Abstract
Individuals with Down syndrome (DS) overexpress many genes on chromosome 21 due to trisomy and have high risk of dementia due to the Alzheimer’s disease (AD) neuropathology. However, there is a wide range of phenotypic differences (e.g., age at onset of AD, amyloid β levels) among adults with DS, suggesting the importance of factors that modify risk within this particularly vulnerable population, including genotypic variability. Previous genetic studies in the general population have identified multiple genes that are associated with AD. This study examined the contribution of polymorphisms in these genes to the risk of AD in adults with DS ranging from 30 to 78 years of age at study entry (N = 320). We used multiple logistic regressions to estimate the likelihood of AD using single-nucleotide polymorphisms (SNPs) in candidate genes, adjusting for age, sex, race/ethnicity, level of intellectual disability and APOE genotype. This study identified multiple SNPs in APP and CST3 that were associated with AD at a gene-wise level empirical p-value of 0.05, with odds ratios in the range of 1.5–2. SNPs in MARK4 were marginally associated with AD. CST3 and MARK4 may contribute to our understanding of potential mechanisms where CST3 may contribute to the amyloid pathway by inhibiting plaque formation, and MARK4 may contribute to the regulation of the transition between stable and dynamic microtubules.
Keywords: Down syndrome, Alzheimer disease, Dementia, Gene mapping, Candidate genes, APP, CST3, MARK4
1. Introduction
Adults with Down syndrome (DS) are at high risk of developing Alzheimer’s disease (AD) (Schupf, 2002; Zigman, 2013; Zigman and Lott, 2007), and many, but not all, will develop dementia by the end of their seventh decade of life (Lai and Williams, 1989; Zigman, 2013). The neuropathological manifestations of AD in DS have been attributed, at least in part, to triplication and overexpression of the gene for amyloid precursor protein (APP) located on chromosome 21 (Rumble et al., 1989), leading to an increased substrate for production of amyloid β (Aβ) peptides (Mehta et al., 1998; Schupf et al., 2001; Tokuda et al., 1997). Of the two major species of Aβ peptides—Aβ40 and Aβ42—generated by sequential proteolytic cleavage by β and γ secretases of the APP (Selkoe, 2001), lower levels of Aβ42 or the Aβ42/Aβ40 ratio in cerebrospinal fluid along with high levels of tau are associated with high risk of AD (Blennow and Hampel, 2003; Jack et al., 2013). However, even among individuals with full trisomy 21, age at onset of AD varies widely, and levels of Aβ40 and Aβ42 and Aβ42/Aβ40 ratio also vary widely even among individuals who are of comparable age (Coppus et al., 2008; Head et al., 2012; Holland et al., 2000; Lai and Williams, 1989; Schupf, 2002; Zigman et al., 2007).
Genetic as well as environmental factors may contribute to the observed variation in age at onset. Multiple genome-wide association studies (GWAS) and meta-analyses have identified at least 20 genes that are significantly associated with AD in the general population (Bertram et al., 2007; Hollingworth et al., 2011; Lambert et al., 2009, 2013; Lee et al., 2011; Naj et al., 2011; Wijsman et al., 2011). To date, however, only 1 genome-wide study of age at the onset of AD in DS based 67 autopsy samples has been reported (Jones et al., 2013). Several studies have examined the relation between single nucleotide polymorphisms (SNPs) and dementia in adults with DS using a candidate gene approach (Jones et al., 2013; Lee et al., 2007b; Liu et al., 2008; Margallo-Lana et al., 2004; Mok et al., 2014; Patel et al., 2011). In addition, mouse models of DS have identified genes that are differentially expressed between AD and controls (Chrast et al., 2000; Cook et al., 2005; Lyle et al., 2004; Prandini et al., 2007). Compared with individuals without DS, triplication and overexpression of genes that are located on chromosome 21, including APP and others, may contribute to AD risk or more general atypical aging in adults with DS. Some of these genes have also been implicated in AD pathogenesis. These include beta amyloid converting enzyme-2 (BACE2), superoxide dismutase (SOD1), and the astrocyte-derived neurotrophic factor S100 beta (S100β). In the present study, we examined SNPs in candidate genes on chromosome 21 as well as a subset of autosomes and chromosome X to determine their contribution to variation in risk for dementia due to AD in a large longitudinal cohort of adults with DS (refer to Supplement Table 1 for a complete list of candidate genes).
2. Materials and methods
2.1. Study participants
We examined 93 individuals with dementia and 227 without dementia for a total of 320 community-residing adults with confirmed DS (Table 1). All individuals were 30 years of age and older at the time of their study enrollment (range 31–78) and resided in New York, Connecticut, New Jersey, or eastern Pennsylvania. Participants were recruited with the help of state and voluntary service provider agencies and were eligible for inclusion in the present study if: (1) a family member or correspondent provided informed consent; (2) he or she either provided consent or assent indicating willingness to participate; and (3) he or she was willing and able to provide blood samples. Recruitment, informed consent, and study procedures were approved by the Institutional Review Boards of the New York State Institute for Basic Research in Developmental Disabilities, Columbia University Medical Center, and the Johns Hopkins University School of Medicine.
Table 1.
Characteristics of the study participants
| Characteristics | Combined | Dementia | No dementia |
|---|---|---|---|
| Number of individuals | 320 | 93 | 227 |
| Mean age at baseline (SD) | 49.9 (7.58) | 55.4 (7.28) | 47.7 (6.48) |
| Level of intellectual disability (n, %) | |||
| Mild/moderate | 186 (58.1) | 47 (50.5) | 139 (61.2) |
| Severe/profound | 134 (42.9) | 46 (49.5) | 88 (38.8) |
| Ethnicity (n, %) | |||
| White | 294 (91.9) | 87 (93.5) | 207 (91.2) |
| Non-White | 26 (8.1) | 6 (6.5) | 20 (8.8) |
| APOE allele frequencya | |||
| ε2 | 0.077 | 0.065 | 0.082 |
| ε3 | 0.807 | 0.801 | 0.809 |
| ε4 | 0.116 | 0.134 | 0.109 |
| Sex (n, %) | |||
| Female | 235 (73.4) | 65 (69.9) | 170 (74.9) |
| Male | 85 (26.6) | 28 (30.1) | 57 (25.1) |
Two subjects missing APOE status.
2.2. Clinical assessment
Assessments were conducted at the time of study entry and were repeated at intervals of approximately 18 months for up to 5 cycles of follow-up (mean duration of follow-up of 4.5 years; SD = 1.89). Assessments included evaluations of cognition and functional abilities, behavioral/psychiatric conditions, and an examination of medical records for information on health status and medication usage. Cognitive function was evaluated with a test battery designed for use with individuals varying widely in their initial levels of intellectual functioning, as previously described (Silverman et al., 2004). Structured interviews were conducted with caregivers to collect information on adaptive behavior and neuropsychiatric conditions. Past and current medical records were reviewed for all participants.
For diagnostic classification of dementia, recommendations of the AAMR-IASSID Working Group for the Establishment of Criteria for the Diagnosis of Dementia in Individuals with Developmental Disability were followed (Aylward et al., 1997; Burt and Aylward, 2000). After each assessment cycle, dementia classification was made based on consensus case conferences relying on empirical evidence of stability or decline in performance profiles over time (Silverman et al., 2004). Each individual was classified as: (1) no dementia, indicating with reasonable certainty that significant impairment was absent; (2) MCI-DS, indicating that there was evidence of mild cognitive or functional decline, but importantly, the observed change did not meet dementia criteria; (3) possible dementia, indicating that some signs and symptoms of dementia were present but declines over time was not entirely convincing; and (4) definite dementia, indicating with reasonable confidence that dementia was present based on substantial decline over time.
2.3. Selection of candidate genes
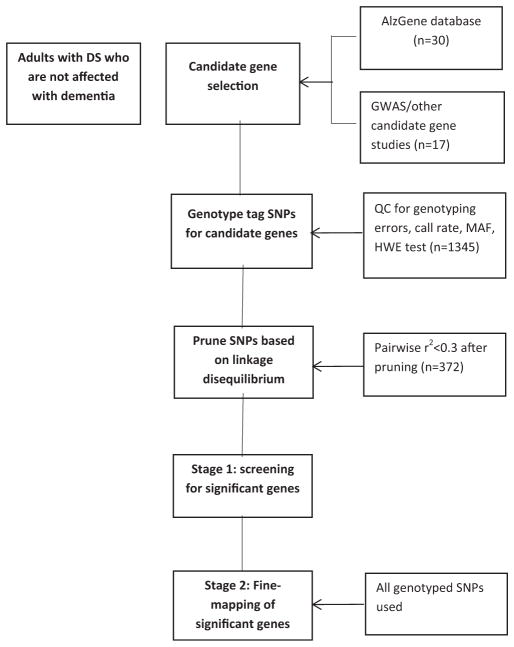
Candidate genes (see Supplement Table 1) were selected based on previous reports of positive associations with AD or dementia, either in adults with DS or the general population. These genes included: (1) SNPs that were found to be significant in other genetic studies of DS; (2) the top candidate genes from the ALZGENE database when the customized SNP chips were being developed for this study between 2012 and 2013; and (3) additional positional candidate genes from published genome-wide linkage and association studies. Due to the limited capacity of the Illumina’s GoldenGate platform, only a subset of candidate genes was examined. For candidate regions from genome-wide linkage or association studies where precise genes have not been identified, we used SNAP (http://www.broadinstitute.org/mpg/snap/ldsearch.php) to identify genes within the candidate regions. This process generated 6 candidates on chromosome 21 and 41 genes on other chromosomes. Candidate genes on chromosome 21 included the genes for amyloid precursor protein (APP), β amyloid converting enzyme-2 (BACE2), the DS critical region-1 (DSCR1; also known as RCAN1), runt-related transcription factor 1 (RUNX1), the astrocyte-derived neurotrophic factor S100β, and CU/Zn superoxide dismutase (SOD-1). Additional candidate genes were on chromosomes 1, 2, 6–11, 15, 17, 19, 20, and X (see Supplement Table 1 for the full list of genes). Fig. 1 provides an overview of SNP selection and SNP analysis performed in this 2-stage candidate gene study.
Fig. 1.
Flow chart for a 2-stage candidate gene study of Alzheimer’s disease.
2.4. SNP selection
We genotyped each gene with a sufficient number of SNPs to provide relatively dense coverage (r2 ~ 0.8), and selected SNPs that had a relatively high minor allele frequency (>0.15) to increase the information content of each SNP, thereby enhancing statistical power. From these SNPs, we used the TAGGER program (de Bakker, 2009) to identify tag SNPs using the Caucasian samples from the HapMap data set (http://hapmap.ncbi.nlm.nih.gov). To ensure that coverage of the gene was relatively complete, we used SNAP (http://www.broadinstitute.org/mpg/snap/ldsearch.php) to check LD patterns across the genic region. For chromosome 21, 231 SNPS from the 6 genes had a median inter-marker distance of 2185 base pairs. For chromosomes other than 21, we identified 1114 SNPs from 41 genes with a median inter-marker distance of 2552 base pairs. In this article, we present top strands from the Illumina-customized platform.
2.5. SNP genotyping: customized SNP array in trisomic samples
Genomic DNA was genotyped using an Illumina GoldenGate custom array. Clustering and genotype calling of Chromosome 21 SNPs and non-Chr21 SNPs was performed using GenomeStudio genotyping module v1.8 which supports polyploidy loci. For SNPs on chromosome 21, the custom cluster option in GenomeStudio genotyping module v1.8 was used to specify 4 clusters and the custom GType was used to display genotype calls for polyploidy loci (AAA, AAB, ABB, or BBB). All genotype calls were then inspected manually by viewing SNP graph cluster plots (Schupf et al., 2015).
2.6. Quality control (QC) assessment
We included SNPs in the allelic association analysis when the Gencall value, a quality score, exceeded 0.25. This quality score was determined from allele cluster definitions for each SNP as determined by the Illumina GenomeStudio Genotyping Module version 3.0. For chromosome 21, we studied SNPs that produced call rate ≥90% (average call rate 98%) and dropped SNPs with call rate <90% (n = 23) or produced no genotypes (n = 9). For chromosomes other than 21, we dropped SNPs with call rates <98% (n = 11; average call rate: 99%). In addition, we randomly selected 15 samples and genotyped in duplicate. The concordance rates for genotyped SNPs in these samples ranged from 91.8% to 100% for chromosome 21 SNPs and from 95.2 to 99.6 for nonchromosome 21 SNPs. Further QC assessments using PLINK (Purcell et al., 2007) excluded 15 additional SNPs with the following characteristics: missing genotyping rate >5%; minimum allele frequency <1%; Hardy-Weinberg Equilibrium test at a p-value <1E-6. Eventually, we analyzed 231 SNPs on chromosome 21 and 1099 SNPs from chromosomes other than 21.
2.7. Population stratification and covariates
We applied the multidimensional scaling method as implemented in PLINK to adjust for population stratification. Using all available SNPs that survived the QC process, genetic similarity across individuals was estimated by computing identity by state. In addition to our own samples, we included Whites (n = 165), Africans (n = 165), and Asians (170) from the Hapmap database (www.hapmap.org) to ensure proper classification of ethnicity. This analysis generated 3 ethno-racial clusters. These clusters, akin to principal components, were included in the multivariate model as covariates. For multivariable models, we adjusted for the following potential confounders: age, sex, level of intellectual disability (mild to moderate [IQ 35–70] vs. severe to profound [IQ <35]), ethno-racial clusters from population stratification analysis, and the presence or absence of an APOE ε4 allele.
2.8. Statistical analyses
To minimize type-1 error rate from multiple testing, we conducted a 2-stage analysis (Fig. 1). In stage 1, we selected tag SNPs to achieve an r2 of 0.3 or below (variance inflation factor of 1.43) using the PLINK algorithm. We then applied a multivariable logistic regression model to examine the association between an SNP and AD, adjusting for confounders. An additive dosage model was used where we compared the risk associated with having none versus 1 versus 2 copies of the SNP. SNP-wise empirical p-value was estimated based on 10,000 replicates. In stage 2, to fine map the genes that had at least one SNP with SNP-wise empirical p-value <0.05, we then used all genotyped SNPs within the gene for further evaluation using the same logistic regression model. To take into account multiple SNP testing within any given gene, we applied the false discovery rate approach proposed by Benjamini and Yekutieli (Benjamini and Yekutieli, 2001) and computed gene-wise empirical p-value. The rationale for the false discovery rate approach was that: (1) the main goal of the present study is to confirm candidate genes for AD or dementia in adults with DS; thus, multiple testing correction at the level of each gene is reasonable and (2) even though this is the largest fine mapping study in DS to date, sample size is still relatively small. For the two most promising non-chromosome 21 genes (the loci that had more than one SNP with marginal significance [p < 0.05]), we performed sliding window haplotype analysis using 3 SNPs at a time to identify haplotypes that may contain functional variants within a gene. R statistical package (http://www.r-project.org/) was used for analysis.
3. Results
3.1. Demographic and clinical characteristics
The average age of the 320 study participants at the time of baseline was 49.9 year old (SD = 7.6), and the mean age of adults at the baseline for adults with dementia was 7 years older than those without (Table 1). The majority of the individuals had mild to moderate intellectual disability. Ethnicity for over 90% of the study participants was reported in the medical charts as non-Hispanic White, and the allele frequency of the APOE ε4 (11.6%) was comparable to other populations of Caucasian ancestry (Table 1).
3.2. Stage 1 screening analysis
In stage 1, we screened genes on chromosome 21 using a set of tag SNPs. Because these tag SNPs represent a group of SNPs in the chromosomal region with high linkage disequilibrium, they reduced the burden of multiple testing. Only APP and RUNX1 had SNPs that had empirical p-values <0.05 (Table 2). rs17588612 in APP had an SNP-wise empirical p-value of 0.0126, and rs4816501 and rs13046934 in RUNX1 had SNP-wise empirical p-values of 0.0308 and 0.0081, respectively. In stage 1, screening of genes on chromosomes other than 21, the following genes had one or more SNPs with a significant association at empirical p < 0.05: MSRA (empirical p = 0.0230), DAPK1 (empirical p = 0.0474), PITRM1 (empirical p = 0.0498), SORCS1 (0.0230 < empirical p < 0.0469), SORL1 (empirical p = 0.0387), TNK1 (empirical p = 0.0477), LDLR (empirical p = 0.0442), ZNF224 (empirical p = 0.0275), MARK4 (empirical p < 0.0298), and CST3 (empirical p = 0.0094) (Table 3).
Table 2.
Chromosome 21 SNPs that are associated with Alzheimer’s disease at empirical p < 0.05
| Chr | Gene | SNPa | BP (Hg19) | Risk allele | MAF | OR | OR_L95 | OR_U95 | Empirical p (pointwise)b | BH adjusted empirical p (genewise) |
|---|---|---|---|---|---|---|---|---|---|---|
| 21 | APP | rs3991 | 27,428,256 | T | 0.236 | 1.893 | 1.384 | 2.618 | 0.0001 | 0.0031 |
| 21 | APP | rs2830031 | 27,429,317 | C | 0.314 | 1.702 | 1.244 | 2.356 | 0.0010 | 0.0122 |
| 21 | APP | rs2830033 | 27,430,925 | A | 0.314 | 1.763 | 1.280 | 2.458 | 0.0004 | 0.0081 |
| 21 | APP | rs2830036 | 27,435,525 | A | 0.182 | 0.558 | 0.365 | 0.829 | 0.0050 | 0.0235 |
| 21 | APP | rs1041420 | 27,443,650 | A | 0.221 | 0.554 | 0.371 | 0.804 | 0.0028 | 0.0203 |
| 21 | APP | rs2830048 | 27,459,674 | C | 0.339 | 0.673 | 0.490 | 0.910 | 0.0135 | 0.0484 |
| 21 | APP | rs2070654 | 27,462,727 | T | 0.298 | 2.044 | 1.439 | 2.948 | 0.0000 | 0.0000 |
| 21 | APP | rs2830050 | 27,464,270 | T | 0.252 | 1.590 | 1.157 | 2.198 | 0.0046 | 0.0234 |
| 21 | APP | rs2830054 | 27,476,104 | G | 0.407 | 1.564 | 1.156 | 2.137 | 0.0030 | 0.0203 |
| 21 | APP | rs2830066 | 27,494,202 | C | 0.496 | 1.488 | 1.122 | 1.993 | 0.0063 | 0.0275 |
| 21 | APP | rs2830076 | 27,502,468 | A | 0.357 | 1.541 | 1.143 | 2.093 | 0.0044 | 0.0234 |
| 21 | APP | rs2830086 | 27,512,956 | T | 0.325 | 1.612 | 1.194 | 2.193 | 0.0018 | 0.0183 |
| 21 | APP | rs2830088 | 27,514,740 | C | 0.487 | 1.433 | 1.085 | 1.909 | 0.0118 | 0.0480 |
| 21 | APPc | rs17588612 | 27,517,203 | C | 0.082 | 1.766 | 1.124 | 2.794 | 0.0126 | 0.0480 |
| 21 | APP | rs17588612 | 27,517,203 | C | 0.082 | 1.766 | 1.124 | 2.794 | 0.0126 | 0.0480 |
| 21 | APP | rs13049230 | 27,521,417 | G | 0.318 | 1.550 | 1.149 | 2.104 | 0.0039 | 0.0234 |
| 21 | APP | rs2830099 | 27,530,610 | C | 0.319 | 1.586 | 1.174 | 2.159 | 0.0023 | 0.0200 |
| 21 | APP | rs2830100 | 27,533,329 | T | 0.334 | 1.658 | 1.228 | 2.261 | 0.0010 | 0.0122 |
| 21 | RUNX1 | rs4816501 | 36,294,539 | A | 0.251 | 1.369 | 1.028 | 1.825 | 0.0308 | 0.4980 |
| 21 | RUNX1 | rs13046934 | 36,371,207 | T | 0.215 | 1.592 | 1.134 | 2.245 | 0.0081 | 0.4980 |
Key: MAF, minor allele frequency; OR, odds ratio; SNPs, single nucleotide polymorphisms.
SNPs with gene-wise FDR-adjusted empirical p-value <0.05 are in boldface.
For empirical p-value, 10,000 replicates were generated.
Positive SNPs from stage 1 are highlighted in gray. SNPs without the gray highlight were added in stage 2 for fine mapping.
Table 3.
Nonchromosome 21 SNPs that are associated with Alzheimer’s disease at empirical p < 0.05
| Chr | Gene | SNPa | BP (Hg19) | Risk allele | Allele freq | OR | (L95-U95) | Empirical p (pointwise)b | BH adjusted empirical p (genewise) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 8 | MSRAc | rs17692624 | 10,070,368 | C | 0.233 | 0.589 | 0.374 | 0.927 | 0.0230 | 0.8470 |
| 9 | DAPK1 | rs2058882 | 90,114,746 | G | 0.2 | 1.579 | 1.003 | 2.486 | 0.0474 | 0.9415 |
| 10 | PITRM1 | rs9423705 | 3,200,155 | G | 0.339 | 1.440 | 1.002 | 2.070 | 0.0498 | 0.4050 |
| 10 | SORCS1 | rs10748921 | 108,390,100 | C | 0.333 | 0.648 | 0.424 | 0.990 | 0.0469 | 0.5532 |
| 10 | SORCS1 | rs1538417 | 108,583,599 | A | 0.283 | 1.536 | 1.025 | 2.301 | 0.0361 | 0.5532 |
| 10 | SORCS1 | rs10787011 | 108,863,228 | G | 0.359 | 1.482 | 1.022 | 2.150 | 0.0371 | 0.5532 |
| 10 | SORCS1 | rs7897974 | 108,893,522 | A | 0.458 | 0.685 | 0.475 | 0.988 | 0.0426 | 0.5532 |
| 10 | SORCS1 | rs7099998 | 108,928,558 | G | 0.2 | 0.566 | 0.345 | 0.929 | 0.0230 | 0.5532 |
| 11 | SORL1 | rs11605969 | 121,430,872 | A | 0.153 | 1.683 | 1.032 | 2.747 | 0.0387 | 0.8816 |
| 17 | TNK1 | rs7219773 | 7,283,144 | A | 0.42 | 1.549 | 1.065 | 2.251 | 0.0257 | 0.0596 |
| 17 | TNK1 | rs12948090 | 7,285,104 | A | 0.416 | 1.458 | 1.013 | 2.098 | 0.0442 | 0.0596 |
| 17 | TNK1 | rs1554948 | 7,286,326 | A | 0.413 | 1.604 | 1.106 | 2.327 | 0.0135 | 0.0596 |
| 17 | TNK1 | rs3744549 | 7,293,715 | G | 0.251 | 0.655 | 0.429 | 0.998 | 0.0477 | 0.0596 |
| 19 | LDLR | rs2738466 | 11,242,765 | G | 0.423 | 1.537 | 1.010 | 2.341 | 0.0442 | 0.2063 |
| 19 | ZNF224 | rs4803675 | 44,589,716 | G | 0.388 | 1.522 | 1.054 | 2.198 | 0.0275 | 0.1925 |
| 19 | MARK4 | rs12976518 | 45,759,344 | G | 0.475 | 1.636 | 1.141 | 2.347 | 0.0089 | 0.0534 |
| 19 | MARK4 | rs2377324 | 45,768,946 | G | 0.282 | 0.551 | 0.355 | 0.854 | 0.0089 | 0.0534 |
| 19 | MARK4 | rs2306660 | 45,802,863 | G | 0.245 | 0.599 | 0.378 | 0.948 | 0.0298 | 0.0715 |
| 20 | CST3 | rs35610040 | 23,616,469 | G | 0.179 | 1.774 | 1.106 | 2.844 | 0.0165 | 0.0297 |
| 20 | CST3 | rs3787498 | 23,616,781 | A | 0.172 | 1.909 | 1.180 | 3.086 | 0.0085 | 0.0211 |
| 20 | CST3 | rs3827142 | 23,617,007 | A | 0.179 | 1.907 | 1.182 | 3.078 | 0.0079 | 0.0211 |
| 20 | CST3 | rs5030707 | 23,618,656 | C | 0.167 | 1.974 | 1.230 | 3.167 | 0.0049 | 0.0211 |
| 20 | CST3 | rs3827143 | 23,619,617 | G | 0.226 | 1.748 | 1.141 | 2.677 | 0.0094 | 0.0211 |
| 20 | CST3 | rs2254635 | 23,622,758 | A | 0.203 | 1.655 | 1.043 | 2.626 | 0.0316 | 0.0406 |
| 20 | CST3 | rs2405367 | 23,622,880 | A | 0.189 | 1.703 | 1.080 | 2.686 | 0.0200 | 0.0300 |
SNPs with gene-wise FDR adjusted empirical p-value <0.05 are in boldface.
For empirical p-value, 10,000 replicates were generated.
Positive SNPs from stage 1 are highlighted in gray. SNPs without the gray highlight were added in stage 2 for fine mapping.
3.3. Stage 2 fine mapping analysis
For the genes that were significant in stage 1, we fine mapped the genes using all genotyped SNPs to better localize SNPs that are associated with AD (Tables 2 and 3). In the stage 2 analysis of chromosome 21, we computed a gene-wise empirical p-value to correct for multiple SNPs within the gene (Table 2). When this more rigorous correction was applied in stage 2, 18 SNPs in the APP gene, located within 100kb, had gene-wise empirical p-values that reached a threshold of p ≤ 0.05, and odds ratios (ORs) for minor allele for these SNPs ranged from 2.04 (rs2070654) to 1.49 (rs2830066). Three minor alleles for the associated SNPs, namely rs2830036, rs1041420, rs2830048, were protective (OR <1). For genes on chromosomes other than 21, we identified 5 SNPs in the CST3 gene that had gene-wise empirical p-value <0.05 (Table 3), and ORs for those SNPs ranged from 1.97 to 1.75. On the other hand, for the MARK4 gene, 3 SNPs barely missed gene-wise empirical p-value of 0.05 (rs12976518, p = 0.0534; rs2377324, p = 0.0534; rs2306660, p = 0.0715). The minor allele for rs12976518 was putative (OR = 1.64), whereas the minor alleles for rs2377324 (OR = 0.55) and rs2306660 (OR = 0.60) were protective.
3.4. Haplotype analysis
To better localize the SNP signals and to potentially guide our future sequencing efforts for the 2 candidate genes that are located on chromosomes other than 21, we performed a 3-mer sliding window haplotype analysis for the two most promising candidate genes: CST3 and MARK4 (Tables 4 and 5; linkage disequilibrium patterns for SNPs in CST3 and MARK4 are shown in Supplement Fig. 1). For CST3, we examined the region containing rs2424577 to rs2405367 and found the strongest evidence in a contiguous 3-mer haplotype G-G-G for rs3787498-rs3827142-rs5030707 (p = 0.00281) to 3-mer haplotype G-A-A for rs2424582-rs2254635-rs2405367 (p = 0.00884). All associated haplotypes in this region were risk haplotypes. For MARK4, the strongest evidence was observed for haplotype A-A-G for rs12976518-rs10445572-rs2377324 (p = 0.00796) and haplotype A-G-A for rs10445572-rs2377324-rs2240672 (p = 0.00924). For APP on chromosome 21, we are currently working to develop an algorithm to generate robust haplotypes for trisomy.
Table 4.
Haplotypes analysis for CST3 and Alzheimer’s disease: 3-SNP window
| SNP seta | Omnibus | Haplotype | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||
| 1 | 2 | 3 | STAT | p | Haplotype | Frequency | OR | STAT | p | ||
| rs2424577 | rs35610040 | rs3787498 | 7.170 | 0.02780 | G | G | A | 0.172 | 1.960 | 7.500 | 0.00617 |
| G | A | G | 0.196 | 0.758 | 1.290 | 0.25600 | |||||
| A | A | G | 0.625 | 0.815 | 1.200 | 0.27300 | |||||
| rs35610040 | rs3787498 | rs3827142 | 6.330 | 0.01190 | G | A | A | 0.172 | 1.960 | 7.500 | 0.00617 |
| A | G | G | 0.818 | 0.544 | 6.330 | 0.01190 | |||||
| rs3787498 | rs3827142 | rs5030707 | 9.100 | 0.02800 | A | A | C | 0.154 | 1.910 | 6.490 | 0.01080 |
| G | G | C | 0.012 | 2.820 | 1.540 | 0.21500 | |||||
| A | A | G | 0.018 | 1.640 | 0.622 | 0.43000 | |||||
| G | G | G | 0.809 | 0.493 | 8.930 | 0.00281 | |||||
| rs3827142 | rs5030707 | rs3827143 | 8.960 | 0.06220 | A | C | G | 0.152 | 2.010 | 7.590 | 0.00587 |
| A | G | G | 0.020 | 1.840 | 0.815 | 0.36700 | |||||
| G | G | G | 0.052 | 0.928 | 0.040 | 0.84100 | |||||
| G | C | A | 0.010 | 1.820 | 0.408 | 0.52300 | |||||
| G | G | A | 0.757 | 0.576 | 6.640 | 0.00997 | |||||
| rs5030707 | rs3827143 | rs2424582 | 10.100 | 0.03820 | C | G | G | 0.152 | 2.010 | 7.590 | 0.00587 |
| G | G | G | 0.020 | 1.020 | 0.001 | 0.97400 | |||||
| G | A | G | 0.019 | 0.212 | 1.950 | 0.16200 | |||||
| G | G | A | 0.052 | 1.090 | 0.058 | 0.80900 | |||||
| G | A | A | 0.742 | 0.613 | 5.260 | 0.02180 | |||||
| rs3827143 | rs2424582 | rs2254635 | 9.000 | 0.06120 | G | G | A | 0.170 | 2.120 | 9.070 | 0.00260 |
| A | G | A | 0.019 | 0.207 | 1.990 | 0.15900 | |||||
| A | A | A | 0.014 | 0.714 | 0.130 | 0.71900 | |||||
| G | A | C | 0.053 | 1.130 | 0.121 | 0.72800 | |||||
| A | A | C | 0.737 | 0.661 | 3.890 | 0.04860 | |||||
| rs2424582 | rs2254635 | rs2405367 | 4.820 | 0.18600 | G | A | A | 0.175 | 1.900 | 6.850 | 0.00884 |
| A | A | A | 0.014 | 0.874 | 0.024 | 0.87600 | |||||
| G | A | G | 0.014 | 0.717 | 0.141 | 0.70700 | |||||
| A | C | G | 0.790 | 0.627 | 3.970 | 0.04640 | |||||
Key: SNP, single nucleotide polymorphism.
SNP(s) most strongly associated with AD are bolded and italicized, and the most significant haplotype is highlighted in gray.
Table 5.
Haplotypes analysis for MARK4 and Alzheimer’s disease: 3-SNP window
| SNP seta | Omnibus | Haplotype | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||
| 1 | 2 | 3 | STAT | p | Haplotype | Frequency | OR | STAT | p | ||
| rs12976518 | rs10445572 | rs2377324 | 10.400 | 0.01530 | A | A | G | 0.278 | 0.548 | 7.040 | 0.00796 |
| G | G | A | 0.386 | 1.610 | 6.520 | 0.01070 | |||||
| G | A | A | 0.086 | 1.510 | 1.660 | 0.19800 | |||||
| A | A | A | 0.241 | 0.816 | 0.860 | 0.35400 | |||||
| rs10445572 | rs2377324 | rs2240672 | 9.780 | 0.02050 | A | G | A | 0.274 | 0.552 | 6.780 | 0.00924 |
| G | A | A | 0.048 | 1.120 | 0.070 | 0.79200 | |||||
| G | A | G | 0.344 | 1.610 | 6.530 | 0.01060 | |||||
| A | A | G | 0.324 | 0.992 | 0.002 | 0.96800 | |||||
| rs2377324 | rs2240672 | rs345409 | 8.370 | 0.03900 | G | A | G | 0.268 | 0.559 | 6.390 | 0.01150 |
| A | A | G | 0.050 | 1.070 | 0.029 | 0.86500 | |||||
| A | G | G | 0.138 | 1.420 | 2.010 | 0.15600 | |||||
| A | G | A | 0.530 | 1.300 | 2.100 | 0.14700 | |||||
| rs2240672 | rs345409 | rs11883302 | 7.410 | 0.19200 | A | G | G | 0.246 | 0.584 | 4.920 | 0.02660 |
| G | G | G | 0.096 | 1.100 | 0.096 | 0.75600 | |||||
| G | A | G | 0.019 | 1.720 | 0.637 | 0.42500 | |||||
| A | G | A | 0.073 | 0.788 | 0.387 | 0.53400 | |||||
| G | G | A | 0.047 | 1.700 | 1.780 | 0.18200 | |||||
| G | A | A | 0.513 | 1.270 | 1.650 | 0.19900 | |||||
Key: SNP, single nucleotide polymorphism.
SNP(s) most strongly associated with AD are bolded and italicized, and the most significant haplotype is highlighted in gray.
3.4.1. Comparison with the general population
When we compared allelic association of the significant candidate SNPs from our study of DS against those in the general population, we observed 3 SNPs—rs2830066 (p = 0.003) and rs2830088 (p = 0.033) in the APP gene, and rs2377324 in the MARK4 gene (p = 0.023)—that had p-values <0.05 in a large GWAS study of adults without DS (Lambert et al., 2013). In addition, rs9423705 (p = 0.074) in the PITRM1 gene and rs2306660 (p = 0.096) in the MARK4 were weakly associated with AD. For this purpose, we used the first stage meta-GWAS data (Lambert et al., 2013) (n = 54,167).
4. Discussion
The present study confirmed that SNPs in APP and CST3 were significantly associated with AD risk for adults with DS, whereas those in MARK4 were suggestively associated; further, 2 SNPs in APP and 1 SNP in MARK4 were also found to be associated with AD in a large-scale GWAS of adults without DS. Our results extend previous findings of a relationship between SNPs in candidate genes located on chromosome 21 and chromosomes other than 21 and risk of AD in adults with DS (Jones et al., 2013; Lee et al., 2007a; Liu et al., 2008; Margallo-Lana et al., 2004; Mok et al., 2014; Patel et al., 2011; Wegiel et al., 2008, 2011). Our analysis revealed that multiple SNPs in APP within a 100kb region on 21q21.3 were associated with AD in adults with DS at gene-wise level. Some of the minor alleles were risk alleles, whereas others were protective alleles. Beyond APP, this study reports significant allelic association for SNPs in CST3, suggesting potential contributions through vascular factors in adults with DS; and also reports suggestive allelic association for SNPs in MARK4, suggesting interaction between excess levels of Aβ peptides and tau.
To date, several studies have examined the role of genes on AD or age at onset of AD in adults with DS. Among genes on chromosome 21, a tetratnucleotide repeat in intron 7 on APP (Jones et al., 2010), SNPs on BACE2 (Mok et al., 2014), and an SNP on RUNX1 (Patel et al., 2011) have been associated with earlier age at the onset of AD, whereas among nonchromosome 21 candidate genes, SNPs in APOE, SORL1, BACE1, ALDH18A1 (Lai et al., 1999; Lee et al., 2007b; Patel et al., 2011; Prasher et al., 2008; Schupf et al., 1998) and PIC-ALM (Jones et al., 2013) have also been associated with earlier age at onset of AD. Subsequently, Patel et al. (2014) examined the relation of 9 GWAS-derived SNPs with risk of AD in DS, including SNPs in CR1, BIN1, CD2AP, EPHA1, CLU MS4A6A/4A, PICALM, ABCA7, and CD33 but found no significant relationship to dementia in adults with DS. Below we discuss 3 genes (APP, CST3, and MARK4) that had the most promising signals for genetic association in adults with DS, plus PICALM, which was previously reported to be associated with age at the onset in the only genome-wide study in autopsy samples from individuals with DS.
4.1. APP
Even though mutations in APP are among mutations in 3 genes that are known to cause AD, along with PSEN1 and PSEN2, only a few variants have been implicated in population-based studies of late onset AD (LOAD; Hindorff et al., 2014; Welter et al., 2014). Guyant-Marechal et al. (2007) found and replicated 1 variant rs463946 located at 27,546,187 bp that are somewhat proximal to the SNP signals in the present study, whereas Margallo-Lana et al. (2004) reported that individuals with 3 tetranucleotide repeats on intron 7 of the APP gene had significantly earlier age at the onset than those who did not, independent of APOE. Subsequently, Jones et al. (2010) confirmed the finding by Margallo-Lana et al. in a larger cohort of adults with DS. In 2002, Athan et al. (2002) reported +37G/C polymorphisms in the promotor region of the APP gene were associated with increased risk of AD. Jonsson et al. (2012) reported that rs63750847, a rare Icelandic mutation with allele frequency <1%, protects against age-related cognitive losses, but this finding has not yet been confirmed in other independent studies. To evaluate the relevance of these 2 previously reported loci, we will need additional genotyping of the 2 loci; however, it is unlikely that the present study of primarily US Caucasians will have sufficient number of carriers, since 2 large publicly available data sets (i.e., ADGC and IGAP) did not observe any individual with this coding mutation.
4.2. CST3
This gene codes for the cystatin c protein and colocalizes with Aβ in vascular walls and in senile plaque cores in the brains of individuals with AD as well as in adults with DS. Even though cystatin c protein binds with Aβ, it is reported to prevent oligomerization, and formation of fibrils in vitro (Kaur and Levy, 2012). Cystatin c protein plays the role of an inhibitor by suppressing the production of cathepsins B and D, where low levels of cathepsins were associated with reduced neuronal damages. Thus, cystatic c may rescue degenerating neurons (Kaur and Levy, 2012; Kaur et al., 2010). This idea was supported by an in vivo knockout mice experiment that showed deletion of cystatin c in knockout mice resulted in an elevated cathepsin B activity, leading to greater neuronal damages (Sun et al., 2008). Some have suggested that low levels of cystatin c protein may be a risk factor for AD (Gauthier et al., 2011).
The findings from genetic studies have been inconsistent in that allelic association between candidate SNPs in CST3 and AD differs by ethnicity. A meta-analysis of SNPs in CST3 using German, US, and European cohorts showed that the minor allele in rs5030707 was associated with an increased risk for AD (OR = 1.28) (Dodel et al., 2002; Finckh et al., 2000). In a separate study, a homozygous missense varint (G allele) in CST3 was associated with both AD and ALS. Among Asian cohorts, however, the relationship between AD and SNPs in CST3 was equivocal (Chuo et al., 2007; Maruyama et al., 2001; Wang et al., 2008). Further studies are needed to better understand the differences in allelic association for SNPs in CST3 among different ethnic groups as these ethnic groups will differ in their genetic background as well as the distribution of environmental risk factors.
4.3. MARK4
Studies have reported that MARK4 may be involved in early tau phosphorylation and/or may play a critical role for the PAR1/MARK-tau axis in mediating the toxic effects of Aβ on synapses and dendritic spines (Lund et al., 2014; Yu et al., 2012). The observed association between SNPs on MARK4 and AD risk in adults with DS supports the possibility that a variant in MARK4 may influence this process. However, the potential mechanism underlying this 2-hit model is not well studied. Interestingly, the GWAS by Naj et al. (2011) observed association between AD and SNPs located in the EXOC2L3, and MARK4 region, but they dismissed the association because it no longer was observed when adjustment was made for APOE ε4. However, it is possible that this gene is a significant modifier only in the presence of elevated levels of Aβ, as in the case of APOE ε4 or in adults with DS with trisomy, consistent with the finding of the association between SNPs in MARK4 and AD for adults with DS after adjusting for APOE ε4.
4.4. PICALM
We examined PICALM, implicated in a GWAS employing autopsy samples of adults with DS (Jones et al., 2013) and in large-scale genome studies (Harold et al., 2009; Lambert et al., 2013; Naj et al., 2011), using 40 SNPs. Based on our more conservative gene-wise empirical p-values, the SNPs (rs2888903, rs7941541, rs10751134 and rs561655) that were associated with AD in the general population, and an earlier age at onset in the DS population (Jones et al., 2013) were not significantly associated with AD in the present study of adults with DS.
4.5. Adults with versus without DS
Of the genes identified from the large-scale international genome-wide association studies in the general population (Lambert et al., 2013), we examined 9, but did not observe significant association.
Our study has several limitations. First, even though this is the largest gene mapping study of AD in adults with DS to date, this study is limited by a relatively small sample size, and lacked sufficient power for SNPs with low allele frequencies and those with small effect sizes. Despite this limitation, this study identified multiple SNPs with modest risk ratios at the gene-wise level, and most likely, the positive findings may be owed to studying a high-risk group that is known for early onset of AD and high levels of Aβ peptides. Second, this study examined the relation between AD and intronic SNPs, which serve as genetic markers to better localize the chromosomal location. Thus, further sequencing of these loci is needed to identify genetic variants that contribute to physiological alterations. Third, we interrogated known AD genes in adults with DS. Therefore, this candidate gene study was not designed to identify novel genes/variants, but rather, to better characterize the role of the SNPs in genes that are already shown to be associated with increased AD risk in adults with DS or in individuals with high levels of Aβ. Finally, study participants without AD were, on average, 7.7 years younger than those who had AD. It is possible that the effect sizes associated with the significant SNPs might be reduced if unaffected individuals were followed for a longer period of time. However, given that our multivariate model had adjusted for age and other potential confounders, the effect of age difference on allelic association would be limited.
In short, the present study reports SNPs in APP, CST3, and MARK4 that are associated with elevated risk of AD in adults with DS. This study illustrates that genetic factors that contribute to AD in the general population are likely to play a similar role in adults with DS, and a genetic study of AD in adults with DS can provide additional insight into the mechanisms for AD as these 2 genes may provide a new understanding for the role of cardiovascular factors and tau in AD. For these genes, further studies of adults with DS are needed to identify functionally relevant variants through extensive sequencing of exons and introns, and to evaluate how these variants may influence levels of gene expression.
Supplementary Material
Acknowledgments
This study is supported by grants R01AG014673 (Schupf) and P01HD035897 and U54 HD079123 (Silverman) from NIA and NICHD, respectively, and by NYS through its Office for People with Developmental Disabilities. The authors thank the study participants and participating agencies from the tristate area that made this study possible.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neurobiolaging.2017.04.018.
Footnotes
Disclosure statement
The authors have no actual or potential conflicts of interest.
References
- Athan ES, Lee JH, Arriaga A, Mayeux RP, Tycko B. Polymorphisms in the promoter of the human APP gene: functional evaluation and allele frequencies in Alzheimer disease. Arch Neurol. 2002;59:1793–1799. doi: 10.1001/archneur.59.11.1793. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Burt DB, Thorpe LU, Lai F, Dalton A. Diagnosis of dementia in individuals with intellectual disability. J Intellect Disabil Res. 1997;41(Pt 2):152–164. doi: 10.1111/j.1365-2788.1997.tb00692.x. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29:1165–1188. [Google Scholar]
- Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- Blennow K, Hampel H. CSF markers for incipient Alzheimer’s disease. Lancet Neurol. 2003;2:605–613. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- Burt DB, Aylward EH. Test battery for the diagnosis of dementia in individuals with intellectual disability. Working Group for the Establishment of Criteria for the Diagnosis of Dementia in Individuals with Intellectual Disability. J Intellect Disabil Res. 2000;44(Pt 2):175–180. doi: 10.1046/j.1365-2788.2000.00264.x. [DOI] [PubMed] [Google Scholar]
- Chrast R, Scott HS, Papasavvas MP, Rossier C, Antonarakis ES, Barras C, Davisson MT, Schmidt C, Estivill X, Dierssen M, Pritchard M, Antonarakis SE. The mouse brain transcriptome by SAGE: differences in gene expression between P30 brains of the partial trisomy 16 mouse model of Down syndrome (Ts65Dn) and normals. Genome Res. 2000;10:2006–2021. doi: 10.1101/gr.10.12.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuo LJ, Sheu WH, Pai MC, Kuo YM. Genotype and plasma concentration of cystatin C in patients with late-onset Alzheimer disease. Demen Geriatr Cogn Disord. 2007;23:251–257. doi: 10.1159/000100021. [DOI] [PubMed] [Google Scholar]
- Cook CN, Hejna MJ, Magnuson DJ, Lee JM. Expression of calcipressin1, an inhibitor of the phosphatase calcineurin, is altered with aging and Alzheimer’s disease. J Alzheimer’s Dis. 2005;8:63–73. doi: 10.3233/jad-2005-8108. [DOI] [PubMed] [Google Scholar]
- Coppus AM, Evenhuis HM, Verberne GJ, Visser FE, Oostra BA, Eikelenboom P, van Gool WA, Janssens AC, van Duijn CM. Survival in elderly persons with Down syndrome. J Am Geriatr Soc. 2008;56:2311–2316. doi: 10.1111/j.1532-5415.2008.01999.x. [DOI] [PubMed] [Google Scholar]
- de Bakker PI. Selection and evaluation of Tag-SNPs using Tagger and Hap-Map. Cold Spring Harbor Protocols. 2009 doi: 10.1101/pdb.ip67. http://dx.doi.org/10.1101/pdb.ip67. [DOI] [PubMed]
- Dodel RC, Du Y, Depboylu C, Kurz A, Eastwood B, Farlow M, Oertel WH, Muller U, Riemenschneider M. A polymorphism in the cystatin C promoter region is not associated with an increased risk of AD. Neurology. 2002;58:664. doi: 10.1212/wnl.58.4.664. [DOI] [PubMed] [Google Scholar]
- Finckh U, von der Kammer H, Velden J, Michel T, Andresen B, Deng A, Zhang J, Muller-Thomsen T, Zuchowski K, Menzer G, Mann U, Papassotiropoulos A, Heun R, Zurdel J, Holst F, Benussi L, Stoppe G, Reiss J, Miserez AR, Staehelin HB, Rebeck GW, Hyman BT, Binetti G, Hock C, Growdon JH, Nitsch RM. Genetic association of a cystatin C gene polymorphism with late-onset Alzheimer disease. Arch Neurol. 2000;57:1579–1583. doi: 10.1001/archneur.57.11.1579. [DOI] [PubMed] [Google Scholar]
- Gauthier S, Kaur G, Mi W, Tizon B, Levy E. Protective mechanisms by cystatin C in neurodegenerative diseases. Front Biosci. 2011;3:541–554. doi: 10.2741/s170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyant-Marechal L, Rovelet-Lecrux A, Goumidi L, Cousin E, Hannequin D, Raux G, Penet C, Ricard S, Mace S, Amouyel P, Deleuze JF, Frebourg T, Brice A, Lambert JC, Campion D. Variations in the APP gene promoter region and risk of Alzheimer disease. Neurology. 2007;68:684–687. doi: 10.1212/01.wnl.0000255938.33739.46. [DOI] [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Hardy J, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schurmann B, Heun R, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frolich L, Hampel H, Hull M, Rujescu D, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Muhleisen TW, Nothen MM, Moebus S, Jockel KH, Klopp N, Wichmann HE, Carrasquillo MM, Pankratz VS, Younkin SG, Holmans PA, O’Donovan M, Owen MJ, Williams J. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head E, Silverman W, Patterson D, Lott IT. Aging and Down syndrome. Curr Gerontol Geriatr Res. 2012;2012:412536. doi: 10.1155/2012/412536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindorff LA, MJEBI, Morales J, (European Bioinformatics Institute), Junkins HA, Hall PN, Klemm AK, Manolio TA. [Accessed March 13, 2017];A Catalog of Published Genome-wide Association Studies. 2014 Available at: http://www.ebi.ac.uk/gwas/
- Holland AJ, Hon J, Huppert FA, Stevens F. Incidence and course of dementia in people with Down’s syndrome: findings from a population-based study. J Intellect Disabil Res. 2000;44(Pt 2):138–146. doi: 10.1046/j.1365-2788.2000.00263.x. [DOI] [PubMed] [Google Scholar]
- Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, Abraham R, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Jones N, Stretton A, Thomas C, Richards A, Ivanov D, Widdowson C, Chapman J, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Beaumont H, Warden D, Wilcock G, Love S, Kehoe PG, Hooper NM, Vardy ER, Hardy J, Mead S, Fox NC, Rossor M, Collinge J, Maier W, Jessen F, Ruther E, Schurmann B, Heun R, Kolsch H, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frolich L, Hampel H, Gallacher J, Hull M, Rujescu D, Giegling I, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Muhleisen TW, Nothen MM, Moebus S, Jockel KH, Klopp N, Wichmann HE, Pankratz VS, Sando SB, Aasly JO, Barcikowska M, Wszolek ZK, Dickson DW, Graff-Radford NR, Petersen RC, van Duijn CM, Breteler MM, Ikram MA, DeStefano AL, Fitzpatrick AL, Lopez O, Launer LJ, Seshadri S, Berr C, Campion D, Epelbaum J, Dartigues JF, Tzourio C, Alperovitch A, Lathrop M, Feulner TM, Friedrich P, Riehle C, Krawczak M, Schreiber S, Mayhaus M, Nicolhaus S, Wagenpfeil S, Steinberg S, Stefansson H, Stefansson K, Snaedal J, Bjornsson S, Jonsson PV, Chouraki V, Genier-Boley B, Hiltunen M, Soininen H, Combarros O, Zelenika D, Delepine M, Bullido MJ, Pasquier F, Mateo I, Frank-Garcia A, Porcellini E, Hanon O, Coto E, Alvarez V, Bosco P, Siciliano G, Mancuso M, Panza F, Solfrizzi V, Nacmias B, Sorbi S, Bossu P, Piccardi P, Arosio B, Annoni G, Seripa D, Pilotto A, Scarpini E, Galimberti D, Brice A, Hannequin D, Licastro F, Jones L, Holmans PA, Jonsson T, Riemenschneider M, Morgan K, Younkin SG, Owen MJ, O’Donovan M, Amouyel P, Williams J Alzheimer’s Disease Neuroimaging Initiative, CHARGE consortium, EADI1 consortium. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011;43:429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EL, Ballard CG, Prasher VP, Arno M, Tyrer S, Moore B, Hanney ML. An intron 7 polymorphism in APP affects the age of onset of dementia in down syndrome. Int J Alzheimer’s Dis. 2010;2011:929102. doi: 10.4061/2011/929102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EL, Mok K, Hanney M, Harold D, Sims R, Williams J, Ballard C. Evidence that PICALM affects age at onset of Alzheimer’s dementia in Down syndrome. Neurobiol Aging. 2013;34:2441.e1–2441.e5. doi: 10.1016/j.neurobiolaging.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, Stefansson H, Sulem P, Gudbjartsson D, Maloney J, Hoyte K, Gustafson A, Liu Y, Lu Y, Bhangale T, Graham RR, Huttenlocher J, Bjornsdottir G, Andreassen OA, Jonsson EG, Palotie A, Behrens TW, Magnusson OT, Kong A, Thorsteinsdottir U, Watts RJ, Stefansson K. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012;488:96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- Kaur G, Levy E. Cystatin C in Alzheimer’s disease. Front Mol Neurosci. 2012;5:79. doi: 10.3389/fnmol.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G, Mohan P, Pawlik M, DeRosa S, Fajiculay J, Che S, Grubb A, Ginsberg SD, Nixon RA, Levy E. Cystatin C rescues degenerating neurons in a cystatin B-knockout mouse model of progressive myoclonus epilepsy. Am J Pathol. 2010;177:2256–2267. doi: 10.2353/ajpath.2010.100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F, Kammann E, Rebeck GW, Anderson A, Chen Y, Nixon RA. APOE genotype and gender effects on Alzheimer disease in 100 adults with Down syndrome. Neurology. 1999;53:331–336. doi: 10.1212/wnl.53.2.331. [DOI] [PubMed] [Google Scholar]
- Lai F, Williams RS. A prospective study of Alzheimer disease in Down syndrome. Arch Neurol. 1989;46:849–853. doi: 10.1001/archneur.1989.00520440031017. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, Letenneur L, Bettens K, Berr C, Pasquier F, Fievet N, Barberger-Gateau P, Engelborghs S, De Deyn P, Mateo I, Franck A, Helisalmi S, Porcellini E, Hanon O, Lendon C, Dufouil C, Jaillard C, Leveillard T, Alvarez V, Bosco P, Mancuso M, Panza F, Nacmias B, Bossu P, Piccardi P, Annoni G, Seripa D, Galimberti D, Hannequin D, Licastro F, Soininen H, Ritchie K, Blanche H, Dartigues JF, Tzourio C, Gut I, Van Broeckhoven C, Alperovitch A, Lathrop M, Amouyel P European Alzheimer’s Disease Initiative Initiative de Pancorbo MM. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, DeStafano AL, Bis JC, Beecham GW, Grenier-Boley B, Russo G, Thorton-Wells TA, Jones N, Smith AV, Chouraki V, Thomas C, Ikram MA, Zelenika D, Vardarajan BN, Kamatani Y, Lin CF, Gerrish A, Schmidt H, Kunkle B, Dunstan ML, Ruiz A, Bihoreau MT, Choi SH, Reitz C, Pasquier F, Cruchaga C, Craig D, Amin N, Berr C, Lopez OL, De Jager PL, Deramecourt V, Johnston JA, Evans D, Lovestone S, Letenneur L, Moron FJ, Rubinsztein DC, Eiriksdottir G, Sleegers K, Goate AM, Fievet N, Huentelman MW, Gill M, Brown K, Kamboh MI, Keller L, Barberger-Gateau P, McGuiness B, Larson EB, Green R, Myers AJ, Dufouil C, Todd S, Wallon D, Love S, Rogaeva E, Gallacher J, St George-Hyslop P, Clarimon J, Lleo A, Bayer A, Tsuang DW, Yu L, Tsolaki M, Bossu P, Spalletta G, Proitsi P, Collinge J, Sorbi S, Sanchez-Garcia F, Fox NC, Hardy J, Deniz Naranjo MC, Bosco P, Clarke R, Brayne C, Galimberti D, Mancuso M, Matthews F, Moebus S, Mecocci P, Del Zompo M, Maier W, Hampel H, Pilotto A, Bullido M, Panza F, Caffarra P, Nacmias B, Gilbert JR, Mayhaus M, Lannefelt L, Hakonarson H, Pichler S, Carrasquillo MM, Ingelsson M, Beekly D, Alvarez V, Zou F, Valladares O, Younkin SG, Coto E, Hamilton-Nelson KL, Gu W, Razquin C, Pastor P, Mateo I, Owen MJ, Faber KM, Jonsson PV, Combarros O, O’Donovan MC, Cantwell LB, Soininen H, Blacker D, Mead S, Mosley TH, Jr, Bennett DA, Harris TB, Fratiglioni L, Holmes C, de Bruijn RF, Passmore P, Montine TJ, Bettens K, Rotter JI, Brice A, Morgan K, Foroud TM, Kukull WA, Hannequin D, Powell JF, Nalls MA, Ritchie K, Lunetta KL, Kauwe JS, Boerwinkle E, Riemenschneider M, Boada M, Hiltuenen M, Martin ER, Schmidt R, Rujescu D, Wang LS, Dartigues JF, Mayeux R, Tzourio C, Hofman A, Nothen MM, Graff C, Psaty BM, Jones L, Haines JL, Holmans PA, Lathrop M, Pericak-Vance MA, Launer LJ, Farrer LA, van Duijn CM, Van Broeckhoven C, Moskvina V, Seshadri S, Williams J, Schellenberg GD, Amouyel P European Alzheimer’s Disease Initiative (EADI) Genetic and Environmental Risk in Alzheimer’s Disease Alzheimer’s Disease Genetic Consortium Cohorts for Heart and Aging Research in Genomic Epidemiology. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45:1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Cheng R, Barral S, Reitz C, Medrano M, Lantigua R, Jimenez-Velazquez IZ, Rogaeva E, St George-Hyslop PH, Mayeux R. Identification of novel loci for Alzheimer disease and replication of CLU, PICALM, and BIN1 in Caribbean Hispanic individuals. Arch Neurol. 2011;68:320–328. doi: 10.1001/archneurol.2010.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Cheng R, Schupf N, Manly J, Lantigua R, Stern Y, Rogaeva E, Wakutani Y, Farrer L, St George-Hyslop P, Mayeux R. The association between genetic variants in SORL1 and Alzheimer disease in an urban, multi-ethnic, community-based cohort. Arch Neurol. 2007a;64:501–506. doi: 10.1001/archneur.64.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Chulikavit M, Pang D, Zigman WB, Silverman W, Schupf N. Association between genetic variants in sortilin-related receptor 1 (SORL1) and Alzheimer’s disease in adults with Down syndrome. Neurosci Lett. 2007b;425:105–109. doi: 10.1016/j.neulet.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Liang Z, Wegiel J, Hwang YW, Iqbal K, Grundke-Iqbal I, Ramakrishna N, Gong CX. Overexpression of Dyrk1A contributes to neurofibrillary degeneration in Down syndrome. FASEB J. 2008;22:3224–3233. doi: 10.1096/fj.07-104539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund H, Gustafsson E, Svensson A, Nilsson M, Berg M, Sunnemark D, von Euler G. MARK4 and MARK3 associate with early tau phosphorylation in Alzheimer’s disease granulovacuolar degeneration bodies. Acta Neuropathol Commun. 2014;2:22. doi: 10.1186/2051-5960-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyle R, Gehrig C, Neergaard-Henrichsen C, Deutsch S, Antonarakis SE. Gene expression from the aneuploid chromosome in a trisomy mouse model of Down syndrome. Genome Res. 2004;14:1268–1274. doi: 10.1101/gr.2090904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margallo-Lana M, Morris CM, Gibson AM, Tan AL, Kay DW, Tyrer SP, Moore BP, Ballard CG. Influence of the amyloid precursor protein locus on dementia in Down syndrome. Neurology. 2004;62:1996–1998. doi: 10.1212/01.wnl.0000129275.13169.be. [DOI] [PubMed] [Google Scholar]
- Maruyama H, Izumi Y, Oda M, Torii T, Morino H, Toji H, Sasaki K, Terasawa H, Nakamura S, Kawakami H. Lack of an association between cystatin C gene polymorphisms in Japanese patients with Alzheimer’s disease. Neurology. 2001;57:337–339. doi: 10.1212/wnl.57.2.337. [DOI] [PubMed] [Google Scholar]
- Mehta PD, Dalton AJ, Mehta SP, Kim KS, Sersen EA, Wisniewski HM. Increased plasma amyloid beta protein 1-42 levels in Down syndrome. Neurosci Lett. 1998;241:13–16. doi: 10.1016/s0304-3940(97)00966-x. [DOI] [PubMed] [Google Scholar]
- Mok KY, Jones EL, Hanney M, Harold D, Sims R, Williams J, Ballard C, Hardy J. Polymorphisms in BACE2 may affect the age of onset Alzheimer’s dementia in Down syndrome. Neurobiol Aging. 2014;35:1513.e1–1513.e5. doi: 10.1016/j.neurobiolaging.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, Gallins PJ, Buxbaum JD, Jarvik GP, Crane PK, Larson EB, Bird TD, Boeve BF, Graff-Radford NR, De Jager PL, Evans D, Schneider JA, Carrasquillo MM, Ertekin-Taner N, Younkin SG, Cruchaga C, Kauwe JS, Nowotny P, Kramer P, Hardy J, Huentelman MJ, Myers AJ, Barmada MM, Demirci FY, Baldwin CT, Green RC, Rogaeva E, St George-Hyslop P, Arnold SE, Barber R, Beach T, Bigio EH, Bowen JD, Boxer A, Burke JR, Cairns NJ, Carlson CS, Carney RM, Carroll SL, Chui HC, Clark DG, Corneveaux J, Cotman CW, Cummings JL, DeCarli C, DeKosky ST, Diaz-Arrastia R, Dick M, Dickson DW, Ellis WG, Faber KM, Fallon KB, Farlow MR, Ferris S, Frosch MP, Galasko DR, Ganguli M, Gearing M, Geschwind DH, Ghetti B, Gilbert JR, Gilman S, Giordani B, Glass JD, Growdon JH, Hamilton RL, Harrell LE, Head E, Honig LS, Hulette CM, Hyman BT, Jicha GA, Jin LW, Johnson N, Karlawish J, Karydas A, Kaye JA, Kim R, Koo EH, Kowall NW, Lah JJ, Levey AI, Lieberman AP, Lopez OL, Mack WJ, Marson DC, Martiniuk F, Mash DC, Masliah E, McCormick WC, McCurry SM, McDavid AN, McKee AC, Mesulam M, Miller BL, Miller CA, Miller JW, Parisi JE, Perl DP, Peskind E, Petersen RC, Poon WW, Quinn JF, Rajbhandary RA, Raskind M, Reisberg B, Ringman JM, Roberson ED, Rosenberg RN, Sano M, Schneider LS, Seeley W, Shelanski ML, Slifer MA, Smith CD, Sonnen JA, Spina S, Stern RA, Tanzi RE, Trojanowski JQ, Troncoso JC, Van Deerlin VM, Vinters HV, Vonsattel JP, Weintraub S, Welsh-Bohmer KA, Williamson J, Woltjer RL, Cantwell LB, Dombroski BA, Beekly D, Lunetta KL, Martin ER, Kamboh MI, Saykin AJ, Reiman EM, Bennett DA, Morris JC, Montine TJ, Goate AM, Blacker D, Tsuang DW, Hakonarson H, Kukull WA, Foroud TM, Haines JL, Mayeux R, Pericak-Vance MA, Farrer LA, Schellenberg GD. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet. 2011;43:436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Rees SD, Kelly MA, Bain SC, Barnett AH, Prasher A, Arshad H, Prasher VP. Genetic variants conferring susceptibility to Alzheimer’s disease in the general population; do they also predispose to dementia in Down’s syndrome. BMC Res Notes. 2014;7:42. doi: 10.1186/1756-0500-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Rees SD, Kelly MA, Bain SC, Barnett AH, Thalitaya D, Prasher VP. Association of variants within APOE, SORL1, RUNX1, BACE1 and ALDH18A1 with dementia in Alzheimer’s disease in subjects with Down syndrome. Neurosci Lett. 2011;487:144–148. doi: 10.1016/j.neulet.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Prandini P, Deutsch S, Lyle R, Gagnebin M, Delucinge Vivier C, Delorenzi M, Gehrig C, Descombes P, Sherman S, Dagna Bricarelli F, Baldo C, Novelli A, Dallapiccola B, Antonarakis SE. Natural gene-expression variation in Down syndrome modulates the outcome of gene-dosage imbalance. Am J Hum Genet. 2007;81:252–263. doi: 10.1086/519248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasher VP, Sajith SG, Rees SD, Patel A, Tewari S, Schupf N, Zigman WB. Significant effect of APOE epsilon 4 genotype on the risk of dementia in Alzheimer’s disease and mortality in persons with Down syndrome. Int J Geriatr Psychiatry. 2008;23:1134–1140. doi: 10.1002/gps.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumble B, Retallack R, Hilbich C, Simms G, Multhaup G, Martins R, Hockey A, Montgomery P, Beyreuther K, Masters CL. Amyloid A4 protein and its precursor in Down’s syndrome and Alzheimer’s disease [see comments] New Engl J Med. 1989;320:1446–1452. doi: 10.1056/NEJM198906013202203. [DOI] [PubMed] [Google Scholar]
- Schupf N. Genetic and host factors for dementia in Down’s syndrome. Br J Psychiatry. 2002;180:405–410. doi: 10.1192/bjp.180.5.405. [DOI] [PubMed] [Google Scholar]
- Schupf N, Kapell D, Nightingale B, Rodriguez A, Tycko B, Mayeux R. Earlier onset of Alzheimer’s disease in men with Down syndrome. Neurology. 1998;50:991–995. doi: 10.1212/wnl.50.4.991. [DOI] [PubMed] [Google Scholar]
- Schupf N, Lee A, Park N, Dang LH, Pang D, Yale A, Oh DKT, Krinsky-McHale SJ, Jenkins EC, Luchsinger JA, Zigman WB, Silverman W, Tycko B, Kisselev S, Clark L, Lee JH. Candidate genes for Alzheimer’s disease are associated with individual differences in plasma levels of beta amyloid peptides in adults with Down syndrome. Neurobiol Aging. 2015;36:2907.e1–2907.e10. doi: 10.1016/j.neurobiolaging.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupf N, Patel B, Silverman W, Zigman WB, Zhong N, Tycko B, Mehta PD, Mayeux R. Elevated plasma amyloid beta-peptide 1-42 and onset of dementia in adults with Down syndrome. Neurosci Lett. 2001;301:199–203. doi: 10.1016/s0304-3940(01)01657-3. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Silverman W, Schupf N, Zigman W, Devenny D, Miezejeski C, Schubert R, Ryan R. Dementia in adults with mental retardation: assessment at a single point in time. Am J Ment Retard. 2004;109:111–125. doi: 10.1352/0895-8017(2004)109<111:DIAWMR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Sun B, Zhou Y, Halabisky B, Lo I, Cho SH, Mueller-Steiner S, Devidze N, Wang X, Grubb A, Gan L. Cystatin C-cathepsin B axis regulates amyloid beta levels and associated neuronal deficits in an animal model of Alzheimer’s disease. Neuron. 2008;60:247–257. doi: 10.1016/j.neuron.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda T, Fukushima T, Ikeda S, Sekijima Y, Shoji S, Yanagisawa N, Tamaoka A. Plasma levels of amyloid beta proteins Abeta1-40 and Abeta1-42(43) are elevated in Down’s syndrome. Ann Neurol. 1997;41:271–273. doi: 10.1002/ana.410410220. [DOI] [PubMed] [Google Scholar]
- Wang B, Xie YC, Yang Z, Peng D, Wang J, Zhou S, Li S, Ma X. Lack of an association between Alzheimer’s disease and the cystatin C (CST3) gene G73A polymorphism in Mainland Chinese. Demen Geriatr Cogn Disord. 2008;25:461–464. doi: 10.1159/000125670. [DOI] [PubMed] [Google Scholar]
- Wegiel J, Dowjat K, Kaczmarski W, Kuchna I, Nowicki K, Frackowiak J, Mazur Kolecka B, Wegiel J, Silverman WP, Reisberg B, Deleon M, Wisniewski T, Gong CX, Liu F, Adayev T, Chen-Hwang MC, Hwang YW. The role of overexpressed DYRK1A protein in the early onset of neurofibrillary degeneration in Down syndrome. Acta Neuropathol. 2008;116:391–407. doi: 10.1007/s00401-008-0419-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel J, Kaczmarski W, Barua M, Kuchna I, Nowicki K, Wang KC, Wegiel J, Yang SM, Frackowiak J, Mazur-Kolecka B, Silverman WP, Reisberg B, Monteiro I, de Leon M, Wisniewski T, Dalton A, Lai F, Hwang YW, Adayev T, Liu F, Iqbal K, Iqbal IG, Gong CX. Link between DYRK1A overexpression and several-fold enhancement of neurofibrillary degeneration with 3-repeat tau protein in Down syndrome. J Neuropathol Exp Neurol. 2011;70:36–50. doi: 10.1097/NEN.0b013e318202bfa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welter D, MacArthur J, Morales J, Burdett T, Hall P, Junkins H, Klemm A, Flicek P, Manolio T, Hindorff L, Parkinson H. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 2014;42:D1001–D1006. doi: 10.1093/nar/gkt1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijsman EM, Pankratz ND, Choi Y, Rothstein JH, Faber KM, Cheng R, Lee JH, Bird TD, Bennett DA, Diaz-Arrastia R, Goate AM, Farlow M, Ghetti B, Sweet RA, Foroud TM, Mayeux R NIA-LOAD/NCRAD Family Study Group. Genome-wide association of familial late-onset Alzheimer’s disease replicates BIN1 and CLU and nominates CUGBP2 in interaction with APOE. PLoS Genet. 2011;7:e1001308. doi: 10.1371/journal.pgen.1001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Polepalli J, Wagh D, Rajadas J, Malenka R, Lu B. A critical role for the PAR-1/MARK-tau axis in mediating the toxic effects of Abeta on synapses and dendritic spines. Hum Mol Genet. 2012;21:1384–1390. doi: 10.1093/hmg/ddr576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman WB. Atypical aging in Down syndrome. Dev Disabil Res Rev. 2013;18:51–67. doi: 10.1002/ddrr.1128. [DOI] [PubMed] [Google Scholar]
- Zigman WB, Lott IT. Alzheimer’s disease in Down syndrome: neurobiology and risk. Ment Retard Dev Disabil Res Rev. 2007;13:237–246. doi: 10.1002/mrdd.20163. [DOI] [PubMed] [Google Scholar]
- Zigman WB, Schupf N, Jenkins EC, Urv TK, Tycko B, Silverman W. Cholesterol level, statin use and Alzheimer’s disease in adults with Down syndrome. Neurosci Lett. 2007;416:279–284. doi: 10.1016/j.neulet.2007.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.