Abstract
Exposure to polychlorinated biphenyls (PCBs), a class of endocrine-disrupting chemicals, can result in altered reproductive behavior in adulthood, especially when exposure occurs during critical periods of brain sexual differentiation in the fetus. Whether PCBs alter other sexually dimorphic behaviors such as those involved in anxiety is poorly understood. To address this, pregnant rat dams were injected twice, on gestational days 16 and 18, with the weakly estrogenic PCB mixture Aroclor 1221 (A1221) at one of two low dosages (0.5 mg/kg or 1.0 mg/kg, hereafter 1.0 and 0.5), estradiol benzoate (EB; 50 μg/kg) as a positive estrogenic control, or the vehicle (3% DMSO in sesame oil). We also conducted a comprehensive assessment of developmental milestones of the F1 male and female offspring. There were no effects of treatment on sex ratio at birth and age at eye opening. Puberty, assessed by vaginal opening in females and preputial separation in males, was not affected in females but was advanced in males treated with A1221 (1.0). Males and females treated with A1221 (both dosages) were heavier in early adulthood relative to controls. The earliest manifestation of this effect developed in males prior to puberty and in females slightly later, during puberty. Anxiety-like behaviors were tested using the light:dark box and elevated plus maze tests in adulthood. In females, anxiety behaviors were unaffected by treatment. Males treated with A1221 (1.0) showed reduced indices of anxiety and increased activity in the light:dark box but not the elevated plus maze. EB failed to replicate the phenotype produced by A1221 for any of the developmental and behavioral endpoints. Collectively, these results indicate that PCBs increase body weight in both sexes, but their effects on anxiety-like behaviors are specific to males. Furthermore, differences between the results of A1221 and EB suggest that the PCBs are likely acting through mechanisms distinct from their estrogenic activity.
Keywords: Aroclor 1221, Development, Fetal exposure, Anxiety, Light:dark box, Elevated plus maze, Endocrine-disrupting chemical, PCB
1. Introduction
Endocrine disrupting chemicals (EDCs) are environmental contaminants that interfere with reproductive, endocrine, and metabolic functions. Fetal exposures to EDCs, even at low dosages, can affect the developmental trajectory of an individual due to the high sensitivity of the developing organism to both natural and synthetic hormones. In the embryonic brain, endogenous gonadal hormones play important roles in neuronal birth, apoptosis, migration, and synaptic connectivity in a sex-specific manner that dictates sex-typical behaviors and functions later in life (Crews et al., 2014; Gore et al., 2015).
Polychlorinated biphenyls (PCB), a class of EDCs, are widespread synthetic organic chlorinated compounds that were used for decades in industry. When improperly stored or disposed of, PCBs entered the environment in soil, water, and air, and were subsequently incorporated into the food chain. Although banned in 1979 (McFarland and Clarke, 1989), nearly all humans and wildlife have measurable amounts of PCBs in their bodies (Meeker et al., 2011; Quinn et al., 2011).
PCB exposure late in fetal development altered developmental milestones (Dickerson et al., 2011a) and affected adult sexual (Chung et al., 2001; Colciago et al., 2009; Steinberg et al., 2007a, 2008) and social (Reilly et al., 2015) behaviors. Moreover, PCBs changed levels of neurotransmitters and their receptors in the brain, including those involved in anxiety and affective states. In Wistar rats, serotonin metabolites in the prefrontal cortex and hippocampus were increased by exposure to PCBs in late gestation (Morse et al., 1996). Work conducted both in vivo and in in vitro brain slices showed that dopamine synthesis, release, and re-uptake were perturbed by PCB treatments (Bemis and Seegal, 1999; Chishti et al., 1996).
Although the literature on links between PCBs and affective behaviors is limited, in male rats, exposure to PCBs during fetal development resulted in increased anxiety behavior and hypothalamic-pituitary-adrenal reactivity to stressful events during adolescence (PND 28–35) (Orito et al., 2007). These results are likely translatable to humans, as epidemiological studies associated increased PCB blood concentrations in aged adults with deficits in learning and memory, and increased depressive symptoms (Fitzgerald et al., 2008). Here, we hypothesized that exposure during sensitive periods of fetal development to low dosages of PCBs would alter anxiety-like behaviors in adulthood in a sex-specific manner. We selected a lightly-chlorinated, weakly estrogenic industrial PCB mixture, Aroclor 1221 (A1221), comparing it to estradiol benzoate (EB), and thereby enabling us to determine whether A1221’s effects, if any, were similar to those of estradiol. In addition, we sought to determine whether these treatments had more general physiological effects on postnatal developmental landmarks influenced by prenatal hormones and EDCs.
2. Materials and methods
2.1. Animals, husbandry, and PCB treatment
Male and female Sprague Dawley rats were purchased from Harlan (Houston, TX) at 2–3 months of age. All animals were housed in standard rat polycarbonate cages (46 cm × 24 cm × 20.5 cm) with ad libitum access to low phytoestrogen rat chow (Harlan, #2019) and tap water, on a 12L:12D cycle (lights off at 1200). Eleven DMSO and EB and ten A1221 (1.0 and 0.5) litters distributed equally across seven cohorts were bred for analysis. All animal work was conducted using humane procedures that were approved by the Institutional Animal Care and Use Committee at the University of Texas at Austin. Following acclimation to the housing facility for 1 week, females (virgins) were tested for receptivity on proestrus. If receptive they were left with a sexually experienced male overnight. The following morning the male was removed and presence of sperm was verified via vaginal smear. If sperm was present, embryonic day (E)1 was noted. Pregnant dams were weighed weekly and checked for health but otherwise left undisturbed until E16. On that date, and again on E18, dams were injected i.p. with the same treatment given on both days: vehicle (3% dimethyl sulfoxide [DMSO] in sesame oil; Sigma), 0.5 mg/kg A1221 (Accustandard), 1.0 mg/kg A1221, or 50 μg/kg estradiol benzoate (EB), within 2 h of lights off. All treatments used 3% DMSO in sesame oil as the vehicle. EB served as a positive estrogenic control for the weakly estrogenic effects of A1221. PCB concentrations in fetal tissue was not measured, but it has previously been predicted that the dosages used in the current study result in a fetal exposure of approximately 2 μg/kg (Dickerson et al., 2011b; Steinberg et al., 2007b; Takagi et al., 1986), an exposure that is relevant to circulating PCBs in humans (Fitzgerald et al., 2008; Centers for Disease Control, 2009). Nesting material was provided to pregnant dams beginning on E18.
On postnatal day (PND) 1, the day after birth, pups were weighed and their anogenital distance (AGD) was measured using a digital microcaliper. The anogenital index (AGI) was calculated (AGI = AGD divided by the cube root of body weight) as it enables proper sexing of neonates and also serves as an indicator of masculinization or feminization (Vandenbergh and Huggett, 1995). Litters were culled based on AGI. The four males and females with AGIs closest to the median of the same sex and litter were kept to achieve equal litter size and sex ratio. All litters, including controls, were culled to a total of 8 pups (4 male and 4 female) to ensure that sex ratio was equal, because a biased sex ratio changes behavioral outcomes (de Medeiros et al., 2010) and to allow us to attain adequate statistical power based on a power analysis. We note that litters of 8 pups, compared to litters of 10 raised similarly in our lab, are about 10% heavier prior to weaning, but that this levels off after weaning, and treatment effects on body weight are similar in litters of 8 or 10 (Unpublished data). AGD was measured once per week until PND 14. Pups were weaned at PND 21 and were housed with same-sex siblings four per cage until PND 49 and then subdivided to two per cage. Beginning on PND 28 and PND 35 individuals were checked daily for vaginal opening and preputial separation, respectively. All animals were weighed and handled for 5 min once per week after PND 21. Two males and two females from each litter were randomly selected for behavioral analysis. Dams were euthanized between 1 and 2 weeks after pups were weaned in order to examine the uterus for the number of implantation sites.
2.2. Testosterone radioimmunoassay in males
Trunk blood was collected from males when they were later euthanized at PND 90. Serum was used to determine circulating testosterone according to the manufacturer’s protocols (MP Biomedical; Testosterone 125I RIA, Catalog #07189102). Littermates that were not behaviorally characterized were included in testosterone analysis. The assay sensitivity was 0.03 ng/ml and the average intra-assay variability was 1.56%. This assay is not sensitive enough to measure serum testosterone in females.
2.3. Behavioral testing
Beginning on PND 60, each animal randomly selected for behavioral testing (2 males and 2 females per litter) was subjected to a battery of 5 behavioral tasks in a randomized order in attempt to minimize the effect of previous tests. Results on tests of sociability and social novelty have been published (Reilly et al., 2015). Tests of sexuality (mate preference and ultrasonic vocalizations) are still under analysis (manuscript under preparation). The tests of anxiety-like behaviors (elevated plus maze and light:dark box) are presented here. We made the decision to publish the 3 categories of behaviors separately as we found that each one told a unique story, and overlapping those stories became unwieldy. Each behavioral task was performed at least 48 h apart and there was no effect of testing order. Estrous cycles of females were monitored daily, but females were tested in the elevated plus maze and light:dark box on a random cycle day. Cycle status was included as a covariate during statistical analysis and confirmed not to affect behavioral outcomes. All behavioral tasks were conducted 1–3 h after lights off, using apparati and protocols modified from work previously published by our lab (Gillette et al., 2014). All work was done with the experimenter blind to treatment, and data uncoded only after analysis was complete.
2.3.1. Light:dark box
The rectangular light:dark apparatus (Stoelting) was bisected into two chambers, one clear and the other opaque black Plexiglas (each 50 × 50 cm), with a passageway between them. The light field was diffusely illuminated with a 60 w incandescent light bulb ~3 m from the top of the enclosure. The dark field was covered with a black Plexiglas transparent lid that permitted red light to pass, allowing observation and video recording of the animal in the dark field. At the beginning of the light:dark box test, the animal was placed at the entrance to the dark field facing the light field and tracked for 5 min.
2.3.2. Elevated-plus maze
The elevated plus apparatus (Stoelting) was raised 40 cm above the ground. The arms of the plus maze were 10 cm wide and 50 cm long. The two closed arms of the elevated plus maze were surrounded by 40 cm walls. Elevated plus testing was conducted under diffuse red light. Animals were allowed 5 min to acclimate to the testing room and then placed at the center of the cross facing an open arm, and recorded for 5 min.
Behavioral tasks were video recorded and subsequently analyzed with Any-Maze (Stoelting). Between tests, the Plexiglas enclosures were wiped down with 70% ethanol and allowed to air dry for 10 min. Any-Maze recorded the position of each animal 30 times per second. The barrier between a closed arm and open arm or the light field and the dark field was drawn virtually within Any-Maze. An entry to a field was automatically scored using 80% of the animal’s total area as viewed by the camera, which corresponded to all 4 paws in the field. The time spent in each field, distance traveled, mean speed, line crossings, and time immobile were recorded.
2.4. Statistics
All analyses were performed in R with the base, nlme, multcomp, heplots, and effsize packages. Graphs were created using the ggplot2 R package and modified for style with Adobe Illustrator CS5.
Male and female rats display different baseline anxiety behaviors in the elevated-plus maze and light:dark box. In general, females tend to show higher mobility and more time in the aversive arena (Palanza, 2001). These predicted sex differences were verified for each test. Vehicle-treated (DMSO) males and females were used as baselines for post hoc pairwise analyses.
2.4.1. Birth outcomes and developmental milestones
Birth outcomes (number of live births, sex ratio, implantation sites, and resorptions) were analyzed using the non-parametric Mann-Whitney U test because the data were not normally distributed. Pairwise comparisons were performed and corrected for multiple comparisons (Benjamini and Hochberg, 1995). The age at eye opening, vaginal opening or preputial separation, and circulating testosterone were analyzed within sex via a one-way ANOVA followed by pairwise post hoc tests to determine group differences.
2.4.2. Repeated measures analyses of body weight and AGI
The sexes were analyzed separately by repeated measures analysis followed by Tukey post hoc tests, the latter reported with Z-value and probability value. Analyses of body weight were subdivided into three life phases to account for different body weight curves: prepubertal (PND 1–21), after weaning through puberty (adolescence, PND 28–56); and in adulthood (PND 63–91). AGI was analyzed similarly but only on PND 1, 7, and 14.
2.4.3. Behavior
For the light:dark box and elevated plus maze, we analyzed the time individuals explored the aversive arena (light box or open arms), line crossings between the arena, and general mobility measures: mean speed, distance traveled, and time immobile. For each of these measures, a two-way ANOVA was used to determine effects of treatment and sex. Pairwise post hoc tests were performed and were corrected for multiple comparisons [Benjamini-Hochberg correction for multiple comparisons (Benjamini and Hochberg, 1995)]. Significance after correction was set at p < 0.05. Effect size comparisons were performed for ANOVA (partial eta-squared, ) and post hoc tests (Cohen’s d, d). Eta-squared represents the proportion of variance that is accounted for by the factor that is tested. An greater than 0.06 is considered medium and an greater than 0.14 is considered a large effect size. A Cohen’s d of 0.8 is considered a large effect size and represents a difference in means that equals 0.8 standard deviations.
2.4.4. Litter usage and sample size
Numbers of litters produced for each treatment were DMSO (10), EB (10), A1221 1.0 (11) and A1221 0.5 (11) based on previous power analyses. For behavioral analysis, 2 animals per litter per sex were used to provide a larger number of individuals without any one litter contributing more than 2 rats to a statistical endpoint. Initial ANCOVAs were run to determine if litter identity had any effect on these outcomes, and it did not, so subsequent analyses included the two littermates as individuals. This resulted in final N’s for behaviors of: Males: DMSO = 21, EB = 22, A1221 (0.5) = 19, A1221 (1.0) = 20, and Females: DMSO = 22, EB = 22, A1221 (0.5) = 20, A1221 (1.0) = 20. For birth outcomes, developmental milestones, and biometric measures, four animals per litter per sex were used when available (Males; DMSO = 40, EB = 43, A1221 (0.5) = 37, A1221 (1.0) = 39, Females; DMSO = 43, EB = 42, A1221 (0.5) = 38, A1221 (1.0) = 38). Serum testosterone in males, measured when they were euthanized at PND 90, was assayed in all animals whether or not they were behaviorally characterized [N = 36, 41, 37, 37 for DMSO, EB, A1221 (0.5) and A1221 (1.0)].
3. Results
3.1. Birth outcomes and developmental milestones
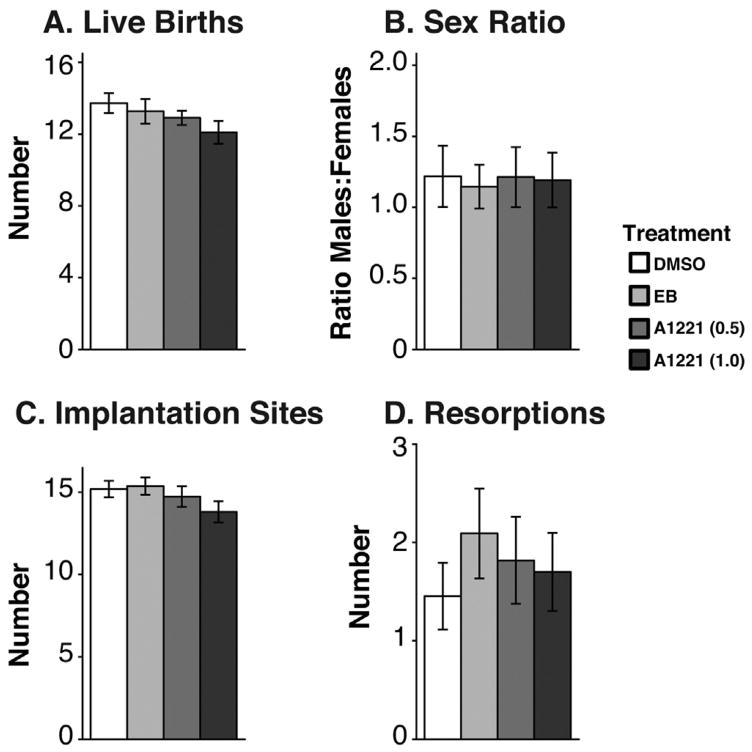
The number of live pups at birth, and sex ratio at birth, were unaffected by treatment (Fig. 1A and B, respectively). The uterus of the dams was examined to ascertain number of implantation sites and to calculate the number of resorptions based on the number of live pups. Treatment did not affect the number of implantation sites or the number of resorptions (Fig. 1C and D, respectively). The age at eye opening was also unaffected by treatment (Table 1). The timing of vaginal opening in females was unaffected by treatment. In males, preputial separation was advanced by treatment (F3,159 = 3.39, p < 0.05, ; Table 1). Post hoc analysis showed that this was driven by the A1221 (1.0) compared to the DMSO males (p < 0.05, d = 0.686; Table 1). Treatment did not have an effect on circulating testosterone (F3,151 = 0.423, p = 0.74; Table 1).
Fig. 1.
Birth outcomes. (A) Numbers of live births per litter. (B) The sex ratio of each litter at birth (males:females). (C) The number of implantation sites counted on the uterus in dams after euthanasia. (D) The number of resorptions per litter calculated as the difference between live births and implantation sites. No effects of treatment were found for any of these measures. Data shown are mean ± SEM.
Table 1.
Developmental milestones. Age at eye opening was not affected by treatment in males or females. Vaginal opening (VO) in females and preputial separation (PPS) in males were used as markers of puberty. VO was unaffected by treatment in females. A1221 (1.0) slightly but significantly advanced PPS compared to DMSO (*p < 0.05). Treatment did not have an effect on circulating testosterone in males. Data shown are mean ± SEM.
| Treatment | Females | Males | |
|---|---|---|---|
|
| |||
| Mean ± SEM | Mean ± SEM | ||
| Eye opening (PND) | DMSO | 15.24 ± 0.10 | 15.35 ± 0.10 |
| EB | 15.08 ± 0.11 | 15.35 ± 0.10 | |
| A1221 (0.5) | 15.01 ± 0.10 | 15.15 ± 0.08 | |
| A1221 (1.0) | 14.97 ± 0.07 | 15.12 ± 0.08 | |
| Puberty (PND) | DMSO | 34.79 ± 0.28 | 43.52 ± 0.26 |
| EB | 34.86 ± 0.32 | 42.93 ± 0.23 | |
| A1221 (0.5) | 34.66 ± 0.19 | 43.00 ± 0.19 | |
| A1221 (1.0) | 34.16 ± 0.31 | 42.51 ± 0.20* | |
| Testosterone (ng/ml) | DMSO | 0.93 ± 0.22 | |
| EB | 0.75 ± 0.11 | ||
| A1221 (0.5) | 0.76 ± 0.09 | ||
| A1221 (1.0) | 0.76 ± 0.10 | ||
3.2. Body weight and AGI
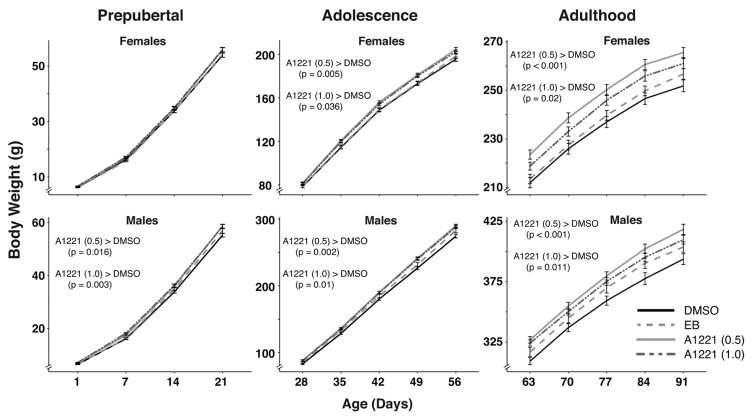
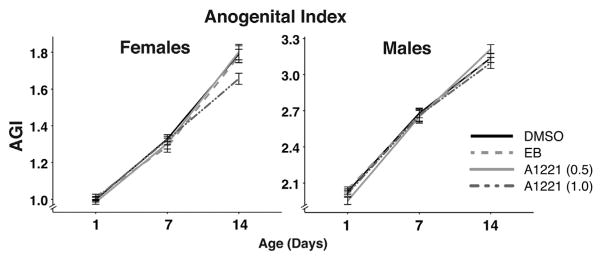
Prepubertal males, but not females, showed an effect of treatment on body weight (males: F3,475 = 5.21, p < 0.005, ; females: F3,499 = 0.99, p = 0.12, ; Fig. 2). Post hoc analysis revealed that both A1221 (0.5; z = 2.96, p < 0.05) and (1.0; z = 3.49, p < 0.005) males were heavier than DMSO males. Both males and females showed an effect of treatment on body weight in adolescence (males: F3,634 = 5.38, p < 0.001, ; females: F3,642 = 5,27, p < 0.005, ). Post hoc analysis in males again revealed that A1221 (0.5 mg/kg; z = 3.54, p < 0.005) and A1221 (1.0 mg/kg; z = 3.27, p < 0.01) animals weighed more than DMSO animals. Similarly, in females, both A1221 (0.5) and (1.0) dosages resulted in heavier rats compared to the DMSO group (0.5 mg/kg: z = 3.32, p < 0.005; 1 mg/kg: z = 2.69, p < 0.05). In adulthood, both males and females continued to have significant treatment effects (males: F3,634 = 6.22, p < 0.001, ; females: F3,643 = 8.37, p < 0.0001, ). Post hoc tests showed that A1221 (0.5) and (1.0) treated rats were heavier than DMSO in males (z = 4.10, p < 0.001; z = 3.08, p < 0.05) and females (z = 4.58, p < 0.001; z = 2.89, p < 0.05). Repeated measures analysis on AGI did not identify any significant effects of treatment from PND 1 to PND 14 in either sex (Fig. 3).
Fig. 2.
Body weight (g) from birth through adulthood are shown in females and males during three developmental windows: prepubertal (PND 1–21), adolescence (PND 28–56) and adulthood (PND 63–91). Females treated with either dosage of A1221 were heavier than DMSO females in adolescence and adulthood. Males treated with either dosage of A1221 were heavier than DMSO males beginning in prepuberty and through adulthood. p-Values for significant differences are listed within each figure. Data shown are mean ± SEM.
Fig. 3.
Anogenital index, calculated as anogenital distance divided by the cube root of body weight, is shown on PND 1, 7 and 14. AGI over the course of neonatal development was unaffected by treatment in females and males. Data shown are mean ± SEM.
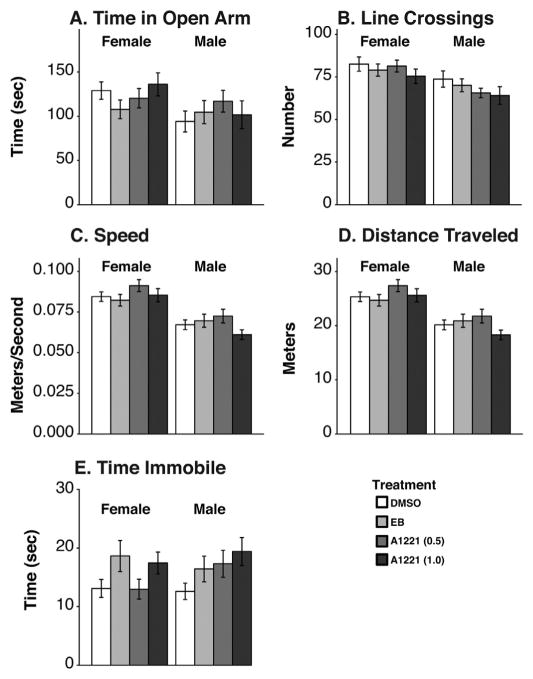
3.3. Behavior in the elevated plus maze
The predicted sex difference in the elevated plus maze was replicated in this study. As adults, females traveled faster and farther (F1,165 = 50.78, p < 0.001, ; F1,165 = 50.92, p < 0.001, , respectively) and crossed more lines than males (F1,165 = 14.71, p < 0.001, ; Fig. 4B, C, and D). Females spent more time in the open arm than males (F1,165 = 9.05, p < 0.005, ; Fig. 4A), but there were no treatment effects. A main effect of treatment was found for time spent immobile, which was the only measure affected by treatment for this apparatus (F3,163 = 2.91 p < 0.05, ; Fig. 4E). Compared to DMSO animals, EB and A1221 (1.0) females and all three male treatment groups showed increased time immobile but post hoc tests failed to differentiate between treatment groups.
Fig. 4.
Elevated plus maze behaviors are shown. (A) Time spent in the open arm of the plus maze, (B) numbers of line crossings, (C) average speed, and (D) total distance traveled, were significantly greater in females than males. (E) Time spent immobile was affected by treatment but post hoc analyses failed to distinguish individual treatment groups. Data shown are mean ± SEM.
3.4. Behavior in the light:dark box
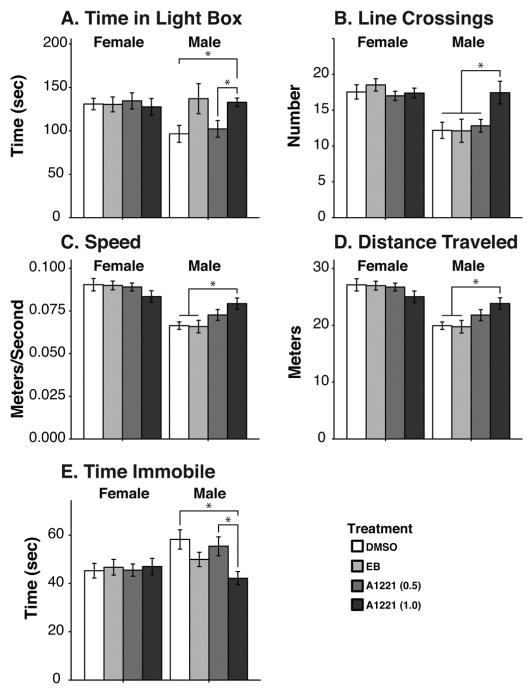
Again, the predicted sex difference in mobility measures was found. Females crossed more lines and traveled faster and farther than males (F1,165 = 24.88, p < 0.001, ; F1,165 = 62.01, p < 0.001, ; F1,165 = 62.49 p ≤ 0.001, , respectively; Fig. 5B, C, and D). Females also spent less time immobile than males (F1,165 = 3.91, p < 0.05, ), attributable to a sex by treatment interaction (F3,163 = 2.92, p < 0.05, ; Fig. 5E). In females, there were no significant effects of treatment on any measures. By contrast, males treated with A1221 (1.0) crossed between the chambers more often and traveled faster and farther than DMSO males (all p < 0.05, d = 0.850, d = 1.021, d = 1.031; Fig. 5B, C, and D). Males exposed prenatally to the A1221 (1.0) dosage also spent less time immobile than control males (p < 0.01, d = 1.023; Fig. 5E). This group also spent more time in the light box than DMSO and A1221 (0.5) males (p < 0.05, d = 1.018; p < 0.05, d = 0.940; Fig. 5A).
Fig. 5.
Light:dark box behaviors are shown. (A) The amount of time spent in the light box was affected only in males. A1221 (1.0) males spent more time in the light box than the DMSO or the A1221 (0.5) males. A sex difference was found for (B) the number of line crossings, (C) mean speed, and (D) distance traveled, all of which were greater in females. There were no treatment effects in females. Males treated with A1221 (1.0) had more line crossings (B), were faster (C), and traveled farther (D), than either the DMSO or the EB males. (E) The total time spent immobile, regardless of location was lower in the A1221 (1.0) males than the DMSO or A1221 (0.5) males. Significant differences determined by post hoc tests are indicated within each figure as brackets, with *p < 0.05. Data shown are mean ± SEM.
4. Discussion
The current study aimed to determine the effects of prenatal exposure to PCBs on birth outcomes, developmental milestones, and anxiety behavior in adulthood. Consistent with our expectations for the low dosages and limited exposure period (two days during the last week of gestation), there were no overt signs of toxicity of the treatments, evidenced by no effects on birth outcomes. While the A1221 mixture had no effect on puberty in females, it caused a small but significant advance in puberty in males. The results on the effects of A1221 on anxiety-like behaviors in adulthood were more interesting, with mixed outcomes depending upon the sex of the animal, and the behavioral test used. We observed the prototypical sex differences in measures of general mobility and time spent in aversive arenas in both the elevated plus maze and the light:dark box, but PCB exposure did not alter any of these behaviors in females. By contrast, males showed decreased anxiety and increased mobility specific to the light:dark box. These results reveal that at least with respect to the endpoints measured, males are more sensitive to PCB exposures in late gestation, and that this is manifested, at least in part, as decreased anxiety in adulthood.
4.1. PCBs and anxiety behaviors
The body of literature from animal models and human epidemiological studies linking EDC exposures to anxiety behaviors is growing, especially for BPA and phthalates (Braun et al., 2011; Carbone et al., 2013; Engel et al., 2010; Kundakovic et al., 2013). However, this literature is relatively limited for PCBs. Although head-to-head comparisons can be difficult to make due to differences in treatment paradigm, age of testing, and endpoints of analysis, prior work showed that PCB exposure altered affective behaviors. Neonatal mice exposed to PCBs during nursing showed increased anxiety behavior during puberty and in adulthood as measured by the elevated plus maze and light:dark box respectively (Elnar et al., 2012). Male Wistar rats exposed to PCB 126, a congener more heavily chlorinated than the A1221 mixture, from E7 –PND 21 via maternal feeding, had increased general mobility in the elevated plus maze and light:dark box when tested in adulthood. Conversely, rats exposed prenatally and lactationally to a higher dose of PCBs than used in the current study (10 mg/kg dam s.c. injection) did not demonstrate altered anxiety behavior when tested in a different arena, the elevated zero maze (Colciago et al., 2009). In humans with higher than average circulating PCBs, depressivity but not anxiety was increased (Gaum et al., 2014). In Inuit children, PCB 153 exposure, another more heavily chlorinated PCB than those used in the current study, was correlated with increased unhappiness and anxiety (Plusquellec et al., 2010). Although this literature is disparate in methodology, it is consistent with our general results that PCB exposure during gestation can lead to altered affective states in a sex-dependent manner.
The mechanism for altered affective behaviors due to fetal PCB exposure is not well-understood, in part because of the complexity of the neurobiology of any complex behavior. In the case of anxiety-like behaviors, a variety of neural circuits are involved, many of which are hormone sensitive and/or sexually dimorphic. Thus, teasing apart which level is affected by a prenatal EDC exposure is exceedingly difficult. Nevertheless, there are some likely candidates. The hypothalamic-pituitary-adrenal (HPA) axis, which mediates anxiety behaviors (Romeo, 2016), is altered by PCB exposure: circulating corticosterone of young (PND 15) Sprague-Dawley rats was decreased by exposure to Aroclor 1254 via maternal ingestion throughout pregnancy. Further, corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone were decreased indicating a hypothalamic basis for the decreased circulating corticosterone concentrations (Meserve et al., 1992). Also, the same rats used in our current study were assayed for circulating corticosterone in a terminal blood sample. Interestingly, females treated with A1221 (0.5), but not males of either dosage, showed increased circulating corticosterone (Reilly et al., 2015). This suggests that the anxiety phenotype of the males in the current study may be due to a different neural substrate other than hypothalamic CRH neurons. Current work is analyzing the brains of animals to investigate other neural systems in brain regions involved in the neurobiology of anxiety behaviors.
4.2. The elevated plus maze and light:dark box measure distinct aspects of anxiety
No single behavioral test can fully represent an animal’s spectrum of affective behavior (Ramos and Mormède, 1998). For this reason, batteries of tasks are often used in an attempt to cover a broad range of reactivity. The current study utilized both the light:dark box and the elevated plus maze as indices of anxiety behavior. Differences in outcomes suggest that each test measures distinct facets of anxiety-like behavior. Non-conditioned behavioral tests for anxiety can be broken down into two general categories: passive (conflict) avoidance and active avoidance. The light:dark box and elevated plus maze tests are both conflict tests in which an animal is driven to approach novel stimuli and, at the same time, avoid a possibly dangerous environment. However, the elevated plus maze also contains elements of active avoidance, which would best be described as retreat or escape from the open arms (Handley and McBlane, 1991). Furthermore, there is evidence that passive and active avoidance behaviors are governed by two separate neural networks: passive avoidance depends primarily on the hippocampus while active avoidance depends primarily on the amygdala, hypothalamus, and periaqueductal grey region of the midbrain (Deakin and Graeff, 1991). Indeed, our previous work with transgenerational exposure to EDCs has shown that some brain nuclei, particularly the CA3 of the hippocampus, are more vulnerable than others (Gillette et al., 2015). It is probable that fetal exposure to PCBs has region-specific effects within the brain and that the consequences of such exposure can manifest differently in independent neural or physiological contexts.
4.3. Sex differences in PCB effects
Males and females differ in their responses to stressful stimuli [reviewed in (Bale and Epperson, 2015)]. Epidemiological studies indicate that the occurrence of anxiety or depressive disorders is much higher in women than in men, although the underlying reasons for this effect are not well-understood (Gorman, 2006). In rodent models, females tend to show more exploration and fewer anxiety-like behaviors (Palanza, 2001). Some of these differences are potentially due to sex differences in the hormonal milieu of fetal males and females, resulting in neural networks and behaviors that are sexually differentiated (Matsumoto, 1991; McCormick et al., 1998; McCormick and Mathews, 2007; Seale et al., 2005). Similar to this literature, the performance of our control males and females in the elevated plus maze and the light:dark box showed the sex differences predicted from the literature (Donner and Lowry, 2013; Johnston and File, 1991). Moreover, we found that anxiety and exploratory behavior of males, but not females, were modified by prenatal exposure to PCBs. Not only did A1221 (1.0) males show reduced anxiety-like behaviors and increased activity in the light:dark box; this group’s behavior went in a direction that made them more similar to females than their male counterparts. It is interesting that the lower dosage of A1221 (0.5) males were not different from the control males. Our data suggest that in the context of anxiety behavior, developing males are more susceptible to PCBs than females, that this is dose-dependent, and that most effects are not mimicked by estradiol.
4.4. Fetal PCB exposure increased body weight in adulthood
Our finding that two injections of A1221 prior to birth significantly increased body weight is intriguing. While this effect occurred in rats of both sexes, the timing of when this was first evident differed, beginning prior to weaning in males, and only after weaning in females. This increased body weight was maintained through adulthood. About 10 years ago, it was first proposed that some EDCs are putative obesogens, with properties that alter the control of adipogenesis and energy balance (Grün and Blumberg, 2006) and lead to increased body weight. Obesogenic EDCs include tributyltin, tolyfluanid, and DES (Grün and Blumberg, 2006; Newbold et al., 2007; Regnier et al., 2015). A body burden of EDCs is also predictive of metabolic disorders and obesity in human populations (Lee et al., 2011).
To our knowledge this is the first study linking prenatal PCBs to increased body weight. That the exposure period, two days prenatally, is shorter than that of most published work, is also interesting and points to that limited developmental period (E16 and E18) as a window of sensitivity. Interestingly, our positive estrogen control group (EB) did not replicate this effect, but it is notable that the body weights of EB-exposed individuals were intermediate to the A1221 and the control animals. Thus, A1221 likely acts through a combination of mechanisms only partially attributable to its estrogenic action. Indeed, PCBs can bind the aryl hydrocarbon receptor (AhR - Kafafi et al., 1993), which is implicated in playing a role in metabolic and obesity disorders (Xu et al., 2015). However, A1221 is lightly chlorinated and not believed to have dioxin-like effects on the AhR (Van den Berg et al., 1998). Therefore, more work is needed to determine the mechanism of action.
5. Summary and implications
In conclusion, our study on anxiety-like behaviors suggests that male rats are particularly vulnerable to PCB exposure during sexual differentiation in late gestation, a result that was dose, and task specific. During brain sexual differentiation, natural sex differences in the release of gonadal hormones in the fetus and infant result in structural changes to the nervous system that in turn lead to functional behavioral differences in adulthood [reviewed in (McCormick and Mathews, 2007)]. During the period when A1221 was administered, on E16 and E18, there is a prenatal surge of testosterone from the gonads of male (but not female) fetuses. In the brain, testosterone can act upon androgen receptors, and also, be aromatized into estradiol, the latter acting upon estrogen receptors. Presumably, the presence of A1221 during this prenatal period perturbed these (and/or other) actions of hormones in the males, a process absent in the females, possibly due to the lack of the prenatal testosterone surge. Further studies on the nature of the neural circuits, neurotransmitters, and hormone receptors targeted in a sex-dependent manner will help to identify underlying mechanisms and potentially inform possible interventions in the case of known EDC exposures.
Our finding that A1221 (1.0) exposure decreased anxiety behavior in adulthood should not be interpreted as either a positive or negative outcome. Rather, this behavioral change may represent increased risk taking behavior in the males. PCBs are found in tissues of humans and readily cross the placental barrier, making our work in prenatal rats of potential concern for humans.
Footnotes
Grant support: NIH RO1 ES020662, NIH RO1 ES023254, NSF GRFP 2011130386 (RG).
References
- Bale TL, Epperson CN. Sex differences and stress across the lifespan. Nat Neurosci. 2015;18:1413–1420. doi: 10.1038/nn.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemis JC, Seegal RF. Polychlorinated biphenyls and methylmercury act synergistically to reduce rat brain dopamine content in vitro. Environ Health Perspect. 1999;107:879–885. doi: 10.1289/ehp.99107879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, Lanphear BP. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. 2011;128:873–882. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone S, Ponzo OJ, Gobetto N, Samaniego YA, Reynoso R, Scacchi P, Moguilevsky JA, Cutrera R. Hormones and behavior. Horm Behav. 2013;63:692–699. doi: 10.1016/j.yhbeh.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. Fourth National Report on Human Exposure to Environmental Chemicals. 2009. [Google Scholar]
- Chishti MA, Fisher JP, Seegal RF. Aroclors 1254 and 1260 reduce dopamine concentrations in rat striatal slices. Neurotoxicology. 1996;17:653–660. [PubMed] [Google Scholar]
- Chung YW, Nunez AA, Clemens LG. Effects of neonatal polychlorinated biphenyl exposure on female sexual behavior. Physiol Behav. 2001;74:363–370. doi: 10.1016/s0031-9384(01)00579-0. [DOI] [PubMed] [Google Scholar]
- Colciago A, Casati L, Mornati O, Vergoni AV, Santagostino A, Celotti F, Negri-Cesi P. Chronic treatment with polychlorinated biphenyls (PCB) during pregnancy and lactation in the rat part 2: effects on reproductive parameters, on sex behavior, on memory retention and on hypothalamic expression of aromatase and 5alpha-reduc-tases in the offspring. Toxicol Appl Pharmacol. 2009;239:46–54. doi: 10.1016/j.taap.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Crews D, Gillette R, Miller-Crews I, Gore AC, Skinner MK. Nature, nurture and epigenetics. Mol Cell Endocrinol. 2014;398:42–52. doi: 10.1016/j.mce.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Medeiros CB, Rees SL, Llinas M, Fleming AS, Crews D. Deconstructing early life experiences: distinguishing the contributions of prenatal and postnatal factors to adult male sexual behavior in the rat. Psychol Sci. 2010;21(10):1494–1501. doi: 10.1177/0956797610382122. [DOI] [PubMed] [Google Scholar]
- Deakin JF, Graeff FG. 5-HT and mechanisms of defence. J Psychopharmacol (Oxf) 1991;5:305–315. doi: 10.1177/026988119100500414. [DOI] [PubMed] [Google Scholar]
- Dickerson SM, Cunningham SL, Gore AC. Prenatal PCBs disrupt early neuroen-docrine development of the rat hypothalamus. Toxicol Appl Pharmacol. 2011a;252:36–46. doi: 10.1016/j.taap.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SM, Cunningham SL, Patisaul HB, Woller MJ, Gore AC. Endocrine disruption of brain sexual differentiation by developmental PCB exposure. Endocrinology. 2011b;152:581–594. doi: 10.1210/en.2010-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner NC, Lowry CA. Sex differences in anxiety and emotional behavior. Pflugers Arch. 2013;465:601–626. doi: 10.1007/s00424-013-1271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elnar AA, Diesel B, Desor F, Feidt C, Bouayed J, Kiemer AK, Soulimani R. Neurodevelopmental and behavioral toxicity via lactational exposure to the sum of six indicator non-dioxin-like-polychlorinated biphenyls (Σ6 NDL-PCBs) in mice. Toxicology. 2012;299:44–54. doi: 10.1016/j.tox.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Engel SM, Miodovnik A, Canfield RL, Zhu C, Silva MJ, Calafat AM, Wolff MS. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ Health Perspect. 2010;118:565–571. doi: 10.1289/ehp.0901470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald EF, Belanger EE, Gomez MI, Cayo M, McCaffrey RJ, Seegal RF, Jansing RL, Hwang SA, Hicks HE. Polychlorinated biphenyl exposure and neuro-psychological status among older residents of upper Hudson River communities. Environ Health Perspect. 2008;116:209–215. doi: 10.1289/ehp.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaum PM, Esser A, Schettgen T, Gube M, Kraus T, Lang J. Prevalence and incidence rates of mental syndromes after occupational exposure to polychlorinated biphenyls. Int J Hyg Environ Health. 2014;217:765–774. doi: 10.1016/j.ijheh.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Gillette R, Miller-Crews I, Nilsson EE, Skinner MK, Gore AC, Crews D. Sexually dimorphic effects of ancestral exposure to vinclozolin on stress reactivity in rats. Endocrinology. 2014;155:3853–3866. doi: 10.1210/en.2014-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette R, Miller-Crews I, Skinner MK, Crews D. Distinct actions of ancestral vinclozolin and juvenile stress on neural gene expression in the male rat. Front Genet. 2015;6:56. doi: 10.3389/fgene.2015.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT. EDC-2: the endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015;36:E1–E150. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman JM. Gender differences in depression and response to psychotropic medication. Gend Med. 2006;3:93–109. doi: 10.1016/s1550-8579(06)80199-3. [DOI] [PubMed] [Google Scholar]
- Grün F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147:S50–S55. doi: 10.1210/en.2005-1129. [DOI] [PubMed] [Google Scholar]
- Handley SL, McBlane JW. 5-HT-the disengaging transmitter? J Psychopharmacol (Oxf) 1991;5:322–326. doi: 10.1177/026988119100500417. [DOI] [PubMed] [Google Scholar]
- Johnston AL, File SE. Sex differences in animal tests of anxiety. Physiol Behav. 1991;49:245–250. doi: 10.1016/0031-9384(91)90039-q. [DOI] [PubMed] [Google Scholar]
- Kafafi SA, Afeefy HY, Ali AH, Said HK, Kafafi AG. Binding of polychlorinated biphenyls to the aryl hydrocarbon receptor. Environ Health Perspect. 1993;101:422–428. doi: 10.1289/ehp.93101422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP, Champagne FA. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. PNAS. 2013;110:9956–9961. doi: 10.1073/pnas.1214056110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Steffes MW, Sjödin A, Jones RS, Needham LL, Jacobs DR. Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipid-emia, and insulin resistance among people free of diabetes. PLoS One. 2011;6:e15977–e15979. doi: 10.1371/journal.pone.0015977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A. Synaptogenic action of sex steroids in developing and adult neuro-endocrine brain. Psychoneuroendocrinology. 1991;16:25–40. doi: 10.1016/0306-4530(91)90069-6. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav. 2007;86:220–233. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Furey BF, Child M, Sawyer MJ, Donohue SM. Neonatal sex hormones have “organizational” effects on the hypothalamic-pituitary-adrenal axis of male rats. Brain Res Dev Brain Res. 1998;105:295–307. doi: 10.1016/s0165-3806(97)00155-7. [DOI] [PubMed] [Google Scholar]
- McFarland VA, Clarke JU. Environmental occurrence, abundance, and potential toxicity of polychlorinated biphenyl congeners: considerations for a congener-specific analysis. Environ Health Perspect. 1989;81:225–239. doi: 10.1289/ehp.8981225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Maity A, Missmer SA, Williams PL, Mahalingaiah S, Ehrlich S, Berry KF, Altshul L, Perry MJ, Cramer DW, Hauser R. Serum concentrations of polychlorinated biphenyls in relation to in vitro fertilization outcomes. Environ Health Perspect. 2011;119:1010–1016. doi: 10.1289/ehp.1002922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meserve LA, Murray BA, Landis JA. Influence of maternal ingestion of Aroclor 1254 (PCB) or FireMaster BP-6 (PBB) on unstimulated and stimulated corticosterone levels in young rats. Bull Environ Contam Toxicol. 1992;48:715–720. doi: 10.1007/BF00195992. [DOI] [PubMed] [Google Scholar]
- Morse DC, Seegal RF, Borsch KO, Brouwer A. Long-term alterations in regional brain serotonin metabolism following maternal polychlorinated biphenyl exposure in the rat. Neurotoxicology. 1996;17:631–638. [PubMed] [Google Scholar]
- Newbold RR, Padilla-Banks E, Snyder RJ, Jefferson WN. Perinatal exposure to environmental estrogens and the development of obesity. Mol Nutr Food Res. 2007;51:912–917. doi: 10.1002/mnfr.200600259. [DOI] [PubMed] [Google Scholar]
- Orito K, Gotanda N, Murakami M, Ikeda T. Prenatal exposure to 3, 3″, 4, 4″, 5-pentachlorobiphenyl (PCB126) promotes anxiogenic behavior in rats. Tohoku J Exp Med. 2007;212:151–157. doi: 10.1620/tjem.212.151. [DOI] [PubMed] [Google Scholar]
- Palanza P. Animal models of anxiety and depression: how are females different? Neurosci Biobehav Rev. 2001;25:219–233. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- Plusquellec P, Muckle G, Dewailly E, Ayotte P, Bégin G, Desrosiers C, Després C, Saint-Amour D, Poitras K. The relation of environmental contaminants exposure to behavioral indicators in Inuit preschoolers in Arctic Quebec. Neurotoxicology. 2010;31:17–25. doi: 10.1016/j.neuro.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Quinn CL, Wania F, Czub G, Breivik K. Investigating intergenerational differences in human PCB exposure due to variable emissions and reproductive behaviors. Environ Health Perspect. 2011;119:641–646. doi: 10.1289/ehp.1002415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos A, Mormède P. Stress and emotionality: a multidimensional and genetic approach. Neurosci Biobehav Rev. 1998;22:33–57. doi: 10.1016/s0149-7634(97)00001-8. [DOI] [PubMed] [Google Scholar]
- Regnier SM, El-Hashani E, Kamau W, Zhang X, Massad NL, Sargis RM. Tribu-tyltin differentially promotes development of a phenotypically distinct adipocyte. Obesity (Silver Spring) 2015;23:1864–1871. doi: 10.1002/oby.21174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly MP, Weeks CD, Topper VY, Thompson LM, Crews D, Gore AC. The effects of prenatal PCBs on adult social behavior in rats. Horm Behav. 2015;73:47–55. doi: 10.1016/j.yhbeh.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD. The impact of stress on the structure of the adolescent brain: implications for adolescent mental health. Brain Res. 2016 doi: 10.1016/j.brainres.2016.03.021. http://dx.doi.org/10.1016/j.brainres.2016.03.021. [DOI] [PubMed]
- Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL. Postnatal masculinization alters the HPA axis phenotype in the adult female rat. J Physiol. 2005;563:265–274. doi: 10.1113/jphysiol.2004.078212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg RM, Juenger TE, Gore AC. The effects of prenatal PCBs on adult female paced mating reproductive behaviors in rats. Horm Behav. 2007a;51:364–372. doi: 10.1016/j.yhbeh.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg RM, Juenger TE, Gore AC. The effects of prenatal PCBs on adult female paced mating reproductive behaviors in rats. Horm Behav. 2007b;51:364–372. doi: 10.1016/j.yhbeh.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg RM, Walker DM, Juenger TE, Woller MJ, Gore AC. Effects of perinatal polychlorinated biphenyls on adult female rat reproduction: development, reproductive physiology, and second generational effects. Biol Reprod. 2008;78:1091–1101. doi: 10.1095/biolreprod.107.067249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y, Aburada S, Hashimoto K, Kitaura T. Transfer and distribution of accumulated (14C)polychlorinated biphenyls from maternal to fetal and suckling rats. Arch Environ Contam Toxicol. 1986;15:709–715. doi: 10.1007/BF01054917. [DOI] [PubMed] [Google Scholar]
- Van den Berg M, Birnbaum L, Bosveld AT, Brunstrom B, Cook P, Feeley M, Giesy JP, Hanberg A, Hasegawa R, Kennedy SW, Kubiak T, Larsen JC, van Leeuwen FX, Liem AK, Nolt C, Peterson RE, Poellinger L, Safe S, Schrenk D, Tillitt D, Tysklind M, Younes M, Waern F, Zacharewski T. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect. 1998;106:775–792. doi: 10.1289/ehp.98106775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbergh JG, Huggett CL. The anogenital distance index, a predictor of the in-trauterine position effects on reproduction in female house mice. Lab Anim Sci. 1995;45:567–573. [PubMed] [Google Scholar]
- Xu CX, Wang C, Zhang ZM, Jaeger CD, Krager SL, Bottum KM, Liu J, Liao DF, Tischkau SA. Aryl hydrocarbon receptor deficiency protects mice from diet-induced adiposity and metabolic disorders through increased energy expenditure. Int J Obes. 2015;39:1300–1309. doi: 10.1038/ijo.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]