Abstract
Mass spectrometry (MS)-based multi-omic measurements, including proteomics, metabolomics, lipidomics, and glycomics, are increasingly transforming our ability to characterize and understand biological systems. Multi-omic analyses and the desire for comprehensive measurement coverage presently have limitations due to the chemical diversity and range of abundances of biomolecules in complex samples. Advances addressing these challenges increasingly are based upon the ability to quickly separate, react and otherwise manipulate sample components for analysis by MS. Here we report on a new approach using Structures for Lossless Ion Manipulations (SLIM) to enable long serpentine path ion mobility spectrometry (IMS) separations followed by MS analyses. This approach provides previously unachieved resolution for biomolecular species, in conjunction with more effective ion utilization, and a basis for greatly improved characterization of very small sample sizes.
Keywords: Structures for Lossless Ion Manipulations (SLIM), Ion Mobility Spectrometry, Mass Spectrometry, Isomers
Multi-omic MS-based analyses generally require long separations resulting in low throughput, extensive sample fractionation, and sample size requirements that preclude many applications. Considerable efforts are being devoted to address these issues, but of great interest is the use of fast IMS separations. In IMS, ions are pulled through a buffer gas by an electric field and separate based on their shapes.[1] IMS separations do not involve a stationary phase, making measurements more robust and reproducible,[2] and have been shown to enable characterization of species indistinguishable by MS alone,[3] such as protein modification sites,[4] protein leucine and isoleucine residues,[5] cis-trans isomers,[6] anomers[7] and diastereomers.[8] Additionally, the coupling of some forms of IMS with MS is now highly effective.[1b, 9]
A key challenge for IMS-MS presently however, is the limited IMS separation power. This limitation results in a restricted range of molecular coverage for each measurement[10] and the desire for the higher IMS resolution needed to both improve analysis coverage and enhance the overall measurement dynamic range.[10] To date these efforts have invariably resulted in either large ion losses (lower sensitivity) or very limited coverage (a very narrow mobility range), or both.[11] Since the separation or resolving power achievable by an IMS separation is proportional to the square root of the IMS path length (L),[12] there have been many attempts at increasing L. However, the long drift path (e. g. 10 to 100 meters) and high voltages needed have been prohibitive. In a notable attempt to address this challenge, Merenbloom et al. created a cyclic multi-pass IMS instrument.[11] One constraint of such a design is the limited path length for each cycle, resulting in a measureable mobility range that decreases with every cycle needed to achieve greater separation. More recently a cyclic design was developed based upon the use of traveling wave IMS that avoided the high voltages needed for effective separations, but was also limited by the similar cyclic path length.[13]
In this work we have addressed these challenges using SLIM, which we have previously shown to enable effective low resolution IMS separations in short 30-cm paths,[14] as well as have capabilities for efficient ion selection,[15] trapping,[16] and accumulation.[16] In SLIM ion conduits are created using electric field generated by arrays of electrodes fabricated using printed circuit board (PCB) technology. By using traveling waves[17] and a compact serpentine ion drift path, we constructed a 13-m SLIM IMS device. This design provides more than an order of magnitude increase in L and greatly improved separations, in addition to having high sensitivity due to the ion confining fields created by RF potentials on the array of SLIM electrodes.[18] Initially, ion trajectory simulations were used to optimize the electrode arrays on two mirror-image PCBs (each 18″ long × 12.8″ wide; Figure 1a) that were used to define the serpentine path. The SLIM IMS device was then tested at 3.0 Torr with a 3-mm PCB gap spacing and voltages similar to those recently reported for the short 30-cm SLIM IMS design[14] (Supplementary Material). A mixture of peptides, lipids and carbohydrates was subjected to electrospray ionization and ions were transported to a 3.0 Torr region where they were injected into the 13-m SLIM IMS (Figure 1a). The SLIM IMS-MS analysis showed all expected ions in the mixture with the different molecular classes clearly separated (Figure 1b).
Figure 1.
a) A schematic and photo of the serpentine 13-m SLIM IMS-MS platform. b) The 2D nested IMS-MS spectrum from the SLIM platform showing drift time (DT) versus m/z for the peptide/carbohydrate/lipid mixture analyzed (supplemental materials). Different molecular classes typically separate by different ‘trend lines’ with the 2+ peptides arriving first (red), then 1+ carbohydrates (blue), 1+ peptides (green) and finally the 1+ lipids (pink) arriving last due to the distinct backbone structures of each molecule type.
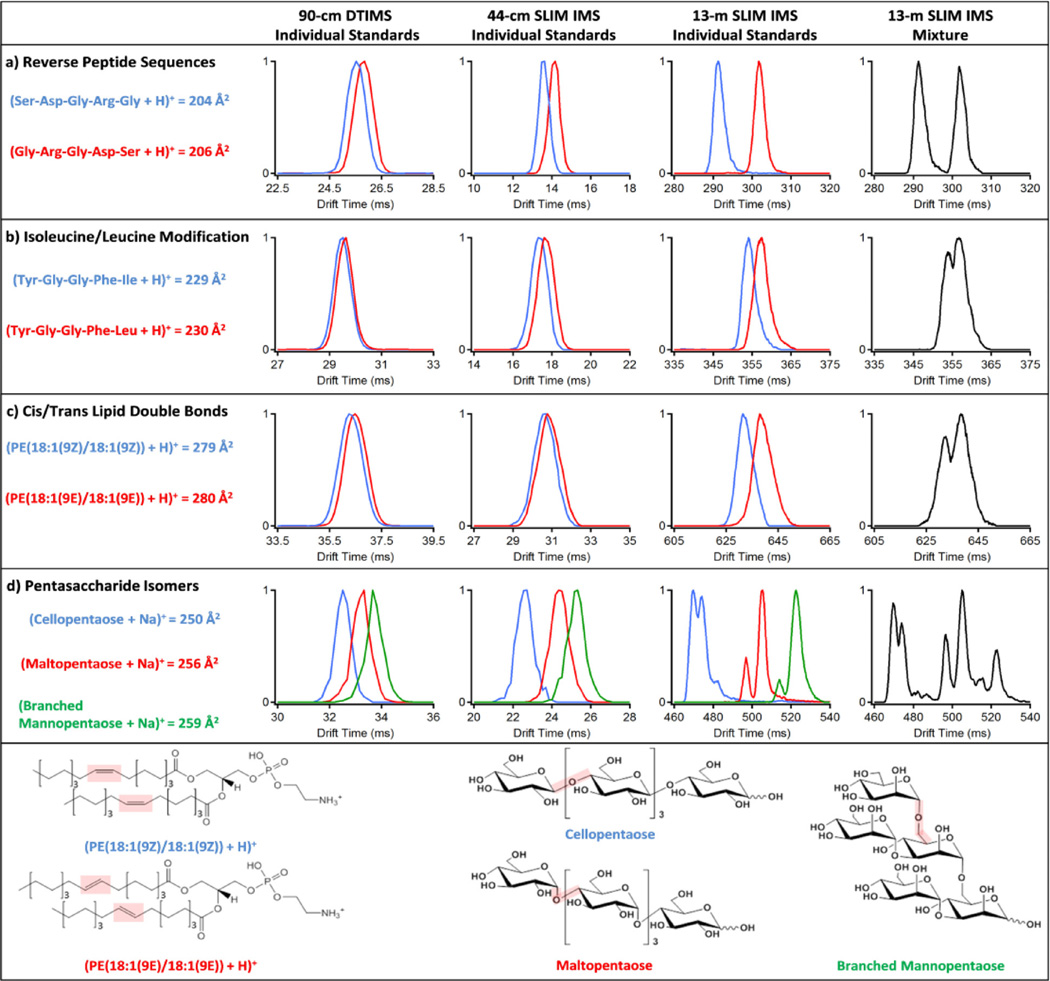
This serpentine SLIM IMS-MS platform was then used to study several peptide, lipid and sugar isomers that are indistinguishable by mass and problematic with currently available IMS-MS platforms. We compared the 13-m SLIM IMS-MS isomer measurements with those using a 90-cm conventional drift tube IMS (DTIMS)-MS[19] and 44-cm TW-SLIM IMS-MS platforms. Figure 2 illustrates four different isomer group separations with the three platforms. Figure 2a shows the separation of two pentapeptides having the same amino acid residue composition, but different sequences (reversed).[13] In both the 90-cm DTIMS and 44-cm SLIM IMS the reverse sequence isomers were only slightly separated (i. e. largely overlapping), but showed complete separation using the serpentine 13-m SLIM IMS. Figure 2b shows separation of two peptides that differ only by a leucine residue being replaced by isoleucine, a case problematic for MS measurements alone.[5] Here the 90-cm DTIMS and 44-cm SLIM IMS provided little separation of the isomers (and indistinguishable in the mixture) compared to that achieved with the new platform, where the separation was sufficient to distinguish these species due to the high reproducibility of IMS. Such improved IMS separations provide the basis for much more routine identification of these isomers, and thus more effective proteomics measurements.
Figure 2.
IMS separations using 90-cm DTIMS, 44-cm SLIM IMS, and 13-m SLIM IMS platforms for a) two reverse peptide sequence isomers (m/z = 491.22), b) pentapeptide isomers with an isoleucine/leucine modification (m/z = 556.28), c) lipid isomers with cis and trans double bond orientations (m/z = 744.55), and d) three pentasaccharide isomers (m/z = 851.26). Each molecule is color coded on the right and its cross section from DTIMS is noted and associated with 0.5% error. Structural isomers for the lipids and carbohydrates are shown at the bottom of the figure.
Lipidomic isomers were then studied with the three platforms to determine the distinguishability of cis- and trans-double bond orientations for complex glycerophosphoethanolamine (PE) lipid isomers (Figure 2c). The relative abundances of cis and trans double bonded species can influence the fluidity and thickness of lipid bilayers,[20] but such separations are currently problematic due to the similar structures.[21] The cis/trans species (Figure 2, bottom) were much better separated with the new platform and sufficient for determining their relative abundances.
Finally, we compared separations for three pentasaccharides with distinct monosaccharide arrangements (cellopentaose, maltopentaose and branched mannopentaose) (Figure 2d). Cellopentaose [(β-D-Glc-[1,4])4-D-Glc] and maltopentaose [(α-D-Glc-[1,4])4-D-Glc] have the same five glucose monomers, but differ by their β and α linkage connectivity, while the branched mannopentaose [α-D-Man-(1,3)(α-D-Man-1,6)-α-D-Man-(1,6)(α-D-Man-1,3)-D-Man] had five mannose units with a branched 3,6 core (Figure 2, bottom). These oligosaccharides have a reducing monosaccharide end that exists in solution in at least two predominant configurations (α and β pyranose). Further, characterization of such carbohydrates is challenging due to the many arrangements and isomeric subunits possible, which create difficulties in understanding disease specific changes. The new platform was able to baseline resolve the pentasaccharide ion conformations not distinguishable with the other platforms in addition to extra structural peaks. These peaks were potentially attributed to the carbohydrates’ α, β or open-chain configurations and the various sites of charge attachment for the sodium cation. Previously, two partially resolved IMS peaks were observed for cellopentaose and only one peak for maltopentaose and branched mannopentaose in a platform with less resolving power;[22] this is the first observation of multiple ion species for all three pentasaccharides.
Improved multi-omic analyses are being driven by developments advancing the coverage, sensitivity, and throughput of MS-based platforms, and promise to rapidly increase the understandings of biological systems. The results illustrated here, and the lossless ion transmission achieved in these measurements (see Supplemental Information), demonstrate the potential for high resolution SLIM IMS-MS platforms for lipidomic, proteomic, glycomic and metabolomic measurements. The serpentine 13-m long path length SLIM IMS achieved much better separations than currently available by alternative platforms, while still maintaining extremely high sensitivity and broad coverage in a highly compact and practical design. The reported SLIM IMS-MS platform opens many opportunities for improved and highly sensitive global multi-omic measurements to understand molecular changes ranging from environmental perturbations to disease onset. While the high resolution global SLIM IMS separations shown are a great improvement over currently available technologies, the flexible nature of SLIM and the potential for creating much more sophisticated devices based upon the robust and mature PCB technology for fabrication of electrode arrays offers further avenues completely precluded at present. These developments include the implementation of much longer path lengths offering even better separations, designs that allow for reaction of ions for better characterization such as possible with dissociation or chemical conversion in ion-ion processes,[23] and finally optimization of ion utilization efficiency by better transferring ions to the MS for more detailed analyses of very small sample sizes (e. g. single cells).
Experimental Section
Methods and any associated references are available in the online version of the paper.
Supplementary Material
Acknowledgments
Portions of this research were supported by grants from the National Institute of General Medical Sciences (P41 GM103493), the U.S. Department of Energy Office of Biological and Environmental Research Genome Sciences Pan-omics Program at Pacific Northwest National Laboratory (PNNL), the Laboratory Directed Research and Development Program at PNNL. This research was performed at the W. R. Wiley Environmental Molecular Sciences Laboratory (EMSL), a DOE national scientific user facility at the Pacific Northwest National Laboratory (PNNL). PNNL is operated by Battelle for the DOE under contract DE-AC05-76RL0 1830.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/slct.201600460
Supplementary information is also available online.
References
- 1.a) Mason E, McDaniel E. Transport Properites of Ions in Gases. Wiley, New York: 1988. [Google Scholar]; b) Guevremont R, Siu KW, Wang J, Ding L. Anal Chem. 1997;69:3959–3965. doi: 10.1021/ac970359e. [DOI] [PubMed] [Google Scholar]
- 2.a) Wyttenbach T, Bowers MT. Top Curr Chem. 2003;225:207–232. [Google Scholar]; b) May JC, McLean JA. Anal Chem. 2015;87:1422–1436. doi: 10.1021/ac504720m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanucara F, Holman SW, Gray CJ, Eyers CE. Nat Chem. 2014;6:281–294. doi: 10.1038/nchem.1889. [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim YM, Shvartsburg AA, Smith RD, Belov ME. Anal Chem. 2011;83:5617–5623. doi: 10.1021/ac200719n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnett DA, Ells B, Guevremont R, Purves RW. J Am Soc Mass Spectr. 1999;10:1279–1284. [Google Scholar]
- 6.a) Bushnell JE, Kemper PR, Bazan GC, Bowers MT. J Phys Chem A. 2004;108:7730–7735. [Google Scholar]; b) Dong L, Shion H, Davis RG, Terry-Penak B, Castro-Perez J, van Breemen RB. Anal Chem. 2010;82:9014–9021. doi: 10.1021/ac101974g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hofmann J, Hahm HS, Seeberger PH, Pagel K. Nature. 2015;526:241–244. doi: 10.1038/nature15388. [DOI] [PubMed] [Google Scholar]
- 8.a) Baker ES, Hong JW, Gidden J, Bartholomew GP, Bazan GC, Bowers MT. J Am Chem Soc. 2004;126:6255–6257. doi: 10.1021/ja039486k. [DOI] [PubMed] [Google Scholar]; b) Flick TG, Campuzano ID, Bartberger MD. Anal Chem. 2015;87:3300–3307. doi: 10.1021/ac5043285. [DOI] [PubMed] [Google Scholar]
- 9.a) Hoaglund CS, Valentine SJ, Sporleder CR, Reilly JP, Clemmer DE. Anal Chem. 1998;70:2236–2242. doi: 10.1021/ac980059c. [DOI] [PubMed] [Google Scholar]; b) Baker ES, Burnum-Johnson KE, Jacobs JM, Diamond DL, Brown RN, Ibrahim YM, Orton DJ, Piehowski PD, Purdy DE, Moore RJ, Danielson WF, 3rd, Monroe ME, Crowell KL, Slysz GW, Gritsenko MA, Sandoval JD, Lamarche BL, Matzke MM, Webb-Robertson BJ, Simons BC, McMahon BJ, Bhattacharya R, Perkins JD, Carithers RL, Jr, Strom S, Self SG, Katze MG, Anderson GA, Smith RD. Mol Cell Proteomics. 2014;13:1119–1127. doi: 10.1074/mcp.M113.034595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a) Dugourd P, Hudgins RR, Clemmer DE, Jarrold MF. Rev Sci Instrum. 1997;68:1122–1129. [Google Scholar]; b) Asbury GR, Hill HH. J Microcolumn Sep. 2000;12:172–178. [Google Scholar]
- 11.Merenbloom SI, Glaskin RS, Henson ZB, Clemmer DE. Anal Chem. 2009;81:1482–1487. doi: 10.1021/ac801880a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rivercomb HE, Mason EA. Anal Chem. 1975;47:970–983. [Google Scholar]
- 13.Giles K, Wildgoose JL, Pringle S, Langridge D, Nixon P, Garside J, Carney P. ASMS conference. St. Louis, MO: 2015. [Google Scholar]
- 14.Hamid AM, Ibrahim YM, Garimella SV, Webb IK, Deng L, Chen TC, Anderson GA, Prost SA, Norheim RV, Tolmachev AV, Smith RD. Anal Chem. 2015;87:11301–11308. doi: 10.1021/acs.analchem.5b02481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webb IK, Garimella SV, Tolmachev AV, Chen TC, Zhang X, Cox JT, Norheim RV, Prost SA, LaMarche B, Anderson GA, Ibrahim YM, Smith RD. Anal Chem. 2014;86:9632–9637. doi: 10.1021/ac502139e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Garimella SV, Prost SA, Webb IK, Chen TC, Tang K, Tolmachev AV, Norheim RV, Baker ES, Anderson GA, Ibrahim YM, Smith RD. Anal Chem. 2015;87:6010–6016. doi: 10.1021/acs.analchem.5b00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giles K, Williams JP, Campuzano I. Rapid Commun Mass Spectrom. 2011;25:1559–1566. doi: 10.1002/rcm.5013. [DOI] [PubMed] [Google Scholar]
- 18.Garimella SV, Ibrahim YM, Webb IK, Tolmachev AV, Zhang X, Prost SA, Anderson GA, Smith RD. J Am Soc Mass Spectrom. 2014;25:1890–1896. doi: 10.1007/s13361-014-0976-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibrahim YM, Baker ES, Danielson WF, 3rd, Norheim RV, Prior DC, Anderson GA, Belov ME, Smith RD. Int J Mass Spectrom. 2015;377:655–662. doi: 10.1016/j.ijms.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holthuis JC, Menon AK. Nature. 2014;510:48–57. doi: 10.1038/nature13474. [DOI] [PubMed] [Google Scholar]
- 21.Kyle JE, Zhang X, Weitz KK, Monroe ME, Ibrahim YM, Moore RJ, Cha J, Sun X, Lovelace ES, Wagoner J, Polyak SJ, Metz TO, Dey SK, Smith RD, Burnum-Johnson KE, Baker ES. Analyst. 2016;141:1649–1659. doi: 10.1039/c5an02062j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Bendiak B, Siems WF, Gang DR, Hill HH., Jr Rapid Commun Mass Spectrom. 2013;27:2699–2709. doi: 10.1002/rcm.6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLuckey SA, Mentinova M. J Am Soc Mass Spectrom. 2011;22:3–12. doi: 10.1007/s13361-010-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.