Abstract
One of the exciting advances in modern medicine and life science is cell-based neurovascular regeneration of damaged brain tissues and repair of neuronal structures. The progress in stem cell biology and creation of adult induced pluripotent stem (iPS) cells has significantly improved basic and pre-clinical research in disease mechanisms and generated enthusiasm for potential applications in the treatment of central nervous system (CNS) diseases including stroke. Endogenous neural stem cells and cultured stem cells are capable of self-renewal and give rise to virtually all types of cells essential for the makeup of neuronal structures. Meanwhile, stem cells and neural progenitor cells are well-known for their potential for trophic support after transplantation into the ischemic brain. Thus, stem cell-based therapies provide an attractive future for protecting and repairing damaged brain tissues after injury and in various disease states. Moreover, basic research on naïve and differentiated stem cells including iPS cells has markedly improved our understanding of cellular and molecular mechanisms of neurological disorders, and provides a platform for the discovery of novel drug targets. The latest advances indicate that combinatorial approaches using cell based therapy with additional treatments such as protective reagents, preconditioning strategies and rehabilitation therapy can significantly improve therapeutic benefits. In this review, we will discuss the characteristics of cell therapy in different ischemic models and the application of stem cells and progenitor cells as regenerative medicine for the treatment of stroke.
Keywords: Stem cells, Transplantation, Regeneration, Survival, Differentiation, Stroke, Pre-conditioning, Trophic support
Introduction
Stem cell therapy has been considered in basic and clinical stroke research fields to be a promising regenerative medical treatment and a more promising approach for brain injury induced by different types of strokes. Stem cells (either endogenous neural stem cells or induced pluripotent stem cells e.g. iPS cells) have the potential of replacing damaged cells in the brain. Cell replacement strategies have been proposed and tested in many stroke models across decades of research in animal models. In addition, multipotent and pluripotent stem cells have shown beneficial paracrine effects, which can reduce cell death and provide growth/trophic support to host cells and regenerative activities in the host brain.
Induction of iPS cells from somatic cells (e.g. fibroblasts) with transcriptional factors (Oct-3/4, Sox-2, c-Myc and Klf-4) has shown promising translational potential. Similar to embryonic stem (ES) cells, iPS cells can be expanded in vitro and induced into neurospheres, neural progenitors, and mature neurons. Neuronally-differentiating ES/iPS cells and ES/iPS-derived neural progenitors have been extensively tested in transplantation therapies for the treatment of stroke, traumatic brain injury (TBI), spinal cord injury (SCI) and neurodegenerative diseases. It is generally agreed that transplanted cells may provide morphological and functional benefits via multiple mechanisms including, but not limited to, cell replacement, trophic support, immunosuppression/anti-inflammation, stimulation of endogenous signaling for neural plasticity and regeneration, and regulatory interactions with endogenous cells such as astrocytes and oligodendrocytes (Horie et al., 2015; McDonald and Howard, 2002; Volpe et al., 2016; Yu et al., 2013). Based on promising results and better understanding on the therapeutic mechanisms, translational studies and clinical trials using stem cells including mesenchymal stem cells (MSCs), umbilical cord stem cells (UCSCs), adipose-derived stem cells (ASCs), iPS cells, and many others are being tested for clinical applications.
In this review, we will focus on neural progenitor cells derived from ES, adult brain and iPS cells, and widely tested MSCs. Their mechanism of action and beneficial effects after stroke in animal models and human studies will be reviewed. Regarding transplantation therapy, we will discuss the efforts to improve the survival, neuronal differentiation and therapeutic potential of transplanted cells, post-transplantation cell distribution, and effects related to repairing the neurovascular unit. Cell delivery methods will be discussed highlighting the recent advances in non-invasive intranasal delivery of cells. We will also introduce functional and behavioral improvements after stem cell transplantation therapy.
Regenerative Medicine for the Treatment of Stroke
Stroke is the fifth leading cause of death and the number one cause of disability in the adult population in the United States (Mozaffarian et al., 2015). With an average of one victim every 40 seconds, almost 795,000 individuals experience a stroke every year in the United States, accounting for approximately one out of every 18 deaths (Mozaffarian et al., 2015; Roger et al., 2012). Of all stroke cases, 87% are ischemic in nature and the rest are hemorrhagic. In ischemic stroke, a clot occludes a brain vessel (most commonly the middle cerebral artery or its branches) and blood flow to the brain region supplied by that vessel is ceased, causing a cascade of pathological events associated with energy failure, acidosis, excessive glutamate release, elevated intracellular Ca2+ levels, generation of free radicals (especially after reperfusion), blood-brain-barrier (BBB) disruption, inflammation and eventually massive excitotoxic cell death composed of necrosis, apoptosis, autophagy and likely concurrent mixed forms of cell death involving hybrid mechanisms (Durukan and Tatlisumak, 2007; Li et al., 2013a; Puyal et al., 2013; Wei et al., 2004; Xiao et al., 2002). Hemorrhagic stroke, on the other hand, occurs when a blood vessel ruptures in the brain leading to intracranial hemorrhage (ICH) (Ferro, 2006). ICH, with the associated edema, makes the outcome of hemorrhagic stroke more serious than ischemic stroke. Approximately 10% of ischemic stroke victims and 38% of hemorrhagic stroke victims die within 30 days.
Despite an estimated cost of more than 70 billion dollars in 2010 (Roger et al.), stroke therapy remains very limited and dependent predominantly on supportive therapies. The only FDA-approved treatment for acute stroke patients is tissue plasminogen activator (t-PA). t-PA administered within the 4.5-hr treatment window breaks down the vessel clot, allowing reperfusion of the infarcted/penumbral area. Late t-PA administration, can cause intracranial hemorrhage and is thus contraindicated in hemorrhagic stroke. As such, t-PA is applicable for only about 2% of stroke patients who meet the criteria for t-PA treatment. Evidently, there is a critical and urgent need for effective therapies for acute as well as chronic stroke patients (Wahlgren et al., 2008).
Many drugs that show significant neuroprotection in animal models have failed to replicate in human clinical trials. Erythropoietin (EPO), for example, has shown significant neuroprotection in animal models of stroke (Bernaudin et al., 1999; Keogh et al., 2007; Leist et al., 2004; Li et al., 2007a; Li et al., 2007b; Prass et al., 2003; Tsai et al., 2006) and has even shown positive protection in a small clinical trial where t-PA was not used (Ehrenreich et al., 2002). However, it failed to improve outcomes and may have detrimental effects in patients receiving systemic thrombolysis (Ehrenreich et al., 2009). Mild and moderate hypothermia (therapeutic hypothermia) has provided significant neuroprotection in animal models of stroke (van der Worp et al., 2007). In contrast, therapeutic hypothermia is considered one of very few therapies that had promising potential as a clinical treatment for stroke patients, based on consistent beneficial effects observed in animal models of ischemic and hemorrhage stroke and traumatic brain injury (TBI). Benefits have also been replicated in clinical settings for the treatment of heart ischemia after cardiovascular arrest, term neonates after hypoxic-ischemic encephalopathy (HIE), and children with TBI (Jeon, 2014; Lampe and Becker, 2011; Luehr and Etz, 2014; Pietrini et al., 2012; Tissier et al., 2012).
Stem Cells Are Pluripotent/Multipotent Cells Capable of Tissue Repair
Emerging research interests have been focused on protective and regenerative strategies using stem cells and neural progenitors cells in the sub-acute and chronic phases after stroke. Stem cells are defined as cells which can both self-renew and differentiate into other cell types. Pluripotent stem cells, such as ES and iPS cells, can differentiate into any cell type of the three germ layers. In contrast, multipotent stem cells, such as hematopoietic stem cells, have a more lineage-restricted differentiation potential. Undifferentiated pluripotent stem cells can theoretically proliferate indefinitely in vitro, avoiding the senescence observed in cultures of adult multipotent stem cells (Gadalla et al., 2015).
Since their first isolation in 1998 by Dr. Thomson (Thomson et al., 1998), storms of ethical disputes have arisen around human ES cells. Due to the fact that they are derived from the inner cell mass of human blastocysts, human ES cells have raised many ethical concerns. Research progress and funding for human ES cells have been significantly hampered and thus, mainstream stem cell research has turned to alternative non-blastocyst derived cell sources. Along these same lines, different cocktails of transcriptions factors and small molecules have been developed whose expression or addition of various gene products can induce a pluripotent state in terminally differentiated somatic cells such as fibroblasts (Takahashi and Yamanaka, 2006; Yu et al., 2007).
Ethical concerns and the fear of rejection when using allogeneic cells have prevented clinical trials using ES cells, but the introduction of iPS cells has spurred research into the personalized use of pluripotent cells as a therapy for neurodegenerative disorders (Crook et al., 2015; Inoue et al., 2014). These induced pluripotent cells can differentiate into cell types of the three germ layers. Recently, novel protocols have been developed for creating neurons or neural precursors directly from fibroblasts, astrocytes or other terminally differentiated cells while avoiding a pluripotent cell state (Caiazzo et al.; Lujan et al., 2012; Marro et al., 2011; Pfisterer et al.; Vierbuchen et al.).
Stem cell transplantation has proven to be beneficial in injury models such as myocardial infarction and heart failure, with promising work in animal models translating to success in human clinical trials (Lee and Terracciano; Wollert and Drexler; Yin et al., 2014). In this review, we use the words “stem cells transplantation” to refer to all cell transplantation using stem cells and stem cell-derived neural progenitor cells. In fact, undifferentiated pluripotent cells such as ES cells are not used directly for transplantation because of their high risk of tumorigenesis. Meanwhile, transplantation of multipotent bone marrow stem cells (BMSCs) has been extensively evaluated for tumorigenesis but no tumor formation has been observed using these cells.
The clinical use of BMSCs in neurologic injury models, such as spinal cord injuries (Watanabe et al., 2015; Yoon et al., 2007) and neurodegenerative diseases such as multiple sclerosis (Ghobrial et al., 2014; Su et al., 2006), have demonstrated beneficial effects. It is unclear whether the observed improvements arise from cell engraftment or the replacement of damaged tissues. Considerable evidence has shown that bone marrow derived stem cells release trophic factors that show pro-survival and pro-regeneration effects in ischemic regions and promote endogenous repair mechanisms, particularly with regard to angiogenesis and neurogenesis. Furthermore, tissue engraftment in Parkinson’s disease has provided a proof-of-concept for cell replacement (Hallett et al., 2014; Lindvall and Kokaia, 2009), leading researchers to believe that stem cell therapies can eventually be used to replace damaged tissue resulting from neurological injuries and disorders.
In rodent models of focal ischemic stroke, endogenous neural stem cells in the sub-ventricular zone (SVZ) are recruited to the infarct site and form newborn neurons (Fig. 1). However, the efficiency of this endogenous repair mechanism is very low and the number of new cells that can survive and differentiate into mature neurons is insufficient for effective repair of damaged brain tissue. Neural stem cells (NSCs) can be isolated from the adult brain and after proliferation and neural differentiation under culture conditions, differentiated neural progenitor cells (NPCs) can be transplanted into the ischemic brain (Fig. 1). These transplanted cells can differentiate into neural and vascular cells and, by secreting trophic factors, reduce cell death and increase endogenous neurogenesis and angiogenesis (Hess and Borlongan, 2008; Wei et al., 2005b; Yu and Silva, 2008). Cells derived from the bone marrow (Chopp and Li, 2002) or umbilical cord blood (Taguchi et al., 2004; Vendrame et al., 2004) have also been used in rodent models of stroke. Improvements observed in such studies seem to have come primarily from trophic effects or enhancement of endogenous regeneration rather than replacement of damaged neuronal tissue. This idea is supported by the fact that functional improvements occur even when the homing of intravenously infused BMSCs is very low (~1%) so it appeared that cells did not need to cross the blood-brain barrier (BBB) to provide beneficial effects (Borlongan et al., 2004).
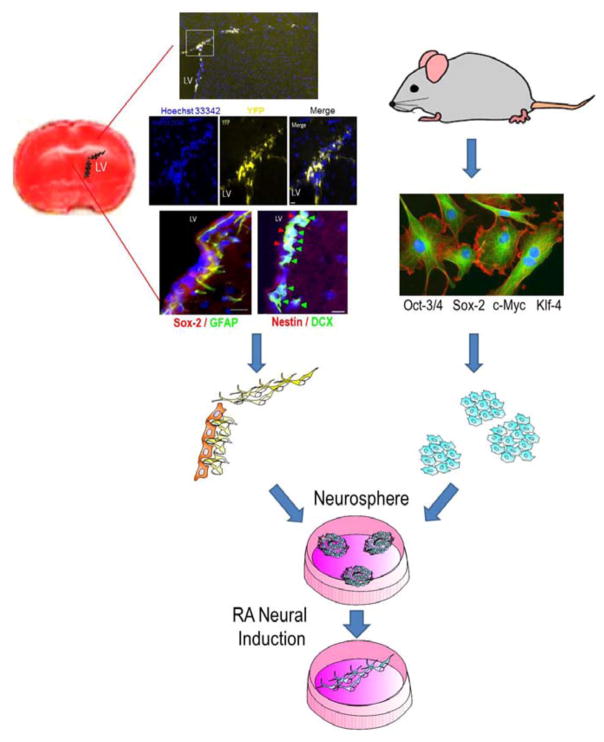
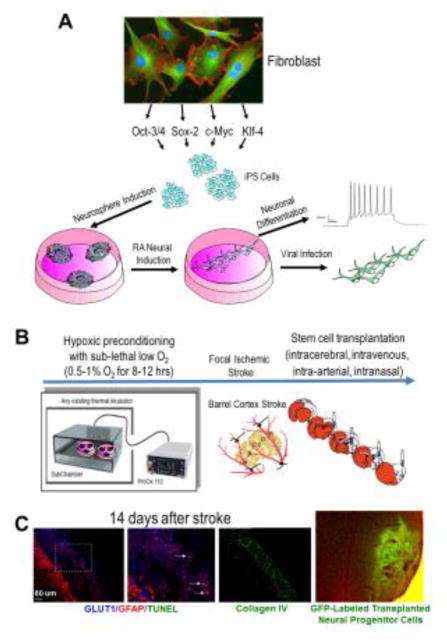
Figure 1. Neural stem cells from the SVZ and induced pluripotent stem cells from somatic cells.
The brain coronal section with TTC staining (red) shows the SVZ region. Immunohistochemical images show multiple markers for neural stem cells in the lateral ventricle SVZ 14 days after an ischemic insult. Using Nestin-Cre-ERT2/Rosa-YFP transgenic mice, the YFP+ cells indicate neural stem cells from the SVZ. Neural progenitors were DCX+ and Nestin+ while neural stem cells were GFAP+ and SOX-2+. Hoechst staining (blue) shows cell nuclei in the lateral ventricle SVZ. These neural stem/progenitors cells can be isolated, cultured and differentiated into neurons. Induced pluripotent stem (iPS) cells can be induced from rodents or humans using a set of reprogramming factors such as Oct-3/4, Sox2, cMyc, and Klf4. iPS cells can also be expanded and differentiated into different cell types including neurons.
Neural precursor cells that have been used in rodent models include conditionally immortalized cell lines derived from human fetal tissue (Pollock et al., 2006; Stevanato et al., 2009), carcinomas (Bliss et al., 2006; Hara et al., 2008), fetal neuronal stem cells (Zhang et al., 2009a), mouse neural precursors derived from the post-stroke cortex (Nakagomi et al., 2009), and precursors derived from mouse (Theus et al., 2008a; Wei et al., 2005; Yanagisawa et al., 2006) or human (Daadi et al., 2008b; Kim et al., 2007b) embryonic stem cells. Undifferentiated pluripotent stem cells are normally treated with a neural induction protocol before transplantation to gain cell lineage direction as well as reduce the risk of tumorigenesis (Newman et al., 2005). After neural induction, neural progenitor cells are either injected into the circulation (intravenously or intra-arterially) or directly into the ischemic brain (intracerebrally, intracranially, or intranasally) and have been shown to improve functional outcomes in rodent models of stroke.
Neuronal Differentiation of Stem Cells
In the absence of differentiation cues, pluripotent stem cells can remain in a state of continuous self-renewal in culture. Murine and human ES and iPS cells have the potential to differentiate into any of the three primary germ layers including the ectodermal layer responsible for neural development (Doetschman et al., 1985). Neural progenitors can further differentiate into any of the neural cell types, including glial cells and neurons (Reubinoff et al., 2001). The differentiation outcome greatly depends on the microenvironment in the cell culture (Watt and Hogan, 2000). Specific neuronal subtypes (dopaminergic, GABAergic, etc.) can be cultivated in a controlled microenvironment with specific substrates and media components. There are many different ways to differentiate mouse pluripotent cells into neurons depending on the neuronal subtype of interest (i.e. midbrain dopaminergic (Lee et al., 2000; Maxwell and Li, 2005), serotonergic (Lee et al., 2000; Lu et al., 2016), forebrain (Jing et al., 2011), radial glia (Glaser and Brustle, 2005; Gorris et al., 2015).
Among different protocols for mouse stem cells, differentiation using the vitamin A derivative, retinoic acid (RA), is the most commonly used method (Bain et al., 1995; Fraichard et al., 1995; Francis and Wei, 2010; Guan et al., 2001; Rohwedel et al., 1999) (Fig. 1B). RA plays a role in the developing mammalian embryo and specifies the anterior-posterior body plan (Horschitz et al., 2015; Kessel and Gruss, 1991). Anteriorly, RA induces Hox gene expression (Kessel and Gruss, 1991; Marshall et al., 1992; Simeone et al., 1991) and has specific effects on rhombomere development in the CNS (Marshall et al., 1992). RA interacts with Cellular RA-Binding Proteins (CRABP) which bind to nuclear RA receptors. There are two families of RA receptors (RARs and RXRs) (Rohwedel et al., 1999). RARs include RAR-α, RAR-β and RAR-γ. During neuroectodermal differentiation, RAR-α and RAR-β mRNA are upregulated suggesting that neural differentiation requires these receptor subtypes (Rohwedel et al., 1999). The binding of RA to its receptor activates the transcription of downstream target genes leading to neural lineage selection. In pluripotent cell differentiation, RA induction activates neural-specific transcription factors, signaling molecules, and RA-inducible genes resulting in the production of different neuron sub-types such as GABAergic and glutamatergic neurons (Guan et al., 2001). The RA neural induction method produces both glial cells and neurons. Neurons derived in this method show mature neuronal morphology, express mature neuronal markers such as neuronal nuclei (NeuN) and neurofilament and exhibit neuronal electrophysiology profiles that include mature sodium currents, potassium currents, and action potentials (Bain et al., 1995).
Undifferentiated mouse ES and iPS cells are regularly maintained in a self-renewing, proliferating state in the presence of leukemia inhibitory factor (LIF) which suppresses differentiation and is required for the maintenance of pluripotency (Vidal et al., 2015; Williams et al., 1988). Neural induction using the “4+/4−” RA protocol takes eight days in vitro. After LIF withdrawal, cells are grown in suspension to form three dimensional spherical aggregates of cells known as embryonic bodies (EB). No RA is included in the medium for the first 4 days of EB culture (termed “4−”), but it is added in the last four days (termed “4+”). This treatment induces EBs to form neurospheres which are then dissociated and plated on an adherent substrate (laminin, Matrigel or fibronectin) to allow terminal neuronal differentiation and neurite extension. These cells will express neuronal markers such as the M subunit of neurofilament and class III β-tubulin protein within a few days. Most of the neurons derived in this fashion will express functional Na+, K+, and Ca2+ channels and fire action potentials (Bain et al., 1995).
Neural cells can also be obtained from pluripotent stem cells through lineage selection (Guan et al., 2001). In this method, differentiation into cell types of all three germs layers (ectoderm, mesoderm, and endoderm) occurs in the EBs (Guan et al., 2001). Neuroectodermal cells are selected with serum withdrawal, as well as the inclusion of basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF) to increase proliferation of neural progenitor cells. Neural differentiation factors and survival-promoting factors (SPF) (interleukin-1β, TGF-β, GDNF and many others) are then added for further differentiation of neural cells into specified neuronal cell types (Guan et al., 2001).
There are also several other protocols for the neuronal differentiation of mouse ES and iPS cells. One particular protocol makes use of mouse stromal supportive cells for neuronal induction (Barberi et al., 2003). Murine bone marrow–derived stromal feeder MS5 cells (Itoh et al., 1989) are mesenchymal stem cells that were originally derived to support the long-term growth and expansion of hematopoietic stem cells, but have also been found to induce a neuro-ectodermal lineage fate in mouse ES cells. In brief, undifferentiated murine ES cells are seeded at a very low density on MS5 cells in serum replacement and cultured for 7 days with bFGF added in the last two days. Co-culture of mouse ES with MS5 cells induces the expression of neural markers (Nestin and Musashi) as early as day 6. Further terminal differentiation can yield different neural subtypes (glial cells, dopaminergic, serotonergic, and GABAergic neurons) depending on the patterning factors used after neural induction. Although RA strongly induces neuronal formation in mouse ES cells, its use in transplantation therapy is unclear. Neuronal induction of the MS5 cell line produces cortical pyramidal neurons that connect with their correct targets in vivo after transplantation (Ideguchi et al., 2010). These graft-to-host axonal connections, however, did not occur when cells were differentiated using RA. The MS5 protocol may thus be more appropriate for use in cell replacement strategies using murine cells.
Human ES and iPS Cell Differentiation
Human ES and iPS cells have the potential to differentiate into all human cell lineages including neurons, making them an attractive cell source for studying disease mechanisms and for drug screening as well as cell transplantation therapy in neurodegenerative diseases such as stroke (Fig. 2). As with mouse pluripotent cells, several approaches have been developed for the neural induction of human pluripotent cells (Suzuki and Vanderhaeghen, 2015). The neuralizing effect of RA, for example, is not the same between human and mouse pluripotent cells. However, like their mouse counterparts, human cells can be differentiated either as EBs in suspension or in adherent culture. We will discuss two differentiation protocols because of their widespread use and proven efficiency.
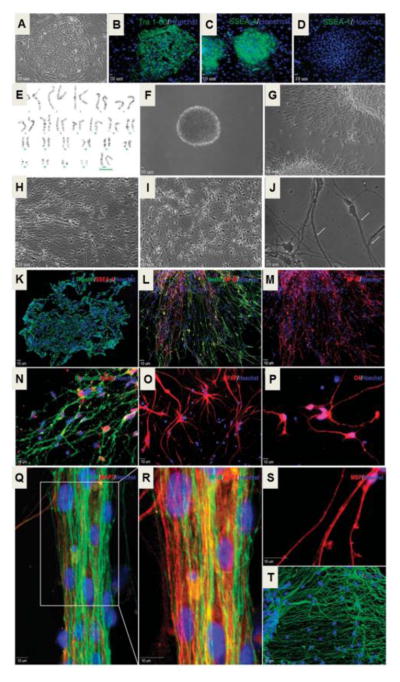
Figure 2. Neural gene expression in differentiating human ES cell-derived neural progenitor cells.
Neural differentiation of UCO6 human ES cells (hESCs). A. Typical colony of hESCs (passage 70). B–D. Colonies express pluripotent markers TRA-1-60 (B) and SSEA4 (C), but are negative for SSEA-1 (D). E. Normal karyotypic analysis of passage 75 UCO6 colonies. F. Twenty-eight day old floating neurospheres (~200 μm in size). G–J. After beginning terminal differentiation, cells exhibit increasingly neuronal morphology after 1 day (G), 7 days (H) and 14 days (I). Cells exhibit significant neurite extensions and many dendritic spines form (white arrows) after 21 days of terminal differentiation (J). K. Cells exhibit high expression of the neural precursor marker Nestin (green) and very low expression of SSEA-4 (red) 1 day after cell adhesion. L and M. Three days into terminal differentiation, Nestin (green) positive, elongated cells begin to express medium chain neurofilament (NF-M; red). N. Seven days after plating, the neuronal markers βIII-tubulin (green) and NeuN (red) were highly expressed. O. Expression of the astrocyte marker GFAP is observed after 7 days. P. Very few O4-positive immunoreactive cells were found in these cells. Q and R. Extensive NF-M (green) and microtubule-associate protein-2 (MAP2; red) immunoreactive projections were present in cells of at 21 days into differentiation. S. Twenty eight days after plating, some projections are positive for myelin-binding protein (MBP), indicating axonal myelination. T. Extensive beds of GFAP-positive astrocytes can also be observed after 28 days into terminal differentiation. All blue staining indicates Hoechst-positive nuclei. Adopted from Figures 1 and 2 in Francis and Wei, 2010 in compliance with the journal’s copyright policy.
After the first isolation of human ES cells in 1998, efforts were focused on their neuronal differentiation based on the knowledge accumulated from the development of the human nervous system. One of the earliest efforts was led by Zhang and Thomson utilizing EB formation (Zhang et al., 2001). In this protocol, human ES cell colonies are dissociated into smaller clusters of cells and grown in human ES cell media without bFGF in low adherence plates. When EBs form, they are grown for four days before being plated on tissue culture plastic in a neural induction media. After neural rosette formation (~8–10 days), rosettes are re-suspended and expanded as neurospheres in medium containing bFGF. Prior to neurosphere formation, cells in the rosette structures express neural markers such as Nestin and Musashi-1. After expansion, these cells are plated on laminin, with mature neuronal markers detectable within 7–10 days. Further differentiation will yield more neurons and, after longer periods in culture, glial cells. This protocol was one of the earliest methods used to generate neural cells from human ES cells and has successfully been applied to human iPS cells (Hu et al., 2010).
Despite its high efficiency, the EB protocol usually involves several weeks of expansion to obtain large numbers of neural precursors before differentiation into neurons and glial cells. In an attempt to overcome this time limitation and avoid the heterogeneous signals encountered by cells in aggregates, Chambers et. al. developed an adherent protocol for the neural differentiation of human ES and iPS cells that produces large numbers of neural precursors in approximately 15 days (Chambers et al., 2009). In brief, human ES or iPS cells are dissociated into single cells and grown on Matrigel in the presence of Rho associated protein kinase (ROCK) inhibitor, which reduces apoptosis and increases human pluripotent cell survival after dissociation (Watanabe et al., 2007). When they become confluent, the cells are grown in a knock-out serum replacement media for five days. This medium is supplemented with two inhibitors of the SMAD signaling pathway, Noggin and SB431542. The media is then sequentially changed to neural induction media (increasing amounts of N2 media) for a total of six days. Consequently, neural rosette-like structures are formed and can be further differentiated into neurons of different subtypes. Unlike the EB based protocol, this adherent protocol yields Pax-6 positive neural precursors in more than 80% of the cells, with little need to separate rosettes from cells that have differentiated into other lineages. When used with hiPS cells, the EB protocol had a significantly reduced efficiency and increased variability as compared to hES cells (Hu et al., 2010). On the contrary, the dual inhibition protocol significantly promotes neural differentiation from multiple human ES and iPS cell lines, (Kim et al., 2010) indicating more consistent results with this protocol. In our investigations, we have shown that Noggin can be replaced with the small molecule dorsomorphin in this protocol, greatly reducing the cost for neuronal differentiation of human cells (Drury-Stewart et al., 2012). Dorsomorphin has been used to enhance neural differentiation in suspension cultures (Kim et al., 2010; Morizane et al., 2011) and has been shown to induce efficient neural differentiation alone in adherent culture (Zhou et al., 2010).
Strategies to Enhance Cell Survival after Transplantation: Genetic Modification and Hypoxic Preconditioning
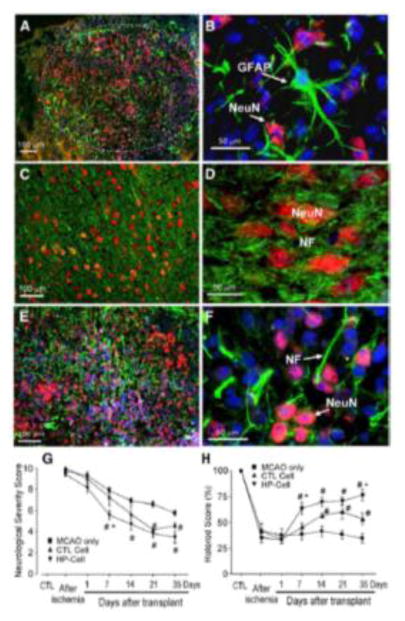
Genetic manipulation of transplanted cells has been tested for enhancing the cell survival after transplantation (Doeppner et al., 2010; Wei et al., 2005). Expression of pro-survival factors shows significant effects on enhancing graft viability after transplantation. For example, we reported in our early research that transplantation of ES cell-derived neural progenitor cells over-expressing the anti-apoptotic gene Bcl-2 survived much better than control cells after ischemic stroke in rats (Wei et al., 2005). Compared to controls, Bcl-2 expressing progenitors also exhibited greater neuronal differentiation and better neurological and behavioral outcomes after stroke (Fig. 3) (Wei et al., 2005). This approach of over-expressing pro-survival genes in transplanted cell has been actively investigated by a number of groups (Chen et al., 2012; Leu et al., 2010; Liu et al., 2011). However, this type of genetic engineering requires the alteration of DNA sequences in stem cells, generally by inserting the gene(s) of interest into the genome. While this method can be highly efficient, manipulating the genome of stem cells increases the risk of tumorigenesis in a population of cells with an already established risk of tumor formation.
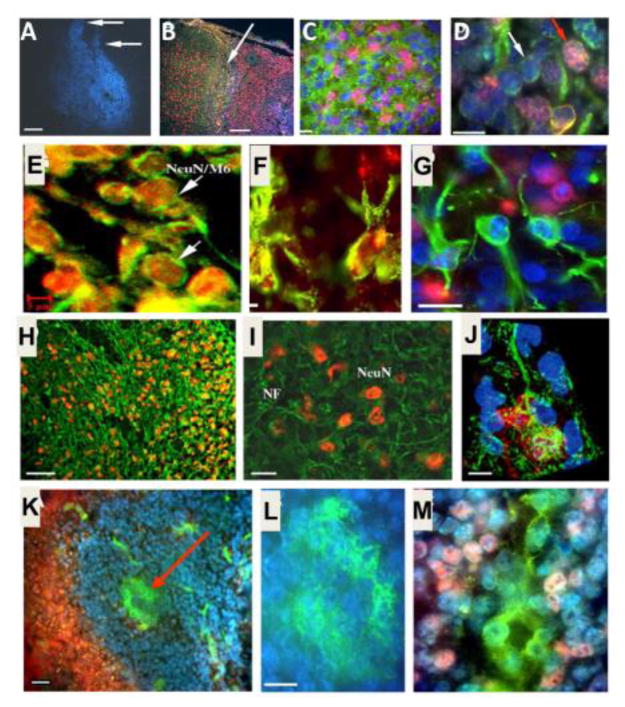
Figure 3. ES cell survival and differentiation after transplantation into the ischemic brain.
Immunohistochemical staining 7 days after intracranial transplantation (14 days post-ischemia). A. Distribution of transplanted ES cells in the post-ischemic basal ganglia revealed by pre-labeled Hoechst (blue) staining. B. Distribution of transplanted ES cells in the post-ischemic cortex. Hoechst-positive ES cells (blue) were present next to undamaged tissue (arrow and the area to the left). NeuN (red) staining shows endogenous neurons in non-ischemic cortical region (left of the arrow) and differentiated neuron-like cells in the ischemic region (right of the arrow). The NeuN staining on the right appears pink in color due to overlap with the blue Hoechst 33342 staining. C. Mouse cell-specific antibodies M2 and M6 (both green) and Hoechst 33342 staining (blue) verified the transplant origin of cells surviving 7 days in the rat host ischemic cortex. NeuN staining (pink) identified differentiated neuronal cells. D. Higher magnification of a confocal image shows the double labeling of Hoechst (blue) and mouse antibody M2/M6 (green) that confirms murine origin of these cells (white arrow). The triple-labeled cells with the additional staining of NeuN (pink) identify ES cell-derived neurons (red arrow). E. Confocal images of NeuN staining (red), mouse antibody M6 staining (green), and overlapped double staining (yellow) show neuronal cells originated from transplanted ES cells in a transplantation area. Scale bar equals 800, 200, 20, 10 and 5 μm, in A, B, C, D and E, respectively. F. and G. Differentiations of transplanted ES cells in the host ischemic cortex and striatum. ES cells were labeled with BrdU or Hoechst 33342 prior to transplantation. The double labeling of BrdU (red) and GFAP (green) identified differentiated astrocytes of ES cell origin (F). An image taken from (G) an 8-μm section shows Hoechst-prelabeled ES cells double stained either with NeuN (red) or GFAP (green), consistent with ES cell differentiation into neurons and astrocytes. Scale bar = 20 μm. H–J. Dendrite growth after ES cell transplantation in the ischemic cortex. NeuN-positive cells and apical dendrite distribution in the ipsilateral cortex. Seven weeks after ES cell transplantation, NeuN and NF double immunostaining in the ischemic core region of the ipsilateral cortex under an inverted fluorescence microscope. Many NeuN-positive cells (yellow or orange) were surrounded by NF labeled processes (green). A confocal image of NeuN/NF-positive ES cell in the 8-Am-thick slice of ischemic cortex. The cell body was positive to NeuN staining (red), cell processes were positive to NF staining (green). The Hoechst labeling (blue) showed several transplanted ES cells. Scale bar = 60 μm in H, 20 μm in I, 10 μm in J. K–M. ES cell-derived Glut-1-positive cells in the ischemic region. Seven days after transplantation, the ischemic core was filled with ES cells (Hoechst-positive, blue) and contained Glut-1-positive vascular-like structures of endothelial cells (green; arrow). Scale bar = 50 μm. Enlarged view of a vascular structure in K (L), positively stained with Glut-1 (green). Scale bar = 10 Am. Glut-1-positive endothelial cells (green) were also co-labeled with Hoechst (blue), verifying their origination from transplanted ES cells (M). The pink color is from neighboring NeuN-positive cells. Images were taken using an inverted fluorescence microscope. Adopted from Figures in Wei et al., 2005 in compliance with the journal’s copyright policy.
Other techniques have been developed to increase survival of stem cell grafts after transplantation. Instead of over-expressing exogenous genes in transplanted cells, we developed an alternative strategy stimulating endogenous mechanisms to promote the survival and regenerative properties of transplanted cells (Pignataro et al., 2007; Wei et al., 2005b). In cardiomyocyte ischemia, an early exposure of the heart to brief episodes of ischemia protected the myocardium against a later prolonged ischemic insult (Murry et al., 1986). We showed that pretreatment of ES or iPS cell-derived neural progenitor cells in low oxygen condition (0.1–1% O2) for 8–12 hrs markedly enhanced their tolerance to OGD insult in vitro and after transplantation into the ischemic brain (Fig. 4). The pro-survival and an enhanced homing to the ischemic brain were later demonstrated using BMSCs (Theus et al., 2008b; Wei et al., 2012; Wei et al., 2015; Yu et al., 2013).
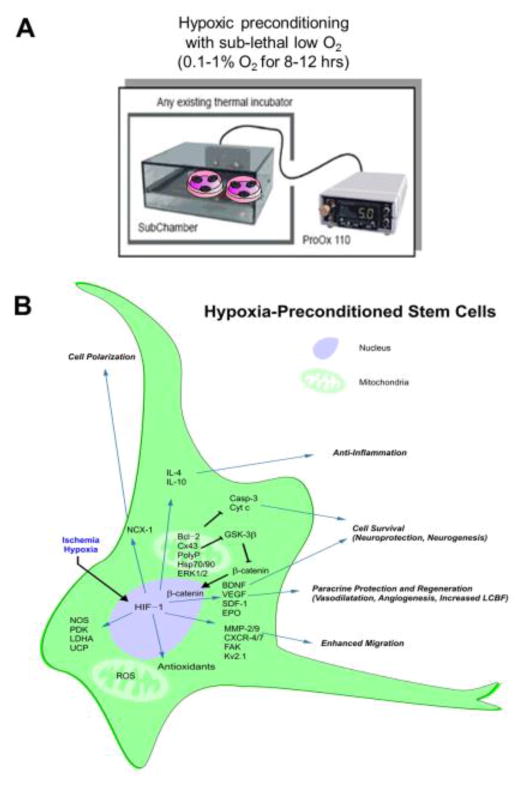
Figure 4. Hypoxic preconditioning of stem cells or neural progeinitor cells and the mechanis of action.
A. Neuronally-differentiated cells or BMSCs were subjected to hypoxic preconditioning priming treatment before to be used for cell therapy. Cells were placed in a hypoxic chamber (0.1–1% O2) for 8–12 hrs before transplantation. B. Potential mechanisms underlying the beneficial effects of hypoxic preconditioning. The hypoxic preconditioning strategy was designed to mimic and utilize endogenous protective mechanisms to promote neuroprotection, tissue regeneration and brain function recovery. Hypoxic preconditioning directly induces HIF-1α/β upregulation that increases BDNF, SDF-1, VEGF, EPO and many other genes which can stimulate neurogenesis, angiogenesis, vasodilatation and increase cell survival. HIF-1 expression regulates antioxidants, survival signals and other genes related to cell adhesion, polarization, migration, and anti-inflammatory responses. Abbreviation in the figure: BDNF, brain-derived neurotrophic factor; Casp, caspase; Cx43, connexin-43; CXCR, CXC chemokine receptor; Cyt c, cytochrome c; EPO, erythropoietin; ERK, extracellular signal-regulated kinase; FAK, focal adhesion kinase; GSK-3β, glycogen synthase kinase-3β; HIF-1, hypoxia-inducible factor-1; Hsp, heat shock protein; IL-4, interleukin-4; IL-10, interleukin-10; MMP, matrix metalloproteinase; NCX-1, sodium-calcium exchanger-1; NOS, nitric oxide synthase; PDK, pyruvate dehydrogenase kinase; polyP, polyphosphate; ROS, reactive oxygen species; SDF-1, stromal-derived factor-1; UCP, uncoupling protein; VEGF, vascular endothelial
Numerous investigations have demonstrated the effectiveness of hypoxic/ischemic preconditioning in virtually all types of cells, organs, and in animals (Gross and Auchampach, 1992; Yu et al., 2006). Preconditioning has been successfully applied in cell therapy to protect the transplanted cells from apoptosis and increase their survival after transplantation in vivo. Hypoxic preconditioning (HP) was performed by exposing cells to non-lethal hypoxia (0.1–1%) for certain hours before transplantation, which is very effective in increasing transplanted cell survival and improving overall functional recovery after stroke or myocardial infarction (MI) (Hu et al., 2011b; Hu et al., 2008; Sun et al., 2015; Theus et al., 2008a; Wei et al., 2012). HP has also shown enhancement of cell differentiation in culture and after transplantation (Sart et al., 2014; Sun et al., 2015). Non-lethal exposure to hypoxic conditions is believed to activate the hypoxia-inducible factor 1α (HIF-1α) pathway, increasing expression of HIF-1α-dependent genes. Expression of a number of trophic factors is increased, including brain-derived and glial cell-derived neurotrophic factors (BDNF and GDNF), vascular endothelial growth factor (VEGF) and its receptor FIK-1, erythropoietin (EPO) and its receptor EPOR, and stromal derived factor-1 (SDF-1) and CXC chemokine receptor 4 (CXCR4) (Wei et al., 2012) (Fig. 4). With a greater translational significance, these beneficial effects of HP have been demonstrated in iPS cell-derived neural progenitor cells (Fig. 5A and 5B).
Figure 5. Neuronal differentiation of iPS cells with hypoxic preconditioning and transplantation into the ischemic brain.
A. Somatic cells such as fibroblasts can be reprogrammed into pluripotent stem cells with specific transcription genes. These cells can be differentiated into mature and fucntional neurons, firing repetitive actiona potentials and can be manipulated with gene modification. B. At the later stage of neural induction, cells are subjected to non-lethal hypoxic preconditioning treatment and then ready for translantation into the ischemic brain. C. Demonstration of implanted cells in the ischemic core and peri-infect region. Immunohistochemial staining show remaining and/or regenerated vasculartures (GLUT1 (blue) and Collagen IV (green) staining) and the formation of a glia score (GAFP, red) surrounding the ischemic core 14 days after sensorimotor cortex stroke. Green flourescence GFP labeled iPS-derived neural progenitor cells were implanted into the ischemic core and peri-infarct regions;. these hypoxic preconditioned cells survived well several weeks after transplantation.
Pharmacological preconditioning is an alternative way by exposing stem cells of pharmacological agents or trophic/growth factors to increase their in vivo survival. Diazoxide (Afzal et al., 2010), minocycline (Sakata et al., 2012), and SDF-1 (Pasha et al., 2008) can all enhance stem cell survival after transplantation in stroke or MI animal models.
In Vitro and Animal Models for the Study of Ischemic Stroke
There are many in vivo and in vitro models used to study stroke and stem cell therapy. While successes in treating stroke in animal models have been unreliable when translated to human clinical trials, ischemic stroke models are necessary to understand the pathophysiology of stroke progression and provide initial information for the development of appropriate therapeutics. In vitro model of oxygen glucose deprivation (OGD) mimics the hypoxic and energy crisis that occur during acute ischemic injury. Hence, this model is widely accepted and tested for studies of cell death, mitochondrial dynamics, and reperfusion/reoxygenation injuries.
In vitro investigations using cell culture models allow the understanding of the basic cellular, molecular and biochemical mechanisms without the systemic influences of an in vivo model. The OGD technique, applied to cultures of pure primary neurons or mixed cultures of glia and neurons, is the most commonly used in vitro model of stroke (Tornabene and Brodin, 2016). Depriving cultured neurons of oxygen and glucose supplies simulates, to a certain extent, in vivo ischemic conditions. Briefly, growth medium is replaced with a physiological solution like Ringer’s solution and cultures are placed in an airtight chamber with low oxygen (0.1 – 1.0%). After certain duration of times (usually one to several hours depending on the degree of hypoxia and cell types), cells are then returned to their normal culture conditions. Cell death and survival are assessed after an appropriate “reperfusion” period, usually in 24 hours. Sometimes, cell death is measured after a prolonged time of OGD without “reperfusion”. Cell death induced by different OGD episodes may be mediated or dominated by distinctive mechanisms, e.g. necrotic and apoptotic pathways (Jones et al., 2004)(Agudo-López et al., 2010). OGD can be used with different cell types, including ES cell-derived neural precursors and glial cells, to study their mechanisms of survival and differentiation under an ischemia-like condition. For example, researchers used OGD to study a nuclear translocator (ARNT) in ES cells in response to hypoxia (Maltepe et al., 1997). In vivo models allow us to study stroke within the scope of a comprehensive biological system. Here, we will discuss some major in vitro and in vivo stroke models used in preclinical research.
Inducing stroke in animals allows researchers to reproduce stroke conditions in vivo together with the related systemic influences. This allows for the study of stroke pathology and pathophysiology and the exploration for optimal conditions (survival, timing, and location) for experimental cell transplantation therapies. Animal models for the study of ischemic stroke primarily vary by their methods of induction, location, duration, and, correspondingly, the severity of ischemic injury and functional deficits (Ingberg et al., 2016; Molinari, 1988). Ischemic stroke models can be divided into two broad categories: focal and global ischemia. Focal ischemia is more commonly seen in clinical cases and widely tested in basic research. It is induced by an acute occlusion of specific vessels to cause damage in affected brain regions. Focal ischemia can be modeled with transient or permanent occlusions and the extent of damage can be small or large depending on the occlusion of the artery and the anatomy of cerebral vassal networks. The word “global ischemia” is used when all cerebral blood flow including that of the vertebral arteries cease. Clinically, global ischemia occurs during suffocation, cardiac arrest or after bilateral common carotid artery (CCA) occlusions. The middle cerebral artery (MCA) is the most commonly affected vessel in stroke patients. Accordingly, the most common models of ischemic stroke in rodents involve the occlusion of MCA. Here, we will discuss several models of ischemic stroke.
Focal Ischemic Stroke Models
Middle cerebral artery occlusion (MCAO) of rodents
The MCAO model is widely used and accepted because it most closely resembles human stroke conditions. The original method for inducing this stroke was the Tamura method for occluding the proximal MCA in the rat, causing ischemic damage in the cortex and basal ganglia (Tamura et al., 1981a, b). This procedure requires an invasive subtemporal craniotomy. The intraluminal method, on the other hand, requires no craniotomy and is thus less invasive (Longa et al., 1989). A neck incision is made and a nylon intraluminal suture is introduced through the external carotid artery to occlude the MCA. This procedure is reversible and can be varied with a thicker (2–0) or thinner (4–0) suture thread (Longa et al., 1989; O’Brien and Waltz, 1973). Both models induce relatively large brain injuries involving cortex and/or subcortical regions or even an entire half hemisphere.
Using MCAO models, stem cell transplantation studies have been extensively performed during the past 20 years. Neuroprotective effects of therapeutic benefits in reducing infarct volume, and improving functional outcomes during acute and chronic phases can be examined. It is difficult, however, to evaluate neurovascular damage and neuronal circuit reestablishment at structural and network levels in severe stroke models. To this end, small stroke modes are more suitable for morphological examinations of neural network structural repair.
Barrel cortex stroke and small stroke models
Barrel cortex stroke is a small focal stroke model, involving occlusions of distal branches of the MCA. According to American Heart Association, small strokes are common in clinical cases (~40% of total cases)(Lloyd-Jones et al., 2010). In recent years, increasing attention has been paid to develop and study small stroke models (Liu et al., 2012; Llovera et al., 2014; Pevsner et al., 2001). This shift appears not only due to clinical relevance, but also for the advantage of the feasibility of examining structural and neural network repair after a well controlled focal cerebral ischemia. Small ischemic strokes can be induced by ligations of the distal branches of MCA. A typical small stroke model in rats was initially reported by Ling Wei in 1995 (Liu et al., 2012) (Fig. 6). In this model, the barrel cortex and its vasculature are visualized using intrinsic optical signal imaging based on the mechanism that stimulation of the contralateral whiskers increases the local cerebral blood flow (LCBF) in the barrel cortex. The distal branches of the MCA around the barrel cortex are identified and then ligated with 10-0 sutures. In recent years, variations of the small barrel stroke models have been reported. In the mouse, the main branch of the MCA is permanently occluded combined with transient bilateral occlusions of the CCA. This simplified surgery procedure is sufficient to reduce the local blood flow and cause infarct formation in the affected sensorimotor cortex. The infarct volume induced by this procedure is relatively larger than the original barrel cortex stroke; it retains all the advantages of a small stroke model but also allows for functional and behavioral tests of the sensorimotor cortex in rodents.
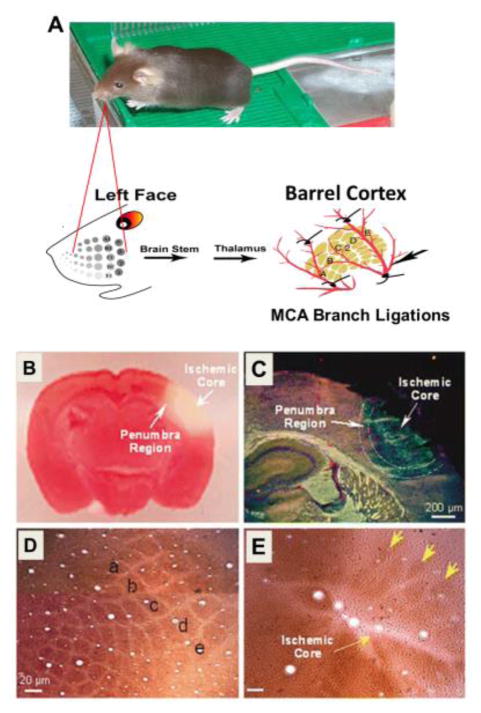
Figure 6. Small stroke model of barrel cortex ischemia in rodents.
A. Focal ischemic insult to the barrel cortex is induced by permanent occlusion of the distal branches of the right or left middle cerebral artery combined with transient ligations of bilateral common carotid arteries. The barrel cortex is identified by the optical imaging in responding to whisker stimulation. B. Ischemia-induced barrel cortex infarct formation and cell death. Middle cerebral artery branch ligations induced a focal ischemic infarct and cell death in the barrel cortex. Selective damage occurred to the right barrel cortex region shown as a negative (white) area in triphenyl tetrazolium staining (red) 24 hrs after ischemia. The pink area between the normal cortex and ischemic core represents the bordering penumbra area. C. In the immunohistochemical image, Glut-1 staining (red) shows vascular endothelial cells in the penumbra and normal brain tissues. NeuN (blue) indicates neurons. Ischemic core shows the autofluorescence associated with damaged tissue. D and E. Cytochrome oxidase staining revealed the barrel column structures in the context of a normal cortex (D) and undergone ischemic damage (E). The lowercase letters “a”–”e” in image D illustrate the characteristic pattern of barrel distribution in the cortex. Fourteen days after ischemia, most of the barrel column structures disappear except a few barrel-like clumps (arrowhead) preserved in the peripheral region of the barrel cortex (E).
Since the whisker-thalamus-barrel cortex neuronal pathway and the relationship between the barrel cortex and whisker activity had been well defined (Woolsey et al., 1996), the barrel cortex and the sensorimotor cortex stroke models are ideal for examining the intracortical and thalamo-cortical neuronal morphological connections and functional recovery. For example, the corner test can be used to test the functional deficit of affected whiskers after stroke (Langhauser et al., 2014; Zhang et al., 2002). In this test, when facing toward a 30° corner wall, normal control animals turn equally to the right or left whereas stroke animals turn more frequently toward the non-impaired side (ipsilateral to infarcted brain)(Zhang et al., 2002). Moreover, our group has used whisker stimulation as a physical rehabilitation treatment during chronic phase of stroke. It was shown that the target-specific peripheral activity reduced cell death and increased trophic/growth factors in the peri-infarct region of the cortex. Consequently, the physical therapy enhanced neurogenesis, angiogenesis, and functional recovery after ischemic stroke (Li et al., 2011; Li et al., 2008a; Whitaker et al., 2007).
Other focal ischemic stroke models in rodents
There are several other ways to induce focal ischemia including the photothrombotic (Schmidt et al., 2015), autologous clot (Jin et al., 2014; Taupin and Gage, 2002), and endothelin-1 (Roome et al., 2014) methods (Table 1). The photothrombotic method is a non-invasive method in which a photosensitive dye is intravenously injected into the animal (Chen et al., 2004; De Ryck et al., 1996; Eichenbaum et al., 2002). To induce thrombotic occlusion, the distal MCA is irradiated by a laser to activate microthromboses. A significant strength of this model is that it allows MCA occlusion in awake animals. In the thromboembolic stroke model, the embolus is created by removing the arterial blood from a donor into tubing of predetermined size. The clots are formed by alternating the blood through different syringes with saline. In the technique described by Zhang and colleagues, the clot was introduced into the external carotid artery (ECA) to be lodged into the origin of the MCA (Zhang et al., 1997). A drawback of thromboembolic models is the difficulty for precise control of reperfusion and larger variations in infarct formation (Wang et al., 2001; Zhang et al., 1997). In the Endothelin-1 model, the vasoconstrictor peptide Endothelin-1 (ET-1) is injected into the brain. This peptide reduces blood flow and induces ischemic injury (Windle et al., 2006). This model can induce transient to semi-permanent focal ischemia, but reperfusion in this model still requires characterization (Biernaskie et al., 2001). It was reported that systemic inflammation continues to impair reperfusion via endothelin dependent mechanisms (Murray et al., 2014). ET-1 can be injected intracerebrally or topically applied onto the brain into an area of interest like the motor cortex (Windle et al., 2006). It can also be injected next to the MCA to induce larger ischemic injury (Biernaskie et al., 2001).
Table 1.
Focal ischemic stroke models.
| Model | Surgical procedures | Species/Ages | Major pathological change | Reference |
|---|---|---|---|---|
| Photothrombosis | intravascular photocoagulation and inducing occlusion of the irradiated vessels with secondary tissue ischemia | Rat/Adult | Localized focal injury that may depend on injection site. Massive T cell and macrophage infiltration, controlled lesion in cortical areas, fast blood-brain barrier disruption and vasogenic edema | (Rosenblum and El-Sabban, 1977); (Watson et al., 1985) |
| MCA Occlusion | thrombembolic MCAO: injecting clots that were formed in vitro or by endovascular instillation of thrombin for in situ clotting | Rat/Adult/Neonate Mouse/Adult Dogs/Adult Sheep/Adult Monkey/Adult | Severe stroke model can cause half hemisphere damage in suture MCAO; Vascular endothelial damage, platelet activation and subsequent thrombotic vessel occlusion | (Tamura et al., 1981a); (Nagasawa and Kogure, 1989); (Chen et al., 1986); (Boltze et al., 2008); (Fan et al., 2012) |
| Endothelin Stroke | cortical application of ET-1 | Rat/Adult | Necrotic, apoptotic and hypertrophic neurons and neurons with pyknosis in surrounding penumbra | (Robinson et al., 1990); Masaki et al (1992); Macrae et al. (1993); (Sharkey et al., 1993); Fuxe et al. (1997) |
| Barrel Cortex Stroke | permanently ligating 3 to 6 branches of MCA, temporarily occluding CCA | Mouse/6–8 wk; Rat/Adult/Neonate | First ischemic model specifically targeting the barrel cortex and suitable for whisker functional studies; Infarct formation in rodent somatosensory barrel cortex, vascular endothelial damage, and platelet activation | (Wei et al., 1995); (Luo et al., 2008); (Jiang et al., 2016) |
Abbreviation in the Table: BBB, blood-brain barrier; BMSC, bone marrow mesenchymal stem cell; CCA, common carotid artery; ES, embryonic stem cells; ET-1, endothelin-1; iPS, induced pluripotent stem cells; LCBF, local cerebral blood flow; MCA, middle cerebral artery; NSC, neural stem cells; PN, postnatal.
Global Ischemia Models
Two-vessel occlusion model
In this model, histopathological ischemic damage to the forebrain is induced by bilateral occlusion of the CCA in conjunction with systemic hypotension (down to 50 mm Hg) to attenuate forebrain blood flow in the animal (Pulsinelli and Brierley, 1979). Hypotension is induced by arterial or central venous exsanguinations along with CCA clamping. The occluding clamps are removed and the shed blood is then used to reperfuse the brain (Smith et al., 1984). Injury to specific brain areas depends on the duration of exposure to the ischemic conditions. The hippocampus, caudate-putamen, and cerebral cortex can all be affected, depending on the duration of the ischemia (Smith et al., 1984). This occlusion technique can produce a reversible and reliable forebrain ischemia.
Four-vessel occlusion model
This model is carried out in two stages. First, reversible clamps are loosely placed around the CCA and the vertebral arteries are electro-cauterized (Pulsinelli and Brierley, 1979). After 24 hours, the CCA clamps are tightened (Pulsinelli and Brierley, 1979). This results in loss of body responsiveness, with approximately 75% of animals losing their righting reflex. Within minutes after CCA clamping, isoelectric activity appears on electroencephalography (EEG). Reperfusion is allowed when the clamps on the CCA are removed, at which point animal activity is restored. Histopathology reveals that neuronal damage occurs within 20 to 30 minutes of ischemia. Damage usually appears in the hippocampus, striatum, and posterior neocortex (Pulsinelli and Brierley, 1979). Unlike the two-vessel method, this method is only partly reversible because the vertebral arteries are cauterized. Another drawback to this model is that it has a higher risk of inducing seizures, which voids data if seizure is not the research target of the study.
Stem Cell/Neural Progenitor Transplantation in Animal Models
An appropriate selection of the animal model is important for reaching the goal of an investigation. For example, different stroke models may be suitable for investigations on neuroprotection or regeneration or neurovascular/neural network repair. An ischemic insult that damaged sub-cortical regions including the hippocampus and dentate gyrus may not be feasible for a study relying on the neurogenesis in the subgranular zone (SGZ). In addition to test the efficacy of cell therapies, one of the goals of preclinical cell transplantation studies is to optimize transplantation parameters so that the transplanted cells survive, migrate, differentiate and integrate with host cells appropriately in vivo. Two important parameters to be considered for transplanting any cell type are the administration route and the timing of delivery. These parameters may vary by cell type and the model of ischemic stroke. Before translating cell therapy into human patients, transplantation parameters need to be verified in different animal models. The STEPS committees have set out guidelines for preclinical studies pointing out the need for a well-characterized cell population, dose-response studies, and tests in different models of at least two species (de Mello et al., 2015).
Intracerebral/Intracranial transplantation
Identifying a favorable microenvironment for stem cell transplantation allows for increased survival of the graft. Some have suggested that transplanting cells into the penumbra is a more successful approach because the penumbra provides a more favorable microenvironment for the graft (Johnston et al., 2001; Theus et al., 2008a; Wei et al., 2005). An alternative for avoiding stem cell transplantation into the cytotoxic stroke core is to transplant cells into the hemisphere contralateral to the infarct (Modo et al., 2002b). It was shown in rats that a greater percentage of cells survived in the contralateral somatosensory cortex and striatum compared to those from ipsilateral or intraventricular injections (Modo et al., 2002b). This strategy may be useful based on the observation that endogenous neural stem cells and exogenous transplanted cells have the ability to migrate to the ischemic infarct region (Arvidsson et al., 2002; Li et al., 2008a; Modo et al., 2004; Zhang et al., 2009b).
Our recent investigation on the cell fate after stroke, however, provided a different rationale for selecting the transplantation site. We showed in the sensorimotor cortex stroke and several other stroke models that inside the ischemic core, normal or even higher levels of BDNF and VEGF remained for many days after stroke. Some NeuN-positive cells with intact ultrastructural features of synapses and axons resided in the core 7–14 days post stroke. BrdU-positive but TUNEL-negative neuronal and endothelial cells were detected in the core where extensive extracellular matrix infrastructure developed. Active regenerative niche was identified inside the core many days after stroke. These data suggest that the ischemic core is an actively regulated brain region with residual and newly formed viable neuronal and vascular cells acutely and chronically after at least some types of ischemic strokes (Jiang et al., 2016). The microenvironment of ischemic core several days and weeks after stroke provides certain cellular support, blood flow supply and strong trophic factor endorsements for cells to survive while the remaining and regenerating neurovascular infrastructure may be utilized as therapeutic targets for repairing damaged neurovascular unit and neuronal networks. With recent progress in priming transplanted cells using the preconditioning strategy, preconditioned cells are more tolerant and survive better even after transplantation into the ischemic core (Theus et al., 2008a; Wei et al., 2013; Wei et al., 2015). It appears that for the purpose of tissue repair, the development of transplanted cells with greater survival and regenerative properties will have the advantage of implanting cells into the brain area(s) where they are needed (Fig. 5C).
Intravascular administration
Stem cells may also be administered systemically through the vasculature. While this strategy is less invasive and does not necessitate craniotomy, vascular administration adds the requirement that cells must cross the blood brain barrier and home to the brain injury site. Vascular delivery of cells can be either intravenous or intra-arterial. There are advantages and disadvantages to both methods. While less invasive than intra-arterial delivery, the intravenous route delivers the cells through the systemic and pulmonary circulations where cells are more likely to be entrapped in other organs (spleen, liver, and lungs). Intra-arterial delivery, on the other hand, circumvents the systemic circulation. Li and colleagues reported that 21% of cells entered the brain with intra-arterial administration compared to only 4% of cells with intravenous administration (Biernaskie et al., 2001). Similarly, Guzman and colleagues reported that 1300 cells/mm2 populate the infarct area after intra-arterial administration compared to only 74 cells/mm2 after intravenous delivery (Guzman et al., 2008). Bone marrow mesenchymal cells systemically infused into ischemic rats have been reported to migrate to the site of injury (Eglitis et al., 1999; Samper et al., 2013). Some reports also showed potential neuronal differentiation of the transplanted cells after intravascular administration (Chen et al., 2001a). The homing of BMSCs to the brain lesion site, however, is generally very low (~1%) (Arbab et al., 2012) and neuronal differentiation is uncertain or difficult for these multipotent cells although some recent work reported improved differentiation from these cells (Kitada, 2012). Intravenous injection of human neural stem cells into an ischemic rat led to incorporation of some cells into the ischemic infarct. However, transplanted stem cells were also trapped in other organs such as the kidney, lung, and spleen. Within the brain, transplanted neural stem/progenitor cells were also found in the cortex, along the corpus callosum, and in the hippocampus (Chu et al., 2003).
Trophic factors may improve the homing of transplanted cells to the brain region. For example, it was demonstrated that the intravascular injection of bone marrow stem cells coupled with subsequent injections of granulocyte-colony stimulating factor (G-CSF) and stem cell factor (SCF) increased cell homing to the brain and neuronal differentiation (Corti et al., 2002; Piao et al., 2009). With G-CSF and SCF, more transplanted bone marrow cells could arrive at the ischemic brain, suggesting that bone marrow-derived stem cell therapy supplemented with other neurotrophic factors is a potential strategy in the stroke treatment.
This is also supported by our observation that a parathyroid hormone (PTH) can mobilize stem cells from the bone marrow and promote the homing of these cells to the brain. We showed that after 6 days of PTH treatment, there was a significant increase in bone marrow derived CD-34/Fetal liver kinase-1 (Flk-1) positive endothelial progenitor cells (EPCs) in the peripheral blood. PTH treatment significantly increased the expression of trophic/regenerative factors including VEGF, SDF-1, BDNF and Tie-1 in the brain peri-infarct region. Angiogenesis, assessed by co-labeled Glut-1 and BrdU vessels, was significantly increased in PTH-treated ischemic brain compared to vehicle controls. PTH treatment also promoted neuroblast migration from the subventricular zone (SVZ) and increased the number of newly formed neurons in the peri-infarct cortex (Wang et al., 2014).
In any event, the route of intravenous administration has been effective in preclinical and clinical trials. In one study, IV administration of cord blood stem cells in rat stroke animals resulted in improvement in motor functional recovery as assessed by the Rota-rod test (Chen et al., 2001b). Similarly, intra-arterial administration of mesenchymal stem cells improved the NSS score and performance in the adhesive-removal test of stroke rats (Biernaskie et al., 2001). IV administration of autologous MSCs has already been shown to be safe and appears to be effective in small clinical trials in human stroke patients (Suarez-Monteagudo et al., 2009; Wise et al., 2014). The PTH-treated mice mentioned above showed significantly better sensorimotor functional recovery compared to stroke controls. It is possible that mobilizing endogenous bone marrow-derived stem cells/progenitor cells is an alternative regenerative treatment after ischemic stroke (Wang et al., 2014).
Intranasal administration
The intranasal delivery route is a non-invasive method for bypassing the blood-brain barrier (BBB). It has been used clinically and in pre-clinical research to administer drugs, peptides/proteins, and a variety of factors (Fig. 7A). In cell transplantation therapy, intranasal delivery of stem cells is a new avenue of investigation. Intranasal delivery offers two major advantages that show great clinical potential for the treatment of stroke. Transplanted cells essentially move from the nasal mucosa into the CNS through the cribriform plate and migrate into the brain parenchyma along the olfactory neural pathways and blood vessels (Fig. 7A). In this procedure, the membrane permeation enhancers (like hyaluronidase) are often needed (Lochhead and Thorne, 2011). It was shown that rat MSCs reached the brain only one hour after intranasal delivery through either the rostral migratory stream (RMS) or the cerebrospinal fluid (CSF) (Danielyan et al., 2009). Complete reproducibility of cell distribution, however, still need verification and improvement for different cells.
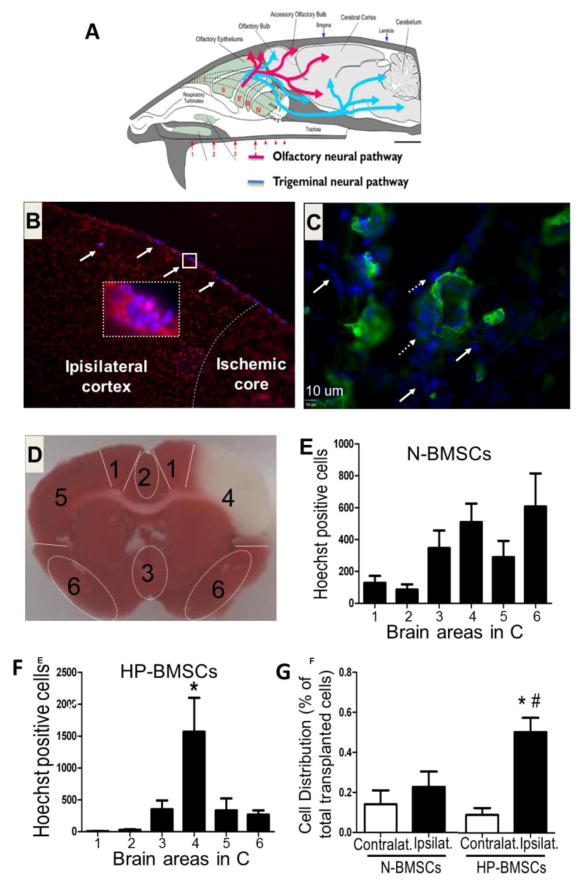
Figure 7. Distribution of BMSCs after intranasal delivery into the ischemic brain and the effect of hypoxic preconditioning.
The intranasal administration is an established method for drug delivery. A. The drawing illustrates how therapeutics may bypass the BBB via from the intranasal space and distribute in brain regions. B. Immunohistochemical staining reveals the distribution of transplanted cells after intranasal delivery of BMSCs in a stroke mouse brain. BMSCs (blue color from pre-labeled Hoechst 33342) were seen in the cortex near the ischemic core. The morphology of pre-labeled cells can be better seen under higher magnification (400 X, magenta cells, inserts images). C. collagen IV (green) was used to show brain blood vessels. Transplanted cells (blue) were found inside vessels, lining vessels (dashed arrows), or deposited outside the vessels (arrows). D. Distribution of BMSCs in different brain regions was measured and quantified by counting Hoechst 33342 dye positive cells in different brain regions. The assigned number for each brain area is shown in the brain section stained with TTC. Area 4 is the ischemic core region. E and F. N-BMSCs or HP-BMSCs (1 X 106) were delivered via intranasal administration 24 hrs after stroke. One and half hrs later, host brain cells (propidium iodide, PI, red, see B) and transplanted BMSCs (Hoechst 33342, blue) were counted. N-BMSCs showed a random distribution pattern (E) while HP-BMSCs (F) selectively home to the ischemic cortex (area 4 in D). G. The bar graphs summarize the percentage of cells that reached the ischemic brain region 1.5 hrs after intranasal delivery compared to all the cells found in the other brain region. Significantly higher percentage of HP-BMSCs was seen in the ispilateral side of the brain. N=6, *. p<0.05 vs. HP-BMSC in contralateral brain, #. p<0.05 vs. N-BMSC in the ispilateral brain (I think * and # need to be clarified). Revised from Figures in Wei et al., 2013 in compliance with the journal’s copyright policy.
We demonstrated for the first time that hypoxic preconditioning is a promising or even essential strategy for intranasal delivery of stem cells and neural progenitor cells (Hu et al., 2011b) (Fig. 7B–7G). In focal ischemic stroke mice, intranasally delivered hypoxia preconditioned (HP) BMSCs arrived at the ischemic cortex and deposited outside of vasculatures only 1.5 hrs after administration. In comparison to non-HP BMSCs prepared under normoxic condition (N-BMSCS), HP-treated BMSCs (HP-BMSCs) expressed high levels of genes associated with migratory activities, including the CXC chemokine receptor type 4 (CXCR4), matrix metalloproteinase 2 (MMP-2), and MMP-9. HP-BMSCs showed enhanced migratory activities in vitro and increased homing to the infarct cortex when compared with N-BMSCs. Three days after transplantation (four days after stroke), BMSC transplantation decreased cell death in the peri-infarct cortex. Mice receiving HP-BMSCs showed significantly reduced infarct volumes compared to stroke mice receiving N-BMSCs. HP-BMSC-treated mice performed much better than N-BMSC- and vehicle-treated animals (Wei et al., 2013). Furthermore, we demonstrated in a neonatal stroke model of rats that intranasally delivered BMSCs showed beneficial effects on brain development after stroke and that rat pups receiving HP-BMSCs developed better sensorimotor activity, olfactory functional recovery, and performed better in social behavioral tests (Wei et al., 2015). Recently, we showed that intranasal delivery of HP-BMSCs enhanced neurogenesis and angiogenesis after intracerebral hemorrhagic stroke in mice (Sun et al., 2015).
Timing of Stem Cell Transplantation
The environment of the brain after stroke is constantly changing. Timing of stem cell delivery is an essential parameter to be considered before transplantation. Unfortunately, the optimal time for transplantation in animal models and consequently in patients is poorly defined. Stem cell transplantation has been performed in the order of hours to weeks after stroke induction depending mainly on the investigator. Early time transplantation (e.g. <24 hours) after stroke has been tested and some reported neuroprotective effects (Chu et al., 2005a; Vendrame et al., 2004). In an investigation on intrastriatal transplantation of human NSCs, it was shown that cells transplanted at 2 days after stroke survived better than those transplanted 6 weeks after stroke (Darsalia et al., 2011). Transplanting greater numbers of grafted NSCs did not result in a greater number of surviving cells or increased neuronal differentiation. It is now widely believed that several days after the onset of stroke is an appropriate time window for cell transplantation therapy. Based on the understanding of the time course of excitotoxicity, brain edema, inflammatory reaction, and trophic factor expressions, we and many others have typically transplanted cells at 7 days after stroke, at which point the extracellular glutamate has returned to lower levels, brain edema subsided, and the microenvironment in the post-stroke brain has entered into a pro-regeneration stage (Schmidt and Minnerup, 2016). Coincidentally, reported clinical trials with autologous MSCs have been limited by the time needed to expand cells to the point that an appropriate cell numbers are available, which normally takes several days or longer (Honmou et al., 2011; Lee et al., 2010). Very delayed time points of cell transplantation (several months after stroke) are also clinically relevant, but few pre-clinical investigations have focused on the chronic phases. More research remains to be carried out to delineate the optimal strategies for cell transplantation for chronic stroke patients.
Mechanisms Underlying Stem Cell Therapy
Neuroprotective effect and reduction of infarct volume
Investigations on transplantation of neural stem/progenitor cells at early and sub-acute phases after stroke report neuroprotective effects of reducing infarct volume (Takahashi et al., 2008; Wei et al., 2012; Wei et al., 2015). The infarct reducing effect can be observed in the acute phase after stroke. Using intracerebral injection and i.v. infusion, we and others have shown that BMSCs have the potential of reducing brain infarction and neuronal cell death and improving functional recovery after ischemic stroke. Since the homing of BMSCs to the brain tissues is very low after i.v. infusion, the mechanism of action of this treatment is likely due to increased diffusible trophic and growth factors such as BDNF, NGF and VEGF (Chau et al., 2014; Sun et al., 2015).
Endogenous repair mechanisms
Stem cell transplantation provides a supplement to the endogenous repair that may occur after ischemia. It is now generally recognized that the adult brain has the ability to regenerate neurons. As early as 1961, Smart first demonstrated that the adult mammalian brain showed neurogenic activity (Smart, 1961). It is apparent now that the adult brain has several niches for neurogenesis, particularly the subventricular zone (SVZ) lining the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus in the hippocampus (Altman, 1962, 1969; Kaplan and Hinds, 1977; Kempermann et al., 2004; Taupin and Gage, 2002). Under normal physiological conditions, neural progenitors from the SVZ migrate to the olfactory bulb (Kornack and Rakic, 2001) comprising the rostral migratory stream (RMS). These neural progenitors migrate in chains and differentiate into interneurons in the olfactory bulb where they functionally integrate themselves into the existing neural circuitry (Altman, 1969).
Several types of brain injuries including seizure, stroke, and traumatic brain injury can up-regulate progenitor proliferation (Ernst and Christie, 2006; Parent et al., 1997; Rola et al., 2006). Under ischemic conditions, proliferation of neural progenitors increases in the SGZ and the SVZ (Kuge et al., 2009; Li et al., 2008b; Li et al., 2010). Neural progenitors from the SGZ do not seem to migrate to areas of injury after stroke, but those from the SVZ are diverted laterally from the RMS to the site of the injury. Neurogenesis peaks at 3 and 4 weeks after ischemic insult in the SVZ and the SGZ, respectively. Migration of progenitors from the SVZ to the site of injury peaks at around 4 weeks (Kuge et al., 2009).
The adult brain’s endogenous neurogenic machinery requires a coordinated effort between the SVZ, chemotactic factors, and the vasculature to replace dead or dying cells. The process of neuroblast migration relies greatly on the upregulation of chemotactic factors at the injury site and the physical scaffolding provided by blood vessels. Cells of the neurovascular unit (neurons, astrocytes, and endothelial cells) and cells recruited from the systemic circulation produce chemotactic factors including but not limited to SDF-1α, BDNF, and VEGF (Behar et al., 1997; Imitola et al., 2004; Pencea et al., 2001; Robin et al., 2006; Thored et al., 2007; Wang et al., 2007). Ohab and colleagues emphasized the importance of the neurovascular niche in which neural progenitors associate with vasculature to migrate to the site of ischemic damage, showing that capillary endothelial cells in the infarct area release factors like SDF-1 and Angiopoietin-1 (Ang-1) (Ohab et al., 2006). Under normal conditions, approximately half of the cells migrating to the olfactory bulb are in close proximity and contact with vasculature (Bovetti et al., 2007) and this association continues when cells are diverted from the RMS. Progenitors migrate to the ischemic injury using blood vessels as a scaffold and chemotactic factors as a guide (Ohab et al., 2006). SDF-1 in particular is a potent chemo-attractant, which is upregulated in the stroke infarct in a gradient fashion to guide migrating cells (Li et al., 2008a). Migration from the SVZ to the infarct occurs in chains and as individual cells (Zhang et al., 2009b). It was shown that neural progenitors extending from the border of the SVZ towards the infarcted striatum at a rate of 28.67±1.04 μm/h (Zhang et al., 2009b). Doublecortin (DCX)-positive migrating progenitor cells move along with and cluster around the vasculature. It was observed that 35% of progenitors were within 5 μm of a blood vessel.
Nonetheless, the neurogenic activity induced new cells usually do not survive well during the migration and after arrival at the injury site (Capowski et al., 2007; Daadi et al., 2008a; Kim et al., 2007a; Roy et al., 2006; Yang et al., 2008). The repair capability of newly formed neurons is very limited and does not suffice for an effective tissue repair after brain injury (Grégoire et al., 2015). For example, it was calculated that about 80% of the newly born neurons die in the striatum stroke model and that endogenous regeneration only replaces about 0.2% of dead striatal neurons (Arvidsson et al., 2002). Thus, the need for novel therapies to replace lost tissue is immense. Using the spatial and cell type specific optogenetic technique, we showed in a transgenic mouse expressing the light-gated channelrhodopsin-2 (ChR2) in glutamatergic neurons that optogenetic stimulation of the glutamatergic activity in the striatum triggered glutamate release into SVZ region, increased proliferation of SVZ neuroblasts mediated by AMPA receptor activation and Ca2+ increases in these cells. After stroke, optogenetic stimuli to the striatum for 8 days promoted cell proliferation and the migration of SVZ neuroblasts into the peri-infarct cortex with increased neuronal differentiation and improved long-term functional recovery. It was suggested that the striatum-SVZ neuronal regulation via a semi-phasic volume transmission mechanism may be a therapeutic target to directly promote SVZ neurogenesis after stroke(Song et al., 2017)(Song et al., 2017).
Trophic support and attenuation of inflammation
Adult and embryonic stem cells can enhance the survival of the surrounding tissue by providing trophic factor support. Trophic factors secreted by transplanted cells can provide trophic support to the injured parenchyma (Calió et al., 2014; Dillon-Carter et al., 2002; Johnston et al., 2001). As an example, a large proportion of neural stem cells transplanted into the murine cochlea were found to express neurotrophins such as GDNF and BDNF. The release of these factors provided protection and support to the surrounding cells (Bang, 2016; Iguchi et al., 2003).
Transplanted adult marrow-derived MSCs release cytokines and trophic or growth factors that have autocrine and paracrine effects (Caplan and Dennis, 2006; Haynesworth et al., 1996; Malgieri et al., 2010). MSCs have been shown to secrete colony-stimulating factor-1 (CSF-1), stem cell factor, bFGF, nerve growth factor (NGF), and VEGF (Chen et al., 2002a; Labouyrie et al., 1999; Laurenzi et al., 1998; Majumdar et al., 1998; Villars et al., 2000). Such secreted factors reduce host cell death and attenuate inflammatory processes (Tse et al., 2003). Interestingly, injecting BMSC-conditioned media into the brain after stroke led to functional benefits, indicating that trophic support is a major mechanism by which MSCs contribute to functional recovery (Caplan and Dennis, 2006; Chen et al., 2002b; Li et al., 2002).
Cell differentiation, replacement and integration after transplantation
Cell differentiation and integration after transplantation is an important aspect of cell therapy. Neural cells have a greater potential to functionally integrate into the existing host circuitry if they differentiate into the appropriate neuronal phenotype (Lam et al., 2014). Several studies have examined the in vivo differentiation of transplanted stem cells (Fig. 8). In one systemic study, murine ES cell-derived neural precursors differentiated into different neuronal sub-types including cholinergic (1.4% of all grafted cells), serotonergic (1.8% of all grafted cells), GABAergic neurons (>50% of all grafted cells), and striatal neurons (1.4% of all grafted cells) after transplantation into the infarcted region. For the reason that is unclear, the percentage of excitatory glutamatergic neurons was not evaluated in this study. At 12 weeks after transplantation, 25% of the remaining transplanted cells were NeuN-positive while 8% were positive for glial fibrillary acidic protein (GFAP), a marker of astrocytes. Transplanted cells also exhibited voltage-gated Na+, Ca2+ and K+ currents. While continued proliferation after transplantation was detected, no tumors were detected at 4 or 12 weeks (Buhnemann et al., 2006).
Figure 8. Neural differentiation of transplantation of ES-NPCs and functional benefits after stroke.
Neural differentiation of transplanted HP-ES-NPCs was examined 14 days after transplantation into the ischemic rat brain. A. Hoechst 33342 staining (blue) illustrated transplanted HP-ES-NPCs in the ischemic region. HP-ES-NPCs differentiated in vivo into NeuN-positive cells (red) and GFAP-positive (green) astrocytes. The circular dotted line demarcates the transplantation area. B. High magnification image showing co-localization of NeuN (red) or GFAP (green) with Hoechst 33342 (blue). C–F. NeuN-positive cells and neurite distribution in the contralateral cortex (C and D) and ipsilateral cortex (E and F). In the contralateral non-ischemic cortex, normal neurons were stained with NeuN, appearing red/orange. Neuronal processes were labeled with neurofilament (NF, green). In the ipsilateral cortex, NeuN or NF double immunostaining with pre-labeled Hoechst 33342 (blue) was apparent under a fluorescence microscope. Many NeuN-positive cells (red or pink when co-labeled with Hoechst 33342) were surrounded by NF-labeled processes (green), although the distribution of these processes was less organized compared to the contralateral side. Note the substantially smaller ES-derived NeuN-positive cells in E and F compared to the native cells in C and D. Neurological and motor functions were examined after transplantation with no-HP control progenitor cells and HP-treated ES-NPCs. G. Neurological Severity Score was tested up to 35 days after transplantation. Normal non-ischemic rats have a NSS near zero while the NSS for rats several days after stroke was 9–10. Transplantation of either control cells or HP-progenitor cells improved functional recovery 21 days after ischemia while animals without ES cell transplantation had limited recovery. HP-ES-NPC transplantation, however, afforded significant functional recovery as early as 7 days after transplantation while at this time rats that received control cells showed no difference from culture media group. H. Cell transplantation enhanced motor ability after stroke. Scores were compared to baseline data generated before stroke and are represented as % of control. Animals receiving HP-ES-NPCs had accelerated motor function recovery; at day 7 and 35 after transplantation the testing score was significantly better than that of medium and control cell groups. #. P< 0.05 compared with MCAO only; *P< 0.05 compared with control cell transplant; n=5–8 rats per group. Adopted from Figure 8 in Theus et al., 2008a in compliance with the journal’s copyright policy.
Another study reported that 30% of transplanted human fetal neural stem cells survived 1 month after stroke in the striatum (Darsalia et al., 2007). Striatal-derived and cortical-derived neural stem cells from human fetal tissue were transplanted into the striatum of stroked rats. About 80% of the cells migrated from the original site of the transplant closer to the lesion. Unlike the aforementioned study with ES cell-derived progenitors, there was little proliferation (less than 1%) as measured by p-H3 and Ki67 staining. A high percentage of the cells differentiated into neurons expressing neural Hu protein D (HuD), calbindin, and parvalbumin, all markers of neuronal differentiation, but very few differentiated into astrocytes or oligodendrocytes (Darsalia et al., 2007).
Combined Approach of Optogenetics and Stem Cell Therapy
Studying the integration of differentiated cells into the host structures after transplantation is essential for a long-term characterization of the therapy. Earlier studies used electrophysiological approaches to investigate whether transplanted stem cell-derived neural (NS) cells functionally integrate within the established host brain neuronal circuits (Opitz et al., 2007; Scheffler et al., 2003; Wernig et al., 2004). However, electrophysiological stimulation and recording paradigms are cumbersome and lack temporal and spatial resolution for studying information processing in living multicellular networks. The advent of optogenetics has provided tools that allow in vivo probing and mapping of neuronal circuits with very high temporal and spatial resolution. These tools allow for accurate control and observation of biological process within target cells using specific light stimulation (Nagel et al., 2003; Nagel et al., 2005). Optogenetics employ two classes of channels that transduce light of specific wavelengths into a biological response. When expressed in cells, these channels open in response to light of the proper wavelength and conduct ions altering the membrane potential. This leads to light-induced excitation (Channelrhodopsin-2, ChR2; 470 nm) or inhibition (halorhodopsin; 580 nm) of neurons with controlled frequency and timing. ChR2 and eNpHR can be genetically targeted to specific populations or sub-populations of cells (providing high spatial resolution), can control cell function on a millisecond scale (high temporal resolution down to a single action potential), and have proven to function in culture, acute brain slices, and living animals.
Optogenetic actuators has been used to accurately map brain circuits (Arenkiel et al., 2007; Arrenberg et al., 2009; Han et al., 2009; Kastanenka and Landmesser, 2010; Kramer et al., 2009), dissect pathways of learning and fear (Huber et al., 2008; Johansen et al., 2010), and understand the role of neural cells in pathology and physiology (Gourine et al., 2010; Kravitz et al., 2010). Stem cell therapy has also benefited from the exponential growth in the optogenetics field. Integrating optogenetics into cell based therapies through the over-expression of ChR2 in stem cell-derived neural precursors, it was demonstrated that ChR2 expressing human ES-cell derived neurons can receive excitatory and inhibitory post-synaptic current in acute brain slices (Weick et al., 2010). In another study where ChR2 was over-expressed in mouse ES cells, optical excitation increased the expression of Nestin and β–III-tubulin in ChR2-ES cell-derived neural precursors and neurons, respectively (Stroh et al., 2011). Optogenetic evidence showed that neural stem cell-derived dopaminergic neurons functionally integrate in the denervated striatum in an animal model of Parkinson’s disease (Tonnesen et al., 2011). This technology offers an invaluable set of tools for studying stem cell differentiation and in vivo integration after transplantation.
Limitations and Potential Adverse Effects
Immune responses in cell translantation therapy
Suppression of inflammation is an important consideration in cell-based treatment of neurological disorders. Cell therapies can modulate the host inflammatory response and establish a favorable microenvironment that prevents further endogenous cell death due to inflammation. By modulating the inflammatory processes, transplanted cells increase their own chances of survival. Otherwise, such processes can result in failure to survive or engraft, bleeding, altered immune responses and can reduce or even eliminate the therapeutic effects of transplantation (Strecker et al., 2016).
Inflammation may affect the ability of transplanted cells to replace damaged neurons. In a study on transplanted neurospheres derived from mouse ES cells into the striatum of allogeneic mice, some mice were treated with cyclosporine A to suppress the immune response. No difference was observed between the groups in graft size or cell survival, but fewer inflammatory cells were attracted to the graft in the cyclosporine A-treated animals (Ideguchi et al., 2008). In addition, engrafted cells were more likely to be positive for NeuN and negative for GFAP in the cyclosporine A-treated animals, suggesting that the immune response created an environment that favored glial differentiation over neuronal differentiation. In in vitro experiments, the addition of the inflammatory factor interleukin 6 (IL-6) to differentiating cells resulted in less cells positive for the β-III tubulin clone Tuj1 and more GFAP-positive cells, further suggesting that inflammation can suppress neuronal differentiation.
Much of the research on the suppression of inflammation by stem cell therapy has focused on MSCs, which have been shown to sup ress innate and adaptive immune responses (Broughton et al., 2015). While there is a concern that the suppression of the immune system may lead to infection or tumor cell proliferation, there is a consensus that human trials should move forward using MSCs in the treatment of multiple sclerosis (MS) (Siatskas et al.). We observed that transplantation of BMSCs had a great effect of suppressing microglia activity in the brain(Wei et al., 2012). MSCs can inhibit inflammation in multiple ways. In vitro, MSCs can reduce proliferation of allogeneic or even xenogeneic peripheral blood mononuclear cells (PBMCs) (Aggarwal and Pittenger, 2005) with specific effects noted on the proliferation of both T-lymphocytes (Augello et al., 2005; Di Nicola et al., 2002; Zappia et al., 2005) and B-lymphocytes (Augello et al., 2005; Corcione et al., 2006). MSCs also reduce tumor necrosis factor alpha (TNF-α) in dendritic cells (DCs) and interferon gamma (INF-γ) in differentiating TH1 cells and natural killer cells (Aggarwal and Pittenger, 2005). They can also increase the anti-inflammatory interleukin IL-10 in DCs (Aggarwal and Pittenger, 2005) and macrophages (Nemeth et al., 2009), as well as IL-4 in differentiating TH2 cells (Aggarwal and Pittenger, 2005). MSCs do not, however, seem to have any modulating effect on the in vitro activation of microglia (Tambuyzer et al., 2009). In vivo, the immunosuppressive effects of MSCs are achieved regardless of how the cells are delivered, whether they are injected into the bloodstream, peritoneum, or parenchyma of the affected tissue (Siatskas et al.).
While most human transplantation studies have used autologous MSCs (Bang et al., 2005b; Honmou et al.), the promising data with allogeneic MSCs suggests that these cells need not come from a genetically identical source. In fact, some clinical trials are using cells from allogenic sources. Additionally, protocols have been devised to derive MSCs from human ES cells (Barberi et al., 2005; Lian et al., 2007; Olivier et al., 2006; Tan et al.). Like MSCs, these hES cell-derived cells can differentiate into bone, cartilage, and fat. In co-culture experiments, they can suppress lymphocyte proliferation and activation (Tan et al.). Interestingly, in a xenogenic transplant model using BALB/C mice with liver inflammation, undifferentiated hESCs induced antibody production, while MSCs derived from those cells did not (Tan et al.). This further supports the idea that MSCs are immune privileged. In addition, MSCs derived from hES cells reduced the infiltration of inflammatory cells to the injured liver area.
Another strategy to enhance cell-based therapies is co-transplantation of MSCs with neural progenitor cells (Liu et al., 2014). Transplanted allogeneic ES cell-derived oligodendrocyte progenitors (OPCs) into a mouse model of MS with MSCs showed better survival of transplanted cells and greater levels of re-myelination were observed compared to the levels of OPCs alone (Cristofanilli et al.). Microglial infiltration was also reduced when MSCs were included, suggesting that syngeneic MSCs do not inhibit microglia. Given the conflicting results, it is unclear whether or not allogeneic MSCs would work well in a co-transplant strategy.
Immune rejection
A potential hurdle in stem cell transplantation is the rejection of transplanted cells. Accumulating evidence suggests that culturing human ES cells on animal-derived substrates or with animal-derived media products increase their immunogenicity because of their increased expression of a non-human sialic acid residue, Neu5Gc (Byrne et al., 2015; Martin et al., 2005). This has pushed companies involved in the biotechnology of human ES cells to create media products and substrates that are fully defined and completely animal-product free. While clinical trials have been carried out with cells expanded in xenogenic serum without any reported ill effects from the animals products (Bang et al., 2005b; Lee et al., 2010), the removal of animal products is still preferred.
As with tissue and organ transplants, allogenic stem cells can be rejected and destroyed by the host immune system. Rejection is modulated by the expression and detection of unmatched human leukocyte antigen (HLA) proteins, specifically major histocompatibility (MHC) proteins (Litchfield et al., 1997). Host T-lymphocytes recognize these proteins, primarily MHC-I, on the donor cells and sensitize the immune system, causing it to attack the transplanted cells. The brain has been historically seen as an immune privileged location, but even with allogenic cells or cells directly injected into the brain (Simpson, 2006; Sloan et al., 1991), rejection might still occur and may require the use of immune suppression for continued benefit (Olanow et al., 2003). On the other hand, an immune suppression treatment may show detrimental effect on endogenous healing. For example, some immune responses can be neuroprotective and necessary for endogenous neurogenesis (Beers et al., 2008; Chiu et al., 2008; Ziv et al., 2006a; Ziv et al., 2006b). Immune suppression may also decrease inflammatory signals that attract cells to the injury site (Ben-Hur et al., 2003). Moreover, immune suppression may increase the risk of tumor formation (Dunn et al., 2002; Dunn et al., 2004). Because of these issues associated with long-term immune suppression (Marcen, 2009), the use of such suppression should be avoided in stem cell transplantation.
In some cases, short-term measurements of immune response to transplanted cells seem to suggest that immune suppression is not necessary and may even reduce graft survival (Modo et al., 2002a). Some transplanted cells have been reported to survive via immune evasion rather than being immune privileged cells such as MSCs (Ankrum et al., 2014). BMSCs and adult neural precursors have been shown to modulate the immune system, a functionality that might be lost with systemic immune suppression (Ben-Hur, 2008; Karussis et al., 2008). However, the immune response can also be delayed as host antigen-presenting cells pick up on the foreign MHC peptides and sensitize the immune system to them (Drukker and Benvenisty, 2004; Gould and Auchincloss, 1999; Rogers and Lechler, 2001). The expression level of MHC proteins is a major determining factor in graft survival and cells expressing fewer mis-matched MHC proteins will likely demonstrate the best survival. Human ES cells express only very low levels of MHC-I in an undifferentiated state. However, MHC-I is upregulated when cells are differentiated and can be highly upregulated in undifferentiated or differentiated cells by interferons (Drukker et al., 2002), which are expressed in endogenous immune responses such as inflammation. It is possible to reduce MHC expression in transplanted cells (Hacke et al., 2009), but genetic changes and viral vectors can introduce their own problems.
Tumor formation
One of the most prominent concerns in cell therapy is the propensity of transplanted cells to form tumors. Due to their cancerous origin, there are some fears that hNT cells can produce tumors, but the retinoic acid treatment used to obtain hNT cells from N2N cells seems to suppress their tumorigenicity (Maerz et al., 1998; Newman et al., 2005). A study has shown that iPS cells derived from B6 mouse embryonic fibroblasts formed teratomas that were immune-rejected after transplantation in B6 mice (Zhao et al., 2011). On the contrary, ES cells derived from B6 mice form teratomas in B6 recipients without any evident immune rejection, suggesting a specific immune response to the iPS cells. Pluripotent cells, such as ES or iPS cells, can form teratomas or teratocarcinomas when implanted in a living host [16, 161]. These two terms (teratomas or teratocarcinomas) have been used interchangeably in the past, but standard definitions have begun to emerge (Blum and Benvenisty, 2009; Damjanov and Andrews, 2007; Lensch and Ince, 2007). Teratomas are benign tumors which contain tissues from all three germ layers and are believed to be formed from undifferentiated cells that remain karyotypically normal. As teratomas mature, they generally stop growing, but the damage done to the surrounding tissues can be fatal. Most of this damage is simply related to tissue displacement and pressure injuries. Teratocarcinomas, on the other hand, are believed to be formed from transformed or culture adapted pluripotent cells. These tumors contain highly proliferative undifferentiated cells known as embryonal carcinomas (ECs) and are aggressively malignant. ECs were first discovered in spontaneous testicular carcinomas (Stevens and Little, 1954) and are believed to be the malignant counterparts to ES cells. In fact, as ES cells become karyotypically abnormal in cell culture, they acquire many of the same mutations as those seen in ECs (Andrews et al., 2005; Baker et al., 2007; Harrison et al., 2007). Human ES cells are routinely screened for karyotypic changes, but small additions and deletions within a chromosome, changes in mitochondrial DNA, and epigenetic changes that occur in culture can also select for populations of undifferentiated stem cells (Maitra et al., 2005; Werbowetski-Ogilvie et al., 2009). Screening for such small mutations will likely be necessary if ES cells are to be used in clinical settings.
iPS cells have also been shown to have a risk of tumorigenicity when transplants are contaminated with undifferentiated cells. The two major concerns for tumorigenicity of iPS cells have been mutagenesis at viral insertion sites and continued expression of exogenous genes used for reprogramming (Robbins et al.). c-Myc has been of particular concern as a possible oncogene (Okita et al., 2007). In order to minimize these issues, transgene-free iPS cells have been created (Gonzalez et al., 2009; Okita et al., 2008; Sommer et al., 2009; Stadtfeld et al., 2008) and some protocols have found ways to induce pluripotency without the use of c-myc (Huangfu et al., 2008; Nakagawa et al., 2008). In addition, the tissue origin of iPS cells seems to play a role in tumorigenicity, with derivation from mouse embryonic fibroblasts or gastric epithelial cells mirroring ES cells and derivation from hepatocytes or tail fibroblasts both increasing the risk of tumor formation (Miura et al., 2009). As with teratocarcinomas, iPS cell tumorigenicity appears to be a result of cells that are resistant to differentiation, possibly because of incomplete reprogramming.
Interestingly, tumor formation from contamination of undifferentiated cells is dependent on the species of both the graft and host (Erdo et al., 2003). When undifferentiated murine cells were xenotransplanted into a rat brain, tumors were small and relatively rare, occurring in 2 out of 22 animals. After transplantation to the contralateral hemisphere, these undifferentiated cells migrated to the ischemic infarct along the corpus callosum and differentiated into neurons. In mouse brain, however, undifferentiated mouse cells produced large malignant teratocarcinomas in the majority of animals, without migration or replacement of ischemic tissue. These tumors formed even after efficient neural differentiation in which a very small portion of cells remained pluripotent.
While xenotransplantation is unlikely as a viable cell therapy strategy in humans, these results do raise doubts about the applicability of xenotransplantation studies. Even if differentiation with a particular protocol is efficient enough to avoid tumor formation in xenotransplantation, a small number of contaminating cells may still induce tumorigenesis in homologous transplantation. Even very small numbers of contaminating undifferentiated ES cells caused tumors in nude mice (Lawrenz et al., 2004), further suggesting that any safe cell therapy using pluripotent cells will have to avoid such contamination.
We have empirically determined from our work that if ES cells are efficiently pre-differentiated into progenitors in vitro, their risk of tumor formation is drastically reduced. Many protocols have been tested to optimize the homogeneity of the transplant. While a few transplantation studies reported tumor formation, most transplantation studies using progenitors found no tumorigenicity (Arnhold et al., 2000; Brustle et al., 1999; Reubinoff et al., 2001; Theus et al., 2008a; Wei et al., 2005). Nonetheless, it is difficult to differentiate pluripotent stem cells with 100% efficiency. Alternatively, methods to remove any undifferentiated cells from the transplant are being developed. In some cases, cells are sorted by staining for a pluripotency marker (Shibata et al., 2006) or by adding GFP-conjugated proteins that allow for the removal of undifferentiated cells (Eiges et al., 2001). Other strategies would cause apoptosis through the use of ceramide analogues (Bieberich et al., 2004) or survivin blockers (Blum et al., 2009), both of which selectively target pluripotent cells. A “suicide gene” may be included in transplanted cells so that they can be induced to undergo apoptosis if a tumor begins (Jung et al., 2007). With further progress in this area, it may be possible that the suicide gene will be turned on only in tumor-forming cells but not normally differentiating cells so the transplantation benefits will be achieved without the adverse consequence of tumor formation.
Stem Cells Transplantation in Stroke Clinical Trials
Human trials with stem cell transplantation for treating stroke are ongoing. An early study demonstrated that hNT human neuronal cells can engraft into the stroke region and survive for more than two years in a human patient (Nelson et al., 2002). Other cell transplantation studies in Parkinson’s disease have reported surviving cells up to 14 years after transplant (Hallett et al., 2014). However, concerns about the cancerous origin and the possibility of tumor formation with hNT cells persist and no organization appears to have moved into larger scale trials with this cell type. Bone marrow derived cells were shown to be promising in terms of safety and initial efficacy (Suarez-Monteagudo et al., 2009). A number of Phase I and II clinical trials using cell populations derived from bone marrow have begun (Table 1). Early results suggest that IV injection of mesenchymal stem cells (MSCs) does not cause significant adverse effects and improves measures of function such as the Barthel Index (BI) (Bang et al., 2005b), modified Rankin Scale (mRS) (Lee et al., 2010), and National Institutes of Health Stroke Score (NIHSS) (Honmou et al., 2011). Conflicting data also suggest that MSC injection may not improve functional outcomes (Prasad et al., 2014). These studies used autologous MSCs that were expanded in culture before use. While no adverse effects from animal products were reported, cells expanded in autologous serum, leading to faster cell expansion and allaying fears of xenogeneic contamination [257].
A Phase 1 trial (ReNeuron trials) is currently being carried out with a conditionally immortalized fetal neural stem cell line (Pollock et al., 2006; Stevanato et al., 2009). No adverse effects have been reported to date. Although the ReNeuron trial is using neural stem cells, these cells are not expected to engraft/replace lost cells in the stroke region. StemCells, Inc., on the other hand, is currently testing a highly purified human neural stem cell product that they expect to engraft in the long-term. These cells are currently bring tested for spinal cord injury and neurodegenerative disorders, with plans for a stroke trial in the future.
Stem cell transplantation in stroke provides a promising avenue of exploration, but further studies are needed before such therapy becomes a reality. The stem cell therapies as an emerging paradigm in stroke meetings have brought together scientists, clinicians, regulators, and industry representatives to create guidelines for preclinical and clinical studies. The initial guidelines were published in 2009 (Therapies, 2009), followed by an update in early 2011 (Savitz et al., 2011). A summary of the use of stem cells in stroke also concludes its potential as an emerging therapy in the near future (Liu et al., 2014).
Stem Cells Transplantation in Stroke Combined with Other Disorders
As a clinically relevant issue, some translational studies have reported adverse effects of BMSC therapy such as atherosclerosis and arteriosclerosis in stroke diabetes animals (Chen et al., 2011). Niaspan was shown to reduce these adverse effects of BMSCs in type one diabetic rats (Yan et al., 2013). In future translational studies, efficacy to treat co-morbidities such as stroke and diabetes should be considered and tested.
Conclusions
The prospect of using stem cell therapy for stroke remains an exciting and promising avenue of exploration. While a great deal of preclinical work has increased knowledge and feasibility of possible cell transplantation therapies for CNS disorders including stroke, the ultimate goals of this research area remains to be satisfactorily achieved. The promise of stem cell therapy offers hope for both protection of neural tissue in the acute phase of stroke and replacement of lost tissues (either through direct replacement or enhancement of endogenous mechanisms) in the chronic phase. As research moves forward, new technologies are consistently bring incorporated into the field. Prior to the derivation of iPS cells, it was difficult to imagine a way to amass enough HLA-matched neural precursor cells for an effective cell therapy, but now, there are many laboratories using these cells in preclinical studies. The recent exciting discovery of direct reprogramming adult somatic cells into mature and functional neurons provides another bright future with an alternative approach of repairing damaged brain tissues using endogenous resources without the need of exogenous cells (An et al., 2016; Guo et al., 2014). A number of clinical trials investigating stem cell therapy for stroke are now ongoing. Clinical challenges may include more complicated situations such as co-morbidities which can affect the efficacy and effectiveness of a cell therapy. It may take coordinated basic and clinical efforts before cell therapy can become a standard stroke treatment.
Table 2.
Cell transplantation therapy research in ischemic and hemorrhage stroke models.
| Administration route | Initiation time point | Cell type/Dose | Species/Model | Outcome | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Intracerebral | 1 months | NT2N line/0.8m | Rat/tMCAO | Motor function recovery | Biobridge, cell replacement, induced growth and trophic support | (Borlongan et al., 1997; Liska et al., 2017) |
| 7 days | hBMSC | Rat/tMCAO | Sensorimotor recovery | Induced growth and trophic support | (Zhao et al., 2002); (Burns et al., 2009); (Kurozumi et al., 2004) | |
| 14 days | MHP36 line/0.2m/8 μl | Rat/tMCAO | Sensorimotor recovery | Cell replacement | (Veizovic et al., 2001); (Modo et al., 2002b) | |
| 7 days | hBMSC | Rat/ICH | Sensorimotor recovery | Induced growth and trophic factors | (Nagai et al., 2007) | |
| 7 days | hNSC/ | Mouse/ICH | Motor function recovery | Cell replacement | (Lee et al., 2007) | |
| 7 days | hNSC/0.8m/2 μl | Rat/Endothelin Stroke | Motor function recovery | Cell replacement | (Abeysinghe et al., 2015) | |
| 7 days | hES/0.2m/4 μl | Mouse/Barrel Stroke | Sensorimotor recovery | Cell replacement | (Drury-Stewart et al., 2013) | |
| 7 days | rESC/0.1m | Rat/MCAO | Survival and differentiation of grafts | Cell replacement | (Buhnemann et al., 2006) | |
| Intracranial | 7 days | miPS/0.4m/4 μl | Rat/Barrel Stroke | Sensorimotor recovery | Cell replacement | (Chau et al., 2014) |
| Intravenous | rBMSC/1m/1 ml | Rat/tMCAO | Reducing hemorrhagic transformation, motor function recovery | Inhibit endothelial dysfunction | (Nakazaki et al., 2017) | |
| 24 hrs | hUCBC/ | Rat/tMCAO | Sensorimotor recovery | Cell replacement | (Chen et al., 2001b) | |
| 24 hrs | rMSC/3m | Rat/tMCAO | Sensorimotor recovery | Reducing cell death, induced growth and trophic factors | (Chen et al., 2003) | |
| - | hNSC | Rat | - | Cell replacement | ; (Chu et al., 2005b) | |
| hNSC/5m/500 μl | Rat/ICH | Sensorimotor recovery | - | (Jeong et al., 2003) | ||
| Intra-arterial | 1 hrs | rBMSC/1m/1 ml | Rat/tMCAO | Reducing infarct | Induced growth and trophic factors | (Toyoshima et al., 2015) |
| 24 hrs | hBMSC/1,000 | Sensorimotor recovery | Reducing inflammation | (Fukuda et al., 2015) | ||
| Intranasal | 6 hrs | rBMSC/1m/100 μl | Rat/Barrel Stroke | Reducing infarct, sensorimotor recovery, improved olfactory functions, social and neuropsychiatric benefits | Induced growth and trophic factors | (Wei et al., 2015) |
Abbreviation in the Table: BMSC, bone marrow mesenchymal stem cell; ES, embryonic stem cells; ICH, intracerebral hemorrhage; iPS, induced pluripotent stem cells; m indicates million cells; MCAO, middle cerebral artery occlusion; NSC, neural stem cells; UCBC, umbilical cord blood cells.
Table 3.
Stroke clinical trials using stem cell therapy.
| Trials | Initiation year | Country/Region | Cell source/Dose | Administration route | Population | Outcome | Status | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| Safety | NT2/D1 | Intracerebral | Basal ganglia stroke | Feasible | Completed | (Kondziolka et al., 2000),(Nelson et al., 2002) | |||
| 2001 | USA | NT2/D1 | Intracerebral | Stroke patients | Feasible with small risk of seizure | Completed | (Kondziolka et al., 2005) | ||
| MSC | Intravenous | MCA | (Bang et al., 2005a) | ||||||
| 2005 | USA | ES | Intracerebral | Ischemic stroke patients | 2/5 patients showed improvements | Terminated | (Savitz et al., 2005) | ||
| 2008 | India | BMMNC | Intrathecal | Stroke patients | Completed | NCT02065778 | |||
| 2009 | Cuba | BMSC | Intracerebral | Stroke patients | Good tolerance and safety | Completed | (Suarez-Monteagudo et al., 2009) | ||
| 2010 | Brazil | BMMNC | Intra-arterial | Nonacute ischemic stroke | Feasible and safe | Completed | (Battistella et al., 2011) | ||
| 2011 | Japan | MSC | Intravenous | Stroke patients | Feasible and safe | Completed | (Honmou et al., 2011) | ||
| 2012 | Hong Kong | UCBMC | Intracranial | Stroke in the middle cerebral artery territory and stable hemiplegia or hemiparesis | N/A | Completed | NCT01673932 | ||
| 2012 | China | BMSC | Intracerebral | Ischemia stroke or ICH patient | No adverse effects observed; 87% functional/neurological improvement | Completed | (Li et al., 2013b) | ||
| 2012 | Brazil | BMMNC | Intra-arterial | MCA acute ischemic stroke | Safe | Completed | (Friedrich et al., 2012) | ||
| 2010 | France | MSC | Intravenous | Carotid ischemic stroke patients | Ongoing | NCT00875654 | |||
| 2010 | UK | NSC | Intracranial | Stroke patients | Ongoing | (Kalladka et al., 2016) | |||
| 2011 | Taiwan | OEC | Intracerebral | Thromboembolic Stroke | NCT01327768 | ||||
| 2012 | China | HSC | Intra-arterial | Internal carotid artery territory infarction | N/A | Recruiting | NCT01518231 | ||
| 2014 | Spain | BMMNC/2m/kg or 5m/kg | Intra-arterial | Moderate-to-severe acute ischemic stroke patients | Appears to be safe; 30% clinical improvement at 90 days | Recruiting | (Moniche et al., 2015) | ||
| 2014 | Mexico | NSC | Intravenous | Ischemic stroke | Ongoing | www.Novastem.mx | |||
| 2014 | China | NSC | Intracerebral | Chronic ischemia stroke | Completed | www.Neuralstem.com | |||
| 2014 | China | EPC | Intravenous | Chronic ischemia stroke | Recruiting | NCT01468064 | |||
| 2015 | China | UCMSC/20m | Intravenous | ICH | Ongoing | NCT02283879 | |||
| 2016 | China | UCMSC | Intravenous | Intracerebral ischemic stroke | - | NCT02580019 | |||
| 2016 | China | MSC | Intracerebral | ICH | Recruiting | NCT02767817 | |||
| 2016 | USA | BMSC | Intravenous/Intranasal | functional damage to CNS/PNS | Recruiting | NCT02795052 | |||
| 2016 | Taiwan | ADSC | Intracerebral | Stroke patients | - | NCT02813512 | |||
| Efficacy | 2008 | Japan | BMMNC/25 ml | Intravenous | Stroke patients | Completed | (Toyoda, 2013) | ||
| 2009 | Taiwan | CD34+ Stem Cell | Intracerebral | Chronic stroke adult patient | Completed | (Mackie a,,nd Losordo, 2011); NCT01438593, NCT01249287 | |||
| 2011 | China | BMSC, EPC/2.5m/kg/100 ml | Intravenous | Ischemic lesion within the MCA territory | Ongoing | NCT01468064 | |||
| 2011 | USA | BMSC/2.5m 5.0m or 10m | Intracranial | Chronic stroke patients | No serious adverse events attributable and significant improvements in motor impairment | Completed | www.san-bio.com | ||
| 2014 | UK | NSC | Intracerebral | Stroke patient Phase II | Strongly positive results for 12 months; Well-tolerated/no serious adverse events | Ongoing | www.reneuron.com; NCT02117635 | ||
| 2014 | India | BMMNC | Intravenous | Ischemic stroke Phase II | Safe but no beneficial effect | Recruiting | (Prasad et al., 2014); NCT02245698 | ||
| 2016 | US/UK | MultiStem cells | Intravenous | Ischemic stroke Phase II | Excellent; 12-month functional improvement | Completed | www.athersys.com | ||
| 2016 | Europe | ADSC/1m/kg | Intravenous | Hemispheric ischemic stroke | Recruiting | NCT02849613 | |||
| Effectiveness | 2013 | China | MSC | Intrathecal | Cerebral palsy | Recruiting | NCT01929434 | ||
This Table may not include all current clinical trials. The reference list includes published papers, searchable websites or ClinicalTrials.gov Identifiers. Abbreviation in the Table: ASC, Adipose-derived Stromal Cells; BMSC, bone marrow mesenchymal stem cell; EPC, endothelial progenitor cells; ES, embryonic stem cells; ICH, intracerebral hemorrhage; iPS, induced pluripotent stem cells; m indicates million cells; MCAO, middle cerebral artery occlusion; NSC, neural stem cells; UCBMC, umbilical cord blood mononuclear cells; UCMSC, umbilical cord mesenchymal stem cells.
Highlights.
A comprehensive review of cell-based regeneration therapy for ischemic stroke.
Summary of different stem cells in the transplantation therapy.
Summary of different stroke models for regenerative research.
Introduction of the mechanism of action of transplanted cells.
Introduction of strategies for promoting cell survival and regenerative properties.
Introduction of innovative approaches in cell transplantation and translational research.
Acknowledgments
This work was supported by NIH grants NS062097 (LW), NS085568 (LW/SPY), and NS091585 (LW), VA National Merit grant RX000666 (SPY), AHA Predoctoral/Postdoctoral Fellowships PRE25710020 (MJ) and POST25710112 (ZZW). We also thank Ryan Chin at Emory University for assistance in literature search and data collection.
Abbreviation
- Ang 1
Angiopoietin 1
- BI
Barthel Index
- bFGF
Basic fibroblast growth factor
- BBB
Blood brain barrier
- BMSCs
Bone marrow stem cells
- BDNF
Brain-derived neurotrophic factors
- CRABP
Cellular RA-Binding Proteins
- ChR2
Channelrhodopsin-2
- CXCR4
Chemokine receptor type 4
- CSF-1
Colony-stimulating factor-1
- CCA
Common carotid artery
- DCX
Doublecortin
- EEG
Electroencephalography
- ET-1
Endothelin-1
- ECs
Embryonal carcinomas
- EB
Embryonic bodies
- ES
Embryonic stem
- EGF
Epidermal growth factor
- EPO
Erythropoietin
- ECA
External carotid artery
- GRID
Gadolinium-RhodamIne Dextran
- GDNF
Glial cell-derived neurotrophic factor
- GFAP
Glial fibrillary acidic protein
- G-CSF
Granulocyte-colony stimulating factor
- HuD
Hu protein D
- HLA
Human leukocyte antigen
- HIE
Hypoxic-ischemic encephalopathy
- HP
Hypoxia preconditioned
- iPS cells
Induced pluripotent stem cells
- IV
Injected intravenously
- INF-γ
Interferon gamma
- ICH
Intracranial hemorrhage
- LIF
Leukemia inhibitory factor
- LCBF
Local cerebral blood flow
- MRI
Magnetic resonance imaging
- MHC
Major histocompatibility
- MSCs
Mesenchymal stem cells
- MMP-2
Metalloproteinase 2
- MCA
Middle cerebral artery
- mRS
Modified Rankin Scale
- MS
Multiple sclerosis
- MI
Myocardial infarction
- NIHSS
National Institutes of Health Stroke Score
- NGF
Nerve growth factor
- ARNT
Nuclear translocator
- PBMCs
Peripheral blood mononuclear cells
- OPCs
Oligodendrocyte progenitors
- OGD
Oxygen-glucose deprivation
- RA
Retinoic acid
- RMS
Rostral migratory stream
- SCF
Stem cell factor
- SGZ
Subgranular zone
- SVZ
Subventricular zone
- SPF
Survival-promoting factors
- t-PA
Tissue plasminogen activator
- TBI
Traumatic brain injury
- TNF-α
Tumor necrosis factor alpha
- VEGF
Vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abeysinghe HC, Bokhari L, Quigley A, Choolani M, Chan J, Dusting GJ, Crook JM, Kobayashi NR, Roulston CL. Pre-differentiation of human neural stem cells into GABAergic neurons prior to transplant results in greater repopulation of the damaged brain and accelerates functional recovery after transient ischemic stroke. Stem Cell Res Ther. 2015;6:186. doi: 10.1186/s13287-015-0175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzal MR, Haider H, Idris NM, Jiang S, Ahmed RP, Ashraf M. Preconditioning promotes survival and angiomyogenic potential of mesenchymal stem cells in the infarcted heart via NF-kappaB signaling. Antioxid Redox Signal. 2010;12:693–702. doi: 10.1089/ars.2009.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- Agudo-López A, Miguel BG, Fernández I, Martínez AM. Involvement of mitochondria on neuroprotective effect of sphingosine-1-phosphate in cell death in an in vitro model of brain ischemia. Neuroscience letters. 2010;470:130–133. doi: 10.1016/j.neulet.2009.12.070. [DOI] [PubMed] [Google Scholar]
- Altman J. Are new neurons formed in the brains of adult mammals? Science. 1962;135:1127–1128. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- An N, Xu H, Gao WQ, Yang H. Direct Conversion of Somatic Cells into Induced Neurons. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-0350-0. [DOI] [PubMed] [Google Scholar]
- Andrews PW, Matin MM, Bahrami AR, Damjanov I, Gokhale P, Draper JS. Embryonic stem (ES) cells and embryonal carcinoma (EC) cells: opposite sides of the same coin. Biochem Soc Trans. 2005;33:1526–1530. doi: 10.1042/BST0331526. [DOI] [PubMed] [Google Scholar]
- Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nature biotechnology. 2014;32:252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbab AS, Thiffault C, Navia B, Victor SJ, Hong K, Zhang L, Jiang Q, Varma NR, Iskander A, Chopp M. Tracking of In-111-labeled human umbilical tissue-derived cells (hUTC) in a rat model of cerebral ischemia using SPECT imaging. BMC medical imaging. 2012;12:1. doi: 10.1186/1471-2342-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ, Ehlers MD, Feng G. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron. 2007;54:205–218. doi: 10.1016/j.neuron.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnhold S, Lenartz D, Kruttwig K, Klinz FJ, Kolossov E, Hescheler J, Sturm V, Andressen C, Addicks K. Differentiation of green fluorescent protein-labeled embryonic stem cell-derived neural precursor cells into Thy-1-positive neurons and glia after transplantation into adult rat striatum. Journal of Neurosurgery. 2000;93:1026–1032. doi: 10.3171/jns.2000.93.6.1026. [DOI] [PubMed] [Google Scholar]
- Arrenberg AB, Del Bene F, Baier H. Optical control of zebrafish behavior with halorhodopsin. Proc Natl Acad Sci U S A. 2009;106:17968–17973. doi: 10.1073/pnas.0906252106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R, Pennesi G. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35:1482–1490. doi: 10.1002/eji.200425405. [DOI] [PubMed] [Google Scholar]
- Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic stem cells express neuronal properties in vitro. Dev Biol. 1995;168:342–357. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- Baker DE, Harrison NJ, Maltby E, Smith K, Moore HD, Shaw PJ, Heath PR, Holden H, Andrews PW. Adaptation to culture of human embryonic stem cells and oncogenesis in vivo. Nat Biotechnol. 2007;25:207–215. doi: 10.1038/nbt1285. [DOI] [PubMed] [Google Scholar]
- Bang OY. Clinical Trials of Adult Stem Cell Therapy in Patients with Ischemic Stroke. Journal of Clinical Neurology. 2016;12:14–20. doi: 10.3988/jcn.2016.12.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005a;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Annals of neurology. 2005b;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- Barberi T, Klivenyi P, Calingasan NY, Lee H, Kawamata H, Loonam K, Perrier AL, Bruses J, Rubio ME, Topf N, Tabar V, Harrison NL, Beal MF, Moore MA, Studer L. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat Biotechnol. 2003;21:1200–1207. doi: 10.1038/nbt870. [DOI] [PubMed] [Google Scholar]
- Barberi T, Willis LM, Socci ND, Studer L. Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med. 2005;2:e161. doi: 10.1371/journal.pmed.0020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistella V, de Freitas GR, da Fonseca LM, Mercante D, Gutfilen B, Goldenberg RC, Dias JV, Kasai-Brunswick TH, Wajnberg E, Rosado-de-Castro PH, Alves-Leon SV, Mendez-Otero R, Andre C. Safety of autologous bone marrow mononuclear cell transplantation in patients with nonacute ischemic stroke. Regen Med. 2011;6:45–52. doi: 10.2217/rme.10.97. [DOI] [PubMed] [Google Scholar]
- Beers DR, Henkel JS, Zhao W, Wang J, Appel SH. CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proc Natl Acad Sci U S A. 2008;105:15558–15563. doi: 10.1073/pnas.0807419105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar TN, Dugich-Djordjevic MM, Li YX, Ma W, Somogyi R, Wen X, Brown E, Scott C, McKay RD, Barker JL. Neurotrophins stimulate chemotaxis of embryonic cortical neurons. Eur J Neurosci. 1997;9:2561–2570. doi: 10.1111/j.1460-9568.1997.tb01685.x. [DOI] [PubMed] [Google Scholar]
- Ben-Hur T. Immunomodulation by neural stem cells. J Neurol Sci. 2008;265:102–104. doi: 10.1016/j.jns.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Ben-Hur T, Ben-Menachem O, Furer V, Einstein O, Mizrachi-Kol R, Grigoriadis N. Effects of proinflammatory cytokines on the growth, fate, and motility of multipotential neural precursor cells. Mol Cell Neurosci. 2003;24:623–631. doi: 10.1016/s1044-7431(03)00218-5. [DOI] [PubMed] [Google Scholar]
- Bernaudin M, Marti HH, Roussel S, Divoux D, Nouvelot A, MacKenzie ET, Petit E. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19:643–651. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- Bieberich E, Silva J, Wang G, Krishnamurthy K, Condie BG. Selective apoptosis of pluripotent mouse and human stem cells by novel ceramide analogues prevents teratoma formation and enriches for neural precursors in ES cell-derived neural transplants. J Cell Biol. 2004;167:723–734. doi: 10.1083/jcb.200405144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernaskie J, Corbett D, Peeling J, Wells J, Lei H. A serial MR study of cerebral blood flow changes and lesion development following endothelin-1-induced ischemia in rats. Magn Reson Med. 2001;46:827–830. doi: 10.1002/mrm.1263. [DOI] [PubMed] [Google Scholar]
- Bliss TM, Kelly S, Shah AK, Foo WC, Kohli P, Stokes C, Sun GH, Ma M, Masel J, Kleppner SR, Schallert T, Palmer T, Steinberg GK. Transplantation of hNT neurons into the ischemic cortex: cell survival and effect on sensorimotor behavior. J Neurosci Res. 2006;83:1004–1014. doi: 10.1002/jnr.20800. [DOI] [PubMed] [Google Scholar]
- Blum B, Bar-Nur O, Golan-Lev T, Benvenisty N. The anti-apoptotic gene survivin contributes to teratoma formation by human embryonic stem cells. Nat Biotechnol. 2009;27:281–287. doi: 10.1038/nbt.1527. [DOI] [PubMed] [Google Scholar]
- Blum B, Benvenisty N. The tumorigenicity of diploid and aneuploid human pluripotent stem cells. Cell Cycle. 2009;8:3822–3830. doi: 10.4161/cc.8.23.10067. [DOI] [PubMed] [Google Scholar]
- Boltze J, Forschler A, Nitzsche B, Waldmin D, Hoffmann A, Boltze CM, Dreyer AY, Goldammer A, Reischauer A, Hartig W, Geiger KD, Barthel H, Emmrich F, Gille U. Permanent middle cerebral artery occlusion in sheep: a novel large animal model of focal cerebral ischemia. J Cereb Blood Flow Metab. 2008;28:1951–1964. doi: 10.1038/jcbfm.2008.89. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Hadman M, Sanberg CD, Sanberg PR. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke. 2004;35:2385–2389. doi: 10.1161/01.STR.0000141680.49960.d7. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Koutouzis TK, Jorden JR, Martinez R, Rodriguez AI, Poulos SG, Freeman TB, McKeown P, Cahill DW, Nishino H, Sanberg PR. Neural transplantation as an experimental treatment modality for cerebral ischemia. Neurosci Biobehav Rev. 1997;21:79–90. doi: 10.1016/0149-7634(95)00063-1. [DOI] [PubMed] [Google Scholar]
- Bovetti S, Hsieh YC, Bovolin P, Perroteau I, Kazunori T, Puche AC. Blood vessels form a scaffold for neuroblast migration in the adult olfactory bulb. J Neurosci. 2007;27:5976–5980. doi: 10.1523/JNEUROSCI.0678-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton BR, Lim R, Arumugam TV, Drummond GR, Wallace EM, Sobey CG. Post-stroke inflammation and the potential efficacy of novel stem cell therapies: focus on amnion epithelial cells. Developing stem cell-based therapies for neural repair. 2015 doi: 10.3389/fncel.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, Duncan ID, McKay RD. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999;285:754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- Buhnemann C, Scholz A, Bernreuther C, Malik CY, Braun H, Schachner M, Reymann KG, Dihne M. Neuronal differentiation of transplanted embryonic stem cell-derived precursors in stroke lesions of adult rats. Brain. 2006;129:3238–3248. doi: 10.1093/brain/awl261. [DOI] [PubMed] [Google Scholar]
- Burns TC, Verfaillie CM, Low WC. Stem cells for ischemic brain injury: a critical review. J Comp Neurol. 2009;515:125–144. doi: 10.1002/cne.22038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G, Lin Y, Du Z, Kogelberg H, McGregor C. Xenogeneic glycans: Human Antibody Reactivity and Their Impact on Xenograft Rejection. The Journal of Heart and Lung Transplantation. 2015;34:S271–S272. [Google Scholar]
- Caiazzo M, Dell’anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, Russo G, Carninci P, Pezzoli G, Gainetdinov RR, Gustincich S, Dityatev A, Broccoli V. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- Calió ML, Marinho DS, Ko GM, Ribeiro RR, Carbonel AF, Oyama LM, Ormanji M, Guirao TP, Calió PL, Reis LA. Transplantation of bone marrow mesenchymal stem cells decreases oxidative stress, apoptosis, and hippocampal damage in brain of a spontaneous stroke model. Free Radical Biology and Medicine. 2014;70:141–154. doi: 10.1016/j.freeradbiomed.2014.01.024. [DOI] [PubMed] [Google Scholar]
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- Capowski EE, Schneider BL, Ebert AD, Seehus CR, Szulc J, Zufferey R, Aebischer P, Svendsen CN. Lentiviral vector-mediated genetic modification of human neural progenitor cells for ex vivo gene therapy. J Neurosci Methods. 2007;163:338–349. doi: 10.1016/j.jneumeth.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau MJ, Deveau TC, Song M, Gu X, Chen D, Wei L. iPSC Transplantation increases regeneration and functional recovery after ischemic stroke in neonatal rats. Stem Cells. 2014;32:3075–3087. doi: 10.1002/stem.1802. [DOI] [PubMed] [Google Scholar]
- Chen F, Suzuki Y, Nagai N, Peeters R, Sun X, Coudyzer W, Marchal G, Ni Y. Rat cerebral ischemia induced with photochemical occlusion of proximal middle cerebral artery: a stroke model for MR imaging research. MAGMA. 2004;17:103–108. doi: 10.1007/s10334-004-0061-9. [DOI] [PubMed] [Google Scholar]
- Chen J, Chen J, Chen S, Zhang C, Zhang L, Xiao X, Das A, Zhao Y, Yuan B, Morris M. Transfusion of CXCR4-primed endothelial progenitor cells reduces cerebral ischemic damage and promotes repair in db/db diabetic mice. PLoS One. 2012;7:e50105. doi: 10.1371/journal.pone.0050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001a;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001b;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- Chen J, Ye X, Yan T, Zhang C, Yang XP, Cui X, Cui Y, Zacharek A, Roberts C, Liu X, Dai X, Lu M, Chopp M. Adverse effects of bone marrow stromal cell treatment of stroke in diabetic rats. Stroke. 2011;42:3551–3558. doi: 10.1161/STROKEAHA.111.627174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- Chen ST, Hsu CY, Hogan EL, Maricq H, Balentine JD. A model of focal ischemic stroke in the rat: reproducible extensive cortical infarction. Stroke. 1986;17:738–743. doi: 10.1161/01.str.17.4.738. [DOI] [PubMed] [Google Scholar]
- Chen X, Katakowski M, Li Y, Lu D, Wang L, Zhang L, Chen J, Xu Y, Gautam S, Mahmood A, Chopp M. Human bone marrow stromal cell cultures conditioned by traumatic brain tissue extracts: growth factor production. J Neurosci Res. 2002a;69:687–691. doi: 10.1002/jnr.10334. [DOI] [PubMed] [Google Scholar]
- Chen X, Li Y, Wang L, Katakowski M, Zhang L, Chen J, Xu Y, Gautam SC, Chopp M. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002b;22:275–279. doi: 10.1046/j.1440-1789.2002.00450.x. [DOI] [PubMed] [Google Scholar]
- Chiu IM, Chen A, Zheng Y, Kosaras B, Tsiftsoglou SA, Vartanian TK, Brown RH, Jr, Carroll MC. T lymphocytes potentiate endogenous neuroprotective inflammation in a mouse model of ALS. Proc Natl Acad Sci U S A. 2008;105:17913–17918. doi: 10.1073/pnas.0804610105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- Chu K, Kim M, Jeong SW, Kim SU, Yoon BW. Human neural stem cells can migrate, differentiate, and integrate after intravenous transplantation in adult rats with transient forebrain ischemia. Neurosci Lett. 2003;343:129–133. doi: 10.1016/s0304-3940(03)00174-5. [DOI] [PubMed] [Google Scholar]
- Chu K, Park KI, Lee ST, Jung KH, Ko SY, Kang L, Sinn DI, Lee YS, Kim SU, Kim M. Combined treatment of vascular endothelial growth factor and human neural stem cells in experimental focal cerebral ischemia. Neuroscience Res. 2005a;53:384–390. doi: 10.1016/j.neures.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- Corti S, Locatelli F, Strazzer S, Salani S, Del Bo R, Soligo D, Bossolasco P, Bresolin N, Scarlato G, Comi GP. Modulated generation of neuronal cells from bone marrow by expansion and mobilization of circulating stem cells with in vivo cytokine treatment. Exp Neurol. 2002;177:443–452. doi: 10.1006/exnr.2002.8004. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Harris VK, Zigelbaum A, Goossens AM, Lu A, Rosenthal H, Sadiq SA. Mesenchymal Stem Cells Enhance the Engraftment and Myelinating Ability of Allogeneic Oligodendrocyte Progenitors in Dysmyelinated Mice. Stem Cells Dev. doi: 10.1089/scd.2010.0547. [DOI] [PubMed] [Google Scholar]
- Crook JM, Wallace G, Tomaskovic-Crook E. The potential of induced pluripotent stem cells in models of neurological disorders: implications on future therapy. Expert Review of Neurotherapeutics. 2015;15:295–304. doi: 10.1586/14737175.2015.1013096. [DOI] [PubMed] [Google Scholar]
- Daadi MM, Maag AL, Steinberg GK. Adherent self-renewable human embryonic stem cell-derived neural stem cell line: functional engraftment in experimental stroke model. PLoS One. 2008a;3:e1644. doi: 10.1371/journal.pone.0001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damjanov I, Andrews PW. The terminology of teratocarcinomas and teratomas. Nat Biotechnol. 2007;25:1212. doi: 10.1038/nbt1107-1212a. discussion 1212. [DOI] [PubMed] [Google Scholar]
- Danielyan L, Schafer R, von Ameln-Mayerhofer A, Buadze M, Geisler J, Klopfer T, Burkhardt U, Proksch B, Verleysdonk S, Ayturan M, Buniatian GH, Gleiter CH, Frey WH., 2nd Intranasal delivery of cells to the brain. Eur J Cell Biol. 2009;88:315–324. doi: 10.1016/j.ejcb.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Darsalia V, Allison SJ, Cusulin C, Monni E, Kuzdas D, Kallur T, Lindvall O, Kokaia Z. Cell number and timing of transplantation determine survival of human neural stem cell grafts in stroke-damaged rat brain. J Cereb Blood Flow Metab. 2011;31:235–242. doi: 10.1038/jcbfm.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsalia V, Kallur T, Kokaia Z. Survival, migration and neuronal differentiation of human fetal striatal and cortical neural stem cells grafted in stroke-damaged rat striatum. Eur J Neurosci. 2007;26:605–614. doi: 10.1111/j.1460-9568.2007.05702.x. [DOI] [PubMed] [Google Scholar]
- de Mello RF, de Souza Santos I, Alencar AP, Benseñor IM, Lotufo PA, Goulart AC. Major Depression as a Predictor of Poor Long-Term Survival in a Brazilian Stroke Cohort (Study of Stroke Mortality and Morbidity in Adults) EMMA study. Journal of Stroke and Cerebrovascular Diseases. 2016;25(3):618–25. doi: 10.1016/j.jstrokecerebrovasdis.2015.11.021. [DOI] [PubMed] [Google Scholar]
- De Ryck M, Keersmaekers R, Duytschaever H, Claes C, Clincke G, Janssen M, Van Reet G. Lubeluzole protects sensorimotor function and reduces infarct size in a photochemical stroke model in rats. J Pharmacol Exp Ther. 1996;279:748–758. [PubMed] [Google Scholar]
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- Dillon-Carter O, Johnston RE, Borlongan CV, Truckenmiller ME, Coggiano M, Freed WJ. T155g-immortalized kidney cells produce growth factors and reduce sequelae of cerebral ischemia. Cell Transplant. 2002;11:251–259. [PubMed] [Google Scholar]
- Doeppner TR, El Aanbouri M, Dietz GP, Weise J, Schwarting S, Bähr M. Transplantation of TAT-Bcl-x L-transduced neural precursor cells: Long-term neuroprotection after stroke. Neurobiology of disease. 2010;40:265–276. doi: 10.1016/j.nbd.2010.05.033. [DOI] [PubMed] [Google Scholar]
- Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- Drukker M, Benvenisty N. The immunogenicity of human embryonic stem-derived cells. Trends Biotechnol. 2004;22:136–141. doi: 10.1016/j.tibtech.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Drukker M, Katz G, Urbach A, Schuldiner M, Markel G, Itskovitz-Eldor J, Reubinoff B, Mandelboim O, Benvenisty N. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99:9864–9869. doi: 10.1073/pnas.142298299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury-Stewart D, Song M, Mohamad O, Guo Y, Gu X, Chen D, Wei L. Highly efficient differentiation of neural precursors from human embryonic stem cells and benefits of transplantation after ischemic stroke in mice. Stem Cell Res Ther. 2013;4:93. doi: 10.1186/scrt292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury-Stewart D, Song M, Mohamad O, Yu SP, Wei L. Small Molecule Promoted Adherent and Feeder Free Differentiation of Functional Neurons from Human Embryonic and Induced Pluripotent Stem Cells. J Stem Cells. 2012;6:1–7. [PubMed] [Google Scholar]
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Durukan A, Tatlisumak T. Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav. 2007;87:179–197. doi: 10.1016/j.pbb.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Eglitis MA, Dawson D, Park KW, Mouradian MM. Targeting of marrow-derived astrocytes to the ischemic brain. Neuroreport. 1999;10:1289–1292. doi: 10.1097/00001756-199904260-00025. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, Rustenbeck HH, Breiter N, Jacob S, Knerlich F, Bohn M, Poser W, Ruther E, Kochen M, Gefeller O, Gleiter C, Wessel TC, De Ryck M, Itri L, Prange H, Cerami A, Brines M, Siren AL. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K, Schellinger PD, Bohn M, Becker H, Wegrzyn M, Jahnig P, Herrmann M, Knauth M, Bahr M, Heide W, Wagner A, Schwab S, Reichmann H, Schwendemann G, Dengler R, Kastrup A, Bartels C. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40:e647–656. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]
- Eichenbaum JW, Pevsner PH, Pivawer G, Kleinman GM, Chiriboga L, Stern A, Rosenbach A, Iannuzzi K, Miller DC. A murine photochemical stroke model with histologic correlates of apoptotic and nonapoptotic mechanisms. J Pharmacol Toxicol Methods. 2002;47:67–71. doi: 10.1016/s1056-8719(02)00215-0. [DOI] [PubMed] [Google Scholar]
- Eiges R, Schuldiner M, Drukker M, Yanuka O, Itskovitz-Eldor J, Benvenisty N. Establishment of human embryonic stem cell-transfected clones carrying a marker for undifferentiated cells. Curr Biol. 2001;11:514–518. doi: 10.1016/s0960-9822(01)00144-0. [DOI] [PubMed] [Google Scholar]
- Erdo F, Buhrle C, Blunk J, Hoehn M, Xia Y, Fleischmann B, Focking M, Kustermann E, Kolossov E, Hescheler J, Hossmann KA, Trapp T. Host-dependent tumorigenesis of embryonic stem cell transplantation in experimental stroke. J Cereb Blood Flow Metab. 2003;23:780–785. doi: 10.1097/01.WCB.0000071886.63724.FB. [DOI] [PubMed] [Google Scholar]
- Ernst C, Christie BR. Temporally specific proliferation events are induced in the hippocampus following acute focal injury. J Neurosci Res. 2006;83:349–361. doi: 10.1002/jnr.20724. [DOI] [PubMed] [Google Scholar]
- Fan X, Qiu J, Yu Z, Dai H, Singhal AB, Lo EH, Wang X. A rat model of studying tissue-type plasminogen activator thrombolysis in ischemic stroke with diabetes. Stroke. 2012;43:567–570. doi: 10.1161/STROKEAHA.111.635250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro JM. Update on intracerebral haemorrhage. J Neurol. 2006;253:985–999. doi: 10.1007/s00415-006-0201-4. [DOI] [PubMed] [Google Scholar]
- Fraichard A, Chassande O, Bilbaut G, Dehay C, Savatier P, Samarut J. In vitro differentiation of embryonic stem cells into glial cells and functional neurons. J Cell Sci. 1995;108( Pt 10):3181–3188. doi: 10.1242/jcs.108.10.3181. [DOI] [PubMed] [Google Scholar]
- Francis KR, Wei L. Human embryonic stem cell neural differentiation and enhanced cell survival promoted by hypoxic preconditioning. Cell Death Dis. 2010;1:e22. doi: 10.1038/cddis.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich MA, Martins MP, Araujo MD, Klamt C, Vedolin L, Garicochea B, Raupp EF, Sartori El Ammar J, Machado DC, Costa JC, Nogueira RG, Rosado-de-Castro PH, Mendez-Otero R, Freitas GR. Intra-arterial infusion of autologous bone marrow mononuclear cells in patients with moderate to severe middle cerebral artery acute ischemic stroke. Cell Transplant 21 Suppl. 2012;1:S13–21. doi: 10.3727/096368912x612512. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Horie N, Satoh K, Yamaguchi S, Morofuji Y, Hiu T, Izumo T, Hayashi K, Nishida N, Nagata I. Intra-arterial transplantation of low-dose stem cells provides functional recovery without adverse effects after stroke. Cell Mol Neurobiol. 2015;35:399–406. doi: 10.1007/s10571-014-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadalla SM, Wang T, Haagenson M, Spellman SR, Lee SJ, Williams KM, Wong JY, De Vivo I, Savage SA. Association between donor leukocyte telomere length and survival after unrelated allogeneic hematopoietic cell transplantation for severe aplastic anemia. Jama. 2015;313:594–602. doi: 10.1001/jama.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghobrial G, Haas C, Maulucci C, Lepore A, Fischer I. Promising Advances in Targeted Cellular Based Therapies: Treatment Update in Spinal Cord Injury. J Stem Cell Res Ther. 2014;4:2. [Google Scholar]
- Glaser T, Brustle O. Retinoic acid induction of ES-cell-derived neurons: the radial glia connection. Trends Neurosci. 2005;28:397–400. doi: 10.1016/j.tins.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Gonzalez F, Barragan Monasterio M, Tiscornia G, Montserrat Pulido N, Vassena R, Batlle Morera L, Rodriguez Piza I, Izpisua Belmonte JC. Generation of mouse-induced pluripotent stem cells by transient expression of a single nonviral polycistronic vector. Proc Natl Acad Sci U S A. 2009;106:8918–8922. doi: 10.1073/pnas.0901471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorris R, Fischer J, Erwes KL, Kesavan J, Peterson DA, Alexander M, Nöthen MM, Peitz M, Quandel T, Karus M. Pluripotent stem cell - derived radial glia - like cells as stable intermediate for efficient generation of human oligodendrocytes. Glia. 2015;63:2152–2167. doi: 10.1002/glia.22882. [DOI] [PubMed] [Google Scholar]
- Gould DS, Auchincloss H., Jr Direct and indirect recognition: the role of MHC antigens in graft rejection. Immunol Today. 1999;20:77–82. doi: 10.1016/s0167-5699(98)01394-2. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grégoire CA, Goldenstein BL, Floriddia EM, Barnabé - Heider F, Fernandes KJ. Endogenous neural stem cell responses to stroke and spinal cord injury. Glia. 2015;63:1469–1482. doi: 10.1002/glia.22851. [DOI] [PubMed] [Google Scholar]
- Gross GJ, Auchampach JA. Blockade of ATP-sensitive potassium channels prevents myocardial preconditioning in dogs. Circ Res. 1992;70:223–233. doi: 10.1161/01.res.70.2.223. [DOI] [PubMed] [Google Scholar]
- Guan K, Chang H, Rolletschek A, Wobus AM. Embryonic stem cell-derived neurogenesis. Retinoic acid induction and lineage selection of neuronal cells. Cell and Tissue Res. 2001;305:171–176. doi: 10.1007/s004410100416. [DOI] [PubMed] [Google Scholar]
- Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell. 2014;14:188–202. doi: 10.1016/j.stem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman R, De Los Angeles A, Cheshier S, Choi R, Hoang S, Liauw J, Schaar B, Steinberg G. Intracarotid injection of fluorescence activated cell-sorted CD49d-positive neural stem cells improves targeted cell delivery and behavior after stroke in a mouse stroke model. Stroke. 2008;39:1300–1306. doi: 10.1161/STROKEAHA.107.500470. [DOI] [PubMed] [Google Scholar]
- Hacke K, Falahati R, Flebbe-Rehwaldt L, Kasahara N, Gaensler KM. Suppression of HLA expression by lentivirus-mediated gene transfer of siRNA cassettes and in vivo chemoselection to enhance hematopoietic stem cell transplantation. Immunol Res. 2009;44:112–126. doi: 10.1007/s12026-008-8088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett PJ, Cooper O, Sadi D, Robertson H, Mendez I, Isacson O. Long-term health of dopaminergic neuron transplants in Parkinson’s disease patients. Cell reports. 2014;7:1755–1761. doi: 10.1016/j.celrep.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Qian X, Bernstein JG, Zhou HH, Franzesi GT, Stern P, Bronson RT, Graybiel AM, Desimone R, Boyden ES. Millisecond-timescale optical control of neural dynamics in the nonhuman primate brain. Neuron. 2009;62:191–198. doi: 10.1016/j.neuron.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Yasuhara T, Maki M, Matsukawa N, Masuda T, Yu SJ, Ali M, Yu G, Xu L, Kim SU, Hess DC, Borlongan CV. Neural progenitor NT2N cell lines from teratocarcinoma for transplantation therapy in stroke. Prog Neurobiol. 2008;85:318–334. doi: 10.1016/j.pneurobio.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Harrison NJ, Baker D, Andrews PW. Culture adaptation of embryonic stem cells echoes germ cell malignancy. Int J Androl. 2007;30:275–281. doi: 10.1111/j.1365-2605.2007.00762.x. discussion 281. [DOI] [PubMed] [Google Scholar]
- Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996;166:585–592. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Hess DC, Borlongan CV. Cell-based therapy in ischemic stroke. Expert Rev Neurother. 2008;8:1193–1201. doi: 10.1586/14737175.8.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honmou O, Houkin K, Matsunaga T, Niitsu Y, Ishiai S, Onodera R, Waxman SG, Kocsis JD. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134:1790–1807. doi: 10.1093/brain/awr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie N, Hiu T, Nagata I. Stem Cell Transplantation Enhances Endogenous Brain Repair after Experimental Stroke. Neurol Med Chir (Tokyo) 55 Suppl. 2015;1:107–112. [PubMed] [Google Scholar]
- Horschitz S, Matthäus F, Groß A, Rosner J, Galach M, Greffrath W, Treede RD, Utikal J, Schloss P, Meyer-Lindenberg A. Impact of preconditioning with retinoic acid during early development on morphological and functional characteristics of human induced pluripotent stem cell-derived neurons. Stem Cell Res. 2015;15:30–41. doi: 10.1016/j.scr.2015.04.007. [DOI] [PubMed] [Google Scholar]
- Hu BY, Weick JP, Yu J, Ma LX, Zhang XQ, Thomson JA, Zhang SC. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci U S A. 2010;107:4335–4340. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Wei L, Taylor TM, Wei J, Zhou X, Wang JA, Yu SP. Hypoxic preconditioning enhances bone marrow mesenchymal stem cell migration via Kv2. 1 channel and FAK activation. American Journal of Physiology-Cell Physiol. 2011a;301:C362–C372. doi: 10.1152/ajpcell.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Wei L, Taylor TM, Wei J, Zhou X, Wang JA, Yu SP. Hypoxic preconditioning enhances bone marrow mesenchymal stem cell migration via Kv2.1 channel and FAK activation. Am J Physiol Cell Physiol. 2011b;301:C362–372. doi: 10.1152/ajpcell.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang JA, Wei L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135:799–808. doi: 10.1016/j.jtcvs.2007.07.071. [DOI] [PubMed] [Google Scholar]
- Huangfu D, Osafune K, Maehr R, Guo W, Eijkelenboom A, Chen S, Muhlestein W, Melton DA. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26:1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- Huber D, Petreanu L, Ghitani N, Ranade S, Hromadka T, Mainen Z, Svoboda K. Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature. 2008;451:61–64. doi: 10.1038/nature06445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ideguchi M, Palmer TD, Recht LD, Weimann JM. Murine embryonic stem cell-derived pyramidal neurons integrate into the cerebral cortex and appropriately project axons to subcortical targets. J Neurosci. 2010;30:894–904. doi: 10.1523/JNEUROSCI.4318-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ideguchi M, Shinoyama M, Gomi M, Hayashi H, Hashimoto N, Takahashi J. Immune or inflammatory response by the host brain suppresses neuronal differentiation of transplanted ES cell-derived neural precursor cells. J Neurosci Res. 2008;86:1936–1943. doi: 10.1002/jnr.21652. [DOI] [PubMed] [Google Scholar]
- Iguchi F, Nakagawa T, Tateya I, Kim TS, Endo T, Taniguchi Z, Naito Y, Ito J. Trophic support of mouse inner ear by neural stem cell transplantation. Neuroreport. 2003;14:77–80. doi: 10.1097/00001756-200301200-00015. [DOI] [PubMed] [Google Scholar]
- Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingberg E, Dock H, Theodorsson E, Theodorsson A, Strom JO. Method parameters’ impact on mortality and variability in mouse stroke experiments: a meta-analysis. 2016;6:21086. doi: 10.1038/srep21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Nagata N, Kurokawa H, Yamanaka S. iPS cells: a game changer for future medicine. The EMBO journal. 2014;33:409–417. doi: 10.1002/embj.201387098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Tezuka H, Sakoda H, Konno M, Nagata K, Uchiyama T, Uchino H, Mori KJ. Reproducible establishment of hemopoietic supportive stromal cell lines from murine bone marrow. Exp Hematol. 1989;17:145–153. [PubMed] [Google Scholar]
- Jäger C, Glaab E, Michelucci A, Binz TM, Köglsberger S, Garcia P, Trezzi JP, Ghelfi J, Balling R, Buttini M. The Mouse Brain Metabolome: Region-Specific Signatures and Response to Excitotoxic Neuronal Injury. Amer J Pathol. 2015;185:1699–1712. doi: 10.1016/j.ajpath.2015.02.016. [DOI] [PubMed] [Google Scholar]
- Jeon SB. Therapeutic Hypothermia in the Intensive Care Unit. J Neurocritical Care. 2014;7:6–15. [Google Scholar]
- Jeong SW, Chu K, Jung KH, Kim SU, Kim M, Roh JK. Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke. 2003;34:2258–2263. doi: 10.1161/01.STR.0000083698.20199.1F. [DOI] [PubMed] [Google Scholar]
- Jiang MQ, Zhao YY, Cao W, Wei ZZ, Gu X, Wei L, Yu SP. Long-term survival and regeneration of neuronal and vasculature cells inside the core region after ischemic stroke in adult mice. Brain Pathol. 2016 doi: 10.1111/bpa.12425. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Zhu X, Li G. Embolic middle cerebral artery occlusion (MCAO) for ischemic stroke with homologous blood clots in rats. JoVE (Journal of Visualized Experiments) 2014:e51956–e51956. doi: 10.3791/51956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y, Machon O, Hampl A, Dvorak P, Xing Y, Krauss S. In vitro differentiation of mouse embryonic stem cells into neurons of the dorsal forebrain. Cell Mol Neurobiol. 2011;31:715–727. doi: 10.1007/s10571-011-9669-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Hamanaka H, Monfils MH, Behnia R, Deisseroth K, Blair HT, LeDoux JE. Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proc Natl Acad Sci U S A. 2010;107:12692–12697. doi: 10.1073/pnas.1002418107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RE, Dillon-Carter O, Freed WJ, Borlongan CV. Trophic factor secreting kidney cell lines: in vitro characterization and functional effects following transplantation in ischemic rats. Brain Res. 2001;900:268–276. doi: 10.1016/s0006-8993(01)02327-7. [DOI] [PubMed] [Google Scholar]
- Jones PA, May GR, McLuckie JA, Iwashita A, Sharkey J. Apoptosis is not an invariable component of in vitro models of cortical cerebral ischaemia. Cell Res. 2004;14:241–250. doi: 10.1038/sj.cr.7290225. [DOI] [PubMed] [Google Scholar]
- Jung J, Hackett NR, Pergolizzi RG, Pierre-Destine L, Krause A, Crystal RG. Ablation of tumor-derived stem cells transplanted to the central nervous system by genetic modification of embryonic stem cells with a suicide gene. Hum Gene Ther. 2007;18:1182–1192. doi: 10.1089/hum.2007.078. [DOI] [PubMed] [Google Scholar]
- Kalladka D, Sinden J, Pollock K, Haig C, McLean J, Smith W, McConnachie A, Santosh C, Bath PM, Dunn L, Muir KW. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet. 2016;388:787–796. doi: 10.1016/S0140-6736(16)30513-X. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Karussis D, Kassis I, Kurkalli BG, Slavin S. Immunomodulation and neuroprotection with mesenchymal bone marrow stem cells (MSCs): a proposed treatment for multiple sclerosis and other neuroimmunological/neurodegenerative diseases. J Neurol Sci. 2008;265:131–135. doi: 10.1016/j.jns.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Kastanenka KV, Landmesser LT. In vivo activation of channelrhodopsin-2 reveals that normal patterns of spontaneous activity are required for motoneuron guidance and maintenance of guidance molecules. J Neurosci. 2010;30:10575–10585. doi: 10.1523/JNEUROSCI.2773-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004;14:186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Keogh CL, Yu SP, Wei L. The effect of recombinant human erythropoietin on neurovasculature repair after focal ischemic stroke in neonatal rats. J Pharmacol Exp Ther. 2007;322:521–528. doi: 10.1124/jpet.107.121392. [DOI] [PubMed] [Google Scholar]
- Kessel M, Gruss P. Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell. 1991;67:89–104. doi: 10.1016/0092-8674(91)90574-i. [DOI] [PubMed] [Google Scholar]
- Kim DY, Park SH, Lee SU, Choi DH, Park HW, Paek SH, Shin HY, Kim EY, Park SP, Lim JH. Effect of human embryonic stem cell-derived neuronal precursor cell transplantation into the cerebral infarct model of rat with exercise. Neurosci Res. 2007a;58:164–175. doi: 10.1016/j.neures.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Kim DS, Lee JS, Leem JW, Huh YJ, Kim JY, Kim HS, Park IH, Daley GQ, Hwang DY, Kim DW. Robust enhancement of neural differentiation from human ES and iPS cells regardless of their innate difference in differentiation propensity. Stem Cell Rev. 2010;6:270–281. doi: 10.1007/s12015-010-9138-1. [DOI] [PubMed] [Google Scholar]
- Kitada M. Mesenchymal cell populations: development of the induction systems for Schwann cells and neuronal cells and finding the unique stem cell population. Anat Sci Int. 2012;87:24–44. doi: 10.1007/s12565-011-0128-4. [DOI] [PubMed] [Google Scholar]
- Kondziolka D, Steinberg GK, Wechsler L, Meltzer CC, Elder E, Gebel J, Decesare S, Jovin T, Zafonte R, Lebowitz J, Flickinger JC, Tong D, Marks MP, Jamieson C, Luu D, Bell-Stephens T, Teraoka J. Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial. J Neurosurg. 2005;103:38–45. doi: 10.3171/jns.2005.103.1.0038. [DOI] [PubMed] [Google Scholar]
- Kondziolka D, Wechsler L, Goldstein S, Meltzer C, Thulborn KR, Gebel J, Jannetta P, DeCesare S, Elder EM, McGrogan M, Reitman MA, Bynum L. Transplantation of cultured human neuronal cells for patients with stroke. Neurol. 2000;55:565–569. doi: 10.1212/wnl.55.4.565. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. The generation, migration, and differentiation of olfactory neurons in the adult primate brain. Proc Natl Acad Sci USA. 2001;98:4752–4757. doi: 10.1073/pnas.081074998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer RH, Fortin DL, Trauner D. New photochemical tools for controlling neuronal activity. Curr Opin Neurobiol. 2009;19:544–552. doi: 10.1016/j.conb.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge A, Takemura S, Kokubo Y, Sato S, Goto K, Kayama T. Temporal profile of neurogenesis in the subventricular zone, dentate gyrus and cerebral cortex following transient focal cerebral ischemia. Neurol Res. 2009;31:969–976. doi: 10.1179/174313209X383312. [DOI] [PubMed] [Google Scholar]
- Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O, Houkin K, Date I, Hamada H. BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol Ther. 2004;9:189–197. doi: 10.1016/j.ymthe.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Labouyrie E, Dubus P, Groppi A, Mahon FX, Ferrer J, Parrens M, Reiffers J, de Mascarel A, Merlio JP. Expression of neurotrophins and their receptors in human bone marrow. Am J Pathol. 1999;154:405–415. doi: 10.1016/S0002-9440(10)65287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J, Lowry WE, Carmichael ST, Segura T. Delivery of iPS - NPCs to the Stroke Cavity within a Hyaluronic Acid Matrix Promotes the Differentiation of Transplanted Cells. Advanced Functional Mat. 2014;24:7053–7062. doi: 10.1002/adfm.201401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe JW, Becker LB. State of the art in therapeutic hypothermia. Annual review of medicine. 2011;62:79. doi: 10.1146/annurev-med-052009-150512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhauser F, Kraft P, Göb E, Leinweber J, Schuhmann MK, Lorenz K, Gelderblom M, Bittner S, Meuth SG, Wiendl H. Blocking of α4 integrin does not protect from acute ischemic stroke in mice. Stroke. 2014;45:1799–1806. doi: 10.1161/STROKEAHA.114.005000. [DOI] [PubMed] [Google Scholar]
- Laurenzi MA, Beccari T, Stenke L, Sjolinder M, Stinchi S, Lindgren JA. Expression of mRNA encoding neurotrophins and neurotrophin receptors in human granulocytes and bone marrow cells--enhanced neurotrophin-4 expression induced by LTB4. J Leukoc Biol. 1998;64:228–234. doi: 10.1002/jlb.64.2.228. [DOI] [PubMed] [Google Scholar]
- Lawrenz B, Schiller H, Willbold E, Ruediger M, Muhs A, Esser S. Highly sensitive biosafety model for stem-cell-derived grafts. Cytotherapy. 2004;6:212–222. doi: 10.1080/14653240410006031. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kim KS, Kim EJ, Choi HB, Lee KH, Park IH, Ko Y, Jeong SW, Kim SU. Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells. 2007;25:1204–1212. doi: 10.1634/stemcells.2006-0409. [DOI] [PubMed] [Google Scholar]
- Lee J, Terracciano CM. Cell therapy for cardiac repair. Br Med Bull. doi: 10.1093/bmb/ldq005. [DOI] [PubMed] [Google Scholar]
- Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28:1099–1106. doi: 10.1002/stem.430. [DOI] [PubMed] [Google Scholar]
- Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol. 2000;18:675–679. doi: 10.1038/76536. [DOI] [PubMed] [Google Scholar]
- Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, Savino C, Bianchi M, Nielsen J, Gerwien J, Kallunki P, Larsen AK, Helboe L, Christensen S, Pedersen LO, Nielsen M, Torup L, Sager T, Sfacteria A, Erbayraktar S, Erbayraktar Z, Gokmen N, Yilmaz O, Cerami-Hand C, Xie QW, Coleman T, Cerami A, Brines M. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- Lensch MW, Ince TA. The terminology of teratocarcinomas and teratomas. Nat Biotechnol. 2007;25:1211. doi: 10.1038/nbt1107-1211a. author reply 1211–1212. [DOI] [PubMed] [Google Scholar]
- Leu S, Lin Y-C, Yuen C-M, Yen C-H, Kao Y-H, Sun C-K, Yip H-K. Adipose-derived mesenchymal stem cells markedly attenuate brain infarct size and improve neurological function in rats. J Transl Med. 2010;8:63. doi: 10.1186/1479-5876-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WL, Fraser JL, Shan PY, Zhu J, Jiang YJ, Wei L. The role of VEGF/VEGFR2 signaling in peripheral stimulation-induced cerebral neurovascular regeneration after ischemic stroke in mice. Exper Brain Res. 2011;214:503–513. doi: 10.1007/s00221-011-2849-y. [DOI] [PubMed] [Google Scholar]
- Li WL, Yu S, Chen D, Yu S, Jiang YJ, Genetta T, Wei L. The regulatory role of NF-κB in autophagy-like cell death after focal cerebral ischemia in mice. Neurosci. 2013a;244:16–30. doi: 10.1016/j.neuroscience.2013.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WL, Yu SP, Ogle ME, Ding XS, Wei L. Enhanced neurogenesis and cell migration following focal ischemia and peripheral stimulation in mice. Developmental Neurobiol. 2008a;68:1474–1486. doi: 10.1002/dneu.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- Li Y, Lu Z, Keogh CL, Yu SP, Wei L. Erythropoietin-induced neurovascular protection, angiogenesis, and cerebral blood flow restoration after focal ischemia in mice. J Cereb Blood Flow Metab. 2007a;27:1043–1054. doi: 10.1038/sj.jcbfm.9600417. [DOI] [PubMed] [Google Scholar]
- Li Y, Lu ZY, Ogle M, Wei L. Erythropoietin prevents blood brain barrier damage induced by focal cerebral ischemia in mice. Neurochem Res. 2007b;32:2132–2141. doi: 10.1007/s11064-007-9387-9. [DOI] [PubMed] [Google Scholar]
- Li Y, Yu S, Mohamad O, Genetta T, Wei L. Sublethal Transient Global Ischemia Stimulates Migration of Neuroblasts and Neurogenesis in Mice. Translational Stroke Res. 2010;1:184–196. doi: 10.1007/s12975-010-0016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZM, Zhang ZT, Guo CJ, Geng FY, Qiang F, Wang LX. Autologous bone marrow mononuclear cell implantation for intracerebral hemorrhage-a prospective clinical observation. Clin Neurol Neurosurg. 2013b;115:72–76. doi: 10.1016/j.clineuro.2012.04.030. [DOI] [PubMed] [Google Scholar]
- Lian Q, Lye E, Suan Yeo K, Khia Way Tan E, Salto-Tellez M, Liu TM, Palanisamy N, El Oakley RM, Lee EH, Lim B, Lim SK. Derivation of clinically compliant MSCs from CD105+, CD24- differentiated human ESCs. Stem Cells. 2007;25:425–436. doi: 10.1634/stemcells.2006-0420. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z. Prospects of stem cell therapy for replacing dopamine neurons in Parkinson’s disease. Trends Pharmacol Sci. 2009;30:260–267. doi: 10.1016/j.tips.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Liska MG, Crowley MG, Nguyen H, Borlongan CV. Biobridge concept in stem cell therapy for ischemic stroke. J Neurosurg Sci. 2017;61:173–179. doi: 10.23736/S0390-5616.16.03791-7. [DOI] [PubMed] [Google Scholar]
- Litchfield TM, Whiteley SJ, Yee KT, Tyers P, Usherwood EJ, Nash AA, Lund RD. Characterisation of the immune response in a neural xenograft rejection paradigm. J Neuroimmunol. 1997;73:135–144. doi: 10.1016/s0165-5728(96)00192-0. [DOI] [PubMed] [Google Scholar]
- Liu N, Zhang Y, Fan L, Yuan M, Du H, Cheng R, Liu D, Lin F. Effects of transplantation with bone marrow-derived mesenchymal stem cells modified by Survivin on experimental stroke in rats. J Transl Med. 2011;9:105–106. doi: 10.1186/1479-5876-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Hu WX, Zu QQ, Lu SS, Xu XQ, Sun L, Zhou WZ, Shi HB. A novel embolic stroke model resembling lacunar infarction following proximal middle cerebral artery occlusion in beagle dogs. Journal of neuroscience methods. 2012;209:90–96. doi: 10.1016/j.jneumeth.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Liu X, Ye R, Yan T, Yu SP, Wei L, Xu G, Fan X, Jiang Y, Stetler RA, Liu G. Cell based therapies for ischemic stroke: from basic science to bedside. Progress in neurobiology. 2014;115:92–115. doi: 10.1016/j.pneurobio.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovera G, Roth S, Plesnila N, Veltkamp R, Liesz A. Modeling stroke in mice: Permanent coagulation of the distal middle cerebral artery. JoVE (Journal of Visualized Experiments) 2014:e51729–e51729. doi: 10.3791/51729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C. Heart disease and stroke statistics—2010 update A report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2011 doi: 10.1016/j.addr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Lu J, Zhong X, Liu H, Hao L, Huang CTL, Sherafat MA, Jones J, Ayala M, Li L, Zhang SC. Generation of serotonin neurons from human pluripotent stem cells. Nature biotechnology. 2016;34:89–94. doi: 10.1038/nbt.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luehr M, Etz CD. Moderate-to-mild hypothermia may not be sufficient to protect the spinal cord during aortic arch surgery. European Journal of Cardio-Thoracic Surgery. 2014;45:767–767. doi: 10.1093/ejcts/ezt349. [DOI] [PubMed] [Google Scholar]
- Lujan E, Chanda S, Ahlenius H, Sudhof TC, Wernig M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci U S A. 2012;109(7):2527–32. doi: 10.1073/pnas.1121003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Wang Z, Li P, Zeng S, Luo Q. A modified mini-stroke model with region-directed reperfusion in rat cortex. J Cereb Blood Flow Metab. 2008;28:973–983. doi: 10.1038/sj.jcbfm.9600591. [DOI] [PubMed] [Google Scholar]
- Mackie AR, Losordo DW. CD34-positive stem cells: in the treatment of heart and vascular disease in human beings. Tex Heart Inst J. 2011;38:474–485. [PMC free article] [PubMed] [Google Scholar]
- Maerz WJ, Baselga J, Reuter VE, Mellado B, Myers ML, Bosl GJ, Spinella MJ, Dmitrovsky E. FGF4 dissociates anti-tumorigenic from differentiation signals of retinoic acid in human embryonal carcinomas. Oncogene. 1998;17:761–767. doi: 10.1038/sj.onc.1201992. [DOI] [PubMed] [Google Scholar]
- Maitra A, Arking DE, Shivapurkar N, Ikeda M, Stastny V, Kassauei K, Sui G, Cutler DJ, Liu Y, Brimble SN, Noaksson K, Hyllner J, Schulz TC, Zeng X, Freed WJ, Crook J, Abraham S, Colman A, Sartipy P, Matsui S, Carpenter M, Gazdar AF, Rao M, Chakravarti A. Genomic alterations in cultured human embryonic stem cells. Nat Genet. 2005;37:1099–1103. doi: 10.1038/ng1631. [DOI] [PubMed] [Google Scholar]
- Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol. 1998;176:57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Malgieri A, Kantzari E, Patrizi MP, Gambardella S. Bone marrow and umbilical cord blood human mesenchymal stem cells: state of the art. Int J Clin Exp Med. 2010;3:248–269. [PMC free article] [PubMed] [Google Scholar]
- Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature. 1997;386:403–407. doi: 10.1038/386403a0. [DOI] [PubMed] [Google Scholar]
- Marcen R. Immunosuppressive drugs in kidney transplantation: impact on patient survival, and incidence of cardiovascular disease, malignancy and infection. Drugs. 2009;69:2227–2243. doi: 10.2165/11319260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Marro S, Pang ZP, Yang N, Tsai MC, Qu K, Chang HY, Sudhof TC, Wernig M. Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell. 2011;9:374–382. doi: 10.1016/j.stem.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall H, Nonchev S, Sham MH, Muchamore I, Lumsden A, Krumlauf R. Retinoic acid alters hindbrain Hox code and induces transformation of rhombomeres 2/3 into a 4/5 identity. Nature. 1992;360:737–741. doi: 10.1038/360737a0. [DOI] [PubMed] [Google Scholar]
- Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11:228–232. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- Maxwell SL, Li M. Midbrain dopaminergic development in vivo and in vitro from embryonic stem cells. J Anat. 2005;207:209–218. doi: 10.1111/j.1469-7580.2005.00453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JW, Howard MJ. Repairing the damaged spinal cord: a summary of our early success with embryonic stem cell transplantation and remyelination. Prog Brain Res. 2002;137:299–309. doi: 10.1016/s0079-6123(02)37023-7. [DOI] [PubMed] [Google Scholar]
- Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K, Nakagawa M, Koyanagi M, Tanabe K, Ohnuki M, Ogawa D, Ikeda E, Okano H, Yamanaka S. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743–745. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- Modo M, Mellodew K, Cash D, Fraser SE, Meade TJ, Price J, Williams SC. Mapping transplanted stem cell migration after a stroke: a serial, in vivo magnetic resonance imaging study. Neuroimage. 2004;21:311–317. doi: 10.1016/j.neuroimage.2003.08.030. [DOI] [PubMed] [Google Scholar]
- Modo M, Rezaie P, Heuschling P, Patel S, Male DK, Hodges H. Transplantation of neural stem cells in a rat model of stroke: assessment of short-term graft survival and acute host immunological response. Brain Res. 2002a;958:70–82. doi: 10.1016/s0006-8993(02)03463-7. [DOI] [PubMed] [Google Scholar]
- Modo M, Stroemer RP, Tang E, Patel S, Hodges H. Effects of implantation site of stem cell grafts on behavioral recovery from stroke damage. Stroke. 2002b;33:2270–2278. doi: 10.1161/01.str.0000027693.50675.c5. [DOI] [PubMed] [Google Scholar]
- Molinari GF. Why model strokes? Stroke. 1988;19:1195–1197. doi: 10.1161/01.str.19.10.1195. [DOI] [PubMed] [Google Scholar]
- Moniche F, Escudero I, Zapata-Arriaza E, Usero-Ruiz M, Prieto-Leon M, de la Torre J, Gamero MA, Tamayo JA, Ochoa-Sepulveda JJ, Maestre J, Carmona M, Pinero P, Calderon-Cabrera C, Jimenez MD, Gonzalez A, Montaner J. Intra-arterial bone marrow mononuclear cells (BM-MNCs) transplantation in acute ischemic stroke (IBIS trial): protocol of a phase II, randomized, dose-finding, controlled multicenter trial. Int J Stroke. 2015;10:1149–1152. doi: 10.1111/ijs.12520. [DOI] [PubMed] [Google Scholar]
- Morizane A, Doi D, Kikuchi T, Nishimura K, Takahashi J. Small-molecule inhibitors of bone morphogenic protein and activin/nodal signals promote highly efficient neural induction from human pluripotent stem cells. J Neurosci Res. 2011;89:117–126. doi: 10.1002/jnr.22547. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després J-P, Fullerton HJ. Heart Disease and Stroke Statistics—2016 Update A Report From the American Heart Association. Circulation, CIR. 2015 doi: 10.1161/CIR.0000000000000350. 0000000000000350. [DOI] [PubMed] [Google Scholar]
- Murray KN, Girard S, Holmes WM, Parkes LM, Williams SR, Parry-Jones AR, Allan SM. Systemic inflammation impairs tissue reperfusion through endothelin-dependent mechanisms in cerebral ischemia. Stroke. 2014;45:3412–3419. doi: 10.1161/STROKEAHA.114.006613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Nagai A, Kim WK, Lee HJ, Jeong HS, Kim KS, Hong SH, Park IH, Kim SU. Multilineage potential of stable human mesenchymal stem cell line derived from fetal marrow. PLoS One. 2007;2:e1272. doi: 10.1371/journal.pone.0001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa H, Kogure K. Correlation between cerebral blood flow and histologic changes in a new rat model of middle cerebral artery occlusion. Stroke. 1989;20:1037–1043. doi: 10.1161/01.str.20.8.1037. [DOI] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Kateriya S, Adeishvili N, Hegemann P, Bamberg E. Channelrhodopsins: directly light-gated cation channels. Biochem Soc Trans. 2005;33:863–866. doi: 10.1042/BST0330863. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- Nakagomi N, Nakagomi T, Kubo S, Nakano-Doi A, Saino O, Takata M, Yoshikawa H, Stern DM, Matsuyama T, Taguchi A. Endothelial cells support survival, proliferation, and neuronal differentiation of transplanted adult ischemia-induced neural stem/progenitor cells after cerebral infarction. Stem Cells. 2009;27:2185–2195. doi: 10.1002/stem.161. [DOI] [PubMed] [Google Scholar]
- Nakazaki M, Sasaki M, Kataoka-Sasaki Y, Oka S, Namioka T, Namioka A, Onodera R, Suzuki J, Sasaki Y, Nagahama H, Mikami T, Wanibuchi M, Kocsis JD, Honmou O. Intravenous infusion of mesenchymal stem cells inhibits intracranial hemorrhage after recombinant tissue plasminogen activator therapy for transient middle cerebral artery occlusion in rats. J Neurosurg. 2017:1–10. doi: 10.3171/2016.8.JNS16240. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Kondziolka D, Wechsler L, Goldstein S, Gebel J, DeCesare S, Elder EM, Zhang PJ, Jacobs A, McGrogan M, Lee VM, Trojanowski JQ. Clonal human (hNT) neuron grafts for stroke therapy: neuropathology in a patient 27 months after implantation. Am J Pathol. 2002;160:1201–1206. doi: 10.1016/S0002-9440(10)62546-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman MB, Misiuta I, Willing AE, Zigova T, Karl RC, Borlongan CV, Sanberg PR. Tumorigenicity issues of embryonic carcinoma-derived stem cells: relevance to surgical trials using NT2 and hNT neural cells. Stem Cells Dev. 2005;14:29–43. doi: 10.1089/scd.2005.14.29. [DOI] [PubMed] [Google Scholar]
- O’Brien MD, Waltz AG. Transorbital approach for occluding the middle cerebral artery without craniectomy. Stroke. 1973;4:201–206. doi: 10.1161/01.str.4.2.201. [DOI] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, Shannon KM, Nauert GM, Perl DP, Godbold J, Freeman TB. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol. 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- Olivier EN, Rybicki AC, Bouhassira EE. Differentiation of human embryonic stem cells into bipotent mesenchymal stem cells. Stem Cells. 2006;24:1914–1922. doi: 10.1634/stemcells.2005-0648. [DOI] [PubMed] [Google Scholar]
- Opitz T, Scheffler B, Steinfarz B, Schmandt T, Brustle O. Electrophysiological evaluation of engrafted stem cell-derived neurons. Nat Protoc. 2007;2:1603–1613. doi: 10.1038/nprot.2007.230. [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasha Z, Wang Y, Sheikh R, Zhang D, Zhao T, Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res. 2008;77:134–142. doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. Journal of Neuroscience. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevsner PH, Eichenbaum JW, Miller DC, Pivawer G, Eichenbaum KD, Stern A, Zakian KL, Koutcher JA. A photothrombotic model of small early ischemic infarcts in the rat brain with histologic and MRI correlation. Journal of pharmacological and toxicological methods. 2001;45:227–233. doi: 10.1016/s1056-8719(01)00153-8. [DOI] [PubMed] [Google Scholar]
- Pfisterer U, Kirkeby A, Torper O, Wood J, Nelander J, Dufour A, Bjorklund A, Lindvall O, Jakobsson J, Parmar M. Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci U S A. 108:10343–10348. doi: 10.1073/pnas.1105135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao CS, Gonzalez-Toledo ME, Xue YQ, Duan WM, Terao S, Granger DN, Kelley RE, Zhao LR. The role of stem cell factor and granulocyte-colony stimulating factor in brain repair during chronic stroke. J Cereb Blood Flow Metab. 2009;29:759–770. doi: 10.1038/jcbfm.2008.168. [DOI] [PubMed] [Google Scholar]
- Pietrini D, Piastra M, Luca E, Mancino A, Conti G, Cavaliere F, De Luca D. Neuroprotection and hypothermia in infants and children. Current drug targets. 2012;13:925–935. doi: 10.2174/138945012800675641. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Studer FE, Wilz A, Simon RP, Boison D. Neuroprotection in ischemic mouse brain induced by stem cell-derived brain implants. Journal of Cerebral Blood Flow & Metabolism. 2007;27:919–927. doi: 10.1038/sj.jcbfm.9600422. [DOI] [PubMed] [Google Scholar]
- Pollock K, Stroemer P, Patel S, Stevanato L, Hope A, Miljan E, Dong Z, Hodges H, Price J, Sinden JD. A conditionally immortal clonal stem cell line from human cortical neuroepithelium for the treatment of ischemic stroke. Exp Neurol. 2006;199:143–155. doi: 10.1016/j.expneurol.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Prasad K, Sharma A, Garg A, Mohanty S, Bhatnagar S, Johri S, Singh KK, Nair V, Sarkar RS, Gorthi SP. Intravenous Autologous Bone Marrow Mononuclear Stem Cell Therapy for Ischemic Stroke A Multicentric, Randomized Trial. Stroke. 2014;45:3618–3624. doi: 10.1161/STROKEAHA.114.007028. [DOI] [PubMed] [Google Scholar]
- Prass K, Scharff A, Ruscher K, Lowl D, Muselmann C, Victorov I, Kapinya K, Dirnagl U, Meisel A. Hypoxia-induced stroke tolerance in the mouse is mediated by erythropoietin. Stroke. 2003;34:1981–1986. doi: 10.1161/01.STR.0000080381.76409.B2. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Brierley JB. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979;10:267–272. doi: 10.1161/01.str.10.3.267. [DOI] [PubMed] [Google Scholar]
- Puyal J, Ginet V, Clarke PG. Multiple interacting cell death mechanisms in the mediation of excitotoxicity and ischemic brain damage: a challenge for neuroprotection. Progress in neurobiology. 2013;105:24–48. doi: 10.1016/j.pneurobio.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A, Ben-Hur T. Neural progenitors from human embryonic stem cells. Nat Biotechnol. 2001;19:1134–1140. doi: 10.1038/nbt1201-1134. [DOI] [PubMed] [Google Scholar]
- Robbins RD, Prasain N, Maier BF, Yoder MC, Mirmira RG. Inducible pluripotent stem cells: not quite ready for prime time? Curr Opin Organ Transplant. 15:61–67. doi: 10.1097/MOT.0b013e3283337196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin AM, Zhang ZG, Wang L, Zhang RL, Katakowski M, Zhang L, Wang Y, Zhang C, Chopp M. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:125–134. doi: 10.1038/sj.jcbfm.9600172. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Macrae IM, Todd M, Reid JL, McCulloch J. Reduction of local cerebral blood flow to pathological levels by endothelin-1 applied to the middle cerebral artery in the rat. Neurosci Lett. 1990;118:269–272. doi: 10.1016/0304-3940(90)90644-o. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2012 update: a report from the american heart association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers NJ, Lechler RI. Allorecognition. Am J Transplant. 2001;1:97–102. [PubMed] [Google Scholar]
- Rohwedel J, Guan K, Wobus AM. Induction of cellular differentiation by retinoic acid in vitro. Cells Tissues Organs. 1999;165:190–202. doi: 10.1159/000016699. [DOI] [PubMed] [Google Scholar]
- Rola R, Mizumatsu S, Otsuka S, Morhardt DR, Noble-Haeusslein LJ, Fishman K, Potts MB, Fike JR. Alterations in hippocampal neurogenesis following traumatic brain injury in mice. Exp Neurol. 2006;202:189–199. doi: 10.1016/j.expneurol.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Roome RB, Bartlett RF, Jeffers M, Xiong J, Corbett D, Vanderluit JL. A reproducible Endothelin-1 model of forelimb motor cortex stroke in the mouse. Journal of neuroscience methods. 2014;233:34–44. doi: 10.1016/j.jneumeth.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Rosenblum WI, El-Sabban F. Platelet aggregation in the cerebral microcirculation: effect of aspirin and other agents. Circ Res. 1977;40:320–328. doi: 10.1161/01.res.40.3.320. [DOI] [PubMed] [Google Scholar]
- Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell–derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nature medicine. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- Sakata H, Niizuma K, Yoshioka H, Kim GS, Jung JE, Katsu M, Narasimhan P, Maier CM, Nishiyama Y, Chan PH. Minocycline-preconditioned neural stem cells enhance neuroprotection after ischemic stroke in rats. J Neurosci. 2012;32:3462–3473. doi: 10.1523/JNEUROSCI.5686-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samper E, Diez-Juan A, Montero J, Sepúlveda P. Cardiac cell therapy: boosting mesenchymal stem cells effects. Stem Cell Reviews and Reports. 2013;9:266–280. doi: 10.1007/s12015-012-9353-z. [DOI] [PubMed] [Google Scholar]
- Sart S, Ma T, Li Y. Preconditioning stem cells for in vivo delivery. BioResearch open access. 2014;3:137–149. doi: 10.1089/biores.2014.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz SI, Chopp M, Deans R, Carmichael ST, Phinney D, Wechsler L. Stem Cell Therapy as an Emerging Paradigm for Stroke (STEPS) II. Stroke. 2011;42:825–829. doi: 10.1161/STROKEAHA.110.601914. [DOI] [PubMed] [Google Scholar]
- Savitz SI, Dinsmore J, Wu J, Henderson GV, Stieg P, Caplan LR. Neurotransplantation of fetal porcine cells in patients with basal ganglia infarcts: a preliminary safety and feasibility study. Cerebrovasc Dis. 2005;20:101–107. doi: 10.1159/000086518. [DOI] [PubMed] [Google Scholar]
- Scheffler B, Schmandt T, Schroder W, Steinfarz B, Husseini L, Wellmer J, Seifert G, Karram K, Beck H, Blumcke I, Wiestler OD, Steinhauser C, Brustle O. Functional network integration of embryonic stem cell-derived astrocytes in hippocampal slice cultures. Development. 2003;130:5533–5541. doi: 10.1242/dev.00714. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Diederich K, Strecker JK, Geng B, Hoppen M, Duning T, Schäbitz WR, Minnerup J. Progressive Cognitive Deficits in a Mouse Model of Recurrent Photothrombotic Stroke. Stroke. 2015;46:1127–1131. doi: 10.1161/STROKEAHA.115.008905. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Minnerup J. Promoting recovery from ischemic stroke. Expert Rev Neurotherapeutics. 2016;16(2):173–86. doi: 10.1586/14737175.2016.1134324. [DOI] [PubMed] [Google Scholar]
- Sharkey J, Ritchie IM, Kelly PA. Perivascular microapplication of endothelin-1: a new model of focal cerebral ischaemia in the rat. J Cereb Blood Flow Metab. 1993;13:865–871. doi: 10.1038/jcbfm.1993.108. [DOI] [PubMed] [Google Scholar]
- Shibata H, Ageyama N, Tanaka Y, Kishi Y, Sasaki K, Nakamura S, Muramatsu S, Hayashi S, Kitano Y, Terao K, Hanazono Y. Improved safety of hematopoietic transplantation with monkey embryonic stem cells in the allogeneic setting. Stem Cells. 2006;24:1450–1457. doi: 10.1634/stemcells.2005-0391. [DOI] [PubMed] [Google Scholar]
- Siatskas C, Payne NL, Short MA, Bernard CC. A consensus statement addressing mesenchymal stem cell transplantation for multiple sclerosis: it’s time! Stem Cell Rev. 6:500–506. doi: 10.1007/s12015-010-9173-y. [DOI] [PubMed] [Google Scholar]
- Simeone A, Acampora D, Nigro V, Faiella A, D’Esposito M, Stornaiuolo A, Mavilio F, Boncinelli E. Differential regulation by retinoic acid of the homeobox genes of the four HOX loci in human embryonal carcinoma cells. Mech Dev. 1991;33:215–227. doi: 10.1016/0925-4773(91)90029-6. [DOI] [PubMed] [Google Scholar]
- Simpson E. A historical perspective on immunological privilege. Immunol Rev. 2006;213:12–22. doi: 10.1111/j.1600-065X.2006.00434.x. [DOI] [PubMed] [Google Scholar]
- Sloan DJ, Wood MJ, Charlton HM. The immune response to intracerebral neural grafts. Trends Neurosci. 1991;14:341–346. doi: 10.1016/0166-2236(91)90159-r. [DOI] [PubMed] [Google Scholar]
- Smart I. Subependymal Layer of Mouse Brain and Its Cell Production as Shown by Radioautography after Thymidine-H3 Injection. J Comp Neurol. 1961;116:325. [Google Scholar]
- Smith ML, Auer RN, Siesjo BK. The density and distribution of ischemic brain injury in the rat following 2–10 min of forebrain ischemia. Acta Neuropathologica. 1984;64:319–332. doi: 10.1007/BF00690397. [DOI] [PubMed] [Google Scholar]
- Sommer CA, Stadtfeld M, Murphy GJ, Hochedlinger K, Kotton DN, Mostoslavsky G. Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells. 2009;27:543–549. doi: 10.1634/stemcells.2008-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Yu SP. Ionic regulation of cell volume changes and cell death after ischemic stroke. Translational stroke research. 2014;5:17–27. doi: 10.1007/s12975-013-0314-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Yu SP, Mohamad O, Cao W, Wei ZZ, Gu X, Jiang MQ, Wei L. Optogenetic stimulation of glutamatergic neuronal activity in the striatum enhances neurogenesis in the subventricular zone of normal and stroke mice. Neurobiol Dis. 2017;98:9–24. doi: 10.1016/j.nbd.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevanato L, Corteling RL, Stroemer P, Hope A, Heward J, Miljan EA, Sinden JD. c-MycERTAM transgene silencing in a genetically modified human neural stem cell line implanted into MCAo rodent brain. BMC Neurosci. 2009;10:86. doi: 10.1186/1471-2202-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens LC, Little CC. Spontaneous Testicular Teratomas in an Inbred Strain of Mice. Proc Natl Acad Sci U S A. 1954;40:1080–1087. doi: 10.1073/pnas.40.11.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecker JK, Olk J, Hoppen M, Gess B, Diederich K, Schmidt A, Schäbitz WR, Schilling M, Minnerup J. Combining Growth Factor and Bone Marrow Cell Therapy Induces Bleeding and Alters Immune Response After Stroke in Mice. Stroke. 2016;47:852–862. doi: 10.1161/STROKEAHA.115.011230. [DOI] [PubMed] [Google Scholar]
- Stroh A, Tsai HC, Wang LP, Zhang F, Kressel J, Aravanis A, Santhanam N, Deisseroth K, Konnerth A, Schneider MB. Tracking stem cell differentiation in the setting of automated optogenetic stimulation. Stem Cells. 2011;29:78–88. doi: 10.1002/stem.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Xu J, Ji BX, Wan SG, Lu CY, Dong HQ, Yu YY, Lu DP. Autologous peripheral blood stem cell transplantation for severe multiple sclerosis. Int J Hematol. 2006;84:276–281. doi: 10.1532/IJH97.A10516. [DOI] [PubMed] [Google Scholar]
- Suarez-Monteagudo C, Hernandez-Ramirez P, Alvarez-Gonzalez L, Garcia-Maeso I, de la Cuetara-Bernal K, Castillo-Diaz L, Bringas-Vega ML, Martinez-Aching G, Morales-Chacon LM, Baez-Martin MM, Sanchez-Catasus C, Carballo-Barreda M, Rodriguez-Rojas R, Gomez-Fernandez L, Alberti-Amador E, Macias-Abraham C, Balea ED, Rosales LC, Del Valle Perez L, Ferrer BB, Gonzalez RM, Bergado JA. Autologous bone marrow stem cell neurotransplantation in stroke patients. An open study. Restor Neurol Neurosci. 2009;27:151–161. doi: 10.3233/RNN-2009-0483. [DOI] [PubMed] [Google Scholar]
- Sun J, Wei ZZ, Gu X, Zhang JY, Zhang Y, Li J, Wei L. Intranasal delivery of hypoxia-preconditioned bone marrow-derived mesenchymal stem cells enhanced regenerative effects after intracerebral hemorrhagic stroke in mice. Experimental neurology. 2015;272:78–87. doi: 10.1016/j.expneurol.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Suzuki IK, Vanderhaeghen P. Is this a brain which I see before me? Modeling human neural development with pluripotent stem cells. Development. 2015;142:3138–3150. doi: 10.1242/dev.120568. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM, Naritomi H, Matsuyama T. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yasuhara T, Shingo T, Muraoka K, Kameda M, Takeuchi A, Yano A, Kurozumi K, Agari T, Miyoshi Y, Kinugasa K, Date I. Embryonic neural stem cells transplanted in middle cerebral artery occlusion model of rats demonstrated potent therapeutic effects, compared to adult neural stem cells. Brain Res. 2008;1234:172–182. doi: 10.1016/j.brainres.2008.07.086. [DOI] [PubMed] [Google Scholar]
- Tambuyzer BR, Bergwerf I, De Vocht N, Reekmans K, Daans J, Jorens PG, Goossens H, Ysebaert DK, Chatterjee S, Van Marck E, Berneman ZN, Ponsaerts P. Allogeneic stromal cell implantation in brain tissue leads to robust microglial activation. Immunol Cell Biol. 2009;87:267–273. doi: 10.1038/icb.2009.12. [DOI] [PubMed] [Google Scholar]
- Tamura A, Graham DI, McCulloch J, Teasdale GM. Focal cerebral ischaemia in the rat: 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1981a;1:53–60. doi: 10.1038/jcbfm.1981.6. [DOI] [PubMed] [Google Scholar]
- Tamura A, Graham DI, McCulloch J, Teasdale GM. Focal cerebral ischaemia in the rat: 2. Regional cerebral blood flow determined by [14C]iodoantipyrine autoradiography following middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1981b;1:61–69. doi: 10.1038/jcbfm.1981.7. [DOI] [PubMed] [Google Scholar]
- Tan Z, Su ZY, Wu RR, Gu B, Liu YK, Zhao XL, Zhang M. Immunomodulative effects of mesenchymal stem cells derived from human embryonic stem cells in vivo and in vitro. J Zhejiang Univ Sci B. 12:18–27. doi: 10.1631/jzus.B1000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P, Gage FH. Adult neurogenesis and neural stem cells of the central nervous system in mammals. J Neurosci Res. 2002;69:745–749. doi: 10.1002/jnr.10378. [DOI] [PubMed] [Google Scholar]
- Therapies SC. Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS): bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke. 2009;40:510–515. doi: 10.1161/STROKEAHA.108.526863. [DOI] [PubMed] [Google Scholar]
- Theus MH, Wei L, Cui L, Francis K, Hu X, Keogh C, Yu SP. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol. 2008a;210:656–670. doi: 10.1016/j.expneurol.2007.12.020. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38:3032–3039. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- Tissier R, Ghaleh B, Cohen MV, Downey JM, Berdeaux A. Myocardial protection with mild hypothermia. Cardiovascular research. 2012;94:217–225. doi: 10.1093/cvr/cvr315. [DOI] [PubMed] [Google Scholar]
- Tonnesen J, Parish CL, Sorensen AT, Andersson A, Lundberg C, Deisseroth K, Arenas E, Lindvall O, Kokaia M. Functional integration of grafted neural stem cell-derived dopaminergic neurons monitored by optogenetics in an in vitro Parkinson model. PLoS One. 2011;6:e17560. doi: 10.1371/journal.pone.0017560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornabene E, Brodin B. Stroke and Drug Delivery In Vitro Models of the Ischemic Blood-Brain Barrier. Journal of pharmaceutical sciences. 2016;105:398–405. doi: 10.1016/j.xphs.2015.11.041. [DOI] [PubMed] [Google Scholar]
- Toyoda K. Epidemiology and registry studies of stroke in Japan. J Stroke. 2013;15:21–26. doi: 10.5853/jos.2013.15.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima A, Yasuhara T, Kameda M, Morimoto J, Takeuchi H, Wang F, Sasaki T, Sasada S, Shinko A, Wakamori T, Okazaki M, Kondo A, Agari T, Borlongan CV, Date I. Intra-Arterial Transplantation of Allogeneic Mesenchymal Stem Cells Mounts Neuroprotective Effects in a Transient Ischemic Stroke Model in Rats: Analyses of Therapeutic Time Window and Its Mechanisms. PLoS One. 2015;10:e0127302. doi: 10.1371/journal.pone.0127302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PT, Ohab JJ, Kertesz N, Groszer M, Matter C, Gao J, Liu X, Wu H, Carmichael ST. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J Neurosci. 2006;26:1269–1274. doi: 10.1523/JNEUROSCI.4480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- van der Worp HB, Sena ES, Donnan GA, Howells DW, Macleod MR. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain. 2007;130:3063–3074. doi: 10.1093/brain/awm083. [DOI] [PubMed] [Google Scholar]
- Veizovic T, Beech JS, Stroemer RP, Watson WP, Hodges H. Resolution of stroke deficits following contralateral grafts of conditionally immortal neuroepithelial stem cells. Stroke. 2001;32:1012–1019. doi: 10.1161/01.str.32.4.1012. [DOI] [PubMed] [Google Scholar]
- Vendrame M, Cassady J, Newcomb J, Butler T, Pennypacker KR, Zigova T, Sanberg CD, Sanberg PR, Willing AE. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke. 2004;35:2390–2395. doi: 10.1161/01.STR.0000141681.06735.9b. [DOI] [PubMed] [Google Scholar]
- Vidal SE, Stadtfeld M, Apostolou E. F-Class Cells: New Routes and Destinations for Induced Pluripotency. Cell stem cell. 2015;16:9–10. doi: 10.1016/j.stem.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villars F, Bordenave L, Bareille R, Amedee J. Effect of human endothelial cells on human bone marrow stromal cell phenotype: role of VEGF? J Cell Biochem. 2000;79:672–685. doi: 10.1002/1097-4644(20001215)79:4<672::aid-jcb150>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Volpe G, Bernstock JD, Peruzzotti-Jametti L, Pluchino S. Modulation of host immune responses following non-hematopoietic stem cell transplantation: Translational implications in progressive multiple sclerosis. J Neuroimmunol. 2016;(16):30312–5. doi: 10.1016/j.jneuroim.2016.12.005. pii: S0165-5728. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Wahlgren N, Ahmed N, Davalos A, Hacke W, Millan M, Muir K, Roine RO, Toni D, Lees KR. Thrombolysis with alteplase 3–4.5 h after acute ischaemic stroke (SITS-ISTR): an observational study. Lancet. 2008;372:1303–1309. doi: 10.1016/S0140-6736(08)61339-2. [DOI] [PubMed] [Google Scholar]
- Wang CX, Yang T, Shuaib A. An improved version of embolic model of brain ischemic injury in the rat. J Neurosci Methods. 2001;109:147–151. doi: 10.1016/s0165-0270(01)00408-3. [DOI] [PubMed] [Google Scholar]
- Wang LL, Chen D, Lee J, Gu X, Alaaeddine G, Li J, Wei L, Yu SP. Mobilization of endogenous bone marrow derived endothelial progenitor cells and therapeutic potential of parathyroid hormone after ischemic stroke in mice. PLoS One. 2014;9:e87284. doi: 10.1371/journal.pone.0087284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jin K, Mao XO, Xie L, Banwait S, Marti HH, Greenberg DA. VEGF-overexpressing transgenic mice show enhanced post-ischemic neurogenesis and neuromigration. J Neurosci Res. 2007;85:740–747. doi: 10.1002/jnr.21169. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Uchida K, Nakajima H, Matsuo H, Sugita D, Yoshida A, Honjoh K, Johnson WE, Baba H. Early Transplantation of Mesenchymal Stem Cells After Spinal Cord Injury Relieves Pain Hypersensitivity Through Suppression of Pain - Related Signaling Cascades and Reduced Inflammatory Cell Recruitment. Stem Cells. 2015;33:1902–1914. doi: 10.1002/stem.2006. [DOI] [PubMed] [Google Scholar]
- Watson BD, Dietrich WD, Busto R, Wachtel MS, Ginsberg MD. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann Neurol. 1985;17:497–504. doi: 10.1002/ana.410170513. [DOI] [PubMed] [Google Scholar]
- Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- Wei L, Cui L, Snider BJ, Rivkin M, Yu SS, Lee CS, Adams LD, Gottlieb DI, Johnson EM, Jr, Yu SP, Choi DW. Transplantation of embryonic stem cells overexpressing Bcl-2 promotes functional recovery after transient cerebral ischemia. Neurobiol Dis. 2005;19:183–193. doi: 10.1016/j.nbd.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Wei L, Fraser JL, Lu ZY, Hu X, Yu SP. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol Dis. 2012;46(3):635–45. doi: 10.1016/j.nbd.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Keogh CL, Whitaker VR, Theus MH, Yu SP. Angiogenesis and stem cell transplantation as potential treatments of cerebral ischemic stroke. Pathophysiology. 2005b;12:47–62. doi: 10.1016/j.pathophys.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Wei L, Rovainen CM, Woolsey TA. Ministrokes in rat barrel cortex. Stroke. 1995;26:1459–1462. doi: 10.1161/01.str.26.8.1459. [DOI] [PubMed] [Google Scholar]
- Wei L, Ying DJ, Cui L, Langsdorf J, Yu SP. Necrosis, apoptosis and hybrid death in the cortex and thalamus after barrel cortex ischemia in rats. Brain research. 2004;1022:54–61. doi: 10.1016/j.brainres.2004.06.080. [DOI] [PubMed] [Google Scholar]
- Wei N, Yu SP, Gu X, Taylor TM, Song D, Liu XF, Wei L. Delayed intranasal delivery of hypoxic-preconditioned bone marrow mesenchymal stem cells enhanced cell homing and therapeutic benefits after ischemic stroke in mice. Cell Transplant. 2013;22:977–991. doi: 10.3727/096368912X657251. [DOI] [PubMed] [Google Scholar]
- Wei ZZ, Gu X, Ferdinand A, Lee JH, Ji X, Ji XM, Yu SP, Wei L. Intranasal delivery of bone marrow mesenchymal stem cells improved neurovascular regeneration and rescued neuropsychiatric deficits after neonatal stroke in rats. Cell transplantation. 2015;24:391–402. doi: 10.3727/096368915X686887. [DOI] [PubMed] [Google Scholar]
- Weick JP, Johnson MA, Skroch SP, Williams JC, Deisseroth K, Zhang SC. Functional control of transplantable human ESC-derived neurons via optogenetic targeting. Stem Cells. 2010;28:2008–2016. doi: 10.1002/stem.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werbowetski-Ogilvie TE, Bosse M, Stewart M, Schnerch A, Ramos-Mejia V, Rouleau A, Wynder T, Smith MJ, Dingwall S, Carter T, Williams C, Harris C, Dolling J, Wynder C, Boreham D, Bhatia M. Characterization of human embryonic stem cells with features of neoplastic progression. Nat Biotechnol. 2009;27:91–97. doi: 10.1038/nbt.1516. [DOI] [PubMed] [Google Scholar]
- Wernig M, Benninger F, Schmandt T, Rade M, Tucker KL, Bussow H, Beck H, Brustle O. Functional integration of embryonic stem cell-derived neurons in vivo. J Neurosci. 2004;24:5258–5268. doi: 10.1523/JNEUROSCI.0428-04.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker VR, Cui L, Miller S, Yu SP, Wei L. Whisker stimulation enhances angiogenesis in the barrel cortex following focal ischemia in mice. J Cerebr Blood F Met. 2007;27:57–68. doi: 10.1038/sj.jcbfm.9600318. [DOI] [PubMed] [Google Scholar]
- Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, Wagner EF, Metcalf D, Nicola NA, Gough NM. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- Windle V, Szymanska A, Granter-Button S, White C, Buist R, Peeling J, Corbett D. An analysis of four different methods of producing focal cerebral ischemia with endothelin-1 in the rat. Exp Neurol. 2006;201:324–334. doi: 10.1016/j.expneurol.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Wise AF, Williams TM, Kiewiet MB, Payne NL, Siatskas C, Samuel CS, Ricardo SD. Human mesenchymal stem cells alter macrophage phenotype and promote regeneration via homing to the kidney following ischemia-reperfusion injury. American Journal of Physiology-Renal Physiology. 2014;306:F1222–F1235. doi: 10.1152/ajprenal.00675.2013. [DOI] [PubMed] [Google Scholar]
- Wollert KC, Drexler H. Cell therapy for the treatment of coronary heart disease: a critical appraisal. Nat Rev Cardiol. doi: 10.1038/nrcardio.2010.1. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Rovainen CM, Cox SB, Henegar MH, Liang GE, Liu D, Moskalenko YE, Sui J, Wei L. Neuronal units linked to microvascular modules in cerebral cortex: response elements for imaging the brain. Cerebral Cortex. 1996;6:647–660. doi: 10.1093/cercor/6.5.647. [DOI] [PubMed] [Google Scholar]
- Xiao AY, Wei L, Xia S, Rothman S, Yu SP. Ionic mechanism of ouabain-induced concurrent apoptosis and necrosis in individual cultured cortical neurons. The Journal of neuroscience. 2002;22:1350–1362. doi: 10.1523/JNEUROSCI.22-04-01350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, Ye X, Chopp M, Zacharek A, Ning R, Venkat P, Roberts C, Lu M, Chen J. Niaspan attenuates the adverse effects of bone marrow stromal cell treatment of stroke in type one diabetic rats. PLoS One. 2013;8:e81199. doi: 10.1371/journal.pone.0081199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa D, Qi M, Kim DH, Kitamura Y, Inden M, Tsuchiya D, Takata K, Taniguchi T, Yoshimoto K, Shimohama S, Akaike A, Sumi S, Inoue K. Improvement of focal ischemia-induced rat dopaminergic dysfunction by striatal transplantation of mouse embryonic stem cells. Neurosci Lett. 2006;407:74–79. doi: 10.1016/j.neulet.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Yang D, Zhang ZJ, Oldenburg M, Ayala M, Zhang SC. Human Embryonic Stem Cell - Derived Dopaminergic Neurons Reverse Functional Deficit in Parkinsonian Rats. Stem Cells. 2008;26:55–63. doi: 10.1634/stemcells.2007-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Battiwalla M, Ito S, Feng X, Chinian F, Melenhorst JJ, Koklanaris E, Sabatino M, Stroncek D, Samsel L. Bone marrow mesenchymal stromal cells to treat tissue damage in allogeneic stem cell transplant recipients: correlation of biological markers with clinical responses. Stem Cells. 2014;32:1278–1288. doi: 10.1002/stem.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SH, Shim YS, Park YH, Chung JK, Nam JH, Kim MO, Park HC, Park SR, Min BH, Kim EY, Choi BH, Park H, Ha Y. Complete spinal cord injury treatment using autologous bone marrow cell transplantation and bone marrow stimulation with granulocyte macrophage-colony stimulating factor: Phase I/II clinical trial. Stem Cells. 2007;25:2066–2073. doi: 10.1634/stemcells.2006-0807. [DOI] [PubMed] [Google Scholar]
- Yu D, Silva GA. Stem cell sources and therapeutic approaches for central nervous system and neural retinal disorders. Neurosurg Focus. 2008;24:E11. doi: 10.3171/FOC/2008/24/3-4/E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HM, Zhi JL, Cui Y, Tang EH, Sun SN, Feng JQ, Chen PX. Role of the JAK-STAT pathway in protection of hydrogen peroxide preconditioning against apoptosis induced by oxidative stress in PC12 cells. Apoptosis. 2006;11:931–941. doi: 10.1007/s10495-006-6578-9. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Yu SP, Wei Z, Wei L. Preconditioning strategy in stem cell transplantation therapy. Translational stroke research. 2013;4:76–88. doi: 10.1007/s12975-012-0251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- Zhang L, Schallert T, Zhang ZG, Jiang Q, Arniego P, Li Q, Lu M, Chopp M. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J Neurosci Methods. 2002;117:207–214. doi: 10.1016/s0165-0270(02)00114-0. [DOI] [PubMed] [Google Scholar]
- Zhang P, Li J, Liu Y, Chen X, Kang Q. Transplanted human embryonic neural stem cells survive, migrate, differentiate and increase endogenous nestin expression in adult rat cortical peri-infarction zone. Neuropathology. 2009a doi: 10.1111/j.1440-1789.2008.00993.x. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Gregg SR, Toh Y, Roberts C, Letourneau Y, Buller B, Jia L, SPND, Zhang ZG. Patterns and dynamics of subventricular zone neuroblast migration in the ischemic striatum of the adult mouse. J Cereb Blood Flow Metab. 2009b;29:1240–1250. doi: 10.1038/jcbfm.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR. A rat model of focal embolic cerebral ischemia. Brain Res. 1997;766:83–92. doi: 10.1016/s0006-8993(97)00580-5. [DOI] [PubMed] [Google Scholar]
- Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low WC. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol. 2002;174:11–20. doi: 10.1006/exnr.2001.7853. [DOI] [PubMed] [Google Scholar]
- Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- Zhou J, Su P, Li D, Tsang S, Duan E, Wang F. High-efficiency induction of neural conversion in human ESCs and human induced pluripotent stem cells with a single chemical inhibitor of transforming growth factor beta superfamily receptors. Stem Cells. 2010;28:1741–1750. doi: 10.1002/stem.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Avidan H, Pluchino S, Martino G, Schwartz M. Synergy between immune cells and adult neural stem/progenitor cells promotes functional recovery from spinal cord injury. Proc Natl Acad Sci U S A. 2006a;103:13174–13179. doi: 10.1073/pnas.0603747103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006b;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]