Abstract
Objectives
In recent years, the anti-cancer properties of several commonly used drugs have been explored, with drugs such as aspirin and beta-blockers associated with improved cancer outcomes. Previous preclinical work demonstrated that tricyclic anti-depressants have antitumor efficacy in lung cancer. Our goal was to examine the association between anti-depressant use and survival in lung cancer.
Materials and Methods
We examined the association between use of common anti-depressants and survival in 1,097 lung cancer patients from the NCI-Maryland lung cancer study. The types of anti-depressants included in the study were norepinephrine and dopamine reuptake inhibitors, serotonin reuptake inhibitors, selective serotonin reuptake inhibitors, non-selective serotonin reuptake inhibitors, and tricyclic anti-depressants. Anti-depressant use was extracted from the medical history section of a detailed interviewer-administered questionnaire. Specific use in the three months before a lung cancer diagnosis was determined. Cox portioned hazards modeling was used to estimate the association between anti-depressant use with lung cancer-specific death with adjustment for potential confounding co-factors.
Results
Anti-depressant use was associated with extended lung cancer-specific survival. In an analysis of specific classes of anti-depressant use, NDRIs and TCAs were associated with improved survival. Importantly, the extended survival associated with anti-depressants was maintained after adjustment for the clinical indications for these drugs, suggestive of a direct effect on lung cancer biology.
Conclusions
Considering the manageable and largely tolerable side effects of anti-depressants, and the low cost of these drugs, these results indicate that evaluation of anti-depressants as adjunct therapeutics with chemotherapy may have a translational effect for lung cancer patients.
Keywords: lung cancer, antidepressants, survival
Introduction
Lung cancer is the leading cause of cancer-related mortality in the United States and worldwide.(1) The 5-year survival rate is approximately 15%. One of the major contributing factors to this dismal survival rate is the stage at which the majority of lung cancers are diagnosed—greater than 50% is diagnosed at stage 3 or 4, a time when both local and systemic treatments are unlikely to be curative. Although early detection of lung cancer among high-risk individuals can reduce lung cancer mortality by up to 20%,(2) there is still an immense need for the development of therapeutic approaches to help lung cancer patients worldwide.
Treatment options for lung cancer patients have recently progressed in the age of genomic medicine. For instance, targeted treatments for EGFR mutations and EML4-ALK rearrangements have extended survival times in advanced stage lung adenocarcinoma patients that carry these genomic alterations.(3) To complement ongoing efforts to improve the efficacy of chemotherapy and identify targeted therapies, the anti-cancer properties of several commonly used drugs have been explored in recent years; intriguingly, drugs such as aspirin and beta-blockers have been associated with improved cancer outcomes.(4-6) While these drugs can have toxicities, they are generally better tolerated than those associated with chemotherapeutic drugs and they can be easily combined with standard-of-care treatment.
Anti-depressants are commonly used in both the general population and cancer patients.(7) Previous studies have yielded contradictory results regarding possible links between the use of anti-depressants and cancer.(7-15) In lung cancer specifically, recent work in mice suggests that tricyclic anti-depressants could be an efficacious treatment strategy in small cell lung cancer and neuroendocrine tumors.(13) Based on these observations, we examined the relationship between anti-depressant use and prognosis in a population of patients diagnosed with lung cancer. We found that lung cancer patients taking NDRIs and TCAs have a significantly improved survival. These results may have significant translational impact for repositioning commonly used drugs as anti-cancer therapy.
Methods
Study population
We conducted a nested case-only analysis of patients with pathologically confirmed lung cancer, recruited from the greater metropolitan area of Baltimore, MD as part of the NCI-MD lung cancer case control study between 1998 and 2010. Written informed consent was obtained from all participants and the study was approved by the Institutional Review Boards of all participating institutions. Inclusion criteria for this on-going case-control study have been previously described.(16) Briefly, participants were United States citizens, English-speaking and non-institutionalized. Participants took part in a detailed questionnaire at the time of their diagnosis that collected extensive information on nutrition, reproductive health, medical history, occupational history, smoking, and alcohol consumption. Never smokers were defined as those who smoked <100 cigarettes during their lifetime. Former smokers were defined as those who reported quitting smoking ≥1 year before the date of interview. Race was self-reported. A summary of the patient characteristics is shown in Table 1.
Table 1.
Characteristics of the population
| Characteristic | All | Not Taking Anti- depressants |
Taking Anti- depressants |
P |
|---|---|---|---|---|
| 1,097 | 890 | 207 | ||
| Age (mean ± S.D.) | 65.8 ± 10.5 | 66.5 ± 10.4 | 63.0 ± 10.4 | <0.001 |
| Gender (%) | 0.004 | |||
| Male | 560 (51%) | 473 (53%) | 87 (42%) | |
| Female | 537 (49%) | 417 (47%) | 120 (58%) | |
| Missing | 0 | 0 | 0 | |
| Race (%) | <0.001 | |||
| African American | 312 (28%) | 279 (31%) | 33 (16%) | |
| European American | 784 (71%) | 611 (69%) | 173 (84%) | |
| Unknown | 1 | 0 | 1 | |
| Smoking Status (%) | 0.003 | |||
| Never | 89 (8%) | 75 (8%) | 14 (7%) | |
| Former | 498 (45%) | 423 (48%) | 75 (36%) | |
| Current | 510 (47%) | 392 (44%) | 118 (57%) | |
| Missing | ||||
| Pack-years (mean ± S.D.) | 41.3 ± 30.0 | 40.9 ± 30.5 | 42.8 ± 27.6 | 0.408 |
| Income | 0.790 | |||
| < $10,000 | 160 (15%) | 131 (17%) | 29 (16%) | |
| $10,000-$29,999 | 287 (26%) | 236 (30%) | 51 (28%) | |
| $30,000-$59,999 | 245 (22%) | 203 (26%) | 42 (23%) | |
| $60,000-$90,000 | 132 (12%) | 102 (13%) | 30 (17%) | |
| >$90,000 | 129 (12%) | 102 (13%) | 27 (15%) | |
| Missing | 144 (13%) | |||
| Education | 0.029 | |||
| 5th or 6th Grade | 24 (2%) | 19 (2%) | 5 (3%) | |
| 7th, 8th, or 9th Grade | 143 (13%) | 123 (14%) | 20 (10%) | |
| 10th or 11th Grade | 128 (12%) | 111 (13%) | 17 (9%) | |
| 12th Grade | 338 (31%) | 272 (32%) | 66 (33%) | |
| Some College | 190 (17%) | 141 (17%) | 49 (25%) | |
| Technical School | 43 (4%) | 30 (4% | 12 (6%) | |
| College | 138 (13%) | 118 (14%) | 20 (10%) | |
| Professional School | 48 (4%) | 37 (4%) | 11 (6%) | |
| Missing | 46 (4%) | |||
| Stage | 0.251 | |||
| I | 382 (35%) | 300 (34%) | 82 (40%) | |
| II | 109 (10%) | 96 (11%) | 13 (6%) | |
| III | 244 (22%) | 197 (22%) | 47 (23%) | |
| IV | 258 (24%) | 211 (24%) | 47 (23%) | |
| Missing | 104 | 86 | 18 | |
| Histology | 0.626 | |||
| Adenocarcinoma | 504 (46%) | 399 (45%) | 105 (51%) | |
| Squamous cell carcinoma | 279 (26%) | 229 (26%) | 50 (24%) | |
| Large cell carcinoma | 24 (2%) | 20 (2%) | 4 (2%) | |
| Other* | 280 (26%) | 234 (26%) | 46 (22%) | |
| Missing | 10 | 8 | 2 | |
| Survival (median years, I.Q.R.) | ||||
| Overall Survival | 2.7, 0.5-5.0 | 2.59, 0.5-5.0 | 3.2, 0.6-5.0 | |
| Lung Cancer Deaths | 647 | 537 | 110 | |
| All Deaths | 698 | 576 | 122 |
S.D. denotes standard deviation, I.Q.R. denotes interquartile range
Includes designation NSCLC and SCLC
Extraction of medication use data
As part of the NCI-MD case controls study, patients took part in a questionnaire that gathered demographic, lifestyle and medical history. For this nested case-only study, we identified 1,097 lung cancer patients in the NCI-MD case control study with questionnaire data relating to medication use (Table 1). The health questionnaire was developed to include the assessment of medication use during the three months prior to interview. Patients were asked: Have you taken any prescription or non-prescription medications in the last 3 months? For those patients that responded yes, the name of the drug was recorded, later extracted and transcribed by hand. Anti-depressant use, including the reasons for its use, was also extracted. Anti-depressant drug classes considered in this analysis were: NDRIs (norepinephrine-dopamine reuptake inhibitors), SNRIs (serotonin and norepinephrine reuptake inhibitors), SSRIs (selective serotonin reuptake inhibitors), SRIs (serotonin reuptake inhibitor), NSSRIs (non-selective serotonin reuptake inhibitors), tricyclics (TCAs), and MAOIs (monoamine oxidase inhibitors). Patients were initially classified as having taken an anti-depressant if they reported using any of these drugs. Subsequently, variables were created to specifically document sole use of each anti-depressant class (Supplementary Table 1). A full list of the drugs within each class from this patient population is outlined in Supplementary Table 2.
Statistical Analysis
To test the magnitude of association between anti-depressant use with lung cancer-specific survival, 5-year lung cancer-specific hazard ratios (HR) were estimated using multivariable Cox proportional hazards regression modeling with adjustment for potential confounders, including age (continuous), sex (male/female), current smoking status (never/former/current), pack-years of smoking, race (African American/European American), histology (adenocarcinoma/squamous cell carcinoma/LCLC/other), stage (stage I/stage II/stage III/stage IV), income, education and drug indication. Survival times in the NCI/MD study were gathered through a query of the National Death Index (last entry 12/31/2012) and determined as time from diagnosis to last known follow-up or date of death. Time from lung cancer diagnosis was used to estimate the survival timescale, and failure was described as lung cancer-specific death. Proportional hazards assumptions were verified by visual inspection of log-log plots and using a nonzero slope test of the Schoenfeld residuals. In this study, causes of death other than lung cancer were censored (n=70). As competing risks are distinct from standard censoring, we performed a competing risks regression based on the method of Fine and Gray (17) using the stcrreg function in STATA. A new variable was generated to specify the competing events (death from cancer and death from another cause).
We also addressed whether or not confounding by medication indication could have contributed to our observed findings. This is because the condition that the drug was initially prescribed for could be associated with lung cancer outcome also. Patients were asked why each drug was prescribed; this was then coded as a variable and included in the regression model. All statistical analyses were performed using STATA (Stata Statistical Software: Release 13. College Station, TX: StataCorp LP).
Results
Characteristics of the study population
This study included 1,097 primary lung cancer cases (Table 1). The median age at diagnosis of the population was 65.8 years. Those taking anti-depressants were diagnosed earlier, more likely to be female, of European American ancestry and current smokers (Table 1). The overall median survival time across all stages was 2.7 years: for those not taking anti-depressants it was 2.6 years and for those who took anti-depressants the median survival was 3.2 years (Table 2). There were 382 patients with stage I lung cancer, 109 with stage II, 244 with stage III and 258 with stage IV; stage information was missing on 104 patients. As shown in Table 1, there were 59, 7, 118, 25, 4, 25, and 1 patients who reported taking NDRIs, SNRIs, SSRIs, SRIs, NSSRIs, TCAs, and MAOIs, respectively (overall, 207 patients reported taking anti-depressants). As there was only one patient that reported taking MAOIs, this patient was removed for single class analyses. Some of the patients in this study reported using more than one type of anti-depressant. The matrix of multiple anti-depressant use is outlined in Supplementary Table 1. The population examined is representative of the general population in the United States, in that the proportion of patients with lung cancer taking anti-depressants was similar to that observed in the general population.(18,19) As previously reported,(18,19) women were more likely to use anti-depressants than men (P=0.004).
Table 2.
Distribution of use by anti-depressant drug class
| N | N | |
|---|---|---|
|
|
||
| Drug Class | (no exclusions) | (excluding other anti-depressant drugs) |
| NDRI | 59 | 46 |
| SNRI | 7 | 5 |
| SSRI | 118 | 93 |
| SRI | 25 | 12 |
| NSSRI | 4 | 3 |
| Tricyclic | 25 | 18 |
| MAOI | 1 | 1 |
NDRIs (norepin ephrine-dopamine r euptake inhibitors),
SNRIs (serotonin and norepinephrine reuptake inhibitors) SSRIs (selective serotonin reuptake inhibitors)
SRIs (serotonin reuptake inhibitor)
NSSRIs (non-selective serotonin reuptake inhibitors)
tricyclics and MAOIs (monoamine oxidase inhibitors)
Prolonged lung cancer survival associated with anti-depressant use
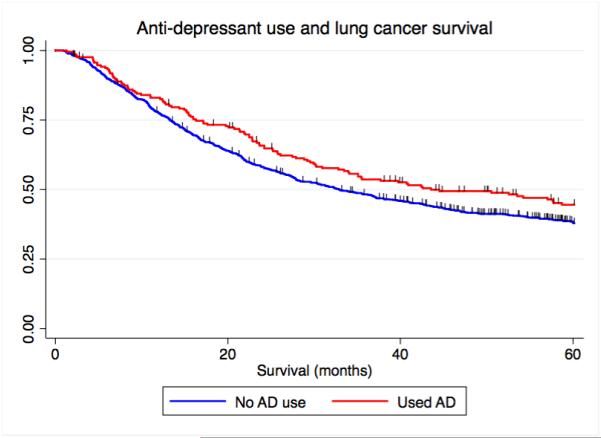
Overall, 19% (207) of the patients in this study had been prescribed one or more anti-depressants by their physician (Table 1 and Supplementary Table 1). One hundred and seventy-eight patients took a single anti-depressant and 29 patients took more than one (Table 1, Supplementary Table 1 and Supplementary Table 2). We found that, independent of the specific subclass, the administration of any anti-depressant was associated with a significantly prolonged survival (unadjusted model HR=0.68, 95% C.I. = 0.49 – 0.95, P=0.05, n=1,097) (Figure 1A) (Table 3).
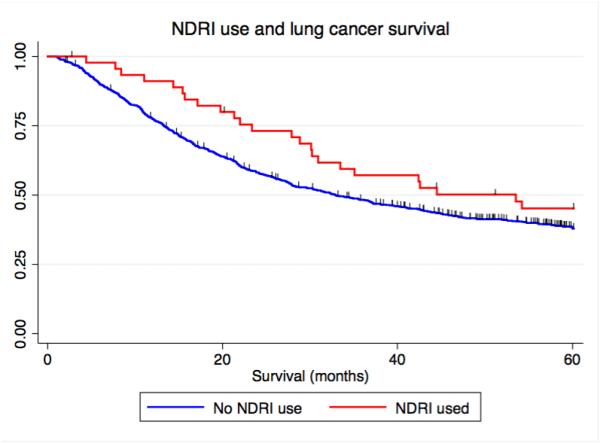
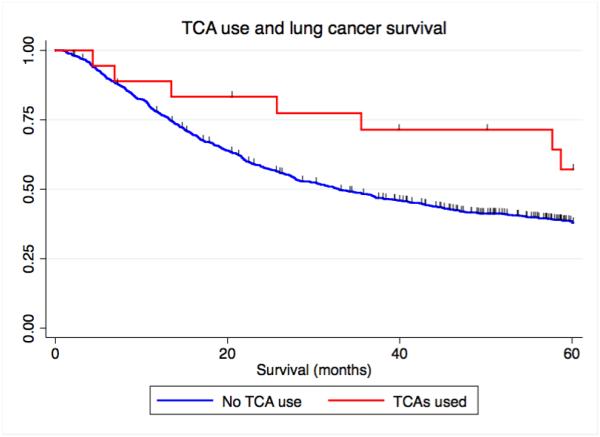
Figure 1.
Kaplan-Meier curves depicting the relationship between antidepressant use and lung cancer-specific survival. A) Overall antidepressant use and lung cancer-specific survival, B) NDRI sole use, and C) TCA sole use. NDRI denotes norepinephrine reuptake inhibitor, TCA denotes tricyclic antidepressant.
Table 3.
Association between anti-depressant use and lung cancer survival
|
|
Univariable |
|
Multivariable* |
|
|||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| H R |
95% (C.I.) |
P | H R |
95% (C.I.) |
P | ||||
|
Not taking anti-
depressants |
1 | Referenc e |
1 | Referenc e |
|||||
|
Taking anti-
depressants |
0.6 8 |
0.49 – 0.95 |
0.05 2 |
0.6 8 |
0.49 - 0.95 |
0.02 5 |
|||
HR denotes hazard ratio; C .I. de notes confidence interval
denotes adjustment for age, race, gender, smoking status, pack-years of smoking, race, stage, histology, income, education level and drug indication
In order to exclude potential bias in our results, we investigated the indication of anti-depressant administration in our cohort of patients. We found that predictors of anti-depressant prescription, such as the presence of comorbidities or pain management, were consistent with previous studies.(20) However, following adjustment for drug indication, and general potential confounders such as age, gender, stage, histology, race, smoking status and pack-years of smoking in our model, anti-depressant use remained associated with a longer survival time (HR=0.68, 95% C.I. = 0.49 – 0.95, P=0.025) (Table 3) (Supplementary Table 4). Our model also included adjustment for metrics of SES including income and education level, as a proxy for insurance status. This was included in the analysis as patients who experience difficulty in paying for medications may be less likely to renew prescriptions. However, these variables did not modify the relationship between anti-depressant use and patient survival.
If patients not treated with anti-depressant drugs died of causes other than lung cancer early in the study, then those patients taking anti-depressants could appear to have an artificially reduced hazard of death. We therefore conducted a competing risks analysis to address this potential factor and found that the association between anti-depressant use and survival was not affected by censoring of the 70 individuals who died of causes other than lung cancer (fully adjusted model, HR: 0.68, 95% C.I. 0.50 – 0.91, P=0.012).
In addition, we also analyzed stage IV patients only, to remove some of the heterogeneity of the population. As shown in Supplementary Figure 1, the association between anti-depressant use and lung cancer specific survival remains in stage IV patients (HR: 0.60, 95% C.I. 0.41 – 0.89, P=0.011):
NDRI and tricyclic anti-depressants are associated with improved lung cancer survival
Anti-depressants comprise a heterogeneous group of drugs and mediate their effects through a range of neurotransmitters. To explore whether specific types of anti-depressants were associated with lung cancer survival or whether the group as a whole was associated with survival, we identified six classes of anti-depressants in the medical records of our patient cohort. We found that the administration of NDRIs (HR: 0.60, 95% C.I. = 0.39 – 0.92, P=0.018) and TCAs (HR: 0.57, 95% C.I. = 0.29 – 1.10, P=0.092) were associated with prolonged lung cancer survival, although the TCA results only approached statistical significance (Table 4).
Table 4.
Association between class of anti-depressant use and lung cancer survival
| HR | 95% (C.I.) | P | HR | 95% (C.I.) | P | |
|---|---|---|---|---|---|---|
| Mixed Class Use | Single Class Use | |||||
| No anti-depressant use | 1 | Reference | 1 | Reference | ||
| NDRI | 0.54 | 0.33 - 0.88 | 0.014 | 0.52 | 0.31 - 0.89 | 0.017 |
| SNRI | 1.39 | 0.53 – 3.68 | 0.498 | 1.63 | 0.56 – 4.78 | 0.374 |
| SSRI | 0.77 | 0.49 – 1.21 | 0.260 | 0.72 | 0.45 – 1.17 | 0.185 |
| SRI | 1.77 | 0.93 - 3.36 | 0.080 | 1.54 | 0.65 - 3.64 | 0.329 |
| NSSRI | 1.87 | 0.55 – 6.36 | 0.312 | 2.21 | 0.51 – 9.61 | 0.289 |
| Tricyclic | 0.58 | 0.29 – 1.13 | 0.107 | 0.40 | 0.17 – 0.92 | 0.031 |
HR denotes hazard ratio; C.I. denotes confidence interval
P denotes adjustment for age, race, gender, smoking status, pack-years of smoking, race, stage, histology, income, education level and drug indication
As patients were sometimes treated with more than one kind of anti-depressant, we conducted an analysis of single anti-depressant class use. We again observed that patients who took only an NDRI (HR=0.56, 95% C.I. = 0.36 – 0.90, P=0.016 or tricyclic (HR=0.40, 95% C.I. = 0.18 – 0.91, P=0.028) had prolonged survival (Table 4) (Figure 1B and Figure 1C). We conducted a competing risks analysis and observed that survival was not affected by censoring of the 70 individuals who died of causes other than lung cancer (fully adjusted model NDRI, HR: 0.51, 95% C.I. 0.32 – 0.81, P=0.004) (fully adjusted model tricyclic, HR: 0.41, 95% C.I. 0.84 – 0.76, P=0.015).
Discussion
Cancer patients are three times more likely to develop major depression compared with the general population(7,21) and there is evidence that depression can contribute to adverse cancer outcomes.(7) Potential mechanisms include a potentially decreased likelihood to adhere to cancer treatments.(7) However, it is also possible that depression can have a pathophysiological effect via neuroendocrine or immunological signaling.(7) In this study, we demonstrated that the administration of anti-depressants to lung cancer patients, particularly of the NDRI and TCA class, is associated with prolonged survival. While the relationship between treatment with TCAs and lung cancer survival was of borderline significance when TCA use was considered with other drugs (some patients who took TCAs were also treated with SSRIs, Supplementary Tables 1 and 2), we found that patients treated with only TCAs had a significantly better survival as compared with patients not treated with anti-depressants. As some patients that took TCAs also took other ant-depressants, this could explain that observation.
Our recent work in mouse models identified TCAs as potential agents for the treatment of lung cancer using an agnostic drug repositioning approach,(13) however a recent phase IIA clinical trial treating stage IV small cell lung cancer patients with the TCA desipramine recently ended due to a lack of efficacy and intolerance among most patients to the drug side effects. TCAs have high affinity for the histamine H1 receptor (H1R), the muscarinic acetylcholine receptor (mAchR), the 5-HT2 serotonin receptor (HTR2; and in particular HTR2a), and the α1-adrenergic receptor (ADRA1a and ADRA1b).(22-26) Interestingly, studies have detected these receptors on epithelial cancer cells.(13) Moreover, improved lung cancer survival among patients treated with beta-adrenergic receptor blockers was previously observed(6) while histamine agonists have also been linked to therapeutic response in breast cancer.(27) In lung cancer, TCAs induce cell death via histamine and adrenergic receptors by blocking Gαs and PKA, CREB and ATF1 signaling.(13) Additional studies have confirmed the induction of cell death by TCAs(10,28) while others found associations with reduced incidence of certain cancers.(29) Also, previous work on models of glioma showed a potential synergistic effect between chlorimipramine (a TCA) and dexamethasone,(11,30) while use of the TCA imipramine in combination with citalopram, an SSRI, induced apoptosis of acute myeloid leukemia cells.(31)
NDRIs were the other class of anti-depressant associated with survival. NDRIs mainly function by inhibiting the reuptake of dopamine and norepinephrine.(22,23) Recent studies have provided mechanistic evidence for a link between these neurotransmitters and cancer.(32-34) An agnostic screen of drugs that target cancer stem cells identified thioridazine(35-38)—an anti-psychotic drug that antagonizes dopamine receptors (specifically the DRD2 class)—as a potential anti-tumor drug that antagonizes VEGFR2/PI3K/mTOR signaling.(35-38) In addition, a recent compound library screen of the NCI-60 cell line repository identified the anti-psychotic trifluoperzine (TFP)—a DRD2 antagonist—as a potential anti-metastatic cancer drug.(12) In lung cancer, TFP inhibits the ß-catenin pathway and diminishes the stem-like phenotype.(39) It also reversed gefitinib-resistance. Functional dopamine receptors are expressed on ovarian, glioblastoma, and breast cancer stem cells.(36,38,40) Norepinephrine was recently shown to inhibit the migratory ability of pancreatic cells(41) while as mentioned earlier, beta-adrenergic receptor blockade is also associated with improved outcomes.(6,42-44) Recent work also found that the dopamine transporter inhibitor, sertraline, induced cell death in renal cell carcinoma,(45) data that collectively implicate catecholamine biology in carcinogenesis.(32,33)
Anti-depressants are widely prescribed for the treatment of conditions other than classical depression, including anxiety, circulatory disorders, insomnia and chronic pain management, while some NRDIs—such as Bupropion—are used to aid smoking cessation. Mortality risk has been linked with smoking,(46,47) raising the possibility that the association between this drug with smoking cessation could confound the association between NDRI use and survival. However, in our population, smoking was not associated with survival (former versus never: HR 0.87, 95% C.I. 0.65-1.16) (current versus never: HR 0.91, 95% C.I. 0.69-1.21). In addition, we adjusted our model for smoking; therefore if the relationship between NDRIs and outcome was confounded by smoking cessation, we would have expected this to be reflected in the statistics. However, the association remained significant after the adjustment.
As mentioned, depression may be linked to cancer development.(48) In addition, anti-depressants are often prescribed to treat conditions other than psychological disorders. It was therefore important to determine whether our results were potentially confounded by drug indication. Our analysis found no association between the specific indication of prescribed anti-depressant and lung cancer outcome.
Interestingly, a recent study highlighted a relationship between anti-depressant use and cancer mortality. In a Danish cancer registry study, anti-depressant use--particularly in the 3-4 months before cancer diagnosis—was associated with an increased risk of mortality.(19) Former anti-depressant use was not associated with mortality. However, anti-depressant use was reported as a single group and therefore it is not possible to determine if our results are concordant with this recently published work. A clinical diagnosis of depression has previously been associated with poor outcomes in both breast and lung cancer(49,50); however it is not clear if, and how, anti-depressant use might have contributed to these observations. Of note, recent work has also highlighted the role of early palliative care in outcomes among patients with metastatic lung cancer. Specifically, patients with metastatic lung cancer that received early palliative care had a significant improvement in both quality of life and mood (51). Moreover, these patients received less aggressive care and also had a longer survival.
Our study has several limitations. Firstly, although we were able to ascertain whether patients took anti-depressants around the time of their diagnosis, we were not able to determine this prospectively, or determine the duration of use. Secondly, we do not have complete treatment data available for this cohort of patients and thus, it was not possible to look at potential drug interactions. Further studies will need to address whether the timing, dose and length of treatment can affect these outcomes. Patients in this study were diagnosed over a 12 year period: thus, given that lung cancer survival has incrementally increased over time(52) and that the prevalence of anti-depressant use varied over time, it is possible that there was a higher prevalence of anti-depressant use among the more recently diagnosed patients. We therefore assessed temporal trends in the prevalence of anti-depressant use during the period of study for this cohort. We found that there was no significant trend, either increasing or decreasing, among our population (Supplementary Figure 2), suggesting that this did not confound our results.
In summary, we have shown that lung cancer patients prescribed especially NDRIs and TCAs have prolonged survival. Although the mechanism of action is not completely clear, evidence suggests that a direct physiologic mechanism of action involving neurotransmitter bioavailability and signaling might be involved.(13,32,33) The physiologic effects of stress and depression can affect neuroendocrine, immune, lymphatic and angiogenic responses, each of which could affect cancer progression.(32,33,53) However, depression may also affect how and when cancer patients interact with the medical system, meaning that an indirect mechanism cannot also be ruled out. Thus, further studies will be needed to assess the role of the sympathetic and parasympathetic nervous systems in carcinogenesis. In recent years, the re-positioning of commonly used FDA-approved drugs has garnered interest as a cost and time efficient strategy to uncover novel drugs for cancer treatment. In fact, considering that only a small percent of oncology drugs used in clinical trials attain FDA approval, and that anti-depressants are likely to be less toxic than traditional chemotherapeutic drugs, our insights suggest that further work should be done to understand the mechanism behind these drugs in the context of cancer and to investigate the safe use and repurposing of this drug class in cancer patients. We recognize that the study power is limited and while the results of this study need to be replicated and extended to a larger population size to include finer detail such as drug interactions, dose and duration of exposure, the association of TCA and other NDRI use with improved survival supports a movement towards repurposing existing drugs for lung cancer indication and may be relevant to other cancer types as well.
Supplementary Material
Microabstract.
Catecholamine signaling is increasingly recognized in connection with cancer
The relationship between antidepressant use and survival was assessed in 1,097 lung cancer patients
Antidepressants, which modulate catecholamines, are associated with lung cancer survival
Of the six drug classes tested, TCAs and NDRIs are the main forms associated with outcome
Both TCAs and NDRIs are associated with prolonged patient survival
Acknowledgments
Disclosure of Potential Conflicts of Interest and Funding Sources:
This work was supported by the Intramural Program of the Center for Cancer Research, National Cancer Institute (B.M.R., D.B., E.B., O.V.) and the Department of Defense (grant LC120252 to J.S.). The authors have no conflicts of interest to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. doi 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.National Lung Screening Trial Research T. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. doi 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korpanty GJ, Graham DM, Vincent MD, Leighl NB. Biomarkers That Currently Affect Clinical Practice in Lung Cancer: EGFR, ALK, MET, ROS-1, and KRAS. Front Oncol. 2014;4:204. doi: 10.3389/fonc.2014.00204. doi 10.3389/fonc.2014.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barron TI, Flahavan EM, Sharp L, Bennett K, Visvanathan K. Recent prediagnostic aspirin use, lymph node involvement, and 5-year mortality in women with stage I-III breast cancer: a nationwide population-based cohort study. Cancer Res. 2014;74(15):4065–77. doi: 10.1158/0008-5472.CAN-13-2679. doi 10.1158/0008-5472.can-13-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flahavan EM, Bennett K, Sharp L, Barron TI. A cohort study investigating aspirin use and survival in men with prostate cancer. Ann Oncol. 2014;25(1):154–9. doi: 10.1093/annonc/mdt428. doi 10.1093/annonc/mdt428. [DOI] [PubMed] [Google Scholar]
- 6.Wang HM, Liao ZX, Komaki R, Welsh JW, O'Reilly MS, Chang JY, et al. Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Annals of Oncology. 2013;24(5):1312–9. doi: 10.1093/annonc/mds616. doi Doi 10.1093/Annonc/Mds616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med. 2010;40(11):1797–810. doi: 10.1017/S0033291709992285. doi 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharpe CR, Collet JP, Belzile E, Hanley JA, Boivin JF. The effects of tricyclic antidepressants on breast cancer risk. Br J Cancer. 2002;86(1):92–7. doi: 10.1038/sj.bjc.6600013. doi 10.1038/sj.bjc.6600013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandes LJ, Arron RJ, Bogdanovic RP, Tong J, Zaborniak CL, Hogg GR, et al. Stimulation of malignant growth in rodents by antidepressant drugs at clinically relevant doses. Cancer Res. 1992;52(13):3796–800. [PubMed] [Google Scholar]
- 10.Daley E, Wilkie D, Loesch A, Hargreaves IP, Kendall DA, Pilkington GJ, et al. Chlorimipramine: a novel anticancer agent with a mitochondrial target. Biochem Biophys Res Commun. 2005;328(2):623–32. doi: 10.1016/j.bbrc.2005.01.028. doi 10.1016/j.bbrc.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 11.Higgins SC, Pilkington GJ. The in vitro effects of tricyclic drugs and dexamethasone on cellular respiration of malignant glioma. Anticancer Res. 2010;30(2):391–7. [PubMed] [Google Scholar]
- 12.Pulkoski-Gross AE, Li J, Zheng C, Li Y, Ouyang N, Rigas B, et al. Repurposing the Anti-psychotic Trifluoperazine as an Anti-metastasis Agent. Mol Pharmacol. 2014 doi: 10.1124/mol.114.096941. doi 10.1124/mol.114.096941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jahchan NS, Dudley JT, Mazur PK, Flores N, Yang D, Palmerton A, et al. A drug repositioning approach identifies tricyclic antidepressants as inhibitors of small cell lung cancer and other neuroendocrine tumors. Cancer Discov. 2013;3(12):1364–77. doi: 10.1158/2159-8290.CD-13-0183. doi 10.1158/2159-8290.cd-13-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu W, Tamim H, Shapiro S, Stang MR, Collet JP. Use of antidepressants and risk of colorectal cancer: a nested case-control study. Lancet Oncol. 2006;7(4):301–8. doi: 10.1016/S1470-2045(06)70622-2. doi 10.1016/s1470-2045(06)70622-2. [DOI] [PubMed] [Google Scholar]
- 15.Toh S, Rodriguez LA, Hernandez-Diaz S. Use of antidepressants and risk of lung cancer. Cancer Causes Control. 2007;18(10):1055–64. doi: 10.1007/s10552-007-9045-1. doi 10.1007/s10552-007-9045-1. [DOI] [PubMed] [Google Scholar]
- 16.Zheng YL, Loffredo CA, Yu Z, Jones RT, Krasna MJ, Alberg AJ, et al. Bleomycin-induced chromosome breaks as a risk marker for lung cancer: a case-control study with population and hospital controls. Carcinogenesis. 2003;24(2):269–74. doi: 10.1093/carcin/24.2.269. [DOI] [PubMed] [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94(446):496–509. doi Doi 10.2307/2670170. [Google Scholar]
- 18.Pratt LA, Brody DJ. Antidepressant use in persons aged 12 and over: United States, 2005–2008. National Center for Health Statistics; Hyattsville, MD: 2011. Q G. [Google Scholar]
- 19.Sun Y, Vedsted P, Fenger-Grøn M, Wu CS, Bech BH, Olsen J, et al. Cancer Mortality in People Treated with Antidepressants before Cancer Diagnosis: A Population Based Cohort Study. PLoS One. 2015;10(9):e0138134. doi: 10.1371/journal.pone.0138134. doi 10.1371/journal.pone.0138134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashbury FD, Madlensky L, Raich P, Thompson M, Whitney G, Hotz K, et al. Antidepressant prescribing in community cancer care. Support Care Cancer. 2003;11(5):278–85. doi: 10.1007/s00520-003-0446-8. doi 10.1007/s00520-003-0446-8. [DOI] [PubMed] [Google Scholar]
- 21.Walker J, Sawhney A, Hansen CH, Symeonides S, Martin P, Murray G, et al. Treatment of depression in people with lung cancer: a systematic review. Lung Cancer. 2013;79(1):46–53. doi: 10.1016/j.lungcan.2012.09.014. doi 10.1016/j.lungcan.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Stahl SM, Pradko JF, Haight BR, Modell JG, Rockett CB, Learned-Coughlin S. A Review of the Neuropharmacology of Bupropion, a Dual Norepinephrine and Dopamine Reuptake Inhibitor. Prim Care Companion J Clin Psychiatry. 2004;6(4):159–66. doi: 10.4088/pcc.v06n0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richelson E. Synaptic effects of antidepressants. J Clin Psychopharmacol. 1996;16(3 Suppl 2):1S–7S. doi: 10.1097/00004714-199606002-00001. discussion S-9S. [DOI] [PubMed] [Google Scholar]
- 24.Petersen RC, Richelson E. Anticholinergic activity of imipramine and some analogs at muscarinic receptors of cultured mouse neuroblastoma cells. Psychopharmacology (Berl) 1982;76(1):26–8. doi: 10.1007/BF00430749. [DOI] [PubMed] [Google Scholar]
- 25.Chen G, Hasanat KA, Bebchuk JM, Moore GJ, Glitz D, Manji HK. Regulation of signal transduction pathways and gene expression by mood stabilizers and antidepressants. Psychosom Med. 1999;61(5):599–617. doi: 10.1097/00006842-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Donati RJ, Rasenick MM. G protein signaling and the molecular basis of antidepressant action. Life Sci. 2003;73(1):1–17. doi: 10.1016/s0024-3205(03)00249-2. [DOI] [PubMed] [Google Scholar]
- 27.Martinel Lamas DJ, Croci M, Carabajal E, Crescenti EJ, Sambuco L, Massari NA, et al. Therapeutic potential of histamine H4 receptor agonists in triple-negative human breast cancer experimental model. Br J Pharmacol. 2013;170(1):188–99. doi: 10.1111/bph.12137. doi 10.1111/bph.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arimochi H, Morita K. Desipramine induces apoptotic cell death through nonmitochondrial and mitochondrial pathways in different types of human colon carcinoma cells. Pharmacology. 2008;81(2):164–72. doi: 10.1159/000111144. doi 10.1159/000111144. [DOI] [PubMed] [Google Scholar]
- 29.Walker AJ, Card T, Bates TE, Muir K. Tricyclic antidepressants and the incidence of certain cancers: a study using the GPRD. Br J Cancer. 2011;104(1):193–7. doi: 10.1038/sj.bjc.6605996. doi 10.1038/sj.bjc.6605996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levkovitz Y, Gil-Ad I, Zeldich E, Dayag M, Weizman A. Differential induction of apoptosis by antidepressants in glioma and neuroblastoma cell lines: evidence for p-c-Jun, cytochrome c, and caspase-3 involvement. J Mol Neurosci. 2005;27(1):29–42. doi: 10.1385/JMN:27:1:029. doi 10.1385/JMN:27:1:029. [DOI] [PubMed] [Google Scholar]
- 31.Xia Z, DePierre JW, Nässberger L. Modulation of apoptosis induced by tricyclic antidepressants in human peripheral lymphocytes. J Biochem Mol Toxicol. 1998;12(2):115–23. doi: 10.1002/(sici)1099-0461(1998)12:2<115::aid-jbt6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 32.Moreno-Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future Oncol. 2010;6(12):1863–81. doi: 10.2217/fon.10.142. doi 10.2217/fon.10.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen S, Rabin BS. Psychologic stress, immunity, and cancer. J Natl Cancer Inst. 1998;90(1):3–4. doi: 10.1093/jnci/90.1.3. [DOI] [PubMed] [Google Scholar]
- 34.Robles AI, Yang P, Jen J, McClary AC, Calhoun K, Bowman ED, et al. A DRD1 Polymorphism Predisposes to Lung Cancer among Those Exposed to Secondhand Smoke during Childhood. Cancer Prev Res (Phila) 2014 doi: 10.1158/1940-6207.CAPR-14-0158. doi 10.1158/1940-6207.capr-14-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu M, Li J, Luo Z, Zhang S, Xue S, Wang K, et al. Roles of dopamine receptors and their antagonist thioridazine in hepatoma metastasis. Onco Targets Ther. 2015;8:1543–52. doi: 10.2147/OTT.S77373. doi 10.2147/OTT.S77373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mao M, Yu T, Hu J, Hu L. Dopamine D2 receptor blocker thioridazine induces cell death in human uterine cervical carcinoma cell line SiHa. J Obstet Gynaecol Res. 2015 doi: 10.1111/jog.12691. doi 10.1111/jog.12691. [DOI] [PubMed] [Google Scholar]
- 37.Park MS, Dong SM, Kim BR, Seo SH, Kang S, Lee EJ, et al. Thioridazine inhibits angiogenesis and tumor growth by targeting the VEGFR-2/PI3K/mTOR pathway in ovarian cancer xenografts. Oncotarget. 2014;5(13):4929–34. doi: 10.18632/oncotarget.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sachlos E, Risueno RM, Laronde S, Shapovalova Z, Lee JH, Russell J, et al. Identification of drugs including a dopamine receptor antagonist that selectively target cancer stem cells. Cell. 2012;149(6):1284–97. doi: 10.1016/j.cell.2012.03.049. doi 10.1016/j.cell.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 39.Yeh CT, Wu AT, Chang PM, Chen KY, Yang CN, Yang SC, et al. Trifluoperazine, an antipsychotic agent, inhibits cancer stem cell growth and overcomes drug resistance of lung cancer. Am J Respir Crit Care Med. 2012;186(11):1180–8. doi: 10.1164/rccm.201207-1180OC. doi 10.1164/rccm.201207-1180OC. [DOI] [PubMed] [Google Scholar]
- 40.Li J, Zhu S, Kozono D, Ng K, Futalan D, Shen Y, et al. Genome-wide shRNA screen revealed integrated mitogenic signaling between dopamine receptor D2 (DRD2) and epidermal growth factor receptor (EGFR) in glioblastoma. Oncotarget. 2014;5(4):882–93. doi: 10.18632/oncotarget.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stock AM, Powe DG, Hahn SA, Troost G, Niggemann B, Zänker KS, et al. Norepinephrine inhibits the migratory activity of pancreatic cancer cells. Exp Cell Res. 2013;319(12):1744–58. doi: 10.1016/j.yexcr.2013.04.015. doi 10.1016/j.yexcr.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 42.Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population-based study. J Clin Oncol. 2011;29(19):2635–44. doi: 10.1200/JCO.2010.33.5422. doi 10.1200/JCO.2010.33.5422. [DOI] [PubMed] [Google Scholar]
- 43.Palm D, Lang K, Niggemann B, Drell TL, Masur K, Zaenker KS, et al. The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by beta-blockers. Int J Cancer. 2006;118(11):2744–9. doi: 10.1002/ijc.21723. doi 10.1002/ijc.21723. [DOI] [PubMed] [Google Scholar]
- 44.Yang EV, Sood AK, Chen M, Li Y, Eubank TD, Marsh CB, et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006;66(21):10357–64. doi: 10.1158/0008-5472.CAN-06-2496. doi 10.1158/0008-5472.CAN-06-2496. [DOI] [PubMed] [Google Scholar]
- 45.Schrödter S, Braun M, Syring I, Klümper N, Deng M, Schmidt D, et al. Identification of the dopamine transporter SLC6A3 as a biomarker for patients with renal cell carcinoma. Mol Cancer. 2016;15:10. doi: 10.1186/s12943-016-0495-5. doi 10.1186/s12943-016-0495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanner NT, Kanodra NM, Gebregziabher M, Payne E, Hughes Halbert C, Warren GW, et al. The Association Between Smoking Abstinence and Mortality in the National Lung Screening Trial. Am J Respir Crit Care Med. 2015 doi: 10.1164/rccm.201507-1420OC. doi 10.1164/rccm.201507-1420OC. [DOI] [PubMed] [Google Scholar]
- 47.Meyer J, Rohrmann S, Bopp M, Faeh D, Group SNCS Impact of Smoking and Excess Body Weight on Overall and Site-Specific Cancer Mortality Risk. Cancer Epidemiol Biomarkers Prev. 2015;24(10):1516–22. doi: 10.1158/1055-9965.EPI-15-0415. doi 10.1158/1055-9965.EPI-15-0415. [DOI] [PubMed] [Google Scholar]
- 48.Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5(10):617–25. doi: 10.1016/S1470-2045(04)01597-9. doi 10.1016/s1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- 49.Chen J, Li W, Cui L, Qian Y, Zhu Y, Gu H, et al. Chemotherapeutic Response and Prognosis among Lung Cancer Patients with and without Depression. J Cancer. 2015;6(11):1121–9. doi: 10.7150/jca.11239. doi 10.7150/jca.11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goodwin JS, Zhang DD, Ostir GV. Effect of depression on diagnosis, treatment, and survival of older women with breast cancer. J Am Geriatr Soc. 2004;52(1):106–11. doi: 10.1111/j.1532-5415.2004.52018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–42. doi: 10.1056/NEJMoa1000678. doi 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 52.Kachroo S, Tong L, Spitz MR, Xing Y, Merriman K, Zhu DK, et al. Trends in prevalence of prognostic factors and survival in lung cancer patients from 1985 to 2004 at a tertiary care center. Cancer Detect Prev. 2008;32(2):101–8. doi: 10.1016/j.cdp.2008.05.009. doi 10.1016/j.cdp.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Le CP, Nowell CJ, Kim-Fuchs C, Botteri E, Hiller JG, Ismail H, et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat Commun. 2016;7:10634. doi: 10.1038/ncomms10634. doi 10.1038/ncomms10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.