Abstract
Development of osteoarthritis commonly involves degeneration of epiphyseal trabecular bone. In previous studies, we observed 30–44% loss of epiphyseal trabecular bone (BV/TV) from the distal femur within one week following non-invasive knee injury in mice. Mechanical unloading (disuse) may contribute to this bone loss, however it is unclear to what extent the injured limb is unloaded following injury, and whether disuse can fully account for the observed magnitude of bone loss. In this study, we investigated the contribution of mechanical unloading to trabecular bone changes observed following non-invasive knee injury in mice (female C57BL/6N). We investigated changes in gait during treadmill walking, and changes in voluntary activity level using Open Field analysis at 4, 14, 28, and 42 days post-injury. We also quantified epiphyseal trabecular bone using μCT and weighed lower-limb muscles to quantify atrophy following knee injury in both ground control and hindlimb unloaded (HLU) mice. Gait analysis revealed a slightly altered stride pattern in the injured limb, with a decreased stance phase and increased swing phase. However, Open Field analysis revealed no differences in voluntary movement between injured and sham mice at any time point. Both knee injury and HLU resulted in comparable magnitudes of trabecular bone loss, however HLU resulted in considerably more muscle loss than knee injury, suggesting another mechanism contributing to bone loss following injury. Altogether, these data suggest that mechanical unloading likely contributes to trabecular bone loss following non-invasive knee injury, but the magnitude of this bone loss cannot be fully explained by disuse.
Keywords: Post-traumatic osteoarthritis, hindlimb unloading, trabecular bone, knee injury, mechanical loading
Introduction
Post-traumatic osteoarthritis (PTOA) is a common consequence of traumatic joint injury, with 20–50% of individuals suffering anterior cruciate ligament (ACL) rupture or meniscectomy developing PTOA within 10–20 yrs [1]. Decreased epiphyseal trabecular bone volume during OA development is commonly observed clinically [2–4] and in animal models [5, 6]. In previous studies, we observed a 30–44% loss of epiphyseal trabecular bone volume fraction (BV/TV) in both the proximal tibia and distal femur compared to uninjured controls following non-invasive knee injury in mice [7–9]. This loss of trabecular bone volume occurs rapidly (within one week following injury), and is maintained for at least 16 weeks post-injury. However, the specific mechanisms driving this rapid bone loss have not been identified. Compensatory unloading or decreased activity level may be expected following this type of injury, and this decreased mechanical loading of the injured limb is a likely contributor to this acute bone loss. It is unclear to what extent the injured limb is unloaded following non-invasive knee injury in mice, and whether disuse accounts for all of the observed bone loss.
Studies of mechanical unloading in humans, such as during prolonged bed rest or long-term space flight, have shown decreases in urinary biomarkers for bone formation and increases in biomarkers for bone resorption, corresponding to loss of cancellous and cortical bone [10–13]. Astronauts have been shown to lose ~2% of their bone mass per month at the proximal femur during space flight [14]. Rodent tail suspension models were developed to model the effects of microgravity on humans, and these models also demonstrate increased bone resorption and decreased bone formation [15–17], as well as decreases in muscle mass [16, 18–20], and decreased interstitial fluid flow [21]. Mice and rats typically lose 23–50% trabecular bone volume in the distal femur and proximal tibia and 10–55% muscle mass (depending on the muscle) during 2 weeks of tail suspension [18–20, 22–26]. These data suggest that tail suspension may provide an important model for understanding the contribution of mechanical loading to bone loss observed following traumatic joint injury.
In this study we investigated changes in gait and voluntary movement in mice following non-invasive knee injury. Further, to determine the role of mechanical loading and altered knee biomechanics on PTOA, we quantified trabecular bone and muscle atrophy in mice following non-invasive knee injury in both ground control and hindlimb unloaded animals. Our goal was to quantify changes in voluntary movement in injured mice following knee injury, and to determine the contribution of mechanical unloading to bone changes observed in the injured joint. We hypothesized that knee injury would result in decreased voluntary movement and gait asymmetry in injured mice. We further hypothesized that hindlimb unloading would cause a loss of trabecular bone and muscle in both injured and sham animals, but that this atrophy would be greater in injured animals.
Methods
Animals
A total of 73 female C57BL/6N mice (10 weeks old at time of injury) were obtained from Harlan Sprague Dawley, Inc. (Indianapolis, IN). Mice underwent a two-week acclimation period before injury. Mice were randomly assigned to experimental groups. Mice were cared for in accordance with the guidelines set by the National Institutes of Health (NIH) on the care and use of laboratory animals. Mice were housed in Tecniplast conventional cages (Tecniplast SPA, Buguggiate, Italy), with Bed-o’ Cob bedding (The Andersons Inc., Maumee, OH), with 3–4 mice per cage prior to injury or sham injury, 12 hour light/dark cycle, 20–26° C ambient temperature. Mice had ad libitum access to food (Harlan irradiated 2918 chow) and autoclaved water, and were provided with Enviro-dri and/or Nestlets for environmental enrichment. Mice were monitored by husbandry staff at least once a day, 7 days a week, with monthly health care checks by a veterinarian. All procedures were approved by our Institutional Animal Care and Use Committee.
Non-invasive knee injury
Knee joint injury was induced as previously described [7]. Briefly, 38 mice were subjected to a single dynamic overload cycle of tibial compression. With the mouse anesthetized under isoflurane in a prone position with right lower leg vertically aligned between two platens, tibia compression was performed at a rate of 1 mm/s using an electromagnetic materials testing machine (Bose ElectroForce 3200, Eden Prairie, MN) until injury. Injury was noted by a release of compressive force, at which point the compressive load was stopped. Compressive force at injury was typically 8–10 N. Our previous studies showed that this loading protocol results in rupture of the anterior cruciate ligament (ACL) without notable damage to other tissues of the joint. An additional 35 mice were subjected to sham injury. Mice in the sham group were anesthetized with isoflurane and placed in the tibial compression system, then a 1–2 N load was applied to the right lower leg. After injury (or sham injury), mice were removed from the testing apparatus, given a subcutaneous injection of buprenorphine (0.01 mg/kg), and returned to individual housing.
Open field analysis of voluntary movement
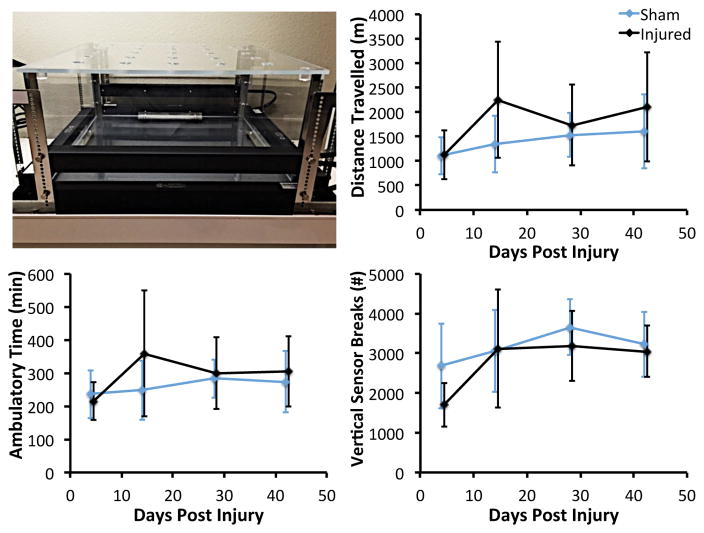
Following non-invasive knee injury or sham injury, 12 mice (6 injured, 6 sham) were transported to the UC Davis Mouse Biology Program (https://www.mousebiology.org/) for Open Field analysis of voluntary movement. Each mouse was tested for a 24-hour period at 4, 14, 28, and 42 days post-injury. During open field testing, each mouse was individually housed in an 18″×18″ enclosure (Opto-Varimex 4, Columbus Instruments, Columbus, OH). Mouse activity was tracked using a grid of infrared photocells placed around the arena; vertical motion was detected by a second array of photocells placed above the animal. Total distance travelled, ambulatory time, vertical sensor breaks (rearing), and other parameters were tracked during each 24-hour testing period using the manufacturer’s analysis software.
Gait analysis
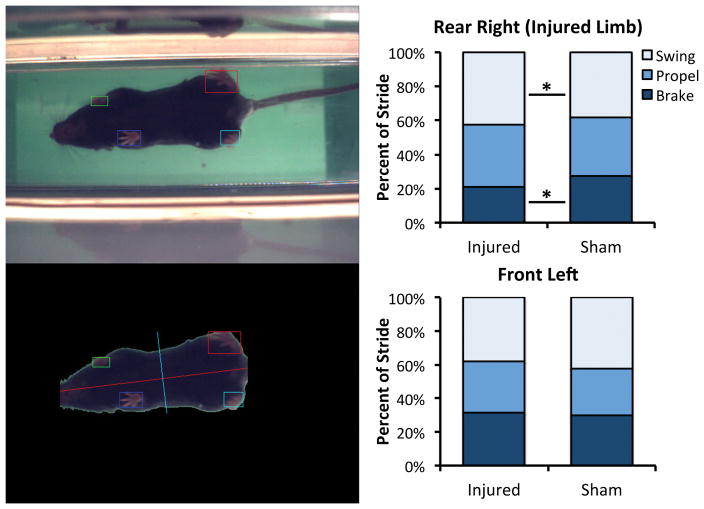
Following non-invasive knee injury or sham injury, 16 mice (8 injured, 8 sham) were transported to the UC Davis Mouse Biology Program for gait analysis. Mice were tested 1 day post-injury using the Treadscan system (CleverSys Inc., Reston, VA) to assess gait during forced locomotion. Running speed for all mice was 14.6 cm/s. Percent stance phase, percent swing phase, stance time, brake time, propel time, swing time, and other gait parameters of each limb were recorded for analysis.
Hindlimb unloading via tail suspension
After injury or sham injury, mice were individually housed in a standard cage (ground control (GC) mice, n = 21), or were individually housed in a custom tail suspension cage (hindlimb unloaded (HLU) mice, n = 24; Techshot Inc., Greenville, IN). HLU mice were tail suspended according to previously described methods [27]. While still under anesthesia following knee injury or sham injury, HLU mice had a metal loop attached near the base of their tail using cyanoacrylate and athletic tape. This loop was then attached to a swivel that allowed for 360 degrees of rotation and was mounted on a bar spanning the top length of the cage. The height of the swivel was adjusted to maintain mice in approximately 30° head down angle, so that the hindlimbs were not able to contact the floor. Absorbent material was placed under a wire mesh flooring to allow the mice to pull themselves about their cage. Hindlimb unloaded mice were given free access to food, water, and nesting materials. Mice were sacrificed 7 days or 14 days after knee injury or sham injury (n = 5–6 mice/group/time point).
Results from one mouse (day 7, injured, HLU) were not included because the mouse escaped the tail suspension setup near the end of the experimental period, and thus was subjected to normal mechanical loading during that time. Another mouse (day 14, uninjured, HLU) was sacrificed during the experimental period due to health concerns.
Trabecular bone analysis by micro-computed tomography imaging
Injured and uninjured knees were imaged with micro-computed tomography (SCANCO μCT 35, Bassersdorf, Switzerland) to quantify trabecular bone structure in the distal femoral epiphysis. Dissected limbs were fixed in 4% paraformaldehyde for 24–48 hours, then transferred to 70% ethanol. Knees were scanned according to the guidelines for micro-computed tomography (μCT) analysis of rodent bone structure [28] (x-ray tube potential = 55 kVp, current = 114 μA, 10 μm isotropic nominal voxel size, integration time = 900 ms, number of projections = 1000/180°). Knees were scanned from above the patella to below the proximal tibial growth plate. Trabecular bone in the distal femoral epiphysis was analyzed by manually drawing contours on 2D transverse slices. The distal femoral epiphysis was designated as the region of trabecular bone enclosed by the growth plate and subchondral cortical bone plate. Bone was segmented from non-bone using a global threshold of 564.6 mg HA/cm3; this threshold was determined based on histograms of pixel brightness for the volumes of interest and visual comparison with unsegmented images. We quantified trabecular bone volume per total volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and apparent bone mineral density (Apparent vBMD; mg HA/cm3 TV) using the manufacturer’s analysis tools. The direct measurement method (not dependent on model assumptions) was used to determine Tb.Th, Tb.N, and Tb.Sp.
Quantification of muscle mass and biomarkers of muscle atrophy
At the time of dissection, muscle mass of the tibialis anterior (TA) and gastrocnemius + soleus (GS) was quantified for each leg of each mouse. After weighing, muscles were flash frozen in liquid nitrogen and retained for analysis of biomarkers of muscle atrophy. Total RNA was extracted from powdered muscles using TRIzol reagent according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). cDNA was then synthesized using a QuantiTech Reverse Transcription Kit (Qiagen, Hilden, Germany) from one μg of total RNA. MuRF1 and MAFbx gene expression was measured by quantitative PCR (qPCR) using Power SYBR Green PCR Master Mix (Life Technologies, Benicia, CA) on an ABI 7900HT thermocycler. Cycling conditions were one cycle at 94°C for 10 min followed by forty cycles at 94°C for 30 s, 59°C for 30 s, and 72°C for 30 s. Each sample was run in triplicate. All data was normalized to GAPDH expression.
Statistical analysis
All data are presented as mean ± standard deviation. Body mass data were compared using repeated measures ANOVA. Open field and gait data were compared using Student’s t-test at each time point. For μCT and muscle data, differences between injured and uninjured (contralateral) limbs were determined using paired t-test. Remaining groups were compared using analysis of variance (ANOVA) with post-hoc analysis by Fisher’s protected least significant difference (PLSD). Significant differences were defined as p < 0.05.
RESULTS
Animal body mass
Knee injury and HLU were generally well tolerated by the animals in this experiment. There were no significant differences in animal body mass between experimental groups at any time point (Fig. 1). In general, animals in all experimental groups gained weight during the experiment. Approximately half of the animals subjected to HLU lost weight from day 0 to day 7, but by day 14 their body mass was increased relative to day 0.
Figure 1.
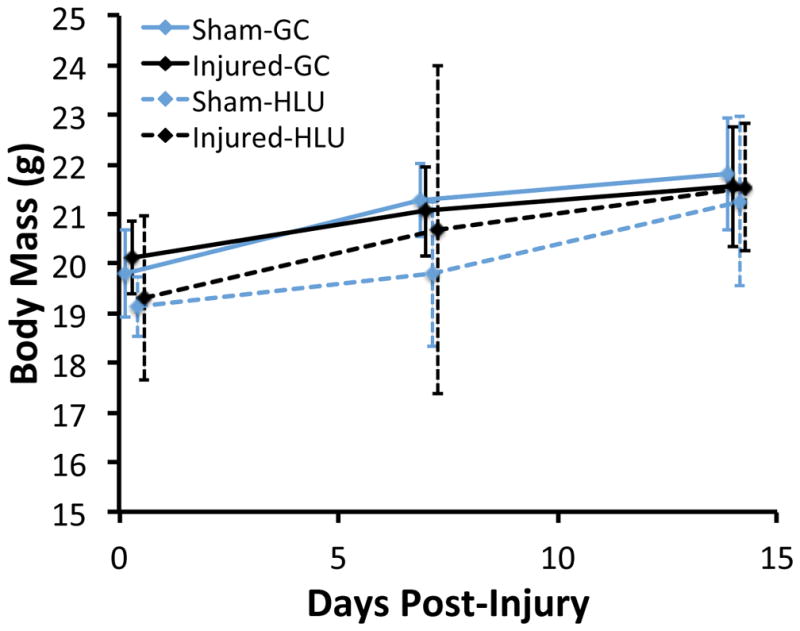
Body mass of hindlimb unloaded (HLU) and ground control (GC) mice following non-invasive knee injury or sham injury. There were no significant differences between any of the experimental groups at any time points. Approximately half of the animals subjected to HLU lost weight from day 0 to day 7, but by day 14 their body mass was increased relative to day 0. Data are presented as mean ± standard deviation.
Open field analysis of voluntary movement
There were no significant differences between injured and sham mice for any parameters measured by open field analysis at any time point (Fig. 2). In fact, injured mice exhibited greater distance travelled and ambulatory time than sham animals, particularly at 14 days post-injury, although these differences were not significant (p = 0.12–0.23). However, injured mice exhibited a lower number of vertical sensor breaks at 4 days post-injury (−49% in injured mice; p = 0.10). Injured mice also spent greater time in the margins of the enclosure (+8.1%) and less time in the center of the enclosure (−58%) than sham mice at 4 days post-injury (p = 0.053).
Figure 2.
Open Field containment for analysis of voluntary movement (top left). Each mouse was tested for a 24-hour period at 4, 14, 28, and 42 days post-injury. No significant differences were observed between injured and sham mice for any parameters at any time point. Data are presented as mean ± standard deviation.
Gait analysis
Gait analysis revealed an altered stride pattern in the injured limb of mice compared to sham animals (Fig. 3). Percent of stride spent in stance phase was decreased 6.0% in injured limbs (p = 0.048), primarily due to a 24% reduction in brake time (p = 0.03). Percent of stride spent in swing phase was increased 9.5% in the injured limb compared to sham (p = 0.048). The stride pattern of the contralateral front limb was also somewhat altered (although not statistically significant), with a 7.3% increase in percent of stride spent in stance phase, and a 10.1% decrease in percent of stride spent in swing phase compared to sham animals (p = 0.10). No significant differences were observed for the contralateral rear limb or the ipsilateral front limb. No significant differences were observed for stride time or stride length for any of the limbs.
Figure 3.
Gait of injured and sham mice was analyzed during treadmill running using the Treadscan system (left). Average percent of stride spent in stance phase was decreased in injured limbs compared to sham, while percent of stride spent in swing phase was increased (right). Mean data for swing, propel, and brake phases are presented.
Trabecular bone analysis by micro-computed tomography imaging
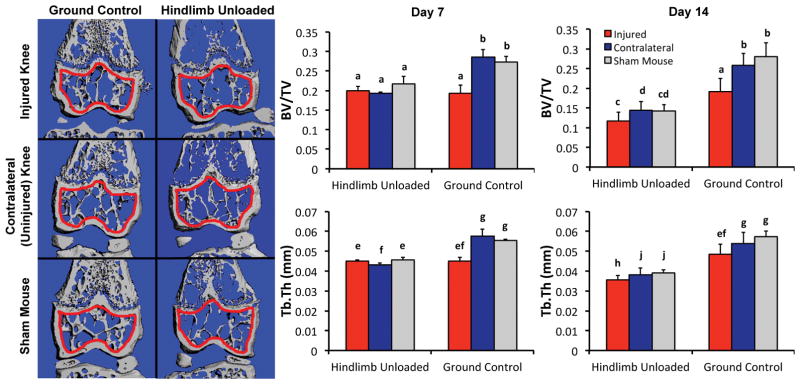
Both knee injury and hindlimb unloading resulted in significantly less epiphyseal trabecular bone volume than sham mice at 7 and 14 days post-injury (Fig. 4). All HLU mice exhibited similar deficits in epiphyseal trabecular bone regardless of injury status, and this decrease in bone volume progressed from day 7 to day 14 of unloading (~25% decrease in BV/TV at day 7 (p < 0.01), ~50% decrease in BV/TV at day 14 (p < 0.01)). Injured limbs of GC mice exhibited a comparable deficit in epiphyseal trabecular bone volume compared to HLU mice at 7 days post-injury, but this deficit did not progress from 7–14 days post-injury. A similar pattern was observed for Tb.Th, with both HLU mice and injured limbs of GC mice exhibiting ~20% lower Tb.Th of the epiphyseal trabecular bone at 7 days post-injury (p < 0.01), and HLU mice exhibiting ~33% lower Tb.Th by 14 days post-injury (p < 0.01) with no further decrease in Tb.Th in GC mice from day 7–14 post-injury. No differences in Tb.N were observed due to HLU, however injured limbs of GC mice had 5.8% and 6.5% lower Tb.N than contralateral limbs at day 7 (p = 0.044) and day 14 (p < 0.01), respectively (data not shown).
Figure 4.
(Left) Representative μCT reconstruction frontal sections of mouse distal femurs showing epiphyseal trabecular bone after 14 days of injury or HLU. The volume of interest for analysis is indicated in red. (Right) Trabecular bone volume fraction (top row) and trabecular thickness (bottom row) after 7 or 14 days of injury and/or HLU. Both HLU and knee injury resulted in comparable trabecular bone loss after 7 days, however HLU resulted in further decreases in trabecular bone volume from 7–14 days, while injured GC knees exhibited no further decreases. Data are presented as mean ± standard deviation. Groups that do not share a letter are significantly different from one another (p < 0.05).
Quantification of muscle mass
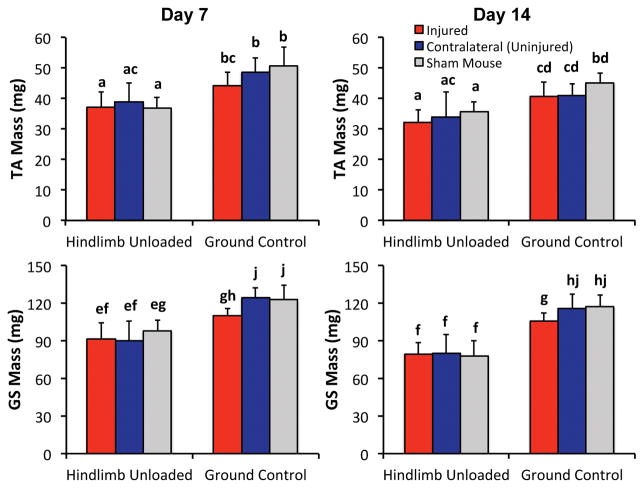
Both hindlimb unloading and knee injury resulted in decreases in muscle mass of the lower limb compared to Sham mice, with HLU resulting in considerably greater decreases than knee injury (Fig. 5). All HLU mice exhibited similar decreases in muscle mass in the lower limbs compared to Sham regardless of injury status (~25% muscle mass loss at day 7 (p = 0.0002–0.023), ~30% muscle mass loss at day 14 (p = 0.0008–0.09)). Injured limbs of GC mice exhibited less of a deficit in muscle mass compared to HLU mice (~10% muscle mass loss relative to contralateral; p = 0.009–0.13). Additionally, sham GC mice exhibited a 4–11% decrease in muscle mass at day 14 compared to day 7, although this difference was not statistically significant (p = 0.11–0.47).
Figure 5.
Muscle mass of the Tibialis Anterior (top row) and Gastrocnemius and Soleus (bottom row) after 7 or 14 days of injury or HLU. All HLU mice lost comparable muscle mass at 7 and 14 days regardless of injury status. Knee injury alone also resulted in decreased muscle mass, but to a much lesser extent than HLU. Data are presented as mean ± standard deviation. Groups that do not share a letter are significantly different from one another (p < 0.05).
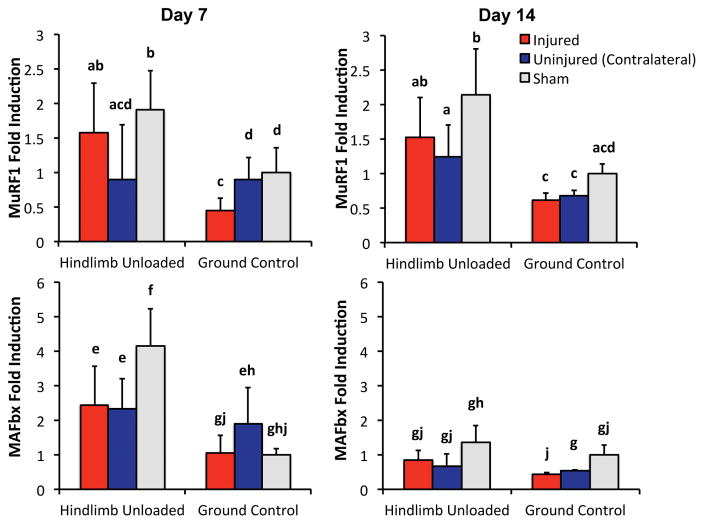
Quantification of biomarkers of muscle atrophy
Hindlimb unloading resulted in increased levels of biomarkers of muscle atrophy MuRF1 and MAFbx relative to GC mice, while knee injury alone did not increase these biomarkers (Fig. 6). For example, 7 days of HLU increased MuRF1 and MAFbx expression 90% and 314%, respectively, in sham HLU mice relative to sham GC mice (p = 0.05 and p = 0.005, respectively). After 14 days, MuRF1 expression remained 93% higher in sham HLU mice relative to sham GC mice (p = 0.016), while MAFbx expression returned to baseline values at this time point. In contrast, non-invasive knee injury did not result in increased levels of MuRF1 or MAFbx expression at either time point, and in fact often decreased expression in injured mice compared to sham mice (p = 0.0008–0.91). Additionally, the combination of HLU and knee injury did not further increase MuRF1 or MAFbx expression relative to HLU alone, and in fact was typically lower for injured HLU mice relative to sham HLU mice (p = 0.074–0.495).
Figure 6.
Expression of biomarkers of muscle atrophy MuRF1 (top row) and MAFbx (bottom row) after 7 or 14 days of injury or HLU. HLU resulted in increased levels of MuRF1 and MAFbx relative to GC mice, while knee injury did not result in increased levels of MuRF1 or MAFbx expression at either time point, and in fact often decreased expression in injured mice compared to sham mice. Data are presented as mean ± standard deviation. Groups that do not share a letter are significantly different from one another (p < 0.05).
Discussion
This study investigated the contribution of mechanical unloading to the loss of trabecular bone volume following non-invasive knee injury in mice. We originally hypothesized that knee injury would result in decreased voluntary movement and gait asymmetry in injured mice, and that hindlimb unloading would cause a loss of trabecular bone and muscle in both injured and sham animals, but that this atrophy would be greater in injured animals. Contrary to this hypothesis, we observed similar magnitudes of trabecular bone and muscle mass loss during HLU for both injured and sham animals. We also observed only minor changes in gait and movement behavior due to injury, with no changes in general activity level. These data indicate that mechanical unloading is a primary determinant of bone loss following joint injury in mice, although other non-mechanical factors must be considered in order to account for the observed magnitude of trabecular bone loss.
Open field analysis indicated that injured mice were on average just as active as sham mice at all time points following injury, although within-group variance was high for all of the groups. The primary behavioral change of injured mice was the decrease in vertical sensor breaks at 4 days post-injury, indicating a reduction in rearing. Additionally, injured mice spent a greater amount of time in the margins of the enclosure and less time in the center of the enclosure at this early time point, indicative of increased stress. These early differences can be attributed to pain and discomfort associated with knee injury, but at later time points no differences in behavior were observed. Similarly, gait analysis at 1 day post-injury indicated that injured animals exhibited a shorter stance phase in the injured limb relative to sham animals, and compensated with a slightly longer stance phase in the contralateral forelimb. These differences can also be attributed to acute knee pain associated with injury. Altogether, these data indicate small differences in gait and behavior of animals at early time points following injury, although the overall activity of the animals is not reduced, and movement at later time points does not differ from sham animals.
Results from μCT analysis were consistent with our previous study, in which the injured limbs of GC mice exhibiting a peak loss of trabecular bone at 7–14 days followed by a partial recovery of bone volume at later time points [7]. HLU resulted in a comparable magnitude of trabecular bone loss from days 0–7, with a further loss of trabecular bone from day 7–14. In our initial hypothesis we postulated that we would observe a greater trabecular bone loss in injured mice subjected to HLU than in Sham HLU mice. This effect was observed at 14 days post-injury, at which point the injured limb of HLU mice had the lowest BV/TV, although this difference was small and not significantly different from Sham HLU mice. This data partially supports our initial hypothesis, but to a much lesser degree than we expected. It is possible that HLU produces a “maximal” bone resorption response, and further bone resorption due to knee injury is not possible. Importantly, it appears that injured GC mice also achieve this “maximal” response from day 0–7 after injury, since the magnitude of trabecular bone loss was comparable to HLU mice at day 7. Unfortunately, this “maximal” bone resorption response precludes us from determining the contribution of mechanical unloading to trabecular bone loss in injured GC mice based on this data. Since the same degree of trabecular bone loss was observed in all HLU mice and the injured limbs of GC mice, it is not possible to determine the individual contributions of mechanical unloading or other non-mechanical factors based on this data. It is also possible that the observed trabecular bone losses in injured GC mice are attributable only to mechanical unloading, which is why the bone loss magnitude is similar to that observed with HLU. However, this is unlikely based on our activity/gait analysis and muscle mass analysis.
Analysis of lower limb muscle mass suggests that disuse in the injured limbs of GC mice was considerably less than in HLU mice, with muscle mass of HLU mice exhibiting a 2-fold greater deficit than injured limbs of GC mice compared to Sham. Consistent with muscle mass data, MuRF1 and MAFbx expression increased only in HLU mice. MuRF1 and MAFbx are E3 ubiquitin ligases that are expressed at relatively low levels in resting muscle and upregulated under a variety of atrophy associated conditions including unloading, inactivity, elevated glucocorticoids, and elevated cytokines [29]. Contrary to our initial predictions, MuRF1 and MAFbx decreased in the injured limbs, which is unexpected since decreases in MuRF1 and MAFbx expression have only been reported with increased loading, such with functional overload [30] and in response to β2-adrenergic agonists [31]. Therefore, the mechanisms underlying the observed decreases in MuRF1 and MAFbx expression with injury remain unknown.
Based on this data, it may be possible to conclude that disuse in the injured limb of GC mice accounts for less than half of the observed trabecular bone volume loss during this time. A limitation of this conclusion is that it assumes that bone and muscle are completely “in phase”, and that these tissues require the same level of mechanical loading to maintain their volume and structure. This may not be the case, and therefore the ability to use muscle mass as a proxy for disuse effects in bone may be limited. However, our observations from open field and gait analysis may support this conclusion, since only slight changes in animal behavior were observed at early time points, with no overall decrease in the activity level of injured animals. These data suggest that some degree of disuse occurs with knee injury, but to a far less extent than total unloading of the hindlimbs.
This study is somewhat limited because we did not collect baseline data for any outcomes, and instead relied on sham-injured GC mice for controls. This is particularly important since we observed some loss of muscle mass in sham GC mice during the experimental period. This is likely due to the fact that GC mice were moved from group housing to individual housing at the beginning of the experimental period in order to control for the single housing of HLU mice. This may have resulted in some loss of muscle mass or trabecular bone volume from day 0–7 that was not quantified in this study. Despite this limitation, we were able to observe significant changes in muscle and bone due to both HLU and non-invasive knee injury, and based on this data we are able to estimate the contribution of mechanical unloading to trabecular bone loss in injured GC mice. Another limitation of the study was the fact that gait analysis was performed at only one time point (1 day post-injury), and that mice were subjected to a prescribed walking speed, rather than a self-selected walking speed. This study was also limited due to gait analysis being performed at day 1, while the first bout of Open Field analysis was not performed until day 4 post-injury. It is possible that voluntary activity of mice could change during this time period, and these changes are not quantified in the current study. A more thorough analysis of gait could investigate self-selected walking speed of mice at multiple time points post-injury. However, despite this limitation we were able to determine small differences in mouse gait soon after injury, and identify this as a possible mechanism contributing to mechanical unloading of injured limbs.
Conclusions
This study investigated the contribution of mechanical unloading to trabecular bone volume loss observed following non-invasive knee injury in mice. We found that gait and behavior of injured mice is slightly altered at early time points post-injury, resulting in some degree of mechanical unloading that contributes to trabecular bone loss. However, using muscle mass as an indicator of disuse, we found that knee injury resulted in less than half of the muscle mass loss observed for mice subjected to hindlimb unloading. Altogether, these data suggest that mechanical unloading likely contributes to the trabecular bone volume loss observed following non-invasive knee injury, but the magnitude of this bone loss cannot be fully explained by unloading. Other non-mechanical factors (e.g., inflammation) likely contribute to this bone loss, and these factors must be investigated and considered for studies of post-traumatic osteoarthritis and the treatment of acute joint injuries.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under Award Number AR062603. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding body was not involved with design, collection, analysis, or interpretation of data; or in the writing of the manuscript. The authors have no conflicts of interest to disclose.
Footnotes
Author Contributions Statement: MJA performed hindlimb unloading and muscle mass analysis. SD performed microCT analysis. LMB performed muscle biomarker analysis. KB assisted with study design and data interpretation, and provided HLU equipment. SCB assisted with study design and data interpretation, and provided resources for biomarker analysis. BAC coordinated all analyses and was primarily responsible for writing the manuscript. All authors have read and approved the final submitted manuscript.
References
- 1.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756–69. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 2.Messent EA, Ward RJ, Tonkin CJ, Buckland-Wright C. Cancellous bone differences between knees with early, definite and advanced joint space loss; a comparative quantitative macroradiographic study. Osteoarthritis Cartilage. 2005;13(1):39–47. doi: 10.1016/j.joca.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Bolbos RI, Zuo J, Banerjee S, et al. Relationship between trabecular bone structure and articular cartilage morphology and relaxation times in early OA of the knee joint using parallel MRI at 3 T. Osteoarthritis and Cartilage. 2008;16(10):1150–1159. doi: 10.1016/j.joca.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burr DB, Gallant MA. Bone remodelling in osteoarthritis. Nat Rev Rheumatol. 2012;8(11):665–73. doi: 10.1038/nrrheum.2012.130. [DOI] [PubMed] [Google Scholar]
- 5.Boyd SK, Muller R, Leonard T, Herzog W. Long-term periarticular bone adaptation in a feline knee injury model for post-traumatic experimental osteoarthritis. Osteoarthritis Cartilage. 2005;13(3):235–42. doi: 10.1016/j.joca.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Moodie JP, Stok KS, Muller R, et al. Multimodal imaging demonstrates concomitant changes in bone and cartilage after destabilisation of the medial meniscus and increased joint laxity. Osteoarthritis Cartilage. 2011;19(2):163–70. doi: 10.1016/j.joca.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Christiansen BA, Anderson MJ, Lee CA, et al. Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2012;20(7):773–82. doi: 10.1016/j.joca.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Lockwood KA, Chu BT, Anderson MJ, et al. Comparison of loading rate-dependent injury modes in a murine model of post-traumatic osteoarthritis. J Orthop Res. 2014;32(1):79–88. doi: 10.1002/jor.22480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khorasani MS, Diko S, Hsia AW, et al. Effect of alendronate on post-traumatic osteoarthritis induced by anterior cruciate ligament rupture in mice. Arthritis Res Ther. 2015;17(1):30. doi: 10.1186/s13075-015-0546-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rambaut PC, Leach CS, Whedon GD. A study of metabolic balance in crewmembers of Skylab IV. Acta Astronaut. 1979;6(10):1313–22. doi: 10.1016/0094-5765(79)90123-1. [DOI] [PubMed] [Google Scholar]
- 11.Inoue M, Tanaka H, Moriwake T, et al. Altered biochemical markers of bone turnover in humans during 120 days of bed rest. Bone. 2000;26(3):281–6. doi: 10.1016/s8756-3282(99)00282-3. [DOI] [PubMed] [Google Scholar]
- 12.Kim H, Iwasaki K, Miyake T, et al. Changes in bone turnover markers during 14-day 6 degrees head-down bed rest. J Bone Miner Metab. 2003;21(5):311–5. doi: 10.1007/s00774-003-0426-6. [DOI] [PubMed] [Google Scholar]
- 13.Vico L, Collet P, Guignandon A, et al. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet. 2000;355(9215):1607–11. doi: 10.1016/s0140-6736(00)02217-0. [DOI] [PubMed] [Google Scholar]
- 14.Lang TF, Leblanc AD, Evans HJ, Lu Y. Adaptation of the proximal femur to skeletal reloading after long-duration spaceflight. J Bone Miner Res. 2006;21(8):1224–30. doi: 10.1359/jbmr.060509. [DOI] [PubMed] [Google Scholar]
- 15.Judex S, Garman R, Squire M, et al. Genetically linked site-specificity of disuse osteoporosis. J Bone Miner Res. 2004;19(4):607–13. doi: 10.1359/JBMR.040110. [DOI] [PubMed] [Google Scholar]
- 16.Dehority W, Halloran BP, Bikle DD, et al. Bone and hormonal changes induced by skeletal unloading in the mature male rat. Am J Physiol. 1999;276(1 Pt 1):E62–9. doi: 10.1152/ajpendo.1999.276.1.e62. [DOI] [PubMed] [Google Scholar]
- 17.Bloomfield SA, Allen MR, Hogan HA, Delp MD. Site- and compartment-specific changes in bone with hindlimb unloading in mature adult rats. Bone. 2002;31(1):149–57. doi: 10.1016/s8756-3282(02)00785-8. [DOI] [PubMed] [Google Scholar]
- 18.Musacchia XJ, Steffen JM, Deavers DR. Rat hindlimb muscle responses to suspension hypokinesia/hypodynamia. Aviat Space Environ Med. 1983;54(11):1015–20. [PubMed] [Google Scholar]
- 19.LeBlanc A, Marsh C, Evans H, et al. Bone and muscle atrophy with suspension of the rat. J Appl Physiol 1985. 1985;58(5):1669–75. doi: 10.1152/jappl.1985.58.5.1669. [DOI] [PubMed] [Google Scholar]
- 20.Fitts RH, Metzger JM, Riley DA, Unsworth BR. Models of disuse: a comparison of hindlimb suspension and immobilization. J Appl Physiol 1985. 1986;60(6):1946–53. doi: 10.1152/jappl.1986.60.6.1946. [DOI] [PubMed] [Google Scholar]
- 21.Stevens HY, Meays DR, Frangos JA. Pressure gradients and transport in the murine femur upon hindlimb suspension. Bone. 2006;39(3):565–72. doi: 10.1016/j.bone.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Barou O, Valentin D, Vico L, et al. High-resolution three-dimensional micro-computed tomography detects bone loss and changes in trabecular architecture early: comparison with DEXA and bone histomorphometry in a rat model of disuse osteoporosis. Invest Radiol. 2002;37(1):40–6. doi: 10.1097/00004424-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Basso N, Jia Y, Bellows CG, Heersche JN. The effect of reloading on bone volume, osteoblast number, and osteoprogenitor characteristics: studies in hind limb unloaded rats. Bone. 2005;37(3):370–8. doi: 10.1016/j.bone.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 24.Judex S, Donahue LR, Rubin C. Genetic predisposition to low bone mass is paralleled by an enhanced sensitivity to signals anabolic to the skeleton. Faseb J. 2002;16(10):1280–2. doi: 10.1096/fj.01-0913fje. [DOI] [PubMed] [Google Scholar]
- 25.Criswell DS, Booth FW, DeMayo F, et al. Overexpression of IGF-I in skeletal muscle of transgenic mice does not prevent unloading-induced atrophy. Am J Physiol. 1998;275(3 Pt 1):E373–9. doi: 10.1152/ajpendo.1998.275.3.e373. [DOI] [PubMed] [Google Scholar]
- 26.Hanson AM, Ferguson VL, Simske SJ, et al. Comparison of tail-suspension and sciatic nerve crush on the musculoskeletal system in young-adult mice. Biomed Sci Instrum. 2005;41:92–6. [PubMed] [Google Scholar]
- 27.Warden SJ, Galley MR, Richard JS, et al. Reduced gravitational loading does not account for the skeletal effect of botulinum toxin-induced muscle inhibition suggesting a direct effect of muscle on bone. Bone. 2013;54(1):98–105. doi: 10.1016/j.bone.2013.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouxsein ML, Boyd SK, Christiansen BA, et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25(7):1468–86. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 29.Bodine SC, Baehr LM. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Metab. 2014;307(6):E469–84. doi: 10.1152/ajpendo.00204.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baehr LM, Tunzi M, Bodine SC. Muscle hypertrophy is associated with increases in proteasome activity that is independent of MuRF1 and MAFbx expression. Front Physiol. 2014;5:69. doi: 10.3389/fphys.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kline WO, Panaro FJ, Yang H, Bodine SC. Rapamycin inhibits the growth and muscle-sparing effects of clenbuterol. J Appl Physiol 1985. 2007;102(2):740–7. doi: 10.1152/japplphysiol.00873.2006. [DOI] [PubMed] [Google Scholar]