Abstract
Introduction
Achilles tendon ruptures affect 15 (women) to 55 (men) per 100,000 people each year, and controversy continues to exist regarding optimal treatment and rehabilitation protocols. The objective of this study was to investigate the temporal effects of surgical repair and immobilization/activity (IM/ACT) on Achilles healing and limb function following complete transection in rodents.
Methods
Injured tendons (n=128) were repaired or left non-repaired, and animals groups immobilized in plantarflexion for 1, 3, or 6 weeks that later resumed cage and treadmill activity for 5, 3, or 0 weeks (IM1/ACT5, IM3/ACT3, IM6/ACT0). Animals were euthanized after 1- or 6-weeks post-injury.
Results
At 6-weeks post injury, IM1/ACT5 groups had increased range of motion and decreased ankle joint toe stiffness compared to IM3/ACT3 groups. IM6/ACT0 had decreased tendon cross sectional area, but increased tendon echogenicity and collagen alignment. Surgical treatment dramatically decreased fatigue cycles to failure in repaired tendons from earlier IM1/ACT5 groups. Normalized comparisons between 6- and 1-week post-injury data demonstrated that changes in healing tendon properties (area, alignment, and echogenicity) were maximized by IM1/ACT5 compared to IM6/ACT0.
Discussion/Conclusion
This study demonstrates how the temporal post-injury healing response of rodent Achilles tendons depends on both surgical treatment and the timing of IM/ACT.
Introduction
Achilles tendon ruptures are debilitating injuries, affecting 15 (women) to 55 (men) per 100,000 people each year, as approximated in Sweden.1 Unfortunately, consensus whether to operate and the timing of rehabilitation remains controversial.2–4 A recent clinical practice guideline issued by the American Academy of Orthopaedic Surgeons cites the “weak” or “inconclusive” evidence surrounding surgical management of Achilles tendon ruptures and period of joint immobilization (IM) post-surgery.5 Although operative treatment has been believed to result in superior function and lower re-rupture rates compared to non-operative treatment,6 there is inadequate scientific evidence to support this belief, and growing evidence suggests near equivalent efficacy of non-operative treatment.4, 5 Additionally, activity (ACT) may be as effective or, in some cases, more beneficial than longer-term cast immobilization7–9 for Achilles tendon healing in humans regardless of repair.10, 11
Recent work has identified benefits of ACT on Achilles tendon healing and function 3-weeks post-injury using an animal model of Achilles blunt transection, plantarflexion cast immobilization, and treadmill exercise.12 This model is clinically advantageous because most previous animal studies13,14 have not controlled for the resting position of the ankle in plantarflexion to maintain tendon apposition post-injury.13 Compared to 3-weeks of cast immobilization, ACT after 1-week of IM resulted in increased cross sectional area, fatigue resilience, dorsiflexion range of motion (ROM), and weight-bearing.12 In short, this study suggested that ACT was beneficial for tendon properties assessed during early healing (3-weeks post-injury). However, it remained unknown if the benefits of early ACT would persist or prove detrimental for later healing (6-weeks post-injury), or also be true in very early healing (1-week post-injury). Additionally, although functional deficits began to resolve by 3-weeks post-injury in activity groups,12 tissue properties remained aberrant at the matrix and cell levels compared to intact tendon,14 indicating that healing was incomplete. Evaluation of tendon properties 1-week and 6-weeks post-injury, in addition to 3-weeks post-injury, is important to evaluate the immediate role of surgical treatment and effect of IM/ACT following cast immobilization on Achilles tendon healing.
The purpose of this study was to investigate the temporal effects of surgical repair and IM/ACT timing on Achilles tendon healing (structural, mechanical, histological, immunohistochemical properties) and limb function (ankle ROM, stiffness, and gait) following complete transection. Previous studies on Achilles tendon healing in rodents have evaluated the healing response 2–3 weeks post-injury15–21; we therefore provide a comprehensive evaluation by studying healing at 1- and 6-weeks post-injury. We hypothesized that non-surgical treatment would lead to superior tendon properties post-injury (superior mechanical properties due to decreased tissue area, increased fiber alignment, spindle-like cell shape, and elevated colI:III ratio), but would not affect limb function. We also hypothesized that for both repaired and non-repaired groups, earlier ACT (shorter IM) would provide superior (but not equivalent) Achilles properties and limb function (increased ankle ROM, decreased ankle stiffness, and increased weight-bearing).
Methods
Study Design and Surgical Procedure
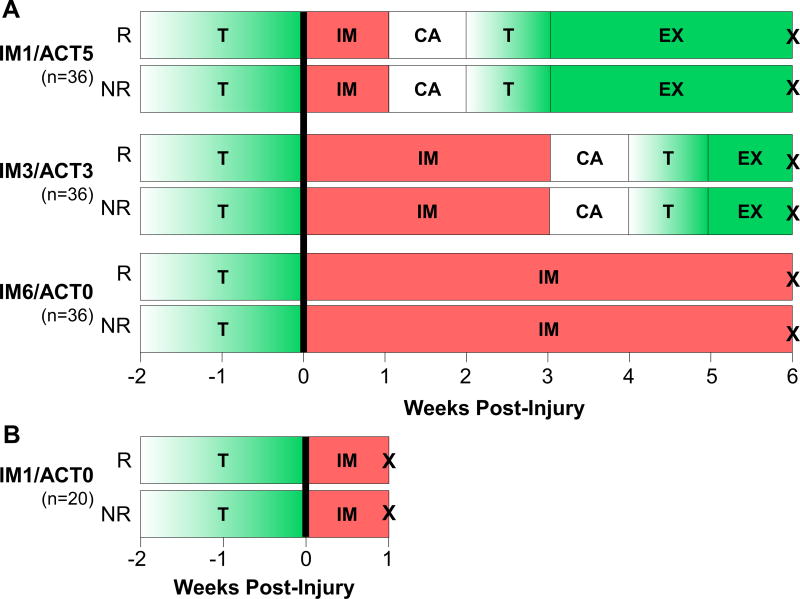
Male Sprague Dawley rats at 16-weeks of age (n=128) (Charles River Laboratories; Malvern, PA) were housed in a conventional facility with 12 hour light/dark cycles, were fed standard chow, and were provided water ad libitum (IACUC approved). All animals first performed two weeks of treadmill training (up to 60 minutes at 10m/min) (Figure 1). Treadmill training prior to injury is necessary to acclimate animals to treadmill activity. Animals then underwent surgery using sterile technique, consisting of anesthesia (isoflurane), blunt transection (reverse cutting side of a #11 blade) of the right Achilles tendon midsubstance with resection of the plantaris longus tendon,22, 12 and subsequent randomization into repaired (n=64) and non-repaired (n=64) groups. Prior to surgery, animals were given a single dose of buprenorphine (0.08 ml, 0.3mg/ml), which was repeated at 12-hour intervals through the third day post-surgery. To evaluate the role of repair and immobilization/activity (IM/ACT) 6-weeks post-injury, animals were randomized into repaired or non-repaired groups, and further randomized into groups immobilized for 1, 3, or 6 weeks that later resumed cage and activity for 5, 3, or 0 weeks (IM1/ACT5, IM3/ACT3, IM6/ACT0) (Figure 1). Repairs were performed with the Urbaniak variant of the Kessler repair23 using 4-0 Tevdek (PTFE coated) suture, as done previously.12 Immobilization procedures consisted of casting the leg from below the knee to the toes in a fully plantarflexed position using silk tape stirrups (3M; St. Paul, MN), webril padding (Hanna Pharmaceutical; Wilmington, DE), a plastic splint (MakerBot Industries, LLC; Brooklyn, NY), CoFlex (Andover Healthcare; Salisbury, MA), and poly(methyl-methacrylate) (Patterson Dental; St. Paul, MN).22, 12 Casts were replaced weekly while animals were under general isoflurane anesthesia.12 To evaluate the role of time on tissue healing, we also investigated tissue properties 1-week post-injury. Animals were similarly randomized into repaired and non-repaired groups prior to sacrifice 1-week post-injury. As all groups had 1-week of cast immobilization following injury, specifically to evaluate the role of IM/ACT following cast removal, we normalized 6-week post-injury data to the corresponding 1-week post-injury pair (R or NR). In doing so, this step removed any differences that may have occurred in healing during the first week of immobilization. For all sacrifices, animals were euthanized via CO2 inhalation.
Figure 1. Study Design.
(A) Following exercise training (T), animals had their right Achilles tendon transected and plantaris removed. Injured tendons were either repaired (R) or left non-repaired (NR). Animals then returned to activity after 1-week (IM1/ACT5), 3-weeks (IM3/ACT3), or 6-weeks (IM6/ACT0) of cast immobilization (IM) prior to sacrifice (X) at 6-weeks. (B) An additional cohort of animals was randomized into R and NR groups, immobilized for 1-week (IM1/ACT0), and sacrificed at 1-week post-injury.
Key: R- repaired; NR- not repaired; T- treadmill training; IM- immobilization; CA- cage activity; EX-exercise; X- sacrifice.
Functional Limb Assessment
Passive functional ankle joint properties (n=10–18/group) were quantified prior to injury, and 3-, 4-, and 6-weeks following injury by a single blinded measurer.12 As torque-angle data were nonlinear, toe and linear regions were defined to quantify ankle joint stiffness and range of motion (ROM) for both dorsiflexion and plantarflexion using custom MATLAB software12 (Mathworks; Natick, MA). Torque cutoffs (dorsiflexion: 8.92 N·mm, plantarflexion: 11.15 N·mm) were employed to ensure that a consistent range of torques was analyzed for all animals.
Hindlimb ground reaction forces (lateral, braking, propulsion, vertical) and spatiotemporal patterns (step width, stride length, velocity) that quantify active limb function were evaluated using an instrumented walkway, as described.12, 24 After acclimation, data were collected prior to injury, and 3-, 4- and 6-weeks after injury in a blinded fashion. All ground reaction force parameters were represented as percent animal body-weight (%BW), as animals were weighed at each time point of ambulatory assessment.
Tendon Sample Preparation
After 1- and 6-weeks post-injury, animals were euthanized (mass (mean±SD): 503±46g) and tendons from all groups were randomized and prepared by a single blinded dissector for high frequency ultrasound (HFUS), mechanical testing, histology, and immunohistochemistry, as described previously.22, 12 For mechanical testing, the Achilles tendon-foot complex was carefully removed en bloc, fine dissected, and measured for cross-sectional area (CSA),25 prior to application of stain dots for optical strain analysis. CSA was measured using a custom laser-based device on hydrated tissues.26 Optical strain was analyzed using texture correlation tracking in MATLAB.12 Sutures were left intact during mechanical testing to mimic the clinical scenario of a repaired tendon where the sutures would contribute to the mechanical load response in the tendon. This was done consistently across all groups in this and our other related studies. The calcaneus-foot complex was then embedded in PMMA, and the free end of the tendon was glued between sandpaper leaving a gage length of 12mm of tested tendon.
High Frequency Ultrasound Assessment of Tissue Structure
For HFUS imaging, tendons were loaded at 1N with the tendon positioned at a 90° angle to the foot in a 1× PBS bath while a series of sagittal B-mode HFUS images were acquired (0.5mm increments) using a 40MHz scanner (MS550D; VisualSonics, CA) (n=9–11/group).12, 27 The mean echogenicity (average gray scale brightness in image (range: 0 to 255)) was evaluated in a region of interest (ROI) selected to contain the zone of injury across 3–4 of the centermost sagittal HFUS images (spaced 0.5 mm apart) using MATLAB. Echogenicity measures may relate to collagen fibril type, orientation, density, hydration, and applied strain.28
Custom MATLAB software was also used to analyze the organization of the fascicle/fiber “bands” within the ex vivo images, as described.12, 27 A power-law function was fit to the resulting intensity versus angle data to determine the phase angle for each pixel bundle. The dispersion of phase angles across all pixel bundles was quantified by computing the circular standard deviation (CSD) and averaged across adjacent images.27
Mechanical Testing
Bone-tendon units were gripped using custom aluminum fixtures so that the foot and Achilles tendon were maintained perpendicular. The fixtures were attached to a testing frame (Electropuls E3000, Instron, Norwood, MA) using a 250N load cell. All specimens (n=9–10/group) remained submerged in a 1× PBS bath maintained at 37°C during testing. The mechanical testing protocol consisted of: (1) preloading (0.1N), (2) preconditioning (0.5–1.5% strain at 0.25Hz for 30 cycles), (3) stress relaxation (6% strain) for 10 minutes, (4) a dynamic frequency sweep (0.125% strain amplitude, at 0.1, 1, 5, and 10Hz, for 10 cycles each), (5) a ramp at 2% strain/s to 35N, and (6) fatigue testing (5–35N at 2Hz; ~10–70% of ultimate failure load) using a sinusoidal waveform until failure.12, 14, 22 During loading, force and displacement data were acquired and analyzed using MATLAB (Mathworks; Natick, MA). Images for optical strain measures (20 microns/pixel) were captured, as done previously.12 Tendon viscoelastic properties were computed, including the percent relaxation, dynamic modulus (|E*|), and tanδ. In addition, the slope of the linear region of the stress-strain curve was quantified to determine the linear modulus (resistance of a material to elastic deformation). Finally, the fatigue properties cycles to failure, laxity (measure of increase in tendon slack length), and hysteresis (measure of energy dissipation) were evaluated.29–32, 12
Histology
Achilles tendons were harvested immediately after sacrifice, and processed using standard paraffin procedures (n=4–8/group). Sagittal sections (7 µm) were collected and stained with Hematoxylin-Eosin (H&E) for cell density and shape, as well as with Safranin-O and Fast Green for glycosaminoglycans (GAGs). Cell density and shape were independently graded by 3 blinded investigators. Graders were provided with previously prepared standard images, and used a scale of 1 to 3 for cellularity (1, low; 2, moderate; 3, high) and 1 to 3 for shape (1, spindle shaped; 2, mixed; 3, rounded) to evaluate images acquired with a 10× objective lens of the H&E stained tendon midsubstance. Tendon length was determined by first stitching together several H&E images throughout the tendon length using an automated 2D motorized stage, and then measuring the tendon’s length with Image J (NIH, v1.48) software. Tendon length was defined as the distance from the proximal calcaneus to the most distal portion of the myotendinous junction present on the posterior aspect of the tendon. GAG content was similarly evaluated by stitching together images of sections stained with Safranin-O and Fast Green throughout the tendon length. Three blinded graders then categorized each image as either containing GAG staining proximal to the fibrocartilagenous insertion or not. In cases of discrepancy between grades, the score of the majority of graders was used. Two to four sections were averaged together per specimen for final analysis.
Immunohistochemistry
The effect of various treatments on the protein expression of the tendons was examined by immunohistochemistry. The same tissue specimens from histology were used and stained for collagen types I and III. The proteins were visualized using 3,3' Diaminobenzidine (DAB) as the chromogen for detection of the antibody-protein conjugate (SIGMAFAST™ from Sigma Aldrich: cat- D4293). Images of the midsubstance of each tendon were captured and further analyzed with Image J (NIH, v1.48). Images were converted to 8-bit images, a threshold was set, and sections were analyzed for percent area stained. Two to four sections were averaged together per specimen for final analysis.
Statistical Analysis
An a priori power analysis determined that 10 tendons were needed per group for mechanical testing,33 with 80% power. Data normality was evaluated with Shapiro-Wilk tests (SPSS, IBM SPSS, Inc., Version 20, Armon, NY). Normally distributed data sets were evaluated with two-way ANOVAs to assess the effect of surgical treatment and IM/ACT time on mechanical, structural, ankle joint functional, and immunohistochemical properties. Significant relationships (α=0.05) and trends (α=0.1) were analyzed with post hoc one-tailed Student’s t-tests with Bonferroni corrections for multiple comparisons. Comparisons between two groups were made using one-tailed Student’s t-tests. Non-normally distributed data sets (histological scoring) were evaluated with Scheirer-Ray-Hare tests34 (non-parametric two-way ANOVA), with post hoc one-tailed Mann-Whitney U-tests. Binary data sets (GAG staining) were evaluated using a binomial logistic regression.34 Briefly, this analysis determines the effect repair and IM/ACT timing have on the likelihood of observing GAG staining in any given section. To evaluate the temporal response following immobilization for the three IM/ACT regimens tested, we normalized 6-week post-injury data to the corresponding 1-week post-injury pair (R or NR). This analysis is necessary to specifically evaluate the role of activity following cast removal, as all groups had 1-week of cast immobilization following injury. In doing so, this step removes any differences in healing during the first week of immobilization.
Results
When evaluating limb function 6-weeks post-injury, IM/ACT was a significant factor on ankle ROM and toe stiffness in both dorsiflexion and plantarflexion (Table 1). Specifically, IM3/ACT3 groups had decreased ROM compared to IM1/ACT5 groups and increased toe stiffness. No changes in the linear stiffness into dorsiflexion or plantarflexion were observed. Additionally, there was no effect of surgical treatment or IM/ACT on any quantitative ambulatory measures 6-weeks post-injury for IM1/ACT5 and IM3/ACT3 groups.
Table 1. Passive ankle joint properties.
Ankle range of motion (ROM) and stiffness during dorsiflexion and plantarflexion were evaluated on anesthetized animals.
| IM1/ACT5 | IM3/ACT3 | ||||
|---|---|---|---|---|---|
| R | NR | R | NR | ||
| Dorsiflexion | ROM (deg) | 53.86 ± 7.54c | 54.41 ± 9.09d | 47.14 ± 9.31a | 45.46 ± 8.79b |
| Toe Stiffness (N*mm/deg) | 0.06 ± 0.02c | 0.06 ± 0.03d | 0.12 ± 0.03a | 0.11 ± 0.05b | |
| Linear Stiffness (N*mm/deg) | 0.60 ± 0.15 | 0.55 ± 0.13 | 0.69 ± 0.20 | 0.64 ± 0.13 | |
|
| |||||
| Plantarflexion | ROM (deg) | 43.42 ± 10.23c | 53.96 ± 9.19d | 32.44 ± 8.64a | 40.46 ± 11.67b |
| Toe Stiffness (N*mm/deg) | 0.07 ± 0.02b,c | 0.06 ± 0.01a,d | 0.10 ± 0.03a,d | 0.10 ± 0.03b,c | |
| Linear Stiffness (N*mm/deg) | 0.27 ± 0.07 | 0.28 ± 0.06 | 0.23 ± 0.02 | 0.23 ± 0.04 | |
denote significant differences between (a) IM1/ACT5 repaired tendons, (b) IM1/ACT5 non-repaired tendons, (c) IM3/ACT3 repaired tendons, and (d) IM3/ACT3 non-repaired tendons after Bonferroni correction (p<0.013).
Zero degrees indicates that the foot is positioned perpendicular to the tibia.
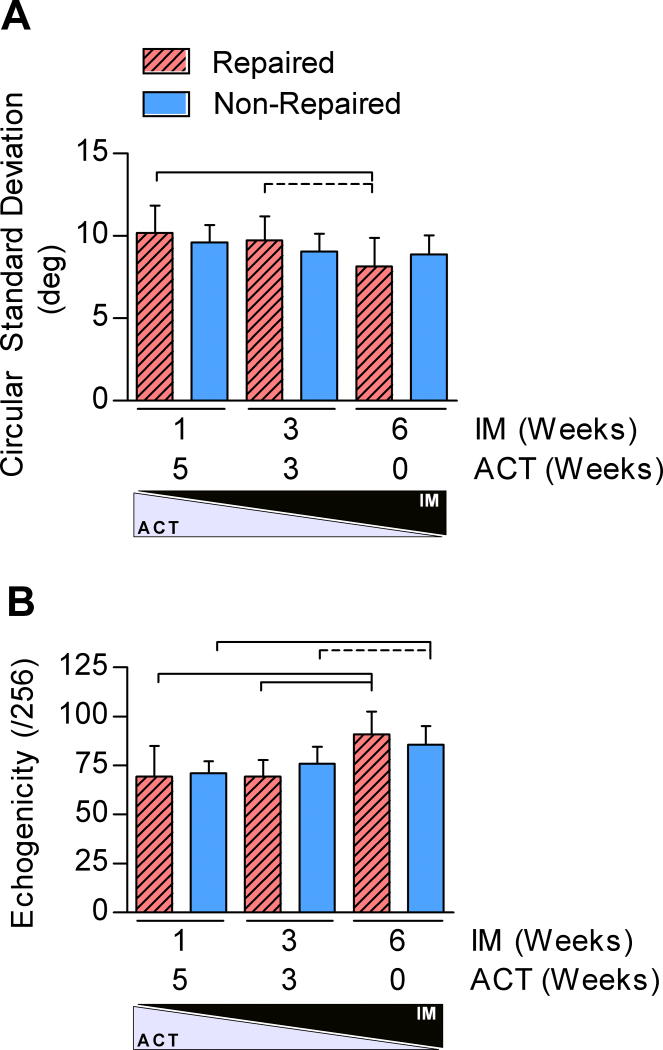
To investigate whether changes in limb function related to tendon structure, we evaluated tendon echogenicity and fiber alignment using HFUS. IM/ACT was a significant factor for both tendon echogenicity and fiber alignment. IM6/ACT0 increased fiber organization in repaired tendons and elevated echogenicity in repaired and non-repaired tendons 6-weeks post-injury (Figure 2).
Figure 2. Structural Evaluation using HFUS.
The region of interest (ROI) analyzed was chosen at the injury site (tendon midsubstance) to quantify the (A) circular standard deviation (CSD), and (B) echogenicity.
*Solid lines indicate significant differences (p<0.0083) and dashed lines indicate trends (p<0.017). Error bars indicate standard deviation.
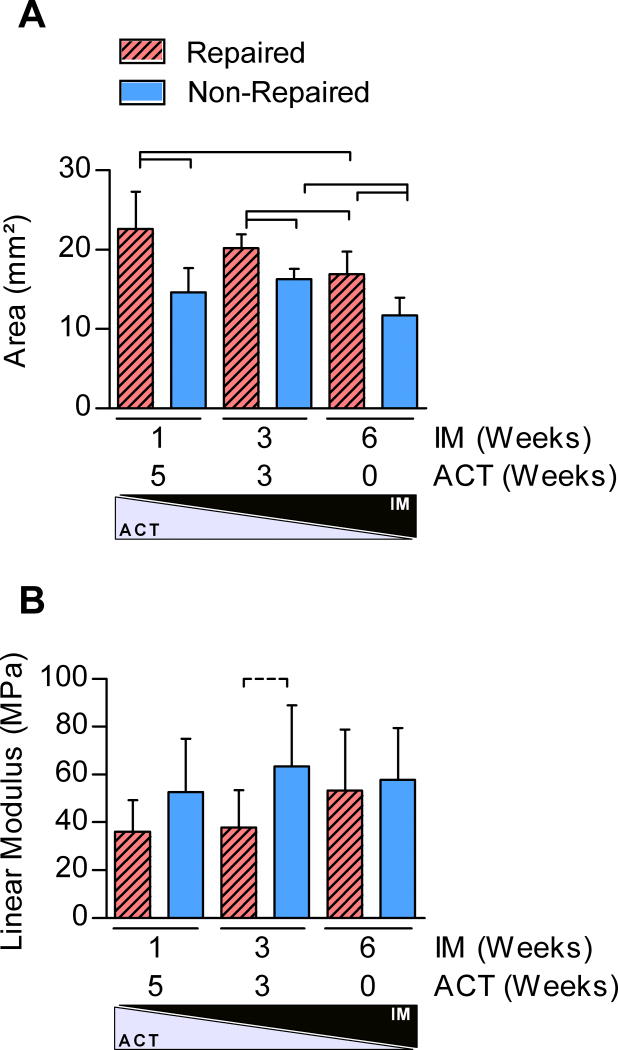
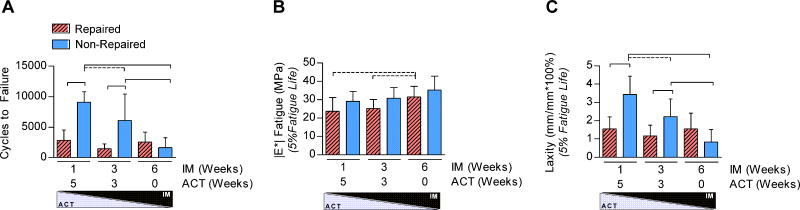
Tissue-level morphological and mechanical properties demonstrated the largest differences with surgical treatment and IM/ACT. Both surgical treatment and IM/ACT were significant factors on tendon CSA; repair and early activity significantly increased tendon CSA compared to non-repair and IM6/ACT0 (Figure 3A). Mechanical testing results found that surgical treatment was a significant factor for the linear modulus (Figure 3B), with a trend toward an increase in non-repaired IM3/ACT3 groups 6-weeks post-injury. Surgical repair and IM/ACT had no effect on tendon relaxation or low-strain viscoelastic properties, |E*| or tanδ. By 6-weeks post-injury, both surgical treatment and IM/ACT were significant determinants of tendon fatigue properties. Specifically, IM6/ACT0 dramatically decreased the number of fatigue cycles until tendon failure and was associated with decreased tendon laxity (at 5% fatigue life) in non-repaired tendons (Figure 4A,C). Repaired tendons exhibited a decreased number of cycles to failure and decreased laxity compared to non-repaired tendons (IM1/ACT5 and IM3/ACT3 groups) at 5% fatigue life. However, at 50% and 95% fatigue life, differences in tendon laxity were only observed between repaired and non-repaired treatments in IM1/ACT5 groups (not shown). IM/ACT was also a significant factor for secant modulus in repaired tendons (Figure 4B), and surgical treatment was a significant factor for tendon hysteresis assessed during fatigue loading.
Figure 3. Tendon area and modulus.
(A) Repair and IM/ACT were significant factors on tendon cross sectional area. (B) Repair was a significant factor on tendon linear modulus.
*Panel A: Solid lines indicate significant differences (p<0.0056).
**Panel B: Solid lines indicate significant differences (p<0.017) and dotted lines indicate trends (p<0.033).
***In both panels, error bars indicate standard deviation.
Figure 4. Fatigue Testing.
Fatigue testing revealed that both surgical repair and IM/ACT affected the (A) number of cycles to failure and (C) tendon laxity. (B) The |E*| was affected by IM/ACT.
*Panels A and C: Solid lines indicate significant differences after Bonferroni correction (p<0.0056) and dashed lines indicate trends (p<0.011).
**Panel B: Dashed lines indicate trends after Bonferroni correction (p<0.017).
****In all panels, error bars indicate standard deviation.
Histological evaluation found that surgical treatment was a significant factor on nuclear shape; however, no differences in cellularity were observed between groups 6-weeks post-injury (Table 2, Figure S1). Interestingly, binomial regression revealed that GAG staining was ~10 times more likely (95% CI: 3–35) to be present in IM1/ACT5 tendons (Figure S2). There was a significant interaction between surgical repair and IM/ACT for the collagen type I:III ratio, which was elevated in IM6/ACT0 repaired groups and in non-repaired IM1/ACT5 tendons (Figure S3). Repair was a significant factor on the tendon length, as repaired tendons were shorter than non-repaired tendons for IM1/ACT5 and IM6/ACT0 groups (Table 2).
Table 2. Histology and Immunohistochemistry.
| IM1/ACT5 | IM3/ACT3 | IM6/ACT0 | ||||
|---|---|---|---|---|---|---|
| R | NR | R | NR | R | NR | |
| Cellularity | 2.75 (1.00) | 2 (0.25) | 2.25 (1.63) | 2.00 (0.50) | 2 (0.75) | 2.00 (1.00) |
| Cell Shape | 2.75 (1.13) | 2 (1.13) | 2.00 (0.75) | 2.00 (0.75) | 2 (1.25) | 2.00 (0.00) |
| Col1:3 Ratio | 0.57 ± 0.27b,e | 0.91 ± 0.13a | 0.90 ± 0.29 | 0.71 ± 0.22 | 0.98 ± 0.14b | 0.89 ± 0.09 |
| Length | 7.97 ± 1.78b | 10.26 ±1.34a | 8.92 ± 1.04 | 9.46 ± 1.17 | 6.95 ± 1.19f | 9.54 ± 0.97e |
denote significant differences between (a) IM1/ACT5 repaired tendons, (b) IM1/ACT5 non-repaired tendons, (c) IM3/ACT3 repaired tendons, (d) IM3/ACT3 non-repaired tendons, (e) IM6/ACT0 repaired tendons, and (f) IM6/ACT0 non-repaired tendons after Bonferroni correction.
For the col1:3 ratio- p<0.0083
For length- p<0.017
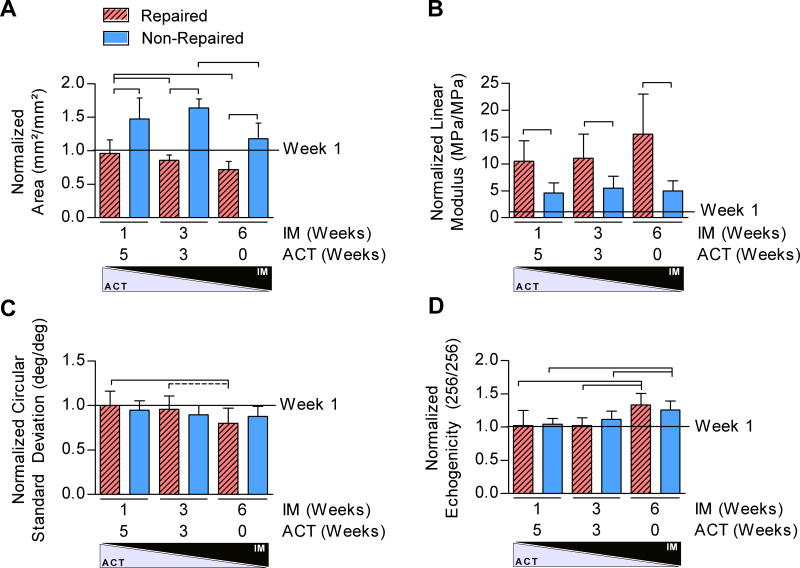
We next investigated the temporal change in healing response from 1- to 6-weeks post-injury. Surgical treatment was a significant factor on the normalized property changes of tendon area and linear modulus from 1 to 6 weeks post-injury (Figure 5A,B). Surprisingly, non-repaired tendons exhibited a greater normalized increase in area but a smaller normalized increase in linear modulus from 1 to 6 weeks post-injury compared to repaired tendons. Additionally, IM/ACT had a significant effect on the normalized property change of tendon area, CSD, and echogenicity from 1 to 6 weeks post-injury (Figure 5A,C,D). Both the normalized area and alignment decreased with decreased ACT in the repaired groups only, whereas the normalized echogenicity increased in both repaired and non-repaired groups.
Figure 5. Temporal Effects of Achilles Healing.
All properties 6-weeks post-injury were normalized to group means for data 1-week post-injury. (A) Both repair and IM/ACT were significant factors for the normalized tendon area. (B) Surgical repair was a significant factor on the normalized linear modulus. (C–D) IM/ACT was a significant factor on the normalized circular standard deviation and echogenicity.
*Panel A: Solid lines indicate significant differences after Bonferroni correction (p<0.0056).
**Panel B: Solid lines indicate trends after Bonferroni correction (p<0.017).
***Panel D: Solid lines indicate significant differences (p<0.0083) and dashed lines indicate trends (p<0.017) after Bonferroni correction.
****In all panels, error bars indicate standard deviation.
Discussion
This study investigated the effects of surgical or non-surgical treatment and varying IM/ACT timing on Achilles tendon healing over time. The animal model system employed incorporates blunt transection of the Achilles tendon, followed by immediate post-injury immobilization of the injured hindlimb in full plantarflexion to minimize tendon gap formation. Novel in vivo and ex vivo methods of tendon healing were used in this study, including quantitative ambulatory analysis, passive ankle joint properties that assess both ROM and joint stiffness, and HFUS-based analysis of tendon structure. This study builds on previous work12 by specifically examining the temporal effects of different IM/ACT regimens following an initial period of post-injury immobilization.
The effects of injury and immobilization on limb function when assessed 3-weeks post-injury12 were drastically improved by ACT when animals were reevaluated an additional 3-weeks later (i.e., 6-weeks post-injury). Although IM (IM3/ACT3 vs. IM1/ACT5) was associated with mild deficits in passive hindlimb ankle ROM and stiffness by 6-weeks post-injury, active hindlimb weight-bearing at 6-weeks was similar in IM3/ACT3 and IM1/ACT5. This indicates that immediate ankle joint stiffness following cast immobilization is able to improve rapidly with IM1/ACT5. Additionally, functional limb properties post-injury were similar between repaired and non-repaired treatments, in agreement with clinical assessment in humans.8, 7, 9, 12
Consistent with findings 3-weeks post-injury,12 6-weeks of immobilization resulted in elevated echogenicity and collagen fiber organization at the injury site. However, although echogenicity increased when normalized to values 1-week post-injury in IM6/ACT0 groups regardless of surgical treatment, collagen fiber organization increased in surgically repaired tendons only. Differences in fiber alignment occurred in concert with the elevated collagen type I:III ratio observed in repaired tendons with IM6/ACT0 6-weeks post-injury, which is typically associated with superior mechanical strength and a more tendon-like phenotype.35 Yet, although repair is commonly thought to reduce tendon lengthening and scar formation,36 repaired tendons also had elevated CSA and displayed overall mechanically inferior properties with IM1/ACT5 than non-repaired tendons. Treadmill exercise promoted mechanical robustness, but only in non-repaired tendons, suggesting that these different treatment paradigms warrant tailored IM/ACT protocols to optimize healing.
Both surgical treatment and IM/ACT greatly affect tendon CSA, further highlighting the known response of Achilles tendons to mechanical loading.37, 38 Although repair and IM1/ACT5 both resulted in increased tendon area by 6-weeks post-injury, normalization to 1-week post- injury values revealed that fold-increases in tendon area were greater in non-repaired tendons (versus repaired) between 1- and 6-weeks. Strikingly, ~5–10 fold-increases in linear modulus were observed between 1- and 6-weeks post-injury, which were greatest in repaired tendons, despite linear modulus at 6-weeks post-injury still ultimately being greater in the non-repaired group. Together, these data indicate that distinct differences in tendon healing are present as early as 1-week post-injury. Moreover, changes in neither area nor tissue structure (e.g., collagen alignment) fully explain the substantial increase in linear modulus, suggesting that there are additional properties contributing to scar robustness.
Surgical treatment greatly affected the mechanical response to fatigue loading, as the number of cycles to failure for IM1/ACT5 and IM3/ACT3 groups was dramatically lower in repaired tendons. This response is consistent with our previous study that also found that surgical treatment resulted in inferior fatigue life.12 Surprisingly, there was no dependence of IM/ACT on the number of cycles to failure for repaired tendons. This data suggests that while repaired tendons exhibit inferior material properties (which may result in less efficient transfer of stress from muscle to bone), the risk of re-rupture of repaired tendons across different IM/ACT paradigms may be similar. As noted, non-repaired tendon area increased with time, unlike repaired tendons, which may allow non-repaired tendons to be more fatigue resilient. Tendon laxity and area may be related to the number of cycles to failure, such that larger tendons capable of dynamic stretch early in fatigue life may be more likely to withstand repetitive loading at the expense of initial additional lengthening. That additional lengthening may affect the ultimate plantarflexion strength, but was not evidenced in differences comparing repaired versus non-repaired treatments in any of our active functional measures.
In agreement with previous work,12 there were no differences in cellularity between groups. Contrary to our hypothesis, even after 6-weeks post injury, cell counts remained high and nuclei remained more rounded compared to uninjured tendon.14 This finding suggests that in our model system at 6-weeks post-injury healing is not yet complete, or alternatively, if healing is complete that a new aberrant tissue phenotype has resulted from injury. If the latter scenario is true, such a failure to regain normal pre-injury values would be supported by a previous study, which demonstrated that ruptured human Achilles tendons may have elevated metabolic activity up to one year post-injury.39 Tendons with IM1/ACT5 were significantly more likely to exhibit GAG staining than IM6/ACT0 tendons, which may suggest a potential compositional downside to returning to sport early. When compared to data 3-weeks post-injury, GAG deposits were more prevalent in tissues 6-weeks post-injury, suggesting that there is a temporal response to GAG accumulation in Achilles tendons following rupture. Changes in GAG accumulation generally occurred in concert with changes in mechanical properties and structure.40, 31 Previous studies have suggested that local mesenchymal stromal cell populations may exhibit a varying predilection for proteoglycan expression, and this variation has been thought to explain differences in glycosaminoglycan staining post-injury.22, 41 It is also possible that a combination of mechanical loading environment42 and time post-injury may drive GAG accumulation, but this remains to be elucidated.
The role of loading environments on Achilles tendon healing has been studied extensively, including tail suspension,16, 43 botox injection,15 whole body vibration,44 and immobilization.12, 19 However, none of these studies evaluated the role of remobilization following more than 1-week of cast immobilization. Our rodent hind limb immobilization technique does not require external fixation, which mimics standard casting techniques used in humans. Previous studies have reported deficits in the Achilles Functional Index, ground reaction forces, and ankle dorsiflexion and plantarflexion (ROM and stiffness) following cast immobilization removal in rodents.19, 12 Such findings are consistent in humans, as early functional rehabilitation can lead to an earlier return to function7 and does not produce functional deficits in the long term after an initial period of casting.9, 10 In one study,10 it was shown that early tensional loading in repaired tendons increased elastic modulus when assessed with roentgen stereophotogrammetric analysis, at both 19- and 52-weeks after cast removal at 7-weeks post-surgery. In contrast, the current study found that IM1/ACT5 decreased the dynamic fatigue modulus and had no effect on the linear modulus in repaired tendons 6-weeks post-injury. Consistently, when assessed at 3-weeks post-injury, IM1/ACT5 did not affect the linear modulus in repaired tendons,12 but had drastic effects on resilience to fatigue loading. This discrepancy may be due to differences in scar formation with early loading or our more direct ex vivo assessment of mechanical properties, which does not rely on measuring the in vivo moment arm of the Achilles tendon.45
There are limitations to this study. First, we recognize that our non-repaired group still requires open surgery to create the injury. Although there were no wound complications, it is possible that the open transection does not fully mimic the injury onset in vivo. This study specifically evaluated the role of an acute injury on Achilles tendon healing with the benefit that this procedure is highly controlled and repeatable. Additionally, alignment properties specifically assess the injury region, which does not capture potential fiber malalignment at the tendon-suture interface in repaired tendons (where failure typically occurs). Thus, it is possible that more localized differences in fiber architecture exist that cannot be detected with HFUS. Future studies will investigate gene expression at these healing time points to provide additional mechanistic insight into the observed responses. Ongoing studies in our laboratory are examining the role of biological sex, aging, angle of immobilization, and adjacent tissues, such as muscle, on Achilles tendon healing.
In conclusion, this study demonstrated that the temporal healing response of Achilles tendons in rodents following injury depends on surgical treatment and IM/ACT time. We revealed that IM1/ACT5 maximizes tissue property changes during healing, particularly in non-repaired tendons. IM/ACT timing can affect concurrent changes in fatigue life, tendon laxity, echogenicity, and ankle function in non-repaired tendons, while modulating primarily structure in repaired tendons. Given the different pattern of healing and qualities of repaired versus non-repaired tendons, there may be two very different healing processes occurring according to the chosen IM/ACT regimens. Furthermore, large functional deficits produced prior to remobilization are transient and may be only necessary for clinical consideration if the individual requires joint mobility immediately. Together, this work continues to evaluate the scientific basis for Achilles tendon treatment methods used clinically following rupture.
Supplementary Material
Figure S1: Histological Evaluation. Hematoxylin and Eosin stained slides of the injury site at the tendon midsubstance comparing the role of repair and activity. Surgical treatment was a significant factor on a rounder nuclear shape; however, no differences in cellularity were observed between groups 6-weeks post-injury.
Figure S2: GAG Staining. Safranin-O and Fast-Green stained slides of the injury site at the tendon midsubstance comparing the role of repair and activity. GAG staining was ~10 times more likely (95% CI: 3–35) to be present in IM1/ACT5 groups.
Figure S3: Immunohistochemistry Collagen type III stained slides of the injury site at the tendon midsubstance comparing the role of repair and activity. No differences in collagen type III staining were observed between groups.
Acknowledgments
Grant Support: This study was funded by NIH/NIAMS R01AR064216 and the NIH/NIAMS supported Penn Center for Musculoskeletal Disorders (P30 AR06919).
References
- 1.Huttunen TT, Kannus P, Rolf C, Fellander-Tsai L, Mattila VM. Acute achilles tendon ruptures: incidence of injury and surgery in Sweden between 2001 and 2012. Am J Sports Med. 2014;42:2419–2423. doi: 10.1177/0363546514540599. [DOI] [PubMed] [Google Scholar]
- 2.Schepull T, Kvist J, Aspenberg P. Early E-modulus of healing Achilles tendons correlates with late function: similar results with or without surgery. Scand J Med Sci Sports. 2012;22:18–23. doi: 10.1111/j.1600-0838.2010.01154.x. [DOI] [PubMed] [Google Scholar]
- 3.Kearney RS, McGuinness KR, Achten J, Costa ML. A systematic review of early rehabilitation methods following a rupture of the Achilles tendon. Physiotherapy. 2012;98:24–32. doi: 10.1016/j.physio.2011.04.349. [DOI] [PubMed] [Google Scholar]
- 4.Soroceanu A, Sidhwa F, Aarabi S, Kaufman A, Glazebrook M. Surgical versus nonsurgical treatment of acute Achilles tendon rupture: a meta-analysis of randomized trials. J Bone Joint Surg Am. 2012;94:2136–2143. doi: 10.2106/JBJS.K.00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.AAOS: Guideline on the Diagnosis and Treatment of Acute Achilles Tendon Rupture. 2009 [Google Scholar]
- 6.Freedman BR, Gordon JA, Soslowsky LJ. The Achilles tendon: fundamental properties and mechanisms governing healing. Muscles Ligaments Tendons J. 2014;4:245–255. [PMC free article] [PubMed] [Google Scholar]
- 7.McCormack R, Bovard J. Early functional rehabilitation or cast immobilisation for the postoperative management of acute Achilles tendon rupture? A systematic review and a meta-analysis of randomised controlled trials. Br J Sports Med. 2015 doi: 10.1136/bjsports-2015-094935. [DOI] [PubMed] [Google Scholar]
- 8.Olsson N, Silbernagel KG, Eriksson BI, et al. Stable Surgical Repair With Accelerated Rehabilitation Versus Nonsurgical Treatment for Acute Achilles Tendon Ruptures: A Randomized Controlled Study. Am J Sports Med. 2013 doi: 10.1177/0363546513503282. [DOI] [PubMed] [Google Scholar]
- 9.Lantto I, Heikkinen J, Flinkkila T, et al. Early Functional Treatment Versus Cast Immobilization in Tension After Achilles Rupture Repair: Results of a Prospective Randomized Trial With 10 or More Years of Follow-up. Am J Sports Med. 2015;43:2302–2309. doi: 10.1177/0363546515591267. [DOI] [PubMed] [Google Scholar]
- 10.Schepull T, Aspenberg P. Early Controlled Tension Improves the Material Properties of Healing Human Achilles Tendons After Ruptures: A Randomized Trial. Am J Sports Med. 2013 doi: 10.1177/0363546513501785. [DOI] [PubMed] [Google Scholar]
- 11.Huang J, Wang C, Ma X, Wang X, Zhang C, Chen L. Rehabilitation regimen after surgical treatment of acute Achilles tendon ruptures: a systematic review with meta-analysis. Am J Sports Med. 2015;43:1008–1016. doi: 10.1177/0363546514531014. [DOI] [PubMed] [Google Scholar]
- 12.Freedman BR, Gordon JA, Bhatt PB, et al. Nonsurgical treatment and early return to activity leads to improved Achilles tendon fatigue mechanics and functional outcomes during early healing in an animal model. J Orthop Res. 2016 doi: 10.1002/jor.23253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suchak AA, Bostick GP, Beaupre LA, Durand DC, Jomha NM. The influence of early weight-bearing compared with non-weight-bearing after surgical repair of the Achilles tendon. J Bone Joint Surg Am. 2008;90:1876–1883. doi: 10.2106/JBJS.G.01242. [DOI] [PubMed] [Google Scholar]
- 14.Pardes AM, Freedman BR, Fryhofer GW, Salka NS, Bhatt PR, Soslowsky LJ. Males have Inferior Achilles Tendon Material Properties Compared to Females in a Rodent Model. Ann Biomed Eng. 2016 doi: 10.1007/s10439-016-1635-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersson T, Eliasson P, Hammerman M, Sandberg O, Aspenberg P. Low-level mechanical stimulation is sufficient to improve tendon healing in rats. J Appl Physiol (1985) 2012;113:1398–1402. doi: 10.1152/japplphysiol.00491.2012. [DOI] [PubMed] [Google Scholar]
- 16.Eliasson P, Andersson T, Aspenberg P. Achilles tendon healing in rats is improved by intermittent mechanical loading during the inflammatory phase. J Orthop Res. 2012;30:274–279. doi: 10.1002/jor.21511. [DOI] [PubMed] [Google Scholar]
- 17.Murrell GA, Lilly EG, 3rd, Collins A, Seaber AV, Goldner RD, Best TM. Achilles tendon injuries: a comparison of surgical repair versus no repair in a rat model. Foot Ankle. 1993;14:400–406. doi: 10.1177/107110079301400706. [DOI] [PubMed] [Google Scholar]
- 18.Murrell GA, Lilly EG, Davies H, Best TM, Goldner RD, Seaber AV. The Achilles Functional Index. J Orthop Res. 1992;10:398–404. doi: 10.1002/jor.1100100313. [DOI] [PubMed] [Google Scholar]
- 19.Murrell GA, Lilly EG, 3rd, Goldner RD, Seaber AV, Best TM. Effects of immobilization on Achilles tendon healing in a rat model. J Orthop Res. 1994;12:582–591. doi: 10.1002/jor.1100120415. [DOI] [PubMed] [Google Scholar]
- 20.Bring DK, Reno C, Renstrom P, Salo P, Hart DA, Ackermann PW. Joint immobilization reduces the expression of sensory neuropeptide receptors and impairs healing after tendon rupture in a rat model. J Orthop Res. 2009;27:274–280. doi: 10.1002/jor.20657. [DOI] [PubMed] [Google Scholar]
- 21.Eliasson P, Andersson T, Aspenberg P. Rat Achilles tendon healing: mechanical loading and gene expression. J Appl Physiol (1985) 2009;107:399–407. doi: 10.1152/japplphysiol.91563.2008. [DOI] [PubMed] [Google Scholar]
- 22.Fryhofer GWFB, Hillin CD, Salka NS, Pardes AM, Weiss SN, Farber DC, Soslowsky LJ. Post-Injury Biomechanics of Achilles Tendon Vary By Sex and Hormone Status. 2016 doi: 10.1152/japplphysiol.00620.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sebastin SJ, Ho A, Karjalainen T, Chung KC. History and evolution of the Kessler repair. J Hand Surg Am. 2013;38:552–561. doi: 10.1016/j.jhsa.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarver JJ, Dishowitz MI, Kim SY, Soslowsky LJ. Transient decreases in forelimb gait and ground reaction forces following rotator cuff injury and repair in a rat model. J Biomech. 2010;43:778–782. doi: 10.1016/j.jbiomech.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Favata M. Bioengineering. University of Pennsylvania; 2006. Scarless Healing in the Fetus: Implications and Strategies for Postnatal Tendon Repair. vol PhD. [Google Scholar]
- 26.Favata M. Bioengineering. Philadelphia: University of Pennsylvania; 2006. Scarless healing in the fetus: Implications and strategies for postnatal tendon repair. vol PhD. [Google Scholar]
- 27.Riggin CN, Sarver JJ, Freedman BR, Thomas SJ, Soslowsky LJ. Analysis of Collagen Organization in Mouse Achilles Tendon Using High-Frequency Ultrasound Imaging. J Biomech Eng. 2013 doi: 10.1115/1.4026285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chamberlain CS, Duenwald-Kuehl SE, Okotie G, Brounts SH, Baer GS, Vanderby R. Temporal Healing in Rat Achilles Tendon: Ultrasound Correlations. Ann Biomed Eng. 2012 doi: 10.1007/s10439-012-0689-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freedman BR, Sarver JJ, Buckley MR, Voleti PB, Soslowsky LJ. Biomechanical and structural response of healing Achilles tendon to fatigue loading following acute injury. J Biomech. 2013 doi: 10.1016/j.jbiomech.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freedman BR, Zuskov A, Sarver JJ, Buckley MR, Soslowsky LJ. Evaluating changes in tendon crimp with fatigue loading as an ex vivo structural assessment of tendon damage. J Orthop Res. 2015;33:904–910. doi: 10.1002/jor.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon J, Freedman B, Zuskov A, Iozzo R, Birk D, Soslowsky L. Achilles tendons from decorin- and biglycan-null mouse models have inferior mechanical and structural properties predicted by an image-based empirical damage model. Journal of Biomechanics. 2014 doi: 10.1016/j.jbiomech.2015.02.058. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peltz CD, Dourte LM, Kuntz AF, et al. The effect of postoperative passive motion on rotator cuff healing in a rat model. J Bone Joint Surg Am. 2009;91:2421–2429. doi: 10.2106/JBJS.H.01121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang TF, Perry SM, Soslowsky LJ. The effect of overuse activity on Achilles tendon in an animal model: a biomechanical study. Ann Biomed Eng. 2004;32:336–341. doi: 10.1023/b:abme.0000017537.26426.76. [DOI] [PubMed] [Google Scholar]
- 34.Dytham C. Choosing and Using Statistics: A Biologist's Guide. 3. Wiley-Blackwell; 2011. p. 298. [Google Scholar]
- 35.Eriksen HA, Pajala A, Leppilahti J, Risteli J. Increased content of type III collagen at the rupture site of human Achilles tendon. J Orthop Res. 2002;20:1352–1357. doi: 10.1016/S0736-0266(02)00064-5. [DOI] [PubMed] [Google Scholar]
- 36.Maquirriain J. Achilles tendon rupture: avoiding tendon lengthening during surgical repair and rehabilitation. Yale J Biol Med. 2011;84:289–300. [PMC free article] [PubMed] [Google Scholar]
- 37.Heinemeier KM, Skovgaard D, Bayer ML, et al. Uphill running improves rat Achilles tendon tissue mechanical properties and alters gene expression without inducing pathological changes. J Appl Physiol (1985) 2012;113:827–836. doi: 10.1152/japplphysiol.00401.2012. [DOI] [PubMed] [Google Scholar]
- 38.Maeda T, Sakabe T, Sunaga A, et al. Conversion of mechanical force into TGF-beta-mediated biochemical signals. Curr Biol. 2011;21:933–941. doi: 10.1016/j.cub.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eliasson P, Couppe C, Lonsdale M, et al. Ruptured human Achilles tendon has elevated metabolic activity up to 1 year after repair. Eur J Nucl Med Mol Imaging. 2016 doi: 10.1007/s00259-016-3379-4. [DOI] [PubMed] [Google Scholar]
- 40.Ahmadzadeh H, Connizzo BK, Freedman BR, Soslowsky LJ, Shenoy VB. Determining the contribution of glycosaminoglycans to tendon mechanical properties with a modified shear-lag model. J Biomech. 2013;46:2497–2503. doi: 10.1016/j.jbiomech.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veronesi F, Della Bella E, Torricelli P, Pagani S, Fini M. Effect of adipose-derived mesenchymal stromal cells on tendon healing in aging and estrogen deficiency: an in vitro co-culture model. Cytotherapy. 2015;17:1536–1544. doi: 10.1016/j.jcyt.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Freedman BR, Bade ND, Riggin CN, et al. The (dys)functional extracellular matrix. Biochim Biophys Acta. 2015;1853:3153–3164. doi: 10.1016/j.bbamcr.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eliasson P, Andersson T, Hammerman M, Aspenberg P. Primary gene response to mechanical loading in healing rat Achilles tendons. J Appl Physiol (1985) 2013;114:1519–1526. doi: 10.1152/japplphysiol.01500.2012. [DOI] [PubMed] [Google Scholar]
- 44.Keller BV, Davis ML, Thompson WR, Dahners LE, Weinhold PS. Varying whole body vibration amplitude differentially affects tendon and ligament structural and material properties. J Biomech. 2013;46:1496–1500. doi: 10.1016/j.jbiomech.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheehan FT. The 3D in vivo Achilles' tendon moment arm, quantified during active muscle control and compared across sexes. J Biomech. 2012;45:225–230. doi: 10.1016/j.jbiomech.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Histological Evaluation. Hematoxylin and Eosin stained slides of the injury site at the tendon midsubstance comparing the role of repair and activity. Surgical treatment was a significant factor on a rounder nuclear shape; however, no differences in cellularity were observed between groups 6-weeks post-injury.
Figure S2: GAG Staining. Safranin-O and Fast-Green stained slides of the injury site at the tendon midsubstance comparing the role of repair and activity. GAG staining was ~10 times more likely (95% CI: 3–35) to be present in IM1/ACT5 groups.
Figure S3: Immunohistochemistry Collagen type III stained slides of the injury site at the tendon midsubstance comparing the role of repair and activity. No differences in collagen type III staining were observed between groups.