Abstract
Background
In sub-Saharan Africa, among patients with advanced human immunodeficiency virus (HIV) infection, the rate of death from infection (including tuberculosis and cryptococcus) shortly after the initiation of antiretroviral therapy (ART) is approximately 10%.
Methods
In this factorial open-label trial conducted in Uganda, Zimbabwe, Malawi, and Kenya, we enrolled HIV-infected adults and children 5 years of age or older who had not received previous ART and were starting ART with a CD4+ count of fewer than 100 cells per cubic millimeter. They underwent simultaneous randomization to receive enhanced antimicrobial prophylaxis or standard prophylaxis, adjunctive raltegravir or no raltegravir, and supplementary food or no supplementary food. Here, we report on the effects of enhanced antimicrobial prophylaxis, which consisted of continuous trimethoprim–sulfamethoxazole plus at least 12 weeks of isoniazid–pyridoxine (coformulated with trimethoprim–sulfamethoxazole in a single fixed-dose combination tablet), 12 weeks of fluconazole, 5 days of azithromycin, and a single dose of albendazole, as compared with standard prophylaxis (trimethoprim–sulfamethoxazole alone). The primary end point was 24-week mortality.
Results
A total of 1805 patients (1733 adults and 72 children or adolescents) underwent randomization to receive either enhanced prophylaxis (906 patients) or standard prophylaxis (899 patients) and were followed for 48 weeks (loss to follow-up, 3.1%). The median baseline CD4+ count was 37 cells per cubic millimeter, but 854 patients (47.3%) were asymptomatic or mildly symptomatic. In the Kaplan–Meier analysis at 24 weeks, the rate of death with enhanced prophylaxis was lower than that with standard prophylaxis (80 patients [8.9% vs. 108 [12.2%]; hazard ratio, 0.73; 95% confidence interval [CI], 0.55 to 0.98; P=0.03); 98 patients (11.0%) and 127 (14.4%), respectively, had died by 48 weeks (hazard ratio, 0.76; 95% CI, 0.58 to 0.99; P=0.04). Patients in the enhanced-prophylaxis group had significantly lower rates of tuberculosis (P=0.02), cryptococcal infection (P=0.01), oral or esophageal candidiasis (P=0.02), death of unknown cause (P=0.03), and new hospitalization (P=0.03). However, there was no significant between-group difference in the rate of severe bacterial infection (P=0.32). There were nonsignificantly lower rates of serious adverse events and grade 4 adverse events in the enhanced-prophylaxis group (P=0.08 and P=0.09, respectively). Rates of HIV viral suppression and adherence to ART were similar in the two groups.
Conclusions
Among HIV-infected patients with advanced immunosuppression, enhanced antimicrobial prophylaxis combined with ART resulted in reduced rates of death at both 24 weeks and 48 weeks without compromising viral suppression or increasing toxic effects. (Funded by the Medical Research Council and others; REALITY Current Controlled Trials number, ISRCTN43622374.)
Although the World Health Organization (WHO) guidelines now recommend universal antiretroviral therapy (ART) regardless of the CD4+ count,1–3 20 to 25% of patients with human immunodeficiency virus (HIV) infection in sub-Saharan Africa present for care with severe immunosuppression (CD4+ count, <100 cells per cubic millimeter).4 Among these patients, approximately 10% die during the first 3 months after ART initiation.5–8 Causes of death are multifactorial and similar between adults and older children,7 with severe bacterial infection,3,9 tuberculosis,8,10,11 and cryptococcal infection12,13 playing prominent roles. The development or exacerbation of such infections has been linked in part to the immune reconstitution inflammatory syndrome (IRIS) associated with the initiation of ART. Current guidelines recommend ruling out tuberculosis and cryptococcal meningitis before the initiation of ART, along with the use of trimethoprim–sulfamethoxazole and isoniazid prophylaxis.1,14,15 The risk of death increases markedly with decreasing CD4+ counts and body-mass index (BMI, the weight in kilograms divided by the square of the height in meters) in both adults and children,7 which suggests the need for additional interventions aimed at preventing infection, accelerating immune recovery (through rapid viral-load reduction), and improving nutritional status.
One approach to preventing infection in all patients is administering preemptive treatment courses for specific high-burden diseases (e.g., tuberculosis) when ART is initiated.16,17 Another is an antimicrobial prophylaxis package targeting dominant pathogens among patients with advanced HIV infection after the clinical exclusion of active infections. Such a pragmatic approach could be universally provided at low-level health facilities. Possible adverse outcomes include toxicity, antimicrobial resistance, and reduced adherence to the ART regimen because of the need to take additional pills.
In the Reduction of Early Mortality in HIV-Infected Adults and Children Starting Antiretroviral Therapy (REALITY) trial, we compared three interventions — enhanced antimicrobial prophylaxis, additional raltegravir, and food supplementation — to reduce early mortality in adults and older children with a CD4+ count of fewer than 100 cells per cubic millimeter in whom ART was initiated in four sub-Saharan African countries. Here, we report the effect of enhanced antimicrobial prophylaxis only.
Methods
Trial Enrollment
From June 2013 through April 2015, in eight urban or periurban centers in Uganda, Zimbabwe, Malawi, and Kenya, we enrolled HIV-infected adults and children who were 5 years of age or older, who had not received previous ART, and who had a CD4+ count of fewer than 100 cells per cubic millimeter. Patients were excluded if they were pregnant or breast-feeding, had received single-dose nevirapine to prevent mother-to-child transmission of HIV, or had any contraindications to the trial drugs. Adult patients and guardians provided written informed consent; older children provided additional assent, according to national guidelines. The trial was approved by ethics committees in Uganda, Zimbabwe, Malawi, Kenya, and the United Kingdom.
Trial Design
All the patients initiated ART with two nucleoside reverse-transcriptase inhibitors and one non-nucleoside reverse-transcriptase inhibitor. They were then randomly assigned in a 1:1 ratio to initiate open-label enhanced antimicrobial prophylaxis or standard prophylaxis. Enhanced prophylaxis consisted of a single dose (400 mg) of albendazole, 5 days of azithromycin (500 mg once daily), 12 weeks of fluconazole (100 mg once daily), and 12 weeks of a fixed-dose combination of trimethoprim–sulfamethoxazole (160 mg of trimethoprim and 800 mg of sulfamethoxazole), isoniazid (300 mg), and pyridoxine (25 mg) as a scored once-daily tablet (total, three tablets per day for 1 to 5 days, then two pills per day for 12 weeks). Doses were halved for children younger than 13 years of age, except for albendazole. Standard prophylaxis consisted of trimethoprim–sulfamethoxazole alone.
After 12 weeks, fluconazole was discontinued and trimethoprim–sulfamethoxazole or the fixed-dose combination was continued in the enhanced-prophylaxis group; trimethoprim–sulfamethoxazole was continued or switched to the fixed-dose combination in the standard-prophylaxis group. The use of isoniazid–pyridoxine beyond the 12-week period depended on national guidelines for the use of isoniazid preventive therapy. Screening for active tuberculosis before randomization was performed with the use of a WHO-based symptom checklist (see the Methods section in the Supplementary Appendix, available with the full text of this article at NEJM.org). Sputum samples were examined and chest radiography was performed in centers where such evaluation was possible. Patients who were already receiving antimicrobial treatment or prophylaxis or who needed such therapy were treated regardless of randomization but received other prophylaxis according to randomization.
Randomization was stratified according to trial center, age (<13 years vs. ≥13 years), and other factorial randomizations (12 weeks of additional raltegravir vs. no raltegravir and 12 weeks of additional ready-to-use supplementary food vs. no supplementary food). A computer-generated sequential randomization list with variably sized permuted blocks was prepared by the trial statistician and incorporated securely into the online trial database. The list was concealed until eligibility was confirmed by staff members at the local center, who then performed the randomization.
Patients discontinued their participation in the trial after 48 weeks. At weeks 2, 4, 8, 12, 18, 24, 36, and 48, a nurse reviewed a symptom checklist and asked patients about their adherence to the trial drugs, and a pharmacist dispensed the trial drugs. At weeks 4, 12, 24, 36, and 48, a physician took a medical history and performed a physical examination; laboratory testing that included a full blood count, CD4+ count, and evaluation of kidney and renal function was performed (with testing of kidney and renal function performed only at weeks 4 and 48); and plasma was stored for retrospective evaluation of the HIV viral load. All the nurses and physicians were aware of the trial-group assignments; all testing was performed in a blinded manner. At the physicians’ discretion, antiretroviral drugs could be substituted in cases of drug toxicity; in cases of first-line drug failure, regimens could be switched to second-line regimens, according to WHO guidelines.18
Following the factorial design, all the patients also underwent randomization in a 1:1 ratio to receive 12 weeks of additional raltegravir or no raltegravir and to receive 12 weeks of ready-to-use supplementary food or no routine supplementation. The results of these analyses are not reported here. Full details regarding the trial design and analyses are provided in the protocol, available at NEJM.org.
Trial Oversight
Gilead Sciences, ViiV Healthcare/GlaxoSmithKline, Cipla, and Merck donated the antiretroviral drugs, Cipla donated the prophylaxis drugs, and ready-to-use supplementary food was purchased from Valid International. Representatives of the drug manufacturers had no role in the trial design, data collection, data analysis, or manuscript preparation. All the authors vouch for the completeness and accuracy of the data and all analyses, and for the fidelity of the trial to the protocol.
Primary and Secondary Outcomes
The primary outcome was death from any cause occurring from randomization to 24 weeks. Secondary outcomes, which were evaluated through 48 weeks, were death from any cause; serious adverse events, grade 4 adverse events, and adverse events leading to modification of ART or other trial drugs; mechanisms of each intervention, including a change in the CD4+ count; incidence of tuberculosis, cryptococcal infection, candidiasis (esophageal or oral), and severe bacterial infections; changes in weight or BMI; hospitalization; and patient-reported adherence to and acceptability of the ART regimen. Adverse events were graded according to the criteria of the National Institutes of Health.19,20 Other outcomes included WHO stage 3 or 4 events.18 An end-point review committee whose members were unaware of trial-group assignments and trial drugs received used protocol-defined criteria and grading tables19,20 to adjudicate all the secondary clinical outcomes that were reported by the trial physicians, along with determining the relatedness of the outcome to a trial drug and compatibility with IRIS. (Details regarding the trial outcomes are provided in the Methods section in the Supplementary Appendix.)
Economic Analysis and Quality of Life
We performed economic analyses to estimate costs and health outcomes during the 48-week trial using data on resources used in the trial and published unit costs for each country. Health was measured on the basis of quality-adjusted life-years, according to the three-level EuroQol Group 5-Dimension Self-Report Questionnaire, which the patients completed at each nurse visit. The value of each health state was assigned with the use of a Zimbabwean value set.21
Statistical Analysis
We determined that the enrollment of 1800 adults and children would provide a power of more than 80% to detect a rate of death from any cause that was 50% lower in the enhanced-prophylaxis group than in the standard-prophylaxis group at 24 weeks (a reduction in mortality from 7.0% to 3.5%) at a two-sided alpha level of 0.05, allowing for a 5% loss to follow-up. An independent data and safety monitoring committee used the Haybittle–Peto approach to review interim data at three annual meetings. We used the intention-to-treat principle to compare the randomized groups using log-rank tests or competing-risks methods for time-to-event outcomes, exact tests for binary outcomes, and generalized estimating equations with independent working correlation for global tests of repeated measures. The primary analyses were stratified according to the factors used to stratify the randomization, with no adjustment for multiple testing. All the analyses were performed with the use of Stata software, version 14.2.
Results
Trial Patients
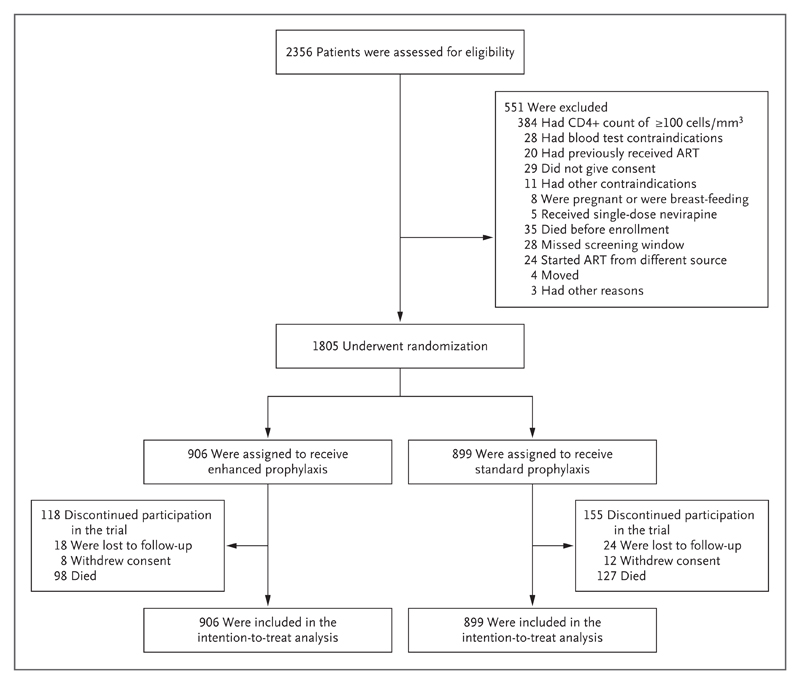
A total of 1805 patients underwent randomization to receive enhanced prophylaxis (906 patients) or standard prophylaxis (899 patients) (Fig. 1). The baseline characteristics of the patients were well balanced in the two groups (Table 1, and Table S1 in the Supplementary Appendix). The median age was 36 years; 72 patients (4.0%) were 5 to 17 years of age. The median CD4+ count was 37 cells per cubic millimeter, and 1300 of 1763 patients (73.7%) had a viral load of at least 100,000 copies per milliliter. Despite these findings, 854 patients (47.3%) were asymptomatic or mildly symptomatic (WHO clinical disease stage, 1 to 2).
Figure 1. Enrollment and Randomization.
Patients could have more than one reason for exclusion; they could also be lost to follow-up without withdrawal of consent and vice versa, so the total numbers of patients with exclusions and discontinuations are lower than the sums of the individual categories. Details regarding the patients’ adherence to treatment are provided in Figure S1 in the Supplementary Appendix.
Table 1. Characteristics of the Patients at Randomization.*.
| Characteristic | Standard Prophylaxis (N = 899) |
Enhanced Prophylaxis (N = 906) |
All Patients (N = 1805) |
|---|---|---|---|
| Age | |||
| Median (IQR) — yr | 36 (30–42) | 36 (29–42) | 36 (29–42) |
| Range — yr | 5–78 | 6–71 | 5–78 |
| 5–17 yr — no. (%) | 33 (3.7) | 39 (4.3) | 72 (4.0) |
| Male sex — no. (%) | 484 (53.8) | 477 (52.6) | 961 (53.2) |
| Median body-mass index (IQR)† | 19.3 (17.4–21.5) | 19.1 (17.1–21.3) | 19.2 (17.2–21.4) |
| Country — no. (%) | |||
| Kenya | 174 (19.4) | 177 (19.5) | 351 (19.4) |
| Malawi | 128 (14.2) | 127 (14.0) | 255 (14.1) |
| Uganda | 313 (34.8) | 317 (35.0) | 630 (34.9) |
| Zimbabwe | 284 (31.6) | 285 (31.5) | 569 (31.5) |
| WHO clinical stage of HIV infection — no. (%)‡ | |||
| 1 | 153 (17.0) | 147 (16.2) | 300 (16.6) |
| 2 | 265 (29.5) | 289 (31.9) | 554 (30.7) |
| 3 | 349 (38.8) | 342 (37.7) | 691 (38.3) |
| 4 | 132 (14.7) | 128 (14.1) | 260 (14.4) |
| Current infection — no. (%) | |||
| Tuberculosis | 135 (15.0) | 136 (15.0) | 271 (15.0) |
| Cryptococcal | 12 (1.3) | 13 (1.4) | 25 (1.4) |
| Candida | 53 (5.9) | 46 (5.1) | 99 (5.5) |
| Median CD4+ count (IQR) — cells/mm3§ | 36 (16–60) | 38 (16–64) | 37 (16–63) |
| HIV viral load ≥100,000 copies/ml — no./total no. (%) | 645/882 (73.1) | 655/881 (74.3) | 1300/1763 (73.7) |
| Initiation of ART — no. (%) | |||
| Efavirenz | 799 (88.9) | 820 (90.5) | 1619 (89.7) |
| Tenofovir–emtricitabine | 706 (78.5) | 716 (79.0) | 1422 (78.8) |
| Medication prescribed at randomization — no. (%) | |||
| Isoniazid | |||
| Prophylaxis | 9 (1.0) | 784 (86.5) | 793 (43.9) |
| Treatment | 104 (11.6) | 118 (13.0) | 222 (12.3) |
| Fluconazole | |||
| Prophylaxis | 1 (0.1) | 863 (95.3) | 864 (47.9) |
| Treatment | 107 (11.9) | 42 (4.6) | 149 (8.3) |
| Azithromycin | |||
| Prophylaxis | 1 (0.1) | 906 (100.0) | 907 (50.2) |
| Treatment | 13 (1.4) | 0 | 13 (0.7) |
| Albendazole | |||
| Prophylaxis as single dose | 1 (0.1) | 906 (100.0) | 907 (50.2) |
| Treatment | 4 (0.4) | 0 | 4 (0.2) |
| Trimethoprim–sulfamethoxazole prophylaxis | 877 (97.6) | 889 (98.1) | 1766 (97.8) |
| Any other antibiotic | 122 (13.6) | 76 (8.4) | 198 (11.0) |
There were no significant differences between the groups at baseline. ART denotes antiretroviral therapy, HIV human immunodeficiency virus, IQR interquartile range, and WHO World Health Organization.
The body-mass index (the weight in kilograms divided by the square of the height in meters) was reported in 1797 patients.
The WHO clinical stage was based on 2006 WHO case definitions.
The median baseline CD4+ count was calculated from the mean of the values that were obtained at screening and at enrollment. Trial eligibility required a screening CD4+ count of fewer than 100 cells per cubic millimeter, but the count at the time of enrollment could have been higher than 100 cells per cubic millimeter.
Before randomization, 174 patients (9.6%) were receiving isoniazid treatment and 196 (10.9%) were receiving fluconazole treatment; 3 (0.2%) and 9 (0.5%), respectively, were receiving the drugs as prophylaxis. More patients in the standard-prophylaxis group than in the enhanced-prophylaxis group were prescribed fluconazole, azithromycin, or other antibiotics at randomization, a difference that probably reflected additional use for treating oral candidiasis or minor bacterial infections. All the patients initiated ART at a median of 5 days after screening, predominantly with first-line tenofovir, emtricitabine, and efavirenz.
A total of 56 patients (3.1%) — 24 in the enhanced-prophylaxis group and 32 in the standard-prophylaxis group — were lost to follow-up (i.e., no clinic attendance for >91 days) (P=0.28). At last follow-up, 1765 patients (97.8%) were still receiving first-line ART, of whom 119 (6.6%) had made within-class substitutions. There was no significant between-group difference in the percentage of patients who missed at least one scheduled visit before death or loss to follow-up (11.6% [105 patients] in the enhanced-prophylaxis group and 11.9% [107 patients] in the standard-prophylaxis group, P=0.84).
Receipt of Prophylaxis and Treatment
During the first 12 weeks after the initiation of ART, patients in the enhanced-prophylaxis group were prescribed isoniazid prophylaxis for 84.4% of person-time and isoniazid treatment for 11.3% of person-time, as compared with 3.6% and 10.7% of person-time, respectively, in the standard-prophylaxis group. Patients in the enhanced-prophylaxis group were prescribed fluconazole prophylaxis for 96.7% of person-time and fluconazole treatment for 1.9% of person-time, as compared with 0.3% and 2.6% of person-time, respectively, in the standard-prophylaxis group (Fig. S1 in the Supplementary Appendix). All the patients in the enhanced-prophylaxis group were prescribed azithromycin and albendazole (Table 1).
At 12 weeks, a substantial proportion of the patients in the standard-prophylaxis group initiated isoniazid preventive therapy (Fig. S2 in the Supplementary Appendix). Thus, from 12 week to 48 weeks, the patients in the enhanced-prophylaxis group were prescribed isoniazid prophylaxis for 46.3% of person-time and isoniazid treatment for 3.2% of person-time, as compared with 54.8% and 3.2% of person-time, respectively, in the standard-prophylaxis group. In contrast, after 12 weeks, patients in the enhanced-prophylaxis group were prescribed fluconazole prophylaxis for 2.3% of person-time and fluconazole treatment for 0.7% of person-time, as compared with 0.5% and 0.8% of person-time, respectively, in the standard-prophylaxis group.
During the first 12 weeks, the patient-reported rate of adherence to prophylaxis was slightly (but significantly) poorer in the enhanced-prophylaxis group than in the standard-prophylaxis group (P=0.004); for example, 7.4% and 5.2% of the patients, respectively, reported that they had missed any doses of prophylaxis drugs (including trimethoprim–sulfamethoxazole) between weeks 8 and 12. However, during weeks 12 to 48, the adherence rates were similar in the two groups (P=0.30), as were rates of patient-reported acceptability of the drugs (Fig. S3B and S3C in the Supplementary Appendix).
Mortality at 24 Weeks and 48 Weeks
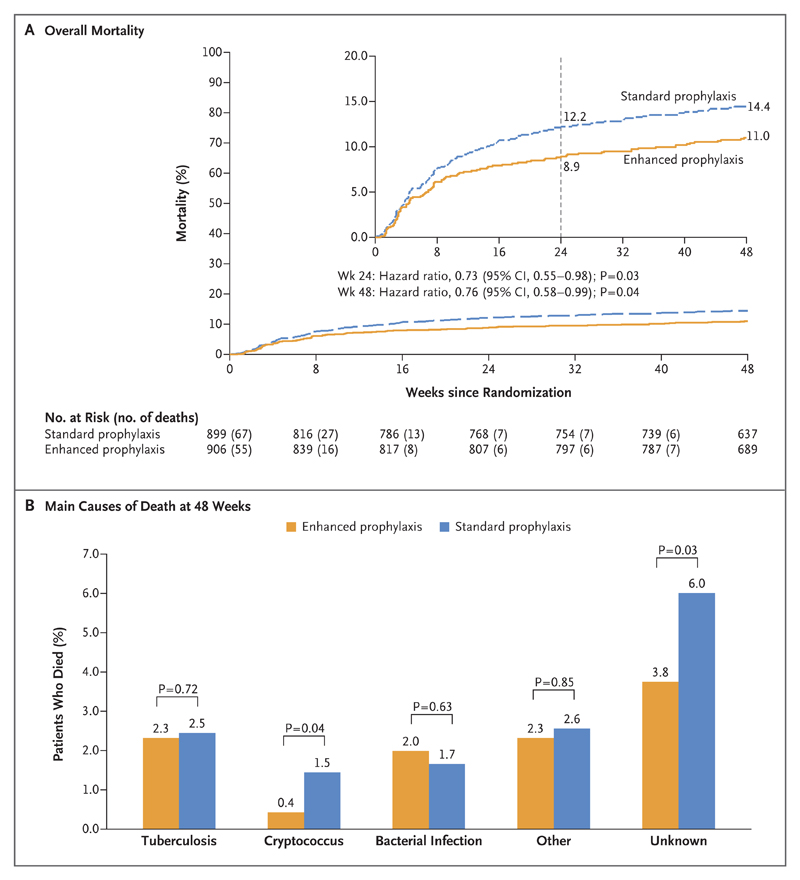
Death by 24 weeks (the primary outcome) was reported in 80 patients in the enhanced-prophylaxis group and in 108 in the standard-prophylaxis group (8.9% vs. 12.2% by Kaplan–Meier analysis; hazard ratio in the enhanced-prophylaxis group, 0.73; 95% confidence interval [CI], 0.55 to 0.98; P=0.03 by the log-rank test) (Fig. 2A). Thus, at 24 weeks, 30 patients would need to have received enhanced prophylaxis to prevent 1 death. A significant survival benefit was maintained through 48 weeks, with deaths reported in 98 patients in the enhanced-prophylaxis group and in 127 in the standard-prophylaxis group (11.0% vs. 14.4% by Kaplan–Meier analysis; hazard ratio, 0.76; 95% CI, 0.58 to 0.99; P=0.04 by the log-rank test) (Fig. 2A). Thus, at 48 weeks, 29 patients would need to have received enhanced prophylaxis to prevent 1 death. There was no evidence that benefits varied over time (P=0.49 for interaction in the comparison of 0 to 24 weeks vs. 24 to 48 weeks) and no evidence of interaction with other factorial randomizations to additional raltegravir or supplementary food (P>0.70).
Figure 2. Overall Mortality and Cause of Death at 48 Weeks.
Panel A shows the results of a Kaplan–Meier analysis of death over 48 weeks in the enhanced-prophylaxis group and the standard-prophylaxis group. The inset shows the same data on an expanded y axis, with Kaplan–Meier estimates of mortality at 24 and 48 weeks. Panel B shows the predominant causes of death in the two groups over 48 weeks.
The most common primary cause of death was infection in 92 of 225 deaths (40.9%) (Table S2 in the Supplementary Appendix). Causes of death were often multifactorial; many occurred at home, and a clear cause was not determined. As adjudicated by the end-point review committee, deaths from cryptococcus infection and from unascertained causes occurred less frequently in the enhanced-prophylaxis group than in the standard-prophylaxis group (P=0.04 and P=0.03, respectively), but there was no evidence of significant between-group differences in the rates of death from tuberculosis (P=0.72), presumptive bacterial infections (P=0.63), or other causes (P=0.85) (Fig. 2B). There was marginal evidence that IRIS-compatible deaths were less common with enhanced prophylaxis than with standard prophylaxis (P=0.06). (Details are provided in the Results section in the Supplementary Appendix.)
Estimated rates of death were highest on day 18, when the absolute difference between enhanced prophylaxis and standard prophylaxis was greatest; these rates then decreased sharply through week 12. The rate of death from unascertained causes followed a similar pattern to that of known causes (Fig. S4 in the Supplementary Appendix).
There was no evidence that the mortality benefit varied across nine preplanned subgroups, including the other randomizations (P>0.20). In particular, there was no evidence that mortality benefits depended on the CD4+ count at the initiation of ART (P=0.29 for the interaction with categories of CD4+ count; P=0.89 for the interaction with the CD4+ count as a continuous variable). Of nine exploratory subgroup analyses, only one suggested that benefits from enhanced prophylaxis might be greater among male patients than among female patients (P=0.048) (Fig. S5 in the Supplementary Appendix).
Secondary Outcomes at 48 Weeks
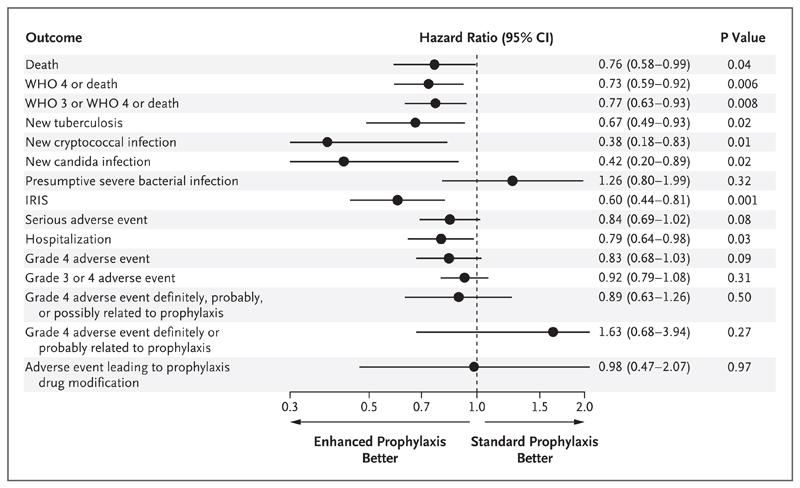
Enhanced prophylaxis was associated with significantly lower rates of WHO stage 3 or 4 events or death than was standard prophylaxis (in 179 patients [19.8%] vs. 224 [24.9%], P=0.008), along with lower rates of a new diagnosis of tuberculosis (in 64 patients [7.1%] vs. 92 [10.2%], P=0.02), cryptococcal infection (9 [1.0%] vs. 23 [2.6%], P=0.01), candidiasis (10 [1.1%] vs. 23 [2.6%], P=0.02), and new hospitalization (154 [17.0%] vs. 186 [20.7%], P=0.03) (Fig. 3, and Table S3 in the Supplementary Appendix).
Figure 3. Secondary and Other Outcomes at 48 Weeks.
Secondary outcomes included death, new tuberculosis, new cryptococcal infection, new candida infection, presumptive severe bacterial infection, serious adverse event, hospitalization, grade 4 adverse event, and adverse event leading to prophylaxis drug modification. IRIS denotes immune reconstitution inflammatory syndrome, and WHO World Health Organization.
The total number of days of hospitalization were 2233 with enhanced prophylaxis and 2819 with standard prophylaxis (P=0.057 by the rank-sum test) during a total of 184 hospitalizations and 247 hospitalizations, respectively (P<0.001 by Poisson regression). There was a significantly lower rate of IRIS-compatible events (as adjudicated by the end-point review committee) with enhanced prophylaxis than with standard prophylaxis (in 67 patients [7.4%] vs. 108 [12.0%], P=0.001). There was no evidence of a between-group difference in the rate of new presumptive severe bacterial infections (in 42 patients [4.6%] vs. 33 [3.7%], P=0.32).
There was marginal evidence of a lower rate of serious adverse events in the enhanced-prophylaxis group than in the standard-prophylaxis group (P=0.08) and of a lower rate of grade 4 adverse events (P=0.09) (Table S3 in the Supplementary Appendix), findings that were strengthened in exploratory analyses that included subsequent events (P=0.002 for serious adverse events and P=0.01 for grade 4 adverse events by Poisson regression) (Table S4 in the Supplementary Appendix). There was no evidence of a between-group difference in grade 3 or 4 adverse events (P=0.31), in grade 4 adverse events that were adjudicated by the end-point review committee as “definitely or probably” related to any prophylaxis drug (P=0.27) or as “definitely, probably, or possibly” related to any prophylaxis drug (P=0.50), or in adverse events leading to the discontinuation of a prophylaxis drug (P=0.97). Enhanced prophylaxis was discontinued in 14 patients (1.5%) because of toxicity involving the liver (in 7 patients), skin (in 4), or blood (in 3) (Table S5 in the Supplementary Appendix).
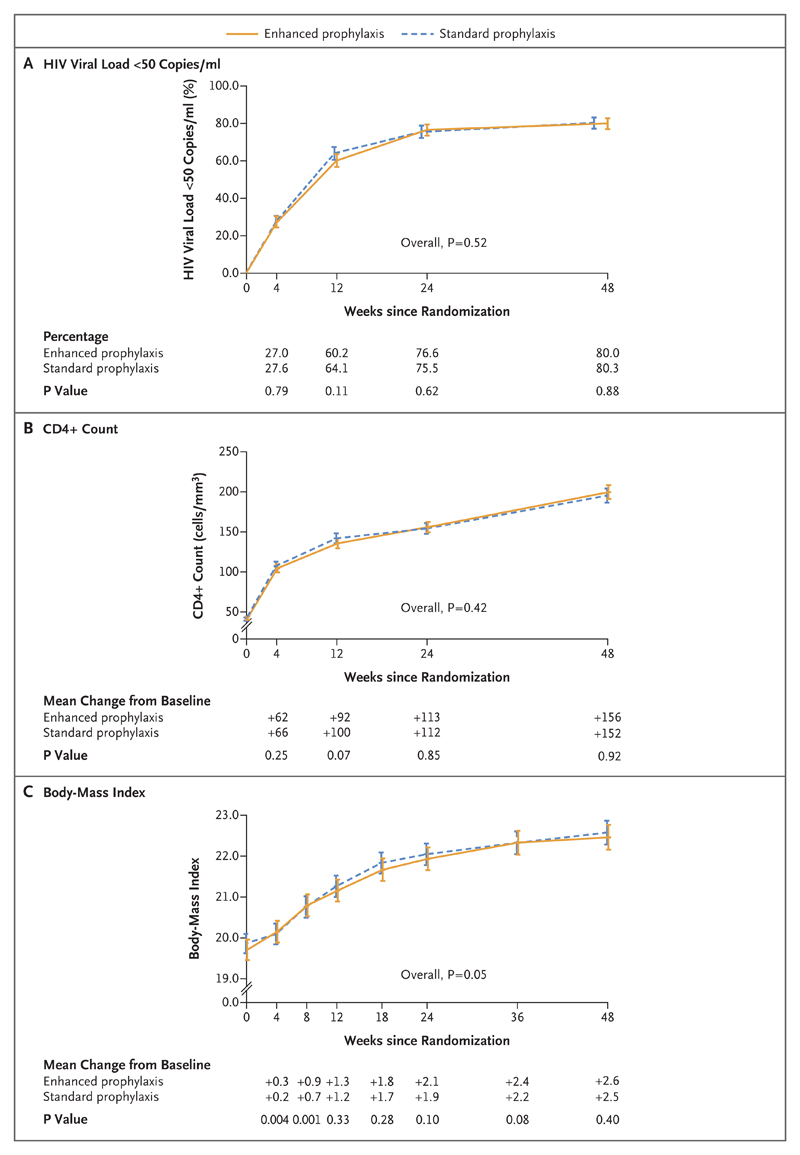
There was no evidence that the patient-reported rate of adherence to ART (based on any missed dose in the previous 4 weeks) differed between the two groups (P=0.31) (Fig. S3D in the Supplementary Appendix). Consistent with this finding, there was no significant between-group difference in the suppression of the HIV viral load to fewer than 50 copies per milliliter (P=0.52) or in the CD4+ count (P=0.42) (Fig. 4A and 4B). At 24 weeks after ART initiation, 601 of 785 patients (76.6%) in the enhanced-prophylaxis group and 557 of 738 (75.5%) in the standard-prophylaxis group had an HIV viral load of fewer than 50 copies per milliliter (P=0.62), and the mean (±SD) increase in the CD4+ count was 113±3.1 cells with enhanced prophylaxis versus 112±3.1 with standard prophylaxis (P=0.85). In adolescents and adults who received enhanced prophylaxis, there were nonsignificantly greater increases in BMI (P=0.053) (Fig. 4C) and weight (P=0.051).
Figure 4. Reduction in HIV Viral Load, Increase in CD4+ Count, and Increase in Body-Mass Index at 48 Weeks.
Shown is the percentage of patients with an HIV viral load of fewer than 50 copies per milliliter (Panel A), the CD4+ count (Panel B), and the body-mass index (BMI, the weight in kilograms divided by the square of the height in meters) (Panel C), according to the week since randomization. Below the graphs, the percentages of patients with an HIV viral load of fewer than 50 copies per milliliter are shown in Panel A, and the mean changes from baseline in the CD4+ count and BMI are shown in Panels B and C. The I bars indicate 95% confidence intervals.
Quality of Life and Cost-Effectiveness
At 48 weeks, patients in the enhanced-prophylaxis group had a higher number of quality-adjusted life-years and life-years gained than did those in the standard-prophylaxis group, improvements that came at a higher financial cost (Tables S6 and S7 in the Supplementary Appendix). Concomitant medications and antiretroviral drugs were more costly with enhanced prophylaxis than with standard prophylaxis because of the use of the intervention drugs and longer survival (mean cost of concomitant medications, $34.79 vs. $16.73); however, hospitalizations were more costly with standard prophylaxis. On the basis of actual within-country drug costs, the cost of enhanced prophylaxis was $761 per quality-adjusted life-year and $613 per life-year gained through 48 weeks; these prices were reduced to $201 and $162, respectively, on the basis of minimum drug costs across the four countries in the trial.
Discussion
Among HIV-infected adults and older children with advanced immunosuppression who initiated ART, the relative rate of death at 24 weeks was 27% lower among those who received an enhanced antimicrobial prophylaxis package than among those who received trimethoprim–sulfamethoxazole alone (standard prophylaxis) for 12 weeks. Benefits were maintained through 48 weeks (24% lower rate), with a number-needed-to-treat of 29 to prevent one death. Patients who received enhanced prophylaxis also had a significantly lower rate of hospitalization, WHO stage 3 or 4 events, IRIS-compatible events, tuberculosis, and cryptococcal and candida infections; the rate of presumptive bacterial infection was similar in the two groups. There was no evidence of increased toxicity, poorer HIV viral-load suppression, or worse adherence to the ART regimen with enhanced prophylaxis; rather, there were nonsignificantly fewer serious adverse events and grade 4 adverse events.
Two trials have shown a lack of efficacy of an alternative approach to reducing early tuberculosis-related mortality through the initiation of preemptive tuberculosis treatment with four drugs at the time of ART initiation in all adults with severe immunosuppression.16,17 A third trial was terminated early because of low enrollment22; a fourth is ongoing.23 Isoniazid prophylaxis is effective and is now recommended in WHO guidelines after the exclusion of active disease,15 but when our trial started, such treatment was not standard care in any of the recruiting countries. At that time, it had been implemented in only 28% of African countries.24 The timing of the initiation of isoniazid prophylaxis is unspecified in the WHO guidelines. Barriers to uptake include poor availability of individual isoniazid–pyridoxine formulations and concern about toxicity, pill burden, and isoniazid resistance. During the first 12 weeks after the administration of single-dose albendazole and azithromycin (one pill once daily for 5 days), the enhanced-prophylaxis regimen in our trial required only one more pill per day than was required for standard prophylaxis; in addition, enhanced prophylaxis was associated with good rates of acceptability and adherence.25 Unfortunately, we could not assess isoniazid resistance in new tuberculosis cases, since these investigations were not routinely performed at the trial centers. Previously, low rates of isoniazid resistance have been reported with isoniazid preventive therapy.26
To prevent cryptococcal disease, the WHO recommends testing for cryptococcal antigen in patients with a CD4+ count of fewer than 100 cells per cubic millimeter.1 Such screening and preemptive treatment reduced early mortality in one trial,27 but screening tests may not be available at lower-level health facilities. We therefore evaluated a lower-cost dose of fluconazole (100 mg) once daily for all patients with immunosuppression, administered during the first 12 weeks of ART, when CD4+ counts remain lowest. This dose was extrapolated from an effective dose of 200 mg three times weekly among patients who tested negative for cryptococcal antigen and who had initiated ART with a CD4+ count of fewer than 200 cells per cubic millimeter.28 The rate of death associated with cryptococcal infection remained significantly elevated for at least 12 weeks in the standard-prophylaxis group, a finding that supports this approach.
One of the limitations of our trial is that we tested an antimicrobial prophylaxis package, which made it difficult to quantify the effect of each component. In the enhanced-prophylaxis group, the rates of death and complications from cryptococcal infection were significantly lower than those in the standard-prophylaxis group, as was the rate of new tuberculosis infection but not the rate of death from tuberculosis. Another limitation of our trial is that although there was no significant between-group difference in the rate of severe bacterial infections, most of the diagnoses were presumptive because many centers lacked facilities for performing microbiologic analyses, and causes of death were frequently not ascertained because the patients died at home soon after randomization. However, the patients who received enhanced prophylaxis had a lower rate of early death from unknown causes. Given the diagnostic challenges posed by severe bacterial infections and their major contribution to HIV-related mortality,3,9 it is likely that such infections contributed to deaths of unknown cause. Thus, azithromycin may provide additional protection over trimethoprim–sulfamethoxazole, given its broad antimicrobial activity, longer half-life, antiinflammatory properties, and activity against nontuberculous mycobacteria.29 Concern regarding antimicrobial resistance30 should be balanced against the potential for a substantial short-term mortality benefit in this high-risk population. Further mechanistic studies will be needed to determine the contribution of each drug to the efficacy of enhanced prophylaxis and to assess whether omitting any component of the package could reduce its efficacy.
The cost of enhanced prophylaxis ranged from $8 to $34 across trial countries (Table S6 in the Supplementary Appendix). However, drug costs varied by a factor of 10, which highlights the importance of ensuring that all countries can access drugs at the lowest prices. At the minimum price, the cost per quality-adjusted life-year falls within recently published cost-effectiveness thresholds for even the lowest-income countries.31 This analysis does not capture the longer-term benefits associated with reduced mortality beyond 48 weeks, and the inclusion of such benefits would further increase the value-for-money of enhanced prophylaxis.
We enrolled both adults and older children or adolescents because the rates and causes of death are similar regardless of age.7 We expected that 300 children over the age of 5 years would be enrolled, but we identified only 72 who had a CD4+ count of fewer than 100 cells per cubic millimeter. This finding may be due to improved coverage of ART to prevent mother-to-child HIV transmission.32 Although the numbers were lower than expected, there is no reason why the enhanced-prophylaxis package would not benefit older children who are vulnerable to tuberculosis, cryptococcal infection, candidiasis, and helminths. In contrast, the trial recruited adults faster than expected, which suggests that large numbers of patients could benefit from enhanced prophylaxis. Nearly half of the patients had minimal symptoms despite having a median CD4+ count of 37 cells per cubic millimeter, which shows the continued importance of obtaining CD4+ counts for the assessment of patients before ART initiation.33 Whether patients who initiate ART with a CD4+ count of 100 to 200 cells per cubic millimeter would also benefit from enhanced prophylaxis is unclear. However, two other groups with potentially low CD4+ counts may benefit from enhanced prophylaxis: those in whom ART has failed, especially if the pre-ART CD4+ count was low,34 and those returning to care after they had been lost to follow-up.
Another limitation of our trial is its openlabel design, as necessitated by the multiple randomizations, weight-based pediatric dosing, and importance of comparing adherence and acceptability in the two groups. However, the primary mortality end point was objective. The pragmatic design meant that the patients who received standard prophylaxis also spent time receiving treatments or secondary prophylaxis as necessary, which probably reduced the differences between the groups. However, any dilution bias would lead to an underestimation of the benefits of enhanced prophylaxis.
In conclusion, we found a survival benefit for multicomponent enhanced antimicrobial prophylaxis in adults and older children with advanced HIV infection who were initiating ART with a CD4+ count of fewer than 100 cells per cubic millimeter — a group that represents a substantial proportion of those starting treatment who are at increased risk for early death.4 The enhanced prophylaxis is relatively inexpensive, has a low pill burden and an acceptable side-effect profile, and would be easy to implement at primary health centers since it relies only on screening for clinical symptoms and testing of CD4+ counts to identify asymptomatic patients with advanced HIV infection.
Supplementary Material
Acknowledgments
Supported by the Joint Global Health Trials Scheme of the Medical Research Council (MRC), the U.K. Department for International Development, and the Wellcome Trust through a grant (G1100693),with additional support from the PENTA Foundation. The MRC Clinical Trials Unit at University College London is supported by grants from the MRC (MC-UU-12023/23 and MC-UU-12023/26). Dr. Prendergast is funded by a grant (108065/Z/15/Z) from the Wellcome Trust, which also funds the Malawi–Liverpool–Wellcome Trust Clinical Research Program at the University of Malawi College of Medicine through a grant (101113/Z/13/Z) and the Kenya Medical Research Institute (KEMRI)–Wellcome Trust Research Program through a grant (203077).
We thank the patients and staff members at all the centers that participated in the trial.
Footnotes
The authors’ full names and academic degrees are as follows: James Hakim, F.R.C.P., Victor Musiime, Ph.D., Alex J. Szubert, M.Sc., Jane Mallewa, M.D., Abraham Siika, M.Med., Clara Agutu, M.B., Ch.B., M.P.H., Simon Walker, M.Sc., Sarah L. Pett, Ph.D., Mutsa Bwakura-Dangarembizi, M.Med., Abbas Lugemwa, M.D., Symon Kaunda, M.B., Ch.B., Mercy Karoney, M.Sc., Godfrey Musoro, M.Sc., Sheila Kabahenda, M.B., Ch.B., Kusum Nathoo, M.B., Ch.B., Kathryn Maitland, Ph.D., Anna Griffiths, Ph.D., Margaret J. Thomason, Ph.D., Cissy Kityo, M.Sc., Peter Mugyenyi, Ph.D., Andrew J. Prendergast, D.Phil., A. Sarah Walker, Ph.D., and Diana M. Gibb, M.D.
The authors’ affiliations are as follows: the University of Zimbabwe Clinical Research Center, Harare, Zimbabwe (J.H., M.B.-D., G.M., K.N.); Joint Clinical Research Center, Kampala (V.M., C.K., P.M.), Mbarara (A.L.), and Fort Portal (S. Kabahenda) — all in Uganda; Medical Research Council Clinical Trials Unit at University College London (A.J.S., S.L.P., A.G., M.J.T., A.S.W., D.M.G.), Wellcome Trust Centre for Clinical Tropical Medicine and Department of Paediatrics, Imperial College (K.M.), and Queen Mary University of London (A.J.P.), London, and the Centre for Health Economics, University of York, York (S.W.) — all in the United Kingdom; the Department of Medicine and Malawi–Liverpool–Wellcome Trust Clinical Research Program, Blantyre, Malawi (J.M., S. Kaunda); and Moi University School of Medicine, Eldoret (A.S., M.K.), and the Kenya Medical Research Institute (KEMRI) Wellcome Trust Research Program, Kilifi (C.A., K.M.) — both in Kenya.
Dr. Hakim reports receiving fees for serving on an advisory board from Mylan Pharmaceuticals and consulting fees from Gilead Sciences and Johnson & Johnson; Dr. Prendergast, receiving fees for preparing educational material from the PENTA Foundation; and Dr. A. Walker, receiving fees paid to her institution for serving on a data and safety monitoring board for Tibotec and lecture fees paid to her institution by Gilead Sciences. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2nd ed. Geneva: World Health Organization; 2016. ( http://www.who.int/hiv/pub/arv/arv-2016/en/) [PubMed] [Google Scholar]
- 2.The INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The TEMPRANO ANRS 12136 Study Group. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373:808–22. doi: 10.1056/NEJMoa1507198. [DOI] [PubMed] [Google Scholar]
- 4.The IeDEA and ART Cohort Collaborations. Immunodeficiency at the start of combination antiretroviral therapy in low-, middle-, and high-income countries. J Acquir Immune Defic Syndr. 2014;65(1):e8–16. doi: 10.1097/QAI.0b013e3182a39979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulle A, Schomaker M, May MT, et al. Mortality in patients with HIV-1 infection starting antiretroviral therapy in South Africa, Europe, or North America: a collaborative analysis of prospective studies. PLoS Med. 2014;11(9):e1001718. doi: 10.1371/journal.pmed.1001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornell M, Grimsrud A, Fairall L, et al. Temporal changes in programme outcomes among adult patients initiating antiretroviral therapy across South Africa, 2002-2007. AIDS. 2010;24:2263–70. doi: 10.1097/QAD.0b013e32833d45c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker AS, Prendergast AJ, Mugyenyi P, et al. Mortality in the year following anti-retroviral therapy initiation in HIV-infected adults and children in Uganda and Zimbabwe. Clin Infect Dis. 2012;55:1707–18. doi: 10.1093/cid/cis797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta A, Nadkarni G, Yang WT, et al. Early mortality in adults initiating anti-retroviral therapy (ART) in low- and middle-income countries (LMIC): a systematic review and meta-analysis. PLoS One. 2011;6(12):e28691. doi: 10.1371/journal.pone.0028691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ford N, Shubber Z, Meintjes G, et al. Causes of hospital admission among people living with HIV worldwide: a systematic review and meta-analysis. Lancet HIV. 2015;2(10):e438–e444. doi: 10.1016/S2352-3018(15)00137-X. [DOI] [PubMed] [Google Scholar]
- 10.Ford N, Matteelli A, Shubber Z, et al. TB as a cause of hospitalization and in-hospital mortality among people living with HIV worldwide: a systematic review and meta-analysis. J Int AIDS Soc. 2016;19:20714. doi: 10.7448/IAS.19.1.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta RK, Lucas SB, Fielding KL, Lawn SD. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS. 2015;29:1987–2002. doi: 10.1097/QAD.0000000000000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–30. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 13.Jarvis JN, Meintjes G, Williams A, Brown Y, Crede T, Harrison TS. Adult meningitis in a setting of high HIV and TB prevalence: findings from 4961 suspected cases. BMC Infect Dis. 2010;10:67. doi: 10.1186/1471-2334-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rangaka MX, Wilkinson RJ, Boulle A, et al. Isoniazid plus antiretroviral therapy to prevent tuberculosis: a randomised double-blind, placebo-controlled trial. Lancet. 2014;384:682–90. doi: 10.1016/S0140-6736(14)60162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. Geneva: World Health Organization; 2011. ( http://apps.who.int/iris/bitstream/10665/44472/1/9789241500708_eng.pdf) [Google Scholar]
- 16.Hosseinipour MC, Bisson GP, Miyahara S, et al. Empirical tuberculosis therapy versus isoniazid in adult outpatients with advanced HIV initiating antiretroviral therapy (REMEMBER): a multicountry open-label randomised controlled trial. Lancet. 2016;387:1198–209. doi: 10.1016/S0140-6736(16)00546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grant A, Charalambous S, Tlali M, et al. Empirical TB treatment in advanced HIV disease: results of the TB Fast Track Trial. Presented at the Conference on Retroviruses and Opportunistic Infections; Boston. February 22–25, 2016; abstract. [Google Scholar]
- 18.Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: World Health Organization; 2013. ( http://www.who.int/hiv/pub/guidelines/arv2013/download/en/index.html) [PubMed] [Google Scholar]
- 19.Division of AIDS table for grading the severity of adult and pediatric adverse events, version 1.0. 2004 Dec; ( https://rsc.tech-res.com/docs/default-source/safety/table_for_grading_severity_of_adult_pediatric_adverse_events.pdf?sfvrsn=6)
- 20.National Institutes of Health Division of Microbiology and Infectious Diseases (DMID) pediatric toxicity tables. 2007 Nov; ( https://www.niaid.nih.gov/sites/default/files/dmidpedtox.pdf)
- 21.Jelsma J, Hansen K, De Weerdt W, De Cock P, Kind P. How do Zimbabweans value health states? Popul Health Metr. 2003;1:11. doi: 10.1186/1478-7954-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ClinicalTrials.gov. Prevention of early mortality by presumptive tuberculosis (TB) treatment (PrOMPT) ClinicalTrials.gov. 2014 no. NCT01417988 ( https://clinicaltrials.gov/ct2/show/NCT01417988) [Google Scholar]
- 23.ClinicalTrials.gov. Systematic empirical vs. test-guided anti-TB treatment impact in severely immunosuppressed HIV-infected adults initiating ART with CD4 cell counts <100/mm3 (STATIS) ClinicalTrials.gov. 2015 no. NCT02057796. ( https://clinicaltrials.gov/ct2/show/NCT02057796) [Google Scholar]
- 24.Global tuberculosis report 2015. 20th ed. Geneva: World Health Organization; 2015. ( http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf?ua=1) [Google Scholar]
- 25.Gibb DM, Bwakura-Dangarembizi M, Abhyankar D, et al. Sulfamethoxazole/trimethoprim/isoniazid/pyridoxine scored tablets are bioequivalent to individual products and are acceptable to patients with advanced HIV infection in the REALITY trial. Presented at the 46th Union World Conference on Lung Health; Cape Town, South Africa. December 2–6, 2015. [Google Scholar]
- 26.Gordin F, Chaisson RE, Matts JP, et al. Rifampin and pyrazinamide vs isoniazid for prevention of tuberculosis in HIV-infected persons: an international randomized trial. JAMA. 2000;283:1445–50. doi: 10.1001/jama.283.11.1445. [DOI] [PubMed] [Google Scholar]
- 27.Mfinanga S, Chanda D, Kivuyo SL, et al. Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open-label, randomised controlled trial. Lancet. 2015;385:2173–82. doi: 10.1016/S0140-6736(15)60164-7. [DOI] [PubMed] [Google Scholar]
- 28.Parkes-Ratanshi R, Wakeham K, Levin J, et al. Primary prophylaxis of cryptococcal disease with fluconazole in HIV-positive Ugandan adults: a double-blind, randomised, placebo-controlled trial. Lancet Infect Dis. 2011;11:933–41. doi: 10.1016/S1473-3099(11)70245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amsden GW. Anti-inflammatory effects of macrolides — an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J Antimicrob Chemother. 2005;55:10–21. doi: 10.1093/jac/dkh519. [DOI] [PubMed] [Google Scholar]
- 30.Coles CL, Mabula K, Seidman JC, et al. Mass distribution of azithromycin for trachoma control is associated with increased risk of azithromycin-resistant Streptococcus pneumoniae carriage in young children 6 months after treatment. Clin Infect Dis. 2013;56:1519–26. doi: 10.1093/cid/cit137. [DOI] [PubMed] [Google Scholar]
- 31.Woods B, Revill P, Sculpher M, Claxton KP. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. CHE research paper 109. York, United Kingdom: University of York Centre for Health Economics; 2015. Mar, pp. 1–24. ( https://www.york.ac.uk/media/che/documents/papers/researchpapers/CHERP109_cost-effectiveness_threshold_LMICs.pdf) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.UNAIDS. Global AIDS response progress reporting 2015. Geneva: World Health Organization; 2015. ( http://www.unaids.org/sites/default/files/media_asset/JC2702_GARPR2015guidelines_en.pdf) [Google Scholar]
- 33.Ford N, Meintjes G, Pozniak A, et al. The future role of CD4 cell count for monitoring antiretroviral therapy. Lancet Infect Dis. 2015;15:241–7. doi: 10.1016/S1473-3099(14)70896-5. [DOI] [PubMed] [Google Scholar]
- 34.Paton NI, Kityo C, Hoppe A, et al. Assessment of second-line antiretroviral regimens for HIV therapy in Africa. N Engl J Med. 2014;371:234–47. doi: 10.1056/NEJMoa1311274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.