Abstract
Background
Elevated body mass index (BMI) and arsenic are both associated with cancer and with non-malignant lung disease. Using a unique exposure situation in Northern Chile with data on lifetime arsenic exposure, we previously identified the first evidence of an interaction between arsenic and BMI for the development of lung cancer.
Objectives
We examined whether there was an interaction between arsenic and BMI for the development of non-malignant lung disease.
Methods
Data on lifetime arsenic exposure, respiratory symptoms, spirometry, BMI, and smoking were collected from 751 participants from cities in Northern Chile with varying levels of arsenic water concentrations. Spirometry values and respiratory symptoms were compared across subjects in different categories of arsenic exposure and BMI.
Results
Adults with both a BMI above the 90th percentile (>33.9 kg/m2) and arsenic water concentrations ≥11 µg/L exhibited high odds ratios (ORs) for cough (OR=10.7, 95% confidence interval (CI): 3.03, 50.1), shortness of breath (OR=14.2, 95% CI: 4.79, 52.4), wheeze (OR=14.4, 95% CI: 4.80, 53.7), and the combined presence of any respiratory symptom (OR=9.82, 95% CI: 4.22, 24.5). In subjects with lower BMIs, respiratory symptom ORs for arsenic water concentrations ≥11 µg/L were markedly lower. In never-smokers, reductions in forced vital capacity associated with arsenic increased as BMI increased. Analysis of the FEV1/FVC ratio in never-smokers significantly increased as BMI and arsenic concentrations increased. Similar trends were not observed for FEV1 alone or in ever-smokers.
Conclusions
This study provides preliminary evidence that BMI may increase the risk for arsenic-related non-malignant respiratory disease.
Keywords: Arsenic, body mass index, spirometry, lung function, Chile
Hundreds of millions of people worldwide are exposed to arsenic through contaminated food or water.1 Multiple studies have linked chronic arsenic exposure to increased risks of cancers including lung, bladder, kidney, and skin cancer, as well as diabetes, cardiovascular disease, and other adverse health outcomes.2–9 The International Agency for Research on Cancer has listed ingested arsenic as a class I human lung carcinogen.10 In the US, it has been estimated that about 12% of all public water systems have arsenic concentrations close to the US and World Health Organization (WHO) regulatory drinking water standard of 10 µg/L.11,12 The US Environmental Protection Agency has estimated that concentrations near this level may be associated with one excess case of cancer for every 120 individuals exposed, which is about 50 times higher than the estimated risk for any other regulated contaminant.13–15
It is increasingly thought that lung cancer is the most common cause of mortality in those with prolonged arsenic exposure, suggesting that the human lung may be particularly susceptible to ingested arsenic.16 Arsenic exposure also increases the risk of non-malignant pulmonary disease and abnormal auscultation findings.17,18 Chronic cough, shortness of breath (SOB), and abnormal lung sounds are increased in those exposed to ingested arsenic.18,19 Multiple studies, many of which involve exposure levels that overlap those in Northern Chile, have also demonstrated a relationship between arsenic exposure and decreases in forced vital capacity (FVC) and forced expiratory volume in the first second (FEV1).18,20–28 FVC and FEV1 are common spirometric measurements, the first being a measurement of the total volume of air forcefully exhaled after one complete inhalation, and the second a measurement of the total volume of air forcefully exhaled in one second after one complete inhalation. These measurements can be used as indicators for varying types of lung dysfunction.29,30
The mechanisms of arsenic pathogenesis are largely unknown, but may involve increased circulation of inflammatory agents and oxidative stress, both of which have been strongly linked to cancer and lung disease progression.31,32 While high arsenic exposure is limited to certain geographic regions, elevated body mass index (BMI) is a globally widespread health concern and also increases systemic inflammation, oxidative stress, and cancer risk.33–43 Both arsenic exposure and elevated BMI have been shown to trigger inflammatory reactions in the lungs.44,45 Obesity is also associated with increased odds of chronic cough and SOB.46 The joint ability of arsenic and elevated BMI to individually cause inflammation, oxidative stress, and potentially alter adipocyte protein synthesis highlights the possibility that the two could interact to increase disease risks.47–50
Our research group has been studying the long-term health effects of arsenic using a uniquely exposed area in Northern Chile. The Antofagasta Region located in the Atacama Desert is one of the driest habitable places on earth, receiving less than two millimeters of rain annually.51–53 Because it is so dry there are few individual water sources, and each city has a single municipal water supply. These water supplies have had a wide range of arsenic concentrations from current-day concentrations of ≤10 µg/L to over 800 µg/L from the late 1950s through the 1960s. Since arsenic records are available on all of the major water sources in this area for many decades in the past, lifetime arsenic exposures can be assessed fairly accurately simply by knowing which city a person has lived in during which years.54 To date, we have identified major increases in lung cancer, bladder cancer, lung symptoms, spirometry decrements, and other health outcomes associated with high arsenic exposures in this area. Most recently, we found that the odds ratios of lung cancer associated with arsenic were over three times greater in people with BMIs above the 90th percentile compared to people with lower BMIs.55 Given these substantial findings for lung cancer and that both arsenic and obesity are associated with increased risks of non-malignant lung symptoms and disease, we used data from this unique study area to evaluate whether arsenic and elevated BMI could interact to increase non-malignant lung disease risks.
Methods
Subject ascertainment
This analysis is part of a parent study of arsenic and non-malignant lung disease conducted in Northern Chile between 2009 and 2011.28 Study participants were adults aged 39 to 60 years who were randomly selected from the Chilean Electoral Registry for three of the four largest cities in Northern Chile, which had variable arsenic water concentrations. Because the focus of the parent study was on the impacts of early-life exposure, all study participants were born between the years of 1958–1970, the high exposure period in Antofagasta. These cities included Arica (population of 157,568; mean arsenic water concentrations near 10 µg/L), Iquique (population of 181,773; mean historical arsenic water concentrations of 60 µg/L), and Antofagasta (population of 391,832). Antofagasta had a distinct period of high exposure (mean arsenic water concentrations of 860 µg/L) beginning in 1958 when two rivers with high arsenic concentrations were diverted to the city for drinking purposes and ending in 1970 when an arsenic treatment plant was installed. Following the installation of this plant, concentrations initially dropped to about 100 µg/L and eventually to 10 µg/L by 2003 with further improvements to the plant.54
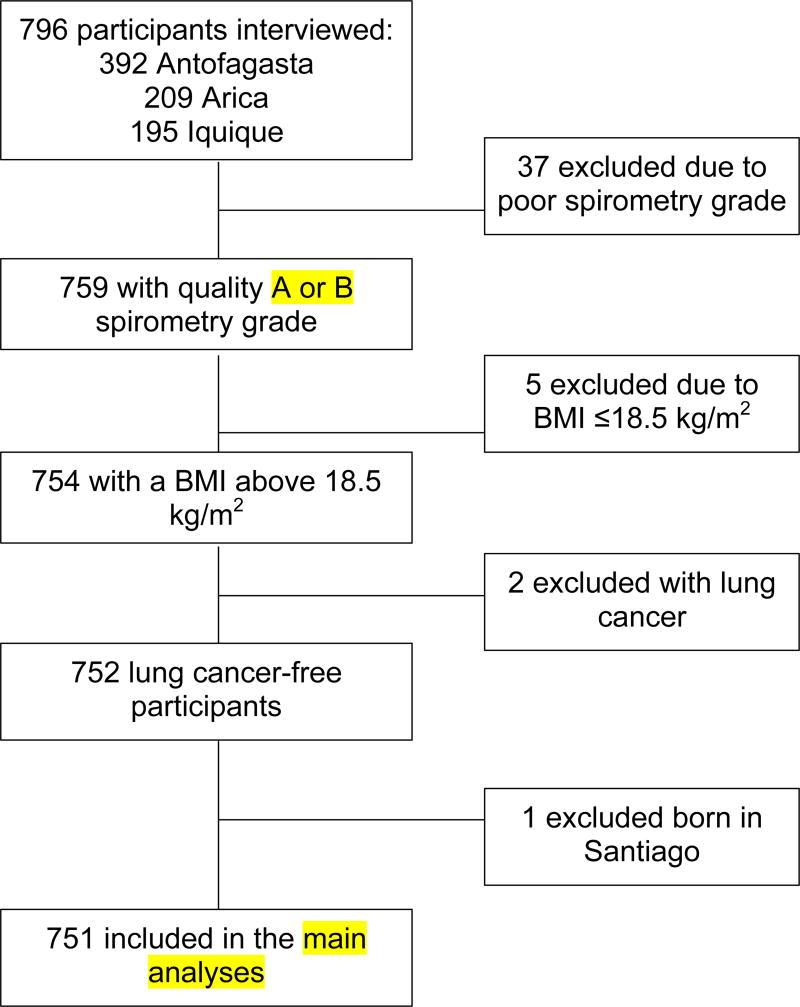
All subjects provided written consent prior to participation. Participants who lived less than 80% of their lives in the city where their interview was conducted, were born in Santiago, exhibited a BMI at the time of interview of <18.5 kg/m2, had lung cancer, or had a poor spirometry grade (greater than 150 mL difference in FEV1 across two best trials) were excluded from the main analyses presented here (Figure 1). Individuals born in Santiago were excluded due to historically high air pollution.56 Since our analyses assumed a unidirectional relationship between lung function and BMI, individuals with a BMI <18.5 kg/m2 were excluded, as this BMI level is associated with higher rates of mortality.57
Figure 1.
Data analysis inclusion criteria
Questionnaires and BMI
Trained staff administered one-time study interviews and pulmonary function tests. Structured questionnaires were administered to collect data on lifetime residential history; occupational history (e.g. mining or smelter work); water sources used (e.g. public water supply, bottled water); typical water intake; ages of and duration of tobacco use; secondhand smoke exposure in childhood and adulthood; specific occupational lung irritant exposures like asbestos, arsenic, and silica; socioeconomic status; typical diet currently and 20 years ago; illnesses like diabetes and hypertension; and ethnicity/race. Socioeconomic status (SES) scores were calculated on a 12-point scale based on self-reported ownership of household appliances, electronics, car, or employment of domestic help. Diet is strongly related to obesity and was assessed as a potential confounder. Participants were also asked about potential pulmonary disease symptoms such as cough, shortness of breath (SOB), wheeze, and phlegm production. Questions on pulmonary symptoms were based on the British Medical Research Council respiratory questionnaire and translated to local Spanish.58 Information on cough was ascertained by asking, “Do you cough almost every day for three consecutive months or more during the year?” and if so, “For how many years have you had this cough?” Participants answering yes to the first were considered to have cough. Participants answering two years or more for the second question were considered to have chronic cough. Participants were also asked about shortness of breath when walking with people their own age on level ground, when hurrying or walking uphill, or when walking quickly on level ground or uphill. They were also asked about duration, frequency, and hospitalizations for wheeze, and about other prior hospitalizations, all medication use, and prior physician-diagnosed asthma, bronchitis, emphysema, and other pulmonary diagnoses. The Spanish version of these questions are shown in Figure S1. Height and weight were measured by the study nurses at the time of interview using standard protocols and were used to calculate BMI.
Spirometry
Spirometry measurements were collected using an EasyOne spirometer in diagnostic mode (NDD Medical Technologies, Zurich, Switzerland) according to protocol guidelines of the American Thoracic Society.59 Participants were asked to provide a minimum of three forced exhalation efforts from the seated position without a nose clip or bronchodilator. Although use of a nose clip is standard protocol, research suggests it has little impact on FVC or FEV1.60 Only participants with spirometry grades A or B (i.e. at least two acceptable maneuvers with FEV1 values less than 150 ml apart) based on the EasyOne spirometry guidelines were included in our main analyses, although separate analyses including all subjects regardless of grade were also done. Each subject’s best effort (largest sum of FEV1 and FVC) was included in the analyses.
Arsenic exposure assessment
Arsenic exposure was assessed using historical municipal drinking water records for all cities and towns in the study area and nearly all subjects reported use of public water supplies. These records were collected from government agencies and water suppliers and were linked to each participant’s residential history in order to create an arsenic exposure estimate for each year of each subjects life, from birth to interview.61 These yearly estimates were then used to develop several exposure metrics including the participant’s highest arsenic water concentration for any single year of their lives; the highest average arsenic water concentration over any contiguous 5, 20, or 40-year period; cumulative exposure (calculated by summing the yearly concentrations); average lifetime arsenic water concentration; and the highest arsenic water concentration during the first 20 years of life. Although some participants lived outside of the three recruitment cities at some point in their lives, almost all of these residences were in Chile and arsenic exposure information was available for almost all of these other cities. Bottled water use and residences without arsenic measurements were assigned values of zero. Overall, arsenic water concentration data were available for 97.9% of all life-years of the study participants.
Data analysis
All statistical analyses were conducted using the open-source statistical analysis software R (Version 0.99.486 2015). The major non-malignant lung disease variables of interest were the presence of respiratory symptoms (coughing, wheezing, SOB) and the spirometric measurements FEV1 and FVC. Age, height, FEV1, and FVC were assessed as continuous variables. Sex, smoking pack-years (by tertile), BMI level (18.5–<25 kg/m2 defined as normal, 25–30 kg/m2 defined as overweight, and >30 kg/m2 defined as obese), and single year lifetime highest arsenic exposure (<11 µg/L, 11–200 µg/L, and ≥200 µg/L) were assessed as categorical variables. These cutoff points for arsenic exposure were based on the highest arsenic concentrations in the drinking water of the largest cities in the study area.28 Participants in the ≥200 µg/L category lived most of their lives in Antofagasta, while all but eight in the 11–200 µg/L group had lived majority of their lives in Iquique. All participants in the <11 µg/L category lived most of their lives in Arica. Although our primary exposure metric was the single highest year, most subjects lived in the area for many years and thus had many years of exposure.
First, we conducted bivariate analysis in order to examine socio-demographic characteristics between the different BMI categories. Odds ratios were calculated to determine potential associations, using people with a normal BMI as the reference category. Next, we calculated odds ratios to evaluate associations between the socio-demographic variables and the prevalence of respiratory symptoms such as cough, wheezing, and SOB, as well a combined measure for any one of these three symptoms.
We then used logistic regression to calculate odds ratios (ORs) for respiratory symptom prevalence in participants with high and low arsenic exposure and with high and low BMI. In these analyses, low and high arsenic exposures were defined as having a single year highest exposure below or ≥11 µg/L, respectively. This cut-off was selected because the WHOs recommended limit for arsenic in water is 10 ug/L.12 Low and high BMI were defined as being below or above the cohort’s 90th percentile, respectively. The 90th percentile was selected for our main analyses since this was the cut-off point used in our previous evaluation of arsenic-BMI interaction for lung cancer, although analyses using other cutoff points (e.g. the median and 30 kg/m2) were also performed. Additional analyses with alternative arsenic exposure cutoff-points and alternative metrics (e.g. cumulative exposure) were also conducted. ORs were adjusted a priori by age, sex, and tertiles of pack-years smoked. In these analyses, arsenic exposure and BMI were each categorized into two groups based on sample size considerations.
Because there were no widely accepted standard reference values for our study area, we used FVC and FEV1 residuals as our primary spirometry outcome metric. Residuals were calculated using multiple linear regression and data from the study subjects themselves. Here, subjects FEV1s or FVCs were entered in the multiple linear regression equation as the dependent variable and their ages, sex, and heights were entered as the independent variables.68 Residuals were then calculated as the difference between the subjects observed FEV1 or FVC and that predicted for them based on the linear regression equation and their age, sex, and height. Race had little impact on results and was not included. The R2 of the linear regression model with FVC as the dependent variable and age, sex, and height as the independent variables was 0.56. For FEV1 this value was 0.51.
FEV1 and FVC residuals were compared across various socio-demographic groups using Student’s t-tests. To analyze whether BMI may impact arsenic-related changes on FVC or FEV1, participants were stratified into groups based on highest single year arsenic exposures of <11, 11–200, and ≥200 µg/L and normal, overweight, and obese BMI levels. Because smoking is a strong predictor of respiratory disease, subjects were further stratified into ever- vs. never-smokers.62 Differences in mean FEV1 and FVC across the various arsenic-BMI groups were then calculated using linear regression models adjusted by age, sex, and height.
Interaction between arsenic and BMI on spirometric outcomes was evaluated in linear regression analyses by adding a product term for arsenic and BMI. Here, BMI was entered as a continuous variable and arsenic as a dichotomous categorical variable (<11 µg/L or ≥11 µg/L). Potential biologic interaction between arsenic and BMI on respiratory symptoms was calculated using synergy indices and the methods described by Andersson et al.63 For synergy calculations, a value of 0.5 was entered for cells with zero subjects. A synergy index greater than one indicates a greater than additive relationship.
Ethics review
The study protocol has approval from the institutional review boards at the University of California, Berkeley and the Pontificia Universidad Católica de Chile.
RESULTS
Participant characteristics
In total, 751 participants were included in the analyses presented here, including 198 from the low-exposure city of Arica, 202 from the middle exposure city of Iquique, and 351 from the high exposure city of Antofagasta. The socio-demographic characteristics of the participants stratified by BMI categories are shown in Table 1. Overall, 178 subjects had normal BMI (18.5 ≤ BMI <25 kg/m2), 375 were overweight (25 ≤ BMI ≤30 kg/m2), and 198 were obese (BMI >30 kg/m2). Males were more likely to be obese than females (OR=1.92, 95% CI: 1.28, 2.90). Those with an occupational exposure to a known lung irritant were more likely to be overweight (OR=1.81, 95% CI: 1.18, 2.78) but not obese (OR=0.94, 95% CI: 0.56, 1.57). There were no associations between highest arsenic exposure and age (p=0.20), pack-years smoked (p=0.62), or SES (p=0.62) (data not shown).
TABLE 1.
Socio-demographic characteristics stratified by BMI category.
| N=751 | Normal BMI (N=178)
|
Overweight BMI (N=375)
|
Obese BMI (N=198)
|
|||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | OR (95% CI) | N | % | OR (95% CI) | |
| Age (years) | ||||||||
| <44 | 44 | 24.7% | 102 | 27.2% | 1.00 (ref) | 59 | 29.8% | 1.00 (ref) |
| 44–49 | 87 | 48.9% | 191 | 50.9% | 0.95 (0.61, 1.45) | 93 | 47.0% | 0.80 (0.49, 1.30) |
| >49 | 47 | 26.4% | 82 | 29.1% | 0.75 (0.45, 1.25) | 46 | 23.2% | 0.73 (0.42, 1.28) |
| Sex | ||||||||
| Female | 107 | 60.1% | 220 | 58.7% | 1.00 (ref) | 87 | 43.9% | 1.00 (ref) |
| Male | 71 | 39.9% | 155 | 41.3% | 1.06 (0.74, 1.53) | 111 | 56.1% | 1.92 (1.28, 2.90) |
| Arsenic exposurea | ||||||||
| <11 µg/L | 46 | 25.8% | 102 | 27.2% | 1.00 (ref) | 50 | 25.3% | 1.00 (ref) |
| 11–200 µg/L | 41 | 23.0% | 88 | 23.5% | 0.97 (0.58, 1.61) | 73 | 36.9% | 1.64 (0.94, 2.85) |
| ≥200 µg/L | 91 | 51.1% | 185 | 49.3% | 0.92 (0.60, 1.41) | 75 | 37.8% | 0.76 (0.46, 1.25) |
| Ever-smoker | ||||||||
| No | 87 | 48.9% | 188 | 50.1% | 1.00 (ref) | 103 | 52.0% | 1.00 (ref) |
| Yes | 91 | 51.1% | 187 | 49.9% | 0.95 (0.67, 1.36) | 95 | 48.0% | 0.88 (0.59, 1.32) |
| Smoking levelb | ||||||||
| Light | 32 | 35.2% | 60 | 32.1% | 0.87 (0.81, 1.13) | 32 | 33.7% | 0.84 (0.48, 1.49) |
| Moderate | 29 | 31.9% | 63 | 33.7% | 1.01 (0.60, 1.67) | 30 | 31.6% | 0.87 (0.49, 1.57) |
| Heavy | 30 | 32.9% | 64 | 34.2% | 0.99 (0.60, 1.63) | 33 | 34.7% | 0.93 (0.52, 1.64) |
| Secondhand smokec | ||||||||
| No | 52 | 59.8% | 114 | 60.6% | 1.00 (ref) | 68 | 66.0% | 1.00 (ref) |
| Yes | 35 | 40.2% | 74 | 39.4% | 0.96 (0.57, 1.62) | 35 | 34.0% | 0.76 (0.42, 1.38) |
| Ethnicity | ||||||||
| Hispanic | 154 | 87.0% | 321 | 85.8% | 1.00 (ref) | 161 | 82.1% | 1.00 (ref) |
| Aymara | 15 | 8.5% | 45 | 12.0% | 1.44 (0.78, 2.66) | 25 | 12.8% | 1.59 (0.81, 3.14) |
| Other | 8 | 4.5% | 8 | 2.2% | 0.48 (0.18, 1.30) | 10 | 5.1% | 1.20 (0.46, 3.11) |
| Education level | ||||||||
| College | 55 | 30.9% | 109 | 29.1% | 1.00 (ref) | 60 | 30.3% | 1.00 (ref) |
| Some college | 84 | 47.2% | 149 | 39.7% | 0.90 (0.59, 1.36) | 83 | 41.9% | 0.91 (0.56, 1.46) |
| High school | 39 | 21.9% | 117 | 31.2% | 1.51 (0.93, 2.46) | 55 | 27.8% | 1.29 (0.75, 2.24) |
| SES scored | ||||||||
| High | 133 | 74.7% | 272 | 72.5% | 1.00 (ref) | 138 | 69.7% | 1.00 (ref) |
| Low | 45 | 25.3% | 103 | 27.5% | 1.12 (0.74, 1.68) | 60 | 30.3% | 1.29 (0.82, 2.02) |
| Occupational exposuree | ||||||||
| No | 143 | 80.3% | 260 | 69.3% | 1.00 (ref) | 161 | 81.3% | 1.00 (ref) |
| Yes | 35 | 19.7% | 115 | 30.7% | 1.81 (1.18, 2.78) | 37 | 18.7% | 0.94 (0.56, 1.57) |
Abbreviations: BMI, body mass index; CI, confidence interval; OR, odds ratio; ref, reference category; SES, socioeconomic status
Single year lifetime highest drinking water arsenic concentration
Light, moderate, and heavy refer to ≤2.4, >2.4 to <8.5, and ≥8.5 pack-years. Never smokers used as the reference group
Amongst never-smokers only
High, upper two tertiles; Low, lower tertile
Includes mining work and exposure to silica, asbestos, wood dust, arsenic, and fiberglass
Table S1 presents respiratory symptoms ORs for various socio-demographic characteristics. Older age (>49 years) was associated with cough (OR=2.28, 95% CI: 1.06, 4.89) and with having any respiratory symptom (OR=1.74, 95% CI: 1.02, 2.96). Obesity was associated with wheeze (OR=2.47, 95% CI: 1.11, 5.49), SOB (OR=1.72, 95% CI: 0.88, 3.34), and any respiratory symptom (OR=1.44, 95% CI: 0.85, 2.45) although only the association with wheeze was statistically significant. A single year lifetime highest arsenic exposure of 11–200 µg/L was associated with SOB (OR=4.94, 95% CI: 1.99, 12.2), wheeze (OR=3.91, 95% CI: 1.55, 9.87), and any symptom (OR=4.07, 95% CI: 2.07, 7.99) when compared to the low exposure group (<11 µg/L). Arsenic exposures >200 µg/L were also associated with increases in cough (OR=10.6, 95% CI: 3.24, 29.2), SOB (OR=4.47, 95% CI: 1.87, 10.7), and any symptom (OR=4.07, 2.15, 7.70) when compared to the low exposure group. Those with cumulative smoking histories of 20 pack-years or more were more likely to report any respiratory symptom (OR=2.40, 95% CI: 1.09, 5.30) compared to never-smokers (not shown).
Table 2 shows the mean sex, age, and height adjusted residuals of FEV1 and FVC by various socio-demographic categories. Compared with BMI of 18.5–<25 kg/m2, the upper category of BMI was associated with decreased FVC and FEV1 residuals although results were not statistically significant. While ≥20 pack-year cumulative smoking histories were also associated with decrements in FVC (127 ml) and FEV1 (70 ml) compared to never smokers (data not shown), these decreases were also not statistically significant.
TABLE 2.
Multiple linear regression analyses of age, gender, and height-adjusted forced vital capacity (FVC) residuals, forced expiratory volume in one second (FEV1) residuals, and FEV1/FVC ratio means by various socio-demographic categories
| FVC residual (ml)
|
FEV1 residual (ml)
|
FEV1/FVC
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | 95% CIa | pb | Mean res. |
95% CIa | pb | Mean | 95% CIa | pb | |
| BMI (kg/m2) | ||||||||||
| 18.5–<25 | 178 | 21 | −63, 104 | Ref | 18 | −55, 90 | Ref | 0.78 | 0.77, 0.79 | Ref |
| 25–30.0 | 375 | 12 | −48, 73 | 0.88 | 13 | −38, 63 | 0.91 | 0.79 | 0.79, 0.80 | 0.01 |
| >30 | 198 | −56 | −136, 25 | 0.20 | −47 | −117, 24 | 0.21 | 0.80 | 0.79, 0.81 | 0.01 |
| As exposure (µg/L)c | ||||||||||
| <11 | 198 | 39 | −35, 113 | Ref | −9 | −68, 51 | Ref | 0.78 | 0.77, 0.79 | Ref |
| 11–200 | 202 | −59 | −137, 19 | 0.07 | −27 | −95, 41 | 0.69 | 0.80 | 0.79, 0.80 | 0.004 |
| ≥200 | 351 | 3 | −63, 69 | 0.48 | 17 | −40, 74 | 0.54 | 0.79 | 0.79, 80 | 0.005 |
| Smokingd | ||||||||||
| Never | 378 | 7 | −53, 68 | Ref | −8 | −60, 44 | Ref | 0.79 | 0.78, 0.80 | Ref |
| Light | 124 | 1 | −99, 101 | 0.92 | 18 | −66, 103 | 0.60 | 0.79 | 0.78, 0.80 | 0.94 |
| Moderate | 122 | −59 | −156, 39 | 0.26 | −21 | −102, 60 | 0.79 | 0.79 | 0.78, 0.80 | 0.58 |
| Heavy | 128 | 16 | −91, 122 | 0.89 | 16 | −74, 107 | 0.64 | 0.79 | 0.78, 0.80 | 0.73 |
| Ethnicity | ||||||||||
| Hispanic | 636 | −14 | −60, 33 | Ref | −1 | −41, 39 | Ref | 0.79 | 0.79, 0.80 | Ref |
| Aymara | 85 | 70 | −37, 178 | 0.16 | 14 | −74, 102 | 0.59 | 0.78 | 0.77, 0.79 | 0.15 |
| Other | 30 | 55 | −123, 233 | 0.45 | 26 | −124, 177 | 0.60 | 0.80 | 0.78, 0.82 | 0.60 |
| Education | ||||||||||
| College | 224 | 64 | −190, 146 | Ref | 39 | −34, 111 | Ref | 0.79 | 0.78, 79 | Ref |
| Some college | 316 | −58 | −119, 2 | 0.02 | −38 | −89, 142 | 0.09 | 0.79 | 0.79, 0.80 | 0.18 |
| High school | 208 | 10 | −70, 90 | 0.36 | 10 | −55, 75 | 0.57 | 0.79 | 0.78, 0.80 | 0.49 |
| SES scoree | ||||||||||
| High | 543 | −9 | −59, 41 | Ref | −12 | −55, 31 | Ref | 0.79 | 0.79, 0.80 | Ref |
| Low | 208 | 11 | −64, 87 | 0.65 | 24 | −39, 87 | 0.35 | 0.79 | 0.78, 0.80 | 0.86 |
| Occupational exposuref | ||||||||||
| No | 564 | 10 | −37, 57 | Ref | 7 | −33, 47 | Ref | 0.79 | 0.79, 0.80 | Ref |
| Yes | 187 | −47 | −139, 44 | 0.27 | −32 | −109, 46 | 0.38 | 0.79 | 0.78, 0.79 | 0.38 |
Abbreviations: BMI, body mass index; CI, confidence interval; Ref, reference group; res, residual; SES, socioeconomic status
95% CIs are for the mean FEV1 or FVC residual for this category
P-value for the difference between the mean FEV1 or FVC residual in the corresponding category and the reference category
Single year lifetime highest arsenic water concentration
Light, moderate, and heavy refer to ≤2.4, >2.4 to <8.5, and ≥8.5 pack years. Never smokers used as the reference group
High, upper two tertiles; Low, lower two tertiles
Includes mining work and exposure to silica, asbestos, wood dust, arsenic, and fiberglass
Arsenic, BMI and respiratory symptoms
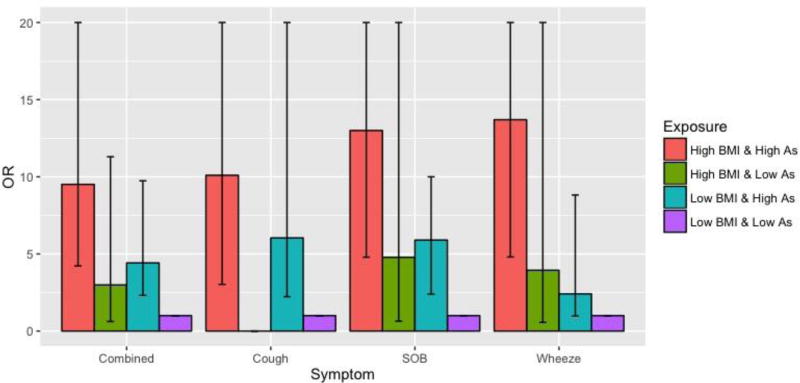
ORs for respiratory symptoms adjusted for sex, age, and smoking by categories of arsenic exposure and BMI, using subjects with lower BMI (<90th percentile of 33.9 kg/m2) and low arsenic exposure (<11 µg/L) as the reference group, are shown in Figure 2. Symptom ORs in subjects never exposed to arsenic concentrations ≥11 µg/L, but having a BMI >33.9 kg/m2 were above 1.0 for SOB, wheeze, and any symptom combined but were not statistically significant. In subjects with low BMIs, arsenic exposures ≥11 ug/L were associated with cough (OR=6.24, 95%CI: 2.23, 26.0), SOB (OR=5.96, 95% CI: 2.39, 19.9), wheeze (OR=2.56, 95% CI: 0.97, 8.82), and any symptom combined (OR=4.46, 95% CI: 2.31, 9.74). Having both an arsenic exposure ≥11 µg/L and a high BMI was associated with even greater increases in cough (OR=10.7, 95% CI: 3.03, 50.1), SOB (OR=14.2, 95% CI: 4.79, 52.4), wheeze (OR=14.4, 95% CI: 4.80, 53.7), and any symptom combined (OR=9.82, 95% CI: 4.22, 24.5). ORs for chronic cough were similar to those for cough. Synergy indexes for cough (S=2.29, 95% CI: 0.92, 5.70), SOB (S=1.49, 95% CI: 0.51, 4.36), wheeze (S=2.76, 95% CI=0.60, 12.7), and any symptom combined (S=1.61, 95% CI: 0.62, 4.17) were all above 1.0 but not statistically significant. Similar findings were seen when other cutoff points for BMI were used, although with overall somewhat lower synergy indices (Table S2). Findings were also similar when other arsenic exposure metrics were used, including lifetime average, cumulative exposure, and cumulative exposure between birth and age 20 (data not shown). No associations with phlegm production or asthma were observed.
Figure 2.
Logistic regression prevalence odds ratios for respiratory symptoms by categories of BMI and single year highest arsenic water concentration
Abbreviations: As, arsenic; BMI, body mass index; CI, confidence interval; OR, odds ratio; SOB, shortness of breath.
Odds ratios were adjusted age, sex, and tertiles of pack-years of smoking; low and high BMI constitutes a BMI below and above the 90th percentile of 33.9 kg/m2, respectively. Low and high arsenic constitutes a single year lifetime highest exposure below and ≥11 µg/L, respectively. All categories were mutually exclusive. Horizontal error bars indicate confidence intervals. The upper limits were capped at 20 for illustrative purposes. Actual upper limit values for the symptom odds ratios were as follows: combined, 24.5 (high BMI & high As); cough, 50.1 (high BMI & high As), 26.0 (low BMI & high As); SOB, 52.4 (high BMI & high As), 27.2 (high BMI & low As); wheeze, 53.7 (high BMI & high As), 24.2 (high BMI & low As).
Arsenic, BMI and spirometry
FVC and FEV1 spirometry results for participants in various arsenic exposure and BMI categories, stratified by smoking status, are presented in Table 3. In never smokers with BMIs >30 kg/m2, arsenic exposures of 11–200 (n=43) and ≥200 µg/L (n=36) were associated with a 311 ml (95% CI: 54, 569) and 353 ml (95% CI: 88, 618) decrease in FVC, respectively, compared to those never exposed to arsenic concentrations ≥11 µg/L (n=24). Given a median FVC value of 3.65 L in never smokers, this 353 ml decrease represents a 9.7% decrease in FVC. In never smokers with BMIs of 25–30 kg/m2, arsenic exposures of 11–200 (n=36) and ≥200 µg/L (n=94) were also associated with decreases in FVC compared to those never exposed ≥11 µg/L (n=58) although the differences were smaller (189 ml and 138 ml, respectively). Similar results for FVC were seen in analyses including all subjects regardless of spirometry grade (Table 3). Clear associations were not seen for FEV1 (Table S3). In analyses adjusted for age, gender, and height, the arsenic-BMI interaction term for FVC in never smokers had a p-value of 0.04 (data not shown). This p-value was lower when all subjects regardless of spirometry grade were included (p=0.01). Clear evidence of arsenic-BMI interaction was not seen in ever-smokers or for FEV1 (data not shown).
TABLE 3.
Age, height, and sex adjusted forced vital capacity (FVC) residuals (ml) by categories of arsenic water concentrations and BMI.
| BMI 18.5–24.99 kg/m2 |
BMI 25–30 kg/m2
|
BMI >30 kg/m2
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As (µg/L)a | N | ß | 95% CI |
p | N | ß | 95% CI |
p | N | ß | 95% CI |
p | |
| Quality spirometry grade A and B only (N=751)b | Never smokers | (N=87) | (N=188) | (N=103) | |||||||||
| 11–200 | 17 | 227 | −131, 584 | 0.21 | 36 | −189 | −437, 60 | 0.14 | 43 | −311 | −569, −54 | 0.02 | |
| ≥200 | 46 | −18 | −131, 275 | 0.90 | 94 | −138 | −335, 58 | 0.17 | 36 | −353 | −618, −88 | 0.01 | |
| Ever smokers | (N=91) | (N=188) | (N=95) | ||||||||||
| 11–200 | 23 | −55 | −427, 316 | 0.77 | 52 | −137 | −371, 98 | 0.25 | 30 | 76 | −189, 341 | 0.57 | |
| ≥200 | 45 | −46 | −370, 278 | 0.78 | 91 | 91 | −58, 365 | 0.15 | 39 | −52 | −301, 196 | 0.68 | |
| All participants (N=787)c | Never smokers | (N=89) | (N=201) | (N=108) | |||||||||
| 11–200 | 17 | 215 | −145, 576 | 0.24 | 37 | −206 | −452, 40 | 0.10 | 43 | −307 | −560, −53 | 0.02 | |
| ≥200 | 48 | −25 | −314, 263 | 0.86 | 105 | −180 | −372, 12 | 0.07 | 41 | −365 | −620, −109 | 0.01 | |
| Ever smokers | (N=93) | (N=197) | (N=99) | ||||||||||
| 11–200 | 23 | −54 | −422, 313 | 0.77 | 53 | −143 | −375, 92 | 0.23 | 30 | 69 | −194, 333 | 0.60 | |
| ≥200 | 47 | −40 | −358, 278 | 0.80 | 99 | 113 | −94, 320 | 0.28 | 43 | −77 | −320, 166 | 0.53 | |
Abbreviations: As, arsenic; BMI, body mass index; β, linear regression coefficient (values represent the difference in adjusted mean FVC between the arsenic exposure categories shown and the reference category of <11 µg/L); CI, confidence interval
Single year lifetime highest arsenic exposure. Subjects with highest exposures <11 µg/L are used as the reference group
Only includes participants who had at least two acceptable trials in which their FEV1 and FVC differed by less than 150 mL
Includes all participants regardless of their spirometry grade
Additional adjustments for mining work, secondhand smoke exposure, occupational lung irritant exposures, socioeconomic status, diabetes, or typical weekly fruit and vegetable consumption had little impact on spirometry and symptom results (Figure S2 and Table S4). Further excluding individuals with emphysema, bronchitis, and asthma from the analysis do not significantly alter the results presented in Table 2, Table 3 or Figure 2, but did decrease the p-value of the arsenic-BMI interaction term for FVC in never-smokers from 0.04 to 0.01. Using other arsenic exposure metrics including cumulative lifetime exposure, cumulative exposure from ages 0 to 20, or yearly lifetime average did not significantly alter the findings.
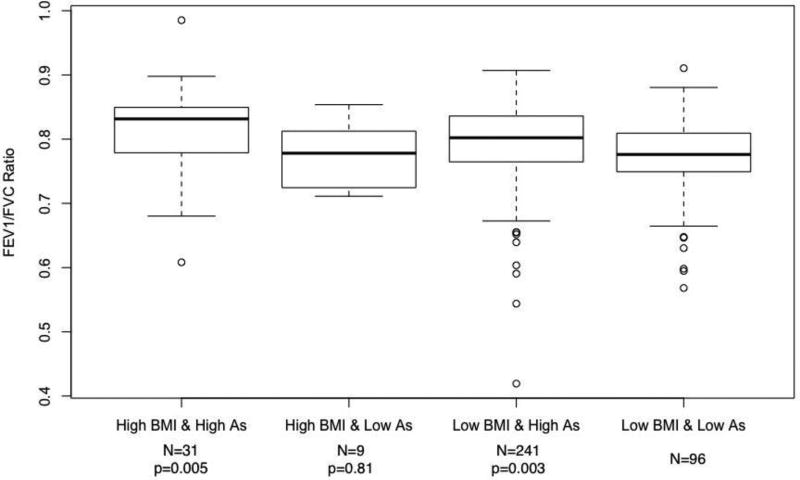
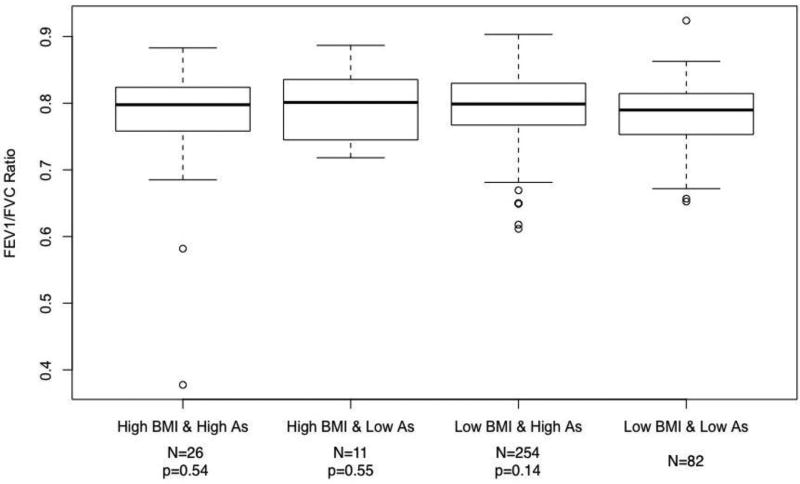
FEV1/FVC ratios for various arsenic-BMI exposure categories are shown in Figure 3 (never smokers) and Figure 4 (smokers). Never-smoking adults with a BMI below the 90th percentile (≤33.9 kg/m2) and a single year highest arsenic exposure below 11 µg/L had a mean ratio of 0.77 (95% CI: 0.76, 0.79). Those with high BMI-low arsenic, low BMI-high arsenic, and high BMI-high arsenic exposures had corresponding ratios of 0.78, 0.79, and 0.81. The p-value for the difference in the mean FEV1/FVC ratio between the low BMI-low arsenic group and the high BMI-high arsenic group was 0.005. In ever smokers, those in the low BMI-low arsenic, high BMI-low arsenic, low BMI-high arsenic, and high BMI-high arsenic groups had FEV1/FVC ratios of 0.79, 0.80, 0.79, and 0.77, respectively. The p-value for the difference between the low BMI-low arsenic group and high BMI-high arsenic group was 0.54.
Figure 3.
Mean FEV1/FVC ratios by categories of BMI and single year lifetime highest arsenic exposure categories in never smokers.
Abbreviations: As, single year lifetime highest arsenic water concentration; BMI, body mass index; FEV1, forced expiratory volume in one second; FVC, forced vital capacity (FVC).
Names along x-axis correspond to categories of arsenic exposure and BMI. Low and high BMI constitutes a BMI below and above the 90th percentile of 33.9 kg/m2, respectively. Low and high arsenic constitutes a single year lifetime highest exposure below and ≥11 µg/L, respectively. All categories were mutually exclusive
Figure 4.
Mean FEV1/FVC ratios by categories of BMI and single year lifetime highest arsenic exposure categories in ever smokers.
Abbreviations: As, single year lifetime highest arsenic water concentration; BMI, body mass index; FEV1, forced expiratory volume in one second; FVC, forced vital capacity (FVC).
Names along x-axis correspond to categories of arsenic exposure and BMI. Low and high BMI constitutes a BMI below and above the 90th percentile of 33.9 kg/m2, respectively. Low and high arsenic constitutes a single year lifetime highest exposure below and ≥11 µg/L, respectively. All categories were mutually exclusive
Discussion
Overall, we identified high ORs for several respiratory symptoms and fairly large decreases in FVC in never-smoking individuals who were exposed to high levels of arsenic and had elevated BMIs. Although synergy indices were not statistically significant, they were above 1.0 and arsenic-associated ORs for SOB, wheeze, and all symptoms combined were markedly higher in subjects with a BMI above the 90th percentile than in those with lower BMIs. Arsenic-associated decrements in FVC were also markedly greater in subjects with higher BMIs, although this relationship was only evident in FVC for never-smokers. We also identified a significant increase in the FEV1/FVC ratio in never-smokers as joint arsenic exposure and BMI increased, which was primarily driven by the decreases in FVC demonstrated in Table 3. Overall, these findings provide preliminary evidence that the combined adverse effects of arsenic and elevated BMI on the lung could be at least additive and possibly greater.
This study is the first to our knowledge to investigate the impact of BMI on lung function and health in arsenic-exposed individuals. Although mechanisms may differ, previous studies have identified somewhat similar associations with other chemical exposures, supporting the idea that the adverse effects of chemical exposures and elevated BMI can be synergistic.64 In our recent study in the same area in Northern Chile, we identified evidence of major synergy (i.e. greater than additive associations) between arsenic exposure and excess BMI for lung and bladder cancer, two outcomes commonly linked with arsenic ingestion.55 In that study, data was available on current BMI, and for BMI based on self-reported height and weights at ages 20 and 40 years. Lung cancer odds ratios for arsenic exposures >800 µg/L were nearly 3-times higher in subjects with a BMI in the upper 90th percentile at age 20 (OR=6.98, 95% CI: 1.84, 26.56) than in those with BMIs below this level (OR=2.31, 95% CI: 1.63, 3.29) (synergy index=4.08, 95% CI: 1.01, 16.4). Even higher odds ratios were seen in subjects whose BMI remained high throughout their adult lives. In the study presented here, data on past BMI were not available so it is unknown whether a similar temporal pattern might occur for non-malignant lung disease. Regardless, the high arsenic-associated respiratory symptom ORs and major arsenic-associated decrements in FVC we identified here in subjects with elevated BMI provide at least some evidence that the overall synergistic relationship we identified for lung cancer might also occur, although possibly to a lesser extent, for non-malignant lung disease.
While the precise mechanism by which arsenic damages the lungs is unknown, arsenic ingestion has been linked to increased levels of inflammatory markers and oxidative stress.65–72 Likewise, obesity also is an established cause of increased inflammation and oxidative stress.33–41,73 More specifically, both arsenic exposure and obesity are known to trigger inflammatory reactions in lung tissue which could lead to additive or possibly even synergistic impacts on lung damage.44,45 In mice, arsenic significantly increased inflammation in overweight mice.74 One or more of many different pathways could be involved. In humans, arsenic exposure may decrease respiratory immune function by suppressing chlorine secretion of epithelial lung tissue.75 Adipocytes specifically respond to inflammatory signals triggered in lung tissue. In particular, lung irritants in mice have been shown to trigger release of interleukin-6 by adipocytes, a cytokine that has been shown to increase metastasis of arsenic-exposed respiratory tissue.67,68,76 Receptors of adiponectin, a cytokine produced by adipose tissue, are expressed in the lungs and play an anti-inflammatory role; however, as more fat tissue accumulates, secretion of adiponectin decreases.77,78 Genetically modified mice unable to produce adiponectin had overall poor lung health, suggesting a link between healthy lung function and the adipocytokine.79 Leptin is another molecule produced by adipocytes, is involved in satiety, energy balance, and fat mass, and is proinflammatory, mitogenic, and antiapoptotic; arsenic has been linked to both increased leptin and to decreased adiponectin levels.49,50,80–82 Overall, these common links between arsenic and BMI on a number of common pathological processes like inflammation or adipocyte expression support the hypothesis that these two factors could produce additive or synergistic effects through a variety of mechanisms. Obesity can also cause a restrictive pattern on spirometry by a purely mechanical pathway. An obese abdomen pushes the diaphragm up and reduces thoracic cavity volume. If arsenic causes decreased lung volumes due to a biological effect on growth of lung function, then obesity could also have an interactive effect with arsenic exposure through a purely mechanical pathway.
Some misclassification of arsenic exposure may have occurred in our study. However, our arsenic exposure data were based primarily on residential history, which can be recalled with good accuracy. Records of arsenic water concentrations covered more than 90% of all person-years in our study subjects, and because arsenic exposure was assessed similarly in all participants, any misclassification of exposure is most likely to have been non-differential and therefore most likely to have biased results towards the null. In addition to water, arsenic may also come from air, food, or workplace exposure. However, arsenic air concentrations were low and similar in all three study cities.83 Because Northern Chile has little rainfall, most food comes from outside the study area where arsenic water concentrations are low.84 As such, arsenic from food is unlikely to have caused major bias in our findings. Misclassification of past diet likely occurred to some extent, but diet was assessed similarly in all participants so this would most likely be non-differential and not likely have caused the positive associations we identified here. Differential recall of diet is possible but we could find no evidence that this would likely cause major bias.
We cannot eliminate the possibility of confounding in our results. For example, the impacts we identified for BMI could be related to some other factor associated with elevated BMI such as diet or an obesity-related disease like diabetes. Adjustments for several factors related to BMI and lung disease including fruit and vegetable intake, occupational exposures to known lung irritants, diabetes, and exposure to second hand smoke had little impact on our results (Table S4 and Figure S2). Specific dietary factors or nutrients were not evaluated here, and their impacts could be evaluated in future research. Air pollution may also impact lung function and increase respiratory symptoms. However, subjects who lived in Santiago were excluded, and air pollution levels (e.g. PM10) are similar in the three study cities and generally below US standards.85
The reason we saw arsenic-associated FVC declines in never-smokers but not in smokers is unknown. It may be the toxic effects of smoking masked those due to arsenic but this is speculative. Several studies have identified synergy between smoking and arsenic for lung cancer but evidence for similar effects for non-malignant lung disease is less consistent.54,86,87 For example, in the large Health Effects of Arsenic Longitudinal Study in Bangladesh, arsenic-associated FVC declines were similar in smokers and non-smokers.26 Further research is needed to more clearly delineate the combined impacts of smoking and arsenic on non-malignant lung disease.
Overall, we found evidence that adults exposed to arsenic with an increased BMI are at an elevated risk for non-malignant respiratory symptoms and overall poorer lung function. Furthermore, our study also suggests that arsenic and obesity could interact, and is the first study to specifically investigate the impact of coupled arsenic exposure and elevated BMI on lung function and health. Lastly, we also observed the highest FEV1/FVC ratios in those with the highest combined arsenic exposure and BMI in never-smokers. Because the results here are novel and the statistical power to evaluate interaction was mostly low, they should be considered preliminary. In addition, our goal was to assess long-term impacts of arsenic, so any impacts occurring in the past that may have resolved or any impacts occurring in children could not be assessed here. Future research involving larger sample sizes, detailed data on past BMI, and markers of potential mechanisms like inflammation and oxidative stress could help clarify the issues we’ve studied here. Given the globally rising rates of obesity and widespread arsenic exposure worldwide identifying risk factors for arsenic-related damage such as BMI could have important and far-reaching public health implications.
Supplementary Material
Highlights.
Arsenic and high BMI both impact inflammation, oxidative stress and immune function.
The human lung is particularly susceptible to arsenic.
Those with elevated BMIs have very high arsenic-associated risks of lung symptoms.
Those with elevated BMIs have higher arsenic-associated declines in lung function.
Preliminary evidence of arsenic-BMI synergy was identified for lung disease.
Acknowledgments
Funding Sources: National Institute of Environmental Health Sciences grants R01ES017463, R01ES014032, and P42ES04705
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Naujokas MF, Anderson B, Ahsan H, et al. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect. 2013;121(3):295–302. doi: 10.1289/ehp.1205875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith AH, Goycolea M, Haque R, Biggs ML. Marked Increase in Bladder and Lung Cancer Mortality in a Region of Northern Chile Due to Arsenic in Drinking Water. Am J Epidemiol. 1998;147(7):660–669. doi: 10.1093/oxfordjournals.aje.a009507. [DOI] [PubMed] [Google Scholar]
- 3.Hopenhayn-Rich C, Biggs ML, Smith AH. Lung and kidney cancer mortality associated with arsenic in drinking water in Córdoba, Argentina. [Accessed January 9, 2016];Int J Epidemiol. 1998 27(4):561–569. doi: 10.1093/ije/27.4.561. http://www.ncbi.nlm.nih.gov/pubmed/9758107. [DOI] [PubMed] [Google Scholar]
- 4.Chen CJ, Chen CW, Wu MM, Kuo TL. Cancer potential in liver, lung, bladder and kidney due to ingested inorganic arsenic in drinking water. [Accessed February 13, 2016];Br J Cancer. 1992 66(5):888–892. doi: 10.1038/bjc.1992.380. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1977977&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tseng WP. Effects and dose--response relationships of skin cancer and blackfoot disease with arsenic. [Accessed February 13, 2016];Environ Health Perspect. 1977 19:109–119. doi: 10.1289/ehp.7719109. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1637425&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benbrahim-Tallaa L, Waalkes MP. Inorganic arsenic and human prostate cancer. Environ Health Perspect. 2008;116(2):158–164. doi: 10.1289/ehp.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuda T, Babazono A, Yamamoto E, et al. Ingested Arsenic and Internal Cancer: A Historical Cohort Study Followed for 33 Years. [Accessed February 13, 2016];Am J Epidemiol. 1995 141(3):198–209. doi: 10.1093/oxfordjournals.aje.a117421. http://aje.oxfordjournals.org/content/141/3/198.short. [DOI] [PubMed] [Google Scholar]
- 8.Maull EA, Ahsan H, Edwards J, et al. Evaluation of the association between arsenic and diabetes: a National Toxicology Program workshop review. Environ Health Perspect. 2012;120(12):1658–1670. doi: 10.1289/ehp.1104579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CJ, Chiou HY, Chiang MH, Lin LJ, Tai TY. Dose-Response Relationship Between Ischemic Heart Disease Mortality and Long-term Arsenic Exposure. Arterioscler Thromb Vasc Biol. 1996;16(4):504–510. doi: 10.1161/01.ATV.16.4.504. [DOI] [PubMed] [Google Scholar]
- 10.Arsenic, Metals, Fibres, and Dusts. Vol. 100. International Agency for Research on Cancer; 2012. [Accessed June 3, 2016]. IARC Working Group on the Evaluation of Carcinogenic Risk. http://www.ncbi.nlm.nih.gov/books/NBK304375/ [Google Scholar]
- 11.Schulman AE. [Accessed February 13, 2016];Arsenic Occurrence in Public Drinking Water Supplies. 2000 https://nepis.epa.gov/Exe/ZyNET.exe/P1004W96.TXT?ZyActionD=ZyDocument&Client=EPA&Index=2000+Thru+2005&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5Czyfiles%5CIndex%20Data%5C00thru05%5CTxt%5C00000021%5CP1004W96.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL.
- 12.The World Health Organization. [Accessed February 13, 2016];WHO | Arsenic. http://www.who.int/mediacentre/factsheets/fs372/en/
- 13.National Research Council. Arsenic in Drinking Water. Washington, DC: National Academies Press; 2001. [DOI] [Google Scholar]
- 14.Smith AH, Lopipero PA, Bates MN, Steinmaus CM. Public health. Arsenic epidemiology and drinking water standards. Science. 2002;296(5576):2145–2146. doi: 10.1126/science.1072896. [DOI] [PubMed] [Google Scholar]
- 15.US EPA National Center for Environmental Assessment IO, Sams R. [Accessed February 13, 2016];IRIS Toxicological Review of Inorganic Arsenic (Cancer) (2010 External Review Draft). http://cfpub.epa.gov/ncea/iris_drafts/recordisplay.cfm?deid=219111.
- 16.Smith AH, Steinmaus CM. Health effects of arsenic and chromium in drinking water: recent human findings. Annu Rev Public Health. 2009;30:107–122. doi: 10.1146/annurev.publhealth.031308.100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Council NR. Critical Aspects of EPA’s IRIS Assessment of Inorganic Arsenic. Washington, DC: National Academies Press; 2014. [DOI] [Google Scholar]
- 18.Sanchez TR, Perzanowski M, Graziano JH. Inorganic arsenic and respiratory health, from early life exposure to sex-specific effects: A systematic review. Environ Res. 2016;147:537–555. doi: 10.1016/j.envres.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazumder DNG, Haque R, Ghosh N, et al. Arsenic in drinking water and the prevalence of respiratory effects in West Bengal, India. Int J Epidemiol. 2000;29(6):1047–1052. doi: 10.1093/ije/29.6.1047. [DOI] [PubMed] [Google Scholar]
- 20.von Ehrenstein OS, Mazumder DNG, Yuan Y, et al. Decrements in lung function related to arsenic in drinking water in West Bengal, India. Am J Epidemiol. 2005;162(6):533–541. doi: 10.1093/aje/kwi236. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh A. Evaluation of chronic arsenic poisoning due to consumption of contaminated ground water in West Bengal, India. [Accessed February 14, 2016];Int J Prev Med. 2013 4(8):976–979. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3775178&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- 22.Smith AH, Yunus M, Khan AF, et al. Chronic respiratory symptoms in children following in utero and early life exposure to arsenic in drinking water in Bangladesh. Int J Epidemiol. 2013;42(4):1077–1086. doi: 10.1093/ije/dyt120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das D, Bindhani B, Mukherjee B, et al. Chronic low-level arsenic exposure reduces lung function in male population without skin lesions. Int J Public Health. 2014;59(4):655–663. doi: 10.1007/s00038-014-0567-5. [DOI] [PubMed] [Google Scholar]
- 24.Nafees AA, Kazi A, Fatmi Z, Irfan M, Ali A, Kayama F. Lung function decrement with arsenic exposure to drinking groundwater along River Indus: a comparative cross-sectional study. Environ Geochem Health. 2011;33(2):203–216. doi: 10.1007/s10653-010-9333-7. [DOI] [PubMed] [Google Scholar]
- 25.Parvez F, Chen Y, Brandt-Rauf PW, et al. A prospective study of respiratory symptoms associated with chronic arsenic exposure in Bangladesh: findings from the Health Effects of Arsenic Longitudinal Study (HEALS) Thorax. 2010;65(6):528–533. doi: 10.1136/thx.2009.119347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parvez F, Chen Y, Yunus M, et al. Arsenic exposure and impaired lung function. Findings from a large population-based prospective cohort study. Am J Respir Crit Care Med. 2013;188(7):813–819. doi: 10.1164/rccm.201212-2282OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dauphiné DC, Ferreccio C, Guntur S, et al. Lung function in adults following in utero and childhood exposure to arsenic in drinking water: preliminary findings. Int Arch Occup Environ Health. 2011;84(6):591–600. doi: 10.1007/s00420-010-0591-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinmaus C, Ferreccio C, Acevedo J, et al. High risks of lung disease associated with early-life and moderate lifetime arsenic exposure in northern Chile. Toxicol Appl Pharmacol. 2016;313:10–15. doi: 10.1016/j.taap.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ranu H, Wilde M, Madden B. Pulmonary function tests. [Accessed June 24, 2016];Ulster Med J. 2011 80(2):84–90. http://www.ncbi.nlm.nih.gov/pubmed/22347750. [PMC free article] [PubMed] [Google Scholar]
- 30.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 31.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49(11):1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114(12):1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aviv A. Telomeres and human aging: facts and fibs. Sci Aging Knowledge Environ. 2004;2004(51):pe43. doi: 10.1126/sageke.2004.51.pe43. [DOI] [PubMed] [Google Scholar]
- 35.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115(5):911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 36.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132(6):2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 37.Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6(6):399–409. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]
- 38.Pischon N, Heng N, Bernimoulin J-P, Kleber B-M, Willich SN, Pischon T. Obesity, Inflammation, and Periodontal Disease. J Dent Res. 2007;86(5):400–409. doi: 10.1177/154405910708600503. [DOI] [PubMed] [Google Scholar]
- 39.de Heredia FP, Gómez-Martínez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. 2012;71(2):332–338. doi: 10.1017/S0029665112000092. [DOI] [PubMed] [Google Scholar]
- 40.Belkina AC, Denis GV. BET domain co-regulators in obesity, inflammation and cancer. Nat Rev Cancer. 2012;12(7):465–477. doi: 10.1038/nrc3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization. [Accessed February 15, 2016];WHO | Obesity and overweight. http://www.who.int/mediacentre/factsheets/fs311/en/ Published 2016.
- 43.Secretan BL, Ph D, Scoccianti C, Ph D, Loomis D, Ph D. Body Fatness and Cancer — Viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Research Council. Subcommittee on Arsenic in Drinking Water. 1999:229–5. https://www.ncbi.nlm.nih.gov/pubmed/25101451.
- 45.Mancuso P, Alexeeff S, Litonjua A, et al. Obesity and lung inflammation. J Appl Physiol. 2010;108(3):722–728. doi: 10.1152/japplphysiol.00781.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jarvis D, Chinn S, Potts J, Burney P European Community Respiratory Health Survey. Association of body mass index with respiratory symptoms and atopy: results from the European Community Respiratory Health Survey. [Accessed October 27, 2016];Clin Exp Allergy. 2002 32(6):831–837. doi: 10.1046/j.1365-2222.2002.01380.x. http://www.ncbi.nlm.nih.gov/pubmed/12047427. [DOI] [PubMed] [Google Scholar]
- 47.Thomas T, Burguera B, Melton LJ, et al. Relationship of serum leptin levels with body composition and sex steroid and insulin levels in men and women. Metabolism. 2000;49(10):1278–1284. doi: 10.1053/meta.2000.9519. [DOI] [PubMed] [Google Scholar]
- 48.Mantzoros CS, Moschos S, Avramopoulos I, et al. Leptin Concentrations in Relation to Body Mass Index and the Tumor Necrosis Factor-α System in Humans. J Clin Endocrinol Metab. 1997;82(10):3408–3413. doi: 10.1210/jcem.82.10.4323. [DOI] [PubMed] [Google Scholar]
- 49.Gossai A, Lesseur C, Farzan S, Marsit C, Karagas MR, Gilbert-Diamond D. Association between maternal urinary arsenic species and infant cord blood leptin levels in a New Hampshire Pregnancy Cohort. Environ Res. 2015;136:180–186. doi: 10.1016/j.envres.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmed S, Mahabbat-e Khoda S, Rekha RS, et al. Arsenic-Associated Oxidative Stress, Inflammation, and Immune Disruption in Human Placenta and Cord Blood. Environ Health Perspect. 2010;119(2):258–264. doi: 10.1289/ehp.1002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McKay CP, Friedmann EI, Gómez-Silva B, Cáceres-Villanueva L, Andersen DT, Landheim R. Temperature and Moisture Conditions for Life in the Extreme Arid Region of the Atacama Desert: Four Years of Observations Including the El Niño of 1997–1998. Astrobiology. 2003;3(2):393–406. doi: 10.1089/153110703769016460. [DOI] [PubMed] [Google Scholar]
- 52.Schwerdtfeger W. Climates of central and South America. Q J R Meteorol Soc. 1976;103(532):219–220. doi: 10.1002/qj.49710343520. [DOI] [Google Scholar]
- 53.Benner SA, Devine KG, Matveeva LN, et al. The missing organic molecules on Mars. Proc Natl Acad Sci. 2000;97(6):2425–2430. doi: 10.1073/pnas.040539497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferreccio C, González C, Milosavjlevic V, Marshall G, Sancha AM, Smith AH. Lung cancer and arsenic concentrations in drinking water in Chile. [Accessed June 20, 2016];Epidemiology. 2000 11(6):673–679. doi: 10.1097/00001648-200011000-00010. http://www.ncbi.nlm.nih.gov/pubmed/11055628. [DOI] [PubMed] [Google Scholar]
- 55.Steinmaus C, Castriota F, Ferreccio C, et al. Obesity and excess weight in early adulthood and high risks of arsenic-related cancer in later life. Environ Res. 2015;142:594–601. doi: 10.1016/j.envres.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ostro B, Sanchez JM, Aranda C, Eskeland GS. Air pollution and mortality: results from a study of Santiago, Chile. [Accessed April 18, 2017];J Expo Anal Environ Epidemiol. 1996 6(1):97–114. http://www.ncbi.nlm.nih.gov/pubmed/8777376. [PubMed] [Google Scholar]
- 57.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess Deaths Associated With Underweight, Overweight, and Obesity. JAMA. 2005;293(15):1861. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 58.Cotes JE. Medical Research Council Questionnaire on Respiratory Symptoms (1986) [Accessed June 20, 2016];Lancet (London, England) 1987 2(8566):1028. doi: 10.1016/s0140-6736(87)92593-1. http://www.ncbi.nlm.nih.gov/pubmed/2889937. [DOI] [PubMed] [Google Scholar]
- 59.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 60.Newall C, McCauley TM, Shakespeare J, Cooper BG. Is it necessary to use a noseclip in the performance of spirometry using a wedge bellows device? Chron Respir Dis. 2007;4(1):53–57. doi: 10.1177/1479972306072889. [DOI] [PubMed] [Google Scholar]
- 61.Borgoño JM, Vicent P, Venturino H, Infante A. Arsenic in the drinking water of the city of Antofagasta: epidemiological and clinical study before and after the installation of a treatment plant. [Accessed February 15, 2016];Environ Health Perspect. 1977 19:103–105. doi: 10.1289/ehp.19-1637404. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1637404&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burchfiel CM, Marcus EB, Curb JD, et al. Effects of smoking and smoking cessation on longitudinal decline in pulmonary function. Am J Respir Crit Care Med. 1995;151(6):1778–1785. doi: 10.1164/ajrccm.151.6.7767520. [DOI] [PubMed] [Google Scholar]
- 63.Andersson T, Alfredsson L, Källberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20(7):575–579. doi: 10.1007/s10654-005-7835-x. [DOI] [PubMed] [Google Scholar]
- 64.Weichenthal S, Hoppin JA, Reeves F. Obesity and the cardiovascular health effects of fine particulate air pollution. Obesity (Silver Spring) 2014;22(7):1580–1589. doi: 10.1002/oby.20748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu MM, Chiou HY, Ho IC, Chen CJ, Lee T-C. Gene expression of inflammatory molecules in circulating lymphocytes from arsenic-exposed human subjects. [Accessed February 1, 2016];Environ Health Perspect. 2003 111(11):1429–1438. doi: 10.1289/ehp.6396. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1241636&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dutta K, Prasad P, Sinha D. Chronic low level arsenic exposure evokes inflammatory responses and DNA damage. Int J Hyg Environ Health. 2015;218(6):564–574. doi: 10.1016/j.ijheh.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 67.Qi Y, Zhang M, Li H, et al. Autophagy inhibition by sustained overproduction of IL6 contributes to arsenic carcinogenesis. Cancer Res. 2014;74(14):3740–3752. doi: 10.1158/0008-5472.CAN-13-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu S, Sun Q, Wang F, et al. Arsenic induced overexpression of inflammatory cytokines based on the human urothelial cell model in vitro and urinary secretion of individuals chronically exposed to arsenic. Chem Res Toxicol. 2014;27(11):1934–1942. doi: 10.1021/tx5002783. [DOI] [PubMed] [Google Scholar]
- 69.Andrew AS, Jewell DA, Mason RA, Whitfield ML, Moore JH, Karagas MR. Drinking-water arsenic exposure modulates gene expression in human lymphocytes from a U.S. population. Environ Health Perspect. 2008;116(4):524–531. doi: 10.1289/ehp.10861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fujino Y, Guo X, Liu J, et al. Chronic arsenic exposure and urinary 8-hydroxy-2’-deoxyguanosine in an arsenic-affected area in Inner Mongolia, China. J Expo Anal Environ Epidemiol. 2005;15(2):147–152. doi: 10.1038/sj.jea.7500381. [DOI] [PubMed] [Google Scholar]
- 71.Weimann A, Belling D, Poulsen HE. Quantification of 8-oxo-guanine and guanine as the nucleobase, nucleoside and deoxynucleoside forms in human urine by high-performance liquid chromatography-electrospray tandem mass spectrometry. [Accessed February 15, 2016];Nucleic Acids Res. 2002 30(2):E7. doi: 10.1093/nar/30.2.e7. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=99846&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jomova K, Jenisova Z, Feszterova M, et al. Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol. 2011;31(2):95–107. doi: 10.1002/jat.1649. [DOI] [PubMed] [Google Scholar]
- 73.Valdes AM, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. Lancet (London, England) 2005;366(9486):662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 74.Tan M, Schmidt RH, Beier JI, et al. Chronic subhepatotoxic exposure to arsenic enhances hepatic injury caused by high fat diet in mice. Toxicol Appl Pharmacol. 2011;257(3):356–364. doi: 10.1016/j.taap.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bomberger JM, Coutermarsh BA, Barnaby RL, Stanton BA. Arsenic promotes ubiquitinylation and lysosomal degradation of cystic fibrosis transmembrane conductance regulator (CFTR) chloride channels in human airway epithelial cells. J Biol Chem. 2012;287(21):17130–17139. doi: 10.1074/jbc.M111.338855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lang JE, Williams ES, Mizgerd JP, Shore SA. Effect of obesity on pulmonary inflammation induced by acute ozone exposure: role of interleukin-6. Am J Physiol Lung Cell Mol Physiol. 2008;294(5):L1013–20. doi: 10.1152/ajplung.00122.2007. [DOI] [PubMed] [Google Scholar]
- 77.Shore SA. Obesity and asthma: Possible mechanisms. J Allergy Clin Immunol. 2008;121(5):1087–1093. doi: 10.1016/j.jaci.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 78.Koerner A, Kratzsch J, Kiess W, et al. Adipocytokines: leptin—the classical, resistin—the controversical, adiponectin—the promising, and more to come. Best Pract Res Clin Endocrinol Metab. 2005;19(4):525–546. doi: 10.1016/j.beem.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 79.Summer R, Little FF, Ouchi N, et al. Alveolar macrophage activation and an emphysema-like phenotype in adiponectin-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2008;294(6):L1035–42. doi: 10.1152/ajplung.00397.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Booth A, Magnuson A, Fouts J, Foster M. Adipose tissue, obesity and adipokines: role in cancer promotion. Horm Mol Biol Clin Investig. 2015;21(1):57–74. doi: 10.1515/hmbci-2014-0037. [DOI] [PubMed] [Google Scholar]
- 81.Ntikoudi E, Kiagia M, Boura P, Syrigos KN. Hormones of adipose tissue and their biologic role in lung cancer. Cancer Treat Rev. 2014;40(1):22–30. doi: 10.1016/j.ctrv.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 82.Huang CF, Yang CY, Chan DC, et al. Arsenic Exposure and Glucose Intolerance/Insulin Resistance in Estrogen-Deficient Female Mice. Environ Health Perspect. 2015;123(11):1138–1144. doi: 10.1289/ehp.1408663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ferreccio C, Sancha AM. Arsenic exposure and its impact on health in Chile. [Accessed January 16, 2017];J Health Popul Nutr. 2006 24(2):164–175. http://www.ncbi.nlm.nih.gov/pubmed/17195557. [PubMed] [Google Scholar]
- 84.Vera R. Country Pasture/Forage Resource Profile: Chile. [Accessed March 15, 2017];Food and Agricultural Organization. http://www.fao.org/ag/agp/agpc/doc/counprof/chile/cile.htm#land. Published 2006.
- 85.Cifuentes LA, Sauma E, Jorquera H, Soto F. Preliminary estimation of the potential co-control benefits for Chile. Integr Environ Strateg. 1999 Nov [Google Scholar]
- 86.Chen CL, Hsu LI, Chiou HY, et al. Ingested Arsenic, Cigarette Smoking, and Lung Cancer Risk. JAMA. 2004;292(24):2984. doi: 10.1001/jama.292.24.2984. [DOI] [PubMed] [Google Scholar]
- 87.Ferreccio C, Yuan Y, Calle J, et al. Arsenic, Tobacco Smoke, and Occupation. Epidemiology. 2013;24(6):898–905. doi: 10.1097/EDE.0b013e31829e3e03. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.