Abstract
Intraflagellar transport (IFT) is a form of motor-dependent cargo transport that is essential for the assembly, maintenance and length-control of cilia, which play critical roles in motility, sensory reception and signal transduction in virtually all eukaryotic cells. During IFT, anterograde kinesin-2 and retrograde IFT-dynein motors drive the bidirectional transport of IFT trains that deliver cargo, for example axoneme precursors such as tubulins as well as molecules of the signal transduction machinery, to their site of assembly within the cilium. Following its discovery in Chlamydomonas, IFT has emerged as a powerful model system for studying general principles of motor-dependent cargo transport and we now appreciate the diversity that exists in the mechanism of IFT within cilia of different cell-types. The absence of heterotrimeric kinesin-2 function, for example, causes a complete loss of both IFT and cilia in Chlamydomonas but following its loss in C. elegans, where its primary function is loading the IFT machinery into cilia, homodimeric kinesin-2-driven IFT persists and assembles a full-length cilium. Generally, heterotrimeric kinesin-2 and IFT-dynein motors are thought to play widespread roles as core IFT-motors whereas homodimeric kinesin-2 motors are accessory motors that mediate different functions in a broad range of cilia, in some cases contributing to axoneme assembly or the delivery of signaling molecules but in many other cases their ciliary functions, if any, remain unknown. In this review, we focus on mechanisms of motor action, motor cooperation and motor-dependent cargo delivery during IFT.
Keywords: Intraflagellar transport, kinesin-2, IFT dynein, motor cooperation
Graphical abstract
Intraflagellar transport is a form of motor-dependent cargo transport that is essential for the assembly, maintenance and length-control of cilia, which play critical roles in motility, sensory reception and signal transduction in virtually all eukaryotic cells. In this review of intraflagellar transport, we focus on mechanisms of motor action, motor cooperation and motor-dependent cargo delivery.
1. Introduction: Basic Mechanism and Functions of IFT
Cilia are microtubule (MT)-based organelles that protrude from the surface of virtually all eukaryotic cells [1]. Motile cilia (also called flagella) are used for cell locomotion or for the generation of fluid flow over a cell surface, whereas non-motile (aka sensory or primary) cilia sense extracellular signals and transmit signals from the cilium to the cytoplasm and nucleus in order to control gene expression and cell behavior, thereby playing several important roles in cell and developmental biology. Cilia consist of a cylindrical, MT-based axoneme, which projects, MT plus ends distal, from a basal body located at the cell surface, surrounded by a specialized membrane that contains various cilium-based signaling molecules (Fig. 1A). Cilia can be found in all branches of the eukaryotic tree indicating that the last eukaryotic common ancestor (LECA) was already equipped with this intriguing cellular antenna [2–4]. The mechanism of cilium assembly (aka cilium biogenesis or ciliogenesis) is currently a cutting-edge topic in cell and molecular biology research [5].
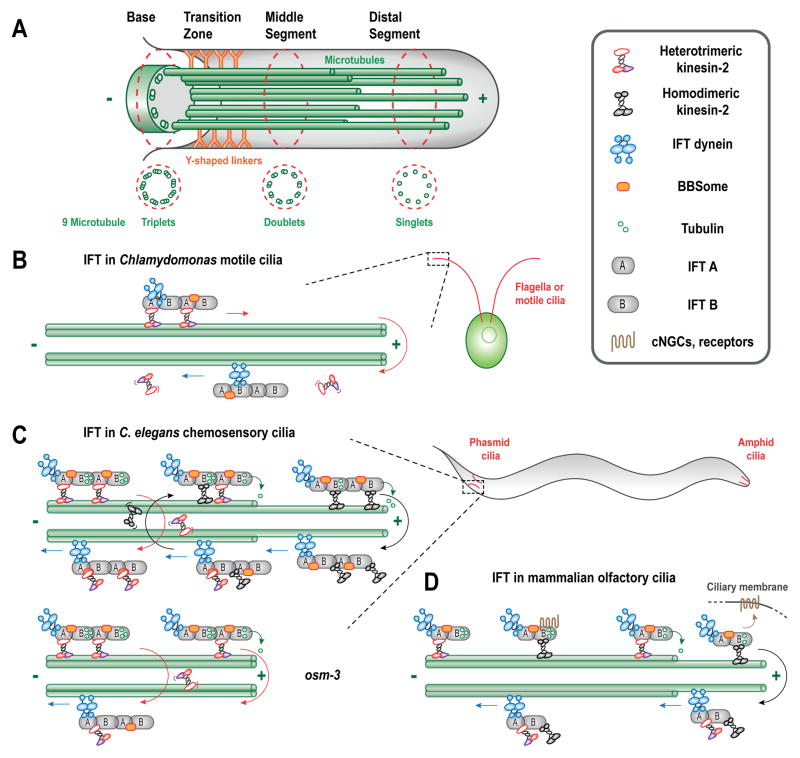
Figure 1.
Intraflagellar transport builds and maintains the cilium. (A) The axoneme is the core structural component of the eukaryotic cilium and it assembles by incorporation of precursors such as tubulin subunits onto MT plus ends at the distal tips of the axonemes (bacterial flagella, which rotate using a chemiosmotic mechanism add flagellin subunits at their distal tip, whereas the flagellum of archaea, the archaellum, rotates using ATP hydrolysis and adds subunits at its base). Shown is the chemosensory cilium of C. elegans neurons. It should be noted, however, that the basal body (BB, comprised of triplet MTs) degenerates in this organism. (B) Heterotrimeric kinesin-2 and IFT dynein are proposed to drive unitary rates of antero- and retrograde IFT, respectively in Chlamydomonas, whereas homodimeric kinesin-2 is absent. (C) IFT in C. elegans is driven by two distinct types of kinesin-2 motors, heterotrimeric kinesin-II and homodimeric OSM-3, delivering cargoes such as tubulin to axoneme tips, while IFT dynein provides the retrograde transport, recycling the kinesin-2 motors. (D) Heterotrimeric kinesin-2 (kinesin-II aka KIF3) builds the axoneme whereas the homodimeric kinesin-2, KIF17 delivers signaling molecules to the ciliary membrane e.g. cNGCs and dopamine receptors in mammalian olfactory cilia, while IFT dynein drives transport towards the base. (A–D, simplified schematics, IFT-A and IFT-B stoichiometry, for example, can vary. See text for further details).
The discovery of intraflagellar transport as a critical component of the mechanism of ciliogenesis was catalyzed by the observation that polystyrene beads could move along the motile cilia of Chlamydomonas in a bidirectional fashion, independent of ciliary beating [6, 7]. This paved the way for a series of pioneering experiments which revealed that ciliary precursors that form in the cell body, e.g. tubulin subunits and pre-assembled motility-related complexes, assemble onto the distal tips of the 10 μm long, motile Chlamydomonas cilium [8]. To test the hypothesis that a mechanism exists for transporting these precursors from the cytoplasm and along the cilium to the distal tip, Rosenbaum and colleagues used differential interference contrast (DIC) microscopy of paralyzed ciliary mutants to visualize the bidirectional movement, i.e. “intraflagellar transport” or “IFT”, of large particles beneath the ciliary membrane [9]. The subsequent purification of these particles, now named IFT trains, opened up the field for molecular analysis [10, 11] [and the discovery of several cilia-related diseases [12]], while the application of GFP-tagging allowed the direct visualization of specific IFT components moving along the cilium in living cells [13]. The rapid progress made by multiple laboratories in understanding the mechanism of IFT has been covered in several reviews [14–23]. The current view is that anterograde IFT trains, which deliver a variety of cargo molecules for incorporation into the ciliary axoneme, membrane and matrix, are moved from the base to the distal tip of the cilium by heterotrimeric and, in some cases, homodimeric motors of the kinesin-2 family, whereas retrograde IFT trains, which recycle turnover products from the tip to the base of the cilium are moved by IFT-dynein (aka dynein-2 or class dhc1b dynein) (Fig. 1B–D) [24, 25].
The elucidation of the cellular functions of IFT, which depends upon the delivery of cargo molecules by the IFT machinery, is an exciting problem in cell-biology research. It has long been recognized that IFT is required for ciliogenesis because it delivers cargo consisting of axonemal precursors such as tubulin subunits, pre-assembled radial-spoke complexes and dynein arms to their site of assembly at the tip of the cilium [17]. The delivery of these same cargo molecules is also relevant to the interesting topic of cilium length control, a topic that has been pioneered by Marshall and others [26–30]. IFT is also thought to be involved in the delivery of specific molecules required for the compartmentalization of the cilium [31] and, via cooperation with the BBSome, to contribute to the biogenesis of the ciliary membrane [32, 33]. Finally, it is now well established that cilia play critical roles in the intracellular signaling that underlies several critical cell and developmental processes, for example in Hedgehog, Notch, Wnt, Planar cell polarity, TGF-β and opsin-dependent signaling, as well as in DNA damage repair pathways, to name but a few [34–38]. A cilium can plausibly do so by serving as a “cellular antenna” that detects extracellular signals from the environment, by serving as a signaling platform that concentrates signaling molecules, and by transmitting signals to the cytoplasm and nucleus to control gene expression and cell function. IFT is thought to play key roles in cilium-based signaling, not only by building the foundation of the ciliary antenna, but also by delivering signaling molecules as cargo to the ciliary membrane and matrix and it possibly also plays a more direct, yet poorly understood role in the mechanism of signal transduction itself. This topic has been nicely covered in several recent reviews e.g. [39, 40].
2. Structure of Cilia and IFT trains
To put in context the topic of IFT as a model system for studying mechanisms of motor-dependent cargo transport, it may be useful to consider the contribution of IFT to the overall process of ciliogenesis and the generation of the complex architecture of cilia, features of which can significantly influence the process of IFT (Fig. 1A).
The basal body and the initiation of ciliogenesis
IFT is thought to begin significantly after the actual initiation of ciliogenesis which begins with the formation and positioning of the basal body (BB), a cylindrical structure consisting of 9 symmetrically arranged triplet MTs (named A, B and C tubules) associated with a complex network of membranes (see fig. 1 in [41]). The BB is derived from the mother centriole which resides at the mitotic spindle pole during M-phase, then translocates to the site of ciliogenesis at the cell surface following mitotic exit [42, 43] in a process that may involve kinesin-2 function [44]. The initial stages of ciliogenesis are characterized by the docking of small “distal appendage vesicles” (DAVs) onto the distal end of this centriole followed by their fusion into a single ciliary vesicle [45, 46]. In a complex, poorly-understood sequence of events, the BB elaborates lateral transitional fibers as its A- and B-tubules elongate, possibly by an IFT-independent mechanism, to form the transition zone doublets, which are crosslinked to the overlying membrane via Y-shaped linkers (Fig 1A) [47–49]. While the exact timing is unclear, it is plausible to think that this is the stage at which IFT engages and begins to assemble the axoneme proper by further elongating the A and B tubules of the transition zone, as the ciliary vesicle fuses with the plasma membrane to form the ciliary membrane, which now surrounds the protruding axoneme (see Fig. 2 of [41]). It should be noted that, in C. elegans, the BB undergoes substantial degeneration after ciliogenesis, leaving behind only the transition fibers (which, based on recent electron microscopy (EM), might actually be flaring MT doublets) [50, 51].
The ciliary axoneme
The axoneme core of the cilium, consisting of 9 doublet MTs extending with their plus ends distal from the A and B tubules of the BB, is assembled and maintained by IFT [52]. The A-tubule of each doublet is a complete MT consisting of 13 protofilaments (pf), whereas the B-tubule is an incomplete MT often consisting of only 10 pfs [53, 54]. Near the ciliary tip, the B-tubules generally terminate before the A-tubules, generating distal singlet MTs that make up the distal segment (ds) whose length varies substantially; in trypanosomes it is non-existent [55], in C. elegans it can be several μm long [50], while in frog olfactory cilia it extends over a hundred microns [56]. It is unclear how the length of the ds relates to ciliary function but it has been hypothesized that it might define a differentiated membrane responsible for specific sensory function [57].
In motile cilia, 9 symmetrically arranged doublet MTs often surround a pair of single MTs, in a so-called 9 + 2 configuration [58]. The doublets in these cilia are linked together via nexin links, and the central MT pair is linked to the A-tubules of the doublets via radial spokes [59, 60]. Outer dynein arms and inner dynein arms are located on the A-tubules of the doublets, allowing the dynein motor domains to contact the neighboring B-tubule in order to generate the forces required for cilium motility [61]. In non-motile cilia, most components required for force generation, such as the single MT pair, are lacking, resulting in a 9 + 0 configuration. In these cilia, the radial spokes, the nexin links and the inner- and outer-arm dynein complexes are also absent. Although the 9 + 2 and the 9 + 0 configurations are the two most commonly found MT structural arrangements, a wide variety of other arrangements exist for both motile and non-motile cilia [62]. In C. elegans, for example, the non-motile chemosensory cilia often have a variable number of singlet MTs at the core of the axoneme [50], which could have associated transport functions although this is unclear. At the tip of the axoneme, ciliary-tip complexes have been observed that might regulate and stabilize axoneme length, and also regulate turnaround of IFT, although further work is required to learn their detailed structure and functions [57].
The axoneme is very stable with only the tips of the A-tubule and B-tubule displaying continuous tubulin turnover [29, 63]. The stability of the axoneme could be the result of various tubulin post-translation modifications (PTMs), which are also known to influence intracellular transport and to facilitate the accumulation of microtubule-inner proteins (MIPs) and outer microtubule-associated proteins (MAPs) [64–66]. Furthermore, the A- and B-tubules can be composed of different tubulin isotypes and can also acquire different post-translation modifications [67]. As described below, these modifications can affect IFT, thereby tuning ciliary function.
Transition zone and the ciliary membrane
The transition zone (TZ) characterized by the Y-shaped linkers between the MT doublets and ciliary membrane, together with the transition fibers and the so-called ciliary necklace at the base of the cilium, form the so-called ciliary gate that provides a physical barrier between the cytoplasm and the ciliary compartment [33, 68]. The TZ contains several protein modules, mutations in which are linked to ciliary diseases [69]. The gate provides structural integrity to the cilium and is thought to influence ciliary entry and exit by physically restricting the size of proteins that can diffuse across it (to about ~10 nm) and by regulating IFT [70–73]. Larger cargoes accumulate in front of the gate before entering the cilium, at the ciliary pocket, which is thought to function as a docking side for ciliary-cargo containing vesicles, and at the transition fibers that can function as docking sites for IFT particles [41, 74]. Interestingly, in the cilia of Tetrahymena, there exists a different physical barrier named the ciliary pore complex (CPC) which consists of a plate containing openings for the MT doublets, the IFT machinery and associated cargo [75].
The ciliary gate also provides a physical barrier between the ciliary membrane and the plasma membrane, and together with the high curvature of the ciliary pocket, it prevents rapid exchange between the two membranes and maintains their distinct lipid and protein compositions thereby permitting their functional differentiation [41, 76]. For example, the dynamic regulation of ciliary membrane composition by the ciliary gate is critical for cilium-specific signaling and recent work suggests that this also depends upon a pathway that utilizes the retrograde IFT-dynein motor in the cytoplasm and the shedding of membrane-bounded ectosomes at the cilium tip [77]. Finally, in addition to its role as a regulator of ciliary membrane composition, the ciliary gate also substantially influences IFT dynamics, as described further below [78].
Structure of IFT trains
IFT trains were first observed in Chlamydomonas cilia using light and electron micoroscpy [9] and similar structures were soon observed in EM images of other types of cilia such as retinal photoreceptor cells [17]. Surprisingly, IFT-train size varies dramatically, with the longest trains being about ~0.7 μm long and displaying a 40-nm periodicity, while the shortest are only ~0.25 μm long and exhibit a 16-nm periodicity [79]. In Chlamydomonas, the size of IFT trains has been shown to scale inversely with ciliary length; trains are longer when the cilium is shorter and vice versa, indicating that regulation of the IFT-train length might represent a crucial ciliary-length control mechanism [80, 81]. More recent work using correlative light and electron microscopy (CLEM) has provided more detailed insight into IFT-train structure [82]. Anterograde- and retrograde IFT-trains appeared to have a similar length of ~0.2 μm, however, anterograde IFT trains, which moved primarily along B-tubules, consisted of more electron-dense structures compared to retrograde trains which ran primarily along A-tubules. An additional, static fraction of IFT trains with a length of ~0.65 μm and 40-nm periodicity was also identified, consistent with the earlier study (Pigino 2009). The authors hypothesized that the selection of B- versus A-tubules by trains moving in opposite directions may avoid collisions between them, although we note that this could also be accomplished by the selective use of the 10–15pf tracks that are available per singlet or through the use of distinct A-tubules [83], and that collisions do not seem to pose a significant problem for IFT trains that move bidirectionally along the distal singlet A-tubules of C. elegans cilia [78]. The precise correlation between the morphologically-defined IFT-trains visible by EM and the biochemically-defined protein components of the IFT machinery (discussed next) is currently an outstanding unresolved problem in the field of cilium biology [81].
3. Biochemistry and Functions of components of the IFT Machinery
The overall molecular composition and structure-function analysis of the IFT machinery has recently been discussed in a scholarly review [84]. This machinery comprises kinesin-2 and IFT-dynein motors that move the IFT trains and associated cargo e.g. tubulin subunits back and forth between the base and tip of the cilium (Fig. 2). In this section we focus on those aspects relevant to motor-dependent transport and cargo delivery.
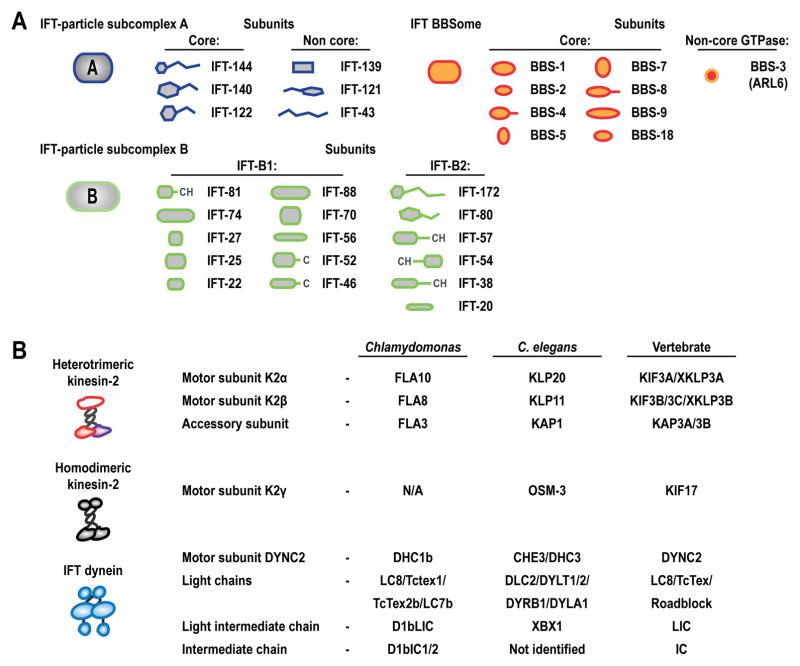
Figure 2.
The subunit composition of the IFT motors and the IFT trains/particles. (A) Detailed description of IFT particles based on a recent review [84]. (B) Description of motors involved in IFT and their subunits in different organisms. For more information on Kinesin-2 subunits see [25] and on IFT-dynein subunits see [24, 105, 143, 144]. See text for details.
IFT trains
Composition
IFT trains are multimeric protein complexes that are related to the protein coats of intracellular transport vesicles [85, 86] and assemble from 16/17S IFT particles, which were first isolated in classic studies based on their temperature-dependent loss from cilia of conditional mutant Chlamydomonas cells lacking kinesin-2 function [10, 11]. Work done in a large number of laboratories supports the hypothesis of Cole et al, that IFT-particles comprise two subcomplexes, namely IFT-A, which contains six subunits (IFT144, 140, 139, 122, 121 and 43) and IFT-B, which contains sixteen subunits (IFT-172, 88, 81, 80, 74, 70, 57, 56, 54, 52, 46, 38, 27, 25, 22 and 20), with many of the subunits displaying characteristic protein-protein interaction motifs such as coiled-coil heptad repeats, tetratrico repeats and WD-40 repeats (Fig. 2A) [84]. The IFT-B complex is further organized into IFT-B1 (aka the IFT-B core comprising ten subunits IFT-88, 81, 74, 70, 56, 52, 46, 27, 25 and 22) and IFT-B2 (aka the peripheral IFT-B complex comprising six subunits, IFT-172, 80, 57, 54, 38 and 20), both of which are capable of assembly into stable complexes [87–91]. Available evidence discussed later suggests that the IFT particles transport axonemal precursors, notably tubulins, to their site of assembly at the axoneme tips.
Cilium membrane biogenesis requires the function of the BBSome, a complex of 8 of the known BBS proteins i.e. subunits BBS-1,-2,-4,-5,-7,-8,-9,-18 that is linked to the ciliary membrane by the small GTPase, ARL6/BBS-3 and is required for normal IFT (Fig. 2A) [92, 93]. Whether the BBSome represents a third integral component of the IFT particles, like IFT-A and IFT-B is debatable. In mammalian and nematode cilia it appears to be present in a 1:1 stoichiometry to the IFT particles and may be required for the structural integrity of IFT particles by holding together IFT-A and IFT-B as they are moved along the cilium by kinesin-2 motors [94, 95]. In Chlamydomonas, however, the BBSome appears to be highly substoichiometric (≈1:6) to IFT-A and IFT-B, and is proposed to serve as an adaptor involved in the export of signaling proteins from the cilium, rather than as an integral component of IFT particles [96]. Loss of BBSome function leads to changes in the protein composition of ciliary membranes consistent with a role for the complex in ciliary membrane protein trafficking [33, 95, 97]. Taken together, the available evidence suggests that the BBSome can function as an IFT regulator, as a core IFT component, or as a cargo adaptor in manners that are apparently cell-type specific.
Cargo Binding to IFT trains
IFT-train subunits are proposed to bind a variety of cargo molecules, including ciliary tubulins, as well as motors such as the dynein arms of motile cilia and IFT motors themselves. For example, IFT dynein is moved base-to-tip along the cilium by anterograde IFT and, in some cilia, kinesin-2 motors are moved back to the cell body by retrograde IFT. IFT is proposed to transport tubulin subunits to their site of incorporation at the tips of the axoneme in low amounts when the assembling cilium has reached its steady-state length [63] but in substantially increased amounts during the elongation phase of ciliogenesis [98]. High-resolution structural studies showed that the –NH2 domains of the IFT-B1 subunits, IFT-81 and IFT-74, form a tubulin-binding module in which the IFT-81 calponin homology (CH) domains serves as a recognition domain. This domain on IFT-81 binds tubulin relatively weakly, but binding is enhanced by the positively charged amino terminus of IFT74, which binds electrostatically to the acidic COOH-terminal tails of tubulin (E-hooks) [99]. In support of this model, disruption of either the CH-domain of IFT-81 or the IFT-74 amino terminal domain required for high affinity binding, led to a reduced rate of assembly of full-length cilia. The disruption of both domains, however, led to the assembly of only highly truncated steady-state cilia [100]. Interestingly, a second tubulin-binding CH-domain was also found in the N-terminal region of the IFT-B2 subunit, IFT-54, potentially allowing the transport of 2 tubulins per IFT-B complex, compatible with the kinetics of ciliogenesis [91]. The dynein arms of motile cilia were many years ago proposed to be delivered by IFT [101] and subsequent work suggests that the IFT-B1 subunit, IFT-46 is specifically involved in binding and transporting the outer, but not the inner, dynein arms as cargo along the cilium [102]. The delivery of inner dynein arms, in contrast, is thought to require the activity of IFT-56 [103].
While there is good evidence that some IFT-cargo molecules such as tubulins are ferried along the cilium via their binding to IFT-particle subunits, there are also indications that some cargo molecules may be delivered to the distal tips of cilia via their direct binding to the anterograde kinesin-2 motors instead, possibly circumventing the participation of IFT trains. These include tubulins [104], subunits of the retrograde IFT-dynein motor [105] and the GLI transcription factors that are involved in Hedgehog-related cilium-based signaling [106]. This interesting possibility merits further work.
Motor Binding to IFT-trains
In C. elegans amphid-channel cilia, heterotrimeric kinesin-2 (aka kinesin-II) appears to bind and transport IFT-A, whereas homodimeric kinesin-2 (OSM-3) binds IFT-B, because in BBSome mutants, the kinesin-II/IFT-A and OSM-3/IFT-B subcomplexes move separately along the axoneme at 0.5 and 1.2 μm/s, respectively [94]. The IFT-B1 subunit, IFT70 (DYF-1) was proposed to be involved in the IFT-B/OSM-3 interaction, which is supported by co-immunoprecipitation experiments in mouse, suggesting that IFT70 binds to multiple IFT-B subunits and to homodimeric kinesin-2 (KIF17) [107]. In contrast to the findings from C. elegans however, co-immunoprecipitation experiments in Chlamydomonas (which lacks homodimeric kinesin-2) and mouse suggest that heterotrimeric kinesin-2 binds directly to IFT-B rather than IFT-A [108], but the significance of this difference is unclear. The retrograde motor, IFT dynein, is proposed to attach to IFT-B and is delivered to the distal tip of the cilium as a passive cargo of anterograde IFT and then docks onto IFT-A to drive retrograde IFT [109, 110]. The latter results, together with observations that loss-of-function mutations in IFT-B and IFT-A subunits often phenocopy mutations in heterotrimeric kinesin-2 and IFT-dynein, respectively, has led to the suggestion that IFT-B and IFT-A function separately in anterograde and retrograde transport along the axoneme [reviewed in [84]]. However, there are several reports that contradict this model, e.g. the requirement of IFT-A for import of some cargo molecules into the cilium and the participation of some IFT-B subunits in retrograde transport [111, 112]. For example an IFT-A subunit, IFT-121, is required for retrograde IFT [113] but also functions in the delivery of several membrane proteins to the cilium [114]. Clearly the biochemistry and functional consequences of the binding IFT-motors to subunits of the anterograde and retrograde IFT trains is a topic that requires additional research.
IFT motors
Heterotrimeric kinesin-2
Anterograde motors that drive the transport of IFT trains and associated ciliary precursors as cargo along the axoneme from the base to the distal tips of the cilium are members of the Kinesin-2 family, which are understood to exist in both heterotrimeric and homodimeric forms (Fig 2B) [reviewed by [25]]. Briefly, heterotrimeric kinesin-2 was first purified from sea urchin eggs as a MT-based motor consisting of a 1:1:1 stoichiometry of subunits 2α, 2β and KAP that moves towards the plus ends of MTs at ≈ 0.4μm/s and is required for ciliogenesis on the blastula stage embryo [115–118]. It is generally assumed to drive IFT along embryonic cilia, but this has not yet, to our knowledge, been confirmed. Similar complexes were subsequently found in several systems, including Chlamydomonas where it is clearly required for IFT and ciliogenesis [10, 119–121] and mouse where it is required for the assembly of nodal cilia and the establishment of left-right asymmetry, although here again, its assumed role in IFT along nodal cilia remains unconfirmed [122, 123]. Current mechanistic studies are aimed at elucidating the mechanical significance of this motor having two distinct motor subunits e.g. [124, 125] and the role of the unique KAP subunit, which contains multiple armadillo repeats and is an essential and specific subunit of heterotrimeric kinesin-2 [118, 126]. For example, in C. elegans cilia, in an osm-3 mutant background, the loss of KAP subunit function results in the axoneme being completely absent, just like loss of motor subunit function [127, 128]. Available evidence suggests that KAP may stabilize the heterodimeric coiled-coil formed between the 2α and 2β motor subunits [129, 130], to target the motor to the base of the cilium and enhance its processive movement along the axoneme [119], and/or to bind IFT train/cargo complexes, although to our knowledge there is no evidence for the latter hypothesis in any cilia.
Homodimeric kinesin-2
Biochemical fractionation of C. elegans extracts suggested that their sensory cilia contain both a heterotrimeric and a homodimeric form of kinesin-2, the latter consisting of two γ (aka OSM-3) motor subunits [131]. The formation of homodimers by purified recombinant mammalian kinesin-2 (aka KIF17) confirmed it oligomeric state and also permitted motility assays, which revealed that homodimeric kinesin-2 moves toward the plus end of MTs at ~1.0 μm/s [132]. Homodimeric forms of kinesin-2 have been found in cilia from a broad range of organisms but they appear to act differently in different cilia, even from the same organism [127]. In C. elegans amphid channel cilia, for example, homodimeric kinesin-2 clearly participates in anterograde IFT and it assembles the distal part of the axoneme, although in the absence of heterotrimeric kinesin-2 function, it is capable of building the full-length axoneme [128]. It also moves along the cilium in mammalian cells [95] and its activity is required for the localization of cyclic nucleotide gated channels and dopamine receptors to ciliary membranes [133, 134], but whether these signaling molecules are actually delivered to the cilium membrane by homodimeric kinesin-2-driven IFT remains unproven. Moreover, in some cilia the loss of homodimeric kinesin-2 function, unlike heterotrimeric kinesin-2, has no detectable effect on ciliary morphology and functions and consequently its ciliary function, if any, remains unclear [44, 135–137].
IFT-dynein
IFT-dynein (aka dynein-2) drives the retrograde transport of IFT-trains from the tip of the axoneme at rates of one to a few microns per second, thereby recycling components of the anterograde IFT machinery and turnover products back to the base of the cilium for export [reviewed by [24]]. The dynein-2 heavy chain (DYNC2 or DHC1b) was identified as a form of cytoplasmic dynein that is upregulated prior to ciliogenesis on the blastula stage sea urchin embryo, suggesting a role in cilium biogenesis [138]. A direct role for this dynein heavy chain isotype in driving retrograde IFT was subsequently supported by work done in Chlamydomonas and C. elegans cilia [139–141]. Earlier work, however, had implicated an accessory dynein light chain, LC8 in retrograde IFT, albeit one that has non-IFT-dynein-related functions as well e.g. stabilization of radial spokes, and inner and outer dynein arms [142]. In fact, IFT-dynein is a complex, multi-subunit motor, much more complex than the kinesin-2 motors, consisting of two DYNC2s and typically 2 each of three types of light chain (LC8, TcTex, roadblock), 2 light intermediate chains and 2 intermediate chains, whose specific functions are largely unclear and may be difficult to tease out [Fig. 2B] [105, 143–148]. For example, in a very rigorous recent study, it was shown that the loss of function of the Chlamydomonas light intermediate chain, D1bLIC, produces a range of phenotypes including decreased IFT-dynein stability, changes in protein expression level and a reduced frequency and velocity, but not a complete loss, of retrograde IFT [149]. This illustrates that much more work is required to understand the specific functions of IFT-dynein’s multiple subunits in IFT and ciliogenesis. With respect to its mechanism, a significant advance was the determination of the crystal structure of the IFT-dynein triple A-containing motor domain in the ADP.Pi-bound pre-power stroke conformation [150]. However, unlike cytoplasmic dynein-1, the in vitro motility properties of purified IFT dynein have not been analyzed extensively, although in one study recombinant dynein-2 was observed to move towards the minus ends of MTs at 0.07 μm/s in vitro, i.e. with the predicted directionality but at a much slower speed than retrograde IFT [151]. Determining the molecular and biophysical mechanism of the IFT-dynein-driven transport of IFT trains is therefore also a topic that merits further attention [152].
Other motors
Several additional MT-based motors are involved in ciliogenesis, but are not thought to function primarily by transporting IFT particles and associated cargo along the axoneme [37, 153, 154]. Of these, a C. elegans kinesin-3 motor clearly modulates IFT and controls the localization of polycystins in the ciliary membrane, possibly via a non-IFT transport pathway [155, 156]. Kinesin-13 MT depolymerases are proposed to act at the axoneme tips or the mother centriole/BB to control ciliary length [157–159] and kinesin-4 controls the organization of the distal tip of the cilium to regulate transcription factors involved in Hedgehog signaling [160]. Because these and other fascinating ciliary motors are not thought to drive IFT, they are not a focus of the current review. Finally, it has been speculated that a novel dynein heavy chain, DHC-3, might represent a second form of IFT-dynein that drives retrograde transport along axonemes in certain C. elegans ciliated neurons, but this idea has not, to our knowledge, been tested [105].
4. Mechanism of Action of IFT-motors
The key drivers of IFT are IFT dynein for retrograde transport and heterotrimeric kinesin-2 for anterograde transport. In several cilia, an accessory homodimeric, kinesin-2 motor is deployed, but as noted above, its role may vary. Current work is aimed at understanding the biophysical mechanisms by which these kinesins and dyneins transduce the chemical energy contained in ATP into mechanical work. These IFT motors do not work on their own since they transport mechanically coupled trains containing multiple motors of either the same or different kinds. The resulting motor cooperation is one of the key areas of research in IFT, which we will address in the following section. First, we will focus on the properties of the individual motors, as obtained from in vitro and in vivo studies.
IFT dynein
As noted above, the slow minus end-directed motility of human recombinant dynein-2 has been documented in vitro [151] but more extensive studies of the mechanism by which IFT dynein generates force and motion are needed. It is known that IFT dynein is more closely related to the multifunctional transport motor, cytoplasmic dynein 1, than it is to axonemal dynein, which drives the beating of motile cilia [161]. Cytoplasmic dynein-1 has been extensively studied in vitro and shown to take sequential ~8nm steps to move processively towards the minus end of MTs [162], although the actual step size varies much more than for kinesins and the processivity strongly depends on the presence or absence of cofactors such as dynactin and cargo adaptors [163, 164]. It remains to be seen if IFT dynein displays similar in vitro motility properties to cytoplasmic dynein 1. Strikingly, the rate of retrograde IFT driven by IFT dynein in vivo varies, ranging between ~0.6 μm/s in mouse primary cilia [165], ~1.2 μm/s in C. elegans chemosensory cilia [128], ~3 μm/s in Chlamydomonas [96, 166], and ~5.6 μm/s in trypanosomes [167]. This remarkable difference in motor velocity between different cells and organisms warrants further study.
Heterotrimeric kinesin-2
As a preliminary step towards testing the role in anterograde IFT of the plus end-directed MT motility of heterotrimeric kinesin-2 observed in MT surface gliding assays [116], the rates of motility in vitro and in vivo have been compared [168]. For example, C. elegans kinesin-II was observed to move at ~0.3 μm/s in vitro in “standard” PIPES buffer concentrations i.e. somewhat slower that in vivo, but faster velocities, very similar to the rates of kinesin-II-driven IFT train transport along sensory cilia (in mutants lacking homodimeric kinesin-2 function), could be obtained by simple variations of the PIPES concentration, supporting the hypothesis that kinesin-II serves as an anterograde IFT motor [168]. Later single-molecule optical tweezers experiments of KLP11/KLP20 motor heterodimers showed that the motor is processive (although substantially less so than kinesin-1), taking 8 nm steps along MTs and being capable of resisting opposing forces of ~5 pN [125]. Remarkably, chimeric constructs consisting of two KLP20 motor domains were processive as well, while constructs containing two KLP11 motor domains were not, indicating that the non-processive KLP11 motor domain is rendered processive by heterodimerization with its KLP20 partner. The authors also found evidence for an autoinhibition mechanism of heterotrimeric kinesin-2, which might be relieved by cargo (i.e. IFT train) binding.
More recently, full-length mouse KIF3A/B, has been studied extensively using single-motor bead tracking and optical tweezer assays [169, 170], yielding an unloaded run length of ~0.4 μm and a velocity of ~0.4 μm/s. Because the neck-linker regions of dimeric kinesin motors are proposed to transmit information about the mechanochemical state of one motor domain to the other, and vice versa, the shorter run length of kinesin-2 was proposed to be a consequence of its longer neck linker (17 amino acids) compared to that of the more processive kinesin-1 (14 aas [171, 172]. A recent study using truncated protein constructs attached to quantum dots, however, reported a very different run length of about 1.6 μm [173]. Further studies are needed to find the source of this apparent discrepancy, which might be due to the different constructs used, the use of quantum-dot versus polystyrene-beads, or other unknown differences in experimental conditions [169, 171–173]. Single-molecule fluorescence assays have further indicated that heterotrimeric kinesin-2 motors are less prone than other kinesins to detach from a MT upon encountering an obstacle e.g. a MAP on their MT track [174]. Stopped-flow and steady-state kinetics experiments on KIF3A/B indicate that, following dissociation, kinesin-2 rebinds to MTs very fast in the ADP-bound state, suggesting that its catalytic properties are optimized to restart a processive run after its progress has been impeded by such an obstacle [124]. A tentative mechanochemical cycle for heterotrimeric kinesin-2 based on these studies is shown in Fig. 3A.
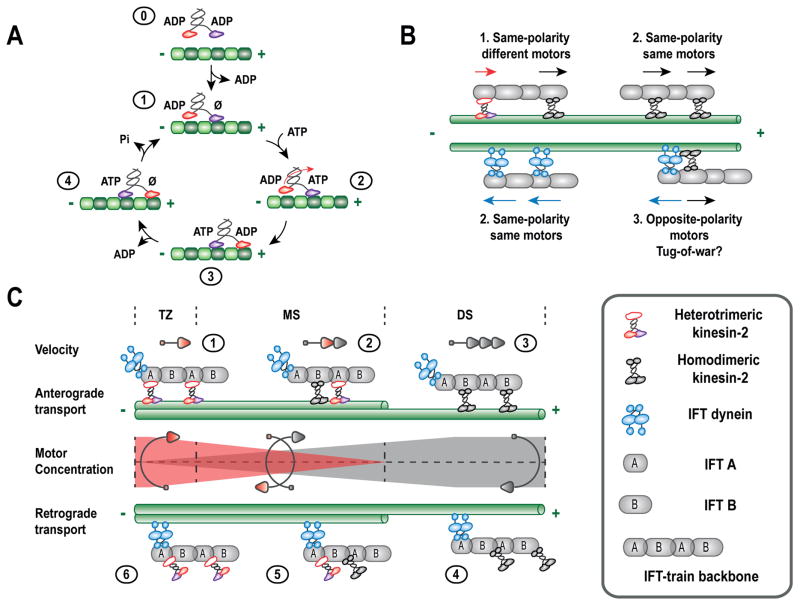
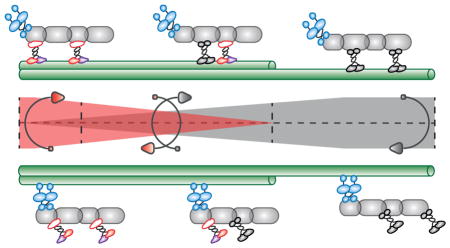
Figure 3.
Motor activity and cooperation of kinesin-2 motors. (A) A simplified mechanochemical cycle showing plus end-directed stepping of a truncated heterotrimeric kinesin-2 along a MT track (based on [124]). (B) Coupling of different molecular motors in a combinatorial fashion to the same cargo determines overall transport dynamics. (C) Cooperation of heterotrimeric and homodimeric kinesin-2 motors in C. elegans chemosensory cilia is determined by gradients of kinesin-2 motors. Numbered steps are; (1) A stable IFT-train backbone is loaded into the cilium and transported through the transition zone (TZ) at ~0.5 μm s−1 mainly by kinesin-II. (2) After navigating the TZ, kinesin-II gradually undocks while, at the same time, OSM-3 motors start docking, resulting in a gradually accelerating IFT train reaching ~1.3 μm s−1 at the end of the middle or proximal segment (PS), and ensuring reliable handover of the IFT-trains. (3) In the distal segment (DS), the train is occupied solely by OSM-3 reaching a terminal velocity of ~1.5 μm s−1. (4) Following turnaround and remodeling at the ciliary tip, the backbone is returned to the base by IFT dynein, recycling OSM-3. (5) OSM-3 undocks from retrograde IFT trains along the PS, while kinesin-II gradually docks. (6) Close to the base, kinesin-II is the main kinesin cargo of retrograde IFT trains. Figure based on [78], see text for details.
Optical tweezers experiments have further demonstrated that opposing forces have relatively little effect on the velocity of heterotrimeric kinesin-2 compared to other kinesins but dramatically reduce its run length [169, 175]. Stopped-flow and steady-state kinetics experiments showed that the kinesin-2 construct, homodimeric KIF3A/A, spends a substantially longer fraction of its ATP hydrolysis cycle in a state with low MT affinity, but that dissociation from the MT in this state is slow under unloaded conditions [176]. This might be the underlying cause of the load dependency of heterotrimeric kinesin-2. In the optical tweezers study of Andreasson et al. [169], artificial homodimeric constructs were also studied, revealing that KIF3B/B is substantially less processive than KIF3A/A and that the properties of KIF3A/B are intermediate between the two homodimeric constructs.
The velocities measured in vitro match those observed for heterotrimeric kinesin-2 working on its own along cilia e.g. anterograde IFT occurs at ~0.5 μm/s in osm-3 mutant C. elegans chemosensory cilia [128] and at ~0.4 μm/s in mouse primary cilia [165]. Remarkably, anterograde IFT in trypanosomes [167] and Chlamydamonas [10, 120, 121] is substantially faster (~2 μm/s). This faster transport might reflect the substantial phylogenic distance of the heterotrimeric kinesins in these organisms with those in mammals and nematodes [25]. Unfortunately, so far no in vitro assays have been performed on purified heterotrimeric kinesins from these latter organisms.
Homodimeric kinesin-2
The mechanism of action of homodimeric kinesin-2 has been less well-studied than that of heterotrimeric kinesin-2, perhaps because its homodimeric architecture resembles that of the extensively studied kinesin-1, suggesting that fundamental new principles are less likely to emerge. As noted above for heterotrimeric kinesin-2, the simple use of trial-and-error variations in the PIPES buffer concentration has revealed conditions in which the rate of plus end-directed MT gliding driven by C. elegans OSM-3 (~1.3 μm/s) matches the rate of transport driven along cilia under conditions when OSM-3 is the sole anterograde motor [128, 168]. Single-molecule fluorescence motility assays further revealed that full-length C. elegans OSM-3 motor constructs bind only very weakly to MTs and do not show processive motion in vitro [177]. This has been attributed to an autoinhibition mechanism that switches off motility in the absence of cargo. Deletion and point-mutation variants of OSM-3 motors devoid of this mechanism were shown to be relatively fast (~1 μm/s) and processive (run length ~1.6 μm). Optical-tweezers assays confirmed the autoinhibition mechanism and indicated that OSM-3’s force-generation capability is comparable to that of kinesin-1.
5. Motor Cooperation and Regulation during IFT
Intracellular transport often depends on groups of motor proteins working together to transport a single cargo (Fig. 3B). During neuronal transport, for example, multiple kinesins with different motility properties and opposite-polarity dynein motors cooperate while transporting the same vesicles along MT tracks [178]. Their association with the same vesicle results in mechanical coupling between the different motors, which can give rise to frequent pausing and direction reversals [179]. In general, the motility properties of motor proteins such as run length and velocity are well known to be load dependent [169, 180]. In situations where multiple motor proteins are mechanically coupled, they can influence each other’s behavior by applying assisting or resisting forces to each other. The cooperation of multiple, mechanically coupled motor proteins is a rich field of research, both theoretically [181–183] and experimentally using in vitro assays [184–188]. Several excellent reviews of this field have been published recently [189, 190]. Experiments and theoretical studies have shown that, depending on the properties of individual motor proteins and the way they are connected, cooperativity between motors can be positive or negative. Of particular note are situations where opposite-directed motors act on the same cargo, resulting in a ‘tug-of-war’, which can result in frequent directional reversals, pausing and even stalling as has also been observed in axonal transport [174, 178, 191].
In IFT, tens of motors act together on the same cargo in a very dense environment. In IFT, however, ‘tug-of-war’ kind of behavior involving reversals and pausing appears to be largely absent, with anterograde IFT trains moving steadily towards the ciliary tip before changing direction, and retrograde IFT trains moving steadily towards the base [94, 128, 192, 193]. This is remarkable since kinesins with different motility properties as well as opposite-polarity motors, the IFT dyneins, are coupled to the same IFT trains. In addition, the motor composition of IFT trains and the motor activity can vary depending on the track position. In C. elegans chemosensory cilia, for example, anterograde IFT-trains close to the base are mainly driven by kinesin-II motors, whereas after a gradual handover of the cargo, OSM-3 propels the trains solely towards the ciliary tip (Fig. 3C) [78]. Dynein-mediated direction reversals mostly occur at the ciliary tip [143], indicating that dynein is locally activated. Kymograph analysis of IFT in various organisms suggests that IFT is a highly orchestrated event with few aberrant movements [78, 94, 128, 192, 193]. Taking into account the number and variety of motor proteins involved in driving the smooth bidirectional movement of IFT-trains strongly suggests that motor activity is highly regulated. The regulation of the IFT motor proteins could occur at several different levels as described in this section.
At the base, the transition fibers serve as an assembly platform where IFT particles dock and form linear arrays of well-defined size [74, 81, 82]. The IFT-train assembly process sets the number of binding sites for IFT motors and also determines motor composition, a process that requires the activity of an additional protein complex, the BBSome [194]. How assembly takes place and how the IFT-train size is controlled is, however, not well understood. A recent study in Chlamydomonas has revealed that the docking of heterotrimeric kinesin-2 to IFT particles at the base is controlled by phosphorylation [108]. The authors showed that phosphorylated kinesin-2 did not bind IFT-particle-subcomplex B, whereas dephosphorylation of the FLA8/KIF3B motor subunit relieved this inhibition. In addition, the KAP subunit of heterotrimeric kinesin-2 could also play a role in IFT-particle docking and controlling motor activity. In Chlamydomonas it has been shown that the FLA3/KAP3 subunit is required for localization of heterotrimeric kinesin-2 to the ciliary base and for its motor activity [119]. Interestingly, in most organisms the KAP subunit is found to be slightly substoichiometric relative to the motor subunits, suggesting an additional form of regulation where the kinesin-2 motor might function as heterodimer [25], possibly having different motility properties. Targeting of the homodimeric kinesin-2, KIF17, to cilia involves a ciliary localization signal (CLS) that resides in the tail of the motor [71]. The same study showed that KIF17 entry into cilia is controlled by a RanGTP gradient and interaction of the motor with importin-β2 [71]. Interestingly, the authors identified the CLS by searching for sequences resembling a nuclear localization signal (NLS), and (as noted by the authors) that the KAP-subunit of heterotrimeric kinesin-2 motor harbors a NLS [195], indicating that IFT motor proteins might harbor (unidentified) NLS/CLS sites required for their cell-cycle dependent localization.
During IFT-train formation another form of motor regulation might come from the intrinsic properties of the motors themselves. In the absence of cargo interaction most kinesins are known to reside in an autoinhibited state, where the tail, which also contains the cargo-binding domain, folds back onto the motor domains, thereby preventing futile ATP hydrolysis, and inhibiting MT and cargo interactions [196]. Cargo binding can relieve this autoinhibitory state upon which the motor extends and becomes active. Both heterotrimeric and homodimeric kinesin-2 can form a compact autoinhibited state [118, 177], which might be relieved upon their association with the IFT particles. In the case of KIF17 the autoinhibitory state is induced by a folded conformation where the tail domain and part of the coiled coil interact with the motor domains [197]. In the case of OSM-3 a single-point mutant (G444E), located in the hinge region of the coiled coil, was shown to relieve the autoinhibition in a series of ATPase and in vitro motility assays [177]. KIF17 is thought to have a similar hinge region, and a single point mutation (G754E) in this region together with mutations in a coiled-coil segment (762–772A) were shown to extend and activate the motor [197]. The hinge region of kinesin-2 motors might act as specific target site for regulatory proteins, which could stabilize the unfolded conformation, for example. In C. elegans the conserved ciliary protein DYF-1 (an IFT-particle B associated component) was shown to affect the autoinhibited state of OSM-3 [94, 198]. Using IFT assays the authors found that DYF-1 is required for targeting of the OSM-3 motor to IFT particles and for relieving its autoinhibition, DYF-1-OSM-3 protein-protein interactions were however not identified.
Once the IFT train is assembled and the kinesin-2 motors activated, the IFT-train has to navigate the structurally dense TZ. In C. elegans, kinesin-II is thought to function as a loader at the base and navigator in the TZ, whereas OSM-3 functions as a long-range transporter once inside the cilium [78]. This means that the localization and activity of both motors has to be precisely tuned via their docking and undocking to and from IFT trains. It is likely that the axonemal track and the IFT particles play important roles in this respect. Recent work in Chlamydomonas has shown that in anterograde direction IFT trains specifically move along the B-tubule of the axoneme, whereas retrograde IFT-trains move along the A-tubule [82]. In C. elegans the early termination of the B-tubule could hence act as a physical barrier to the kinesin-II motor, preventing the motor from entering the distal segment. The OSM-3 motor, however, is required to move along the A-tubule in order to enter the distal segment. It is therefore tempting to speculate that OSM-3 might preferably associate with the A-tubule and not the B-tubule. In this regard it is well known that tubulin PTMs and MAPs can affect motor protein activity [66, 196]. In C. elegans, for example, detyrosination of α-tubulin affects the activity of OSM-3, increasing its run length twofold without affecting its velocity [66], and tubulin deglutamylation has been shown to affect the ciliary localization of the kinesin-3, KLP-6, and the motility of OSM-3, but not that of kinesin-II [199]. Interestingly, the A- and B-tubule of the axonemal doublet can be modified in different ways. In Chlamydomonas, for example, the B-tubule is more detyrosinated in comparison to the A-tubule [67]. In zebrafish, the C. elegans DYF-1 homoloque fleer affects polyglutamylation of sensory cilia and its absence causes ultrastructural defects in the B-tubule [200]. [201]Although the axonemes of different organisms could be differently modified it is likely that tubulin PTMs play crucial roles in regulating the activity of IFT motor proteins.
While the IFT trains move along the axoneme, the IFT particles in concert with specific MAP kinases, regulate the docking of IFT motors to and from IFT trains thereby affecting motor activity and IFT dynamics. As mentioned above, the IFT-B associated DYF-1 can regulate OSM-3 motor activity by relieving its autoinhibition [94], whereas another IFT-B associated protein, DYF-2, is known to regulate turnaround of IFT particles at the ciliary tip [194]. In C. elegans chemosensory cilia the mitogen-activated protein (MAP) kinase DYF-5 is known to affect the undocking of the kinesin-II from IFT trains [202], and in Chlamyodomonas undocking of heterotrimeric kinesin-2 at the ciliary tip has been shown to require its phosphorylation [108]. Moreover, plus-end associated proteins such as EB1 localize to the tips of axonemal MTs and might interact with IFT machinery [63, 203, 204]. Regulation of IFT dynein in comparison to the regulation of the IFT kinesins is less well understood, but is likely to be controlled by its different subunits such as the light intermediate chain [149, 161]. It is clear that several layers of regulation orchestrate motor activity during IFT, and that further studies are required to unravel the intricate complexity of the different regulatory systems that control IFT in more detail.
In anterograde IFT in C. elegans chemosensory cilia, two different kinesin-2 motors, kinesin-II and OSM-3, cooperate [78, 128]. At the ciliary base, the IFT trains consist mainly of kinesin-II motors and move at a relatively slow velocity of 0.5 μm/s. This IFT-train motor composition might give rise to the dynamical properties required for loading IFT particles into the cilium and navigating the structurally dense TZ [78]. Along the MT doublets of the middle segment, the IFT trains, consisting of fewer kinesin-II and more OSM-3 motors, travel at an intermediate velocity of 0.7 μm/s, while along the distal segment IFT trains containing OSM-3 alone, travel at a terminal velocity of 1.3 μm/s [78, 128]. In vitro studies using well-defined ratios of kinesin-2 motors in MT gliding assays (performed in a pre-determined concentration of PIPES buffer as noted above for either type of kinesin-2 motor alone) initially showed that the rates of anterograde transport observed in cilia could be mimicked without the requirement for additional factors [168]. The intermediate velocity was explained by each motor influencing the other’s motility parameters via alternating action or through mechanical competition but a recent in vivo study led to a refinement of this picture. We found that kinesin-II and OSM-3 motors gradually undock and dock to IFT trains, respectively, giving rise to a gradual increase in the velocity of anterograde IFT trains that travel along the middle segment [78]. This behavior can be explained by the introduction of a “bias parameter” which reflects additional factors that influence motor cooperation and cause an almost 10-fold increase in the contribution of OSM-3 relative to kinesin-II motors. It is not clear, however, what factors could create such a bias. It could involve regulatory processes (see above), but might also be the result of distinct load dependencies of the two motor proteins. This latter aspect could be studied using in vitro studies employing optical tweezers.
In Chlamydomonas, assemblies of heterotrimeric kinesin-2 motors move IFT trains at velocities well over 2.0 μm/s [10, 120, 121]. It has been shown that these IFT-motor assemblies can generate over 20 pN of force [193], well exceeding the 5 pN and 6 pN stall forces measured in vitro for the kinesin-2 family motors, kinesin-II and OSM-3, respectively [125, 177]. This indicates that kinesin-2 motor cooperation might be required to generate the forces for Chlamydomonas surface gliding. However, in vitro motor studies of kinesin-2 are required to obtain a deeper understanding of kinesin-2 cooperation during IFT in Chlamydomonas.
6. Cargo Delivery by IFT
Cargo transport by IFT is required for cilium assembly, maintenance and function [17] and it appears to fulfil two basic roles [23]. First, it is involved in selectively transporting proteins across the TZ, which controls the protein composition of the cilium and second it can move proteins against a concentration gradient, e.g. axonemal precursors that are produced in the cell body and are incorporated at the ciliary tip. Cargo molecules that are transported in cilia include the complex IFT machinery itself, axonemal building blocks, such as tubulins, signaling molecules and, in motile cilia, outer-arm dyneins [23]. In Chlamydomonas, IFT also drives gliding of the organism over surfaces that it adheres to. It is important to realize that, in contrast to many other intracellular transport mechanisms [205, 206], IFT cargoes are not contained in membranous vesicles, but in most cases comprise proteins that directly attach to the IFT machinery. Several cargo proteins are, however, membrane-bounded proteins, and in particular the BBSome appears to play a role in facilitating the transport of such proteins into and along the cilium by IFT [32]. For only a few cargoes, molecular details about their interaction with IFT trains are known. Above, we have discussed in detail the molecular basis of tubulin interacting with IFT components. For many other cargoes it is not yet understood how they interact with the IFT machinery. Interactions might be less tight, less specific and might involve unknown cargo adaptors [23]. The loading of cargo most likely happens at the ciliary base, where IFT proteins are enriched, apparently ‘waiting’ for assembly into trains and connection to cargo. Many aspects of train formation and cargo loading are unclear, but dephosphorylation of one of the heterotrimeric kinesin-2 subunits appears to be an important trigger [108]. IFT trains then depart in the anterograde direction and traverse the dense TZ, which has been shown to slow down transport [78]. Many cargoes are thought to be delivered in one go at the ciliary tip, where the trains disassemble and reassemble into retrograde trains [108, 167]. Certain components, however, including axonemal components in Chlamydomonas [98, 207] and kinesin-2 motors in C. elegans [78] have been shown to detach or attach to trains along the cilium. Below we discuss several specific cargoes in more detail.
IFT components
The IFT machinery consists of many proteins, including subunits of IFT trains as well as anterograde and retrograde motors. IFT trains are assembled at the base, remain intact as they move in the anterograde direction, disassemble at the distal tip then reassemble into retrograde trains that move all the way back to the base. Anterograde and retrograde trains appear different in EM images [82], but it is unclear whether this reflects a different conformation or composition. Anterograde motion is driven by kinesin-2 motors and IFT-dynein subunits have been identified as cargoes on anterograde moving trains in C. elegans [105, 143] and Chlamydomonas [149]. Remarkably, different subunits showed differences in their frequency of transport and location of turnaround [143]. In C. elegans, both homodimeric and heterotrimeric kinesin-2 motor proteins have been observed to be cargoes on retrograde trains [128], with homodimeric OSM-3 shuttling mostly between ciliary tip and end of the transition zone, and heterotrimeric kinesin-II from just beyond the TZ to the base [78]. Remarkably, in Chlamydomomas cilia, where heterotrimeric kinesin-II is the sole anterograde motor, no IFT-dynein-driven active retrograde movement of kinesin-2 has been observed, suggesting that this motor diffuses back to the base [208]. This notable difference in retrograde transport of kinesin motors deserves further investigation.
Tubulin and other axoneme building blocks
The best characterized cargo of IFT is tubulin, the major building block of the axoneme that forms the core of the cilium. Based on studies of IFT mutants in Chlamydomonas it has long been thought that this IFT-based tubulin delivery for incorporation at the distal tip is required to build the cilium and maintain its length [17]. Indeed, careful studies in Chlamydomonas have resulted in the influential “balance point” model directly connecting IFT to ciliary length control [80]. More recent studies have revealed that length control might be more complex, involving upregulation of IFT during cilium growth [98] and kinase regulation of IFT speeds [209]. Direct evidence that tubulin is a cargo of IFT came from in vivo imaging of Chlamydomonas and C. elegans expressing fluorescent tubulin [63, 98] [although it has also been proposed that, in some cases, heterotrimeric kinesin-2 could directly bind and transport tubulin subunits along cilia independent of any IFT-particle subunits [104]]. In Chlamydomonas, additional components of the axoneme that have been shown to be IFT cargos include the radial spokes, the central-pair proteins, outer-arm dyneins and various regulatory complexes [23, 207].
Proteins involved in signalling
Cilia are important signaling hubs and IFT is thought to play a key role in delivering the signaling proteins involved in cilium-based signaling. Some signaling proteins, including TRP channels OSM-9 and OCR-2 in C. elegans, and PKD-2 in Chlamydomonas have been directly shown to be transported by the IFT machinery [210, 211]. In mouse olfactory cilia and human primary cilia, a more complex picture has been presented in which signaling proteins such as Arl, transmembrane olfactory signaling proteins [95], somatostatin receptor 3 [212] and smoothened [212, 213]) largely move by diffusion and appear to be only transiently connected to the IFT machinery. It could be that IFT is mostly involved in transporting these proteins across the TZ, while their distribution throughout the rest of cilium is mostly driven by diffusion. Further studies will be required to unravel what role IFT plays in localizing these signaling proteins.
IFT drives surface motility in Chlamydomonas
Chlamydomonas can use its flagella to adhere to surfaces and to glide along them [214]. Surface gliding is driven by IFT, mediated by flagellar membrane glycoproteins in a Ca2+-dependent manner [193, 215]. The glycoproteins adhere to the surface transiently. Dynein motors then transport the glycoproteins to the ciliary base, driving the gliding of the algae over the surface in the opposite direction.
7. IFT in different Model Systems
The variety of model organisms that are being used to study cilia represent a fairly broad range of eukaryotes (Figs 1 and 4), as discussed in a comprehensive recent review [216]. These organisms range from protists such as the basal eukaryote Giardia [217] through plants where the IFT motor, kinesin-2 is thought to participate in ciliogenesis in mosses [218, 219] to vertebrates such as Xenopus and mouse [123, 220]. Here we discuss only a few of the systems where direct assays of IFT have yielded mechanistic information about motor-dependent cargo transport in IFT.
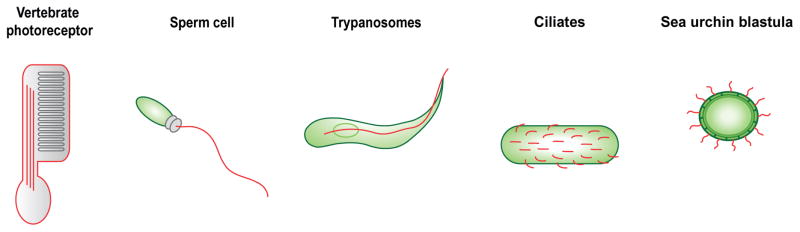
Figure 4.
Cilia of various cells and model systems. For motile cilia (aka flagella) of swimming Chlamydomonas cells and sensory cilia of C. elegans neurons, see Figure 1. Vertebrate photoreceptors are elaborate sensory cilia that detect photons of light. Spermatozoa, trypanosomes, ciliated protists and sea urchin blastula-stage embryos use motile cilia (aka flagella in sperm and trypanosomes) to swim through a fluid medium. Cilia or flagella are indicated in red. The three red lines inside the photoreceptor cilium represent the axonemal microtubules. See text for details.
Chlamydomonas
The green alga, Chlamydomonas uses its two motile cilia to swim through fluid and also as sensory organelles. Work in this system yielded a simple, and still very plausible, model for the mechanism of IFT in which heterotrimeric kinesin-2 moves IFT rafts and associated cargo from the base to the tip of the cilium and IFT dynein transports IFT subunits and turnover products back to the base of the cilium (Fig. 1B) [15, 17, 18]. The observation that the inactivation of the FLA10 subunit of heterotrimeric kinesin-2 using conditional mutants leads to a gradual cessation of IFT and defects in motile cilium assembly or maintenance, supports the hypothesis that it drives the anterograde transport of IFT trains [10, 120, 121]. However, it remains puzzling that the rate of anterograde IFT in this system occurs at 2 μm/s, much faster than the rate of anterograde motility driven by all purified heterotrimeric kinesin-2 motors so far studied [25]. Unfortunately the rate of motility driven by purified Chlamydomonas heterotrimeric kinesin-2, which may be unusually fast, has not to our knowledge been determined. Alternatively, although heterotrimeric kinesin-2 is clearly required for IFT in this system and can be observed moving along the cilium [119] it remains formally possible that this motor functions only to load the IFT machinery into the cilium (as in C. elegans [78]) and then an unidentified faster motor takes over and moves the IFT trains and heterotrimeric kinesin-2 as cargo the rest of the way along the cilium. Most researchers would be dismissive of this idea, but it should be noted that there does exist evidence for multiple ciliary kinesins in the cilia of this organism [221, 222]. If the former, arguably more likely alternative is correct, it may be that the presence of roadblocks such as dynein arms and radial spokes all along the axoneme may necessitate the use of only heterotrimeric kinesin-2 throughout, because of this motors’ ability to circumnavigate roadblocks, thereby providing a rationale for the apparent difference with C. elegans (see next section).
In this system, the kinesin-2 motors are thought to transport inactive IFT dynein as cargo to the ciliary tip, but it is then returned to the base of the cilium via diffusion rather than as cargo of the very well-characterized retrograde IFT machinery [208] and it is thought that the switch from anterograde to retrograde IFT involves an unknown control mechanism that mediates reciprocal switching between ensembles of heterotrimeric kinesin-2 and IFT-dynein motors, thereby eliminating mechanical competition between them [223]. There is good evidence that IFT delivers both axonemal precursors such as tubulins and membrane proteins such as opsins to their site of assembly in the Chlamydomonas cilium [77, 98, 224].
C. elegans sensory cilia
This system is of interest because heterotrimeric and homodimeric kinesin-2 motors cooperate with one another and with IFT dynein to mediate the assembly of C. elegans amphid channel cilia on chemosensory neurons (Fig. 1C) [225]. The axonemes of C. elegans amphid channel cilia have a bipartite structure consisting of the axoneme core consisting of 9 doublet MTs from which 9 distal singlet MTs emanate and are required for certain forms of chemosensory signaling. The assembly of these axonemes normally involves a subtle pattern of functional collaboration between the heterotrimeric kinesin-2, kinesin-II, and the homodimeric kinesin-2, OSM-3. Based on fluorescence microscopy assays of IFT in cilia of living worms of different mutant background, it was initially proposed that the assembly of the axoneme core involves both motors working together to transport IFT trains along the MT doublets whereas the assembly of the distal singlets depends only on OSM-3 transporting IFT trains along these singlets [94, 127, 128, 168]. Thus, in wild-type animals, kinesin-II and OSM-3 function redundantly to build the axoneme core, whereas OSM-3 alone builds the distal singlets and, moreover, in this system it was possible to obtain a good correlation between the rates of anterograde IFT and the rates of MT motility driven by purified kinesin-2 motors [25, 168]. The IFT trains are thought to deliver cargo consisting of axonemal tubulin subunits as well as subunits of the IFT-dynein complex to the tips of the axoneme [63, 105]. At the distal tip of the axoneme, there is a switch from anterograde to retrograde transport after which the kinesin-2 motors are recycled to the basal body as cargo of IFT dynein, rather than by diffusion [141]. It should also be noted that the pattern of motor collaboration seen in these cilia can be modulated to generate cilia with diverse sensory repertoires on neurons with different functions in the animal
Ever since the involvement of two anterograde motors in IFT was proposed [128], it was unclear why two different forms of kinesin-2 that move with the same polarity yet different speeds were deployed to move the same IFT-trains along the axoneme core. Recently, improved methods of genetic manipulation and fluorescence microscopic IFT assays led to a significant revision of the original model, with the recognition that there exists a functional differentiation between the two types of kinesin-2 motors [78]. Specifically it is now thought that the slower, less processive heterotrimeric kinesin-2, which is adapted to circumnavigate obstacles on the MT track, imports IFT trains and their associated cargo from the base of the cilium through the transition zone, where multiple roadblocks are encountered, into the cilium. There, homodimeric kinesin-2 gradually replaces heterotrimeric kinesin-2 as the IFT particles continue to move along the so-called “handover zone”, then it alone drives the long-range transport of these trains along the relatively obstacle-free axoneme to the distal tip of the cilium [78]. The new results are significant in explaining why the two distinct motors are used, because they are now seen to be functionally differentiated and it suggests why Chlamydomonas may use only heterotrimeric kinesin-2, because this motor is best suited to get around the roadblocks that exist along the full length of the axoneme in that system. In contrast, such obstacles are found mainly around the base of the cilium/transition zone in C. elegans and this is where this motor functions in these cilia. It is worth emphasizing that the new work supports the view that, along the handover zone, both heterotrimeric and homodimeric kinesin-2 attach to and move the same IFT particles, but their mole ratio varies with position. This gives rise to a range of intermediate velocities that could be due to either the alternating action and mechanical competition mechanisms proposed earlier [78, 168]. It is also notable that homodimeric kinesin-2 contributes to ciliary assembly and functions in other organisms and cell-types as well, although whether it does so by driving IFT is unclear.
Other invertebrates
In other invertebrates, the contribution of IFT to ciliogenesis varies, although direct assays of IFT are often lacking. As noted above, heterotrimeric kinesin-2 is thought to function alone to assemble the full-length axoneme in Chlamydomonas whereas in C. elegans amphid channel cilia, this motor can only build the axoneme core and in order to assemble the entire axoneme it must cooperate with the ‘accessory’ homodimeric kinesin-2. When heterotrimeric kinesin-2 is inhibited in another invertebrate model system where ciliogenesis and ciliary function have been extensively studied, the developing sea urchin embryo [226–228], the assembly of motile ciliary axonemes is impaired, but a short immotile ‘procilium’ is still assembled, plausibly by homodimeric kinesin-2 which is encoded by the sea urchin genome [117]. In contrast, the cilia on olfactory receptor neurons in Drosophila also have a bipartite organization and develop via a 2-step pathway, yet in this case heterotrimeric kinesin-2 alone appears to be sufficient to assemble the entire axoneme [229]. Thus this work shows that diverse means of deploying kinesin-2 motors seem to have evolved to build cilia, but working out the detailed mechanism of IFT in the sea urchin and fly systems would benefit from the development of IFT assays and to our knowledge, this has not yet been accomplished.
Protists
IFT can be assayed in several protists, including trypanosomes and ciliates (Fig. 4) [167, 230]. For example, the protist Trypanosoma brucei which causes African sleeping sickness contains a cilium that can be as long as ~40 μm and represents a powerful model system for studying IFT and ciliary function [231]. The trypanosome cilium contains a typical complement of IFT-A and B components, but is proposed to be unusual in containing IFT dynein with heterodimerized heavy chains and in lacking the KAP subunit of heterotrimeric kinesin-2 [148]. IFT has been directly visualized by GFP tagging IFT-particle and IFT-dynein subunits combined with RNA interference to knock-down specific proteins, with IFT-B knock-down usually blocking axoneme assembly and IFT-A knock-downs producing truncated cilia filled with IFT-particle subunits. In this system, once the cilium is assembled, IFT is required to maintain its molecular composition e.g. the presence of the regulatory subunit of PKA and the distribution of kinesin-9, but not ciliary length [232]. The IFT machinery displays complex dynamics along the cilia of trypanosomes and the availability of assays of IFT provide a powerful opportunity for dissecting how IFT-motors contribute to the process [167].
Vertebrates
A number of vertebrate model systems are available for studying IFT and ciliogenesis, including, for example, zebrafish, Xenopus and mouse. Malicki and others are systematically studying the functions of putative anterograde IFT motors of the kinesin-2 family and IFT-particle subunits in zebrafish with some interesting and surprising results [44, 233, 234]. For example, based on the mutant phenotype of the motor subunit of the heterotrimeric kinesin-2 motor, KIF3A, this motor is indispensable for ciliogenesis in all cells, but in some cells including photoreceptors, it seems to function primarily in basal body positioning prior to ciliogenesis per se. In some mechanosensory hair cell cilia, however, it is dispensible for basal body positioning and following its loss, short procilia assemble [44], superficially similar to those seen following heterotrimeric kinesin-2 inhibition in sea urchin embryos [117]. In the zebrafish system, homodimeric kinesin-2 is apparently dispensible for ciliogenesis although subtle sensory functions cannot be ruled out [44]. The role of kinesin-2 motors and IFT in mouse photoreceptor morphogenesis is also an active area of investigation [35] and the heterotrimeric kinesin-2-driven transport of opsins and axoneme precursors, as well as the functions of homodimeric kinesin-2, if any, are being investigated intensively [135, 136, 235, 236]. It is worth commenting that the connecting cilium of vertebrate photoreceptors corresponds to the transition zone of more conventional cilia. The application of fluorescence microscopy IFT assays to these photoreceptors would be very useful for sorting out the roles of specific IFT motors in IFT and ciliogenesis, and the development of rigorous FRAP techniques is a significant step forward [235].
Light-microscopy assays allowing direct visualization of IFT have been developed for the cilia of Xenopus [220, 237] and of mouse olfactory sensory neurons (OSN) [95], where complex dynamics are observed. In the latter system, as in C. elegans amphid channel cilia, there is a 1:1 stoichiometry of the BBSome to IFT particles and both heterotrimeric (KIF3) and homodimeric (KIF17) kinesin-2 motors traffic along the OSN cilia, but unlike in C. elegans amphids, homodimeric kinesin-2 is not required to build the distal region of the axoneme [95]. Some evidence supports the hypothesis that KIF17 may be transported as passive cargo associated with IFT-train subcomplex B to the distal tip of the cilium, where its function is unknown [137]. Other studies, on the other hand, provide evidence that KIF17 may actively cooperate with IFT-B to deliver cyclic nucleotide gated channels and dopamine receptors to the membranes of primary cilia [133, 134]. This is consistent with a differential function model in which heterotrimeric kinesin-2 builds the ciliary axoneme and homodimeric kinesin-2 delivers signaling molecules to the ciliary membrane (Fig. 1D).
8. Concluding remarks
IFT is an outstanding system for studying the biophysical and molecular mechanisms of motor-dependent cargo transport. Much has already been learned from this system using a combination of in vitro motility assays and live-cell imaging in various IFT-protein mutant backgrounds, leading to plausible models for how motors move IFT trains along cilia in order to deliver cargo molecules to assemble functional cilia, but, as noted throughout, many questions remain. For example, there are outstanding questions concerning the exact timing of the initiation of IFT during ciliogenesis as well as the relationship between the biochemistry and EM ultrastructure of IFT trains. We would like to know if the elegant functional differentiation of cooperating kinesin-2 motors seen in C. elegans is a special case, or if it is deployed more broadly, not only in cilia but also in other intracellular transport systems where same-polarity motors cooperate [25, 78]. Related questions concern what are the functions of homodimeric kinesin-2 in various types of cilia and why is this motor present is some cilia where it has no apparent function [44]? What is the full spectrum of cargo molecules that are moved by the IFT machinery and how do they and the IFT motors bind to and dissociate from the IFT raft subunits? To answer some of these questions, an atomic level understanding of the structure of the IFT machinery, including the IFT-particles and motors, will be important, an area where good progress is being made [84]. In addition, high-resolution single-molecule biophysical assays done at nanometer and millisecond resolution, combined with transient-state kinetic analysis of IFT-motor function, will be vital [173]. A major advance would be the reconstitution of the entire IFT machinery, including IFT trains, IFT motors and cargo molecules, from purified components, in a way that allows the high-resolution assay of its motility and a dissection of the role of individual components in the mechanism of IFT motor-dependent cargo transport.
Acknowledgments
We acknowledge financial support from the Netherlands Organisation for Scientific Research (NWO) via a Vici and an ALW Open Program grant, via the FOM programmes ‘Barriers in the Brain’ and ‘The Signal is the Noise’ (E.J.G.P.), and from an NIH grant, number GM50718 (J.M.S.).
List of defined abbreviations
- BB
Basal body
- CLEM
Correlative light electron microscopy
- CLS
Ciliary localization signal
- CPC
Ciliary pore complex
- DAVs
Distal appendage vesicles
- DIC
Differential interference contrast
- DS
Distal segment
- EM
Electron microscopy
- IFT
Intraflagellar transport
- LECA
Last eukaryotic common ancestor
- MAP
Mitogen-activated protein
- MAPs
Microtubule associated proteins
- MIPs
Microtubule inner proteins
- MT
Microtubule
- NLS
Nuclear localization signal
- PFs
Protofilaments
- PTMs
Post-translational modifications
- TZ
Transition zone
References
- 1.Pedersen LB, Schroder JM, Satir P, Christensen ST. The Ciliary Cytoskeleton. Compr Physiol. 2012;2:779–803. doi: 10.1002/cphy.c110043. [DOI] [PubMed] [Google Scholar]
- 2.Cavalier-Smith T. The phagotrophic origin of eukaryotes and phylogenetic classification of protozoa. Int J Syst Evol Micr. 2002;52:297–354. doi: 10.1099/00207713-52-2-297. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell DR. Evolution of Cilia. Cold Spring Harb Perspect Biol. 2016:9. doi: 10.1101/cshperspect.a028290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Satir P, Mitchell DR, Jekely G. How Did the Cilium Evolve? Curr Top Dev Biol. 2008;85:63–82. doi: 10.1016/S0070-2153(08)00803-X. [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa H, Marshall WF. Ciliogenesis: building the cell’s antenna. Nat Rev Mol Cell Biol. 2011;12:222–34. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- 6.Bloodgood RA. Motility Occurring in Association with Surface of Chlamydomonas Flagellum. J Cell Biol. 1977;75:983–989. doi: 10.1083/jcb.75.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kozminski KG. Intraflagellar transport-the “new motility” 20 years later. Mol Biol Cell. 2012;23:751–753. doi: 10.1091/mbc.E11-11-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson KA, Rosenbaum JL. Polarity of flagellar assembly in Chlamydomonas. J Cell Biol. 1992;119:1605–11. doi: 10.1083/jcb.119.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci U S A. 1993;90:5519–23. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piperno G, Mead K. Transport of a novel complex in the cytoplasmic matrix of Chlamydomonas flagella. Proc Natl Acad Sci U S A. 1997;94:4457–62. doi: 10.1073/pnas.94.9.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown JM, Witman GB. Cilia and Diseases. Bioscience. 2014;64:1126–1137. doi: 10.1093/biosci/biu174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orozco JT, Wedaman KP, Signor D, Brown H, Rose L, Scholey JM. Movement of motor and cargo along cilia. Nature. 1999;398:674. doi: 10.1038/19448. [DOI] [PubMed] [Google Scholar]
- 14.Blacque OE, Cevik S, Kaplan OI. Intraflagellar transport: from molecular characterisation to mechanism. Front Biosci. 2008;13:2633–52. doi: 10.2741/2871. [DOI] [PubMed] [Google Scholar]
- 15.Cole DG. The intraflagellar transport machinery of Chlamydomonas reinhardtii. Traffic. 2003;4:435–42. doi: 10.1034/j.1600-0854.2003.t01-1-00103.x. [DOI] [PubMed] [Google Scholar]
- 16.Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol. 2008;85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- 17.Rosenbaum JL, Cole DG, Diener DR. Intraflagellar transport: the eyes have it. J Cell Biol. 1999;144:385–8. doi: 10.1083/jcb.144.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–25. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 19.Scholey JM. Intraflagellar transport. Annu Rev Cell Dev Biol. 2003;19:423–43. doi: 10.1146/annurev.cellbio.19.111401.091318. [DOI] [PubMed] [Google Scholar]
- 20.Silverman MA, Leroux MR. Intraflagellar transport and the generation of dynamic, structurally and functionally diverse cilia. Trends Cell Biol. 2009;19:306–16. doi: 10.1016/j.tcb.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Qin H. Regulation of intraflagellar transport and ciliogenesis by small G proteins. Int Rev Cell Mol Biol. 2012;293:149–68. doi: 10.1016/B978-0-12-394304-0.00010-5. [DOI] [PubMed] [Google Scholar]
- 22.Broekhuis JR, Leong WY, Jansen G. Regulation of cilium length and intraflagellar transport. Int Rev Cell Mol Biol. 2013;303:101–38. doi: 10.1016/B978-0-12-407697-6.00003-9. [DOI] [PubMed] [Google Scholar]
- 23.Lechtreck KF. IFT-Cargo Interactions and Protein Transport in Cilia. Trends Biochem Sci. 2015;40:765–78. doi: 10.1016/j.tibs.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou Y, Witman GB. Dynein and intraflagellar transport. Exp Cell Res. 2015;334:26–34. doi: 10.1016/j.yexcr.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scholey JM. Kinesin-2: a family of heterotrimeric and homodimeric motors with diverse intracellular transport functions. Annu Rev Cell Dev Biol. 2013;29:443–69. doi: 10.1146/annurev-cellbio-101512-122335. [DOI] [PubMed] [Google Scholar]
- 26.Avasthi P, Marshall WF. Stages of ciliogenesis and regulation of ciliary length. Differentiation. 2012;83:S30–42. doi: 10.1016/j.diff.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ludington WB, Ishikawa H, Serebrenik YV, Ritter A, Hernandez-Lopez RA, Gunzenhauser J, Kannegaard E, Marshall WF. A systematic comparison of mathematical models for inherent measurement of ciliary length: how a cell can measure length and volume. Biophys J. 2015;108:1361–79. doi: 10.1016/j.bpj.2014.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall WF, Qin H, Rodrigo Brenni M, Rosenbaum JL. Flagellar length control system: testing a simple model based on intraflagellar transport and turnover. Mol Biol Cell. 2005;16:270–8. doi: 10.1091/mbc.E04-07-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marshall WF, Rosenbaum JL. Intraflagellar transport balances continuous turnover of outer doublet microtubules: implications for flagellar length control. J Cell Biol. 2001;155:405–14. doi: 10.1083/jcb.200106141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keeling J, Tsiokas L, Maskey D. Cellular Mechanisms of Ciliary Length Control. Cells. 2016:5. doi: 10.3390/cells5010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J, Chung YD. Ciliary subcompartments: how are they established and what are their functions? BMB Rep. 2015;48:380–7. doi: 10.5483/BMBRep.2015.48.7.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin H, White SR, Shida T, Schulz S, Aguiar M, Gygi SP, Bazan JF, Nachury MV. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell. 2010;141:1208–19. doi: 10.1016/j.cell.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nachury MV, Seeley ES, Jin H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol. 2010;26:59–87. doi: 10.1146/annurev.cellbio.042308.113337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–44. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldberg AF, Moritz OL, Williams DS. Molecular basis for photoreceptor outer segment architecture. Prog Retin Eye Res. 2016;55:52–81. doi: 10.1016/j.preteyeres.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan J, Wang Q, Snell WJ. Cilium-generated signaling and cilia-related disorders. Lab Invest. 2005;85:452–63. doi: 10.1038/labinvest.3700253. [DOI] [PubMed] [Google Scholar]
- 37.He M, Agbu S, Anderson KV. Microtubule Motors Drive Hedgehog Signaling in Primary Cilia. Trends Cell Biol. 2016 doi: 10.1016/j.tcb.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson CA, Collis SJ. Ciliogenesis and the DNA damage response: a stressful relationship. Cilia. 2016;5:19. doi: 10.1186/s13630-016-0040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mourao A, Christensen ST, Lorentzen E. The intraflagellar transport machinery in ciliary signaling. Curr Opin Struct Biol. 2016;41:98–108. doi: 10.1016/j.sbi.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Pedersen LB, Mogensen JB, Christensen ST. Endocytic Control of Cellular Signaling at the Primary Cilium. Trends Biochem Sci. 2016;41:784–97. doi: 10.1016/j.tibs.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Rohatgi R, Snell WJ. The ciliary membrane. Curr Opin Cell Biol. 2010;22:541–546. doi: 10.1016/j.ceb.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carvalho-Santos Z, Azimzadeh J, Pereira-Leal JB, Bettencourt-Dias M. Tracing the origins of centrioles, cilia, and flagella. J Cell Biol. 2011;194:165–175. doi: 10.1083/jcb.201011152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marshall WF. Basal Bodies: Platforms for Building Cilia. Curr Top Dev Biol. 2008;85:1–22. doi: 10.1016/S0070-2153(08)00801-6. [DOI] [PubMed] [Google Scholar]
- 44.Pooranachandran N, Malicki JJ. Unexpected Roles for Ciliary Kinesins and Intraflagellar Transport Proteins. Genetics. 2016;203:771–85. doi: 10.1534/genetics.115.180943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu Q, Insinna C, Ott C, Stauffer J, Pintado PA, Rahajeng J, Baxa U, Walia V, Cuenca A, Hwang YS, Daar IO, Lopes S, Lippincott-Schwartz J, Jackson PK, Caplan S, Westlake CJ. Early steps in primary cilium assembly require EHD1/EHD3-dependent ciliary vesicle formation. Nat Cell Biol. 2015;17:228-+. doi: 10.1038/ncb3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorokin S. Centrioles and Formation of Rudimentary Cilia by Fibroblasts and Smooth Muscle Cells. J Cell Biol. 1962;15:363-&. doi: 10.1083/jcb.15.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reiter JF, Blacque OE, Leroux MR. The base of the cilium: roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. Embo Rep. 2012;13:608–618. doi: 10.1038/embor.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei Q, Ling K, Hu JH. The essential roles of transition fibers in the context of cilia. Curr Opin Cell Biol. 2015;35:98–105. doi: 10.1016/j.ceb.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dean S, Moreira-Leite F, Varga V, Gull K. Cilium transition zone proteome reveals compartmentalization and differential dynamics of ciliopathy complexes. Proc Natl Acad Sci U S A. 2016;113:E5135–43. doi: 10.1073/pnas.1604258113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant Sensory Cilia in the Nematode Caenorhabditis-Elegans. Dev Biol. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- 51.Doroquez DB, Berciu C, Anderson JR, Sengupta P, Nicastro D. A high-resolution morphological and ultrastructural map of anterior sensory cilia and glia in Caenorhabditis elegans. Elife. 2014:3. doi: 10.7554/eLife.01948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ishikawa T. Axoneme Structure from Motile Cilia. Cold Spring Harb Perspect Biol. 2016 doi: 10.1101/cshperspect.a028076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nicastro D, Fu XF, Heuser T, Tso A, Porter ME, Linck RW. Cryo-electron tomography reveals conserved features of doublet microtubules in flagella. P Natl Acad Sci USA. 2011;108:E845–E853. doi: 10.1073/pnas.1106178108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sui HX, Downing KH. Molecular architecture of axonemal microtubule doublets revealed by cryo-electron tomography. Nature. 2006;442:475–478. doi: 10.1038/nature04816. [DOI] [PubMed] [Google Scholar]
- 55.Vaughan S, Gull K. Basal body structure and cell cycle-dependent biogenesis in Trypanosoma brucei. Cilia. 2015;5:5. doi: 10.1186/s13630-016-0023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reese TS. Olfactory Cilia in the Frog. J Cell Biol. 1965;25:209–30. doi: 10.1083/jcb.25.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fisch C, Dupuis-Williams P. Ultrastructure of cilia and flagella - back to the future! Biol Cell. 2011;103:249–270. doi: 10.1042/BC20100139. [DOI] [PubMed] [Google Scholar]
- 58.Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- 59.Nicastro D, Schwartz C, Pierson J, Gaudette R, Porter ME, McIntosh JR. The molecular architecture of axonemes revealed by cryoelectron tomography. Science. 2006;313:944–948. doi: 10.1126/science.1128618. [DOI] [PubMed] [Google Scholar]
- 60.Pigino G, Ishikawa T. Axonemal radial spokes: 3D structure, function and assembly. Bioarchitecture. 2012;2:50–58. doi: 10.4161/bioa.20394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.King SM. Axonemal Dynein Arms. Cold Spring Harb Perspect Biol. 2016:8. doi: 10.1101/cshperspect.a028100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitchell DR. Speculations on the evolution of 9+2 organelles and the role of central pair microtubules. Biol Cell. 2004;96:691–696. doi: 10.1016/j.biolcel.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hao L, Thein M, Brust-Mascher I, Civelekoglu-Scholey G, Lu Y, Acar S, Prevo B, Shaham S, Scholey JM. Intraflagellar transport delivers tubulin isotypes to sensory cilium middle and distal segments. Nat Cell Biol. 2011;13:790–8. doi: 10.1038/ncb2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gaertig J, Wloga D. Ciliary Tubulin and Its Post-Translational Modifications. Curr Top Dev Biol. 2008;85:83–113. doi: 10.1016/S0070-2153(08)00804-1. [DOI] [PubMed] [Google Scholar]
- 65.Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Bio. 2011;12:773–786. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- 66.Sirajuddin M, Rice LM, Vale RD. Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat Cell Biol. 2014;16:335-+. doi: 10.1038/ncb2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johnson KA. The axonemal microtubules of the Chlamydomonas flagellum differ in tubulin isoform content. J Cell Sci. 1998;111:313–320. doi: 10.1242/jcs.111.3.313. [DOI] [PubMed] [Google Scholar]
- 68.Gilula NB, Satir P. Ciliary Necklace - Ciliary Membrane Specialization. J Cell Biol. 1972;53:494-&. doi: 10.1083/jcb.53.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Williams CL, Li CM, Kida K, Inglis PN, Mohan S, Semenec L, Bialas NJ, Stupay RM, Chen NS, Blacque OE, Yoder BK, Leroux MR. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J Cell Biol. 2011;192:1023–1041. doi: 10.1083/jcb.201012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Breslow DK, Nachury MV. Primary Cilia: How to Keep the Riff-Raff in the Plasma Membrane. Curr Biol. 2011;21:R434–R436. doi: 10.1016/j.cub.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 71.Dishinger JF, Kee HL, Jenkins PM, Fan SL, Hurd TW, Hammond JW, Truong YNT, Margolis B, Martens JR, Verhey KJ. Ciliary entry of the kinesin-2 motor KIF17 is regulated by importin-beta 2 and RanGTP. Nat Cell Biol. 2010;12:703-U164. doi: 10.1038/ncb2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu QC, Nelson WJ. Ciliary Diffusion Barrier: The Gatekeeper for the Primary Cilium Compartment. Cytoskeleton. 2011;68:313–324. doi: 10.1002/cm.20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kee HL, Dishinger JF, Blasius TL, Liu CJ, Margolis B, Verhey KJ. A size-exclusion permeability barrier and nucleoporins characterize a ciliary pore complex that regulates transport into cilia. Nat Cell Biol. 2012;14:431-+. doi: 10.1038/ncb2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deane JA, Cole DG, Seeley ES, Diener DR, Rosenbaum JL. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr Biol. 2001;11:1586–1590. doi: 10.1016/s0960-9822(01)00484-5. [DOI] [PubMed] [Google Scholar]
- 75.Ounjai P, Kim KD, Liu HC, Dong M, Tauscher AN, Witkowska HE, Downing KH. Architectural Insights into a Ciliary Partition. Curr Biol. 2013;23:339–344. doi: 10.1016/j.cub.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pazour GJ, Bloodgood RA. Targeting Proteins to the Ciliary Membrane. Curr Top Dev Biol. 2008;85:115–149. doi: 10.1016/S0070-2153(08)00805-3. [DOI] [PubMed] [Google Scholar]
- 77.Cao M, Ning J, Hernandez-Lara CI, Belzile O, Wang Q, Dutcher SK, Liu Y, Snell WJ. Uni-directional ciliary membrane protein trafficking by a cytoplasmic retrograde IFT motor and ciliary ectosome shedding. Elife. 2015:4. doi: 10.7554/eLife.05242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Prevo B, Mangeol P, Oswald F, Scholey JM, Peterman EJ. Functional differentiation of cooperating kinesin-2 motors orchestrates cargo import and transport in C. elegans cilia. Nat Cell Biol. 2015;17:1536–45. doi: 10.1038/ncb3263. [DOI] [PubMed] [Google Scholar]
- 79.Pigino G, Geimer S, Lanzavecchia S, Paccagnini E, Cantele F, Diener DR, Rosenbaum JL, Lupetti P. Electron-tomographic analysis of intraflagellar transport particle trains in situ. J Cell Biol. 2009;187:135–148. doi: 10.1083/jcb.200905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Engel BD, Ludington WB, Marshall WF. Intraflagellar transport particle size scales inversely with flagellar length: revisiting the balance-point length control model. J Cell Biol. 2009;187:81–89. doi: 10.1083/jcb.200812084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vannuccini E, Paccagnini E, Cantele F, Gentile M, Dini D, Fino F, Diener D, Mencarelli C, Lupetti P. Two classes of short intraflagellar transport train with different 3D structures are present in Chlamydomonas flagella. J Cell Sci. 2016;129:2064–2074. doi: 10.1242/jcs.183244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stepanek L, Pigino G. Microtubule doublets are double-track railways for intraflagellar transport trains. Science. 2016;352:721–724. doi: 10.1126/science.aaf4594. [DOI] [PubMed] [Google Scholar]
- 83.Kuhns S, Blacque OE. Cilia Train Spotting. Dev Cell. 2016;37:395–396. doi: 10.1016/j.devcel.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 84.Taschner M, Lorentzen E. The Intraflagellar Transport Machinery. Cold Spring Harb Perspect Biol. 2016 doi: 10.1101/cshperspect.a028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jekely G, Arendt D. Evolution of intraflagellar transport from coated vesicles and autogenous origin of the eukaryotic cilium. Bioessays. 2006;28:191–8. doi: 10.1002/bies.20369. [DOI] [PubMed] [Google Scholar]
- 86.van Dam TJ, Townsend MJ, Turk M, Schlessinger A, Sali A, Field MC, Huynen MA. Evolution of modular intraflagellar transport from a coatomer-like progenitor. Proc Natl Acad Sci U S A. 2013;110:6943–8. doi: 10.1073/pnas.1221011110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lucker BF, Behal RH, Qin H, Siron LC, Taggart WD, Rosenbaum JL, Cole DG. Characterization of the intraflagellar transport complex B core: direct interaction of the IFT81 and IFT74/72 subunits. J Biol Chem. 2005;280:27688–96. doi: 10.1074/jbc.M505062200. [DOI] [PubMed] [Google Scholar]
- 88.Lucker BF, Miller MS, Dziedzic SA, Blackmarr PT, Cole DG. Direct interactions of intraflagellar transport complex B proteins IFT88, IFT52, and IFT46. J Biol Chem. 2010;285:21508–18. doi: 10.1074/jbc.M110.106997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Taschner M, Bhogaraju S, Vetter M, Morawetz M, Lorentzen E. Biochemical mapping of interactions within the intraflagellar transport (IFT) B core complex: IFT52 binds directly to four other IFT-B subunits. J Biol Chem. 2011;286:26344–52. doi: 10.1074/jbc.M111.254920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Taschner M, Kotsis F, Braeuer P, Kuehn EW, Lorentzen E. Crystal structures of IFT70/52 and IFT52/46 provide insight into intraflagellar transport B core complex assembly. J Cell Biol. 2014;207:269–82. doi: 10.1083/jcb.201408002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Taschner M, Weber K, Mourao A, Vetter M, Awasthi M, Stiegler M, Bhogaraju S, Lorentzen E. Intraflagellar transport proteins 172, 80, 57, 54, 38, and 20 form a stable tubulin-binding IFT-B2 complex. EMBO J. 2016;35:773–90. doi: 10.15252/embj.201593164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–13. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 93.Blacque OE, Reardon MJ, Li C, McCarthy J, Mahjoub MR, Ansley SJ, Badano JL, Mah AK, Beales PL, Davidson WS, Johnsen RC, Audeh M, Plasterk RH, Baillie DL, Katsanis N, Quarmby LM, Wicks SR, Leroux MR. Loss of C. elegans BBS-7 and BBS-8 protein function results in cilia defects and compromised intraflagellar transport. Genes Dev. 2004;18:1630–42. doi: 10.1101/gad.1194004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ou G, Blacque OE, Snow JJ, Leroux MR, Scholey JM. Functional coordination of intraflagellar transport motors. Nature. 2005;436:583–7. doi: 10.1038/nature03818. [DOI] [PubMed] [Google Scholar]
- 95.Williams CL, McIntyre JC, Norris SR, Jenkins PM, Zhang L, Pei Q, Verhey K, Martens JR. Direct evidence for BBSome-associated intraflagellar transport reveals distinct properties of native mammalian cilia. Nat Commun. 2014;5:5813. doi: 10.1038/ncomms6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lechtreck KF, Johnson EC, Sakai T, Cochran D, Ballif BA, Rush J, Pazour GJ, Ikebe M, Witman GB. The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. J Cell Biol. 2009;187:1117–32. doi: 10.1083/jcb.200909183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lechtreck KF, Brown JM, Sampaio JL, Craft JM, Shevchenko A, Evans JE, Witman GB. Cycling of the signaling protein phospholipase D through cilia requires the BBSome only for the export phase. J Cell Biol. 2013;201:249–61. doi: 10.1083/jcb.201207139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Craft JM, Harris JA, Hyman S, Kner P, Lechtreck KF. Tubulin transport by IFT is upregulated during ciliary growth by a cilium-autonomous mechanism. J Cell Biol. 2015;208:223–37. doi: 10.1083/jcb.201409036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bhogaraju S, Cajanek L, Fort C, Blisnick T, Weber K, Taschner M, Mizuno N, Lamla S, Bastin P, Nigg EA, Lorentzen E. Molecular basis of tubulin transport within the cilium by IFT74 and IFT81. Science. 2013;341:1009–12. doi: 10.1126/science.1240985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kubo T, Brown JM, Bellve K, Craige B, Craft JM, Fogarty K, Lechtreck KF, Witman GB. Together, the IFT81 and IFT74 N-termini form the main module for intraflagellar transport of tubulin. J Cell Sci. 2016;129:2106–19. doi: 10.1242/jcs.187120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Piperno G, Mead K, Henderson S. Inner dynein arms but not outer dynein arms require the activity of kinesin homologue protein KHP1(FLA10) to reach the distal part of flagella in Chlamydomonas. J Cell Biol. 1996;133:371–9. doi: 10.1083/jcb.133.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hou Y, Qin H, Follit JA, Pazour GJ, Rosenbaum JL, Witman GB. Functional analysis of an individual IFT protein: IFT46 is required for transport of outer dynein arms into flagella. J Cell Biol. 2007;176:653–65. doi: 10.1083/jcb.200608041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ishikawa H, Ide T, Yagi T, Jiang X, Hirono M, Sasaki H, Yanagisawa H, Wemmer KA, Stainier DY, Qin H, Kamiya R, Marshall WF. TTC26/DYF13 is an intraflagellar transport protein required for transport of motility-related proteins into flagella. Elife. 2014;3:e01566. doi: 10.7554/eLife.01566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Girotra M, Srivastava S, Kulkarni A, Barbora A, Bobra K, Ghosal D, Devan P, Aher A, Jain A, Panda D, Ray K. The C-terminal tails of heterotrimeric kinesin-2 motor subunits directly bind to alpha-tubulin1: Possible implications for cilia-specific tubulin entry. Traffic. 2017;18:123–133. doi: 10.1111/tra.12461. [DOI] [PubMed] [Google Scholar]
- 105.Hao L, Efimenko E, Swoboda P, Scholey JM. The retrograde IFT machinery of C. elegans cilia: two IFT dynein complexes? PLoS One. 2011;6:e20995. doi: 10.1371/journal.pone.0020995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Carpenter BS, Barry RL, Verhey KJ, Allen BL. The heterotrimeric kinesin-2 complex interacts with and regulates GLI protein function. J Cell Sci. 2015;128:1034–50. doi: 10.1242/jcs.162552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Howard PW, Jue SF, Maurer RA. Interaction of mouse TTC30/DYF-1 with multiple intraflagellar transport complex B proteins and KIF17. Exp Cell Res. 2013;319:2275–81. doi: 10.1016/j.yexcr.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liang Y, Pang Y, Wu Q, Hu Z, Han X, Xu Y, Deng H, Pan J. FLA8/KIF3B phosphorylation regulates kinesin-II interaction with IFT-B to control IFT entry and turnaround. Dev Cell. 2014;30:585–97. doi: 10.1016/j.devcel.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 109.Pedersen LB, Geimer S, Rosenbaum JL. Dissecting the molecular mechanisms of intraflagellar transport in chlamydomonas. Curr Biol. 2006;16:450–9. doi: 10.1016/j.cub.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 110.Williamson SM, Silva DA, Richey E, Qin H. Probing the role of IFT particle complex A and B in flagellar entry and exit of IFT-dynein in Chlamydomonas. Protoplasma. 2012;249:851–6. doi: 10.1007/s00709-011-0311-4. [DOI] [PubMed] [Google Scholar]
- 111.Mukhopadhyay S, Wen X, Chih B, Nelson CD, Lane WS, Scales SJ, Jackson PK. TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia. Genes Dev. 2010;24:2180–93. doi: 10.1101/gad.1966210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huet D, Blisnick T, Perrot S, Bastin P. The GTPase IFT27 is involved in both anterograde and retrograde intraflagellar transport. Elife. 2014;3:e02419. doi: 10.7554/eLife.02419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Blacque OE, Li C, Inglis PN, Esmail MA, Ou G, Mah AK, Baillie DL, Scholey JM, Leroux MR. The WD repeat-containing protein IFTA-1 is required for retrograde intraflagellar transport. Mol Biol Cell. 2006;17:5053–62. doi: 10.1091/mbc.E06-06-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fu W, Wang L, Kim S, Li J, Dynlacht BD. Role for the IFT-A Complex in Selective Transport to the Primary Cilium. Cell Rep. 2016;17:1505–1517. doi: 10.1016/j.celrep.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cole DG, Cande WZ, Baskin RJ, Skoufias DA, Hogan CJ, Scholey JM. Isolation of a sea urchin egg kinesin-related protein using peptide antibodies. J Cell Sci. 1992;101(Pt 2):291–301. doi: 10.1242/jcs.101.2.291. [DOI] [PubMed] [Google Scholar]
- 116.Cole DG, Chinn SW, Wedaman KP, Hall K, Vuong T, Scholey JM. Novel heterotrimeric kinesin-related protein purified from sea urchin eggs. Nature. 1993;366:268–70. doi: 10.1038/366268a0. [DOI] [PubMed] [Google Scholar]
- 117.Morris RL, Scholey JM. Heterotrimeric kinesin-II is required for the assembly of motile 9+2 ciliary axonemes on sea urchin embryos. J Cell Biol. 1997;138:1009–22. doi: 10.1083/jcb.138.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wedaman KP, Meyer DW, Rashid DJ, Cole DG, Scholey JM. Sequence and submolecular localization of the 115-kD accessory subunit of the heterotrimeric kinesin-II (KRP85/95) complex. J Cell Biol. 1996;132:371–80. doi: 10.1083/jcb.132.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mueller J, Perrone CA, Bower R, Cole DG, Porter ME. The FLA3 KAP subunit is required for localization of kinesin-2 to the site of flagellar assembly and processive anterograde intraflagellar transport. Mol Biol Cell. 2005;16:1341–54. doi: 10.1091/mbc.E04-10-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kozminski KG, Beech PL, Rosenbaum JL. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J Cell Biol. 1995;131:1517–27. doi: 10.1083/jcb.131.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Walther Z, Vashishtha M, Hall JL. The Chlamydomonas FLA10 gene encodes a novel kinesin-homologous protein. J Cell Biol. 1994;126:175–88. doi: 10.1083/jcb.126.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yamazaki H, Nakata T, Okada Y, Hirokawa N. Cloning and characterization of KAP3: a novel kinesin superfamily-associated protein of KIF3A/3B. Proc Natl Acad Sci U S A. 1996;93:8443–8. doi: 10.1073/pnas.93.16.8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–37. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 124.Albracht CD, Guzik-Lendrum S, Rayment I, Gilbert SP. Heterodimerization of Kinesin-2 KIF3AB Modulates Entry into the Processive Run. J Biol Chem. 2016;291:23248–23256. doi: 10.1074/jbc.M116.752196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brunnbauer M, Mueller-Planitz F, Kosem S, Ho TH, Dombi R, Gebhardt JC, Rief M, Okten Z. Regulation of a heterodimeric kinesin-2 through an unprocessive motor domain that is turned processive by its partner. Proc Natl Acad Sci U S A. 2010;107:10460–5. doi: 10.1073/pnas.1005177107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gindhart JG, Jr, Goldstein LS. Armadillo repeats in the SpKAP115 subunit of kinesin-II. Trends Cell Biol. 1996;6:415–6. doi: 10.1016/s0962-8924(96)20037-6. [DOI] [PubMed] [Google Scholar]
- 127.Evans JE, Snow JJ, Gunnarson AL, Ou G, Stahlberg H, McDonald KL, Scholey JM. Functional modulation of IFT kinesins extends the sensory repertoire of ciliated neurons in Caenorhabditis elegans. J Cell Biol. 2006;172:663–9. doi: 10.1083/jcb.200509115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Snow JJ, Ou G, Gunnarson AL, Walker MR, Zhou HM, Brust-Mascher I, Scholey JM. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat Cell Biol. 2004;6:1109–13. doi: 10.1038/ncb1186. [DOI] [PubMed] [Google Scholar]
- 129.Vukajlovic M, Dietz H, Schliwa M, Okten Z. How kinesin-2 forms a stalk. Mol Biol Cell. 2011;22:4279–87. doi: 10.1091/mbc.E11-02-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Doodhi H, Ghosal D, Krishnamurthy M, Jana SC, Shamala D, Bhaduri A, Sowdhamini R, Ray K. KAP, the accessory subunit of kinesin-2, binds the predicted coiled-coil stalk of the motor subunits. Biochemistry. 2009;48:2248–60. doi: 10.1021/bi8018338. [DOI] [PubMed] [Google Scholar]
- 131.Signor D, Wedaman KP, Rose LS, Scholey JM. Two heteromeric kinesin complexes in chemosensory neurons and sensory cilia of Caenorhabditis elegans. Mol Biol Cell. 1999;10:345–60. doi: 10.1091/mbc.10.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Setou M, Nakagawa T, Seog DH, Hirokawa N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science. 2000;288:1796–802. doi: 10.1126/science.288.5472.1796. [DOI] [PubMed] [Google Scholar]
- 133.Jenkins PM, Hurd TW, Zhang L, McEwen DP, Brown RL, Margolis B, Verhey KJ, Martens JR. Ciliary targeting of olfactory CNG channels requires the CNGB1b subunit and the kinesin-2 motor protein, KIF17. Curr Biol. 2006;16:1211–6. doi: 10.1016/j.cub.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 134.Leaf A, Von Zastrow M. Dopamine receptors reveal an essential role of IFT-B, KIF17, and Rab23 in delivering specific receptors to primary cilia. Elife. 2015:4. doi: 10.7554/eLife.06996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jiang L, Tam BM, Ying G, Wu S, Hauswirth WW, Frederick JM, Moritz OL, Baehr W. Kinesin family 17 (osmotic avoidance abnormal-3) is dispensable for photoreceptor morphology and function. FASEB J. 2015;29:4866–80. doi: 10.1096/fj.15-275677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jiang L, Wei Y, Ronquillo CC, Marc RE, Yoder BK, Frederick JM, Baehr W. Heterotrimeric kinesin-2 (KIF3) mediates transition zone and axoneme formation of mouse photoreceptors. J Biol Chem. 2015;290:12765–78. doi: 10.1074/jbc.M115.638437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Funabashi T, Katoh Y, Michisaka S, Terada M, Sugawa M, Nakayama K. Ciliary entry of KIF17 is dependent on its binding to the IFT-B complex via IFT46-IFT56 as well as on its nuclear localization signal. Mol Biol Cell. 2017 doi: 10.1091/mbc.E16-09-0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gibbons BH, Asai DJ, Tang WJ, Hays TS, Gibbons IR. Phylogeny and expression of axonemal and cytoplasmic dynein genes in sea urchins. Mol Biol Cell. 1994;5:57–70. doi: 10.1091/mbc.5.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pazour GJ, Dickert BL, Witman GB. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J Cell Biol. 1999;144:473–81. doi: 10.1083/jcb.144.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Porter ME, Bower R, Knott JA, Byrd P, Dentler W. Cytoplasmic dynein heavy chain 1b is required for flagellar assembly in Chlamydomonas. Mol Biol Cell. 1999;10:693–712. doi: 10.1091/mbc.10.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Signor D, Wedaman KP, Orozco JT, Dwyer ND, Bargmann CI, Rose LS, Scholey JM. Role of a class DHC1b dynein in retrograde transport of IFT motors and IFT raft particles along cilia, but not dendrites, in chemosensory neurons of living Caenorhabditis elegans. J Cell Biol. 1999;147:519–30. doi: 10.1083/jcb.147.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pazour GJ, Wilkerson CG, Witman GB. A dynein light chain is essential for the retrograde particle movement of intraflagellar transport (IFT) J Cell Biol. 1998;141:979–92. doi: 10.1083/jcb.141.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li W, Yi P, Ou G. Somatic CRISPR-Cas9-induced mutations reveal roles of embryonically essential dynein chains in Caenorhabditis elegans cilia. J Cell Biol. 2015;208:683–92. doi: 10.1083/jcb.201411041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Asante D, Stevenson NL, Stephens DJ. Subunit composition of the human cytoplasmic dynein-2 complex. J Cell Sci. 2014;127:4774–87. doi: 10.1242/jcs.159038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Perrone CA, Tritschler D, Taulman P, Bower R, Yoder BK, Porter ME. A novel dynein light intermediate chain colocalizes with the retrograde motor for intraflagellar transport at sites of axoneme assembly in chlamydomonas and Mammalian cells. Mol Biol Cell. 2003;14:2041–56. doi: 10.1091/mbc.E02-10-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schafer JC, Haycraft CJ, Thomas JH, Yoder BK, Swoboda P. XBX-1 encodes a dynein light intermediate chain required for retrograde intraflagellar transport and cilia assembly in Caenorhabditis elegans. Mol Biol Cell. 2003;14:2057–70. doi: 10.1091/mbc.E02-10-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Patel-King RS, Gilberti RM, Hom EF, King SM. WD60/FAP163 is a dynein intermediate chain required for retrograde intraflagellar transport in cilia. Mol Biol Cell. 2013;24:2668–77. doi: 10.1091/mbc.E13-05-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Blisnick T, Buisson J, Absalon S, Marie A, Cayet N, Bastin P. The intraflagellar transport dynein complex of trypanosomes is made of a heterodimer of dynein heavy chains and of light and intermediate chains of distinct functions. Mol Biol Cell. 2014;25:2620–33. doi: 10.1091/mbc.E14-05-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Reck J, Schauer AM, VanderWaal Mills K, Bower R, Tritschler D, Perrone CA, Porter ME. The role of the dynein light intermediate chain in retrograde IFT and flagellar function in Chlamydomonas. Mol Biol Cell. 2016;27:2404–22. doi: 10.1091/mbc.E16-03-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schmidt H, Zalyte R, Urnavicius L, Carter AP. Structure of human cytoplasmic dynein-2 primed for its power stroke. Nature. 2015;518:435–8. doi: 10.1038/nature14023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ichikawa M, Watanabe Y, Murayama T, Toyoshima YY. Recombinant human cytoplasmic dynein heavy chain 1 and 2: observation of dynein-2 motor activity in vitro. FEBS Lett. 2011;585:2419–23. doi: 10.1016/j.febslet.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 152.Schmidt H, Carter AP. Review: Structure and mechanism of the dynein motor ATPase. Biopolymers. 2016;105:557–67. doi: 10.1002/bip.22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Verhey KJ, Dishinger J, Kee HL. Kinesin motors and primary cilia. Biochem Soc Trans. 2011;39:1120–5. doi: 10.1042/BST0391120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hu Z, Liang Y, Meng D, Wang L, Pan J. Microtubule-depolymerizing kinesins in the regulation of assembly, disassembly, and length of cilia and flagella. Int Rev Cell Mol Biol. 2015;317:241–65. doi: 10.1016/bs.ircmb.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 155.Morsci NS, Barr MM. Kinesin-3 KLP-6 regulates intraflagellar transport in male-specific cilia of Caenorhabditis elegans. Curr Biol. 2011;21:1239–44. doi: 10.1016/j.cub.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Peden EM, Barr MM. The KLP-6 kinesin is required for male mating behaviors and polycystin localization in Caenorhabditis elegans. Curr Biol. 2005;15:394–404. doi: 10.1016/j.cub.2004.12.073. [DOI] [PubMed] [Google Scholar]
- 157.Piao T, Luo M, Wang L, Guo Y, Li D, Li P, Snell WJ, Pan J. A microtubule depolymerizing kinesin functions during both flagellar disassembly and flagellar assembly in Chlamydomonas. Proc Natl Acad Sci U S A. 2009;106:4713–8. doi: 10.1073/pnas.0808671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dawson SC, Sagolla MS, Mancuso JJ, Woessner DJ, House SA, Fritz-Laylin L, Cande WZ. Kinesin-13 regulates flagellar, interphase, and mitotic microtubule dynamics in Giardia intestinalis. Eukaryot Cell. 2007;6:2354–64. doi: 10.1128/EC.00128-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kobayashi T, Tsang WY, Li J, Lane W, Dynlacht BD. Centriolar kinesin Kif24 interacts with CP110 to remodel microtubules and regulate ciliogenesis. Cell. 2011;145:914–25. doi: 10.1016/j.cell.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 160.He M, Subramanian R, Bangs F, Omelchenko T, Liem KF, Jr, Kapoor TM, Anderson KV. The kinesin-4 protein Kif7 regulates mammalian Hedgehog signalling by organizing the cilium tip compartment. Nat Cell Biol. 2014;16:663–72. doi: 10.1038/ncb2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cianfrocco MA, DeSantis ME, Leschziner AE, Reck-Peterson SL. Mechanism and regulation of cytoplasmic dynein. Annu Rev Cell Dev Biol. 2015;31:83–108. doi: 10.1146/annurev-cellbio-100814-125438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Reck-Peterson SL, Yildiz A, Carter AP, Gennerich A, Zhang N, Vale RD. Single-molecule analysis of dynein processivity and stepping behavior. Cell. 2006;126:335–48. doi: 10.1016/j.cell.2006.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.McKenney RJ, Huynh W, Tanenbaum ME, Bhabha G, Vale RD. Activation of cytoplasmic dynein motility by dynactin-cargo adapter complexes. Science. 2014;345:337–41. doi: 10.1126/science.1254198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schlager MA, Hoang HT, Urnavicius L, Bullock SL, Carter AP. In vitro reconstitution of a highly processive recombinant human dynein complex. EMBO J. 2014;33:1855–68. doi: 10.15252/embj.201488792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tran PV, Haycraft CJ, Besschetnova TY, Turbe-Doan A, Stottmann RW, Herron BJ, Chesebro AL, Qiu H, Scherz PJ, Shah JV, Yoder BK, Beier DR. THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nat Genet. 2008;40:403–10. doi: 10.1038/ng.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Iomini C, Babaev-Khaimov V, Sassaroli M, Piperno G. Protein particles in Chlamydomonas flagella undergo a transport cycle consisting of four phases. J Cell Biol. 2001;153:13–24. doi: 10.1083/jcb.153.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Buisson J, Chenouard N, Lagache T, Blisnick T, Olivo-Marin JC, Bastin P. Intraflagellar transport proteins cycle between the flagellum and its base. J Cell Sci. 2013;126:327–38. doi: 10.1242/jcs.117069. [DOI] [PubMed] [Google Scholar]
- 168.Pan X, Ou G, Civelekoglu-Scholey G, Blacque OE, Endres NF, Tao L, Mogilner A, Leroux MR, Vale RD, Scholey JM. Mechanism of transport of IFT particles in C. elegans cilia by the concerted action of kinesin-II and OSM-3 motors. J Cell Biol. 2006;174:1035–45. doi: 10.1083/jcb.200606003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Andreasson JO, Shastry S, Hancock WO, Block SM. The Mechanochemical Cycle of Mammalian Kinesin-2 KIF3A/B under Load. Curr Biol. 2015;25:1166–75. doi: 10.1016/j.cub.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Muthukrishnan G, Zhang Y, Shastry S, Hancock WO. The processivity of kinesin-2 motors suggests diminished front-head gating. Curr Biol. 2009;19:442–7. doi: 10.1016/j.cub.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shastry S, Hancock WO. Neck linker length determines the degree of processivity in kinesin-1 and kinesin-2 motors. Curr Biol. 2010;20:939–43. doi: 10.1016/j.cub.2010.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shastry S, Hancock WO. Interhead tension determines processivity across diverse N-terminal kinesins. Proc Natl Acad Sci U S A. 2011;108:16253–8. doi: 10.1073/pnas.1102628108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Guzik-Lendrum S, Rank KC, Bensel BM, Taylor KC, Rayment I, Gilbert SP. Kinesin-2 KIF3AC and KIF3AB Can Drive Long-Range Transport along Microtubules. Biophys J. 2015;109:1472–82. doi: 10.1016/j.bpj.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hoeprich GJ, Thompson AR, McVicker DP, Hancock WO, Berger CL. Kinesin’s neck-linker determines its ability to navigate obstacles on the microtubule surface. Biophys J. 2014;106:1691–700. doi: 10.1016/j.bpj.2014.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Schroeder HW, 3rd, Hendricks AG, Ikeda K, Shuman H, Rodionov V, Ikebe M, Goldman YE, Holzbaur EL. Force-dependent detachment of kinesin-2 biases track switching at cytoskeletal filament intersections. Biophys J. 2012;103:48–58. doi: 10.1016/j.bpj.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chen GY, Arginteanu DF, Hancock WO. Processivity of the kinesin-2 KIF3A results from rear head gating and not front head gating. J Biol Chem. 2015;290:10274–94. doi: 10.1074/jbc.M114.628032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Imanishi M, Endres NF, Gennerich A, Vale RD. Autoinhibition regulates the motility of the C-elegans intraflagellar transport motor OSM-3. J Cell Biol. 2006;174:931–937. doi: 10.1083/jcb.200605179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hendricks AG, Perlson E, Ross JL, Schroeder HW, 3rd, Tokito M, Holzbaur EL. Motor coordination via a tug-of-war mechanism drives bidirectional vesicle transport. Curr Biol. 2010;20:697–702. doi: 10.1016/j.cub.2010.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Moughamian AJ, Holzbaur ELF. Dynactin Is Required for Transport Initiation from the Distal Axon. Neuron. 2012;74:331–343. doi: 10.1016/j.neuron.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Visscher K, Schnitzer MJ, Block SM. Single kinesin molecules studied with a molecular force clamp. Nature. 1999;400:184–9. doi: 10.1038/22146. [DOI] [PubMed] [Google Scholar]
- 181.Campas O, Kafri Y, Zeldovich KB, Casademunt J, Joanny JF. Collective dynamics of interacting molecular motors. Phys Rev Lett. 2006:97. doi: 10.1103/PhysRevLett.97.038101. [DOI] [PubMed] [Google Scholar]
- 182.Jamison DK, Driver JW, Diehl MR. Cooperative Responses of Multiple Kinesins to Variable and Constant Loads. Journal of Biological Chemistry. 2012;287:3357–3365. doi: 10.1074/jbc.M111.296582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Klumpp S, Lipowsky R. Cooperative cargo transport by several molecular motors. Proc Natl Acad Sci U S A. 2005;102:17284–9. doi: 10.1073/pnas.0507363102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ali MY, Kennedy GG, Safer D, Trybus KM, Sweeney HL, Warshaw DM. Myosin Va and myosin VI coordinate their steps while engaged in an in vitro tug of war during cargo transport. P Natl Acad Sci USA. 2011;108:E535–E541. doi: 10.1073/pnas.1104298108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Derr ND, Goodman BS, Jungmann R, Leschziner AE, Shih WM, Reck-Peterson SL. Tug-of-War in Motor Protein Ensembles Revealed with a Programmable DNA Origami Scaffold. Science. 2012;338:662–665. doi: 10.1126/science.1226734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Furuta K, Furuta A, Toyoshima YY, Amino M, Oiwa K, Kojima H. Measuring collective transport by defined numbers of processive and nonprocessive kinesin motors. Proc Natl Acad Sci U S A. 2013;110:501–6. doi: 10.1073/pnas.1201390110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jamison DK, Driver JW, Rogers AR, Constantinou PE, Diehl MR. Two Kinesins Transport Cargo Primarily via the Action of One Motor: Implications for Intracellular Transport. Biophysical Journal. 2010;99:2967–2977. doi: 10.1016/j.bpj.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Leduc C, Pavin N, Julicher F, Diez S. Collective Behavior of Antagonistically Acting Kinesin-1 Motors. Phys Rev Lett. 2010:105. doi: 10.1103/PhysRevLett.105.128103. [DOI] [PubMed] [Google Scholar]
- 189.Holzbaur EL, Goldman YE. Coordination of molecular motors: from in vitro assays to intracellular dynamics. Curr Opin Cell Biol. 2010;22:4–13. doi: 10.1016/j.ceb.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.McLaughlin RT, Diehl MR, Kolomeisky AB. Collective dynamics of processive cytoskeletal motors. Soft Matter. 2016;12:14–21. doi: 10.1039/c5sm01609f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Encalada SE, Goldstein LS. Biophysical challenges to axonal transport: motor-cargo deficiencies and neurodegeneration. Annu Rev Biophys. 2014;43:141–69. doi: 10.1146/annurev-biophys-051013-022746. [DOI] [PubMed] [Google Scholar]
- 192.Ludington WB, Wemmer KA, Lechtreck KF, Witman GB, Marshall WF. Avalanche-like behavior in ciliary import. P Natl Acad Sci USA. 2013;110:3925–3930. doi: 10.1073/pnas.1217354110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Shih SM, Engel BD, Kocabas F, Bilyard T, Gennerich A, Marshall WF, Yildiz A. Intraflagellar transport drives flagellar surface motility. Elife. 2013:2. doi: 10.7554/eLife.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wei Q, Zhang YX, Li YJ, Zhang Q, Ling K, Hu JH. The BBSome controls IFT assembly and turnaround in cilia. Nat Cell Biol. 2012;14:950-+. doi: 10.1038/ncb2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Morris RL, English CN, Lou JE, Dufort FJ, Nordberg J, Terasaki M, Hinkle B. Redistribution of the kinesin-II subunit KAP from cilia to nuclei during the mitotic and ciliogenic cycles in sea urchin embryos. Dev Biol. 2004;274:56–69. doi: 10.1016/j.ydbio.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 196.Verhey KJ, Hammond JW. Traffic control: regulation of kinesin motors. Nat Rev Mol Cell Bio. 2009;10:765–777. doi: 10.1038/nrm2782. [DOI] [PubMed] [Google Scholar]
- 197.Hammond JW, Blasius TL, Soppina V, Cai DW, Verhey KJ. Autoinhibition of the kinesin-2 motor KIF17 via dual intramolecular mechanisms. J Cell Biol. 2010;189:1013–1025. doi: 10.1083/jcb.201001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ou G, Koga M, Blacque OE, Murayama T, Ohshima Y, Schafer JC, Li CM, Yoder BK, Leroux MR, Scholey JM. Sensory ciliogenesis in Caenorhabditis elegans: Assignment of IFT components into distinct modules based on transport and phenotypic profiles. Mol Biol Cell. 2007;18:1554–1569. doi: 10.1091/mbc.E06-09-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.O’Hagan R, Piasecki BP, Silva M, Phirke P, Nguyen KCQ, Hall DH, Swoboda P, Barr MM. The Tubulin Deglutamylase CCPP-1 Regulates the Function and Stability of Sensory Cilia in C. elegans. Curr Biol. 2011;21:1685–1694. doi: 10.1016/j.cub.2011.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Pathak N, Obara T, Mangos S, Liu Y, Drummond IA. The zebrafish fleer gene encodes an essential regulator of cilia tubulin polyglutamylation. Mol Biol Cell. 2007;18:4353–4364. doi: 10.1091/mbc.E07-06-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Verhey KJ, Gaertig J. The tubulin code. Cell Cycle. 2007;6:2152–2160. doi: 10.4161/cc.6.17.4633. [DOI] [PubMed] [Google Scholar]
- 202.Burghoorn J, Dekkers MPJ, Rademakers S, de Jong T, Willemsen R, Jansen G. Mutation of the MAP kinase DYF-5 affects docking and undocking of kinesin-2 motors and reduces their speed in the cilia of Caenorhabditis elegans. P Natl Acad Sci USA. 2007;104:7157–7162. doi: 10.1073/pnas.0606974104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Harris JA, Liu Y, Yang PF, Kner P, Lechtreck KF. Single-particle imaging reveals intraflagellar transport-independent transport and accumulation of EB1 in Chlamydomonas flagella. Mol Biol Cell. 2016;27:295–307. doi: 10.1091/mbc.E15-08-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Pedersen LB, Geimer S, Sloboda RD, Rosenbaum JL. The microtubule plus end-tracking protein EB1 is localized to the flagellar tip and basal bodies in Chlamydomonas reinhardtii. Curr Biol. 2003;13:1969–1974. doi: 10.1016/j.cub.2003.10.058. [DOI] [PubMed] [Google Scholar]
- 205.Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10:682–96. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- 206.Vale RD. The molecular motor toolbox for intracellular transport. Cell. 2003;112:467–80. doi: 10.1016/s0092-8674(03)00111-9. [DOI] [PubMed] [Google Scholar]
- 207.Wren KN, Craft JM, Tritschler D, Schauer A, Patel DK, Smith EF, Porter ME, Kner P, Lechtreck KF. A differential cargo-loading model of ciliary length regulation by IFT. Curr Biol. 2013;23:2463–71. doi: 10.1016/j.cub.2013.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Engel BD, Ishikawa H, Wemmer KA, Geimer S, Wakabayashi K, Hirono M, Craige B, Pazour GJ, Witman GB, Kamiya R, Marshall WF. The role of retrograde intraflagellar transport in flagellar assembly, maintenance, and function. J Cell Biol. 2012;199:151–67. doi: 10.1083/jcb.201206068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Broekhuis JR, Verhey KJ, Jansen G. Regulation of cilium length and intraflagellar transport by the RCK-kinases ICK and MOK in renal epithelial cells. PLoS One. 2014;9:e108470. doi: 10.1371/journal.pone.0108470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Huang K, Diener DR, Mitchell A, Pazour GJ, Witman GB, Rosenbaum JL. Function and dynamics of PKD2 in Chlamydomonas reinhardtii flagella. J Cell Biol. 2007;179:501–14. doi: 10.1083/jcb.200704069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Qin H, Burnette DT, Bae YK, Forscher P, Barr MM, Rosenbaum JL. Intraflagellar transport is required for the vectorial movement of TRPV channels in the ciliary membrane. Curr Biol. 2005;15:1695–9. doi: 10.1016/j.cub.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 212.Ye F, Breslow DK, Koslover EF, Spakowitz AJ, Nelson WJ, Nachury MV. Single molecule imaging reveals a major role for diffusion in the exploration of ciliary space by signaling receptors. Elife. 2013;2:e00654. doi: 10.7554/eLife.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Milenkovic L, Weiss LE, Yoon J, Roth TL, Su YS, Sahl SJ, Scott MP, Moerner WE. Single-molecule imaging of Hedgehog pathway protein Smoothened in primary cilia reveals binding events regulated by Patched1. Proc Natl Acad Sci U S A. 2015;112:8320–5. doi: 10.1073/pnas.1510094112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bloodgood RA. Dynamic properties of the flagellar surface. Symp Soc Exp Biol. 1982;35:353–80. [PubMed] [Google Scholar]
- 215.Collingridge P, Brownlee C, Wheeler GL. Compartmentalized calcium signaling in cilia regulates intraflagellar transport. Curr Biol. 2013;23:2311–8. doi: 10.1016/j.cub.2013.09.059. [DOI] [PubMed] [Google Scholar]
- 216.Vincensini L, Blisnick T, Bastin P. 1001 model organisms to study cilia and flagella. Biol Cell. 2011;103:109–30. doi: 10.1042/BC20100104. [DOI] [PubMed] [Google Scholar]
- 217.Dawson SC, House SA. Life with eight flagella: flagellar assembly and division in Giardia. Curr Opin Microbiol. 2010;13:480–90. doi: 10.1016/j.mib.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lee YR, Qiu W, Liu B. Kinesin motors in plants: from subcellular dynamics to motility regulation. Curr Opin Plant Biol. 2015;28:120–6. doi: 10.1016/j.pbi.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 219.Tomei EJ, Wolniak SM. Kinesin-2 and kinesin-9 have atypical functions during ciliogenesis in the male gametophyte of Marsilea vestita. BMC Cell Biol. 2016;17:29. doi: 10.1186/s12860-016-0107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Toriyama M, Lee C, Taylor SP, Duran I, Cohn DH, Bruel AL, Tabler JM, Drew K, Kelly MR, Kim S, Park TJ, Braun DA, Pierquin G, Biver A, Wagner K, Malfroot A, Panigrahi I, Franco B, Al-Lami HA, Yeung Y, Choi YJ, Duffourd Y, Faivre L, Riviere JB, Chen J, Liu KJ, Marcotte EM, Hildebrandt F, Thauvin-Robinet C, Krakow D, Jackson PK, Wallingford JB University of Washington Center for Mendelian G. The ciliopathy-associated CPLANE proteins direct basal body recruitment of intraflagellar transport machinery. Nat Genet. 2016;48:648–56. doi: 10.1038/ng.3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Bernstein M, Rosenbaum JL. Kinesin-like proteins in the flagella of Chlamydomonas. Trends Cell Biol. 1994;4:236–40. doi: 10.1016/0962-8924(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 222.Fox LA, Sawin KE, Sale WS. Kinesin-related proteins in eukaryotic flagella. J Cell Sci. 1994;107(Pt 6):1545–50. doi: 10.1242/jcs.107.6.1545. [DOI] [PubMed] [Google Scholar]
- 223.Laib JA, Marin JA, Bloodgood RA, Guilford WH. The reciprocal coordination and mechanics of molecular motors in living cells. Proc Natl Acad Sci U S A. 2009;106:3190–5. doi: 10.1073/pnas.0809849106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Awasthi M, Ranjan P, Sharma K, Veetil SK, Kateriya S. The trafficking of bacterial type rhodopsins into the Chlamydomonas eyespot and flagella is IFT mediated. Sci Rep. 2016;6:34646. doi: 10.1038/srep34646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Mijalkovic J, Prevo B, Peterman EJ. Why motor proteins team up - Intraflagellar transport in C. elegans cilia. Worm. 2016;5:e1170275. doi: 10.1080/21624054.2016.1170275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Stephens RE. Ciliogenesis, ciliary function, and selective isolation. ACS Chem Biol. 2008;3:84–6. doi: 10.1021/cb8000217. [DOI] [PubMed] [Google Scholar]
- 227.Stephens RE. Ciliogenesis in sea urchin embryos--a subroutine in the program of development. Bioessays. 1995;17:331–40. doi: 10.1002/bies.950170409. [DOI] [PubMed] [Google Scholar]
- 228.Warner JF, Miranda EL, McClay DR. Contribution of hedgehog signaling to the establishment of left-right asymmetry in the sea urchin. Dev Biol. 2016;411:314–24. doi: 10.1016/j.ydbio.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Jana SC, Girotra M, Ray K. Heterotrimeric kinesin-II is necessary and sufficient to promote different stepwise assembly of morphologically distinct bipartite cilia in Drosophila antenna. Mol Biol Cell. 2011;22:769–81. doi: 10.1091/mbc.E10-08-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Jiang YY, Lechtreck K, Gaertig J. Total internal reflection fluorescence microscopy of intraflagellar transport in Tetrahymena thermophila. Methods Cell Biol. 2015;127:445–56. doi: 10.1016/bs.mcb.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Broadhead R, Dawe HR, Farr H, Griffiths S, Hart SR, Portman N, Shaw MK, Ginger ML, Gaskell SJ, McKean PG, Gull K. Flagellar motility is required for the viability of the bloodstream trypanosome. Nature. 2006;440:224–7. doi: 10.1038/nature04541. [DOI] [PubMed] [Google Scholar]
- 232.Fort C, Bonnefoy S, Kohl L, Bastin P. Intraflagellar transport is required for the maintenance of the trypanosome flagellum composition but not its length. J Cell Sci. 2016;129:3026–41. doi: 10.1242/jcs.188227. [DOI] [PubMed] [Google Scholar]
- 233.Zhao C, Omori Y, Brodowska K, Kovach P, Malicki J. Kinesin-2 family in vertebrate ciliogenesis. Proc Natl Acad Sci U S A. 2012;109:2388–93. doi: 10.1073/pnas.1116035109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Raghupathy RK, Zhang X, Alhasani RH, Zhou X, Mullin M, Reilly J, Li W, Liu M, Shu X. Abnormal photoreceptor outer segment development and early retinal degeneration in kif3a mutant zebrafish. Cell Biochem Funct. 2016;34:429–40. doi: 10.1002/cbf.3205. [DOI] [PubMed] [Google Scholar]
- 235.Trivedi D, Colin E, Louie CM, Williams DS. Live-cell imaging evidence for the ciliary transport of rod photoreceptor opsin by heterotrimeric kinesin-2. J Neurosci. 2012;32:10587–93. doi: 10.1523/JNEUROSCI.0015-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Marszalek JR, Liu X, Roberts EA, Chui D, Marth JD, Williams DS, Goldstein LS. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell. 2000;102:175–87. doi: 10.1016/s0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]
- 237.Brooks ER, Wallingford JB. Control of vertebrate intraflagellar transport by the planar cell polarity effector Fuz. J Cell Biol. 2012;198:37–45. doi: 10.1083/jcb.201204072. [DOI] [PMC free article] [PubMed] [Google Scholar]