Abstract
Disordered sleep has been linked to impaired emotional functioning in healthy and depressed individuals (Bower et al, 2010; van der Helm & Walker, 2010). Little is known, however, about how chronic sleep problems influence emotional reactivity in everyday life. Participants with major or minor unipolar depressive disorder (n = 60) and healthy controls (n = 35) reported on sleep and emotional responses to daily life events using a computerized Experience Sampling Method. We examined whether impaired sleep quality influenced emotional reactivity to daily events, and if this relationship was altered by unipolar mood disorders. Among healthy individuals, sleep difficulties were associated with enhanced negative affect to unpleasant events and a dulled response to neutral events. However, among mood-disordered persons, sleep difficulties were associated with higher negative affect across all types of everyday life events. Impaired sleep quality differentially affects daily life emotional reactions as a function of depression.
Keywords: major depression, subjective sleep quality, ambulatory mood, disordered sleep, emotional reactivity
Disordered sleep has been associated with several psychological problems, including internalizing disorders (Touchette et al., 2012), suicidal thoughts and anhedonia (Urrila et al., 2012), and mood problems (Gujar, Yoo, Hu, & Walker, 2011). Although sleep difficulties can result from psychological symptoms, some evidence indicates that sleep problems can also contribute to the onset of psychological symptoms (Alvaro, Roberts, & Harris, 2013).
One reason sleep problems may contribute to psychological problems is that sleep is closely tied to affective functioning. Chronic poor sleep has been linked both to prevailing moods (Bower, Bylsma, Morris, & Rottenberg, 2010), and to acute emotional reactions (Durmer & Dinges, 2005). Additionally, poor sleep quality over the past week has been shown to impair emotion regulation (Mauss, Troy, & LeBourgeois, 2013). Mirroring these findings, acute sleep deprivation has also been shown to increase negative emotion (Durmer & Dinges, 2005; van der Helm & Walker, 2010). In certain populations (e.g., fibromyalgia), chronic poor sleep has also been shown to delay affective recovery from negative events (Hamilton et al., 2008).
Although laboratory designs predominate in the study of sleep and affect, sleep problems ultimately manifest in everyday life, as witnessed by the high social and economic burdens of sleep disorders (Kessler et al., 2011). Surprisingly, only recently has research used the Experience Sampling Method (ESM; or Ecological Momentary Assessment, Ebner-Priemer & Trull, 2009) to examine how sleep quality impacts everyday life emotion. ESM is advantageous because it documents unfolding real-life emotional reactions of participants. A previous study by our research group with the present sample assessed participants’ affect 10 times daily for 3 consecutive days and self-reported sleep quality among those with major depressive disorder (MDD), minor depression (mD), and healthy controls (Bower, Bylsma, Morris, & Rottenberg, 2010); in this sample, results indicated that poor reported sleep quality predicted lower daily positive affect across the groups. Other findings using ESM show poorer reported sleep quality to be associated with higher negative and lower positive affect (for a review, see Baglioni, Spiegelhalder, Lombardo, & Riemann, 2010).
We are, however, unaware of studies that utilize ESM to examine sleep and reactivity to specific types of daily life emotional events. Such relationships would be particularly important to examine in mood-disordered individuals, who are known to exhibit both sleep problems and altered emotional reactivity. Given that disordered sleep experiences are normative in depressed individuals, sleep may play a role in the blunted emotional reactivity that has been observed among mood-disordered individuals in the laboratory and in the field (Emotion Context Insensitivity; Rottenberg, 2005; Peeters, Nicolson, Berkhof, Delespaul, & deVriew, 2003).
Evidence on the influence of sleep on emotional reactivity in everyday life is indirect. For instance, sleep deprivation paradigms may model the effects of acute sleep loss (Talbot, McGlinchey, Kaplan, Dahl, & Harvey, 2010; Hamilton et al., 2008), but may not be informative about the effects of chronically poor sleep quality on emotional functioning. Moreover, results from deprivation studies have been conflicting (sleep deprivation enhances negative emotional reactivity: (Franzen, Buysse, Dahl, Thompson, & Siegle, 2009; Rosales-Lagarde et al., 2012; Prather, Bogdan, & Hariri, 2013); sleep deprivation reduces negative emotional reactivity: (Baran, Pace-Schott, Ericson, & Spencer, 2012; Schwarz et al., 2013). In sum, the existing literature does not offer strong guidance, with no study to date directly examining the relationship between chronic poor sleep quality and emotional reactivity.
Interestingly, among persons with mood disorders, acute sleep deprivation improves mood, at least temporarily (Kuhs & Tolle, 1991). This has been attributed to REM sleep suppression, which differs in depressed and healthy individuals (for a review, see Adrien, 2002). It is intriguing that acute sleep deprivation may have different effects on the emotional experiences of mood-disordered persons than that of healthy individuals. Sleep deprivation appears to render healthy individuals more sensitive to negative emotional events in particular; however, depression is characterized by deficits in REM-sleep, which has also been associated with enhanced stress response in general (Vandekerckhove & Cluydts, 2010). Overall, it is conceivable that poor sleep quality has different effects on everyday life emotional reactivity in mood disorders than it does in healthy individuals.
There are several ways of measuring sleep quality (e.g., actigraphy, self-report). The Pittsburgh Sleep Quality Index (PSQI) is a self-report measure that has been validated across populations and has been tied to sleep-relevant outcomes (e.g. disorders, Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). Additionally, several PSQI subscales (e.g., sleep duration, sleep efficiency) have been more strongly or uniquely associated with outcomes such as suicidality and positive affect (Agargun, Kara, & Solmaz, 1997; Bower et al., 2010) than the overall PSQI scale score. Indeed, several studies demonstrate the value of examining PSQI subscales (e.g. Nunes et al., 2008; Osorio, Gallinaro, Lorenzi-Filho, & Lage, 2006). Sleep duration, for example, might be expected to impact emotional reactivity given what we know from deprivation designs (e.g. Franzen et al., 2009; Schwarz et al., 2013). Likewise, sleep disturbances, which are common in depression (Medina, Lechuga, Escandón, & Moctezuma, 2014), are associated with poorer performances in emotional information processing (Soffer-Dudek, Sadeh, Dahl, & Rosenblat-Stein, 2011). Finally, sleep efficiency has been suggested as a proxy for REM sleep, which is associated with emotional information processing performance (Wagner, Hallschmid, Verleger, & Born, 2003; also common in MDD, Medina et al., 2014).
The present study examined how impaired sleep quality influences emotional reactivity in everyday life. Specifically, we investigated how problems with sleep quality influence emotional reactivity to everyday life events and whether these effects are influenced by the presence of a current mood disorder.
We sought to test two hypotheses.
Hypothesis 1
As sleep changes have historically been more tied to negative affect (for example, reactivity responses to negative, but not positive images; Franzen et al., 2011), we expected to find effects that were specific to negative emotional reactivity. To address specificity, we ran an overall model to test effects on negative and positive reactivity, and expected to find a three-way interaction between sleep, group, and type of event. To decompose this interaction, we predicted that significant two way interactions would be found only for negative reactivity to unpleasant events, and not for positive reactivity to pleasant events, consistent with our expectation that effects would be specific to negative reactivity (negative affect to unpleasant events).
Hypothesis 2
We predicted that healthy controls’ reactivity to everyday unpleasant events would be more enhanced by poor sleep quality than mood-disordered persons’. This prediction was based on initial evidence that depressed individuals sometimes have paradoxical responses to sleep loss (i.e., decreased negative mood), and on findings of an association between acute sleep deprivation in healthy persons and enhanced physiological reactivity (e.g. Hori et al., 2011), particularly to unpleasant stimuli (e.g. Rosales-Lagarde et al., 2012). Finally, chronic poor sleep quality has been associated with a higher intensity and more frequent report of negative affect (for review, see Baglioni, Spiegelhalder, Lombardo, & Riemann, 2010).
While the main hypotheses pertain to between-person differences (specifically, mood-disordered versus healthy control), follow-up analyses examined within-person differences within groups. These within-person analyses examined how worsening sleep problems were associated with everyday life reactivity within each group.
Method
Participants
Participants were recruited through advertisements placed throughout the Tampa community and were required to meet diagnostic criteria for current Major Depressive Disorder (MDD), current minor Depression (mD), or free of an Axis I diagnosis using DSM-IV criteria. Those with an mD diagnosis with past MDD were included if they had a period of > 8 weeks with no residual depression symptoms between the past episode and onset of current minor depressive episode (see Bylsma, Taylor-Clift, & Rottenberg, 2011 for details about recruiting procedures). Phone screens determined preliminary eligibility and screened out psychosis, bipolar mood conditions, and current substance abuse. Out of 502 screened, 271 met criteria to come into the lab; of those, 164 were assessed with the full Structured Clinical Interview for DSM-IV-TR for Axis 1 Disorders (SCID; First, Spitzer, Gibbon, & Williams, 2002). One hundred and seven of these interviewed individuals were invited to participate. Because of drop out and data lost to technical problems, the final sample size was 96 individuals: those with a current MDD episode (n = 35), a current mD episode (n = 25) or no history of psychopathology (n = 36). Groups were matched on age, ethnicity, gender, education level, income, and marital status.
ESM Procedure
Participants carried a Palm Pilot (Palm Z22) with the Experience Sampling Program (Barrett & Feldman Barrett, 2004), on 3 consecutive weekdays: Tuesday, Wednesday and Thursday. After practicing with the Palm to ensure accuracy and comprehension, participants reported throughout the day about their activities, affect, and emotional events when the Palm Pilots alarmed pseudo-randomly (10 times a day, roughly every 1.5 hours with 15 minutes to respond for each; see Bylsma et al., 2011 and Bower et al., 2010 for more procedural details). Compliance with ESM procedures was adequate and similar to other protocols with a rate of 65% (see Bower et al., 2010; Bylsma et al., 2011 for discussion).
Measures
Event appraisals
Event Type ratings were derived from participants’ cognitive appraisals. The pleasantness and unpleasantness of events were rated on two sliding scales that ranged from 1 to 100 for each event. Up to 30 events were rated, leading to a total of 1,903 event episodes. An event was coded pleasant or unpleasant if the event was rated > 80 on the corresponding scale (see Peeters et al., 2003; Bylsma et al., 2011, for procedural details). For analyses, events were initially dichotomized (e.g. unpleasant versus non-unpleasant). To test Event Type within the first hypothesis, a three-category “Event Type” variable was created. Pleasant events were coded as events that were rated high on pleasantness and low on unpleasantness; Unpleasant events were coded as rated as high on unpleasantness and low on pleasantness. Neutral events were events not rated high on pleasantness or unpleasantness.
Ambulatory affect (PA and NA)
Current positive (PA) and negative (NA) affect and events were reported up to 10 times a day for 3 days. For each measurement episode, participants were asked to rate on a sliding scale from 1 ‘not at all’ to 100 ‘very’ 14 different mood adjectives: 7 positive (talkative, enthusiastic, confident, cheerful, energetic, satisfied, and happy) and 7 negative (tense, anxious, distracted, restless, irritated, depressed, and guilty). The composite scores for both PA and NA were calculated using the sum scores of the mood ratings for the seven positive or negative adjectives, respectively. We chose those terms to be comparable to those used in successful previous ESM studies (e.g. Peeters et al., 2003) and have already been found to be reliable for NA (α = .94) and PA (α = .97) (Bylsma et al., 2011; due to criticisms of reliabilities in a multilevel context, reliability was calculated using a multilevel approach, specifically person-level reliability estimates; Nezlek, 2007). Emotional reactivity within this model is defined as affect ratings that are tied to a specific event (e.g. negative affect to an unpleasant event). To connect to previous emotional reactivity literature, we utilized the same operational definitions as Bylsma (2011). Emotional reactivity to negative and positive events was defined as NA and PA in response to negative and positive daily events, respectively.
Sleep quality
The Pittsburgh Sleep Quality Index (PSQI) is considered to be the strongest self-report instrument for evaluating sleep quality (Buysse et al., 1989). The measure is composed of facets of sleep problems within the last month that can be evaluated separately, or combined for an overall sleep quality score (α = .74 in our sample). The PSQI contains seven widely used subscales (Nunes et al., 2008; Osorio et al., 2006): subjective sleep quality, sleep duration, sleep latency, sleep efficiency, sleep disturbances, daytime dysfunctions, and medicine for sleep. Based on previous findings, we focused on three subscales: sleep duration, sleep efficiency, and sleep disturbances. Sleep duration was simply the reported average time asleep; sleep efficiency was the ratio of time spent asleep versus time spent in bed; sleep disturbances referred to the frequency of disruptions during the night. For all subscales, higher scores (from 0 to 3) indicated worse sleep.
Hypothesis Testing
The nested data for the primary analyses was analyzed with multi-level modeling and the pleasant, unpleasant, and neutral events were analyzed separately for two-way interactions (Bylsma et al., 2011). Missing data was handled, as is the default for mixed models, using maximum likelihood estimates (McCulloch & Neuhaus, 2001). The Level 1 model was as follows:
In the Level 1 model, event type was either pleasant, unpleasant or neutral events. Y denotes the outcome of affect, i the prompt, and j the individual.
The Level 2 model was as follows:
For the Level 2 models, we evaluated the effect of depressive group status, sleep quality subscales (sleep duration, sleep efficiency, and sleep disturbances in separate models) and their interaction as predictors of daily positive and negative emotional reactivity (controlling for affect during the previous event as per Thompson et al., 2012; not shown in above equations). To test Hypothesis 1 (specificity), we created a model with a three-way interaction term with sleep, group, and event type (utilizing three levels of event type: unpleasant, pleasant, and neutral), with NA and PA as dependent variables. To test Hypothesis 2, we ran further full models testing the influence of group and sleep on reactivity to unpleasant versus neutral events. To aid in interpretation, we broke down significant sleep * reactivity interactions within each diagnostic group separately.
Results
Preliminary Analyses
The MDD and mD groups did not differ on overall positive or negative affect or on negative emotional reactivity to pleasant events (as reported in Bylsma et al, 2011). Since there were no differences in ambulatory positive or negative affect, the MDD’s and mD’s were analyzed together as a single mood-disordered group to maximize power. While sleep quality was unrelated to demographic variables, mood-disordered individuals reported worse sleep quality overall than healthy controls (sample demographic characteristics are listed by diagnostic group in Table 1).
Table 1.
Descriptive Statistics of the Sample
| Characteristic | Mood-disordered (n = 60) | Controls (n = 36) | P-Value |
|---|---|---|---|
| Age in years (SD) | 28.50 (9.32) | 28.45 (8.73) | .57 |
| Caucasian (%) | 64.1 | 50.8 | .91 |
| Female (%) | 78 | 79.2 | .70 |
| Income (SD) | 5.14 (3.08) | 6.80 (3.58) | .30 |
| BDI-II (SD) | 26.86 (9.84) | 2.91 (4.36) | < .01 |
| BAI (SD) | 16.43 (9.64) | 1.76 (1.83) | < .01 |
| PSQI (0–21) (SD) | 9.11 (3.70) | 4.62 (2.06) | < .01 |
| Sleep Disturbances (0–3) (SD) | 1.39 (.56) | 1.10 (.48) | <. 01 |
| Sleep Efficiency (0–3) (SD) | .71 (.92) | .43 (.69) | .07 |
| PA (SD) | 15.65 (7.86) | 24.84 (8.37) | < .01 |
| NA (SD) | 21.59 (7.90) | 7.73 (6.51) | < .01 |
BDI-II = Beck Depression Inventory II; BAI = Beck Anxiety Inventory; PSQI = Pittsburgh Sleep Quality Index; PA = Positive Affect; NA = Negative Affect
Hypothesis 1: Sleep and Emotional Reactivity
Consistent with our expectation of specificity, we found a three-way interaction between sleep, event type, and group was significant for NA (sleep efficiency: B = −3.19, p < .001; sleep disturbances: B = −2.14, p < .001) and PA (sleep efficiency: B = 2.60, p < .001; sleep disturbances: B = 2.46, p < .001). However, the follow up analysis of positive reactivity, defined as PA reactivity to positive everyday life events, revealed no interactions between sleep and group (all p’s > .05). By contrast, results for two-way interactions in negative emotional reactivity are displayed below. Thus, all of our observed sleep effects were specific to negative reactivity.
Hypothesis 2: Sleep and Negative Emotional Reactivity
Sleep Efficiency
Parameter estimates for the models testing hypothesis 2 are shown in Table 2. In these two models, sleep efficiency was the main predictor variable and also interacted with group status for both unpleasant and neutral events. For the unpleasant event model, the main effect of group was significant (p<.001), while sleep efficiency was not a significant main effect predictor of negative emotion (p>.05). In the neutral event model, the main effect of group was significant (p<.001), but there was no main effects for sleep efficiency (p>.05). Because of the higher order sleep efficiency by group interactions, main effects are reported, but not interpreted.
Table 2.
Summary of Multilevel Models on Negative Affect with Sleep Efficiency
| Unpleasant | Neutral | |||||
|---|---|---|---|---|---|---|
| B | SE | p | B | SE | p | |
| Intercept | 6.11 | .82 | <.001 | 5.96 | .84 | <.001 |
| Group | 9.84 | 1.03 | <.001 | 9.89 | 1.03 | <.001 |
| Sleep Efficiency | −1.54 | 1.12 | n.s. | −1.77 | 1.10 | n.s. |
| Group*Sleep Efficiency | 3.15 | 1.28 | <.05 | 3.04 | 1.28 | <.05 |
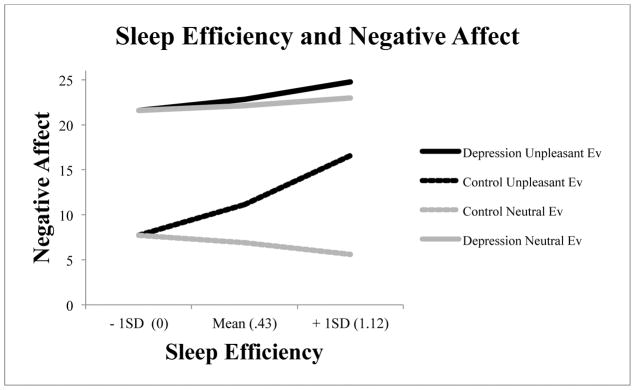
To follow up the significant sleep efficiency by diagnostic group interactions for unpleasant emotion, we ran models for each group individually; model estimates are displayed in Figure 1. In order to unpack the significant group by sleep efficiency interaction to unpleasant events alone, we estimated the within-group impact of sleep efficiency on reactivity for both neutral and unpleasant events. Worse sleep efficiency was associated with enhanced negative emotional reactivity to unpleasant events for both controls (B = 7.88, 95% CI [−3.64 – 19.40]) and for mood-disordered individuals (B = 2.82, 95% CI [1.09 – 4.55]). For visual interpretability, an inspection of the plots shows that, for controls, a two standard deviation change in sleep efficiency was associated with a 15-point increase in negative emotional reactivity. By contrast, in mood-disordered individuals, a two standard deviation change in sleep efficiency was associated with only a 5-point increase in negative emotional reactivity.
Figure 1.
The context-dependent effect of group and sleep efficiency on negative reactivity (higher scores indicate worse sleep, scores limited to 0 minimum for practicality).
For neutral events, a different patterns of results emerged. Impairments in sleep efficiency for mood-disordered individuals were similar across neutral and unpleasant events (B =1.25, 95% CI [−.15 – 2.64] for neutral events and B = 2.82, 95% CI [1.09 – 4.55] for unpleasant events as stated above). By contrast, impairments in sleep efficiency for healthy controls had different effects on reported negative affect for neutral events (B = −1.90, 95% CI [−3.88 – .08]) versus unpleasant events (B = 7.88, 95% CI [−3.64 – 19.40]). In essence, healthy controls with worse sleep efficiency reported decreased negative emotional reactivity to neutral events, but increased negative emotional reactivity to unpleasant events. In other words, among healthy subjects, impaired sleep efficiency was associated with sensitized reactions to unpleasant events and dulled reactions to neutral events. When considering sleep efficiency and reactivity to daily life, event appraisal mattered for healthy individuals, but not for the mood-disordered.
Sleep Disturbances
To test Hypothesis 2, group by sleep interaction models were entered into both models (see Table 3); sleep disturbances were used as the sleep variable as described above. As the sleep disturbances by group interaction was significant, main effects were reported, but not interpreted. For the unpleasant event model, the main effects of group and sleep disturbances were significant (p’s<. 01). In the neutral event model, the main effects of group and sleep disturbances were also significant (p<. 01).
Table 3.
Summary of Multilevel Models on Negative Affect with Sleep Disturbances
| Unpleasant | Neutral | |||||
|---|---|---|---|---|---|---|
| B | SE | p | B | SE | p | |
| Intercept | 5.47*** | .84 | <.001 | 5.33*** | .87 | <.001 |
| Group | 10.38*** | 1.05 | <.001 | 10.32*** | 1.04 | <.001 |
| Sleep Disturbances | −3.95** | 1.48 | <.01 | −4.09** | 1.47 | <.01 |
| Group*Sleep Disturbances | 6.19*** | 1.79 | <.001 | 6.40*** | 1.78 | <.001 |
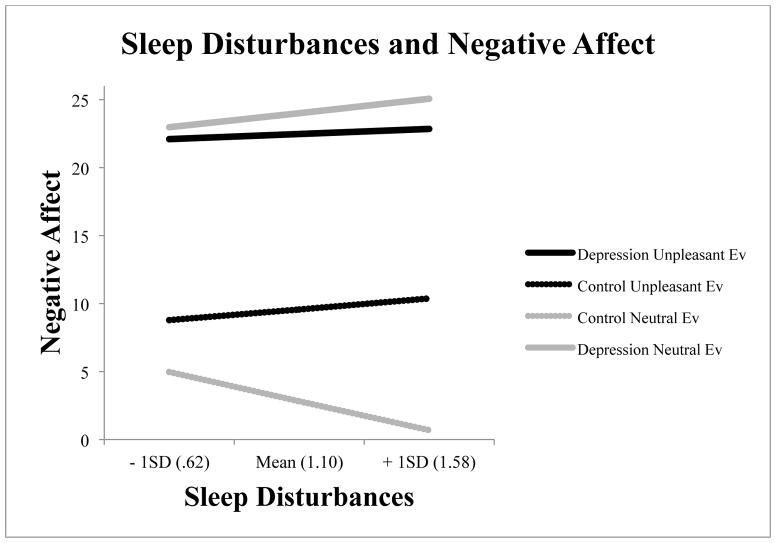
For both neutral and unpleasant events, sleep disturbances interacted with diagnostic group when predicting negative reactivity (see Table 3). To provide a visual display of the data, we ran models in each group individually, with model estimates displayed in Figure 2. To unpack the significant group by sleep disturbances interaction to unpleasant events, we separately modeled the impact of sleep disturbances on reactivity for both neutral and unpleasant events.
Figure 2.
The context-dependent effect of group and sleep disturbances on negative reactivity.
For healthy controls and mood-disordered individuals sleep disturbances were associated with enhanced negative emotional reactivity to unpleasant events. As sleep disturbances increased, both control and mood-disordered groups exhibited a similar increase in negative affect to unpleasant everyday life events (controls at B = 1.68, 95% CI [−5.98 – 9.33] and for mood-disordered at B = .80, 95% CI [−2.27 – 3.87]). Although we could not compare slopes directly, among controls, a two standard deviation increase in sleep disturbance was associated with a 4 point increase in negative emotional reactivity; for mood-disordered individuals, a two standard deviation increase was associated with only a 2 point increase in negative emotional reactivity. As sleep disturbances increased, both healthy controls and mood-disordered individuals exhibited sensitized reactions to unpleasant events.
Likewise for sleep disturbances, different events had different effects on negative emotional reactivity for the two groups. For the mood-disordered group, sleep disturbances had similar effects on negative emotional reactivity to unpleasant events (B = .80, 95% CI [−2.27 – 3.87]) and to neutral events (B =2.20, 95% CI [−.10 – 4.49]). In controls, greater sleep disturbances were associated with sensitized reactivity to unpleasant events (B = 1.68, 95% CI [−5.98 – 9.33], but diminished negative emotional reactivity to neutral events (B =−4.46, 95% CI [−6.87 – −2.05]). In healthy controls, sleep disturbances, like sleep efficiency, were associated with sensitized reactions to unpleasant events, but diminished reactions to neutral events; mood-disordered individuals did not display this event-contingent sensitized response.
Sleep duration
Sleep duration did not interact with group, nor were there any main effects of sleep on negative reactivity (all p’s>.05).
Discussion
Disordered sleep often impairs emotional functioning in healthy and depressed individuals (Bower et al, 2010; van der Helm & Walker, 2010), yet we know little concerning how chronic sleep problems influence emotional reactivity in everyday life. This study was the first to test whether mood disorders alter relationships between disordered sleep and emotional reactivity to everyday life events. Results indicated that, indeed, impairments in sleep quality had different effects on the everyday life reactivity of healthy persons than they do for mood-disordered persons. Specifically, among healthy individuals, sleep difficulties were associated with enhanced negative affect in response to unpleasant events and a dulled response to neutral events. However, among mood disordered persons sleep difficulties were associated with high negative affect to both negative and neutral types of everyday life events. In other words, poor sleep quality in healthy individuals prompted emotional reactions contingent on the environmental stimulus – sensitivity to unpleasant events in particular; by contrast, poor sleep quality in the mood-disordered group was associated with nonspecific, heightened negative emotion.
While this study does not establish the responsible mechanisms for our findings, the present results are interpretable in light of previous knowledge. Differences in REM sleep, for example, are implicated in many psychiatric disorders, including depression (Buysse et al., 2004). The impairments in brain activity associated with emotion and behavior following sleep disruptions are complex and potentially differentially affected in major depression (Goldstein & Walker, 2014). In fact, it has been argued that nonspecific reactivity to negative, neutral, and positive images are a byproduct of depression-related REM sleep differences (Ritchey, Dolcos, Eddington, Strauman, & Cabeza, 2011; Goldstein & Walker, 2014). By contrast, brain-imaging data has shown that healthy individuals with worse sleep show enhanced neurological responses to negative stimuli in the amygdala (Yoo, Gujar, Hu, Jolesz, & Walker, 2007), a result consistent with what was observed in healthy persons.
Within our data, effects were unique to negative emotional reactivity. This fits with prior results (Franzen et al., 2009), as other researchers have noted the special impact that disordered sleep has on negative emotions (Hamilton et al., 2008). Although power in our study was adequate for determining medium effects, it is still nevertheless possible that smaller effects for positive emotion were not detected in our design.
Our results show that specific aspects of chronic sleep disturbance (as opposed to acute sleep disturbance) impact emotional reactivity: while sleep duration did not significantly interact with group to predict negative emotional reactivity, interactions were repeatedly found with sleep efficiency and sleep disturbances. Findings for sleep efficiency and sleep disturbances suggest that problematic events during sleeping hours may be more important than the simple duration of sleep time, also a recent theme in emotion research (e.g. Soffer-Dudek, 2011). Sleep disturbances, for example, may uniquely impair REM sleep with direct consequences for emotional reactivity. It is also noteworthy that post-hoc analyses revealed that overall sleep quality did not significantly interact with depression to affect negative emotional reactivity (p = .13), reinforcing the idea that specific sleep characteristics may have more predictive power than global indices (e.g. Nunes et al., 2008).
Limitations
Although this report extends our knowledge of sleep and depression, our study was not without limitations. First, our design was cross-sectional, which limits our ability to make definitive statements about the direction of association between sleep and emotional reactivity. Our preferred interpretation, that sleep quality predicts emotional disturbances, is guided by previous literature (i.e. Alvaro et al., 2013). At the same time, we cannot rule out that daily life events are impacting sleep characteristics, given evidence for bidirectional relationships between sleep and emotion (Kahn et al., 2013). Sleep quality as a construct was assessed solely via self-report, which may yield different results than objective measures of sleep (e.g., Alfano, Patriquin, & De Los Reyes, 2015). Moreover, in our design, self-reported sleep quality for the previous month was assessed only once during the lab session, while affect was assessed moment to moment throughout the course of three days following the lab session. Clearly, it would be fruitful in future work to examine whether these results hold when sleep is measured in other ways (e.g. on a night-by-night basis).
Additionally, clinical populations can raise concerns about floor and ceiling effects on sleep measures (with controls showing better sleep quality on average compared to the mood disordered group; Bower et al., 2010; Buysse et al., 1989). Mitigating this concern, the groups did not differ in their variance on any subscale sleep dimensions. It should also be noted that the mood-disordered group included those with both major and minor depression. Though there were no obvious differences between these groups, larger sample sizes are needed to address this issue conclusively. Finally, although sleep disorders are common in depression (Buysse, 2004), we did not formally assess sleep disorders. It may be that those with sleep disorders respond similarly to those in the mood-disordered group (given the high co-occurrence between sleep disorders and depression, Buysse, 2004); future work is needed to illuminate the relationship between sleep disorders and everyday life emotional reactivity.
Regardless, this study represents an important first step in the field in demonstrating how sleep quality influences everyday emotional functioning. Our results suggest that the quality of nighttime sleep plays a critical role in response to normal daytime events and that the effects of sleep differ as a function of whether a mood disorder is present.
Acknowledgments
The authors thank the members of the Mood and Emotion Laboratory for feedback on the ideas discussed in this article. Jonathan Rottenberg was supported by National Institute of Mental Health Grant MH77669.
Footnotes
The authors declare no conflicts of interest.
References
- Adrien J. Neurobiological bases for the relation between sleep and depression. Sleep Medicine Review. 2002;6:341–351. [PubMed] [Google Scholar]
- Aĝargün M, Kara H, Solmaz M. Subjective sleep quality and suicidality in patients with major depression. Journal of Psychiatric Research. 1997;31:377–381. doi: 10.1016/S0022-3956(96)00037-4. [DOI] [PubMed] [Google Scholar]
- Alfano CA, Patriquin MA, De Los Reyes A. Subjective–Objective Sleep Comparisons and Discrepancies Among Clinically-Anxious and Healthy Children. Journal of Abnormal Child Psychology. 2015:1–11. doi: 10.1007/s10802-015-0018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvaro PK, Roberts RM, Harris JK. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep: Journal of Sleep and Sleep Disorders Research. 2013;36:1059–1068. doi: 10.5665/sleep.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglioni C, Spiegelhalder K, Lombardo C, Riemann D. Sleep and emotions: A focus on insomnia. Sleep Medicine Reviews. 2010;14:227–238. doi: 10.1016/j.smrv.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Baran B, Pace-Schott EF, Ericson C, Spencer RC. Processing of emotional reactivity and emotional memory over sleep. The Journal of Neuroscience. 2012;32:1035–1042. doi: 10.1523/JNEUROSCI.2532-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett DJ, Feldman Barrett L. The Experience –Sampling Program (ESP) 2004 Available: ( http://www.experience-sampling.org/esp/)
- Bower B, Bylsma LM, Morris BH, Rottenberg J. Poor reported sleep quality predicts low positive affect in daily life among healthy and mood-disordered persons. Journal of Sleep Research. 2010;19:323–332. doi: 10.1111/j.1365-2869.2009.00816. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index (PSQI): A new instrument for psychiatric research and practice. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Buysse DJ. Insomnia, depression and aging. Assessing sleep and mood interactions in older adults. Geriatrics. 2004;59:47–51. [PubMed] [Google Scholar]
- Bylsma LM, Taylor-Clift A, Rottenberg J. Emotional reactivity to daily events in major and minor depression. Journal of Abnormal Psychology. 2011;120:155–167. doi: 10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Durmer JS, Dinges DW. Neurocognitive consequences of sleep deprivation. Seminars in Neurology. 2005;21:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Ebner-Priemer UW, Trull TJ. Ecological momentary assessment of mood disorders and mood dysregulation. Psychological Assessment. 2009;21:463. doi: 10.1037/a0017075. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Franzen PL, Buysse DJ, Dahl RE, Thompson W, Siegle GJ. Sleep deprivation alters pupillary reactivity to emotional stimuli in healthy young adults. Biological Psychology. 2009;80:300–305. doi: 10.1016/j.biopsycho.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AN, Walker MP. The role of sleep in emotional brain function. Annual Review of Clinical Psychology. 2014;10:679–708. doi: 10.1146/annurev-clinpsy-032813-153716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujar N, Yoo S, Hu P, Walker MP. Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. The Journal of Neuroscience. 2011;31:4466–4474. doi: 10.1523/JNEUROSCI.3220-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NA, Affleck G, Tennen H, Karlson C, Luxton D, Preacher KJ, Templin JL. Fibromyalgia: The role of sleep in affect and in negative event reactivity and recovery. Health Psychology. 2008;27:490. doi: 10.1037/0278-6133.27.4.490. [DOI] [PubMed] [Google Scholar]
- Hori H, Teraishi T, Sasayama D, Ozeki Y, Matsuo J, Kawamoto Y, … Kunugi H. Poor sleep is associated with exaggerated cortisol response to the combined dexamethasone/CRH test in a non-clinical population. Journal of psychiatric research. 2011;45:1257–1263. doi: 10.1016/j.jpsychires.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Kahn M, Sheppes G, Sadeh A. Sleep and emotions: Bidirectional links and underlying mechanisms. International Journal of Psychophysiology. 2013;89:218–228. doi: 10.1016/j.ijpsycho.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund PA, Coulouvrat C, et al. Insomnia and the performance of US workers: results from the America Insomnia Survey. Sleep. 2011;34:1161–1171. doi: 10.5665/SLEEP.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhs H, Tölle RR. Sleep deprivation therapy. Biological Psychiatry. 1991;29:1129–1148. doi: 10.1016/0006-3223(91)90255-K. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Troy AS, LeBourgeois MK. Poorer sleep quality is associated with lower emotion-regulation ability in a laboratory paradigm. Cognition & Emotion. 2013;27:567–576. doi: 10.1080/02699931.2012.727783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch CE, Neuhaus JM. Generalized linear mixed models. John Wiley & Sons, Ltd; 2001. [Google Scholar]
- Medina AB, Lechuga DA, Escandón OS, Moctezuma JV. Update of sleep alterations in depression. Sleep Science. 2014 doi: 10.1016/j.slsci.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezlek JB. Multilevel modeling in research on personality. In: Robins R, Fraley RC, Krueger R, editors. Handbook of research methods in personality psychology. New York, NY: Guilford Press; 2007. pp. 502–523. [Google Scholar]
- Nunes DM, Mota RS, Machado MO, Pereira EB, de Bruin VS, de Bruin PC. Effect of melatonin administration on subjective sleep quality in chronic obstructive pulmonary disease. Brazilian Journal of Medical and Biological Research. 2008;41:926–931. doi: 10.1590/S0100-879X2008001000016. [DOI] [PubMed] [Google Scholar]
- Osorio CD, Gallinaro AL, Lorenzi-Filho G, Lage LV. Sleep Quality in patients with fibromyalgia using the Pittsburgh Sleep Quality Index. Journal of Rheumatology. 2006;33:1863–5. [PubMed] [Google Scholar]
- Peeters F, Nicolson NA, Berkhof J, Delespaul P, deVriew M. Effects of daily events on mood states in major depressive disorder. Journal of Abnormal Psychology. 2003;112:203–211. doi: 10.1037/0021-843x.112.2.203. [DOI] [PubMed] [Google Scholar]
- Prather AA, Bogdan R, Hariri AR. Impact of sleep quality on amygdala reactivity, negative affect, and perceived stress. Psychosomatic Medicine. 2013;75:350–358. doi: 10.1097/PSY.0b013e31828ef15b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M, Dolcos F, Eddington KM, Strauman TJ, Cabeza R. Neural correlates of emotional processing in depression: changes with cognitive behavioral therapy and predictors of treatment response. Journal of Psychiatric Research. 2011;45:577–87. doi: 10.1016/j.jpsychires.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales-Lagarde A, Armony JL, del Río-Portilla Y, Trejo-Martínez D, Conde R, Corsi-Cabrera M. Enhanced emotional reactivity after selective REM sleep deprivation in humans: An fMRI study. Frontiers in Behavioral Neuroscience. 2012;6 doi: 10.3389/fnbeh.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg J. Mood and emotion in major depression. Current Directions in Psychological Science. 2005;14:167–170. [Google Scholar]
- Schwarz JA, Popp R, Haas J, Zulley J, Geisler P, Alpers GW, … Eisenbarth H. Shortened night sleep impairs facial responsiveness to emotional stimuli. Biological Psychology. 2013;93:41–44. doi: 10.1016/j.biopsycho.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Soffer-Dudek N, Sadeh A, Dahl RE, Rosenblat-Stein S. Poor sleep quality predicts deficient emotion information processing over time in early adolescence. Sleep: Journal of Sleep and Sleep Disorders Research. 2011;34:1499–1508. doi: 10.5665/sleep.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot LS, McGlinchey EL, Kaplan KA, Dahl RE, Harvey AG. Sleep deprivation in adolescents and adults: Changes in affect. Emotion. 2010;10:831. doi: 10.1037/a0020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RJ, Mata J, Jaeggi SM, Buschkuehl M, Jonides J, Gotlib IH. The everyday emotional experience of adults with major depressive disorder: Examining emotional instability, inertia, and reactivity. Journal of Abnormal Psychology. 2012;121:819–829. doi: 10.1037/a0027978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touchette E, Chollet A, Galéra C, Fombonne E, Falissard B, Boivin M, Melchior M. Prior sleep problems predict internalizing problems later in life. Journal of Affective Disorders. 2012;143:166–171. doi: 10.1016/j.jad.2012.05.049. [DOI] [PubMed] [Google Scholar]
- Urrila AS, Karlsson L, Kiviruusu O, Pelkonen M, Strandholm T, Marttunen M. Sleep complaints among adolescent outpatients with major depressive disorder. Sleep Medicine. 2012;13:816–823. doi: 10.1016/j.sleep.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove M, Cluydts R. The emotional brain and sleep: an intimate relationship. Sleep Medicine Review. 2010;14:219–226. doi: 10.1016/j.smrv.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Van der Helm E, Walker MP. The role of sleep in emotional brain regulation. In: Kring AM, Sloan DM, editors. Emotion regulation and psychopathology: A transdiagnostic approach to etiology and treatment. New York, NY US: Guilford Press; 2010. pp. 253–279. [Google Scholar]
- Wagner U, Hallschmid M, Verleger R, Born J. Signs of REM sleep dependent enhancement of implicit face memory: A repetition priming study. Biological Psychology. 2003;62:197–210. doi: 10.1016/S0301-0511(02)00125-4. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep – a prefrontal amygdala disconnect. Current Biology. 2007;17:877–878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]