Abstract
Research has demonstrated that humans detect threatening stimuli more rapidly than nonthreatening stimuli. Although the literature presumes that biases for threat should be normative, present early in development, evident across multiple forms of threat, and stable across individuals, developmental work in this area is limited. Here, we examine the developmental differences in infants’ (4- to 24-month-olds) attention to social (angry faces) and nonsocial (snakes) threats using a new age-appropriate dot-probe task. In Experiment 1, infants’ first fixations were more often to snakes than to frogs, and they were faster to fixate probes that appeared in place of snakes vs. frogs. There were no significant age differences, suggesting that a perceptual bias for snakes is present early in life and stable across infancy. In Experiment 2, infants fixated probes more quickly after viewing any trials that contained an angry face compared to trials that contained a happy face. Further, there were age-related changes in infants’ responses to face stimuli, with a general increase in looking time to faces before the probe and an increase in latency to fixate the probe after seeing angry faces. Together, this work suggests that different developmental mechanisms may be responsible for attentional biases for social vs. nonsocial threats.
The ability to recognize and detect threatening stimuli has been of interest to researchers for decades. Countless studies have now shown that adult humans and nonhuman primates detect threat-relevant stimuli such as snakes, spiders, and angry faces more quickly than benign control displays (see LoBue & Rakison, 2013; for a review). Researchers have suggested that attentional biases for threat are the result of dedicated brain circuitry activated automatically in the presence of threat (Öhman & Mineka, 2001) and that they are normative, present early in development, and stable across individuals.
Despite these assumptions about development, there is little work on attentional biases for threat in infants and young children. Developmental research on this topic showed that preschool-aged children detect snakes, spiders, and angry faces more quickly than a variety of control stimuli in visual search tasks (LoBue, 2009, 2010a; LoBue & DeLoache, 2008, 2011). Further, two studies on attentional biases to threat in infants have demonstrated that 9- to 12-month-olds turn more quickly to look at images of snakes vs. flowers and angry faces vs. happy faces (LoBue & DeLoache, 2009) and that 5-month-olds look longer at schematic images of spiders than at scrambled versions of the same stimuli (Rakison & Derringer, 2008).
These data provide support for the widely cited and broadly accepted view that humans have a normative visual bias for the detection of threatening stimuli. However, other developmental work suggests that this account is incomplete. Empirical work with both adults and children indicates that visual biases for threatening stimuli can be learned. For example, while adults demonstrate a bias for various modern threats such as syringes and knives (Blanchette, 2006; Brosch & Sharma, 2005), preschool-aged children only demonstrate a bias for those modern threats with which they have had previous negative experience (e.g., syringes) (LoBue, 2010b). Further, 8- and 9-year-old children can develop a visual bias for novel animals after being taught to pair these animals with fearful faces (Reynolds, Field, & Askew, 2014). Research with children of various temperaments has also shown that there are individual differences in attentional biases for social threats (e.g., angry faces) that are first evident between the ages of 2 and 5 (LoBue & Pérez-Edgar, 2014; Pérez-Edgar et al., 2010, 2011).
Unfortunately, no research to date has attempted to document the developmental trajectory of humans’ attention to threat-relevant stimuli over the course of infancy. As such, the extent to which such biases develop and become differentiated over time is still largely unknown. Given the intermingling of normative patterns of attention, individual differences in attention linked to specific traits, and the role of experience- and exposure-dependent learning, it is likely that trajectories of bias may differ across categories of threat-relevant stimuli (Field & Lester, 2010).
Although preschoolers demonstrate a bias for both nonsocial threats, such as snakes and spiders, and social threats, such as angry faces, these stimuli differ on a number of dimensions. For example, while infants acquire a great deal of experience with emotional facial expressions in the first year of life, it is unlikely that they gain the same experience with nonsocial stimuli such as snakes and spiders. Based on these differences, infants’ responses to snakes might be driven by basic perceptual properties of the stimuli (e.g., LoBue, 2014; LoBue & DeLoache, 2011), while their responses to threatening faces might be attributed to a growing understanding of the emotional valence of the stimuli (Leppänen & Nelson, 2006). Thus, it is possible that there are different trajectories for the development of attentional biases for social threats and nonsocial threats.
In the current research, our goal was to document developmental differences in infants’ attention to both social and nonsocial threats using a new age-appropriate variant of a standard attention bias task extensively used in the literature with adults and older children (Roy, Dennis, & Warner, 2015). Our “baby dot-probe” task employs eye-tracking technology to capture attention bias patterns typically assessed through behavioral reaction time (RT) responses. Compared to reaction time methods used in previous research, eye tracking has the advantage of allowing us to capture precise visual fixations to various threatening stimuli and to do so with infants as young as 4 months of age. In two experiments, 4- to 24-month-old infants were presented with two images side by side on a screen—one threatening and one nonthreatening—for a very brief period of time (1000 ms), followed by a probe (an asterisk) that appeared in the location of one of the two images. We used a desk-mounted eye tracker to measure infants’ overall attention to threatening stimuli before the probe (i.e., dwell time to each stimulus), and their response before and after the onset of the probe (i.e., the number of first fixations to each stimulus, latency to first fixate the probe).
In Experiment 1, we examined infants’ responses when probes replaced a nonsocial threat (snake) compared to probes that replaced a neutral distracter (frog). In Experiment 2, we examined infants’ responses when probes replaced a social threat (angry faces) vs. a social nonthreat (happy faces) compared to probes that replaced a neutral (neutral face) distracter. Consistent with previous work, we expected to find evidence of a bias in attention for both types of threatening stimuli by at least 9 months of age (e.g., LoBue & DeLoache, 2009). However, we used a broad age range to capture the potential developmental differences between the two types of threatening stimuli in both younger and older infants.
EXPERIMENT 1—NONSOCIAL THREAT
Method
Participants
Participants in this study (N = 55, 31 males, Meanage = 13.5 months; SDage = 5.8, Rangeage = 4.0 to 23.8 months) were recruited via mailings sent to parents identified in a university-based database of families interested in research, as well as community advertisement, as part of a larger multiphase study. The local Institutional Review Board (IRB) approved all methods, and parents gave written consent for their child’s participation. Parents were compensated with $50, and infants were given a t-shirt.
The initial sample (N = 203) was predominantly Caucasian (88.7%), reflecting the surrounding semirural community. The remaining 11.3% of families self-identified as Asian American, African American, Native American, or Hispanic. All families reported that English was spoken at home, while 16 infants were also exposed to a second language. All but two children were living with a biological parent. Infants were born within 3 weeks of their due date, had no major birth complications, and had adequate birth weight (Meanweight = 7.63 lbs, SDweight = 0.99). Families reported that infants were meeting motor milestones (e.g., rolling over, crawling, and walking) within the normal developmental windows.
Of the 203 infants enrolled in the larger study, 83 attempted this task. Infants who could not complete the task because of fussiness or failure to calibrate (N = 14), or had unreliable data (N = 14), were excluded from the analyses. Data were deemed unreliable if calibration fixations deviated on average more than 4 degrees from the points’ location or eye-tracking ratio, the amount of time when the tracker captured the eyes was <15%, or if the total number of fixations was ±2 standard deviations from the overall sample mean (M = 131, SD = 58). As such, eye-tracking data were available from 55 infants. Among infants who attempted the task, there was no significant age difference between infants who provided useable data and those who did not (12.61 vs. 13.66 months), t(61) = .812, p = .42, d = .21. The two groups of children did not differ in any other demographic variables (p’s > .34).
Infant dot-probe task
The dot-probe task consisted of 20 experimental trials. Each trial began with a central fixation (a clip from a children’s movie), which was presented until the infant fixated for at least 100 ms. The fixation stimulus was then followed by a pair of photographs from LoBue and DeLoache (2008). For 16 trials, infants were presented with a snake and frog (the equivalent of neutral-threat) side by side. A different snake and frog photograph was used in each trial. For four trials, infants were presented with two frogs (neutral–neutral). The neutral–neutral trials were fillers and were only included to ensure that the infants did not expect to see a threatening image in every trial. Consistent with previous research, these trials were not analyzed (e.g., Mogg, Philippot, & Bradley, 2004). Each animal picture measured 13.0 × 16.0 cm and was presented side by side, with a distance of 27.0 cm between their centers.
Although much of the dot-probe literature has focused on 500 ms presentation times (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & Van Ijzendoorn, 2007; Van Bockstaele et al., 2014), many studies have shortened/lengthened this time to capture variations in attention bias patterns (Mogg, Bradley, De Bono, & Painter, 1997). Given the young sample in this study, stimuli were presented for 1000 ms to provide sufficient time to capture eye-gaze patterns for even the youngest participants. The photographs were then removed and followed by a probe (a black asterisk centered on a white screen), which remained on screen for 500 ms. Trials were designated as congruent if the probe appeared in the same location as the snake and incongruent if the probe appeared in the location of the frog. The task was designed so that animal, trial congruency, and probe location (right/left) were counterbalanced throughout. The intertrial interval was 1000 ms. Task presentation was controlled by Experiment Center (SensoMotoric Instruments, Teltow, Germany).
Eye-tracking procedure
The eye-tracking data were obtained using a RED-m Eye Tracking System (SensoMotoric Instruments) and an integrated 22-inch presentation monitor (8.5 cm by 6.3 cm screen). Infants were seated 60 cm from the monitor on an adjustable highchair (N = 38), regular chair (N = 1), or their parent’s lap (N = 16), such that their eye gaze was centered on the screen. The eye tracker monitor has cameras embedded that record the reflection of an infrared light source on the cornea relative to the pupil from both eyes (data from the right eye were analyzed). The average accuracy of this eye-tracking system is in the range of 0.5–1°, which approximates to a 0.5–1 cm area on the screen with a viewing distance of 60 cm. The testing procedure began with a two-point and four-corner calibration procedure using an animated multicolored circle. Testing continued until all 20 trials had been presented, or the infant’s attention could no longer be maintained. Gaze information was sampled at 60 Hz and collected by Experiment Center.
Areas of interest (AOI) that delineated the top, bottom, and contour of the left and right animal and probe locations were created using BeGaze (SensoMotoric Instruments). Subsequent analyses were based on gaze data within the specified AOIs. Gaze data were extracted with BeGaze, supplemented with custom-made Python (Python Software Foundation, http://www.python.org/) and MATLAB (The MathWorks Inc., Natick, MA, USA) scripts to extract study variables by trial. Data were subsequently analyzed using SPSS v22 (Chicago, IL, USA).
Statistical analyses
Data extraction focused on measures of attention and emotion processing: (1) dwell time to each animal, and to parallel the behavioral dot-probe literature, and (2) the number of first fixations to each stimulus and latency to first fixate the probe (Shechner et al., 2013, 2017). A fixation was defined as gaze for at least 80 ms within a 100 pixel area. This threshold was chosen for several reasons. First, 80 ms is standard in the literature (e.g., Benedetto, Pedrotti, & Bridgeman, 2011; Patla & Vickers, 1997) and provides sufficient time for face processing. Indeed, studies with subliminal presentation of faces show that presentation times much faster than our 80-ms threshold (e.g., 17 ms) provides enough time to preliminarily process the faces (e.g., Boyer et al., 2006; Mogg, Bradley, & Williams, 1995; Mogg, Bradley, Williams, & Mathews, 1993; Monk et al., 2008). Further, most of our fixations were well above the 80-ms threshold, with an average dwell time of 248.21 ms (SD = 93.65) for snakes and 233.77 ms (SD = 88.72) for frogs. Finally, we used two converging measures of initial attention (latency to fixate probe and the number of first fixations) and dwell time to differentiate between initial and sustained patterns of attention.
Trials were included for analysis if there was at least one fixation within an AOI (animal or probe) and included for probe fixation latency analysis if there was a probe fixation between 30 (to eliminate fixations that were unlikely to have been directed to the probe) and 1000 ms after probe onset. Repeated measures ANOVA was used to analyze infants’ average dwell time and total first fixations to each stimulus before the probe. A mixed-effects model was used to analyze infants’ first fixations to the probe on each trial. A mixed model was used for latency to fixate the probe (as opposed to GLM) because infants did not fixate the probe on every trial, and a mixed-effects model allowed us to analyze all usable fixation data. Mixed models do not produce standard effect sizes as variance parameters are estimated directly using maximum likelihood. As such, we provided the estimated effect sizes for latency data based on GLM. In line with the sample size and the previous literature noting maturational (Colombo, 1995; Frick, Colombo, & Saxon, 1999), but not gender-linked (e.g., LoBue, 2009, 2010a; LoBue & DeLoache, 2008, 2009, 2011), differences in looking time and gaze patterns in previous threat detection tasks, age was included as a continuous variable in each analysis.
Results
First, we analyzed dwell time to each stimulus before the probe. According to repeated measures ANCOVA with animal (snake vs. frog) as a within-subjects factor and age as a covariate, there was no significant main effect of animal, F(1, 52) = 0.09. p = .771, , or age, F(1, 52) = 1.58. p = .215, , and there was no animal by age interaction, F(1, 52) = 2.19. p = .145, , demonstrating that infants spent an equal amount of time looking at the snakes (M = 248.21 ms; SD = 93.65) and frogs (M = 233.77 ms; SD = 88.72) prior to the onset of the probe.
We also ran repeated measures ANCOVA on the number of first fixations to each of the animals before the probe, with animal (snake vs. frog) as a within-subjects factor and age as a covariate. The results demonstrated that infants more often fixated the snakes first (M = 7.11 first looks; SD = 2.4) when compared to the frogs (M = 5.09 first looks; SD = 2.32), F(1, 53) = 9.29, p = .004, . There was no effect of age, F(1, 53) = 1.56, p = .217, , and no age by condition interaction, F(1, 53) = 0.63, p = .432, .
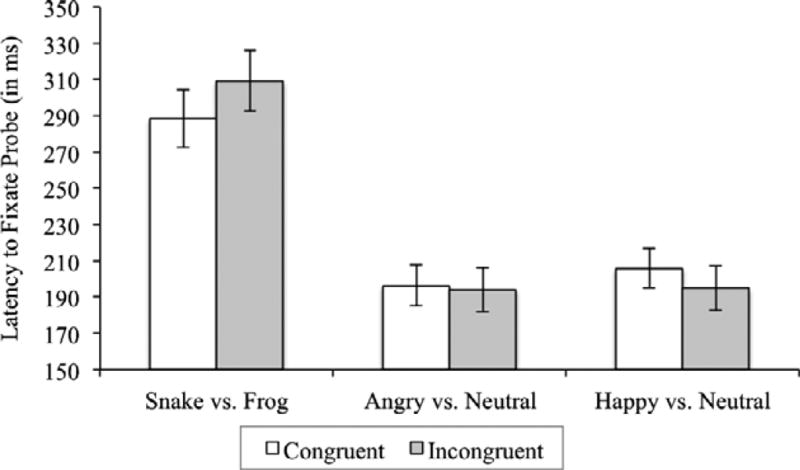
Finally, we analyzed infants’ first fixations to the probe after they saw the pairs of animals. There were 395 total first fixations to the probe across participants (208 following congruent and 187 following incongruent trials). A linear mixed model on latency to first fixate the probe for each condition (congruent vs. incongruent) with age as a covariate resulted in a nonsignificant trend for condition, F(1, 391) = 3.8, p = .053 (estimated ), suggesting that infants might be faster to fixate the probe after it appeared in place of snakes (M = 288.48 ms; SD = 229.42) than frogs (M = 309.25 ms; SD = 228.93) (see Figure 1). There was no main effect of age, F(1, 391) = 0.06, p = .815, and no age by condition interaction, F(1, 391) = 3.0, p = .082 (estimated ), with a nonsignificant trend for latency to first fixate probes appearing in the place of snakes (but not frogs) to be longer as the age of the infants increased.
Figure 1.
Latency data from experiments 1 and 2 by condition (congruent vs. incongruent). In Experiment 1, there was a trend for infants to more quickly fixate probes that appeared in the place of snakes (congruent) than probes that appeared in place of frogs (incongruent). In Experiment 2, regardless of condition, infants fixated probes after seeing face pairs that contained an angry face than face pairs that contained a happy face.
Discussion
These results are consistent with previous work, suggesting that snakes more readily capture infants’ attention when compared to other putatively nonthreatening animal stimuli (LoBue & DeLoache, 2009). Infants, particularly infants as young as 4 months of age, do not have knowledge about the threatening properties of snakes, so it is unlikely that these results can be attributed to the valence of the snake stimuli. Indeed, while previous work suggests that snakes capture infants’ attention, it also suggests that snakes are not necessarily an aversive or negatively valenced stimulus for infants (LoBue, Bloom Pickard, Sherman, Axford, & DeLoache, 2013; Thrasher & LoBue, 2016). Thus, the current results are likely due to the attention-capturing properties of the stimuli, such as their curvy shapes, and not due to emotional properties. Together, the results of Experiment 1 suggest that infants indeed have an attentional bias for snake stimuli—a bias that is evident early in infancy and present throughout the first 2 years of life.
EXPERIMENT 2—SOCIAL THREAT
The results of Experiment 1 suggest an attentional bias for snake stimuli (i.e., nonsocial threat) when compared to nonsnake stimuli, consistent with the previous research. In Experiment 2, we used a similar infant dot-probe procedure to examine infants’ attention to facial signals of threat—namely angry faces. Threatening or angry faces are a social signal of threat and play a greater role in infants’ daily experiences. While infants’ responses to snakes are likely driven by basic attentional mechanisms (e.g., LoBue, 2014; LoBue & DeLoache, 2011), infants recognize and differentiate between several emotional facial expressions by 7 months of age (e.g., Kestenbaum & Nelson, 1990). Although the age range used here is broader, it captures the age range during which infants begin to recognize and differentiate between several emotional expressions and integrate these expressions with their own life experiences. Thus, infants’ responses in a dot-probe paradigm may be substantially different for nonsocial vs. social threats: Whereas a bias for nonsocial threats might be based on specific perceptual features of the stimuli, a bias for social threats might be driven by the stimuli’s socioemotional message, which emerges with age.
Method
Participants
Participants in the current study (N = 117, 65 males, Meanage = 13.1 months; SDage = 5.3, Rangeage = 4.0 to 24.3 months) were recruited from the same, large ongoing study as Study 1. A subset of these children (N = 55) also provided data in Study 1. Note that the dot-probe task with the social stimuli was always presented first, followed by the task with the nonsocial stimuli. In most cases, the tasks were completed on the same day (N = 32), but in some cases (N = 23), the task with social stimuli was completed on the first visit to the laboratory and the task with nonsocial stimuli was completed during a subsequent visit. We compared data for the infants who participated in both tasks to data from participants who only participated in the task with faces, and there were no significant differences between the groups on any of our dependent variables (p’s > .05).
Of the 203 infants enrolled in the larger study, 187 attempted this task. Infants who could not complete the task because of fussiness or failure to calibrate (N = 48), or had unreliable data (SD of calibration >4 or eye-gaze fixations ±2 SD from mean, N = 22), were excluded from the analyses. As such, eye-tracking data were available from 117 infants. Among the infants who attempted the task, the participants who provided good data were older than participants who did not (13.09 vs. 9.63 months), t(185) = −4.33, p < .001, d = .64. The two groups of children did not differ in any other demographic variables (p’s > .30).
Infant dot-probe task and eye-tracking procedure
Again, infants were seated 60 cm from the monitor on an adjustable highchair (N = 82), regular chair (N = 2), or their parent’s lap (N = 33), such that their eye gaze was centered on the screen. The dot-probe task and eye-tracking procedure were identical to Experiment 1 with two exceptions. First, instead of presenting infants with photographs of snakes and frogs, the fixation stimulus was followed by pairs of face stimuli taken from the NimStim set (Tottenham et al., 2009). Different pairs of faces were used for each trial. The second exception was the affective contrast of the experimental trials. In Experiment 1, images of snakes were presented alongside images of frogs. While snakes are presumed to be threatening, neither image projects a clear valence signal. Angry and happy faces, in contrast, communicate a clear emotional message and valence. In line with the child and adult literature (Bantin, Stevens, Gerlach, & Hermann, 2016; Shechner et al., 2013), angry and happy face stimuli were presented as emotion–neutral face pairs. This resulted in three types of face pairs: angry–neutral, happy–neutral, and neutral–neutral. There were 10 faces used (half male), all presented once in each face pair type, for a total of 30 trials. The face pictures were each 14.0 × 19.0 cm and were presented side by side, with a distance of 26.5 cm between their centers.
Results
We first analyzed dwell time to the happy vs. angry faces before the probe. For the repeated measures ANCOVA on average dwell time for each of the emotional facial expressions (angry vs. happy) with age as a covariate, there was a nonsignificant trend for emotion, F(1, 109) = 3.78, p = .054, , suggesting that infants looked longer at the happy faces (M = 244.39 ms, SD = 112.47) than the angry faces (M = 240.92 ms, SD = 102.04). There was also a nonsignificant trend for age, F(1, 109) = 3.66, p = .058, , indicating that dwell time to each of the faces might increase with the age of the infants. There was no significant interaction, F(1, 109) = 2.75, p = .100, .
Next, we ran repeated measures ANCOVA on the number of first fixations to each of the emotional facial expressions before the probe, with emotion (angry vs. happy) as a within-subjects factor and age as a covariate. Infants were no more likely to fixate to the angry faces than the happy faces first, F(1, 115) = 1.84, p = .178, ; there was no main effect of age, F(1, 115) = 7.19, p = .008, , and no age by face interaction, F(1, 115) = 0.69, p = .409, .
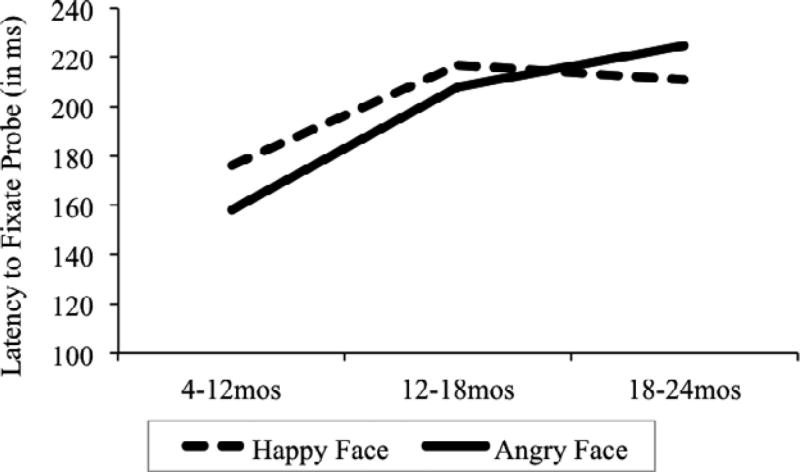
Finally, we analyzed infants’ first fixations to the probe after they saw the face stimuli. There were 775 total first fixations to the probe across trials (383 following the angry face trials and 392 following the happy face trials). A 2 (emotion: happy vs. angry) by 2 (condition: congruent vs. incongruent) mixed-effects model on latency to first fixation to the probe with age as a covariate resulted in a main effect of emotion, F(1, 762) = 3.91, p = .048 (estimated ), a main effect of age, F(1, 762) = 16.20, p < .001 (estimated ), and a nonsignificant trend toward an age by emotion interaction, F(1, 762) = 3.69, p = .055 (estimated ). Importantly, there was no significant effect of condition, F(1, 762) = 0.5, p = .461, and no emotion by condition interaction, F(1, 762) = 1.3, p = .254. Infants were significantly faster to fixate the probe after angry trials (M = 195.32 ms; SD = 161.38) than after happy trials (M = 200.73 ms; SD = 160.98), but there was no difference based on whether the trials were congruent or incongruent (see Figure 1). The main effect of age demonstrates that older infants responded to the probe more slowly than the younger infants. However, breakdown of the nonsignificant interaction suggests that this pattern might only hold for trials with angry faces, F(1, 379) = 16.77, p < .001, and although the same trend was evident for happy faces, it was not statistically significant, F(1, 387) = 2.44, p = .119 (see Figure 2).
Figure 2.
Latency data from Experiment 2 by age. There was a significant increase in latency to fixate to probes by age. The increase was most pronounced for face pairs containing an angry face. Note that although age was analyzed continuously, ages are binned by 4–12, 12–18, and 18– 24 months in this figure for ease of presentation.
Discussion
Unlike our results for snakes, it does not appear that infants have a bias for angry faces that is driven by attention—there was no effect of condition, and infants were not faster to fixate probes that appeared in place of the angry vs. happy faces. They were also no more likely to fixate to the angry faces than the happy faces first. However, they did fixate the probe faster after seeing trials containing an angry face than trials containing a happy face, suggesting that seeing an angry face potentiated the deployment of attention to the probe. Further, it is noteworthy that fixations to the probe were slower in older infants than in younger infants. This finding is not indicative that older infants necessarily lost interest in the task, as overall dwell time before the probe increased with age. Rather, this pattern may reflect general attention mechanisms, as looking duration to nonemotional stimuli is positively related to attention disengagement latency (Colombo, 1995; Frick et al., 1999). Of greater interest, the slowing in latency to fixate to the probe by age was only significant for trials containing an angry face, suggesting the possibility that as infants’ interest in looking at the faces increased, their attentional resources for detecting the probe decreased disproportionately for threatening, or angry face trials. The findings also point to overlapping and complex relations in fixations in general and the impact of dwell time on subsequent fixation patterns.
GENERAL DISCUSSION
We examined developmental differences in humans’ visual biases for threatening stimuli in a large cross-sectional group of infants, ranging from 4 to 24 months of age. Despite previous research demonstrating a similar pattern in adults’ responses to both snakes and angry faces, our results suggest that there are different patterns for the development of attentional biases for social vs. nonsocial threats over the first 2 years of life. When presented with nonsocial threats such as snakes in Experiment 1, there was a nonsignificant trend for infants to more quickly fixate probes that appeared in place of snakes vs. frogs, and their first fixations were significantly more often to snakes than to frogs. There were no age differences, suggesting that a perceptual bias for snakes is present early in life and stable across infancy.
When presented with angry faces in Experiment 2, infants showed a different pattern of responding altogether: They fixated probes more quickly after viewing any trials that contained an image of an angry face compared to trials that contained an image of a happy face. This suggests that seeing an angry face elicits rapid detection of subsequent stimuli, which is more indicative of an arousal or vigilance response than a perceptual bias. Further, there were age-related changes in infants’ responses to the face stimuli, with a general increase in looking time to faces before the probe and a general increase in latency to fixate the probe after seeing an angry face. This suggests that as infants’ interest in emotional faces increases with age, and their attentional resources for detecting a neutral stimulus that follows (e.g., a probe) might decrease.
Together, these findings provide suggestive evidence that different developmental mechanisms may be responsible for humans’ attentional biases for social vs. nonsocial threats in infancy. More specifically, perceptual mechanisms draw infants’ attention to snake stimuli early in development. This perceptual bias is stable and maintained throughout infancy. In contrast, seeing an image of a social threat increases attention to all stimuli, and such a response becomes attenuated as infants get older. These findings are not surprising when considering infants’ experiences with social vs. nonsocial threats in the first 2 years of life.
Most infants likely have little experience with the threatening and emotional properties of snakes, so it is unlikely that valence-driven responses to snakes would be evident early in development. As mentioned above, while snakes capture infants’ attention, snakes are not necessarily an aversive or negatively valenced stimulus for infants or young children (LoBue et al., 2013; Thrasher & LoBue, 2016). Thus, the current results are likely due to the attention-capturing visual properties of snake stimuli, such as their curvy shapes (e.g., LoBue, 2014; LoBue & DeLoache, 2011). These perceptual biases may serve as the foundation for the later acquisition of fear responses often documented with older children (LoBue & Rakison, 2013).
In contrast, infants recognize and differentiate between various emotional facial expressions by 7 months of age (e.g., Kestenbaum & Nelson, 1990), likely through daily experience and exposure. Further, previous research measuring children’s behavioral responses to various threatening stimuli demonstrates that their responses to social threats in particular (but not to nonsocial threats) predict dysregulated fear (Buss, 2011). Thus, it is not surprising that infants’ behavior toward social threats appears to be more in line with arousal or emotion-related responding than with a perceptual bias. In addition, increased experience with faces over time would then translate into age-based differences in attention patterns.
Although our data suggest that infants’ early responses to snakes and angry faces are different, it is possible that the end point of the developmental process for these stimuli is still quite similar once individuals reach adolescence or adulthood. Indeed, parallel findings for the detection of snakes and angry faces have been reported in the adult literature and implicate both perceptual and emotional factors in driving rapid detection (for reviews, see LoBue, 2016; LoBue & Rakison, 2013). In fact, research is beginning to suggest that multiple mechanisms—including perceptual, cognitive, and emotional domains—can all lead to a visual bias for threat, playing potentially interacting and additive roles (LoBue, 2014, 2016). It is possible that as children learn about the threatening properties of snakes and develop emotional responses to their presence, a bias that was once perceptually driven begins to develop into an emotional bias similar to what we see for angry faces. In the same way, an emotional response for angry faces might result in learning to detect their perceptual features very quickly as well.
Given that previous research has demonstrated more rapid orienting to angry vs. happy faces in 9- to 12-month-olds (LoBue & DeLoache, 2009), it was surprising that infants did not first fixate angry faces more often than happy faces and that there was a trend for longer dwelling on happy vs. angry faces before the onset of the probe. There are several possible explanations for these seemingly inconsistent findings. First, while LoBue and DeLoache (2009) found a bias for angry faces in initial orienting speed, we found a bias for happy faces in average dwell time. Although these findings seem inconsistent, they are based on different measures of attention. Indeed, while previous research on threat biases has generally focused on probability or speed of initial orienting, initial orienting may not necessarily be consistent with sustained orienting. In fact, based on vigilance–avoidance theory, early vigilance may even lead to later avoidance. In other words, after initial orienting, individuals may direct their attention away from threatening stimuli (Mogg, Bradley, Miles, & Dixon, 2004). This hypothesis is consistent with the current findings, demonstrating a trend for longer dwelling to happy (nonthreatening) vs. angry (threatening) faces. Further, sustained attention to happy faces when compared to angry faces is consistent with previous research in other domains (e.g., Todd, Evans, Morris, Lewis, & Taylor, 2010).
Although finding longer dwell time to happy vs. angry faces is not necessarily inconsistent with previous research, it is still surprising that we did not find an early orienting bias for angry vs. happy faces. It is unclear why such a bias was not found. However, we used a much wider age range (4–24 months) when compared to previous studies (e.g., 9– 12 months in LoBue & DeLoache, 2009), which might have contributed to differences in our results. Further investigation using our new baby dot-probe paradigm with more concentrated age groups could help to further elucidate these differences.
This research provides the first evidence of differing trajectories in the development of attentional biases for threat. It also introduces a new paradigm that can be used effectively to study these biases in infants as young as 4 months of age. Despite these strengths, the current research also has some limitations. First, we used a cross-sectional sample, so we were unable to study developmental change over time. Further, some of the differences we found were small and only marginally significant, and our inclusion criteria for a fixation were relatively lenient. Thus, future research using a longitudinal design might provide stronger results and help elucidate when and how developmental changes take place and how individual differences might play a role in shaping biased attention.
Second, the methods used here for experiments 1 and 2 had some differences that limit our ability to make direct comparisons across studies. For example, in our study design only a subset of participants completed both tasks, and the task using face stimuli was always presented before the task using animal stimuli. Further, our dot-probe task differed from previous visual search tasks in which infants were simply presented with various stimuli and researchers measured how quickly the infants turned to look (e.g., LoBue & DeLoache, 2009). Thus, the current results cannot be directly compared to previous infant studies on threat detection. Future research using our new baby dot-probe task, coupled with other eye-tracking tasks, might help clarify some of these remaining issues.
In conclusion, the current work provides a first step in elucidating the developmental mechanisms that drive what are considered our most basic attentional biases and provides a basis for future investigations of how early attentional biases for various threats develop in infancy and affect later behavior. Given the potential predictive power of attentional biases for the development of anxiety (Pérez-Edgar et al., 2010, 2011), future longitudinal research on how individual differences in attentional biases for threat affect later behavior might be important in guiding intervention strategies that aim to prevent the development of anxiety disorders at an early age. This future direction would also be important in clarifying some of the age-related changes we found here for the detection of angry faces, which could involve gains in attentional control and the expression of individual differences in emotional responding.
Contributor Information
Vanessa LoBue, Rutgers University.
Kristin A. Buss, Pennsylvania State University
Bradley C. Taber-Thomas, Pennsylvania State University
Koraly Pérez-Edgar, Pennsylvania State University.
References
- Bantin T, Stevens S, Gerlach AL, Hermann C. What does the facial dot-probe task tell us about attentional processes in social anxiety? A systematic review. Journal of Behavior Therapy and Experimental Psychiatry. 2016;50:40–51. doi: 10.1016/j.jbtep.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, Van Ijzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Benedetto S, Pedrotti M, Bridgeman B. Microsaccades and exploratory saccades in a naturalistic environment. Journal of Eye Movement Research. 2011;4:1–10. [Google Scholar]
- Blanchette I. Snakes, spiders, guns, and syringes: How specific are evolutionary constraints on the detection of threatening stimuli? The Quarterly Journal of Experimental Psychology. 2006;59:1484–1504. doi: 10.1080/02724980543000204. [DOI] [PubMed] [Google Scholar]
- Boyer MC, Compas BE, Stanger C, Colletti RB, Konik BS, Morrow SB, Thomsen AH. Attentional biases to pain and social threat in children with recurrent abdominal pain. Journal of Pediatric Psychology. 2006;31:209–220. doi: 10.1093/jpepsy/jsj015. [DOI] [PubMed] [Google Scholar]
- Brosch T, Sharma D. The role of fear-relevant stimuli in visual search: A comparison of phylogenetic and ontogenetic stimuli. Emotion. 2005;5:360–364. doi: 10.1037/1528-3542.5.3.360. [DOI] [PubMed] [Google Scholar]
- Buss KA. Which fearful toddlers should we worry about? Context, fear regulation, and anxiety risk. Developmental Psychology. 2011;47:804–819. doi: 10.1037/a0023227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo J. On the neural mechanisms underlying developmental and individual differences in visual fixation in infancy: Two hypotheses. Developmental Review. 1995;15:97–135. [Google Scholar]
- Field AP, Lester KJ. Is there room for ‘development’ in developmental models of information processing biases to threat in children and adolescents? Clinical Child and Family Psychology Review. 2010;13:315–332. doi: 10.1007/s10567-010-0078-8. [DOI] [PubMed] [Google Scholar]
- Frick JE, Colombo J, Saxon TF. Individual and developmental differences in disengagement of fixation in early infancy. Child Development. 1999;70:537–548. doi: 10.1111/1467-8624.00039. [DOI] [PubMed] [Google Scholar]
- Kestenbaum R, Nelson CA. The recognition and categorization of upright and inverted emotional expressions by 7-month-old infants. Infant Behavior and Development. 1990;13:497–511. [Google Scholar]
- Leppänen JM, Nelson CA. The development and neural bases of recognizing of facial emotion. In: Kail R, editor. Advances in child development and behavior. London: Elsevier Press; 2006. pp. 207–246. [DOI] [PubMed] [Google Scholar]
- LoBue V. More than just a face in the crowd: Detection of emotional facial expressions in young children and adults. Developmental Science. 2009;12:305–313. doi: 10.1111/j.1467-7687.2008.00767.x. [DOI] [PubMed] [Google Scholar]
- LoBue V. And along came a spider: Superior detection of spiders in children and adults. Journal of Experimental Child Psychology. 2010a;107:59–66. doi: 10.1016/j.jecp.2010.04.005. [DOI] [PubMed] [Google Scholar]
- LoBue V. What’s so scary about needles and knives? Examining the role of experience in threat detection. Cognition and Emotion. 2010b;24:80–87. [Google Scholar]
- LoBue V. Deconstructing the snake: The relative roles of perception, cognition, and emotion on threat detection. Emotion. 2014;14:701–711. doi: 10.1037/a0035898. [DOI] [PubMed] [Google Scholar]
- LoBue V. When is a face no longer a face? A problematic dichotomy in visual detection research. Emotion Review. 2016;8:250–257. [Google Scholar]
- LoBue V, Bloom Pickard M, Sherman K, Axford C, DeLoache JS. Young children’s interest in live animals. British Journal of Developmental Psychology. 2013;31:57–69. doi: 10.1111/j.2044-835X.2012.02078.x. [DOI] [PubMed] [Google Scholar]
- LoBue V, DeLoache JS. Detecting the snake in the grass: Attention to fear-relevant stimuli by adults and young children. Psychological Science. 2008;19:284–289. doi: 10.1111/j.1467-9280.2008.02081.x. [DOI] [PubMed] [Google Scholar]
- LoBue V, DeLoache JS. Superior detection of threat-relevant stimuli in infancy. Developmental Science. 2009;13:221–228. doi: 10.1111/j.1467-7687.2009.00872.x. [DOI] [PubMed] [Google Scholar]
- LoBue V, DeLoache JS. What so special about slithering serpents? Children and adults rapidly detect snakes based on their simple features. Visual Cognition. 2011;19:129–143. [Google Scholar]
- LoBue V, Pérez-Edgar K. Sensitivity to social and non-social threats in temperamentally shy children at-risk for anxiety. Developmental Science. 2014;17:239–247. doi: 10.1111/desc.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoBue V, Rakison D. What we fear most: A developmental advantage for threat-relevant stimuli. Developmental Review. 2013;33:285–303. [Google Scholar]
- Mogg K, Bradley BP, De Bono J, Painter M. Time course of attentional bias for threat information in non-clinical anxiety. Behaviour Research and Therapy. 1997;35:297–303. doi: 10.1016/s0005-7967(96)00109-x. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley B, Miles F, Dixon R. Brief report time course of attentional bias for threat scenes: Testing the vigilance-avoidance hypothesis. Cognition and Emotion. 2004;18:689–700. [Google Scholar]
- Mogg K, Bradley BP, Williams R. Attentional bias in anxiety and depression: The role of awareness. British Journal of Clinical Psychology. 1995;34:17–36. doi: 10.1111/j.2044-8260.1995.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Williams R, Mathews A. Subliminal processing of emotional information in anxiety and depression. Journal of Abnormal Psychology. 1993;102:304. doi: 10.1037//0021-843x.102.2.304. [DOI] [PubMed] [Google Scholar]
- Mogg K, Philippot P, Bradley BP. Selective attention to angry faces in clinical social phobia. Journal of Abnormal Psychology. 2004;113:160–165. doi: 10.1037/0021-843X.113.1.160. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, Pine DS. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhman A, Mineka S. Fears, phobias, and preparedness: Toward and evolved module of fear and fear learning. Psychological Review. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Patla AE, Vickers JN. Where and when do we look as we approach and step over an obstacle in the travel path. Neuroreport. 1997;8:3661–3665. doi: 10.1097/00001756-199712010-00002. [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K, Bar-Haim Y, McDermott JM, Chronis-Tuscano A, Pine DS, Fox NA. Attention biases to threat and behavioral inhibition in early childhood shape adolescent social withdrawal. Emotion. 2010;10:349–357. doi: 10.1037/a0018486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Reeb-Sutherland BC, McDermott JM, White LK, Henderson HA, Degnan KA, Fox NA. Attention biases to threat link behavioral inhibition to social withdrawal over time in very young children. Journal of Abnormal Child Psychology. 2011;39:885–895. doi: 10.1007/s10802-011-9495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakison DH, Derringer JL. Do infants possess an evolved spider-detection mechanism? Cognition. 2008;107:381–393. doi: 10.1016/j.cognition.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Reynolds G, Field AP, Askew C. Effect of vicarious fear learning on children’s heart rate responses and attentional bias for novel animals. Emotion. 2014;14:995–1006. doi: 10.1037/a0037225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Dennis TA, Warner CM. A critical review of attentional threat bias and its role in the treatment of pediatric anxiety disorders. Journal of Cognitive Psychotherapy. 2015;29:171–184. doi: 10.1891/0889-8391.29.3.171. [DOI] [PubMed] [Google Scholar]
- Shechner T, Jarcho JM, Britton JC, Leibenluft E, Pine DS, Nelson EE. Attention bias of anxious youth during extended exposure to emotional face pairs: An eye-tracking study. Depression and Anxiety. 2013;30:14–21. doi: 10.1002/da.21986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T, Jarcho JM, Wong S, Leibenluft E, Pine DS, Nelson EE. Threats, rewards, and attention deployment in anxious youth and adults: An eye tracking study. Biological Psychology. 2017;122:121–129. doi: 10.1016/j.biopsycho.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrasher C, LoBue V. Do infants find snakes aversive? Infants’ physiological responses to “fear-relevant” stimuli. Journal of Experimental Child Psychology. 2016;142:382–390. doi: 10.1016/j.jecp.2015.09.013. [DOI] [PubMed] [Google Scholar]
- Todd RM, Evans JW, Morris D, Lewis MD, Taylor MJ. The changing face of emotion: Age-related patterns of amygdala activation to salient faces. Social Cognitive and Affective Neuroscience. 2010;6:16–23. doi: 10.1093/scan/nsq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele B, Verschuere B, Tibboel H, De Houwer J, Crombez G, Koster EH. A review of current evidence for the causal impact of attentional bias on fear and anxiety. Psychological Bulletin. 2014;140:682–721. doi: 10.1037/a0034834. [DOI] [PubMed] [Google Scholar]