically mirroring those obtained for endogenously detected antibodies. Additionally, a macaque (HL03) was infused with unlabeled Gamunex-C IgG with biopsies collected 1-week post-antibody administration. Staining for human IgG in vaginal samples containing these unlabeled antibodies revealed that the fluorescent tags did not alter antibody distribution when compared to both endogenous and labeled-Ab distribution (Fig 2F). Likewise, rectal biopsies taken from infused macaques showed a similar profile to the vaginal biopsies with antibody located within the lamina propria (Fig 2G). Unfortunately, rhesus macaque rectal biopsies that underwent Immunofluorescent staining for unlabeled Gamunex-C, did not provide convincing results. This was due to the inherent muco-adhesive properties of these tissues, which results in secondary antibodies non-specifically binding to rectal mucus (data not shown). Taken together these experiments validate the use of fluorophore-labeled IgG for IV infusion to study antibodies in vivo, by illustrating similar turnover and biodistribution between endogenous, and infused labeled and unlabeled IgG.
3.4. Fluorophore-conjugated antibodies in plasma, vaginal Weck-Cel fluid, cervicovaginal mucus and vaginal/rectal tissue biopsies have consistent distribution between Cy5 and Cy3 labeled antibodies
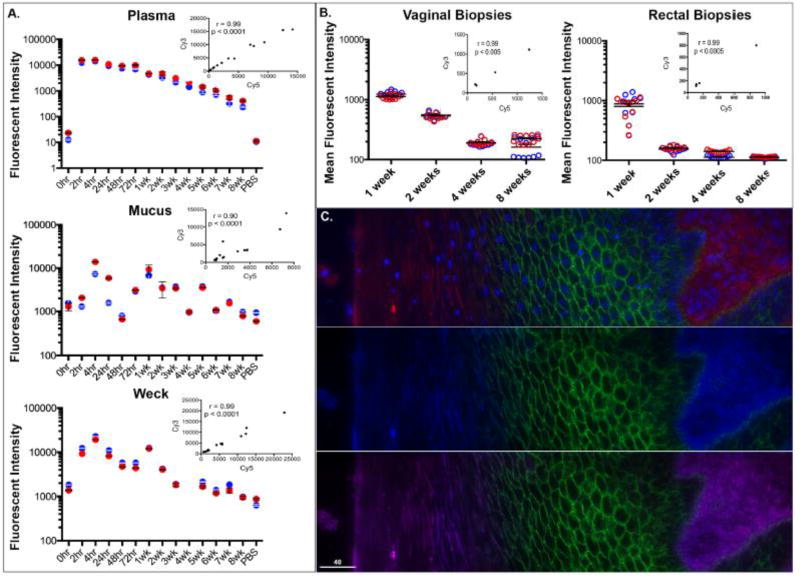
Due to the sensitive and specific nature of this direct labeling technique we were able to assess IgG levels in several tissues and fluids. We examined plasma, vaginal Weck-Cel fluid, cervicovaginal mucus, and vaginal/rectal tissue biopsies out to 8 weeks. However, since the levels of antibodies differ by compartment, the FLUOstar gain setting for plasma, Weck-Cel and mucus were adjusted to show the antibody decay over the full 8 weeks. Plasma antibodies peaked around 4 hours and declined steadily whereas mucosal antibodies peaked at 4 hours though the decay trend out to 8 weeks was noisier (Fig 3A). Similarly, fluorophore-labeled antibodies reached a steady state at 1 week and decreased after 2 weeks in vaginal and rectal biopsies (Fig 3B). With deconvolution microscopy, it was also noted that there were no differences between Cy5 and Cy3 fluorophore-labeled Gamunex-C IgG localization in vaginal (Fig 3C) and rectal (data not shown) biopsy tissues. These combined data illustrate that these labels do not affect biodistribution and stability of the antibody.
Figure 3. Fluorophore-conjugated antibodies have consistent distribution between Cy5 and Cy3 fluorophores.
Fluostar fluorescent intensity measurements for Gamunex-C Cy5(red) and Gamunex-C Cy3(blue) over time for plasma, cervico-vaginal mucus, and vaginal-weck cel fluid with spearman correlation inset (A). Temporal analyses of mean fluorescent intensities of Gamunex-C Cy5 (red) and Gamunex-C Cy3 (blue) in macaque vaginal biopsies (left) and rectal biopsies (right) (B) each displayed dot represents individual images analyzed. Fluorescent deconvolution images (40×) of infused Cy5 and Cy3 labeled Gamunex-C in macaque vaginal tissue(C). Top: Gamunex-C Cy5 (red), E-cadherin (green), and DAPI (blue) Middle: Gamunex-C Cy3 (blue), E-cadherin (green) Bottom: Gamunex-C Cy5 (red), Gamunex-C Cy3 (blue), E-cadherin (green). Size bars are 40 µm.
Discussion
Here, we present and validate the use of fluorophore labeled IgGs as a reliable method of direct visualization of IV-infused antibodies in rhesus macaques. Importantly, we illustrate that antibody turnover and biodistribution are similar between labeled and unlabeled Gamunex-C. Antibody binding to protein A was not altered with the addition of fluorophores as shown through SPR experiments, thus verifying that these antibodies can retain at least some functions. These antibodies contain 1–2 labeled primary amines that are likely randomly distributed between the 25–35 lysines that will be present in these polyclonal antibody populations in Gamunex-C. Even if some of these lysines are functionally important, they will only be labeled in a small percentage of the populations and be difficult to see such a loss in the resolution of the presented experiments. In addition, we were able to detect labeled IgG in very small samples of plasma, vaginal Weck-Cel fluid, cervicovaginal mucus and in vaginal/rectal biopsies. In these samples there was similar localization and turnover when comparing the antibodies with different fluorophores used, Cy5 or Cy3. Our results show that labeling with different fluorophores can be used to compare turnover and localization of different antibodies independently of the fluorophore used.
Any concerns that fluorophore labeling may prove immunoreactive upon IV infusion are resolved by these experiments, as the turnover rates of the antibodies with and without the fluorophore (Fig. 2A–B) were similar. Additionally, neither of the specific fluorophores, Cy5 or Cy3, altered the turnover or localization as is shown by fluorescent intensities in several compartments (Fig 3A–B). This was to be expected since Cy5 and Cy3 are chemically related and it would also be expected that other cyanine dyes would yield similar results, allowing for multiplexing beyond just two fluorophore-conjugated antibodies infused. There was more variability in the plasma human IgG levels in the IgG ELISA(Fig. 2A) compared with the fluorescent intensity measurements(Fig. 3A), highlighting the importance of this new method. It should be noted that due to the low number of animals in this study, conclusive two-compartment pharmacokinetics could not be determined. Therefore, we can only say that the label did not have an overall deleterious effect on antibody levels following IV infusion and the overall kinetic profile was similar. Finally, the health of the rhesus macaques receiving the IV infusion of antibodies was not affected by fluorophores attached to the antibodies.
Importantly, this method could allow for the assessment of a panel of antibodies with subtle amino acid changes within the same animal. Whereas in previous methods secondary antibodies rely on the conserved Fc region, which would not change between antibody mutants, this novel method only relies on the conjugated fluorophore for detection. Here we present results with two fluorophores, but a larger panel of antibody mutants would also be feasible. The number of antibody mutants that could be evaluated in a single animal would only limited by the ability to spectrally distinguish the fluorophores; therefore, advanced microscopy and computational techniques such as spectral imaging with linear unmixing are very promising in this regard.
In conclusion, the method presented here provides robust direct visualization of IV-infused fluorophore conjugated IgG in plasma, tissue and at mucosal surfaces. This will aid in assessing the kinetics of antibody movement following infusion. This method has the potential to monitor stability and biodistribution, and helps to predict efficacy of therapeutic antibodies within the same animal. Through a better assessment of therapeutic antibody circulation following IV infusion, antibodies can be better formulated for optimal bioavailability and efficacy in order to combat a variety of human diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Coons AH, Creech HJ, Jones RN. Immunological properties of an antibody containing a fluorescent group. Proceedings of the Society for Experimental Biology and Medicine. 1941;47(2):200–202. [Google Scholar]
- Coons AH, Creech HJ, Jones RN, Berliner E. The demonstration of pneumococcal antigen in tissues by the use of fluorescent antibody. Journal of Immunology. 1942;45(3):159–170. [Google Scholar]
- Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. MAbs. 2015;7(1):9–14. doi: 10.4161/19420862.2015.989042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber HJ, Hahn CD, Kada G, Riener CK, Harms GS, Ahrer W, Dax TG, Knaus HG. Anomalous fluorescence enhancement of Cy3 and cy3.5 versus anomalous fluorescence loss of Cy5 and Cy7 upon covalent linking to IgG and noncovalent binding to avidin. Bioconjug Chem. 2000;11(5):696–704. doi: 10.1021/bc000015m. [DOI] [PubMed] [Google Scholar]
- Guven E, Duus K, Lydolph MC, Jorgensen CS, Laursen I, Houen G. Non-specific binding in solid phase immunoassays for autoantibodies correlates with inflammation markers. J Immunol Methods. 2014;403(1–2):26–36. doi: 10.1016/j.jim.2013.11.014. [DOI] [PubMed] [Google Scholar]
- Hristodorov D, Fischer R, Linden L. With or without sugar? (A)glycosylation of therapeutic antibodies. Mol Biotechnol. 2013;54(3):1056–1068. doi: 10.1007/s12033-012-9612-x. [DOI] [PubMed] [Google Scholar]
- Ivell R, Teerds K, Hoffman GE. Proper application of antibodies for immunohistochemical detection: antibody crimes and how to prevent them. Endocrinology. 2014;155(3):676–687. doi: 10.1210/en.2013-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson JE, Ekman T. Gut mucosa barrier preservation by orally administered IgA-IgG to patients undergoing bone marrow transplantation: a randomised pilot study. Bone Marrow Transplant. 1999;24(1):35–39. doi: 10.1038/sj.bmt.1701821. [DOI] [PubMed] [Google Scholar]
- Linkert M, Rueden CT, Allan C, Burel JM, Moore W, Patterson A, Loranger B, Moore J, Neves C, Macdonald D, Tarkowska A, Sticco C, Hill E, Rossner M, Eliceiri KW, Swedlow JR. Metadata matters: access to image data in the real world. J Cell Biol. 2010;189(5):777–782. doi: 10.1083/jcb.201004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman B, Herbert GA, Cherry WB, Taylor GC. The quantitation of nonspecific staining as a guide for improvement of fluorescent antibody conjugates. J Immunol. 1967;98(6):1196–1203. [PubMed] [Google Scholar]
- Tabrizi M, Bornstein GG, Suria H. Biodistribution mechanisms of therapeutic monoclonal antibodies in health and disease. AAPS J. 2010;12(1):33–43. doi: 10.1208/s12248-009-9157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terato K, Do CT, Cutler D, Waritani T, Shionoya H. Preventing intense false positive and negative reactions attributed to the principle of ELISA to re-investigate antibody studies in autoimmune diseases. J Immunol Methods. 2014;407:15–25. doi: 10.1016/j.jim.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Vira S, Mekhedov E, Humphrey G, Blank PS. Fluorescent-labeled antibodies: Balancing functionality and degree of labeling. Anal Biochem. 2010;402(2):146–150. doi: 10.1016/j.ab.2010.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng K, Bantog C, Bayer R. The impact of glycosylation on monoclonal antibody conformation and stability. MAbs. 2011;3(6):568–576. doi: 10.4161/mabs.3.6.17922. [DOI] [PMC free article] [PubMed] [Google Scholar]