Abstract
Purpose
Atrial fibrillation with rapid ventricular response (RVR) is common during critical illness. In this study, we explore the comparative effectiveness of three commonly used drugs (metoprolol, diltiazem, and amiodarone) in the management of atrial fibrillation with RVR in the intensive care unit (ICU).
Methods
Data pertaining to the first ICU admission were extracted from the Medical Information Mart for Intensive Care (MIMIC) III database. Patients who received one of the above pharmacologic agents while their heart rate was >110bpm and had atrial fibrillation documented in the clinical chart were included. Propensity score weighting using a generalized boosted model was used to compare medication failure rates (second agent prior to termination of RVR). Secondary outcomes included time to control, control within 4-hours, and mortality.
Results
1,646 patients were included: 736 received metoprolol, 292 received diltiazem, and 618 received amiodarone. Compared to those who received metoprolol, failure rates were higher amongst those who received amiodarone (OR 1.39, 95%CI 1.03–1.87, p=0.03) and there was a trend towards increased failure rates in patients who received diltiazem (OR 1.35, CI 0.89–2.07, p=0.16). Amongst patients who received a single agent, patients who received diltiazem were less likely to be controlled at 4-hours than those who received metoprolol (OR 0.64, CI 0.43–097, p=0.03). Initial agent was not associated with in-hospital mortality.
Conclusions
In this study, metoprolol was the most commonly used agent for atrial fibrillation with RVR. Metoprolol had a lower failure rate than amiodarone and was superior to diltiazem in achieving rate control at 4-hours.
Keywords: Critical Care, Big Data, Machine Learning, Beta Blocker, Calcium Channel Blocker, Amiodarone
Introduction
Atrial fibrillation is common among critically ill patients in the intensive care unit (ICU) and has been associated with increased mortality.(1) In one large epidemiologic study, atrial fibrillation was found in 25.5% of 60,209 hospitalizations for sepsis.(2) Rapid ventricular response (RVR), a potential sequela of atrial fibrillation that can lead to hemodynamic instability, may be triggered by the high-sympathetic state of critical illness as well as by catecholamine-based vasopressor agents and volume resuscitation.(3) Atrial fibrillation with RVR leads to loss of atrial kick, shortened ventricular filling time, increased myocardial oxygen demand, and potentially tachycardia-induced cardiomyopathies.(4)
To date, the optimal pharmacologic approach to atrial fibrillation with RVR in the ICU has not been well defined. Several small studies have attempted to compare various rate and rhythm control agents with mixed results. (5,6) In a recent retrospective, propensity-matched analysis of a large administrative database, calcium channel blockers (CCB) were identified as the most frequently used class of intravenous pharmacologic agents for atrial fibrillation during sepsis.(7) Beta-blockers, however, were associated with improved hospital mortality when compared with CCBs, digoxin, and amiodarone. No study has directly compared the commonly used pharmacologic agents metoprolol, diltiazem, and amiodarone with regard to their effectiveness in atrial fibrillation with RVR in the ICU.
In the present study, we leverage a high temporal resolution ICU database (including time-stamps of medication administration) and a propensity score technique with a generalized boosted model to compare intravenous amiodarone, metoprolol, and diltiazem with regards to rate control of atrial fibrillation with RVR in a large cohort of critically ill patients.
Materials and Methods
Study Population
The study population was drawn from the Medical Information Mart for Intensive Care (MIMIC-III) database, an initiative from the Laboratory of Computational Physiology at Massachusetts Institute of Technology (MIT) and Beth Israel Deaconess Medical Center (BIDMC). MIMIC-III contains data from 32,810 adult patients who were admitted to surgical or medical ICUs at BIDMC. The database contains high temporal resolution data from information systems, including bedside monitors, laboratory system, electronic documentation of clinical notes and nursing flow sheets, hospital administrative data and Social Security death records, for patients admitted to a BIDMC ICU between 2001 and 2012.(8) The use of the MIMIC III database for research is approved by the Institutional Review Boards of BIDMC and MIT.
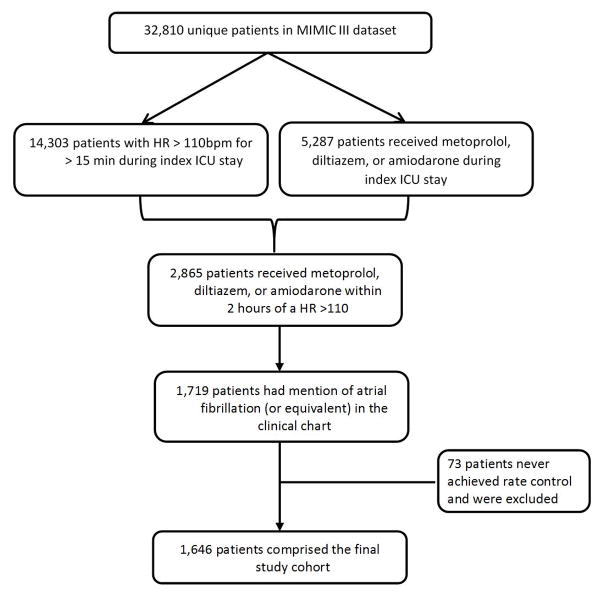
In order to identify those patients with atrial fibrillation and RVR in the dataset, we explored both structured and unstructured data. Specifically, structured data included heart rate (HR) and nursing medication administration documentation. Unstructured data included clinician progress notes and discharge summaries. We used the HR as captured by the bedside monitor, extracted as an 8-beat median rate by a software and verified by nurses, in order to identify patients who had a HR over 110 beats per minute (bpm) for more than 15 minutes (n=14,303). We arbitrarily chose a 15-minute duration as a significant episode of rapid HR to reduce the noise from transient tachycardia or artifact. Only the first episode of HR >110 bpm was evaluated for inclusion in the analysis. After identifying an episode of rapid HR, we next determined whether the patient received an intravenous pharmacologic agent of interest (metoprolol, diltiazem, or amiodarone) within 2 hours of the identified episode. The 2-hour window was used because medication data and HR data come from different information systems, and the time stamps may not perfectly align. Furthermore, the time stamps for medications are subject to inaccurate manual data entry at the time of drug administration. This window was arbitrarily determined; a smaller window would increase specificity but decrease the sensitivity for identifying the cohort. A total of 2,865 patients were identified.
Of these 2,865 patients, 1,719 had mention of ‘atrial fibrillation,’ ‘afib,’ ‘a-fib,’ ‘atrial fib,’ ‘atrial fib/flutter,’ ‘atrial fibrillation/flutter’ in their hospital discharge summary. Finally, 73 patients never achieved sustained rate control prior to death or discharge from the ICU and were excluded. 1,646 patients comprised the final study cohort. (Figure 1)
Figure 1.
Cohort Selection Flow Diagram from the Medical Information Mart for Intensive Care (MIMIC) database
Raw data was extracted from MIMIC using the Oracle version of SQL and was further processed using Python (for analysis of free text data) and MatLab (for processing and visualizing serial HR data and establishing temporal relationship between RVR and medication administration). Covariates, including patient demographic information, other vital signs, laboratory results, past medical history, home medications prior to admission, disease severity score, type of admission, and types of ICU, were extracted using SQL.
Outcomes
The primary outcome was medication ‘failure’ as defined by the use of a second agent prior to the end of the RVR episode. Second agents were not limited to metoprolol, diltiazem, and amiodarone but also included digoxin, ibutilide, esmolol, procainamide, and propafenone. Secondary outcomes included the overall duration of RVR when only one pharmacologic agent was used, rate control within 4 hours, increase in vasopressor requirement, and in-hospital mortality.
The resolution of the RVR episode was defined as sustained HR below 110/min for at least 4 hours, based on the approximate half-life of intravenous metoprolol and diltiazem.(9) The algorithm identifies the start of the RVR as the time the HR exceeded >110 bpm and the end when the HR went below 110 bpm for at least 90% of the recorded measurements.
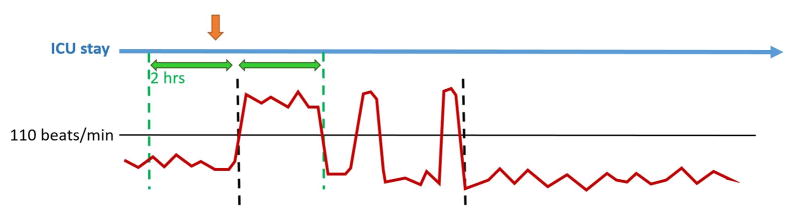
Data visualization techniques were employed to assist in verifying the RVR episode as well as its duration. An example of one episode of RVR is shown in Figure 2.
Figure 2.
Graphical representation of an episode of atrial fibrillation with rapid ventricular response.
Legend - Graphical representation of an episode of atrial fibrillation with rapid ventricular response. a) orange arrow represents first pharmacologic agent administration time b) blue arrow represents the duration of ICU stay c) green arrows represent 2-hour time window for first drug administration d) red line represents heart rate in bpm e) dashed black lines indicate the start and end points of the RVR episode.
Statistical Analysis
A machine-learning algorithm (generalized boosted model) was employed to build a regression tree for the estimation of propensity score (a non-parametric method). The model iteratively combined many simple regression trees until the pre-determined metrics (standardized bias or Kolmogorov-Smirnov statistics) for assessing between group pretreatment covariate imbalance reached a minimum. This method has been previously employed in implementing propensity score weighting in studies with multiple treatment groups.(10) Included covariates were selected based on clinician consensus.
After the propensity score weight was generated, weighted regression was performed, which allowed for exploring interaction terms and adjusting for variables that had heavier effects on the outcomes that could not be fully eliminated by using propensity score alone. Depending on the nature of the outcome variable, weighted logistic regression was used for binary outcomes, and weighted liner regression was used for continuous outcomes. Stabilized weights were used to eliminate extreme weights. All covariates included in the model and results of the propensity weighting are reported in the Supplementary Table (e1). An α<0.05 was considered statistically significant.
In general, propensity score analysis has been used to compare two treatment groups, i.e. treatment vs. control group. Stratification (using propensity score as a covariate in regression model) and propensity score matching (creating treatment and control group of similar pre-treatment attribute and thus mimicking randomized trials) are two common uses of propensity scores. However, stratification (conventional regression model) typically leads to imbalanced strata at both ends of the groupings. Propensity score matching excludes subjects without a match and may introduce bias by doing so. In addition, matching propensity scores of 3 or more treatment groups requires calculating 2 or more dimensional distances for each matched group of subjects, which can be mathematically challenging and lacks supporting theory. Therefore, we chose machine-generated regression trees for our propensity score, optimizing the balance of covariates across the groups, and used a propensity score weighted regression model for outcome effect. This non-parametric approach avoided the limitations and biases introduced by model specification when using parametric methods.
Sensitivity Analysis
Patients initially admitted to the cardiovascular surgery ICU (CVICU) were more likely to receive amiodarone as their first pharmacologic agent as compared to patients admitted to other ICUs. Further, this group of patients may represent a unique subgroup of patients with different pathophysiology as it relates to atrial fibrillation with RVR. As such, a sensitivity analysis was performed excluding patients whose initial ICU disposition was the CVICU.
Results
A total of 1,646 patients were included in the final study cohort. Of these, 736 received intravenous metoprolol, 292 received intravenous diltiazem, and 618 received intravenous amiodarone. To review the baseline characteristics of patients in each group, please refer to Table 1.
Table 1.
Cohort Characteristics and Outcomes
| All | Metoprolol | Amiodarone | Diltiazem | |
|---|---|---|---|---|
| Frequencies | ||||
| Total (n) | 1646 | 736 | 618 | 292 |
| Demographics | ||||
| Gender (% Male) | 56.4 | 57.2 | 57.6 | 52.1 |
| Age (Mean, SD) | 72.7 (12.3) | 73.4 (12.5) | 71.8 (12.1) | 73.0 (12.3) |
| Ethnicity (% Caucasian) | 74.5 | 76.1 | 71.2 | 77.4 |
| Past Medical History (%) | ||||
| Asthma | 3.4 | 3.3 | 1.9 | 6.9 |
| COPD | 18.4 | 17.4 | 15.4 | 27.4 |
| CHF | 46.1 | 45.3 | 44.5 | 51.7 |
| CKD | 14.5 | 14.7 | 14.4 | 14.4 |
| Liver Dx | 3.0 | 3.9 | 2.1 | 2.1 |
| Valvular Dx | 22.1 | 20.2 | 28.3 | 13.4 |
| Diabetes Mellitus | 26.4 | 25.5 | 27.7 | 25.7 |
| Malignancy | 20.0 | 20.9 | 15.7 | 25.3 |
| Atrial Fibrillation | 28.6 | 34.6 | 18.3 | 35.0 |
| Home use of Beta-Blocker | 55.5 | 57.6 | 58.1 | 44.2 |
| Calcium Channel blocker | 12.0 | 10.9 | 9.0 | 20.9 |
| Amiodarone | 4.4 | 3.3 | 6.1 | 3.6 |
| ICU Type (%) | ||||
| MICU | 31.2 | 36.3 | 17.5 | 47.26 |
| SICU | 3.0 | 3.9 | 1.8 | 3.4 |
| CCU | 23.6 | 22.9 | 24.9 | 23.0 |
| CVICU | 42.2 | 37.0 | 55.8 | 26.4 |
| Physiologic Parameters (Mean, SD) | ||||
| Mean arterial pressure, mmHg | 78.2 (16.3) | 80.3 (17.0) | 74.3 (14.3) | 81.4 (16.9) |
| Oxygen Saturation, % | 96.5 (4.4) | 96.8 (3.2) | 96.5 (4.4) | 95.7 (5.7) |
| Temp, F | 98.7 (1.6) | 98.6 (1.5) | 98.9 (1.5) | 98.6 (1.7) |
| Hear Rate, bpm | 124.0 (12.8) | 121.9 (11.4) | 125.3 (13.5) | 126.3 (14.0) |
| Laboratory Values (Mean, SD) | ||||
| Hemoglobin, g/dL | 10.5 (1.6) | 10.6 (1.7) | 10.3 (1.5) | 10.5 (1.7) |
| White blood cell count, K/uL | 13.4 (7.0) | 13.0 (6.9) | 13.7 (6.7) | 13.8 (8.0) |
| Platelet count, K/uL | 202.9 (119.5) | 212.0 (125.6) | 183.2 (105.8) | 224.5 (126.3) |
| Sodium, mg/dL | 139.1 (4.8) | 139.5 (5.0) | 138.3 (4.3) | 139.8 (4.9) |
| Potassium, mg/dL | 4.2 (0.6) | 4.1 (0.6) | 4.3 (0.6) | 4.1 (0.6) |
| Chloride, mg/dL | 105.3 (5.9) | 105.7 (6.1) | 105.1 (5.4) | 104.6 (6.2) |
| Bicarbonate, mg/dL | 25.0 (5.0) | 25.1 (4.8) | 24.4 (4.6) | 26.1 (5.9) |
| Blood urea nitrogen, mg/dL | 31.7 (22.5) | 31.3 (23.5) | 31.1 (21.1) | 34.2 (23.0) |
| Creatinine, mg/dL | 1.5 (1.4) | 1.4 (1.4) | 1.6 (1.5) | 1.6 (1.5) |
| Glucose, mg/dL | 138.1 (51.4) | 135.2 (50.0) | 140.8 (53.7) | 140.0 (49.9) |
| Disease Severity | ||||
| Vasopressors, % | 9.5 | 3.3 | 20.0 | 3.5 |
| SOFA Score (Mean, SD) | 6.9 (4.2) | 6.2 (3.7) | 8.2 (4.5) | 6.1 (3.8) |
Primary Outcome
Compared to patients who received metoprolol as their initial pharmacologic agent, patients who received amiodarone (OR 1.39, CI 1.03–1.87, p=0.03) as their initial agent were more likely to require the addition of a second agent prior to the end of the RVR episode. Amongst patients who initially received diltiazem, there was a trend towards increased failure rates as compared to patients who initially received metoprolol (OR 1.35, CI 0.89–2.07, p=0.16).
Secondary Outcomes
A total of 1207 patients received only a single pharmacologic agent while in atrial fibrillation with RVR. Of those, 567 (47.0%) received metoprolol, 204 (16.9%) received diltiazem, and 436 (36.1%) received amiodarone. In this subgroup of patients, diltiazem had the longest time until control, however this difference did not reach statistical significance in the fully adjusted model (metoprolol 297.3±29.0 minutes vs. amiodarone 247.3±34.1 minutes vs. diltiazem 345.0±47.9 minutes). Patients who received diltiazem were less likely to be controlled at 4-hours than those who received metoprolol (OR 0.64, CI 0.43–0.97, p=0.03).
Patients who initially received amiodarone were more likely to have been receiving vasopressors prior to drug administration and were more likely to have required an increase in dose of vasopressor or the initiation of a new vasopressor as compared to metoprolol (OR 3.34, CI 1.67–6.70, p<0.01). There was no difference with regard to change in vasopressors requirement when metoprolol was compared to diltiazem. Overall in-hospital mortality was highest for patients who received diltiazem as their initial pharmacologic agent (24.0% vs. 19.7% for amiodarone and 20.4% for metoprolol) however the difference did not reach statistical significance in the adjusted model.
Sensitivity Analyses
Of the 1,646 patients included in the study, 694 (42.2%) were admitted initially to the CVICU. Of those patients initially admitted to ICUs other than the CVICU (n=952), those who initially received diltiazem were more likely to require the addition of a second agent when compared to those who initially received metoprolol (OR 1.76, CI 1.06–2.91, p=0.03). There was no difference in ‘medication failure’ rate when metoprolol was compared to amiodarone (OR 1.19, CI 0.68–2.09, p=0.53). Among patients who received only a single pharmacologic agent, those patients who received diltiazem had a trend towards longer durations of RVR as compared to those who received metoprolol (396.8±61.4 minutes vs. 291.4±29.1 minutes, p=0.09) and were less likely to be controlled at 4 hours (OR 0.43, CI 0.25–0.74, p<0.01).
Discussion
In a large population of critically ill patients with atrial fibrillation and RVR, metoprolol was the most commonly used agent. Medication ‘failure,’ defined as the use of a second pharmacologic agent prior to the end of the RVR episode, was more common in patients who received amiodarone as compared to those who received metoprolol. Among patients who received only a single agent, those who received intravenous metoprolol were more likely to be controlled at 4-hours than those who received diltiazem. Excluding patients in the CVICU, patients who received metoprolol had a lower medication ‘failure’ rate and were more likely to be controlled at 4-hours as compared to those who received diltiazem. In-hospital mortality did not differ based on the initial choice of agent.
Atrial fibrillation with RVR is common during critical illness as patients are frequently exposed to high sympathetic tone, intravenous catecholamines, and large fluid shifts.(4) Although it is generally accepted that atrial fibrillation with RVR is associated with worse outcomes in critically ill patients, there is no standard treatment strategy and practice varies across providers. A number of small, single-center trials have compared intravenous metoprolol and diltiazem for the control of atrial fibrillation with RVR in the emergency department (ED). In contrast to the results presented here, these studies have favored diltiazem as a more efficacious agent for slowing heart rate.(11,12) One ED study compared diltiazem, amiodarone, and digoxin. That study found that diltiazem was superior in both time to control and number of patients controlled.(13) Similarly, in a trial comparing diltiazem and digoxin in the ED, diltiazem achieved control faster than digoxin.(14) In contrast, a number of small studies outside the ED suggest beta-blocking agents are superior to CCBs for rate control.(15–17) Only one study has directly compared amiodarone with diltiazem in a critically ill ICU population. In that trial, diltiazem was found to provide better heart rate control but was more likely to lead to hypotension.(18) Recent reviews exploring the management of atrial fibrillation in the ICU have concluded that there is insufficient evidence to select one pharmacologic agent over another in most circumstances.(5,6,19)
Most recently, Walkey et. al. explored practice patterns and outcomes in the treatment of atrial fibrillation during sepsis using a large, administrative database. Among nearly 40,000 patients, the investigators found that CCB were the most commonly used intravenous medication. In a propensity-matched analysis, those patients who received beta-blockers had a lower mortality when compared to CCBs, digoxin, and amiodarone. (7) While that study drew from a nationwide sample and therefore has good generalizability, the physiologic variables used in generating the propensity scores were taken from the day of admission and not from the time of atrial fibrillation onset. Further, due to the nature of the administrative data, the authors were unable to focus on ventricular rate during atrial fibrillation and could only compare overall mortality rates. Leveraging the high-resolution of the MIMIC III database, our propensity score draws from data at the time of the event of interest and allows for analysis of proximal outcomes.
Encouragingly, despite differences in methodology, variables available for analysis, and primary outcomes the present study and the study by Walkey et. al. reached similar conclusions (i.e. metoprolol may have advantages over diltiazem and amiodarone). Given concerns about reproducibility in science, (20) cross-validation of results using different databases and different methodologies is critical. Still, despite the promise of ‘Big Data’ to fuel knowledge creation where randomized trials are not feasible or too expensive, there are inherent limitations to the secondary analysis of electronic health records (e.g. missing data, limited number of available confounders etc) and the findings presented here should be further evaluated using prospective techniques.
The strengths of our study include the relatively large sample size, use of high temporal resolution ICU data, and a sophisticated propensity score weighting analysis. Our study, however, has a number of limitations. Foremost, we did not include medication dosage in our analysis. Further, the MIMIC III database does not reliably include time-stamps of oral drug administration and, therefore, only parenterally administered drugs were considered in this study. Electrocardiogram waveform analysis at the time of RVR was not available for the majority of patients thus we were not able to distinguish between rate and rhythm control. Likewise, the use of electrocardioversion was not included in the analysis. Given that the management of atrial fibrillation with RVR in the ICU includes non-pharmacologic supportive measures (e.g. fluid resuscitation, electrolyte repletion), we rely on the clinical judgement of the prescribing physician with regards to the need for pharmacologic agents. Finally, the single center nature of the study limits generalizability and we encourage the replication of this study in a multi-center database.
Conclusions
In this secondary analysis of electronic health data from the high temporal resolution MIMIC III database, metoprolol was the most commonly used pharmacologic agent for control of atrial fibrillation with RVR in the ICU. Metoprolol had a lower ‘failure’ rate as compared to amiodarone and was more likely than diltiazem to achieve rate control at 4-hours. There was no significant difference in hospital mortality based on choice of initial agent.
Supplementary Material
Balance Assessment Before and After Propensity Weighting
Acknowledgments
Funding Information: Funding for MIMIC III is from the National Institute of Health through the National Institute of Biomedical Imaging and Bioengineering grant (R01EB017205-01A1). Dr. Moskowitz is supported by NIH (2T32HL007374-37)
Authors AM and KPC had full access to the data and take responsibility for the integrity of the data and the accuracy of the data analysis. AM and LAC conceptualized the project. KPC performed the statistical analyses. AM, KPC, AZC, AC, MMG, and LAC contributed substantially to the study design, interpretation of the data, and writing of the manuscript.
Footnotes
Conflicts of Interest: None of the authors have conflicts of interest to report.
References
- 1.Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306(20):2248–2254. doi: 10.1001/jama.2011.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walkey AJ, Greiner MA, Heckbert SR, Jensen PN, Piccini JP, Sinner MF, Curtis LH, Benjamin EJ. Atrial fibrillation among Medicare beneficiaries hospitalized with sepsis: incidence and risk factors. American heart journal. 2013;165(6):949–955.e943. doi: 10.1016/j.ahj.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaver CM, Chen W, Janz DR, May AK, Darbar D, Bernard GR, Bastarache JA, Ware LB. Atrial Fibrillation Is an Independent Predictor of Mortality in Critically Ill Patients. Critical care medicine. 2015;43(10):2104–2111. doi: 10.1097/CCM.0000000000001166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capucci A, Villani GQ, Aschieri D. Risk of complications of atrial fibrillation. Pacing Clin Electrophysiol. 1997;20(10 Pt 2):2684–2691. doi: 10.1111/j.1540-8159.1997.tb06117.x. [DOI] [PubMed] [Google Scholar]
- 5.Kanji S, Stewart R, Fergusson DA, McIntyre L, Turgeon AF, Hebert PC. Treatment of new-onset atrial fibrillation in noncardiac intensive care unit patients: a systematic review of randomized controlled trials. Critical care medicine. 2008;36(5):1620–1624. doi: 10.1097/CCM.0b013e3181709e43. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida T, Fujii T, Uchino S, Takinami M. Epidemiology, prevention, and treatment of new-onset atrial fibrillation in critically ill: a systematic review. Journal of intensive care. 2015;3(1):19. doi: 10.1186/s40560-015-0085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walkey AJ, Evans SR, Winter MR, Benjamin EJ. Practice Patterns and Outcomes of Treatments for Atrial Fibrillation During Sepsis: A Propensity-Matched Cohort Study. Chest 1. 2016;49(1):74–83. doi: 10.1378/chest.15-0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saeed M, Villarroel M, Reisner AT, Clifford G, Lehman LW, Moody G, Heldt T, Kyaw TH, Moody B, Mark RG. Multiparameter Intelligent Monitoring in Intensive Care II: a public-access intensive care unit database. Critical care medicine. 2011;39(5):952–960. doi: 10.1097/CCM.0b013e31820a92c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lexicomp Online® L-D. Hudson, Ohio: Lexi-Comp, Inc; [Accessed: February 8th, 2016]. [Google Scholar]
- 10.McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Statistics in medicine. 2013;32(19):3388–3414. doi: 10.1002/sim.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fromm C, Suau SJ, Cohen V, Likourezos A, Jellinek-Cohen S, Rose J, Marshall J. Diltiazem vs. Metoprolol in the Management of Atrial Fibrillation or Flutter with Rapid Ventricular Rate in the Emergency Department. The Journal of emergency medicine. 2015;49(2):175–182. doi: 10.1016/j.jemermed.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Demircan C, Cikriklar HI, Engindeniz Z, Cebicci H, Atar N, Guler V, Unlu EO, Ozdemir B. Comparison of the effectiveness of intravenous diltiazem and metoprolol in the management of rapid ventricular rate in atrial fibrillation. Emergency medicine journal. 2005;22(6):411–414. doi: 10.1136/emj.2003.012047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siu CW, Lau CP, Lee WL, Lam KF, Tse HF. Intravenous diltiazem is superior to intravenous amiodarone or digoxin for achieving ventricular rate control in patients with acute uncomplicated atrial fibrillation. Critical care medicine. 2009;37(7):2174–2179. doi: 10.1097/CCM.0b013e3181a02f56. [DOI] [PubMed] [Google Scholar]
- 14.Schreck DM, Rivera AR, Tricarico VJ. Emergency management of atrial fibrillation and flutter: intravenous diltiazem versus intravenous digoxin. Annals of emergency medicine. 1997;29(1):135–140. doi: 10.1016/s0196-0644(97)70319-6. [DOI] [PubMed] [Google Scholar]
- 15.Mooss AN, Wurdeman RL, Mohiuddin SM, Reyes AP, Sugimoto JT, Scott W, Hilleman DE, Seyedroudbari A. Esmolol versus diltiazem in the treatment of postoperative atrial fibrillation/atrial flutter after open heart surgery. American heart journal. 2000;140(1):176–180. doi: 10.1067/mhj.2000.106917. [DOI] [PubMed] [Google Scholar]
- 16.Balser JR, Martinez EA, Winters BD, Perdue PW, Clarke AW, Huang W, Tomaselli GF, Dorman T, Campbell K, Lipsett P, et al. Beta-adrenergic blockade accelerates conversion of postoperative supraventricular tachyarrhythmias. Anesthesiology. 1998;89(5):1052–1059. doi: 10.1097/00000542-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Platia EV, Michelson EL, Porterfield JK, Das G. Esmolol versus verapamil in the acute treatment of atrial fibrillation or atrial flutter. The American journal of cardiology. 1989;63(13):925–929. doi: 10.1016/0002-9149(89)90141-0. [DOI] [PubMed] [Google Scholar]
- 18.Delle Karth G, Geppert A, Neunteufl T, Priglinger U, Haumer M, Gschwandtner M, Siostrzonek P, Heinz G. Amiodarone versus diltiazem for rate control in critically ill patients with atrial tachyarrhythmias. Critical care medicine. 2001;29(6):1149–1153. doi: 10.1097/00003246-200106000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Sleeswijk ME, Van Noord T, Tulleken JE, Ligtenberg JJ, Girbes AR, Zijlstra JG. Clinical review: treatment of new-onset atrial fibrillation in medical intensive care patients--a clinical framework. Critical care. 2007;11(6):233. doi: 10.1186/cc6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ioannidis JP. Why most published research findings are false. PLoS medicine. 2005;2(8):e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Balance Assessment Before and After Propensity Weighting