Abstract
Background
Medicare cost-saving initiatives focus on specific conditions; little is known about patient-centered markers of costs that cut across conditions.
Objectives
Given the prevalence and impact of functional impairment on outcomes for community-dwelling seniors, we assessed effects of pre-admission functional impairment on Medicare costs of post-acute care up to 365 days after hospital discharge.
Study Design, Participants, and Setting
We created a nationally-representative sample of 16,673 Medicare hospitalizations for 8,559 community-dwelling seniors from 2000–2012 using the Health and Retirement Study (HRS).
Main Outcome and Measurements
Main outcome was total Medicare costs in the year after hospital discharge assessed by Medicare claims data. Main predictor was functional impairment (level of difficulty or dependence in Activities of Daily Living) determined from HRS interview preceding hospitalization. We performed multivariable linear regression adjusted for age, race, gender, income, and net worth, comorbidities, with clustering at patient level to characterize the association of functional impairment and costs of post-acute care.
Results
Unadjusted mean Medicare costs for one year after discharge increased with severity of impairment in a dose-response fashion (p<.001 for trend): 68% had no functional impairment ($25,931); 17% had 1 ADL difficulty ($32,501), 7% had 1 ADL dependency ($39,928), and 8% had dependency in ≥2 ADLs ($45,895). Compared to those with no impairment, the most severely-impaired cost 77% more; adjusted analyses showed attenuated effect size (33% more) but no change in trend. Considering costs attributable to comorbidities, only specific 3 conditions were more expensive than severe functional impairment (lymphoma, metastatic cancer, and paralysis).
Conclusions
Functional impairment is associated with increased Medicare costs for post-acute care and may be an unmeasured but important marker of long-term costs that cuts across conditions.
Keywords: Costs of Care, Medicare, post-acute care, functional impairment
BACKGROUND
Costs of care for Medicare seniors are a top priority for healthcare reform and an issue of increasing concern in broader public policy debates on overall government spending in the United States.1 Recent studies have attributed rising per-patient Medicare costs to increasing prevalence of chronic conditions2,3,4 with multi-morbid patients representing a disproportionate share.5 Accordingly, current Medicare policies for cost containment prioritize specific conditions such as heart failure. To date, identifying patient-level factors that drive utilization and costs use across multiple conditions and care settings has proven elusive.
Functional status is an excellent barometer of overall disease burden6 and a robust literature suggests it may tell us more about overall health status than condition-specific markers as it is a direct measure of the end impact of all the patient’s illnesses.7,8,9,10,11 Recent research by our group and others has suggested functional impairment outperforms comorbidity for predicting outcomes of acute care such as readmission;12,13,14,15,16 however, very few studies have examined post-acute costs associated with functional impairment. These studies have shown increased cost with higher levels of functional impairment, but they have been limited to patients admitted for acute stroke.17, 18 While functional assessment is often part of routine care for these patients, functional status is not routinely assessed in acute care hospitals and is not reported to Medicare in hospital claims data. Given the strong associations between functional status and other outcomes of hospitalization across many conditions, it may be that functional status is an “unmeasured” patient-level marker for post-acute utilization and costs in the general population of hospitalized Medicare seniors.
Acute care transitions are a major focus of cost-reduction reforms in the Affordable Care Act as hospital admissions are high-cost episodes of care.19 These programs, such as readmission penalties and bundled payments, focus on the hospital stay and a transition period up to 30 days after discharge.20 This focus, however, likely captures only a portion of post-acute costs which are now the fastest-growing and most geographically-variable component of Medicare spending.21,22,23 Moreover, incident hospitalization identifies a subset of high-cost Medicare patients who are likely to require repeated episodes of acute and post-acute care yet there is limited data about what drives costs in these patients over time. To date, only a handful of studies have directly examined the relationship between functional impairment and costs in community (non-hospital) settings24,25 and none have explored functional impairment as a marker of Medicare costs for hospitalized seniors during the post-acute phase of care.
To address these gaps, we utilized longitudinal, nationally-representative survey data from the Health and Retirement Study (HRS) which includes uniform functional assessments of community-dwelling seniors linked to Medicare claims from 2000–2012. We used this dataset to create a cohort of hospitalized Medicare seniors and examine the effects of functional impairment on total costs to Medicare of post-acute care up to 365 days after an incident hospitalization. We hypothesized that functional impairment would be associated with higher costs of care and that severity of impairment would be correlated with higher costs even after adjustments for clinical and demographic features. Greater understanding of functional impairment is crucial to reducing costs of care and increasing attention to unmeasured functional impairment in older, hospitalized adults.
METHODS
Participants
The Health and Retirement Study (HRS) was designed to examine changes in health and wealth as people age.26 HRS is an ongoing nationally-representative longitudinal study of participants age 50 and older with follow up surveys administered to all participants in waves every 2 years; response rates range from 80–90% and over 85% of participants agree to have their responses linked to their Medicare claims data. The study started in 1992 and new community-dwelling participants are recruited every six years to remain representative of the aging US population.27
We created a cohort of community-dwelling participants age 65 or older who were admitted to a hospital between January 1, 2000 and December 31, 2011 to allow for 365 days of follow-up through 2012. To identify hospital admissions, we linked HRS survey data to Medicare claims and searched for inpatient claims in Medicare files. We identified 11,793 participants with at least one admission to an eligible hospital (acute care hospitals only; no rehabilitation or PPS-exempt cancer hospitals) during the sampling frame. We excluded patients for the following reasons: 1. Transition to HMO plan after discharge as complete claims data is not available for managed care admissions (1,924; 16%); 2. Death in hospital or within 30 days of discharge (714; 6%); 3. Less than 12 months of Medicare claims prior to admission required to determine comorbidities from ICD-9 codes (249; 2%); 4. no HRS interview within the preceding 2 survey waves (347; 3%).
Since our objective was to characterize total costs of care for a period of 365 days after an index hospitalization and our study followed patients over 12 years, we allowed multiple 365-day observation periods for individual patients provided these periods did not overlap. Thus, if a subject were admitted again after completing a 365-day observation period, this admission could become another index admission, provided the same inclusion/exclusion criteria above were met for that admission. See figure 1 below demonstrating how various events might occur within an initial 365-day observation period. Our final sample contained 16,673 index admissions from 8,559 participants.
Figure 1.
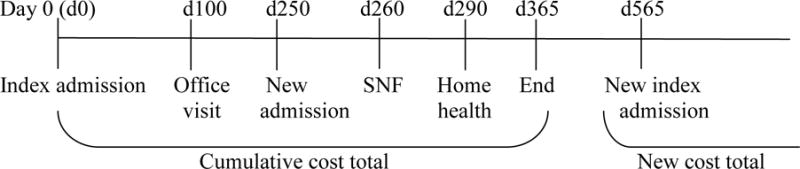
Enrollment timeline
Measures
Primary Predictor: functional impairment
We employed a widely-used measure of disability in older adults (Activities of Daily Living, ADL) obtained from the HRS interview that most closely preceding the index hospital admission. The ADL are a series of self-care activities essential to living independently in the community28, 29 which include bathing, dressing, transferring, toileting, and eating. We created an ordinal variable for ADLs reflecting the clinical continuum and natural history of impairment30 with 4 levels: no impairment, difficulty with any ADL task, dependency with 1 ADL task, and dependency in ≥2 ADL tasks. Difficulty in any ADL indicates the task is burdensome but can be accomplished without assistance from another person whereas dependency in any ADL indicates the individual cannot accomplish that task without assistance from another person. The timing of ADL assessments relative to hospitalization varied among participants from 0 to 24 months prior to admission with an inter-quartile range 202–614 days and an average 423 days. To assess whether this timing was instrumentally important to any association between pre-admission functional status and post-acute costs of care, we performed a sensitivity analysis restricted to subjects with functional assessments within 6 months preceding admission.
Main Outcome: post-acute costs of care to Medicare
To avoid focusing narrowly on costs of care that occur within the transitional period after discharge, we took an all-inclusive approach and defined post-acute care costs as any cost of care billed to Medicare within one year of discharge from an incident hospital admission. We used Medicare data to calculate total costs paid by Medicare after each index admission for up to 365 days after discharge. We used a cumulative variable in the Medicare claims dataset created by summing all costs paid from the Medicare trust fund for services covered by the claim record for each beneficiary in any given year. The amount for each claim is calculated by the financial intermediary or carrier and represents what was actually paid to the institutional provider, physician, or supplier. This summary variable includes costs for Part A (hospital care), Part B (outpatient care such as clinics and home health, as well as provider or “carrier” claims), and Part C (durable medical equipment) but does not include Part D (prescriptions). We adjusted the cost variable for inflation (2012 dollars) based on the medical expenditures component of the Consumer Price Index by the U.S. Bureau of Labor Statistics.3,1
Other Measurements
We considered health and demographic factors shown to impact costs of care in prior studies that could introduce confounding into our analyses. Demographic factors included age, gender, race and/or ethnicity, marital status, education, income and wealth. Health factors included the Elixhauser comorbidity score calculated from ICD-9 codes and any hospitalization within one year prior to the index admission. Comorbidities for the Elixhauser index were determined using Medicare claims data from the 365 days preceding the index admission. The Elixhauser comorbidity index consists of 30 individual conditions which have been shown to predict mortality 1 year after hospitalization more accurately than the Charlson Score3,2 and is similar to the proprietary Hierarchical Conditions Category (HCC) used by Medicare to adjust for comorbidity.3,3 All other data above was derived from the HRS survey immediately preceding hospitalization.
Statistical Analysis
Given multiple admissions per HRS participant, we used admissions rather than individual participants as our unit of analysis. This analytical decision also reflects the clinical reality that many older adults face multiple admissions over time and mirrors the analytic approach used by CMS for cost reduction initiatives such as the Hospital Readmission Reduction Program. We examined the relationship between functional impairment and post-acute costs using unadjusted and adjusted generalized linear models. Specifically, we used a log link and a gamma family, which is often called a gamma regression model. Gamma models are often preferred when using cost as an outcome because they accurately reflect the long-tailed distribution of the data and are able to better adjust for participants with zero costs.3,4 We used gamma regression to adjust outcomes for all demographic and health risk factors described above. We used robust variance estimation (sandwich estimator) to adjust for repeated admission for the same individual, and report unadjusted and adjusted costs from the gamma regression models using the recycled predictions method.3,5 We also performed goodness of fit analyses to calculate c-statistics for our models with and without functional impairment included to determine any marginal predictive benefit beyond that provided by comorbidity and other predictors in the models.
Since longer time from functional measurement and index admission might influence results, we also performed a sensitivity analysis limited to admissions with functional measurements taken within the preceding 6 months. To determine what proportion of total costs were attributable to different settings (e.g. inpatient, skilled nursing facility or SNF, outpatient), we matched dates of service for provider claims (“carrier” costs from Medicare Part B claims) to sites of care whenever possible. For example, if we found physician claim dates that corresponded to a hospital admission, we combined the costs of those claims with costs for the hospital stay (Part A claims). We followed the same procedure for provider (Part B) claims with dates that matched claims for SNF, clinic, home health, etc. (in such cases, both claims are found in Part B but are still separate costs attributable to the same visit).
To determine the relative costs of functional impairment compared to costs attributable to specific comorbidities, we determined the adjusted mean costs for each Elixhauser comorbidity (30 total), for each level of functional impairment. We determined the adjusted cost difference for each comorbidity and impairment level and ranked these differences from greatest to least. Given recent studies suggesting functional status may be more important than comorbidity in predicting outcomes such as readmission,36,3,7 our premise for this analysis was to explore functional impairment as if it were a comorbid condition in order to make more direct comparisons regarding associated costs. We also determined the prevalence-attributable cost for each comorbidity and impairment level to account for the varying levels of prevalence for each.3,8 All computations were performed using Stata 12.
RESULTS
As shown in Table 1, complete data were available for 16,673 index admissions from 8,559 participants. Ages ranged from 65–105 (mean 78.3, ±7.8); 58% were female, 85% were White, average number of Elixhauser comorbidities was 4.9 (±2.7, out of 30 possible total), and 60% had ≥1 hospitalization in the year preceding their index hospital admission. Overall, 4,478 subjects (53%) contributed >1 period of observation to our final sample (average observations per subject was 1.95). The mean costs associated with each index admission and corresponding post-acute care was f $29,586 (STD $30,055). Approximately 20% of this mean cost ($6,003) was spent within the first 30 days of discharge with the remaining 80% ($23,583) was spent in days 31–365.
Table 1.
Participant Characteristics at Time of Index Hospitalization, 2000–2012
| Total (N=16,673) |
||
|---|---|---|
| Demographics | ||
| Age | 78.3 ± 7.8 | |
| <75 | 6617 | 38% |
| 75–85 | 6494 | 41% |
| ≥ 85 | 3574 | 22% |
| Male | 9613 | 58% |
| Race/Ethnicity | ||
| White | 13272 | 85% |
| Black | 2168 | 9% |
| Latino | 977 | 5% |
| Other/Unknown | 266 | 2% |
| Married/Partnered | 8759 | 53% |
| Living Alone | 5335 | 32% |
| Income (median, IQR) | 25K (14K-45K) | |
| Wealth | 150K (35K-407K) | |
| Education less than HS | 5321 | 32% |
| Health Variables | ||
| Self-Rated Health (Fair or Poor) |
7538 | 45% |
| Number of Elixhauser comorbidities | 4.9 ± 2.7 | |
| Any Hospitalization in year prior to Index Hospitalization | 9996 | 60% |
| Baseline Function | ||
| Functional Level | ||
| No Impairment of any kind | 11263 | 68% |
| Difficulty with ≥1 ADL | 2822 | 17% |
| Dependency* in 1 ADL | 1177 | 7% |
| Dependency* in ≥2 ADL | 1411 | 8% |
Dependency is the need for help with ADLs
Total Medicare costs of care for one year after discharge increased as the severity of impairment increased in a dose-response fashion (p<.001 for trend): 68% had no functional impairment (mean cost $25,931); 17% had 1 ADL difficulty ($32,501); 7% had 1 ADL dependency ($39,928), and 8% with dependency in ≥2 ADLs ($45,895). See Table 2. Compared to those with no impairment, the most severely-impaired cost 77% more; adjusted analyses showed that functional status remained an important predictor of Medicare costs even after accounting for comorbidity and demographic characteristics. The effect size was attenuated compared to the unadjusted costs, but still substantial (33% more, p for trend <.001; Table 2). Adjusted costs in a sensitivity analysis restricted to admissions with functional assessment within 6 months of hospitalization showed similar results ($26,665 vs. 40,636 or 52% more; data not shown). Adjusted total costs attributable to 30 Elixhauser comorbidities as compared with functional impairment showed that only 3 Elixhauser conditions were more expensive than severe functional impairment: lymphoma, metastatic cancer, and paralysis (Table 3). We also considered prevalence-attributable cost for each Elixhauser condition and level of functional impairment: severe functional impairment alone (8% prevalence) ranked 10th and any functional impairment (mild, intermediate, and severe combined; 32% prevalence) ranked 5th.
Table 2.
Association of Functional Impairments with Post-acute Costs of Care
| Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|
| Functional Impairment | N | Mean Predicted Cost ($) | 95% CI | Mean Predicted Cost ($) | 95% CI |
| Overall | 16,673 | ||||
| No Impairments | 11,263 | $25,931 | (24,817–27,045) | $28,228 | (27,166–29,289) |
| Difficulty with ≥1 ADL | 2,822 | $32,501 | (31,055–33,947) | $30,951 | (29,250–32,651) |
| Dependency in 1 ADL | 1,177 | $39,928 | (36,198–43,659) | $34,266 | (31,757–36,775) |
| Dependency in ≥2 ADL | 1,411 | $45,895 | (42,638–49,153) | $37,164 | (34,727–39,601) |
Adjusted for age, gender, race, marital status, income, wealth, education, Elixhauser score, hospitalizations in the prior year
Table 3.
Rank Order of Adjusted Costs* by Comorbid Conditions** and Functional Impairment
| Elixhauser Condition or Functional Impairment Level |
Adjusted Cost ($) | Adjusted Cost Rank | Prevalence | Prevalence-Attributable Cost (PAC) | PAC Rank |
|---|---|---|---|---|---|
| Lymphoma | 20,623 | 1 | 1.8% | 388 | 20 |
| Metastatic Cancer | 15,594 | 2 | 4.2% | 664 | 11 |
| Paralysis | 12,450 | 3 | 2.4% | 303 | 21 |
|
Severe Functional Impairment (≥2 ADL dependencies) |
8,936 | 4 | 7.7% | 693 | 10 |
| Neurological Disorders | 8,823 | 5 | 12.7% | 1116 | 7 |
| Renal Failure | 7,296 | 6 | 13.7% | 1003 | 8 |
| Coagulopathy | 7,251 | 7 | 7.8% | 566 | 15 |
| Weight Loss | 6,469 | 8 | 8.6% | 560 | 16 |
| Intermediate Functional Impairment (1 ADL dependency) | 6,038 | 9 | 7.0% | 424 | 19 |
| Peripheral Vascular Disorders | 5,754 | 10 | 26.8% | 1,547 | 4 |
| Congestive Heart Failure | 5,608 | 11 | 31.3% | 1,753 | 2 |
| Fluid and Electrolyte Disorders | 4,990 | 12 | 32.1% | 1,601 | 3 |
| Any Functional Impairment | 4,844 | 13 | 31.9% | 1,545 | 5 |
| Complicated Diabetes | 4,661 | 14 | 13.0% | 607 | 13 |
| Deficiency Anemia | 4,197 | 15 | 13.7% | 574 | 14 |
| Drug Abuse | 4,080 | 16 | 15.0% | 613 | 12 |
| Solid Tumor without Metastasis | 3,956 | 17 | 18.1% | 714 | 9 |
| Uncomplicated Diabetes | 3,788 | 18 | 33.4% | 1,264 | 5 |
| Chronic Pulmonary Disease | 3,071 | 19 | 37.3% | 1,145 | 6 |
| Rheumatoid Arthritis/Collagen Disease | 2,956 | 20 | 8.7% | 257 | 22 |
|
Mild Functional Impairment (ADL difficulty) |
2,723 | 21 | 17.1% | 466 | 17 |
| Hypertension | 2,161 | 22 | 82.9% | 1,790 | 1 |
Costs adjusted for age, gender, race, marital status, income, wealth, education, Elixhauser score, and hospitalizations in the prior year.
Data for Elixhauser conditions not independently associated with increased cost not shown above: Alcohol Abuse, AIDS/HIV, Pulmonary Circulation Disorders, Valvular Disease, Blood Loss Anemia, Depression, Liver Disease, Psychoses, Hypothyroidism, Cardiac Arrhythmia, Obesity, and Peptic Ulcer Disease.
Considering mean total costs by setting of care (Figure 2), costs for re-hospitalization were higher for patients with severe functional impairment but proportions were similar (47% vs. 51%, respectively). Differences in proportional and total costs by level of functional impairment were more pronounced for SNF and home health care. Costs of SNF care were nearly 3 times higher for patients with severe impairment ($9,266) compared to those with no impairment ($3,334). Home health care costs for patients with severe impairment ($5,678) were also approximately triple those for those with no impairment ($1,902). Overall, 3,338 (39%) participants had home health costs, 2140 (25%) had SNF costs, and 599 (7%) had hospice costs; 4,365 (51%) had at least one of the three above.
Figure 2.
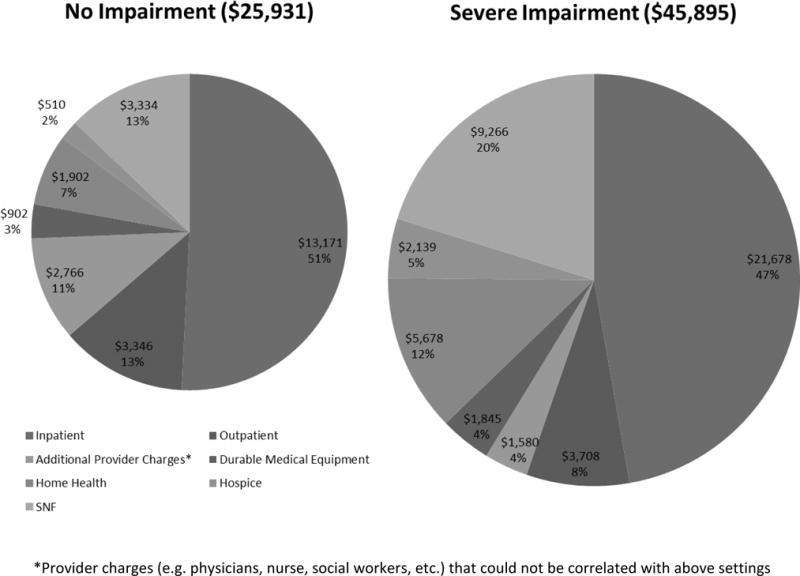
Proportion of total costs according by setting of care
Analyses of variance explained by modeling functional impairment compared to Elixhauser comorbidities did not show significant differences. The c-statistic for patients in highest-20th percentile of overall costs with function in the model was 0.71 and 0.70 without. Similarly, the R-squared for a linear model of log-transformed costs did not show significant differences with functional impairment included or excluded from the models 0.21 vs. 0.20, respectively.
DISCUSSION
In this 12-year longitudinal, nationally-representative study of post-acute costs of among Medicare seniors, approximately one-third had some level of functional impairment which was associated with higher overall costs to Medicare. These costs increased in a dose-response fashion as the degree of impairment increased: the most functionally-impaired patients had costs nearly 80% higher than those with no impairments ($46 vs. $26 thousand). These unadjusted associations demonstrate the value of functional status as a single and simple predictor of the most resource-intensive episodes of post-acute care. The persistence of this association after adjustment for clinical and demographic characteristics highlights functional impairment as an independent predictor of costs that is currently unmeasured in routine hospital care and unreported in Medicare claims data.
Indeed, severe functional impairment is very expensive even when compared to other individual comorbidities – only patients with lymphoma, metastatic cancer, and paralysis had higher adjusted costs. Recent interest in identifying frail, vulnerable, or otherwise “at-risk” seniors in the hospital has increased attention to the specific role of functional impairment in this population; however, very few studies with small sample sizes have explored associations with these concepts and costs of care.39,40 Moreover, it is not clear to what extent such associations are driven by functional issues that are part of frailty or vulnerability scales. Our findings build on this small but important literature and suggest that functional impairment, in addition to comorbidity, is an important, but overlooked marker of costs of care in this population. Current efforts to reduce cost target common diagnoses (e.g. heart failure, hip fractures, etc.) but most older adults have multiple comorbidities and functional status is the end impact of these disease processes which cuts across diagnoses. Interventions targeted towards Medicare seniors according to level of function may be a more effective way of targeting high costs as function status is a barometer of disease impact on older patients, especially after an episode of acute care.
In addition to the high cost of functional impairment to Medicare for medical care, it should be noted that there considerable out of pocket costs to patients and their caregivers not captured in our analysis.41,42 Given the high medical utilization rates for patients with functional impairment,7–16 it may be that certain events such as readmission are related to unmet needs or “missing pieces” of the post-acute plan for recovery.43 Indeed, disability has been broadly defined as “any gap between personal capability and situational demand”44 and many so-called “social admissions” may be driven by unrecognized functional impairment that has created or worsened such a gap to an extent which prompts return to the hospital to meet these needs. Older patients are particularly at risk for poor outcomes due to such gaps in the post-acute period45 and, unfortunately, each successive hospital admission increases the risk of accelerated functional decline and permanent disability.46 Moreover, at least half of permanent disability in older adults begins with functional decline during hospitalization.47 This vicious cycle of worsening functional impairment and increased utilization underscores the urgent need to incorporate functional assessments into routine hospital care. Currently, acute care hospitals are not required or incentivized by Medicare or other payers to collect and report any measures of functional status in hospitalized seniors. In contrast, post-acute care settings such as skilled nursing facilities, inpatient rehabilitation facilities, and home health care, providers are required to report functional status. We believe functional status should be assessed during acute care as well – ideally on admission and discharge at minimum.
Our study has several limitations. First, given the prospective nature of the HRS study, the time from our measurements of functional impairment and hospitalization were not uniform among HRS subjects (inter-quartile range 202–614 days, average 423 days). Although a sub-analysis of admissions with functional assessments within 6 months preceding admission was not significantly different than our main results, our analysis may under-estimate the effects of functional impairments at the actual time of hospital admission as functional status typically declines in the setting of acute illness.48 Second, the correlations we found between functional impairment and cost do not demonstrate causation; further research is needed to understand the mechanisms which result in higher costs for these patients. Third, our analysis adjusts for co-morbidity (the presence or absence of various conditions) but does not account for multi-morbidity (the interactions between multiple morbidities); it is possible that adjustment for these interactions could mute the effect of functional impairment we found. Fourth, although we did not find that functional status explained significantly more variance in costs than predicted from the Elixhauser index alone, clinicians can assess the former much more easily than the latter at the bedside and apply it to the patient and family in front of them when thinking about costs of post-acute care. Finally, perhaps the most important limitation is that we are only able to analyze costs paid by Medicare; functional impairment imposes tremendous out-of-pockets costs on patients and caregivers, thus, our findings are likely a gross underestimate of total costs. Future studies should attempt to combine these patient and caregiver costs with billing data to attain a more accurate understanding of the aggregate costs of functional impairment on society.
In conclusion, functional impairment is associated with increased Medicare costs of post-acute care in seniors with disproportionate spending for SNF and home health compared to those with no impairment. Our findings suggest the need for Medicare policy to expand beyond the traditional focus on chronic disease management and explore initiatives to manage advanced functional impairment in order to reduce overall costs of post-acute care for community-dwelling seniors. Functional impairment on admission may be an overlooked but highly suitable target for interventions to cost of post-acute care in Medicare seniors.
Acknowledgments
Funding information:
Dr. Greysen is supported by the National Institutes of Health (NIH), National Institute of Aging (NIA) through the Claude D. Pepper Older Americans Independence Center (P30AG021342 NIH/NIA), a Career Development Award (5K23AG045338-04) and the NIH-NIA Loan Repayment Program. Dr. Covinsky is supported by the NIA through a K-24 Career Mentoring Award and an R01 from the National Institute for Nursing Research.
Dr. Greysen is supported by the National Institutes of Health (NIH), National Institute of Aging (NIA) through the Claude D. Pepper Older Americans Independence Center (P30AG021342 NIH/NIA), a Career Development Award (1K23AG045338-01) and the NIH-NIA Loan Repayment Program. Dr. Covinsky is supported by the NIA through a K-24 Career Mentoring Award and an R01 from the National Institute for Nursing Research.
Footnotes
Presentation at meetings:
This paper was presented as an oral presentation at the 2015 Annual Meeting of the Society for Hospital Medicine (awarded Best Research Presentation) as well as oral presentation at the 2015 Annual Meeting of the Society for General Internal Medicine.
The first author (SRG) had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
No sponsor (the National Institute on Aging or the National Institute for Nursing Research) had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures:
The authors have no conflicts of interest to declare relative to this study.
Author Contributions:
S.R. Greysen: study conception and design, analysis and interpretation of data; drafting and revising of manuscript; final approval of manuscript for publication.
I.S. Cenzer: data interpretation; manuscript revision; final approval for publication.
W.J. Boscardin: data interpretation; manuscript revision; final approval for publication.
K.E. Covinsky: study conception and design, analysis and interpretation of data; revising of manuscript; final approval of manuscript for publication.
References
- 1.Blumenthal D, Stremikis K, Cutler D. Health care spending–a giant slain or sleeping? N Engl J Med. 2013 Dec 26;369(26):2551–7. doi: 10.1056/NEJMhpr1310415. [DOI] [PubMed] [Google Scholar]
- 2.Thorpe KE, Howard DH. The rise in spending among Medicare beneficiaries: the role of chronic disease prevalence and changes in treatment intensity. Health Aff (Millwood) 2006 Sep-Oct;25(5):w378–88. doi: 10.1377/hlthaff.25.w378. [DOI] [PubMed] [Google Scholar]
- 3.Thorpe KE, Ogden LL, Galactionova K. Chronic conditions account for rise in Medicare spending from 1987 to 2006. Health Aff (Millwood) 2010 Apr;29(4):718–24. doi: 10.1377/hlthaff.2009.0474. [DOI] [PubMed] [Google Scholar]
- 4.Thorpe KE. Treated disease prevalence and spending per treated case drove most of the growth in health care spending in 1987–2009. Health Aff (Millwood) 2013 May;32(5):851–8. doi: 10.1377/hlthaff.2012.0391. [DOI] [PubMed] [Google Scholar]
- 5.Bodenheimer T, Berry-Millett R. Follow the money–controlling expenditures by improving care for patients needing costly services. N Engl J Med. 2009 Oct 15;361(16):1521–3. doi: 10.1056/NEJMp0907185. [DOI] [PubMed] [Google Scholar]
- 6.Stuck AE, Walthert JM, Nikolaus T, et al. Risk factors for functional status decline in community-living elderly people: a systematic literature review. Soc Sci Med. 1999 Feb;48(4):445–69. doi: 10.1016/s0277-9536(98)00370-0. [DOI] [PubMed] [Google Scholar]
- 7.Barnes DE, Mehta KM, Boscardin WJ, et al. Prediction of recovery, dependence or death in elders who become disabled during hospitalization. J Gen Intern Med. 2013 Feb;28(2):261–8. doi: 10.1007/s11606-012-2226-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003 Apr;51(4):451–8. doi: 10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]
- 9.Ferrucci L, Guralnik JM, Pahor M, et al. Hospital diagnoses, Medicare charges, and nursing home admissions in the year when older persons become severely disabled. JAMA. 1997;277:728–734. [PubMed] [Google Scholar]
- 10.Rozzini R, Sabatini T, Cassinadri A, et al. Relationship between functional loss before hospital admission and mortality in elderly persons with medical illness. J Gerontol A Biol Sci Med Sci. 2005 Sep;60(9):1180–3. doi: 10.1093/gerona/60.9.1180. [DOI] [PubMed] [Google Scholar]
- 11.Inouye SK, Peduzzi PN, Robison JT, et al. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998 Apr 15;279(15):1187–93. doi: 10.1001/jama.279.15.1187. [DOI] [PubMed] [Google Scholar]
- 12.Greysen SR, Stijacic Cenzer I, Auerbach AD, Covinsky KE. Functional impairment and hospital readmission in medicare seniors. JAMA Intern Med. 2015 Apr;175(4):559–65. doi: 10.1001/jamainternmed.2014.7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soley-Bori M, Soria-Saucedo R, Ryan CM, et al. Functional Status and Hospital Readmissions Using the Medical Expenditure Panel Survey. J Gen Intern Med. 2015 Feb;:18. doi: 10.1007/s11606-014-3170-9. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerrard P, Goldstein R, et al. Functional Status Outperforms Comorbidities in Predicting Acute Care Readmissions in Medically Complex Patients. J Gen Intern Med. 2015 May;:9. doi: 10.1007/s11606-015-3350-2. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoyer EH, Needham DM, Atanelov L, et al. Association of impaired functional status at hospital discharge and subsequent rehospitalization. J Hosp Med. 2014 Feb 26; doi: 10.1002/jhm.2152. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Depalma G, Xu H, Covinsky KE, et al. Hospital readmission among older adults who return home with unmet need for ADL disability. Gerontologist. 2013 Jun;53(3):454–61. doi: 10.1093/geront/gns103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caro JJ, Huybrechts KF. Stroke treatment economic model (STEM): predicting long-term costs from functional status. Stroke. 1999 Dec;30(12):2574–9. doi: 10.1161/01.str.30.12.2574. [DOI] [PubMed] [Google Scholar]
- 18.Arling G, Ofner S, Reeves MJ, et al. Care Trajectories of Veterans in the 12 Months After Hospitalization for Acute Ischemic Stroke. Circ Cardiovasc Qual Outcomes. 2015 Oct 8;6(Suppl 3):S131–40. doi: 10.1161/CIRCOUTCOMES.115.002068. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Medicare & Medicaid Services (CMS), HHS. Medicare program; hospital inpatient value-based purchasing program. Final rule Fed Regist. 2011 May 6;76(88):26490–547. [PubMed] [Google Scholar]
- 20.Kocher RP, Adashi EY. Hospital readmissions and the Affordable Care Act: paying for coordinated quality care. JAMA. 2011 Oct 26;306(16):1794–5. doi: 10.1001/jama.2011.1561. [DOI] [PubMed] [Google Scholar]
- 21.Chandra A, Dalton MA, Holmes J. Large Increases In Spending On Postacute Care In Medicare Point To The Potential For Cost Savings In These Settings. Health Affairs. 2013;32(5):864–872. doi: 10.1377/hlthaff.2012.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mechanic R. Post-acute care–the next frontier for controlling Medicare spending. N Engl J Med. 2014;370(8):692–4. doi: 10.1056/NEJMp1315607. [DOI] [PubMed] [Google Scholar]
- 23.Newhouse JP, Garber AM. Geographic variation in Medicare services. N Engl J Med. 2013;368:1465–1468. doi: 10.1056/NEJMp1302981. [DOI] [PubMed] [Google Scholar]
- 24.Fried TR, Bradley EH, Williams CS, Tinetti ME. Functional disability and health care expenditures for older persons. Arch Intern Med. 2001 Nov 26;161(21):2602–7. doi: 10.1001/archinte.161.21.2602. [DOI] [PubMed] [Google Scholar]
- 25.Chernew ME, Goldman DP, Pan F, Shang B. Disability and health care spending among medicare beneficiaries. Health Aff (Millwood) 2005;24(Suppl 2):W5R42–52. doi: 10.1377/hlthaff.w5.r42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juster FT, Suzman R. An overview of the Health and Retirement Study. J Hum Resour. 1995;30(suppl):S7–56. [Google Scholar]
- 27.The Health and Retirement Study. Sample Sizes and Response Rates. http://hrsonline.isr.umich.edu/sitedocs/sampleresponse.pdf) and Survey Design and Sample Evolution ( http://hrsonline.isr.umich.edu/sitedocs/surveydesign.pdf) Both cites accessed August 7, 2015.
- 28.Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged: the index of ADL: a standarized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 29.Covinsky KE, Hilton J, Lindquist K, Dudley RA. Development and validation of an index to predict activity of daily living dependence in community-dwelling elders. Med Care. 2006 Feb;44(2):149–157. doi: 10.1097/01.mlr.0000196955.99704.64. [DOI] [PubMed] [Google Scholar]
- 30.Fried LP, Guralnik JM. Disability in older adults: evidence regarding significance, etiology, and risk. J Am Geriatr Soc. 1997;45(1):92–100. doi: 10.1111/j.1532-5415.1997.tb00986.x. [DOI] [PubMed] [Google Scholar]
- 31.The Consumer Price Index. United States Department of Labor, Bureau of Labor Statistics. Available at: http://www.bls.gov/data. Site accessed June 30, 2015.
- 32.Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Med Care. 2012 Dec;50(12):1109–18. doi: 10.1097/MLR.0b013e31825f64d0. [DOI] [PubMed] [Google Scholar]
- 33.Li P, Kim MM, Doshi JA. Comparison of the performance of the CMS Hierarchical Condition Category (CMS-HCC) risk adjuster with the Charlson and Elixhauser comorbidity measures in predicting mortality. BMC Health Serv Res. 2010 Aug 20;10:245. doi: 10.1186/1472-6963-10-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diehr P, Yanez D, Ash A, et al. Methods for Analyzing Health Care Utilization and Costs. Annu Rev Public Health. 1999;20:125–44. doi: 10.1146/annurev.publhealth.20.1.125. [DOI] [PubMed] [Google Scholar]
- 35.Basu A, Rathouz PJ. Estimating marginal and incremental effects on health outcomes using flexible link and variance function models. Biostatistics. 2005;6(1):93–109. doi: 10.1093/biostatistics/kxh020. [DOI] [PubMed] [Google Scholar]
- 36.Shih SL, Gerrard P, Goldstein R, et al. Functional Status Outperforms Comorbidities in Predicting Acute Care Readmissions in Medically Complex Patients. J Gen Intern Med. 2015 Nov;30(11):1688–95. doi: 10.1007/s11606-015-3350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shih SL, Zafonte R, Bates DW, et al. Functional Status Outperforms Comorbidities as a Predictor of 30-Day Acute Care Readmissions in the Inpatient Rehabilitation Population. J Am Med Dir Assoc. 2016 Oct 1;17(10):921–6. doi: 10.1016/j.jamda.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Zanutto EL. A Comparison of Propensity Score and Linear Regression Analysis of Complex Survey Data. J Data Science. 2006;4:67–91. [Google Scholar]
- 39.Comans TA, Peel NM, Hubbard RE, et al. The increase in healthcare costs associated with frailty in older people discharged to a post-acute transition care program. Age Ageing. 2016 Mar;45(2):317–20. doi: 10.1093/ageing/afv196. [DOI] [PubMed] [Google Scholar]
- 40.Edmans J, Bradshaw L, Gladman JR, et al. The Identification of Seniors at Risk (ISAR) score to predict clinical outcomes and health service costs in older people discharged from UK acute medical units. Age Ageing. 2013 Nov;42(6):747–53. doi: 10.1093/ageing/aft054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelley AS, McGarry K, Fahle S, et al. Out-of-pocket spending in the last five years of life. J Gen Intern Med. 2013 Feb;28(2):304–9. doi: 10.1007/s11606-012-2199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldman DP, Zissimopoulos JM. High out-of-pocket health care spending by the elderly. Health Aff (Millwood) 2003 May-Jun;22(3):194–202. doi: 10.1377/hlthaff.22.3.194. [DOI] [PubMed] [Google Scholar]
- 43.Greysen SR, Hoi-Chen D, Garcia V, et al. “Missing Pieces:” Social and Functional Barriers to Recovery for Vulnerable Older Adults Transitioning from Hospital to Home. J Am Geriatr Soc. 2014 Aug;62(8):1556–61. doi: 10.1111/jgs.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verbrugge LM, Jette AM. The disablement process. Soc Sci Med. 1994;38(1):1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 45.Krumholz HM. Post-hospital syndrome–an acquired, transient condition of generalized risk. N Engl J Med. 2013 Jan 10;368(2):100–2. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gill TM, Gahbauer EA, Han L, Allore HG. The role of intervening hospitaladmissions on trajectories of disability in the last year of life: prospective cohort study of older people. BMJ. 2015 May 20;350:h2361. doi: 10.1136/bmj.h2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gill TM, Allore HG, Gahbauer EA, Murphy TE. Change in disability after hospitalization or restricted activity in older persons. JAMA. 2010;304(17):1919–1928. doi: 10.1001/jama.2010.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003 Apr;51(4):451–458. doi: 10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]


