Abstract
High-density lipoproteins (HDL, or “good cholesterol”) are heterogeneous nanoparticles that remove excess cell cholesterol and protect against atherosclerosis. The cardioprotective action of HDL and its major protein, apolipoprotein A-I (apoA-I), is well-established, yet the function of the second major protein, apolipoprotein A-II (apoA-II), is less clear. In this review, we postulate an ensemble of apolipoprotein conformations on various HDL. This ensemble is based on the crystal structure of Δ(185–243)apoA-I determined by Mei and Atkinson combined with the “double-hairpin” conformation of apoA-IIdimer proposed in the cross-linking studies by Silva’s team, and is supported by the wide array of low-resolution structural, biophysical, and biochemical data obtained by many teams over decades. The proposed conformational ensemble helps integrate and improve several existing HDL models, including the “buckle-belt” conformation of apoA-I on the midsize disks and the “trefoil/tetrafoil” arrangement on spherical HDL. This ensemble prompts us to hypothesize that endogenous apoA-II (i) helps confer lipid surface curvature during conversion of nascent discoidal HDL(A-I) and HDL(A-II) containing either apoA-I or apoA-II to mature spherical HDL(A-I/A-II) containing both proteins, and (ii) hinders remodeling of HDL(A-I/A-II) by hindering the expansion of the apoA-I conformation. Also, we report that, although endogenous apoA-II circulates mainly on the midsize spherical HDL(A-I/A-II), exogenous apoA-II can bind to HDL of any size, thereby slightly increasing this size and stabilizing the HDL assembly. This suggests distinctly different effects of the endogenous and exogenous apoA-II on HDL. Taken together, the existing results and models prompt us to postulate a new structural and functional role of apoA-II on human HDL.
Graphical Abstract
High-density lipoproteins (HDL, or “good cholesterol”) are noncovalent assemblies of lipids and specific proteins (termed apolipoproteins) that remove excess cell cholesterol via the reverse cholesterol transport (RCT) pathway and protect from inflammation, oxidation, and thrombosis.1–7 Plasma HDL form subclasses differing in particle shape (nascent discoidal or mature spherical), diameter (d = 7.5–11.5 nm), composition, and functional properties.3,6–8 This heterogeneity reflects remodeling of HDL by plasma factors, which is key to HDL metabolism.9,10 Although the inverse correlation between the plasma levels of HDL and the risk of atherosclerosis has long been established, the current consensus is that both the quantity and the quality of HDL are important for cardioprotection.3,6,11,12 Thus, the efficiency of RCT in preventing atherosclerosis depends not only on the steady-state level of plasma HDL but also on the rate of cholesterol flux from arterial macrophages to the liver for excretion via bile.3 This rate depends upon the structural, stability, and functional properties of HDL subclasses. Elucidating factors that modulate these properties is the current thrust in the search for new HDL-based biomarkers and therapies for atherosclerosis. 6,7,12–14 One such important factor is HDL protein composition.6–8,15
Apolipoproteins, which are located in a cholesterol-containing phospholipid monolayer on the particle surface, confer structural integrity to HDL assembly and direct its metabolism by binding to lipid transporters, lipid transfer proteins, lipophilic enzymes, and lipoprotein receptors.4 The major HDL proteins, apoA-I (243 amino acids) and apoA-II (two 77-amino acid molecules forming a homodimer in humans disulfide-linked via Cys6), comprise ~70% and ~20% of the total HDL protein, respectively, with an average apoA-I:apoA-II molar ratio of 2:1.15–18 Amino acid sequences of these and other exchangeable (water-soluble) apolipoproteins contain 11/22-mer tandem repeats with a strong propensity to form amphipathic α-helices whose large apolar faces are optimized for lipid surface binding.19,20 Most plasma apoA-I and apoA-II circulate on two HDL subclasses, HDL(A-I) that contains apoA-I but no apoA-II and HDL(A-I/A-II) that contains both apoA-I and apoA-II.15,21 The cardioprotective action of apoA-I is well-established and is largely due to its key role in RCT,1–3,22 yet the role of apoA-II is less clear. Mouse model studies report that apoA-II can be pro- or anti-atherogenic depending on the model, and epidemiologic studies ascribe pro- or anti-atherogenic properties to apoA-II. 16–18,23 The consensus is that apoA-II, which is more hydrophobic than apoA-I, is more strongly associated with HDL and modulates HDL metabolism by displacing apoA-I or altering its conformation.15,18,24–27 These and other effects of apoA-II15,28,29 suggest that it modulates lipid metabolism via multiple pathways whose molecular origins are unclear and depend, in part, on the structure and function of apoA-I on HDL(A-I/A-II).25,27
BIOGENESIS AND FUNCTIONAL PROPERTIES OF APOA-II-CONTAINING HDL
At the first rate-limiting step of RCT, apoA-I interacts with the ATP-binding cassette transporter, ABCA1, on the cell surface to form nascent HDL(A-I).12 These discoidal particles are thought to comprise a cholesterol-containing phospholipid bilayer, with two copies of apoA-I wrapped around the perimeter in a largely α-helical antiparallel “double-belt” conformation.4,30–32 ApoA-I on nascent HDL(A-I) activates lecithin:cholesterol acyltransferase (LCAT) whose apolar products, cholesterol esters (CE), move from the surface to the core of the particle, converting it to a mature corecontaining spherical HDL. ApoA-II is secreted on small discoidal HDL(A-II) particles33 that contain two copies of apoA-IIdimer wrapped around the perimeter in a highly α-helical antiparallel conformation that is proposed to form a “double hairpin”.27 Although nascent HDL(A-II) particles are only ~1% as efficient as HDL(A-I) in activating LCAT, they contribute to HDL maturation by rapidly fusing with HDL(A-I) to form spherical HDL(A-I/A-II).34,35 Fusion of discoidal HDL(A-II) with HDL(A-I) is mediated by the action of LCAT on HDL(A-I). The resulting mature HDL(A-I/A-II) particles contain two copies of apoA-I and one to three copies of apoA-IIdimer and carry >95% of the total plasma apoA-II.36 Alternatively, fusion of discoidal HDL(A-I) via the LCAT reaction can produce spherical HDL(A-I) particles that contain two to five copies of apoA-I36 probably arranged in a “trefoil/tetrafoil” fashion.37,38
HDL remodeling during RCT is essential for lipid metabolism and depends critically on the interactions of apoA-I with its ligands.39 Such remodeling involves lipid transfer and lipolysis, apolipoprotein adsorption and desorption, HDL fusion, etc., and is distinct for HDL(A-I) and HDL(A-I/A-II).26,27,39–43 Compared to HDL(A-I) particles, HDL(A-I/A-II) particles are hydrolyzed less readily by hepatic lipase24,41 but more readily by endothelial lipase, presumably because of a specific apoA-I conformation that activates this lipase.27 Furthermore, HDL(A-I/A-II) particles are less prone to remodeling by phospholipid and cholesterol ester transfer proteins.42–44 At the final step of RCT, cholesterol ester and other core lipids are taken up by the hepatic scavenger receptor, SR-BI, leading to HDL disintegration. HDL(A-I/A-II) particles are less efficient than HDL(A-I) particles in mediating cholesterol ester uptake by the receptor.45–47 The structural basis underlying these functional differences is unknown.
The trend emerging from these studies is that human HDL(A-I/A-II) particles are more resistant to metabolic remodeling than HDL(A-I) particles.16,39,40,47 This is supported by the observation that human plasma HDL(A-I/A-II) particles are much more homogeneous in size and composition than HDL(A-I) particles; this prompted Silva’s team to propose that apoA-II modulates RCT by locking apoA-I in a conformation compatible only with the midsize HDL (d ~ 9–10 nm) and thereby indirectly affecting interactions of apoA-I with its ligands.36 The structural details of apoA-I and apoA-II on HDL(A-I/A-II) are unknown because of the paucity of high-resolution structural information about these flexible hydrophobic proteins.
In this review, we postulate a conformational ensemble of apoA-I on various plasma HDL, including the midsize spherical human HDL(A-I/A-II). As a starting model, we use the X-ray crystal structure of the C-terminally truncated lipid-free human apoA-I, Δ(185–243)apoA-I, recently determined by Mei and Atkinson to 2.2 Å resolution,48 together with the low-resolution model of human apoA-IIdimer proposed in the cross-linking studies by Silva and colleagues.36 The conformations of apoA-I on HDL are derived on the basis of the assumptions that (i) the apoA-I dimer forms a closed double belt on HDL (refs 31, 48, and 52 and references therein), (ii) this double belt undergoes minimal structural rearrangements upon accommodation to HDL of various shapes and sizes, thereby minimizing free energy barriers separating these HDL,10,49,50 and (iii) these rearrangements are facilitated by flexible Gly-containing hinge regions. The resulting structural model is correlated with numerous cross-linking, spectroscopic, electron microscopic, biochemical, and other experimental data obtained from various HDL by many teams over the years. Furthermore, we analyze human HDL subclasses that have been enriched with apoA-II to test whether binding of exogenous apoA-II affects HDL size and stability. Thermal denaturation is used as an experimental model that mimics key aspects of the metabolic HDL remodeling, including apolipoprotein dissociation and HDL fusion, rupture, and release of apolar lipids.10,49 The results suggest a simple structural basis underlying some of the complex metabolic effects of apoA-II on HDL.
ENDOGENOUS AND EXOGENOUS APOA-II HAS DISTINCT EFFECTS ON HDL STRUCTURE
Human plasma HDL were separated into five fractions on the basis of particle size or density as described in the Supporting Information (Figures S1 and S3). Each fraction that was either native or enriched with various amounts of exogenous apoA-II was analyzed for structure and stability. Earlier we showed that thermal stability of human HDL correlates inversely with particle diameter,50 yet the difference between the size-matched HDL(A-I) and HDL(A-I/A-II) was unclear because apoA-II comprises only ~20% of the HDL protein.15–18 In an attempt to amplify the effects of apoA-II on HDL structure and stability, HDL fractions were incubated with apoA-II using 1:1 to 1:4 weight ratios of excess apoA-II to total HDL protein. The results of these studies are consistent with the earlier work and are reported in Figures S1–S6 of the Supporting Information. Our main findings are that, even though human apoA-II circulates mainly on the midsize spherical HDL (Figures S1 and S3 of the Supporting Information), exogenous apoA-II can bind to HDL of any diameter (Figure S4 of the Supporting Information). Such binding produces no large changes in the particle size distribution and, hence, no lipid repacking into larger and/or smaller particles (Figure S4 of the Supporting Information). However, apoA-II binding stabilizes spherical HDL against fusion and release of core lipids (Figures S5 and S6 of the Supporting Information), which probably reflects the high hydrophobicity of apoA-II compared to that of apoA-I (Figure S7 of the Supporting Information).
We speculate that apoA-II enrichment can stabilize HDL via two mechanisms. First, additional apoA-II that adsorbs to the HDL surface [which manifests itself as a small increase in the particle size observed by gel filtration (Figure S4 of the Supporting Information)] is expected to seal the hydrophobic packing defects, thereby hampering HDL rupture and release of core lipids. In addition, deep penetration of the apoA-II α- helices into the surface monolayer (Figure S7 of the Supporting Information) can help stabilize spherical HDL.
These effects of exogenous apoA-II are distinct from the role of endogenous apoA-II in native HDL. Our results (Figure S3 of the Supporting Information) agree with the work by Silva’s team showing that apoA-II circulates mainly on the midsize mature HDL(A-I/A-II) particles (d ≅ 9–10.5 nm) that are much more homogeneous in size and composition than HDL(A-I) particles.36 These findings, together with the well-established inhibitory effects of apoA-II on HDL remodeling by plasma factors,26,27,34,39,41,42,45 prompted Silva’s team to propose that endogenous apoA-II locks apoA-I in a conformation that is compatible only with the midsize HDL.36 This hypothesis appears to be at odds with the demonstrated ability of exogenous apoA-II to bind to HDL of any diameter (Figure S4 of the Supporting Information) and thereby induce conformational changes in apoA-I and, eventually, displace apoA-I from HDL.15,18,24–27 To resolve this apparent contradiction, we postulate that endogenous and exogenous apoA-II have distinctly different effects on HDL structure, and propose a structural basis for these effects.
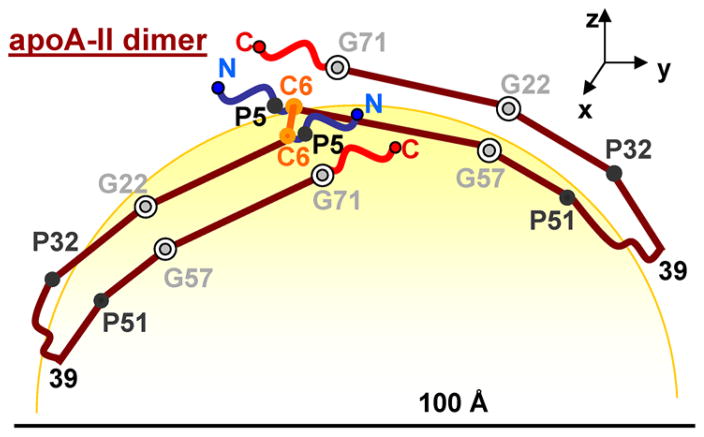
First, we speculate that binding of exogenous apoA-II to HDL with various radii (Figure S4 of the Supporting Information) is probably augmented by the flexibility of the protein molecule that may result, in part, from the concerted motion around the aligned Gly hinges and Pro kinks in the antiparallel helices in the double-hairpin conformation of apoA-IIdimer on HDL (Figure 1).27 Second, because apoA-I alone can form spherical HDL(A-I) of various diameters in vivo and in vitro,3,36 the formation of homogeneous midsize spherical HDL(A-I/A-II) in vivo must involve synergistic action of apoA-I and apoA-IIdimer. Third, because binding of exogenous apoA-II does not confer the medium size to human HDL (Figure S4 of the Supporting Information), such synergy must occur during, rather than after, HDL formation. The latter is consistent with earlier studies showing that HDL assembly is kinetically trapped10,49–51 and, hence, depends upon the pathway of HDL formation. A pathway that generates almost exclusively midsize spherical HDL(A-I/A-II) in vivo and in vitro34,36 is the LCAT reaction that induces fusion of nascent discoidal HDL(A-I) with HDL(A-II) to generate mature spherical HDL(A-I/A-II).34 Below, we postulate apoA-I conformations at several key steps in this pathway and propose a hypothetical molecular mechanism for the synergistic action of apoA-I and apoA-II on HDL.
Figure 1.
Cartoon illustrating the possible conformation of apoA-IIdimer on the HDL surface, which is based on the double-hairpin model proposed in the protein cross-linking and MS studies of human apoA-II on rHDL.27 Line segments show predicted amphipathic α-helices (residues 9–30, 39–50, and 51–70); wavy lines show predicted irregular structure.27 The intermolecular disulfide link via Cys6 is colored orange. The positions of Gly and Pro are indicated. The possible alignment of Gly22 with Gly57 and of Pro32 with Pro51 suggests that the helical kinks at these positions may help confer a curved shape to the apoA-II molecule on the HDL surface. The yellow area is the HDL surface.
CONFORMATIONAL ENSEMBLE OF APOA-I ON HDL OF VARIOUS SIZES
To envision the arrangement of apoA-I on HDL, we took advantage of the modular primary structure of apoA-I comprised of 10 11/22-mer tandem repeats (1–10) punctuated by Pro or Gly and flanked by the N-terminal G* repeat.19,20 The starting model was provided by the 2.2 Å resolution X-ray crystal structure of the C-terminally truncated lipid-free human apoA-I, Δ(185–243)apoA-I, determined by Mei and Atkinson. 48 Briefly, in the crystal, Δ(185–243)apoA-I forms an antiparallel dimer in an ~80% α-helical semicircular conformation with a diameter d of 11 nm and central repeats 5 (residues 121–142) in registry, in agreement with the doublebelt model of apoA-I on HDL.4,31,32 The stability of the crystallographic dimer is conferred by two four-segment bundles, each containing three segments encompassing residues 8–120 (repeats G* and 1–4) from molecule 1 (Figure 2, solid line) and the fourth helical segment encompassing residues 144–184 (repeats 6 and 7) from molecule 2 (dotted line). This structure suggests strongly that the conformational adaptation of apoA-I to HDL of different diameters involves sequential unhinging of the N-terminal bundle.48
Figure 2.
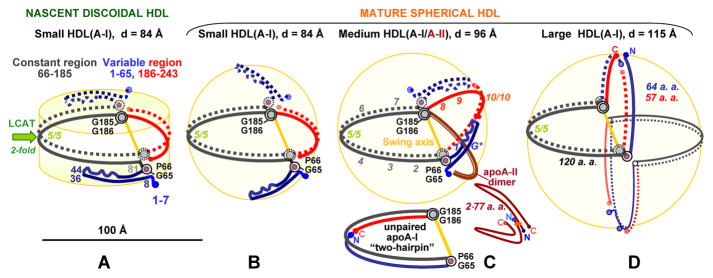
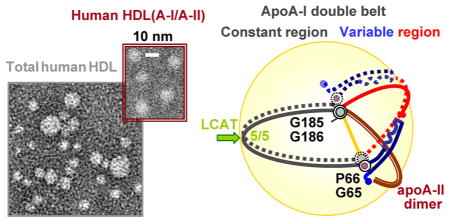
Cartoon illustrating the proposed conformational ensemble of apoA-I and the role of apoA-II on HDL. Protein conformations on four distinct HDL subclasses are shown. (A) Small nascent discoidal and (B) small mature spherical HDL(A-I) (d ~ 8.4 nm) containing two copies of apoA-I. (C) Midsize spherical HDL (d ~ 9.6 nm) containing either three copies of apoA-I on HDL(A-I) (the unpaired copy of apoA-I in a hypothetical hairpin conformation on HDL is shown at the bottom) or two copies of apoA-I and one to three copies of apoA-IIdimer on HDL(A-I/A-II). One copy of apoA-IIdimer on the HDL surface is shown and is illustrated in greater detail at the bottom right; additional copies can be envisioned bound in a similar tetrafoil/pentafoil manner on the surface of spherical HDL. (D) Large spherical HDL(A-I) (d ~ 11.5 nm) containing four copies of apoA-I paired in two bent double belts. In panels A–D, two molecules of apoA-I (one as a solid line and one as a dotted line) are paired in an antiparallel double-belt conformation with central repeats 5 (residues 121–143) in registry. The crystal structure of Δ(185–243)apoA-I48 reveals that the 2-fold axis linking the two apoA-I molecules in the double belt passes through the middle of repeat 5 (green arrow, left), and that repeat pair 5/5 forms the site of the LCAT reaction (indicated). The proposed constant region of the apoA-I double belt (residues 66–185, sequence repeats 2–7) is colored black. The variable region is comprised of N-terminal residues 1–65 (repeats G* and 1, blue) and C-terminal residues 186–243 (repeats 8–10, red). Sequence repeat numbers in one copy of apoA-I are shown in panel C in italics. ApoA-IIdimer is colored brown (C); apoA-IIdimer in the double-hairpin conformation27 and the unpaired apoA-I molecule in a hairpin conformation on the midsize HDL are shown at the bottom of panel C. The conformation of apoA-I on the small HDL (A and B) is based on the crystal structure of Δ(185–243)apoA-I, with the four-segment bundle partially open into two segment pairs for binding the lipid surface.48 ApoA-I on the midsize HDL closely resembles the “buckle-belt” conformation proposed in the cross-linking and MS studies of the discoidal 9.6 nm rHDL.52 The molecular arrangement on the midsize and large HDL (C and D) follows the trefoil/tetrafoil model,37,38 with one important difference. In the existing trefoil/tetrafoil model, the swing axis connects the middles of repeats 5 and 10 (green arrow, left), which we think is a drawback because repeat 10/10 registry appears to be uniquely suited for the midsize particles (C).53 In contrast, the swing axis in our model (yellow line) connects two flexible hinges encompassing the G65–P66 and G185–G186 pairs (gray circles), which are aligned on HDL of various shapes and sizes (A–D) and are highly conserved across the species, indicating their key role in the structural flexibility of apoA-I20 and suggesting that they form a swing axis.
This and other aspects of the structural adaptation of full-length apoA-I to plasma HDL of various shapes and sizes are reflected in the structural models depicted in Figure 2. In these hypothetical models, two copies of apoA-I fully encircle HDL in a closed double-belt conformation whose variable part contains two to four segments depending on the particle diameter (fewer on larger particles). Importantly, molecular packing in the central half of the apoA-I double belt encompassing residues 66–185 from repeats 2–7 (“constant region”) is largely conserved during the maturation and growth of HDL (black arcs, Figure 2A–C). In contrast, the variable half containing N-terminal repeats G* and 1 (residues 1–65) and C-terminal repeats 8–10 (residues 186–243) repacks to adapt to the increasing lipid load in HDL (red and blue arcs, Figure 2). Such repacking probably involves incremental opening of the N-terminal “buckle” around two flexible hinge regions, one encompassing G35–G39 and another near the G65–P66 pair.48 We hypothesize that this is accompanied by incremental shifts in registry of the flexible C-terminal segment (repeats 8–10), to facilitate an increase in the perimeter of the closed double belt.
Several lines of evidence independently support the idea that adaptation of the apoA-I double belt to HDL of various sizes involves conformational changes in the N- and C-terminal regions. First, the cross-linking and mass spectrometry studies by Thomas and co-workers concluded that “the incremental changes in the interaction between the N- and C-terminal ends of apoA-I... allow it to unfold and sequester discrete amounts of phospholipids”.52 Second, electron paramagnetic resonance studies by Oda’s group report the key role of the N-terminal part of apoA-I in its adaptation to particles of various sizes (ref 54 and references therein). Third, mutagenesis studies by Phillips and co-workers indicate the importance of the hydrophobic C-terminal bundle as well as the stability of the N-terminal bundle for the apoA-I–lipid interactions.55,56 Additional support comes from the immunochemical and electron microscopic studies reporting that apoA-I forms a relatively compact conformation on small HDL but expands on larger particles.57,58 The NMR analysis shows that such expansion involves tertiary but not secondary structural changes.58 Taken together, these results support the idea that the double-belt adaptation to HDL of increasing radii occurs, at least in part, via the incremental unhinging of the N-terminal buckle accompanied by changes in the C-terminal part. In Figure 2, the key hinge regions in this adaptation encompass G35–G39 and the G65–P66 pair. Notably, G39 and G65 are highly conserved in vertebrates, indicating the importance of these locations for molecular flexibility.20
CONFORMATIONAL ADAPTATION OF APOA-I TO HDL DISKS AND SPHERES
The two existing X-ray crystal structures of the N- or C-terminally truncated apoA-I30,48 and the ensuing models31,32 show juxtaposed G65–P66 and G185–G186 pairs on the two antiparallel molecules within the double belt. Moreover, sequence analysis shows that Gly residues in these locations are highly conserved, suggesting that their flexibility is necessary for function.20 Our model suggests that the alignment of the G65–P66 and G185–G186 pairs in the two copies of full-length apoA-I is conserved in the double belts with various diameters. This facilitates the concerted out-of-plane swing motion of the variable part of the double belt upon maturation of HDL from a disk to a sphere (Figure 2, yellow axis). In addition, folding around these hinges can produce a “hairpin” conformation of the apoA-I monomer (Figure 2C, bottom) that can help accommodate the unpaired odd copies of apoA-I on HDL.37
Although the conformational ensemble shown in Figure 2 is hypothetical, it is supported by the results of the cross-linking and mass spectrometry studies by Silva and colleagues reporting that the overall conformation of apoA-I on HDL is similar regardless of the particle shape and size.37 Figure 2 shows that the intermolecular packing of the “constant” half of the apoA-I double belt is conserved on the particles of various shapes and sizes, which is expected to reduce the free energy barriers for HDL remodeling during RCT.59 Furthermore, the protein arrangement depicted in panels C and D of Figure 2 is reminiscent of the trefoil/tetrafoil models of spherical HDL proposed by Davidson’s team,36,37 with two important differences. First, in Davidson’s model, which is based on the low-resolution crystal structure of Δ(1–43)apoA-I,30 the double belt encompasses residues 44–243 in a fully expanded helical conformation but does not include N-terminal residues 1–43. The size of the resulting belt is comparable to that of our midsize double belt with repeat 10/10 registry. Second, the hinge axis in Davidson’s model passes through the middle of repeats 5 and 10. According to our model, such a hinge axis is suitable for only the midsize double belt that shows both 5/5 and 10/10 repeat registry (described in the next section). In contrast, the G65/G185 registry and, hence, the G65–G185 hinge axis are conserved on the double belts of any shape and size. Therefore, it is uniquely suited for the adaptation of the protein to the constantly changing HDL geometry. These differences notwithstanding, the model of spherical HDL depicted in Figure 2 agrees with the trefoil/tetrafoil concept and helps extend it to full-length apoA-I on HDL of various sizes.
In summary, the conformational ensemble depicted in Figure 2 helps integrate and refine several existing HDL models that were derived on the basis of the low-resolution crystallographic and cross-linking data obtained by other groups. This includes the trefoil/tetrafoil model of spherical plasma HDL37,38 and the buckle-belt conformation of apoA-I on the midsize discoidal recombinant HDL52 described below. The distinct features of our proposed ensemble are as follows: (i) the constant and variable halves of the apoA-I “double belt” delineated by the two flexible hinges encompassing the G65–P66 and G185– G186 pairs, (ii) incremental unhinging of the N-terminal buckle segments48 with concomitant repacking of C-terminal repeats 8–10, to increase the double-belt perimeter, and (iii) pivoting of the double belt around the G65–P66 and G185–G186 hinges, to confer two-dimensional (2D) curvature to the surface of spherical HDL or for accommodation of unpaired copies of apoA-I on this surface.
Additional details of the proposed conformational ensemble and its experimental validation, including future studies using protein mutagenesis and/or cross-linking, will be reported elsewhere. Here, we focus on the implications for the structural and functional role of apoA-II on HDL. Because endogenous apoA-II is found mainly on the midsize spherical HDL (Figures S1 and S3 of the Supporting Information),8,36 below we summarize experimental evidence for the proposed model of the midsize particles (Figure 2C).
EXPERIMENTAL EVIDENCE SUPPORTING THE STRUCTURAL MODEL OF THE MIDSIZE HDL
The 9.6 nm discoidal rHDL containing two copies of apoA-I are the best-studied rHDL, and the results of their studies provide multiple lines of evidence validating our model (Figure 2C). First, Tian and Jonas reported that the A232C substitution in the middle of repeat 10, which facilitates intermolecular disulfide between the juxtaposed repeats 10,30,32 locks the particle in the 9.6 nm diameter and prevents its rearrangement to other sizes.53 This implies that repeat 10/10 registry is uniquely suited for the 9.6 nm rHDL, in excellent agreement with Figure 2. Second, Lys cross-linking studies of the 9.6 nm discoidal rHDL identified several intermolecular distances under 12 Å, giving rise to the buckle-belt model for the 9.6 nm particle that is very similar to ours, except for a slightly different buckle position. Notably, the conformation of apoA-I on the midsize HDL depicted in Figure 1C readily accommodates the cross-links reported by Bhat et al.,37 including K12–K182 and K118–K140 (intermolecular) and K40–K239 (intramolecular) cross-links, further supporting our model. Additional support comes from the spin-labeling and electron paramagnetic resonance study of the 98 N-terminal residues in apoA-I on rHDL disks of various sizes by Oda’s team; residues 34–41 (tip of the buckle in Figure 2) were found to be particularly sensitive to changes in the particle size and have increased molecular accessibility on larger disks.54 This result is in excellent agreement with Figure 2 but not with Bhat’s model in which the tip of the buckle is centered at L44. In summary, the model of the 9.6 nm particle shown in Figure 2C is in good agreement with the buckle-belt model and is strongly supported by numerous structural studies (refs 30, 32, 37, 48, 53, and 54 and references therein). Here, we use this model to propose a new structural and functional role for apoA-II on HDL.
ENDOGENOUS APOA-II HINDERS EXPANSION OF THE CONFORMATION OF APOA-I ON HDL(A-I/A-II)
The structural ensemble of apoA-I depicted in Figure 2, together with the double-hairpin conformation of apoA-IIdimer proposed by Silva’s group (Figure 1), helps explain why human apoA-II circulates mainly on the midsize spherical HDL. We hypothesize that during the maturation and growth of HDL (Figure 2A–C), the variable region of the apoA-I double belt swings out of the plane of the constant region, thereby vacating the opposite side of the spherical particle to accommodate additional protein that is necessary to confer 2D lipid surface curvature. On midsize HDL, such additional protein can comprise one to three copies of apoA-IIdimer that are found on plasma HDL(A-I/A-II).36 Alternatively, it can comprise one apoA-I molecule, probably in a hairpin conformation (Figure 2C, bottom), which accounts for the total of three copies of apoA-I found on the midsize human HDL(A-I). Notably, the length of apoA-IIdimer (2 × 77 amino acids) approximately matches the length of the apoA-I variable region in the midsize double belt (~85 amino acids). We speculate that this length complementarity helps accommodate apoA-II on the midsize HDL via the indirect lipid-mediated interactions.
We hypothesize that on small spherical HDL, the N-terminal part of apoA-I can swing out of plane of the double belt to confer 2D surface curvature (Figure 2B). Such a three-dimensional conformation of apoA-I, along with the adequate surface coverage provided by two copies of apoA-I on the small HDL, eliminates the need for apoA-II and helps explain its absence on the small spherical HDL in humans.8,36 ApoA-II is also absent from the large spherical HDL8,36 (Figures S1 and S3 of the Supporting Information) that contain multiple copies of apoA-I. According to the model in Figure 2D, apoA-I on large HDL adopts a fully expanded α-helical conformation that limits the maximal diameter of HDL to <12 nm, in good agreement with that of the large HDL found in human plasma. We hypothesize that apoA-II is not found on the large spherical HDL because apoA-II hampers the expansion of apoA-I from the midsize buckle belt (Figure 2C) to the large fully expanded double belt (Figure 2D).
EXOGENOUS APOA-II CAN INDUCE CONTRACTION OF THE CONFORMATION OF APOA-I ON HDL
If endogenous human apoA-IIdimer is optimally suited for the midsize spherical HDL(A-I/A-II) (Figure 2C), how can exogenous apoA-II bind to HDL of any diameter (Figure S4 of the Supporting Information)? We hypothesize that such binding can induce incremental contraction of the N-terminal region of apoA-I into a more compact conformation (Figure 2, from D to A), thereby vacating the surface of HDL for apoA-II. We posit that such incremental contraction of apoA-I, starting from its N-terminal region, ultimately leads to dissociation of apoA-I from HDL in a compact monomeric lipid-poor or lipid-free state.48,60
In summary, the model in Figure 2 suggests that the effect of apoA-II on human HDL is more nuanced than simply locking apoA-I in the midsize conformation. First, we hypothesize that apoA-II hampers the expansion of apoA-I from the midsize buckle belt to the large double belt. We posit that this effect is, at least in part, lipid-mediated and does not necessarily require direct interactions between the two proteins (Figure 2), even though such interactions cannot be excluded.39,61 Second, we propose that progressive expansion of the apoA-I double belt to a diameter approaching 12 nm disrupts the belt-closing contacts between the N- and C-termini, leading to the rupture of HDL and release of core lipids. This suggests that the stabilizing effect of apoA-II on spherical HDL may, at least in part, result from the impaired expansion of the apoA-I double belt on HDL(A-I/A-II). Third, we posit that binding of exogenous apoA-II to the HDL surface can induce contraction and, ultimately, dissociation of apoA-I in a compact lipid-poor or lipid-free state.48,60 Experimental evidence supporting the contraction of apoA-I on rHDL(A-I/A-II) that is based on the work of Silva’s group61 is presented in the Supporting Information. Such a contraction and ultimate displacement of apoA-I can probably occur in vitro upon enrichment of HDL with exogenous apoA-II (Figure S4 of the Supporting Information). We speculate that a similar contraction and displacement of apoA-I can possibly occur in vivo upon binding of exogenous lipophilic proteins to the HDL surface, such as serum amyloid protein that displaces apoA-I from HDL in acute inflammation.62,63
APOA-II HAS DISTINCT EFFECTS ON THE STABILITY OF DISCOIDAL AND SPHERICAL HDL
In contrast to spherical HDL, discoidal rHDL show a gradual reduction in stability with an increase in the apoA-II:apoA-I ratio.50,51,64 What is the origin of these distinct effects of apoA-II on the stability of spherical and discoidal HDL? A key factor that is thought to contribute to differential effects of apoA-II and apoA-I on HDL stability is protein hydrophobicity.16,18,24 The fraction of the apolar lipid-binding surface of the amphipathic apolipoprotein α-helices determines the depth of their insertion into the phospholipid monolayer: the larger the apolar surface fraction, the deeper the insertion (Figure S7A of the Supporting Information). In apoA-II, which is the most hydrophobic of the exchangeable apolipoproteins, the apolar face comprises ~50% of the helical surface, as compared to ~30% in apoA-I (Figure S7 of the Supporting Information). As a result, apoA-II inserts more deeply into the phospholipid monolayer.15,16,18,24 This is expected to help impose the monolayer curvature not only along the α-helical axis but also across it and thereby stabilize the 2D surface of spherical HDL (Figure S7A of the Supporting Information). In addition, deeper insertion of apoA-II may enhance the interactions with the core lipids and thereby contribute to HDL stability.
In contrast to the arrangement of the protein on spherical HDL, in discoidal HDL a substantial part of the protein is thought to be wrapped around the edge of the phospholipid bilayer rather than inserted at its top (Figure S7B of the Supporting Information). In this geometry, the relatively large apolar helical faces in apoA-II do not necessarily confer additional HDL stability as compared to that conferred by apoA-I.50,51 Furthermore, dissociation of smaller proteins from the particle surface involves disruption of fewer protein–lipid contacts and hence a lower activation energy (enthalpy), which translates into a lower free energy of stability of discoidal rHDL(A-II) as compared to rHDL(A-I).50,51 In summary, we propose that the depth of insertion of the apolipoprotein into the phospholipid surface, which is defined by the fraction of the apolar helical face, is important for the stability of spherical HDL, whereas the length of the protein is the major determinant for the stability of discoidal HDL. These distinct effects of apoA-II on the stability of discoidal and spherical HDL may have potentially important functional implications.
POTENTIAL METABOLIC IMPLICATIONS
Although most plasma apoA-II circulates on spherical HDL(A-I/A-II), a small amount is present on nascent discoidal HDL(A-II), a transient form in which apoA-II is secreted.33 Nascent HDL(A-II) rapidly fuse with nascent HDL(A-I) to form mature spherical HDL(A-I/A-II) via the LCAT reaction (ref 33 and references therein). We hypothesize that in this reaction (i) rapid fusion of nascent HDL(A-I) with HDL(A-II) is facilitated by the relatively low structural stability of discoidal HDL(A-II) 51,64 and (ii) apoA-II augments the maturation of HDL by conferring 2D surface curvature to the spherical HDL(A-I/A-II) (Figure 2C). Notably, apoA-II is not required for HDL maturation and can be replaced with an additional copy of apoA-I to form midsize spherical HDL(A-I) (Figure 2C). This helps explain why patients lacking apoA-II have normal plasma HDL profiles.65 Because the maturation of HDL via the LCAT reaction is not rate-limiting in normal RCT (reviewed in ref 2), the role of apoA-II in the maturation of HDL probably has little effect on the overall rate of cholesterol removal in normal RCT.
Taken together, the results of this (Figures S1 and S3 of the Supporting Information) and earlier studies36 support the idea that apoA-II on mature human HDL(A-I/A-II) selects the conformation of apoA-I that is compatible with the midsize particles. The model in Figure 2C suggests that this apoA-I conformation is the midsize buckle belt. The restrictions imposed by apoA-II on the expansion of the apoA-I buckle belt and the ensuing particle growth help explain why HDL remodeling by most plasma factors is impaired in HDL(A-I/A-II) versus that in HDL(A-I).21,40–44 At the same time, we hypothesize that addition of exogenous apoA-II (or, potentially, other lipophilic proteins) can shift apoA-I to a more compact conformation and eventually displace it from HDL.15,18,24–27
Cumulative evidence suggests that plasma HDL(A-I/A-II) particles form relatively homogeneous particles that are structurally and functionally inert compared to HDL(A-I) particles. We hypothesize that apoA-II augments formation of the midsize spherical HDL but hampers their remodeling into large lipid-loaded HDL that are preferred ligands for HDL receptor SR-BI.66 If correct, this implies that, at normal levels of apoA-I, increased levels of apoA-II may help raise the quantity of good cholesterol but not necessarily its quality as a vehicle for cholesterol removal.
Supplementary Material
Acknowledgments
Funding
This work was supported by National Institutes of Health Grants HL026335 and GM067260.
We thank Cheryl England and Michael Gigliotti for help with isolation of human plasma HDL, Donald L. Gantz and Jing Wang for help with electron microscopy, Minjing Liu for help with the sample preparation, and David Plotkin for editorial help. We are indebted to Dr. Xiaohu Mei and Dr. David Atkinson for sharing the privileged information about the crystal structure of the C-terminally truncated apoA-I prior to publication.
ABBREVIATIONS
- HDL
high-density lipoprotein
- rHDL
recombinant HDL
- apo
apolipoprotein
- HDL(A-I)
HDL containing apoA-I but not apoA-II
- HDL(A-II)
HDL containing apoA-II but not apoA-I
- HDL(A-I/A-II)
HDL containing both apoA-I and apoA-II
- RCT
reverse cholesterol transport
- LCAT
lecithin:cholesterol acyltransferase
Footnotes
Notes
The authors declare no competing financial interest.
Structural and stability studies of human HDL subclasses homogeneous in size and density before and after enrichment with exogenous apoA-II (Figures S1–S6), evidence of the apoA-II-induced contraction of apoA-I on the surface of HDL based on the work by Gauthamadasa et al.,61 and a cartoon illustrating the possible arrangement of the amphipathic α-helices of apoA-I and apoA-II on the surface of HDL disks and spheres (Figure S7). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Fielding CJ, Fielding PE. Molecular physiology of reverse cholesterol transport. J Lipid Res. 1995;36:211–228. [PubMed] [Google Scholar]
- 2.Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2009;50(Suppl):S189–S194. doi: 10.1194/jlr.R800088-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothblat GH, Phillips MC. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr Opin Lipidol. 2010;21:229–238. doi: 10.1097/mol.0b013e328338472d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lund-Katz S, Phillips MC. High density lipoprotein structure-function and role in reverse cholesterol transport. Subcell Biochem. 2010;51:183–227. doi: 10.1007/978-90-481-8622-8_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scanu AM, Edelstein C. HDL: Bridging past and present with a look at the future. FASEB J. 2008;22(12):4044–4054. doi: 10.1096/fj.08-117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon SM, Hofmann S, Askew DS, Davidson WS. High density lipoprotein: It’s not just about lipid transport anymore. Trends Endocrinol Metab. 2011;22(1):9–15. doi: 10.1016/j.tem.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kontush A, Chapman MJ. High-density lipoproteins: Structure, metabolism, function and therapeutics. John Wiley and Sons; New York: 2012. [Google Scholar]
- 8.Gordon SM, Deng J, Lu LJ, Davidson WS. Proteomic characterization of human plasma high density lipoprotein fractionated by gel filtration chromatography. J Proteome Res. 2010;9(10):5239–5249. doi: 10.1021/pr100520x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rye KA, Clay MA, Barter PJ. Remodelling of high density lipoproteins by plasma factors. Atherosclerosis. 1999;145(2):227–238. doi: 10.1016/s0021-9150(99)00150-1. [DOI] [PubMed] [Google Scholar]
- 10.Guha M, Gao X, Jayaraman S, Gursky O. Correlation of structural stability with functional remodeling of high-density lipoproteins: The importance of being disordered. Biochemistry. 2008;47(44):11393–11397. doi: 10.1021/bi8014746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arsenault BJ, Lemieux I, Despres JP, Gagnon P, Wareham NJ, Stroes ES, Kastelein JJ, Khaw KT, Boekholdt SM. HDL particle size and the risk of coronary heart disease in apparently healthy men and women: The EPIC-Norfolk prospective population study. Atherosclerosis. 2009;206:276–281. doi: 10.1016/j.atherosclerosis.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 12.Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. HDL, ABC transporters, and cholesterol efflux: Implications for the treatment of atherosclerosis. Cell Metab. 2008;7:365–375. doi: 10.1016/j.cmet.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Joy T, Hegele RA. Is raising HDL a futile strategy for atheroprotection? Nat Rev Drug Discovery. 2008;7(2):143–155. doi: 10.1038/nrd2489. [DOI] [PubMed] [Google Scholar]
- 14.Gao X, Yuan S. High density lipoproteins-based therapies for cardiovascular disease. J Cardiovasc Dis Res. 2010;1(3):99–103. doi: 10.4103/0975-3583.70898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yetukuri L, Huopaniemi I, Koivuniemi A, Maranghi M, Hiukka A, Nygren H, Kaski S, Taskinen MR, Vattulainen I, Jauhiainen M, Orešič M. High density lipoprotein structural changes and drug response in lipidomic profiles following the long-term fenofibrate therapy in the FIELD substudy. PLoS One. 2011;6(8):e23589. doi: 10.1371/journal.pone.0023589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blanco-Vaca F, Escolà-Gil JC, Martín-Campos JM, Julve J. Role of apoA-II in lipid metabolism and atherosclerosis: Advances in the study of an enigmatic protein. J Lipid Res. 2001;42(11):1727–1739. [PubMed] [Google Scholar]
- 17.Tailleux A, Duriez P, Fruchart JC, Clavey V. Apolipoprotein A-II, HDL metabolism and atherosclerosis. Atherosclerosis. 2002;164(1):1–13. doi: 10.1016/s0021-9150(01)00751-1. [DOI] [PubMed] [Google Scholar]
- 18.Kalopissis AD, Pastier D, Chambaz J. Apolipoprotein A-II: Beyond genetic associations with lipid disorders and insulin resistance. Curr Opin Lipidol. 2003;14:165–172. doi: 10.1097/00041433-200304000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Segrest JP, Garber DW, Brouillette CG, Harvey SC, Anantharamaiah GM. The amphipathic α helix: A multifunctional structural motif in plasma apolipoproteins. Adv Protein Chem. 1994;45:303–369. doi: 10.1016/s0065-3233(08)60643-9. [DOI] [PubMed] [Google Scholar]
- 20.Bashtovyy D, Jones MK, Anantharamaiah GM, Segrest JP. Analyses of sequence conservation of apolipoprotein A-I afford novel insights into HDL structure-function. J Lipid Res. 2011;52(3):435–450. doi: 10.1194/jlr.R012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheung MC, Albers JJ. Characterization of lipoprotein particles isolated by immunoaffinity chromatography. Particles containing A-I and A-II and particles containing A-I but no A-II. J Biol Chem. 1984;259(19):12201–122219. [PubMed] [Google Scholar]
- 22.Curtiss LK, Valenta DT, Hime NJ, Rye KA. What is so special about apolipoprotein AI in reverse cholesterol transport? Arterioscler, Thromb, Vasc Biol. 2006;26(1):12–19. doi: 10.1161/01.ATV.0000194291.94269.5a. [DOI] [PubMed] [Google Scholar]
- 23.Birjmohun RS, Dallinga-Thie GM, Kuivenhoven JA, Stroes ES, Otvos JD, Wareham NJ, Luben R, Kastelein JJ, Khaw KT, Boekholdt SM. Apolipoprotein A-II is inversely associated with risk of future coronary artery disease. Circulation. 2007;116:2029–2035. doi: 10.1161/CIRCULATIONAHA.107.704031. [DOI] [PubMed] [Google Scholar]
- 24.Edelstein C, Halari M, Scanu AM. On the mechanism of the displacement of apolipoprotein A-I by apolipoprotein A-II from the high density lipoprotein surface. Effect of concentration and molecular forms of apolipoprotein A-II. J Biol Chem. 1982;257(12):7189–7195. [PubMed] [Google Scholar]
- 25.Durbin DM, Jonas A. The effect of apolipoprotein A-II on the structure and function of apolipoprotein A-I in a homogeneous reconstituted high density lipoprotein particle. J Biol Chem. 1997;272(50):31333–31339. doi: 10.1074/jbc.272.50.31333. [DOI] [PubMed] [Google Scholar]
- 26.Boucher J, Ramsamy TA, Braschi S, Sahoo D, Neville TA, Sparks DL. Apolipoprotein A-II regulates HDL stability and affects hepatic lipase association and activity. J Lipid Res. 2004;45(5):849–858. doi: 10.1194/jlr.M300431-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Silva RA, Schneeweis LA, Krishnan SC, Zhang X, Axelsen PH, Davidson WS. The structure of apolipoprotein A-II in discoidal high density lipoproteins. J Biol Chem. 2007;282(13):9713–9121. doi: 10.1074/jbc.M610380200. [DOI] [PubMed] [Google Scholar]
- 28.Sánchez-Quesada JL, Antón R, Camacho M, Julve J, Escolà-Gil JC, Vila L, Ordóñez-Llanos J, Blanco-Vaca F. Human apolipoprotein A-II enrichment displaces paraoxonase from HDL and impairs its antioxidant properties: A new mechanism linking HDL protein composition and antiatherogenic potential. Circ Res. 2004;95(8):789–797. doi: 10.1161/01.RES.0000146031.94850.5f. [DOI] [PubMed] [Google Scholar]
- 29.Julve J, Escolà-Gil JC, Rotllan N, Fiévet C, Vallez E, de la Torre C, Ribas V, Sloan JH, Blanco-Vaca F. Human apolipoprotein A-II determines plasma triglycerides by regulating lipoprotein lipase activity and high-density lipoprotein proteome. Arterioscler, Thromb, Vasc Biol. 2010;30(2):232–238. doi: 10.1161/ATVBAHA.109.198226. [DOI] [PubMed] [Google Scholar]
- 30.Borhani DW, Rogers DP, Engler JA, Brouillette CG. Crystal structure of truncated human apolipoprotein A-I suggests a lipid-bound conformation. Proc Natl Acad Sci USA. 1997;94:12291–12296. doi: 10.1073/pnas.94.23.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Segrest JP, Jones MK, Klon AE, Sheldahl CJ, Hellinger M, De Loof H, Harvey SC. A detailed molecular belt model for apolipoprotein A-I in discoidal high density lipoprotein. J Biol Chem. 1999;274:31755–31758. doi: 10.1074/jbc.274.45.31755. [DOI] [PubMed] [Google Scholar]
- 32.Brouillette CG, Anantharamaiah GM, Engler JA, Borhani DW. Structural models of human apolipoprotein A-I: A critical analysis and review. Biochim Biophys Acta. 2001;1531:4–46. doi: 10.1016/s1388-1981(01)00081-6. [DOI] [PubMed] [Google Scholar]
- 33.Gillard BK, Lin HY, Massey JB, Pownall HJ. Apolipoproteins A-I, A-II and E are independently distributed among intracellular and newly secreted HDL of human hepatoma cells. Biochim Biophys Acta. 2009;1791(12):1125–1132. doi: 10.1016/j.bbalip.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clay MA, Pyle DH, Rye KA, Barter PJ. Formation of spherical, reconstituted high density lipoproteins containing both apolipoproteins A-I and A-II is mediated by lecithin:cholesterol acyltransferase. J Biol Chem. 2000;275(12):9019–9025. doi: 10.1074/jbc.275.12.9019. [DOI] [PubMed] [Google Scholar]
- 35.Hime NJ, Drew KJ, Wee K, Barter PJ, Rye KA. Formation of high density lipoproteins containing both apolipoprotein A-I and A-II in the rabbit. J Lipid Res. 2006;47(1):115–122. doi: 10.1194/jlr.M500284-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Gauthamadasa K, Rosales C, Pownall HJ, Macha S, Jerome WG, Huang R, Silva RA. Speciated human high-density lipoprotein protein proximity profiles. Biochemistry. 2010;49(50):10656–10665. doi: 10.1021/bi1015452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva RA, Huang R, Morris J, Fang J, Gracheva EO, Ren G, Kontush A, Jerome WG, Rye KA, Davidson WS. Structure of apolipoprotein A-I in spherical high density lipoproteins of different sizes. Proc Natl Acad Sci USA. 2008;105:12176–12181. doi: 10.1073/pnas.0803626105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang R, Silva RA, Jerome WG, Kontush A, Chapman MJ, Curtiss LK, Hodges TJ, Davidson WS. Apolipoprotein A-I structural organization in high-density lipoproteins isolated from human plasma. Nat Struct Mol Biol. 2011;18(4):416–422. doi: 10.1038/nsmb.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rye KA, Wee K, Curtiss LK, Bonnet DJ, Barter PJ. Apolipoprotein A-II inhibits high density lipoprotein remodeling and lipid-poor apolipoprotein A-I formation. J Biol Chem. 2003;278(25):22530–22536. doi: 10.1074/jbc.M213250200. [DOI] [PubMed] [Google Scholar]
- 40.Calabresi L, Lucchini A, Vecchio G, Sirtori CR, Franceschini G. Human apolipoprotein A-II inhibits the formation of pre-β high density lipoproteins. Biochim Biophys Acta. 1996;1304(1):32–42. doi: 10.1016/s0005-2760(96)00102-6. [DOI] [PubMed] [Google Scholar]
- 41.Hime NJ, Barter PJ, Rye KA. The influence of apolipoproteins on the hepatic lipase-mediated hydrolysis of high density lipoprotein phospholipid and triacylglycerol. J Biol Chem. 1998;273:27191–27198. doi: 10.1074/jbc.273.42.27191. [DOI] [PubMed] [Google Scholar]
- 42.Pussinen PJ, Jauhiainen M, Metso J, Pyle LE, Marcel YL, Fidge NH, Ehnholm C. Binding of phospholipid transfer protein (PLTP) to apolipoproteins A-I and A-II: Location of a PLTP binding domain in the amino terminal region of apoA-I. J Lipid Res. 1998;39(1):152–161. [PubMed] [Google Scholar]
- 43.Lagrost L, Persegol L, Lallemant C, Gambert P. Influence of apolipoprotein composition of high density lipoprotein particles on cholesteryl ester transfer protein activity: Particles containing various proportions of apolipoproteins AI and AII. J Biol Chem. 1994;269:3189–3197. [PubMed] [Google Scholar]
- 44.Huang Y, von Eckardstein A, Wu S, Assmann G. Cholesterol efflux, cholesterol esterification, and cholesteryl ester transfer by LpA-I and LpA-I/A-II in native plasma. Arterioscler, Thromb, Vasc Biol. 1995;15(9):1412–1418. doi: 10.1161/01.atv.15.9.1412. [DOI] [PubMed] [Google Scholar]
- 45.Pilon A, Briand O, Lestavel S, Copin C, Majd Z, Fruchart JC, Castro G, Clavey V. Apolipoprotein AII enrichment of HDL enhances their affinity for class B type I scavenger receptor but inhibits specific cholesteryl ester uptake. Arterioscler, Thromb, Vasc Biol. 2000;20(4):1074–1081. doi: 10.1161/01.atv.20.4.1074. [DOI] [PubMed] [Google Scholar]
- 46.Rinninger F, Brundert M, Budzinski RM, Fruchart JC, Greten H, Castro GR. Scavenger receptor BI (SR-BI) mediates a higher selective cholesteryl ester uptake from LpA-I compared with LpA-I:A-II lipoprotein particles. Atherosclerosis. 2003;166(1):31–40. doi: 10.1016/s0021-9150(02)00311-8. [DOI] [PubMed] [Google Scholar]
- 47.de Beer MC, Castellani LW, Cai L, Stromberg AJ, de Beer FC, van der Westhuyzen DR. ApoA-II modulates the association of HDL with class B scavenger receptors SR-BI and CD36. J Lipid Res. 2004;45(4):706–715. doi: 10.1194/jlr.M300417-JLR200. [DOI] [PubMed] [Google Scholar]
- 48.Mei X, Atkinson D. Crystal structure of C-terminal truncated apolipoprotein A-I reveals the assembly of HDL by dimerization. J Biol Chem. 2011;286(44):38570–38582. doi: 10.1074/jbc.M111.260422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jayaraman S, Gantz DL, Gursky O. Effects of salt on thermal stability of human plasma high-density lipoproteins. Biochemistry. 2006;45:4620–4628. doi: 10.1021/bi0524565. [DOI] [PubMed] [Google Scholar]
- 50.Gao X, Yuan S, Jayaraman S, Gursky O. Differential stability of high-density lipoprotein subclasses: Effects of particle size and protein composition. J Mol Biol. 2009;387(3):628–638. doi: 10.1016/j.jmb.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jayaraman S, Gantz DL, Gursky O. Kinetic stabilization and fusion of apolipoprotein A-2:DMPC disks: Comparison with apoA-1 and apoC-1. Biophys J. 2005;88(4):2907–2918. doi: 10.1529/biophysj.104.055921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhat S, Sorci-Thomas MG, Alexander ET, Samuel MP, Thomas MJ. Intermolecular contact between globular N-terminal fold and C-terminal domain of apoA-I stabilizes its lipidbound conformation: studies employing chemical cross-linking and mass spectrometry. J Biol Chem. 2005;280:33015–33025. doi: 10.1074/jbc.M505081200. [DOI] [PubMed] [Google Scholar]
- 53.Tian S, Jonas A. Structural and functional properties of apolipoprotein A-I mutants containing disulfide-linked cysteines at positions 124 or 232. Biochim Biophys Acta. 2002;1599:56–64. doi: 10.1016/s1570-9639(02)00377-1. [DOI] [PubMed] [Google Scholar]
- 54.Lagerstedt JO, Cavigiolio G, Budamagunta MS, Pagani I, Voss JC, Oda MN. Structure of apolipoprotein A-I N terminus on nascent high density lipoproteins. J Biol Chem. 2011;286(4):2966–2975. doi: 10.1074/jbc.M110.163097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vedhachalam C, Chetty PS, Nickel M, Dhanasekaran P, Lund-Katz S, Rothblat GH, Phillips MC. Influence of apolipoprotein (Apo) A-I structure on nascent high density lipoprotein (HDL) particle size distribution. J Biol Chem. 2010;285(42):31965–3173. doi: 10.1074/jbc.M110.126292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanaka M, Dhanasekaran P, Nguyen D, Nickel M, Takechi Y, Lund-Katz S, Phillips MC, Saito H. Influence of N-terminal helix bundle stability on the lipid-binding properties of human apolipoprotein A-I. Biochim Biophys Acta. 2011;1811(1):25–30. doi: 10.1016/j.bbalip.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calabresi L, Meng QH, Castro GR, Marcel YL. Apolipoprotein A-I conformation in discoidal particles: Evidence for alternate structures. Biochemistry. 1993;32(25):6477–6484. doi: 10.1021/bi00076a023. [DOI] [PubMed] [Google Scholar]
- 58.Chen B, Ren X, Neville T, Jerome WG, Hoyt DW, Sparks D, Ren G, Wang J. Apolipoprotein AI tertiary structures determine stability and phospholipid-binding activity of discoidal high-density lipoprotein particles of different sizes. Protein Sci. 2009;18(5):921–935. doi: 10.1002/pro.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gursky O. Apolipoprotein structure and dynamics. Curr Opin Lipidol. 2005;16(3):287–294. doi: 10.1097/01.mol.0000169348.61191.ac. [DOI] [PubMed] [Google Scholar]
- 60.Jayaraman S, Cavigiolio G, Gursky O. Folded functional lipid-poor apolipoprotein A-I obtained by heating of high-density lipoproteins: Relevance to HDL biogenesis. Biochem J. 2012;442(3):703–712. doi: 10.1042/BJ20111831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gauthamadasa K, Vaitinadin NS, Dressman JL, Macha S, Homan R, Greis KD, Silva RA. Apolipoprotein A-II mediated conformational changes of apolipoprotein A-I in discoidal high density lipoproteins. J Biol Chem. 2012;287(10):7615–7625. doi: 10.1074/jbc.M111.291070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Beer MC, Webb NR, Wroblewski JM, Noffsinger VP, Rateri DL, Ji A, van der Westhuyzen DR, de Beer FC. Impact of serum amyloid A on high density lipoprotein composition and levels. J Lipid Res. 2010;51(11):3117–31125. doi: 10.1194/jlr.M005413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jahangiri A, de Beer MC, Noffsinger V, Tannock LR, Ramaiah C, Webb NR, van der Westhuyzen DR, de Beer FC. HDL remodeling during the acute phase response. Arterioscler, Thromb, Vasc Biol. 2009;29(2):261–267. doi: 10.1161/ATVBAHA.108.178681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reijngoud DJ, Phillips MC. Mechanism of dissociation of human apolipoproteins A-I, A-II, and C from complexes with dimyristoylphosphatidylcholine as studied by thermal denaturation. Biochemistry. 1984;23(4):726–734. doi: 10.1021/bi00299a022. [DOI] [PubMed] [Google Scholar]
- 65.Deeb SS, Takata K, Peng RL, Kajiyama G, Albers JJ. A splice-junction mutation responsible for familial apolipoprotein A-II deficiency. Am J Hum Genet. 1990;46:822–827. [PMC free article] [PubMed] [Google Scholar]
- 66.de Beer MC, Durbin DM, Cai L, Jonas A, de Beer FC, van der Westhuyzen DR. Apolipoprotein A-I conformation markedly influences HDL interaction with scavenger receptor BI. J Lipid Res. 2001;42(2):309–313. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.