Abstract
Background
Opioid agonist treatments (OAT) are widely-used, evidence-based strategies for treating opioid dependence and reducing HIV transmission. The positive benefits of OAT are strongly correlated with time spent in treatment, making retention a key indicator for program quality. This study assessed patient retention and associated factors in Ukraine, where OAT was first introduced in 2004.
Methods
Data from clinical records of 2,916 patients enrolled in OAT at thirteen sites from 2005–2012 were entered into an electronic monitoring system. Survival analysis methods were used to determine the probability of retention and its correlates.
Results
Twelve-month retention was 65.8%, improving from 27.7% in 2005, to 70.9% in 2011. In multivariable analyses, the correlates of retention were receiving medium and high doses of medication (compared to low doses, dropout aHR=0.57 for both medium and high doses), having not been tested for HIV and tuberculosis (compared to being tested, dropout aHR=4.44 and 3.34, respectively), and among those who were tested – a negative TB test result (compared to receiving a positive test result, dropout aHR=0.67).
Conclusion
Retention in Ukrainian OAT programs, especially in recent years, is comparable to other countries. The results confirm the importance of adequate OAT dosing (≥ 60mg of methadone, ≥8mg of buprenorphine). Higher dosing, however, will require interventions that address negative attitudes toward OAT by patients and providers. Interruption of OAT, in the case developing tuberculosis, should incorporate continuity of OAT for TB patients through integrated care delivery systems.
Keywords: HIV, Opioid Use Disorders, Medication-Assisted Treatment, methadone, buprenorphine, retention
1. Introduction
Ukraine has one of the most volatile HIV epidemics in Eastern Europe and worldwide (Joint United Nations Programme on HIV/AIDS (UNAIDS), 2016a). Explosive growth in HIV transmission started in the mid-1990s, primarily among people who inject drugs (PWIDs), and peaked in the mid-2000s (Kruglov et al., 2008). Though governmental sources suggest HIV incidence has stabilized (MoH of Ukraine, 2013; UNAIDS, 2013), the majority of new cases are still occurring among PWIDs and their sexual partners (Vitek et al., 2014) with extraordinarily high HIV incidence in some regions (Booth et al., 2016). Among the 310,000 estimated PWIDs in Ukraine (Berleva et al., 2012), most of whom are using home-made opioids (Dumchev et al., 2009), HIV prevalence ranges from 1.3% to 40.2% (Balakireva, Bondar, Sereda, & Sazonova, 2012). Governmental sources suggest HIV prevention program coverage covers up to 60% of PWID (The State Service of Ukraine, 2014), but access to HIV and addiction treatment services remains markedly inadequate (Degenhardt et al., 2014; Joint United Nations Programme on HIV/AIDS (UNAIDS), 2016b; Wolfe, Carrieri, & Shepard, 2010).
Since its advent, combination antiretroviral therapy (cART) has proven effective in various subgroups of patients, radically reducing co-morbidity and mortality (Moore, Keruly, & Chaisson, 2004; Wood et al., 2003). ART coverage in Ukraine, however, has grown insufficiently to meet the rapidly increasing needs. According to official reports, as of the end of 2014, 46.7% of the 137,970 registered HIV patients were receiving ART; while this share was disproportionately low at 13% among HIV+ PWID (MoH of Ukraine, 2015).
Opioid agonist therapies (OAT), primarily with methadone (MMT) or buprenorphine (BMT) maintenance therapy, is internationally recognized as the most effective form of treatment for chronic opioid dependence and is also among the most effective available primary and secondary HIV prevention strategies (Altice, Kamarulzaman, Soriano, Schechter, & Friedland, 2010; Meyer, Althoff, & Altice, 2013). OAT is also highly effective in reducing HIV risk behaviors and transmission (Altice et al., 2006; Metzger et al., 1993), increasing access to antiretroviral treatment (ART) (Altice et al., 2011; Lucas et al., 2010; Uhlmann et al., 2010), and improving retention in HIV care and HIV treatment outcomes (Altice et al., 2011; Low et al., 2016; Palepu et al., 2006).
Ukraine introduced BMT in 2004 in two sites and expanded it to six sites in 2005 (Bruce, Dvoryak, Sylla, & Altice, 2007). In 2008, it introduced MMT (Schaub, Chtenguelov, Subata, Weiler, & Uchtenhagen, 2010), rapidly increasing coverage to over 7,000 patients at 143 sites by mid-2012 (WHO, 2013). Procurement of OAT was conducted mostly through the grants from the Global Fund to fight AIDS, Tuberculosis and Malaria (GFATM). Treatment is primarily provided at government-funded treatment settings. Using implementation data from this context defining the individual and organization barriers to OAT (Bojko et al., 2017; Bojko et al., 2015; Bojko et al., 2016; I. Makarenko et al., 2016; J. Makarenko et al., 2017; yMazhnaya et al., 2016), policies changed in March 2016 that now allows OAT to be prescribed in non-specialty settings, dispensed in pharmacies and OAT to be self-administrated at home.
Several OAT studies in Ukraine have confirmed its effectiveness, namely reductions in drug use and HIV risk (Schaub et al., 2010) and improved quality of life (Dvoriak et al., 2014) in PWID. These idealized efficacy studies achieved a 6-month retention of 85%, which is much higher than the average for countries newly introducing OAT (Feelemyer, Des Jarlais, Arasteh, Abdul-Quader, & Hagan, 2013); however, as selection of sites and participants was not random, there is a possibility of enrollment bias.
In general, though due to a number of structural and political reasons (Bojko, Dvoriak, & Altice, 2013; Bojko et al., 2015; Bojko et al., 2016; Golovanevskaya, Vlasenko, & Saucier, 2012; Kutsa et al., 2016; I. Makarenko et al., 2016; A. Mazhnaya et al., 2016) OAT enrollment in Ukraine has been low and attrition has been high; currently less than 2.7% of the 310,000 PWID in Ukraine receive treatment, which is far below international standards and potentially jeopardizing Ukraine’s targets to address HIV prevention and treatment needs (UNAIDS, 2009; Wolfe et al., 2010). Cost-effectiveness modeling of OAT expansion in Ukraine confirmed that methadone is the single most cost-effective means of controlling the national HIV epidemic (Alistar, Owens, & Brandeau, 2011), including for PWID who become incarcerated (Altice et al., 2016).
A significant body of evidence demonstrates that the benefits of OAT are strongly correlated with time spent in treatment (i.e., retention). Retention is key to successful social rehabilitation, and reduces relapse and criminal activity (del Rio, Mino, & Perneger, 1997; Strike et al., 2005). Numerous studies investigated predictors of dropout and retention in OAT. Notable factors include psychiatric co-morbidity, polysubstance use, number of previous treatment attempts, treatment readiness/motivation, availability of psychosocial support and adequate dosing (Anderson & Warren, 2004; Babst, Chambers, & Warner, 1971; del Rio et al., 1997; Greenfield et al., 2007; Strike et al., 2005).
Despite exceedingly high retention early after the introduction of OAT in Ukraine (Schaub et al., 2010), very little is known about OAT outcomes and retention in more recent years. Therefore, the current study was conducted to examine retention and associated correlates using routine retrospective clinical data from a sample of Ukrainian OAT programs. Results will be used to inform programming and advocacy for HIV prevention and treatment of PWID in Ukraine and other similar countries where HIV is concentrated in PWID.
2. Methods
2.1. Data collection
The study analyzed the data exported from an electronic Simple Treatment Monitoring Application (STMA), developed by the authors for the purposes of routine clinical data management and reporting. The database was designed to routinely track OAT patients, including their patient drug use history, treatment admission and discharge dates, medication prescription and dosing, and a wide variety of clinical and laboratory assessments and psychosocial services provided.
Since 2008, the STMA was made available to Ukrainian OAT providers who were interested in using electronic monitoring systems. Use of the database was not mandatory and the choice of parameters to be entered was left to the discretion of clinic. Data from source medical documentation were entered into the database routinely by clinical staff.
In June 2011, all OAT facilities using the STMA were invited to submit de-identified data to develop an integrated database. Other OAT programs used either simplistic electronic spreadsheets or paper-based charts that were unsuitable for inclusion in the cohort. Data collection was completed by March 2012. In Ukraine, all patients entering OAT sign an informed consent form, which stipulates that their de-identified clinical data may be used for scientific analysis and programmatic improvement.
2.2. Site selection and participants
To be invited to integrate their data into the retrospective cohort, the OAT sites had to meet the following criteria: 1) systematic use of the STMA; 2) having entered basic demographics and treatment history on all patients treated since the site began operation; and 3) willingness to submit a de-identified dataset for analysis. Among 140 OAT clinics in Ukraine at the time of data collection, 20 systematically used the STMA, and 13 agreed to study participation and analysis of data.
Participating clinics were large, located mostly in eastern and central Ukraine where the HIV epidemic is concentrated (The State Service of Ukraine, 2014): Donetsk (N=1), Luhansk (N=1), Crimea (N=1), Dnipro (N=4), Kyiv City (N=2), Vinnitsya (N=2), and Poltava (N=2). These sites were also early OAT sites (Kyiv, Dnipropetrovsk, Donetsk and Crimea started OAT in 2004–2005) and larger (mean=224 patients per site versus 49 nationally). The types of facilities where OAT was provided included one general hospital, one TB hospital, two HIV clinics, and nine addiction specialty treatment (“narcological”) hospitals.
From the opening of the first site until March 2012, when data collection was completed, the 13 sites enrolled 2,916 patients. The entire sample was included in the study as a retrospective cohort.
During the data collection period, patients were eligible for treatment if they were at least 18 years of age and had a diagnosis of opioid dependence confirmed by a board of three Narcology physicians.
2.3. Data
All sites entered basic demographics (sex and age), treatment history, and other variables, which were not mandatorily assessed. Of 13 sites, 11 sites entered HIV testing data, 7 entered medication dosage and TB testing, and 6 entered hepatitis testing information. Drug injection duration is recorded at admission and was available for 6 sites. Other baseline characteristics, such as number of previous treatment attempts and treatment readiness are not always documented in patient charts and were not entered in the STMA. Assessment of psychiatric co-morbidity and polysubstance use (using urine screening or other methods) are also not assessed systematically in Ukrainian OAT programs.
2.4. Variables
2.4.1. Exposure
The final dataset included basic demographic data (age and gender), the last HIV test result recorded (rapid test, ELISA, or Western blot), TB test (sputum microscopy, chest X-ray, or culture), HBV test (HBsAg or anti-HBc), and HCV test (anti-HCV antibody). Despite both TB and HIV testing being required for all patients entering OAT, not all patients had results recorded. Screening for HCV and HBV is encouraged, but seldom done because it is not free. “Not tested” was considered a valid response if HIV, TB and viral hepatitis testing was not performed.
The type of medication (methadone or buprenorphine) and last prescribed dose in milligrams also was included. For analysis purposes, the dose was categorized as low, medium or high (<60, 60–100, >100mg for methadone, and <8, 8–12, >12mg for buprenorphine). The lower cutoff was based on WHO recommendations (WHO, 2009), and the higher cutoff was arbitrarily set to ensure closer-to-normal distribution across categories.
2.4.2. Outcome
Retention in treatment, defined as the time between OAT initiation and discontinuing treatment for 10 or more consecutive days, was the primary outcome. Discharge may occur as a result of transfer to another OAT clinic, voluntary dose tapering, administrative discharge because of violation of program rules, incarceration, death, or voluntary dropout (no-show). Policies regulating the discharge in case of patient no-show vary across institutions, but according to national OAT guidelines, discharge is officially registered if no OAT is dispensed 10 days after the last patient’s visit. There is no defined algorithm for establishing the reason for treatment discontinuation though cases of death or incarceration are verified by family members or social workers. If verification is not ascertained, discharge reason is registered as voluntary dropout.
Similarly to other studies (Cox, Allard, Maurais, Haley, & Small, 2013), if a patient re-started OAT within 7 days of an officially registered dropout, the two consecutive treatment episodes were analyzed as a single episode. When multiple treatment episodes were recorded for one patient, only the first episode was analyzed.
2.5. Data analysis
Since exact dates of admission and discharge were available, it was possible to conduct time-to-event (survival) analysis using Kaplan-Meier and Cox proportional hazards methods to describe treatment retention and identify the predictors of patient dropout.
Person-time began at the date of admission and ended at the date of discharge or date of censoring. Observations were “censored”: at the date of site data submission, if the patient was not yet discharged; and at the date of transfer to another site, if the site was not included in the study. All other observations were regarded as having the outcome of interest.
Because of partially incomplete data (see Section 2.3), it was not possible to perform all analyses using the entire dataset. Complete treatment history on all patients was a requirement for inclusion of a site-level dataset to the study, therefore, calculation of patient retention in OAT, the main outcome of the study, was done on the entire sample of all 13 sites (N=2,916). The descriptive and univariable analyses, however, were done using the subsamples from the sites that submitted data on respective variables (see Table 1 for details). The multivariable analysis was based on the data from 6 sites (N=1,035 from 2 sites in Vinnitsya and 4 sites in Dnipropetrovsk), which provided complete data on demographics, medication, and testing for HIV, tuberculosis and viral hepatitis (HCV and HBV). Tarone-Ware test was used for equality across strata for categorical variables and Cox proportional hazard regression for continuous variables. For HIV, TB and viral hepatitis screening variables, an additional test for equality was done excluding patients that were not screened.
Table 1.
Descriptive and univariate analyses results.
| Categorical variables | Available records (Included sites) | |||
|---|---|---|---|---|
| N total | N (%) | p-value (Tarone-Ware) | ||
| Sex | 2916 (13) | 0.007 | ||
| Males | 2271 (77.9%) | |||
| Females | 645 (22.1%) | |||
| Medication | 1067 (7) | 0.053 | ||
| Buprenorphine | 182 (17.1%) | |||
| Methadone | 885 (82.2%) | |||
| Last prescribed dose | 1065 (7) | <0.0001 | ||
| Low | 385 (36.2%) | |||
| Medium | 466 (43.8%) | |||
| High | 214 (20.1%) | |||
| Last HIV test result | 1981 (11) | <0.0001 | ||
| no record | 205 (10.3%) | <0.0001* | ||
| positive | 1089 (55.0%) | (61.3%)* | ||
| negative | 687 (34.7%) | (38.7%)* | ||
| Last TB test result | 1077 (7) | <0.0001 | ||
| no record | 145 (13.5%) | <0.0001* | ||
| positive | 197 (18.3%) | (21.1%)* | ||
| negative | 735 (68.2%) | (78.9%)* | ||
| Last HCV test result | 1035(6) | <0.0001 | ||
| no record | 591 (57.1%) | 0.763* | ||
| positive | 372 (35.6%) | (83.8%)* | ||
| negative | 72 (7.0%) | (16.2%)* | ||
| Last HBV test result | 1035(6) | <0.0001 | ||
| no record | 682 (65.9%) | 0.125* | ||
| positive | 119 (11.5%) | (33.7%)* | ||
| negative | 234 (22.6%) | (66.3%)* | ||
| Year of admission | 2916 (13) | <0.0001 | ||
| 2004 | 5 (0.2%) | |||
| 2005 | 111 (3.8%) | |||
| 2006 | 228 (7.8%) | |||
| 2007 | 204 (7.0%) | |||
| 2008 | 773 (26.5%) | |||
| 2009 | 703 (24.1%) | |||
| 2010 | 516 (17.7%) | |||
| 2011 | 359 (12.3%) | |||
| 2012 | 17 (0.6%) | |||
| Continuous variables | ||||
| Mean (SD) | p-value (Cox) | |||
| Age, years | 2916 (13) | 36.4 (7.9) | 0.711 | |
| Duration of drug use prior to admission, years | 804 (6) | 15.9 (8.0) | 0.883 | |
The calculation excludes the “no record” observations.
In order to compare correlates of retention among the patients who comply with baseline HIV and TB testing requirement and those who do not, two approaches to multivariable Cox proportional hazard modeling were used. The first one treated the absence of HIV, TB or hepatitis testing record as a valid “not tested” value (N=1,021), whereas the second one was limited to the analysis of cases with available results for HIV and TB testing (N=846). Hepatitis testing variables were excluded from the second modeling approach due to the majority of patients not having a testing record for either HCV or HBV (N=273 cases with available record for all testing variables).
Variable selection and estimation of regression coefficients and standard errors were performed using Bayesian Model Averaging (BMA) method (Hoeting, Madigan, Raftery, & Volinsky, 1999). A 50% variable inclusion probability threshold was used to select variables in the final subset, and both hazard ratios and their respective standard errors were estimated unconditionally (averaged over all evaluated models with the covariate coefficient being considered 0 in case a particular model did not include that covariate), since this approach correctly accounts for model uncertainty and performs estimation conservatively (Morozova, Levina, Uuskula, & Heimer, 2015).
The STMA database and export files were developed in MS Access; the final dataset with de-identified personal data was analyzed using SPSS version 21 for Windows and R Statistical Analysis Software (Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Descriptive
The characteristics of the sample are described in Table 1. Overall, the 2,916 patients were primarily male (77.9%), in their mid-30s (mean=36.4; range 18–70 years ) long-term injectors (mean=15.9, range 1–45 years) and primarily received MMT (82.2%). Mean dosages for methadone and buprenorphine were 73mg (range 5–350mg) and 10mg (range 2–22mg), respectively.
Of 1,776 patients with recorded HIV testing results, 61.3% were positive. Among 932 patients with TB testing information (positive chest X-ray or sputum culture), 21.1% has findings suggestive of active TB disease, with most (18.1% of the total and 86.2% of those with likely TB disease) being HIV/TB co-infected.
3.2. Retention
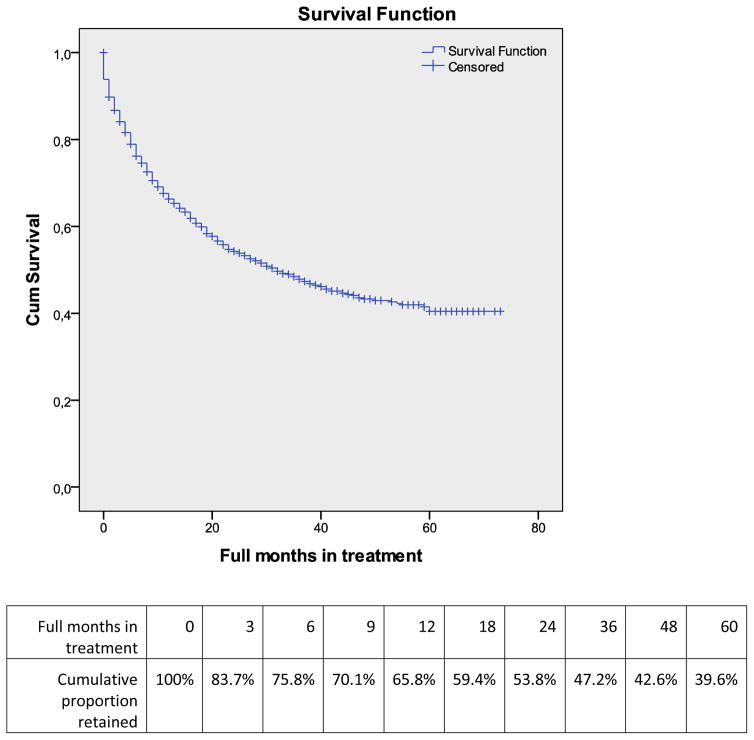
Overall, 12-month retention at all sites after admission was 65.8%, slowly declining to 39.6% by 60 months (Figure 1). After excluding patients receiving OAT at a TB dispensary site, retention across all other sites varied from 59.8% to 74.4% at 12 months, but the difference was not significant. Of note, retention at 12 months was 0% for patients at TB site.
Figure 1.
Survival curve and cumulative proportion retained by month of opioid agonist treatment.
When stratified by year of admission, 12-month retention increased over time from 27.7% among patients enrolled in 2005 to 70.9% among those enrolled in 2011. Generally, the highest attrition from treatment (14.5%) occurred during the first month of treatment, with no significant change by year of admission (data not shown).
Of 1,148 recorded discharge instances (excluding transfers to other sites), the largest proportion (35.4%) of patients were discharged because of administrative reasons. Other major reasons included voluntary dropout without dose tapering (25.7%), voluntary discontinuation with dose tapering (11.5%), death (12.9%), and incarceration (9.7%).
3.3. Multivariable analysis
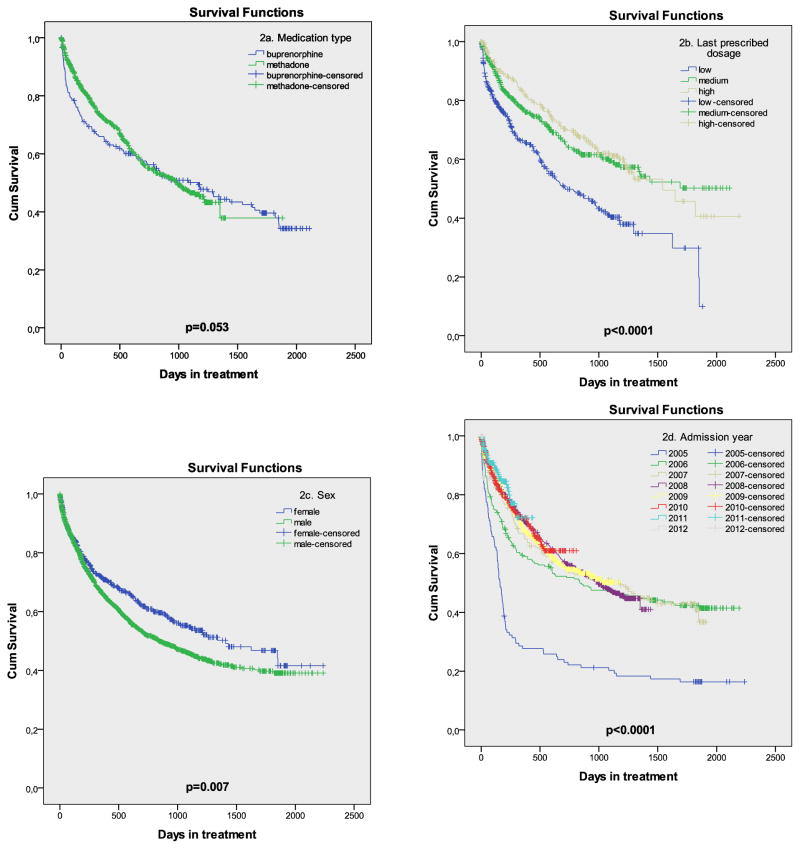
Figure 2 and Table 1 show the results of the univariable analysis, while the full multivariable modelling results using two different approaches are presented in Table 2.
Figure 2.
Survival curves for medication, dosage level, sex and year of admission.
Table 2.
Correlates of dropout from Opioid Agonist Treatment: results of multivariable Cox regression with Bayesian Model Averaging.
| Covariates | Model 1 (N=1,021) | Model 2 (N=846) | ||||||
|---|---|---|---|---|---|---|---|---|
| Inclusion Probability (%) | aHR | 95.0% CI for aHR | Inclusion Probability (%) | aHR | 95.0% CI for aHR | |||
| Lower | Upper | Lower | Upper | |||||
| Sex (male vs female) | 3.7 | 1.00 | 0.94 | 1.07 | 3 | 1.00 | 0.95 | 1.06 |
| Age | 3.9 | 1.00 | 1.00 | 1.00 | 4 | 1.00 | 1.00 | 1.00 |
| Medication type (buprenorphine vs methadone) | 2.5 | 1.00 | 0.94 | 1.06 | 3.6 | 1.00 | 0.92 | 1.07 |
| Last prescribed dosage (medium vs low) | 100 | 0.57 | 0.45 | 0.71 | 100 | 0.56 | 0.43 | 0.72 |
| Last prescribed dosage (high vs low) | 100 | 0.57 | 0.44 | 0.75 | 100 | 0.54 | 0.40 | 0.73 |
| Last TB test result (negative vs positive) | 65.3 | 0.78 | 0.51 | 1.18 | 90.4 | 0.67 | 0.47 | 0.96 |
| Last TB test result (not tested vs positive) | 100 | 3.34 | 2.23 | 5.01 | ||||
| Last HIV test result (negative vs positive) | 2.2 | 1.00 | 0.96 | 1.04 | 3.8 | 1.00 | 0.94 | 1.07 |
| Last HIV test result (not tested vs positive) | 100 | 4.44 | 3.10 | 6.35 | ||||
| Last HCV test result (negative vs positive) | 2.7 | 1.00 | 0.92 | 1.08 | ||||
| Last HCV test result (not tested vs positive) | 35.7 | 1.11 | 0.81 | 1.53 | ||||
| Last HBV test result (negative vs positive) | 34.6 | 0.88 | 0.59 | 1.30 | ||||
| Last HBV test result (not tested vs positive) | 34.3 | 1.12 | 0.80 | 1.57 | ||||
| Site (DPSPC vs DPGP5) | 37.1 | 1.17 | 0.74 | 1.85 | 4.5 | 0.99 | 0.88 | 1.12 |
| Site (PNDKR vs DPGP5) | 53 | 0.82 | 0.53 | 1.26 | 27.9 | 0.92 | 0.68 | 1.24 |
| Site (PNDN7 vs DPGP5) | 21.8 | 0.93 | 0.69 | 1.25 | 4.6 | 1.01 | 0.92 | 1.10 |
| Site (VOND1 vs DPGP5) | 50.6 | 1.23 | 0.78 | 1.92 | 2.5 | 1.00 | 0.95 | 1.05 |
| Site (VOND2 vs DPGP5) | 6.1 | 1.02 | 0.87 | 1.19 | 3.3 | 1.00 | 0.93 | 1.09 |
| Admission year (2010 vs 2011–12) | 2.1 | 1.00 | 0.95 | 1.05 | 18.1 | 1.06 | 0.79 | 1.43 |
| Admission year (2009 vs 2011–12) | 4 | 0.99 | 0.92 | 1.07 | 4.1 | 0.99 | 0.92 | 1.08 |
| Admission year (2008 vs 2011–12) | 2.7 | 1.00 | 0.96 | 1.05 | 4.5 | 0.99 | 0.93 | 1.07 |
| Admission year (2007 vs 2011–12) | 4.9 | 1.01 | 0.88 | 1.17 | 3.8 | 1.01 | 0.90 | 1.12 |
| Admission year (2006 vs 2011–12) | 2.2 | 1.00 | 0.94 | 1.06 | 6.9 | 0.98 | 0.82 | 1.18 |
| Admission year (2005 vs 2011–12) | 100 | 3.39 | 2.04 | 5.63 | 100 | 4.03 | 2.45 | 6.64 |
The first approach resulted in four variables being included in the final subset based on 50% inclusion probability threshold in BMA analysis: OAT dosage, TB testing, HIV testing and admission year. Adjusted hazard ratios (aHR), comparing to the low dose referent, were 0.57 (95% CI 0.45–0.71) for medium dosage and 0.57 (95% CI 0.44–0.75) for high dosage. Changing the reference category for dosage to high showed that the difference between medium and high OAT dosage was not significant (aHR=1.00, 95% CI 0.96–1.04).
Additionally, significant aHR for dropout was 3.34 (95% CI 2.23–5.01) for not having been tested for TB, and 4.44 (95% CI 3.10–6.35) for not having been tested for HIV, compared to having been tested for each respective condition. Having been tested for TB with a negative result compared to a positive one was included into the selected subset for the first modeling approach (with the inclusion probability of 65.3%), however was not significant (aHR=0.78, 95% CI 0.51–1.18). Patients admitted in 2005 had a significantly higher probability of dropout, but other years were not significantly different. Sex, age, medication type, HCV and HBV testing, and site were not associated with retention in our analysis.
The second modelling approach, excluding patients who had no record of HIV or TB test, produced similar results. Negative result of HIV test (compared to positive) remained not significant with aHR=1.00 (95%CI 0.94–1.07), whereas negative TB test (compared to positive) became a significant correlate of retention with aHR=0.67 (95%CI 0.47–0.96). The aHR for medium and OAT high dosage, compared to low, remained nearly identical to those in the first modeling strategy (0.56 and 0.54 respectively).
Due to a large number of missing values for HCV and HBV testing, these variables were not included in the second modeling approach (see Methods), however when we performed similar analyses using N=273 cases with complete data on all testing variables, neither HCV nor HBV test result was associated with treatment retention (aHR=1.00, 95% CI 0.93–1.07; and aHR = 0.97, 95% CI 0.78–1.22 for HCV and HBV negative test result respectively; data not shown).
The patients at the 6 sites included into multivariable analysis compared to those that were excluded were not different by gender (Chi-square p=0.354), medication dosage (Chi-square p=0.243), and retention (Tarone-Ware p=0.246), but were significantly older (average age 38.2 vs. 35.4, t-test p<0.0001).
4. Discussion
To our knowledge, this is the first assessment of patient retention in OAT programs and associated factors in Ukraine since the first 391 patients were assessed over a 6-month period (Schaub et al., 2010). This current retrospective cohort analysis using routine real-world clinical data from 2,916 patients accessing OAT program at 13 sites examines the factors related to treatment retention and provides some important insights into how to optimize OAT scale-up.
First, 12-month retention (65.8%) at the participating sites is comparable with that in other countries (Timko, Schultz, Cucciare, Vittorio, & Garrison-Diehn, 2015), although leaving considerable room for improvement. The fact that retention improved over time may speak to increased experience of staff and improved quality of the programs.
In contrast to other studies (Mattick, Breen, Kimber, & Davoli, 2014; Timko et al., 2015), methadone was not associated with higher retention with BMT patients. This may be explained in part, because prior to 2008 only BMT was available in Ukraine, and improvements in program quality and retention in later years may have diminished the effect of the medication itself. Additionally, BMT coverage remained unchanged and limited to only 700 patients country-wide, making this a preferred treatment option for opioid-dependent PWID (I. Makarenko et al., 2016).
Adequate medication dosing that exceeds 60 mg/day for methadone (National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction, 1998) and 8 mg/day for buprenorphine (WHO, 2009), has been found to be a strong predictor of retention by multiple studies (Anderson & Warren, 2004; Hillhouse, Canamar, & Ling, 2013; Lambdin et al., 2014; Villafranca, McKellar, Trafton, & Humphreys, 2006; Wei et al., 2013), including a meta-analysis of Chinese MAT program (Bao et al., 2009) and in a recent Cochrane Review (Mattick et al., 2014); however a recent meta-analysis of OAT studies in low- and middle-income countries (Feelemyer et al., 2013) failed to confirm this association. In this study, receiving low-dose OAT was significantly correlated with lower retention, whereas the effect of medium and high OAT doses did not differ significantly. Average daily doses in the sample (73mg for methadone and 10mg for buprenorphine) were almost identical to the national average doses (77mg and 10mg respectively, as of 01.01.2015) routinely reported by all programs to the Ukrainian Center for Socially Dangerous Diseases Control. These levels are at the lower end of the range of 60–120mg for methadone and 8–32mg for buprenorphine recommended by WHO (WHO, 2009). The proportion of patients receiving suboptimal doses in this study was substantial (34.9%). Interventions are therefore crucial for expanding and improving the quality of OAT within Ukraine, including the use of informed decision-making tools (Braddock, Edwards, Hasenberg, Laidley, & Levinson, 1999; Braddock, Fihn, Levinson, Jonsen, & Pearlman, 1997). Such tools must not only target negative attitudes toward OAT by patients and providers (I. Makarenko et al., 2016; Polonsky et al., 2015; Polonsky, Rozanova, et al., 2016), but address higher dosages for patients (Bojko et al., 2016; I. Makarenko et al., 2016). Because OAT was introduced in Ukraine primarily for HIV prevention, not for treatment of opioid dependence, addiction treatment specialists and others in Ukraine have not fully adopted evidence-based treatment strategies using OAT due to a legacy of Soviet-style Narcology that promotes extraordinarily negative attitudes toward PWID and OAT(Elovich & Drucker, 2008; Latypov, 2011; Polonsky et al., 2015; Polonsky, Rozanova, et al., 2016).
Being tested for HIV and TB was a significant correlate of retention in OAT programs. One explanation is that being tested was a surrogate of more comprehensive commitment to programmatic recommendations, but could also be explained by the extent to which the program provided integrated treatment services. Further data and additional inquiry, however, would be needed to establish causality. On one hand, higher testing coverage may be a proxy for better quality of care or, alternatively, patients dropping out soon after enrollment may not have had enough time to get tested.
Reactive tests for HIV and tuberculosis testing suggesting active TB were significant predictors of dropout in univariable analyses. After inclusion of these variables into multivariable models, only having testing suggestive of active TB remained significant. The presence of active TB (7.7% among HIV- and 29.1% among HIV+ patients) negatively influences retention at all types of OAT sites. Explanations for this finding might include that Ukrainian guidelines requires inpatient hospitalization for all TB patients initially and involuntary discharge from OAT because TB hospitals may not provide onsite OAT. Alternatively, drop-out may be higher in TB patients due to high levels of MDR-TB and death in these patients (Morozova, Dvoryak, & Altice, 2013). Irrespective of etiology, the healthcare reform should shift to more integrated care settings (Bachireddy et al., 2014; Sylla, Bruce, Kamarulzaman, & Altice, 2007) that incorporate treatment for HIV, TB and opioid dependence, including in hospitalized settings. Important in our findings is that none of the patients receiving OAT in a TB hospital were retained for 12-months, either due to extraordinary high mortality in HIV/TB co-infected patients or inability to transfer patients to alternative OAT sites (Morozova et al., 2013).
Of interest is the finding that higher dropout occurred during the first month of treatment. Though MMT treatment guidelines now recommend achieving a dose of 60 mg per day within the first 30 days of treatment, dose escalation was markedly slower during the time of this study observation (Bojko et al., 2016; Polonsky, Rozanova, et al., 2016). Alternative explanations include of psychosocial support, inability of some patients to comply with strict program regulations or treatment expectations that OAT was intended as a long-term strategy. Until March 2016, no take-home doses were allowed in Ukraine, requiring OAT patients to travel to OAT sites daily where sites had restricted dispensing times (Bojko et al., 2015; Bojko et al., 2016; The State Service of Ukraine, 2014; UNAIDS, 2009) and where police often harassed OAT clients (Izenberg et al., 2013; Polonsky, Azbel, et al., 2016). Subsequent policy reforms in 2016 have allowed both prescription and take-home dosing for buprenorphine and methadone, although this practice has not yet been widely implemented.
4.1. Limitations
Though there were several key findings, some limitations remain. First, selection of sites for this data analysis was not random, and there is a possibility that the 13 sites that agreed to participate in this research had better record-keeping and potentially provided better services. Due to incomplete data, 7 out of 13 sites were excluded from the multivariable analyses, which further limits the ability to extrapolate the results. The finding that the clients in the included and excluded sites did not differ on major predictor variables, however, somewhat reduces this concern. Because of the cross-sectional nature of national OAT reporting, it is not possible to estimate the total cumulative number of patients who received OAT in Ukraine, but the sample included 1,766 (26.4%) out of 6,678 patients who were currently receiving OAT in Ukraine as of March 2012. This sizeable proportion, may also improve generalizability of the findings. Second, because only the last recorded OAT dose was included in the dataset, dosing at discharge may have reflected patients who were voluntarily tapering off OAT. The number of such patients, however, was small (132, or 8.6% of all discharged), and excluding them from analysis did not meaningfully change the results. Last, because medical record data were used, there was insufficient ability to identify other potential mediators of treatment dropout, such as psychiatric co-morbidity, polysubstance use, psychological and behavioral patient characteristics. These variables could have substantial effect if they were available for analysis and should be considered in future studies of OAT retention.
4.2. Conclusions
In Ukraine, a country with a PWID-driven epidemic, OAT programs have shown feasibility and effectiveness, covering 8,264 patients by year-end 2015. As a strategy to prevent HIV transmission and improve engagement into and adherence to ART, MAT should remain a priority for Ukrainian National HIV Program. Retention is a critical factor for success of OAT, and Ukrainian OAT programs demonstrate satisfactory short-term retention, which improved over time. Results of this study confirm the importance of adequate dosing, which remains suboptimal for many patients. This should be addressed through continued provider education and program quality monitoring.
Existing policies, mandating inpatient treatment for patients diagnosed with TB and absence of OAT in TB treatment facilities contribute to dropout from OAT programs. This finding calls for wider integration of MAT and revision of TB treatment guidelines.
Given the utility and ease of use of electronic monitoring instruments, such analysis of retention should become a regular practice for national bodies responsible for MAT implementation.
Acknowledgments
The authors thank the following people who coordinated data collection for this study: Irina Veretko, M.D. (Vinnitsya Narcological Dispensary), Elmira Mamedova, M.D. (Kyiv City AIDS Center), Leonid Vlasenko, M.D. (Clinton Health Access Initiative), Dmitry Chekhov (Dnipropetrovsk AIDS Center), Vyacheslav Solonskyi, M.D. (Krivyi Rih Psychoneurological Dispensary), Andriy Mandybura, M.D. (Crimea Republican Narcological Dispensary), Vadim Klorfine, M.D. (Poltava Narcological Dispensary), Volodymyr Yaryi, M.D. (Kyiv City Narcological Hospital).
Funding for this research was supported by World Health Organization Country Office in Ukraine and through research from the National Institutes on Drug Abuse (R01 DA033679 (Altice), R01 DA029910 (Altice), K24 DA017072 (Altice), R36 DA042643 (Morozova)). The funding agencies played no role in decisions to publish or the content of the manuscript.
Footnotes
Conflict of interest statement
No author reports a conflict of interest, financial or otherwise, relevant to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alistar SS, Owens DK, Brandeau ML. Effectiveness and cost effectiveness of expanding harm reduction and antiretroviral therapy in a mixed HIV epidemic: a modeling analysis for Ukraine. PLoS Med. 2011;8(3):e1000423. doi: 10.1371/journal.pmed.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Azbel L, Stone J, Brooks-Pollock E, Smyrnov P, Dvoriak S, … Vickerman P. The perfect storm: incarceration and the high-risk environment perpetuating transmission of HIV, hepatitis C virus, and tuberculosis in Eastern Europe and Central Asia. Lancet. 2016;388(10050):1228–1248. doi: 10.1016/S0140-6736(16)30856-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Bruce RD, Lucas GM, Lum PJ, Korthuis PT, Flanigan TP, … Bhives Collaborative HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S22–32. doi: 10.1097/QAI.0b013e318209751e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Kamarulzaman A, Soriano VV, Schechter M, Friedland GH. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet. 2010;376(9738):367–387. doi: 10.1016/S0140-6736(10)60829-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Sullivan LE, Smith-Rohrberg D, Basu S, Stancliff S, Eldred L. The potential role of buprenorphine in the treatment of opioid dependence in HIV-infected individuals and in HIV infection prevention. Clin Infect Dis. 2006;43(Suppl 4):S178–183. doi: 10.1086/508181. [DOI] [PubMed] [Google Scholar]
- Anderson JF, Warren LD. Client retention in the British Columbia Methadone Program, 1996–1999. Can J Public Health. 2004;95(2):104–109. doi: 10.1007/BF03405776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst DV, Chambers CD, Warner A. Patient characteristics associated with retention in a methadone maintenance program. Br J Addict Alcohol Other Drugs. 1971;66(3):195–204. doi: 10.1111/j.1360-0443.1971.tb02386.x. [DOI] [PubMed] [Google Scholar]
- Bachireddy C, Soule MC, Izenberg JM, Dvoryak S, Dumchev K, Altice FL. Integration of health services improves multiple healthcare outcomes among HIV-infected people who inject drugs in Ukraine. Drug Alcohol Depend. 2014;134:106–114. doi: 10.1016/j.drugalcdep.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakireva OM, Bondar TV, Sereda YV, Sazonova YO. Behavior Monitoring and HIV Prevalence among Injecting Drug Users as a Component of Second Generation Sentinel Surveillance. 2012 Retrieved from http://www.aidsalliance.org.ua/ru/library/our/2012/me/idu_en_2011.pdf.
- Bao YP, Liu ZM, Epstein DH, Du C, Shi J, Lu L. A meta-analysis of retention in methadone maintenance by dose and dosing strategy. Am J Drug Alcohol Abuse. 2009;35(1):28–33. doi: 10.1080/00952990802342899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berleva G, Dumchev K, Kasianchuk M, Nikolko M, Saliuk T, Shvab I, Yaremenko O. Estimation of the Size of Populations Most-at-Risk for HIV Infection in Ukraine. 2012 Retrieved from http://www.aidsalliance.org.ua/ru/library/our/2013/SE_2012_Eng.pdf.
- Bojko MJ, Dvoriak S, Altice FL. At the crossroads: HIV prevention and treatment for people who inject drugs in Ukraine. Addiction. 2013;108(10):1697–1699. doi: 10.1111/add.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojko MJ, Madden L, Farnum S, Mazhnaya A, Fomenko T, Marcus R, … Altice FL. Using Nominal Group Technique to Assess Barriers to Scale Up of Opioid Agonist Therapy (OAT) in Ukraine: The Providers’ Perspective. International J Drug Policy. 2017 doi: 10.1016/j.drugpo.2017.07.025. in press. [DOI] [PubMed] [Google Scholar]
- Bojko MJ, Mazhnaya A, Makarenko I, Marcus R, Dvoriak S, Islam Z, Altice FL. “Bureaucracy & Beliefs”: Assessing the Barriers to Accessing Opioid Substitution Therapy by People Who Inject Drugs in Ukraine. Drugs (Abingdon Engl) 2015;22(3):255–262. doi: 10.3109/09687637.2015.1016397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojko MJ, Mazhnaya A, Marcus R, Makarenko I, Islam Z, Filippovych S, … Altice FL. The Future of Opioid Agonist Therapies in Ukraine: A Qualitative Assessment of Multilevel Barriers and Ways Forward to Promote Retention in Treatment. J Subst Abuse Treat. 2016;66:37–47. doi: 10.1016/j.jsat.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth RE, Davis JM, Dvoryak S, Brewster JT, Lisovska O, Strathdee SA, Latkin CA. HIV incidence among people who inject drugs (PWIDs) in Ukraine: results from a clustered randomised trial. Lancet HIV. 2016;3(10):e482–489. doi: 10.1016/S2352-3018(16)30040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braddock CH, 3rd, Edwards KA, Hasenberg NM, Laidley TL, Levinson W. Informed decision making in outpatient practice: time to get back to basics. JAMA. 1999;282(24):2313–2320. doi: 10.1001/jama.282.24.2313. [DOI] [PubMed] [Google Scholar]
- Braddock CH, 3rd, Fihn SD, Levinson W, Jonsen AR, Pearlman RA. How doctors and patients discuss routine clinical decisions. Informed decision making in the outpatient setting. J Gen Intern Med. 1997;12(6):339–345. doi: 10.1046/j.1525-1497.1997.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce RD, Dvoryak S, Sylla L, Altice FL. HIV treatment access and scale-up for delivery of opiate substitution therapy with buprenorphine for IDUs in Ukraine--programme description and policy implications. Int J Drug Policy. 2007;18(4):326–328. doi: 10.1016/j.drugpo.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J, Allard R, Maurais E, Haley N, Small C. Predictors of methadone program non-retention for opioid analgesic dependent patients. J Subst Abuse Treat. 2013;44(1):52–60. doi: 10.1016/j.jsat.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Mathers BM, Wirtz AL, Wolfe D, Kamarulzaman A, Carrieri MP, … Beyrer C. What has been achieved in HIV prevention, treatment and care for people who inject drugs, 2010–2012? A review of the six highest burden countries. Int J Drug Policy. 2014;25(1):53–60. doi: 10.1016/j.drugpo.2013.08.004. [DOI] [PubMed] [Google Scholar]
- del Rio M, Mino A, Perneger TV. Predictors of patient retention in a newly established methadone maintenance treatment programme. Addiction. 1997;92(10):1353–1360. [PubMed] [Google Scholar]
- Dumchev KV, Soldyshev R, Qian HZ, Zezyulin OO, Chandler SD, Slobodyanyuk P, … Schumacher JE. HIV and hepatitis C virus infections among hanka injection drug users in central Ukraine: a cross-sectional survey. Harm Reduct J. 2009;6:23. doi: 10.1186/1477-7517-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoriak S, Karachevsky A, Chhatre S, Booth R, Metzger D, Schumacher J, … Woody G. Methadone maintenance for HIV positive and HIV negative patients in Kyiv: acceptability and treatment response. Drug Alcohol Depend. 2014;137:62–67. doi: 10.1016/j.drugalcdep.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovich R, Drucker E. On drug treatment and social control: Russian narcology’s great leap backwards. Harm Reduct J. 2008;5:23. doi: 10.1186/1477-7517-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feelemyer J, Des Jarlais D, Arasteh K, Abdul-Quader AS, Hagan H. Retention of participants in medication-assisted programs in low- and middle-income countries: an international systematic review. Addiction. 2013 doi: 10.1111/add.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovanevskaya M, Vlasenko L, Saucier R. In control?: Ukrainian opiate substitution treatment patients strive for a voice in their treatment. Subst Use Misuse. 2012;47(5):511–521. doi: 10.3109/10826084.2012.644117. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Brooks AJ, Gordon SM, Green CA, Kropp F, McHugh RK, … Miele GM. Substance abuse treatment entry, retention, and outcome in women: a review of the literature. Drug Alcohol Depend. 2007;86(1):1–21. doi: 10.1016/j.drugalcdep.2006.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillhouse M, Canamar CP, Ling W. Predictors of outcome after short-term stabilization with buprenorphine. J Subst Abuse Treat. 2013;44(3):336–342. doi: 10.1016/j.jsat.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeting JA, Madigan D, Raftery AE, Volinsky CT. Bayesian Model Averaging: A Tutorial. Statistical Science. 1999;14(4):382–401. [Google Scholar]
- Izenberg JM, Bachireddy C, Soule M, Kiriazova T, Dvoryak S, Altice FL. High rates of police detention among recently released HIV-infected prisoners in Ukraine: implications for health outcomes. Drug Alcohol Depend. 2013;133(1):154–160. doi: 10.1016/j.drugalcdep.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint United Nations Programme on HIV/AIDS (UNAIDS) Global AIDS Update 2016. Geneva, Switzerland: 2016a. [Google Scholar]
- Joint United Nations Programme on HIV/AIDS (UNAIDS) Prevention Gap Report. Geneva, Switzerland: 2016b. [Google Scholar]
- Kruglov YV, Kobyshcha YV, Salyuk T, Varetska O, Shakarishvili A, Saldanha VP. The most severe HIV epidemic in Europe: Ukraine’s national HIV prevalence estimates for 2007. Sex Transm Infect. 2008;84(Suppl 1):i37–i41. doi: 10.1136/sti.2008.031195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsa O, Marcus R, Bojko MJ, Zelenev A, Mazhnaya A, Dvoriak S, … Altice FL. Factors associated with physical and sexual violence by police among people who inject drugs in Ukraine: implications for retention on opioid agonist therapy. J Int AIDS Soc. 2016;19(4 Suppl 3):20897. doi: 10.7448/IAS.19.4.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambdin BH, Masao F, Chang O, Kaduri P, Mbwambo J, Magimba A, … Bruce RD. Methadone Treatment for HIV Prevention-Feasibility, Retention, and Predictors of Attrition in Dar es Salaam, Tanzania: A Retrospective Cohort Study. Clin Infect Dis. 2014;59(5):735–742. doi: 10.1093/cid/ciu382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latypov AB. The Soviet doctor and the treatment of drug addiction: “A difficult and most ungracious task”. Harm Reduct J. 2011;8:32. doi: 10.1186/1477-7517-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low AJ, Mburu G, Welton NJ, May MT, Davies CF, French C, … Vickerman P. Impact of Opioid Substitution Therapy on Antiretroviral Therapy Outcomes: A Systematic Review and Meta-Analysis. Clin Infect Dis. 2016 doi: 10.1093/cid/ciw416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas GM, Chaudhry A, Hsu J, Woodson T, Lau B, Olsen Y, … Moore RD. Clinic-based treatment of opioid-dependent HIV-infected patients versus referral to an opioid treatment program: A randomized trial. Ann Intern Med. 2010;152(11):704–711. doi: 10.7326/0003-4819-152-11-201006010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarenko I, Mazhnaya A, Polonsky M, Marcus R, Bojko MJ, Filippovych S, … Altice FL. Determinants of willingness to enroll in opioid agonist treatment among opioid dependent people who inject drugs in Ukraine. Drug Alcohol Depend. 2016;165:213–220. doi: 10.1016/j.drugalcdep.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarenko J, Mazhnaya A, Marcus R, Bojko MJ, Madden L, Fillipovich S, … Altice FL. Willingness to Pay for Opioid Agonist Treatment among Opioid Dependent People Who Inject Drugs in Ukraine. International J Drug Policy. 2017 doi: 10.1016/j.drugpo.2017.05.037. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;2:CD002207. doi: 10.1002/14651858.CD002207.pub4. [DOI] [PubMed] [Google Scholar]
- Mazhnaya A, Bojko MJ, Marcus R, Filippovych S, Islam Z, Dvoriak S, Altice FL. In Their Own Voices: Breaking the Vicious Cycle of Addiction, Treatment and Criminal Justice Among People who Inject Drugs in Ukraine. Drugs (Abingdon Engl) 2016;23(2):163–175. doi: 10.3109/09687637.2015.1127327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazhnaya A, Marcus R, Bojko MJ, Zelenev A, Makarencko J, Pykalo I, … Altice FL. The Influence of Opioid Agonist Treatments on the HIV Treatment Cascade in People Who Inject Drugs in Ukraine. Lancet HIV. 2016 doi: 10.1097/QAI.0000000000001827. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger DS, Woody GE, McLellan AT, O’Brien CP, Druley P, Navaline H, … Abrutyn E. Human immunodeficiency virus seroconversion among intravenous drug users in- and out-of-treatment: an 18-month prospective follow-up. J Acquir Immune Defic Syndr. 1993;6(9):1049–1056. [PubMed] [Google Scholar]
- Meyer JP, Althoff AL, Altice FL. Optimizing care for HIV-infected people who use drugs: evidence-based approaches to overcoming healthcare disparities. Clin Infect Dis. 2013;57(9):1309–1317. doi: 10.1093/cid/cit427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MoH of Ukraine. HIV infection in Ukraine Informational Bulletin #39. 2013 Retrieved from http://ucdc.gov.ua/pages/diseases/hiv_aids/monitoring/information-bulletins.
- MoH of Ukraine. HIV infection in Ukraine Informational Bulletin #43. 2015 Retrieved from http://ucdc.gov.ua/pages/diseases/hiv_aids/monitoring/information-bulletins.
- Moore RD, Keruly JC, Chaisson RE. Differences in HIV disease progression by injecting drug use in HIV-infected persons in care. J Acquir Immune Defic Syndr. 2004;35(1):46–51. doi: 10.1097/00126334-200401010-00006. [DOI] [PubMed] [Google Scholar]
- Morozova O, Dvoryak S, Altice FL. Methadone treatment improves tuberculosis treatment among hospitalized opioid dependent patients in Ukraine. Int J Drug Policy. 2013;24(6):e91–98. doi: 10.1016/j.drugpo.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozova O, Levina O, Uuskula A, Heimer R. Comparison of subset selection methods in linear regression in the context of health-related quality of life and substance abuse in Russia. BMC Med Res Methodol. 2015;15:71. doi: 10.1186/s12874-015-0066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction. Effective medical treatment of opiate addiction. JAMA. 1998;280(22):1936–1943. [PubMed] [Google Scholar]
- Palepu A, Tyndall MW, Joy R, Kerr T, Wood E, Press N, … Montaner JS. Antiretroviral adherence and HIV treatment outcomes among HIV/HCV co-infected injection drug users: the role of methadone maintenance therapy. Drug Alcohol Depend. 2006;84(2):188–194. doi: 10.1016/j.drugalcdep.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Polonsky M, Azbel L, Wegman MP, Izenberg JM, Bachireddy C, Wickersham JA, … Altice FL. Pre-incarceration police harassment, drug addiction and HIV risk behaviours among prisoners in Kyrgyzstan and Azerbaijan: results from a nationally representative cross-sectional study. J Int AIDS Soc. 2016;19(4 Suppl 3):20880. doi: 10.7448/IAS.19.4.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonsky M, Azbel L, Wickersham JA, Taxman FS, Grishaev E, Dvoryak S, Altice FL. Challenges to implementing opioid substitution therapy in Ukrainian prisons: Personnel attitudes toward addiction, treatment, and people with HIV/AIDS. Drug Alcohol Depend. 2015;148:47–55. doi: 10.1016/j.drugalcdep.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonsky M, Rozanova J, Azbel L, Bachireddy C, Izenberg J, Kiriazova T, … Altice FL. Attitudes Toward Addiction, Methadone Treatment, and Recovery Among HIV-Infected Ukrainian Prisoners Who Inject Drugs: Incarceration Effects and Exploration of Mediators. AIDS Behav. 2016 doi: 10.1007/s10461-016-1375-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub M, Chtenguelov V, Subata E, Weiler G, Uchtenhagen A. Feasibility of buprenorphine and methadone maintenance programmes among users of home made opioids in Ukraine. Int J Drug Policy. 2010;21(3):229–233. doi: 10.1016/j.drugpo.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Strike CJ, Gnam W, Urbanoski K, Fischer B, Marsh DC, Millson M. Factors predicting 2-year retention in methadone maintenance treatment for opioid dependence. Addict Behav. 2005;30(5):1025–1028. doi: 10.1016/j.addbeh.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Sylla L, Bruce RD, Kamarulzaman A, Altice FL. Integration and co-location of HIV/AIDS, tuberculosis and drug treatment services. Int J Drug Policy. 2007;18(4):306–312. doi: 10.1016/j.drugpo.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The State Service of Ukraine. Assessment of Implementation of the National AIDS Programme For Years 2009–2013 in Ukraine: Synthesis Report. 2014 Retrieved from http://www.undp.org/content/dam/ukraine/docs/PR/Assessment%20of%20implementation%20of%20the%20National%20AIDS%20Programme_2009_2013.pdf.
- Timko C, Schultz NR, Cucciare MA, Vittorio L, Garrison-Diehn C. Retention in Medication-Assisted Treatment for Opiate Dependence: A Systematic Review. J Addict Dis. 2015 doi: 10.1080/10550887.2016.1100960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann S, Milloy MJ, Kerr T, Zhang R, Guillemi S, Marsh D, … Wood E. Methadone maintenance therapy promotes initiation of antiretroviral therapy among injection drug users. Addiction. 2010;105(5):907–913. doi: 10.1111/j.1360-0443.2010.02905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS. Comprehensive External Evaluation of the National AIDS Response in Ukraine. 2009 Retrieved from http://www.unicef.org/evaldatabase/files/Ukraine_National_AIDS.pdf.
- UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2013. 2013 Retrieved from http://files.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf.
- Villafranca SW, McKellar JD, Trafton JA, Humphreys K. Predictors of retention in methadone programs: a signal detection analysis. Drug Alcohol Depend. 2006;83(3):218–224. doi: 10.1016/j.drugalcdep.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Vitek CR, Cakalo JI, Kruglov YV, Dumchev KV, Salyuk TO, Bozicevic I, … Rutherford GW. Slowing of the HIV epidemic in Ukraine: evidence from case reporting and key population surveys, 2005–2012. PLoS One. 2014;9(9):e103657. doi: 10.1371/journal.pone.0103657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Wang L, Wang X, Li J, Li H, Jia W. A Study of 6-Year Retention in Methadone Maintenance Treatment Among Opioid-Dependent Patients in Xi’an. J Addict Med. 2013 doi: 10.1097/ADM.0b013e31829da05b. [DOI] [PubMed] [Google Scholar]
- WHO. Guidelines for the Psychosocially Assisted Pharmacological Treatment of Opioid Dependence. Geneva: 2009. [PubMed] [Google Scholar]
- WHO. Opioid treatment in Ukraine risks losing momentum. Bull World Health Organ. 2013;91(2):87–88. doi: 10.2471/BLT.13.020213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe D, Carrieri MP, Shepard D. Treatment and care for injecting drug users with HIV infection: a review of barriers and ways forward. Lancet. 2010;376(9738):355–366. doi: 10.1016/S0140-6736(10)60832-X. [DOI] [PubMed] [Google Scholar]
- Wood E, Montaner JS, Yip B, Tyndall MW, Schechter MT, O’Shaughnessy MV, Hogg RS. Adherence and plasma HIV RNA responses to highly active antiretroviral therapy among HIV-1 infected injection drug users. CMAJ. 2003;169(7):656–661. [PMC free article] [PubMed] [Google Scholar]