Abstract
We report G-quadruplex formation between peptide nucleic acids (PNAs) composed of LKγ-PNA-G monomers and a known portion of human telomeric DNA that adopts three G3 tracts via intramolecular hydrogen bonding. The resulting complex is a bimolecular PNA–DNA heteroquadruplex. In this report, we show that introduction of a γ-modification and addition of a peptide ligand does not disrupt the heteroquadruplex. Although the unmodified PNA1 forms a quadruplex with itself, the γ-substituted PNAs (PNA2 – PNA6) do not form G-quadruplexes on their own, at even high concentrations. The selectivity of these PNAs could influence the design of new quadruplex-targeting molecules or allow the quadruplex structure to be used as a scaffold for multivalent display of protein binding ligands.
Keywords: Quadruplex, DNA, PNA
Graphical abstract
Guanine-rich oligonucleotides have shown the ability to form alternative secondary structures by utilizing Hoogesten base pairs to form guanine tetrads that are stabilized in the presence of potassium or sodium cations (Figure 1a).1, 2 These guanine tetrads are able to πstack, thereby forming stable secondary structures referred to as guanine quadruplexes (G-Quadruplexes).3, 4 These G-Quadruplexes have been proposed to assist in biological functions such as gene regulation5–8 and chromosomal stability.9, 10 Several studies have explored the biophysical and structural properties of quadruplexes both in vivo9, 11 as well as in vitro.10, 12, 13 There are many strategies for targeting quadruplexes, including small molecules14, engineered DNA-binding proteins15, 16, and complementary oligonucleotides such as locked nucleic acids (LNAs)17, 18 and peptide nucleic acids (PNAs).19–21
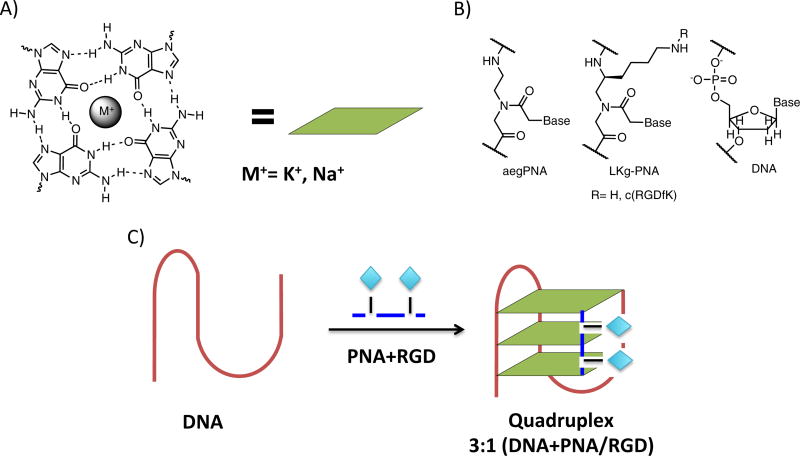
Figure 1.
A) Arrangement of guanines around a central cation (M+) resulting in guanine tetrad eliciting G-quadruplex formation. B) Comparison of aminoethylgycine (aeg) PNA, LKγ-PNA and DNA. C) Proposed PNA-RGD conjugate binding to G-rich DNA region.
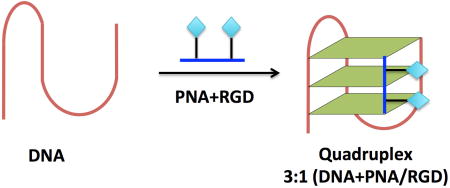
In addition to complimentary binding, a quadruplex can be targeted by exploiting homologous recognition. By replacing one or more G3 tracts with a short, guanine-rich PNA, a stable heteroquadruplex can be formed. An assortment of heteroquadruplexes have been reported with various structures and stoichiometries (i.e. DNA2:PNA2).19, 22–28 Previous studies have investigated an alternative stoichiometry where a short G3 PNA can form a heteroquadruplex with a DNA containing three G3 tracts.29 This asymmetric design suggests the possibility of building scaffolds with ligands appended to the PNA.
Much research in recent years has focused on improving the original design of aegPNA.30–32 Current research has focused on improving the binding properties while eliminating some of the inherent weaknesses (i.e. solubility, bioavailability, and functionality). Some common strategies include conjugating moieties to PNA termini21 and/or modifying the PNA backbone.33–37 Modifications at the -position of the PNA backbone have been well-documented to increase binding affinity and solubility, as well as to provide a convenient handle to attach additional functionality. Previously, we have shown that LKγ-PNA (Figure 1b) is a versatile scaffold to attach a range of functional groups and small molecules without compromising the ability of PNAs to bind to a complementary nucleic acid sequence.38–41 Previous work has demonstrated the advantageous properties of using a PNA to target proteins via multvalent display.38, 39, 42–47
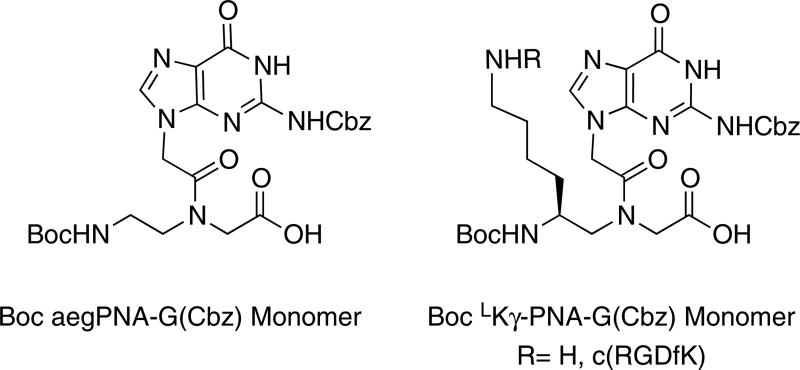
A family of short G-rich LKγ-PNAs with a varying number of -modifications was designed to explore the effect on a 3+1 bimolecular quadruplex binding (PNA 4–6). Furthermore, to explore the possibility of PNA-DNA heteroquadruplexes as scaffolds for ligand display, an integrin-targeting ligand, cRGDfK, was attached to the free amine at the position (PNA 2–3) and the effects on the bimolecualar G-quadruplex with a truncated telomeric DNA (DNA1) were studied. A LKγ-PNA-G monomer (Figure 2) was synthesized and conjugated to a small peptide (cRGDfK) via the free amine of the side chain. Then, the potential for several different PNAs (both with and without a ligand on the LKγ-PNA sidechains) to bind to the telomeric DNA was investigated.
Figure 2.
The Boc-aeg PNA-G(Cbz) and Boc LKγ-PNA-G(NH2/(mPEG)2-RGD) monomers suitable for Boc mediated solid-phase peptide synthesis.
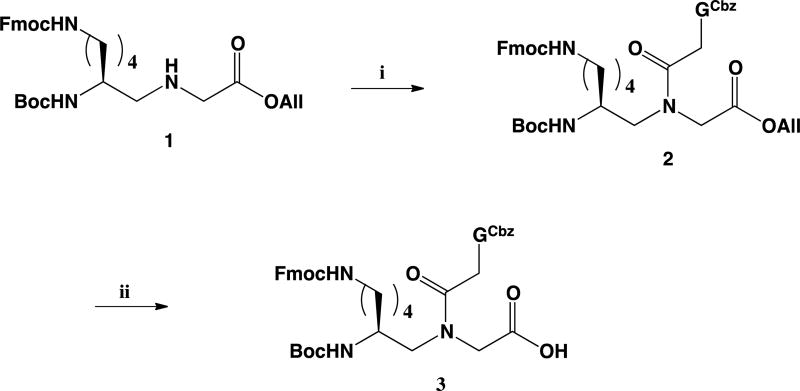
The LKγ-PNA backbone (1) was synthesized as reported previously.25 The G(Cbz)-acetic acid was then coupled to LKγ-PNA backbone (1) producing the LKγ-PNA-G monomer ester (2) in high yield (70%). Removal of the allyl group using catalytic palladium provided the LKγ-PNA-G monomer (3) (Scheme 1). The family of PNAs (Table 1) was synthesized using a standard PNA procedure on Applied BioSystems 433A automated peptide synthesizer, purified by HPLC, and characterized by mass spectrometry as previously reported.26 To attach the ligand, the purified PNA was reacted with diethyl squarate to form an amide with the amines on the -sidechains. Then, the purified intermediate was reacted with an amine-modified cRGDfK ligand to yield the RGD-PNA conjugate.
Scheme 1.
Reagents and conditions: (i) 2-N-(Benzhydryloxycarbonyl)guanine-9-acetic acid, DHBT, EDC, DMF, 40°C, 12h, 87%; (ii) Pd(PPh3)4, N-ethyl aniline, THF, RT, 3h, 70%.
Table 1.
List of DNA/PNA used in this study.
| DNA/PNA | Sequencea | # of γ units |
|---|---|---|
| DNA 1 | d(GGGTTAGGGTTAGGGT) | N/A |
| PNA 1 | Ac-HN -TGGGT-Lys-CONH2 | 0 |
| PNA 2 | Ac-HN -TGGRGT-Lys-CONH2 | 1 |
| PNA 3 | Ac-HN -TGRGGRT-Lys-CONH2 | 2 |
| PNA 4 | Ac-HN-TGGGT-Lys-CONH2 | 1 |
| PNA 5 | Ac-HN-TGGGT-Lys-CONH2 | 2 |
| PNA 6 | Ac-HN-TGGGT-Lys-CONH2 | 3 |
PNA oligomers are written from N terminus to C terminus. The LKγ-PNA-G-RGD monomers (GR) are bold and underlined for clarity, LKγ-PNA-G monomers (G) have a free amine on the side chain.
The thermodynamic properties were assessed using UV melting curve analysis monitoring the hypochromic shift at 295 nm and 305 nm. The Tm was defined as the temperature at which half of the oligonucleotides were folded and was determined by the minimum of the 1st derivative. First, the thermal stability of the truncated telomeric DNA (DNA1) alone was determined to be 58.9 °C. Then, the truncated DNA1 was hybridized to the unmodified PNA1 (1:1 / PNA1 : DNA1), and the Tm was 57.3 °C. The Tm values are consistent with results obtained by Paul. Et al.29 Next, the effect of increasing - modifications with PNA 4–6 was analyzed. When annealed with DNA1, PNAs with increasing LKγ-PNA-G monomers bearing a free amine at the - position (PNA4, PNA5, PNA6) showed a melting transition (Tm = 60.2, 58.4, and 59.1 °C, respectively). The recorded Tm’s were similar, suggesting that a - modification has minimal effect on the quadruplex stability. Introducing one or two LKγ-PNA-G-RGD monomers (GR) in PNA (PNA2 and PNA3) has very minor effects on the melting transition when annealed with DNA1 (Tm = 58.7 °C and Tm = 57.3 °C, respectively). Overall, the UV melting curve analysis indicates that the bimolecular quadruplex was formed and addition of a LKγ-PNA-G monomer and/or a ligand does not perturb the stability.
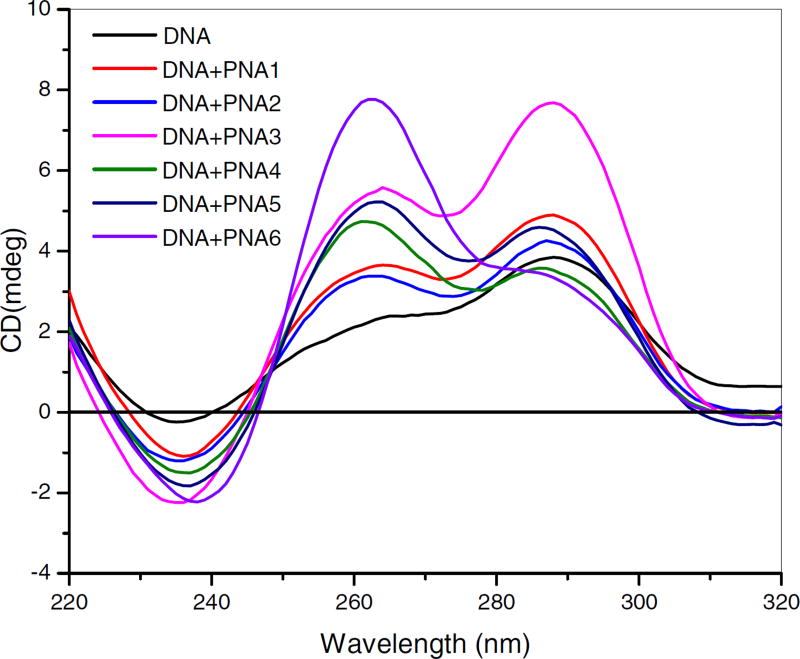
The secondary structures of the oligonucleotides were analyzed using CD spectropolarimetry. DNA1 exhibited a CD signature with two maxima at λ260 and λ295 nm, which are characteristic of a mixture of parallel and antiparallel G-quadruplex conformations, respectively (Figure 5). The CD profile of the unmodified PNA1-DNA1 quadruplex showed a slightly larger maxima at λ 260 nm and λ 295 nm compared to DNA1 alone (Figure 3). Likewise, the CD profile of PNA2-DNA1 and PNA3-DNA1 showed strong maxima at λ260 nm and λ295 nm, although PNA2 and PNA3 also exhibited strong CD signals alone (Figure S1). The CD profile of PNA4, PNA5, and PNA6, in complex with DNA1 showed strong maxima at λ 260 nm and λ295 nm and weaker signals in the absence of DNA, thus indicating formation of a hybrid PNA-DNA quadruplex structure.
Figure 3.
CD spectra DNA1 and DNA1:PNA complexes (20 µM) in 100 mM potassium phosphate buffer containing 100 mM KCl at pH 7.4 (x-axis is wavelength (nm), y-axis is millidegrees).
It was observed that PNA1 forms quadruplex with itself at high concentrations, (Figure S3) and does not have a significant CD signal (Figure S4). These properties were consistent with the literature48. Interestingly, the UV-temperature profile of PNA2 and PNA3 at 295 nm indicates that they do not form quadruplexes in the same buffer at concentrations up to 200 µM. Likewise, the UV-temperature profiles of PNA4, PNA5, and PNA6 at 295 nm indicate they do not form quadruplexes in the absence of DNA1, even at high concentrations.
We have reported the synthesis of LKγ-PNA-G monomers to target a human telomeric DNA sequence, and we have shown that γ-substituted PNA can form a hybrid quadruplex with the DNA. Interestingly, LKγ-PNAs do not form G-quadruplexes alone, even at high concentrations, while unmodified PNA1 forms a quadruplex at 200 µM. The selectivity of these PNAs could open up new possibilities to design quadruplex-targeting ligands. This work broadens the types of secondary structures that can be used for ligand display and future studies should explore the efficacy of these PNA-DNA heteroquadruplexes as scaffolds for ligand display.
Supplementary Material
Table 2.
Melting temperatures (Tm) of DNA and DNA/PNA quadruplexes in 100 mM potassium phosphate buffer + 100 mM KCl (pH 7.4). [DNA]= 20µM; [PNA]= 20µM.
| Complex | TM [°C] |
|---|---|
| DNA1 | 58.9±0.3 |
| DNA1 + PNA1 | 57.3±0.4 |
| DNA1 + PNA2 | 58.7±2.2 |
| DNA1 + PNA3 | 57.3±0.3 |
| DNA1 + PNA4 | 60.2±1.2 |
| DNA1 + PNA5 | 58.4±0.3 |
| DNA1 + PNA6 | 59.1±0.7 |
Acknowledgments
This research was supported by the Intramural Research Program of NIDDK, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hud NV, Smith FW, Anet FAL, Feigon J. Biochemistry. 1996;35:15383. doi: 10.1021/bi9620565. [DOI] [PubMed] [Google Scholar]
- 2.Williamson JR, Raghuraman MK, Cech TR. Cell. 1989;59:871. doi: 10.1016/0092-8674(89)90610-7. [DOI] [PubMed] [Google Scholar]
- 3.Bochman ML, Paeschke K, Zakian VA. Nat. Rev Genet. 2012;13:770. doi: 10.1038/nrg3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel DJ, Phan AT, Kuryavyi V. Nucleic Acids Res. 2007;35:7429. doi: 10.1093/nar/gkm711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfe AL, Singh K, Zhong Y, Drewe P, Rajasekhar VK, Sanghvi VR, Mavrakis KJ, Jiang M, Roderick JE, Van der Meulen J, Schatz JH, Rodrigo CM, Zhao C, Rondou P, de Stanchina E, Teruya-Feldstein J, Kelliher MA, Speleman F, Porco JA, Jr, Pelletier J, Ratsch G, Wendel HG. Nature. 2014;513:65. doi: 10.1038/nature13485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belotserkovskii BP, Mirkin SM, Hanawalt PC. Chem. Rev. 2013;113:8620. doi: 10.1021/cr400078y. [DOI] [PubMed] [Google Scholar]
- 7.Kumari S, Bugaut A, Huppert JL, Balasubramanian G. Nat. Chem. Bio. 2007;3:218. doi: 10.1038/nchembio864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huppert JL, Balasubramanian S. Nucleic Acids Res. 2007;35:406. doi: 10.1093/nar/gkl1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paeschke K, Simonsson T, Postberg J, Rhodes D, Lipps HJ. Nat. Struct. Mol. Biol. 2005;12:847. doi: 10.1038/nsmb982. [DOI] [PubMed] [Google Scholar]
- 10.Zahler AM, Williamson JR, Cech TR, Prescott DM. Nature. 1991;350:718. doi: 10.1038/350718a0. [DOI] [PubMed] [Google Scholar]
- 11.Biffi G, Di Antonio M, Tannahill D, Balasubramanian S. Nat. Chem. 2014;6:75. doi: 10.1038/nchem.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sundquist WI, Klug A. Nature. 1989;342:825. doi: 10.1038/342825a0. [DOI] [PubMed] [Google Scholar]
- 13.Neidle S, Parkinson GN. Biochimie. 2008;90:1184. doi: 10.1016/j.biochi.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Monchaud D, Teulade-Fichou MP. Org. Biomol. Chem. 2008;6:627. doi: 10.1039/b714772b. [DOI] [PubMed] [Google Scholar]
- 15.Ladame S, Schouten JA, Roldan J, Redman JE. Biochemistry. 2006;45:1393. doi: 10.1021/bi050229x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaffitzel C, Postberg J, Paeschke K, Lipps H. In: G-Quadruplex DNA: Methods and Protocols. Baumann P, editor. Vol. 608. Humana Press; 2010. p. 159. [DOI] [PubMed] [Google Scholar]
- 17.Kumar N, Patowary A, Sivasubbu S, Petersen M. Biochemistry. 2008;27:13179. doi: 10.1021/bi801064j. [DOI] [PubMed] [Google Scholar]
- 18.Kumar N, Maiti S. J. Phys. Chem. B. 2007;111:12328. doi: 10.1021/jp072705u. [DOI] [PubMed] [Google Scholar]
- 19.Marin VL, Armitage BA. Biochemistry. 2006;45:1745. doi: 10.1021/bi051831q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amato J, Oliviero G, Pauw DE, Gabelica V. Biopolymers. 2009;91:244. doi: 10.1002/bip.21124. [DOI] [PubMed] [Google Scholar]
- 21.Amato J, Pagano B, Borbone N, Oliviero G, Gabelica V, De Pauw E, D’Errico S, Piccialli V, Varra M, Giancola C, Piccialli G, Mayo L. Bioconjug. Chem. 2011;22:654. doi: 10.1021/bc100444v. [DOI] [PubMed] [Google Scholar]
- 22.Datta B, Armitage BA. J. Am. Chem. Soc. 2001;123:9612. doi: 10.1021/ja016204c. [DOI] [PubMed] [Google Scholar]
- 23.Datta B, Schmitt C, Armitage BA. J. Am. Chem. Soc. 2003;125:4111. doi: 10.1021/ja028323d. [DOI] [PubMed] [Google Scholar]
- 24.Marin VL, Armitage BA. J. Am. Chem. Soc. 2005;127:8032. doi: 10.1021/ja051102y. [DOI] [PubMed] [Google Scholar]
- 25.Lusvarghi S, Murphy CT, Roy S, Tanious FA, Sacui I, Wilson DW, Ly DH, Armitage BA. J. Am. Chem. Soc. 2009;121:18415. doi: 10.1021/ja907250j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roy S, Zanotti KJ, Murphy CT, Tanious FA, Wilson WD, Ly DH, Armitage BA. Chem. Commun. 2011;47:8524. doi: 10.1039/c1cc12805a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petraccone L, Pagano B, Esposito V, Randazzo A, Piccialli G, Barone G, Mattia CA, Giancola C. J. Am. Chem. Soc. 2005;127:16215. doi: 10.1021/ja0545923. [DOI] [PubMed] [Google Scholar]
- 28.Krishnan-Ghosh Y, Stephens E, Balasubramanian G. J. Am. Chem. Soc. 2004;126:5944. doi: 10.1021/ja031508f. [DOI] [PubMed] [Google Scholar]
- 29.Paul A, Sengupta P, Krishnan Y. Chem. Eur. J. 2008;14:8682. doi: 10.1002/chem.200800605. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen PE, Egholm M, Berg RH, Buchardt O. Science. 1991;254:1497. doi: 10.1126/science.1962210. [DOI] [PubMed] [Google Scholar]
- 31.Egholm M, Buchardt O, Christensen L, Behrens C, Freier SM, Driver DA, Berg RH, Kim SK, Norden B, Nielsen PE. Nature. 1993;365:566. doi: 10.1038/365566a0. [DOI] [PubMed] [Google Scholar]
- 32.Porcheddu A, Giacomelli G. Curr. Med. Chem. 2005;12 doi: 10.2174/092986705774370664. [DOI] [PubMed] [Google Scholar]
- 33.Abibi A, Protozanova E, Demidov VV. Biophys. J. 2004;86:3070. doi: 10.1016/S0006-3495(04)74356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pokorski PK, Witschi MA, Purnell BL, Appella DH. J. Am. Chem. Soc. 2004;126:15067. doi: 10.1021/ja046280q. [DOI] [PubMed] [Google Scholar]
- 35.Sahu B, Sacui I, Rapireddy S, Zanotti KJ, Bahal R, Armitage BA, Ly DH. J. Org. Chem. 2011;76:5614. doi: 10.1021/jo200482d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar VA, Ganesh KN. Acc. Chem. Res. 2005;38:404. doi: 10.1021/ar030277e. [DOI] [PubMed] [Google Scholar]
- 37.N. Tilani S, De Costa JMH. PloS one. 2013;8 [Google Scholar]
- 38.Sibley DR, Appella DH, Dix AV, Conroy JL, George Rosenker KM. ACS Med. Chem. Lett. 2015;6:425. doi: 10.1021/ml500478m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Englund EA, Wang D, Fujigaki H, Sakai H, Micklitsch CM, Ghirlando R, Martin-Manso G, Pendrak ML, Roberts DD, Durell SR, Appella DH. Nat. Commun. 2012;3 doi: 10.1038/ncomms1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Englund EA, Appella DH. Angew. Chem. Int. Ed. 2007;46:1414. doi: 10.1002/anie.200603483. [DOI] [PubMed] [Google Scholar]
- 41.Englund EA, Appella DH. Org. Lett. 2005;7:3465. doi: 10.1021/ol051143z. [DOI] [PubMed] [Google Scholar]
- 42.Fasting C, Schalley CA, Weber M, Seitz O, Hecht S, Koksch B, Dernedde J, Graf C, Knapp EW, Haag R. Angew. Chem. Int. Ed. 2012;51:10472. doi: 10.1002/anie.201201114. [DOI] [PubMed] [Google Scholar]
- 43.Scheibe C, Bujotzek A, Dernedde J, Weber M, Seitz O. Chem. Sci. 2011;2:770. [Google Scholar]
- 44.Diezmann F, Seitz O. Chem. Soc. Rev. 2011;40:5789. doi: 10.1039/c1cs15054e. [DOI] [PubMed] [Google Scholar]
- 45.Diezmann F, von Kleist L, Haucke V, Seitz O. Org. Biomol. Chem. 2015;13:8008. doi: 10.1039/c5ob00943j. [DOI] [PubMed] [Google Scholar]
- 46.Eberhard H, Diezmann F, Seitz O. Angew. Chem. Int. Ed. 2011;123:4232. doi: 10.1002/anie.201007593. [DOI] [PubMed] [Google Scholar]
- 47.Winssinger N. Chimia. 2013;67:340. doi: 10.2533/chimia.2013.340. [DOI] [PubMed] [Google Scholar]
- 48.Chen J, Dalal RV, Petrov AN, Tsai A, O'Leary SE, Chapin K, Cheng J, Ewan M, Hsiung PL, Lundquist P, Turner SW, Hsu DR, Puglisi JD. Proc. Natl. Acad. Sci. U S A. 2014;111:664. doi: 10.1073/pnas.1315735111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.