Abstract
Intensive Care Unit (ICU) anemia is an extreme version of anemia of inflammation (AI) that occurs commonly in critically ill patients and is associated with increased morbidity and mortality. Currently available therapies for ICU anemia have shown inconsistent efficacies in clinical trials. We conducted a systematic study of the effects of early versus delayed iron (Fe) and/or erythropoietin (EPO) therapy in our previously characterized mouse model of ICU anemia based on an injection of heat-killed Brucella abortus (BA). To study the effects of ongoing inflammation on the response to therapy, inflamed WT and hepcidin knockout (HKO) mice were treated at either early (days 1&2) or delayed (days 7&8) time points after the inflammatory stimulus. In the early treatment group, Fe and/or EPO therapy did not increase hemoglobin (Hgb) levels or reticulocyte production in either the inflamed WT or HKO groups. In the delayed treatment group, combination Fe+EPO therapy did increase Hgb and reticulocyte production in WT mice (mean ΔHgb in WT saline group −9.2 g/dL vs. Fe/EPO −5.5 g/dL; p<0.001). The HKO mice in the delayed treatment group did not improve their Hgb, but HKO mice in all treatment groups developed a milder anemia than the WT mice. Our findings indicate that combination Fe+EPO therapy is effective in partially reversing ICU anemia when administered after the phase of acute inflammation. Hepcidin ablation alone was more effective in attenuating ICU anemia than Fe+EPO therapy, which indicates the potential of antihepcidin therapeutics in treating ICU anemia.
Keywords: ICU anemia, antihepcidin, anemia of inflammation, anemia therapeutics, hepcidin knockout
INTRODUCTION
Anemia of inflammation (AI) is an anemia seen in the context of infections and systemic inflammatory disorders, including rheumatologic disorders, inflammatory bowel diseases, malignant neoplasms, and chronic kidney disease (1). AI is a normocytic normochromic anemia with a shortened erythrocyte lifespan and suppression of erythropoiesis that can occur despite adequate levels of erythropoietin (EPO) (2). Perhaps the most characteristic feature of AI is dysregulation of iron homeostasis characterized by a reduction of circulating iron despite intact tissue iron stores (1), resulting in decreased iron availability for hemoglobin synthesis and erythrocyte production. This inflammation-induced change in systemic iron metabolism is thought to have evolved as a host defense mechanism to limit iron availability to microbes during infections (3).
The principal regulator of systemic iron homeostasis is hepcidin, a small peptide hormone produced primarily by hepatocytes (4). Hepcidin acts by binding to ferroportin, the only known cellular iron exporter that is expressed on the surface of macrophages, hepatocytes, and duodenal enterocytes. Hepcidin binding causes ferroportin endocytosis and degradation, inhibiting gut iron absorption and the efflux of stored cellular iron necessary for erythropoiesis (3). Inactivation of the hepcidin gene in mice causes a phenotype of severe iron overload (5), while transgenic hepcidin mice with hepcidin overexpression develop the opposite phenotype of iron-restricted anemia (6). Hepcidin expression is strongly stimulated during times of inflammation or infection, largely by IL-6 via the JAK-STAT pathway, with some contribution by the BMP pathway (7).
An extreme version of AI is a condition known as ICU (Intensive Care Unit) anemia that develops in critically ill patients within days (8). About two-thirds of ICU patients present with a hemoglobin <12g/dL on admission, and a remarkable 97% of patients are anemic by day 8 (8–10). Similar to chronic inflammatory anemia syndromes 8-1, ICU anemia is associated with adverse outcomes. These include prolonged mechanical ventilation (11), myocardial infarction, prolonged hospital stay, and mortality (8).
Despite its prevalence and significance, ICU anemia is likely undertreated because there is little consensus on the safety and efficacy of available therapies. Red blood cell (RBC) transfusions are the mainstay of therapy in the ICU, but carry the risks of immunosuppressive effects, transmission of infections, and transfusion reactions (8). RBC transfusions have also been shown to be an independent risk factor for increased mortality (8, 9). Iron therapy has shown mixed efficacy in ICU anemia, with one trial of 200 patients showing no significant improvement in mortality, but a significant decrease in the rate of transfusions (12). A second study using intravenous (IV) iron supplementation for anemia of traumatic critical illness showed no discernible effect on transfusion requirement, length of stay, or mortality (13). Studies investigating the use of erythropoiesis-stimulating agents (ESAs) in ICU anemia have also shown mixed results, with one landmark study showing that weekly epoetin alfa administration in critically ill patients was associated with a nonsignificant trend towards decreased mortality, but also with an increase in thrombotic events (14). While these clinical studies have been illuminating, they are plagued with complex patient demographics and inconsistent treatment regimens. There have been no studies illustrating the specific effects of varying treatment regimens using an animal model.
The current paper is a systematic study of the effects of early versus delayed iron (Fe) and/or EPO therapy in a mouse model of acute and severe inflammation. Our group recently published a detailed characterization of a mouse model of ICU anemia that served as the vehicle for the current studies. With a single intraperitoneal injection of heat-killed Brucella abortus (BA), mice develop an acute and severe anemia with iron restriction despite increased tissue iron stores, erythropoietic suppression, and a shortened erythrocyte lifespan. Hepcidin deletion causes a partial but significant correction of the resulting anemia, accompanied by an accelerated recovery (15). In short, this model displays all the major characteristics seen in ICU anemia, and is an effective platform for testing any potential interventions for acute and severe AI.
MATERIALS AND METHODS
Animal models
Animal studies were approved by the Animal Research Committee at the University of California, Los Angeles (UCLA). For the wild-type (WT) experiments, C57BL/6 mice were obtained from Charles River Laboratories (Wilmington, MA) or The Jackson Laboratory (Bar Harbor, ME). Although the regulation of iron metabolism is similar in both genders, male C57BL/6 mice have lower iron stores and lower hepcidin compared to female mice, thus only male mice were used in this study to minimize the variability in baseline iron parameters and hepcidin concentration (16). WT mice were fed standard chow (~270 ppm iron; Harlan Teklad; Indianopolis, IN) from the time of weaning until ~6 weeks of age, after which they were switched to an iron-sufficient diet (50 ppm iron; Harlan Teklad, Indianapolis IN) for two weeks prior to BA injection. This dietary conditioning was applied because the high iron content of standard chow maximally stimulates hepcidin expression, making it unresponsive to inflammatory stimuli (17). In addition, dietary iron absorption in humans accounts for ~5–10% of the daily iron fluxes but as much at ~50% in mice fed standard chow (18). Reducing the dietary iron content of mouse chow was designed to model iron fluxes of human homeostasis.
In order to evaluate the role of hepcidin in the response to Fe/EPO therapy, we used male hepcidin-1 knockout (HKO) mice. HKO mice were originally provided to our laboratory by Dr. Sophie Vaulont (5) and were backcrossed onto the C57BL/6 background as previously described (19) using marker-assisted accelerated backcrossing. HKO mice are already iron loaded by the time they are weaned, and require dietary conditioning to maintain iron levels comparable to those of WT mice. For this study, HKO mice were placed on a low-iron diet (4ppm) shortly after weaning for ~2 weeks prior to BA injection. This regimen allows for adequate iron depletion without development of iron-deficiency anemia.
To induce AI, animals were injected intraperitoneally (IP) with 5 × 108 particles/mouse of heat-killed BA (lots 5-1101 and 5-1304; US Department of Agriculture, Animal and Plant Health Inspection Service, National Veterinary Services Laboratories) as previously described (20). Control WT mice were injected IP with an equivalent volume of normal saline, then treated with Fe/EPO on days 1&2 after saline injection. Both inflamed WT and HKO mice underwent Fe/EPO treatments at either “early” (days 1&2) or “delayed” (days 7&8) time points. See Supplemental Digital Content - Figure 1 for experimental timeline schematics. Both groups received subcutaneous (SC) injections of 1mg of Fe dextran (Sigma-Aldrich; St. Louis, MO) and/or 1200 units of EPO (Epogen; Amgen; Thousand Oaks, CA) (Procrit; Janssen Pharmaceuticals; Titusville, NJ) (600 units/day X 2 days). Saline treatment groups underwent SC injections of equivalent volumes of saline. Both WT and HKO mice (5–10 evaluable per genotype per treatment group) were analyzed before and 2 weeks after BA or saline treatment. At the time of sacrifice, mouse blood, liver, and spleen were collected for analysis.
Hematologic studies
Blood hemoglobin levels and mean corpuscular volume (MCV) values were obtained using a HemaVet blood analyzer (Drew Scientific; Waterbury, CT). To measure iron-restricted erythropoiesis, zinc protoporphyrin (ZPP) levels were measured using a hematofluorometer (AVIV; Lakewood, NJ) (21). Wet spleen weights were obtained from a subset of the mice as a measure of extramedullary erythropoiesis.
Reticulocyte production was measured using flow cytometry at the UCLA Jonsson Comprehensive Cancer Center and Center for AIDS Research Flow Cytometry Core Facility. Blood samples of 5 μL was added to 1 mL of thiazole orange in phosphate buffered solution with 0.1% sodium azide (PBS-azide; BD Bioscience; San Jose, CA), then incubated at room temperature for 1–3 hours. For unstained controls, equivalent volumes of blood were added to PBS-azide without thiazole orange. The results are expressed as the reticulocyte product index: RPI = Retic % X Final Hgb / Baseline Hgb.
Measurement of iron parameters and serum hepcidin
Serum iron and liver non-heme iron concentrations were measured using a colorimetric assay for iron quantification (Sekisui Diagnostics; Lexington, MA) as previously described (18). Mouse serum hepcidin-1 was measured by a sandwich ELISA (provided by B. Sasu and K. Cooke; Amgen; Thousand Oaks, CA). The assay was validated in our laboratory as previously described (15).
Statistics
All statistics were performed using SigmaStat (Systat Software; Point Richmond, CA). Statistical analyses of various treatment effects on a single genotype were performed using One Way ANOVA. Parametric data was compared using Holm-Sidak Method, and nonparametric data was compared using Dunn’s method or Tukey test. Multivariate analyses of various treatment effects on the two genotypes were performed using Two Way ANOVA, and the data was compared using Holm-Sidak method. P<0.05 was considered statistically significant.
RESULTS
EPO treatment increases erythropoiesis and circulating iron availability in uninflamed control WT mice
In order to verify appropriate dosages of our Fe and EPO treatment interventions, we performed Fe and/or EPO injections in uninflamed control WT mice. To mimic the conditions in the later experiment that include an injection of BA, these mice were injected with an equivalent volume of normal saline (rather than BA). In selecting the appropriate dose of supplemental iron, we aimed to provide significant iron supplementation without causing overt iron overload and toxicity. Previous murine data have shown that iron doses of 0.5 g/kg body weight result in iron overload and end-organ toxicities (22). Standard medical practices of iron supplementation for patients with AI use doses of 1–2 grams of IV iron (~0.02 g/kg body weight) in various formulations (23). Accounting for differences in IV versus SC absorption, we selected a dosing regimen of Fe dextran 0.04 g/kg body weight. The EPO dosing regimen was based on previous work by our group that showed that 600 units total of recombinant human EPO given over 3 days was sufficient to produce changes in hematologic and iron parameters in uninflamed mice (24). Because inflamed mice are likely to be EPO-resistant, we increased the EPO dose in the current experiment to 1200 units of EPO given over 2 days.
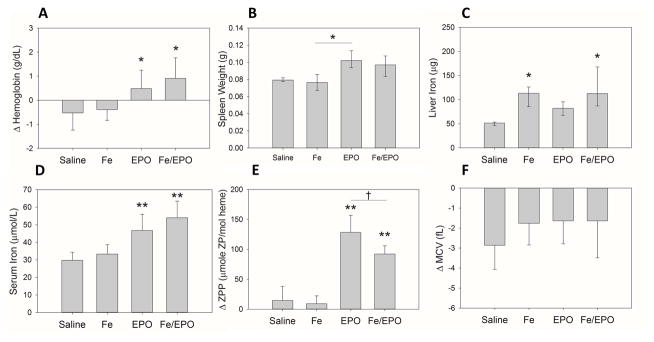
The uninflamed mice were injected with saline on day 0, underwent Fe and/or EPO treatments on days 1&2, and were analyzed on day 14 (Supplemental Digital Content - Figure 1A). Relative to the saline group on day 14, the Fe-only group did not increase their hemoglobin, whereas EPO treatment without Fe supplementation increased hemoglobin by 1.0 g/dL, and EPO with Fe supplementation increased hemoglobin by 1.4 g/dL (Figure 1A, p<0.05 for each EPO treatment group vs. saline). Spleen weights were measured as a marker of extramedullary erythropoiesis, and showed trends toward increased spleen weights in EPO and Fe/EPO-treated mice. (Figure 1B) (median spleen weight in saline group 0.08 g vs. Fe-only 0.08 g vs. EPO-only 0.10 g vs. Fe/EPO 0.10 g). The results confirm the stimulatory erythropoietic effect of our EPO treatment regimen in uninflamed control mice. The addition of Fe did not appear to further increase erythropoiesis in these mice.
Figure 1. The hematologic and iron parameters of uninflamed control WT mice after treatment with exogenous Fe Dextran and/or EPO.
(A) Δ Hemoglobin. (B) Spleen weights. (C) Liver iron. (D) Serum iron. (E) Δ Serum ZPP. (F) Δ MCV. Panel A: Treatment groups each included 8 evaluable male mice; means ± SD are shown; *p<0.05 compared to saline group, by Holm-Sidak method. Panel B: Treatment groups each included 4 male mice; medians ± 75th/25th percentile are shown; *p<0.05 by Tukey test. Panel C: Treatment groups each included 8 male mice; medians ± 75th/25th percentile are shown; *p<0.05 compared to saline group, by Dunn’s method. Panels D–F: Treatment groups each included 7–8 male mice; means ± SD are shown; **p<0.001 compared to saline group, †p<0.05, by Holm-Sidak method.
We measured multiple iron parameters in order to evaluate the status of iron stores and circulating iron in each of our treatment groups. Liver iron levels were increased in mice treated with Fe (± EPO), confirming that our dose of Fe Dextran administration was adequate to increase tissue iron stores (Figure 1C) (median liver iron in saline group 51 μg vs. Fe-only 113 μg vs. EPO-only 82 μg vs. Fe/EPO 113 μg; p<0.05 for each Fe-treatment group vs. saline). Serum iron levels increased in both EPO treatment groups, but not in the Fe-only group (Figure 1D) (mean serum iron in saline group 30 μmol/L vs. Fe-only 33 μmol/L vs EPO-only 47 μmol/L vs. Fe/EPO 54 μmol/L; p<0.001 for each EPO treatment group vs. saline). This rise in serum iron seen in EPO-treated mice likely reflects erythropoiesis-mediated suppression of hepcidin expression resulting in increased enteral dietary iron absorption and efflux of intracellular iron from storage (24).
Serum ZPP measurements were obtained as a marker of iron-restricted erythropoiesis. When insufficient iron is available for erythropoiesis, increased amounts of zinc are incorporated into the protoporphyrin ring and result in increased serum ZPP levels (21). Both EPO and Fe/EPO groups had significantly higher increases in their ZPP levels than control mice (Figure 1E) (mean ΔZPP in saline group 15 vs. Fe-only 9 vs. EPO-only 128 vs. Fe/EPO 92; p<0.001 for each EPO treatment group vs. saline), despite the increased serum iron levels in these same EPO treatment groups. We postulate that the rise in ZPP levels occurs early as robust erythropoiesis is induced by exogenous EPO and exceeds plasma iron availability. The relatively smaller ΔZPP of the Fe/EPO group as compared to the EPO group demonstrates the dampening of this effect with iron supplementation. Δ mean corpuscular volume (MCV) measurements showed no significant differences among the control and treatment groups (Figure 1F).
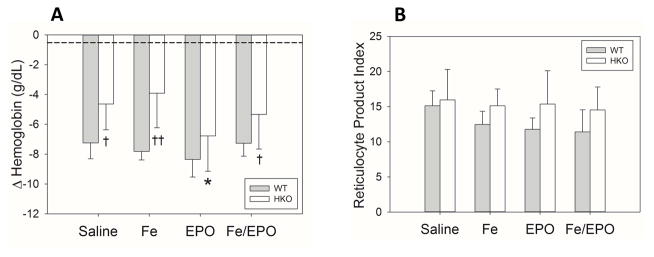
Early Fe and/or EPO treatment of acutely inflamed WT and HKO mice does not increase erythropoiesis and leads to minor changes in iron status
After establishing an appropriate dosing regimen of Fe and EPO treatments for increasing erythropoiesis and circulating iron levels as described above, we repeated the treatment regimen using our previously characterized mouse model of acute inflammation with heat-killed BA (15). In order to investigate the role of hepcidin in the response to therapy, we also repeated the experiment in HKO mice. Both WT and HKO mice were injected with BA on day 0, underwent Fe and/or EPO injections on days 1&2, and were analyzed on day 14 (Supplemental Digital Content - Figure 1B). In neither WT nor HKO treatment groups was there any significant effect of treatment on Hgb compared to their respective saline counterparts (Figure 2A). However, when HKO were compared to WT mice, inflamed HKO mice had smaller hemoglobin drops than their WT counterparts in most treatment groups, confirming the significant role of hepcidin in the development of AI similar to our previous characterization of the BA mouse model describing the protective effect of hepcidin deletion (15). RPI measurements showed no significant increase in erythropoiesis in any of the treatment groups of either genotype. However, the RPI measurements did show a trend towards increased reticulocytosis in the HKO mice in all treatment groups (Figure 2B), consistent with the observation that the lack of hepcidin attenuates the development of anemia during acute inflammation.
Figure 2. Treatment of acutely inflamed WT and HKO mice with Fe and/or EPO does not increase hemoglobin levels or reticulocytosis.
(A) Δ Hemoglobin. (B) Reticulocyte product index. The dashed lines represent the mean values from the uninflamed control WT mice of Figure 1. Treatment groups for each genotype included 5–10 evaluable male mice. Means ± SD are shown; *p<0.05 compared to saline group, †p<0.05 and ††p<0.001 compared to genotype counterpart, all by Holm-Sidak method.
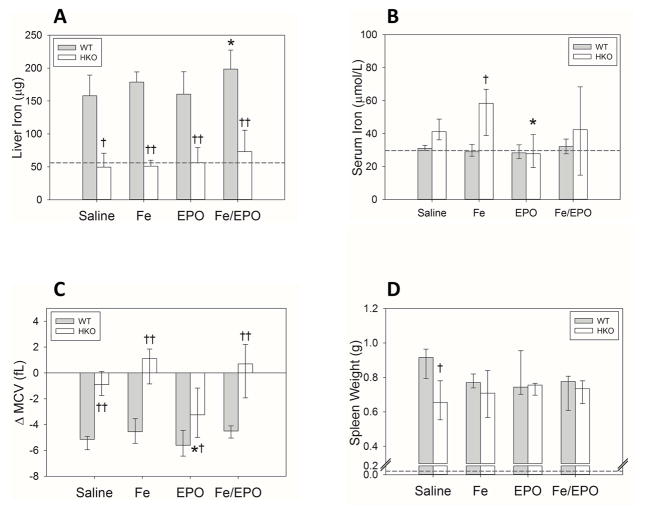
Multiple iron parameters were measured in the WT and HKO mice that had received Fe and/or EPO treatments during acute inflammation. The HKO mice had consistently lower liver iron levels than the WT mice in all treatment groups, confirming that the HKO mice were adequately iron-depleted by their iron-deficient diet (Figure 3A). The WT mice had a subtle trend towards increased liver iron levels in the Fe and Fe/EPO groups (mean liver iron in WT saline group 158 μg vs. WT Fe-only 179 μg vs. WT EPO-only 160 μg vs. WT Fe/EPO 199 μg; p<0.05 for Fe/EPO vs. saline), and the HKO mice had no significant changes with Fe and/or EPO supplementation. In contrast to the uninflamed WT mice, the BA-injected WT mice did not show large increases in iron stores with iron treatments. As illustrated in our previous paper describing the BA-injected mouse model of AI, these acutely inflamed mice develop hepcidin-mediated iron dysregulation with iron-restricted anemia despite significantly increased tissue iron stores (15). Thus, the inflamed WT mice already have markedly elevated baseline liver iron levels and would likely require higher doses of iron supplementation to detect a significant increase.
Figure 3. Iron status and utilization in inflamed WT and HKO mice undergoing early treatment with Fe and/or EPO.
(A) Liver iron. (B) Serum iron. (C) Δ MCV. (D) Spleen weights. The dashed lines represent the mean values from the uninflamed control WT mice of Figure 1. Panels A–C: Treatment groups for each genotype included 5–10 evaluable male mice. Panel D: Treatment groups for WT mice included 3 male mice; Treatment groups for HKO mice included 8–10 male mice. Means ± SD (Panel A) or medians ± 75th/25th percentile (Panels B–D) are shown; *p<0.05 compared to saline group, †p<0.05 and ††p<0.001 compared to genotype counterpart, all by Holm-Sidak method.
Serum iron measurements showed a trend towards increased levels in HKO mice as compared to WT mice (Figure 3B), and significantly higher serum iron in the Fe-only group. This observation is to be expected, as hepcidin deletion allows the free efflux of iron from intracellular storage into circulation. The WT mice showed no significant differences in serum iron levels in the treatment groups, reflecting the decreased availability of circulating iron in times of acute inflammation with increased hepcidin expression. The HKO EPO-treated mice had a small drop in their serum iron levels compared to the saline HKO group (median HKO EPO serum iron 27.8 μmol/L vs. HKO saline serum iron 41.1 μmol/L; p<0.05) of unclear etiology. This fall in serum iron possibly reflects an early increase in erythropoiesis and iron utilization (without the benefit of supplementary Fe) that does not manifest in a hemoglobin improvement at the analyzed time point.
ΔMCV measurements were taken as a second measure of iron restriction, and mirrored the findings of serum iron measurements. ΔMCVs were increased in HKO mice compared to WT mice in all treatment groups (Figure 3C), again reflecting the attenuation of circulating iron restriction that occurs with hepcidin deletion. Within each genotype, however, there was no significant effect of treatments on ΔMCV values, except that EPO-treated HKO mice showed a small but statistically significant drop in ΔMCV values, possibly secondary to an early increase in iron utilization for erythropoiesis.
Spleen weights in all mouse groups were dramatically increased as compared to uninflamed mice, likely as a result of stress extramedullary erythropoiesis (Figure 3D). There were no significant differences with the various treatments in either the WT or HKO groups. The saline-treated WT mice did have higher spleen weights than the HKO saline group, with unclear significance and etiology, perhaps indicating a difference in medullary and extramedullary erythropoiesis between untreated inflamed WT vs. HKO mice.
Together, these results indicated that administering iron and/or EPO shortly after the inflammatory stimulus did not improve any hematological parameters in either WT or HKO mice compared to their own control group, but that HKO mice were less anemic than WT mice regardless of the treatment.
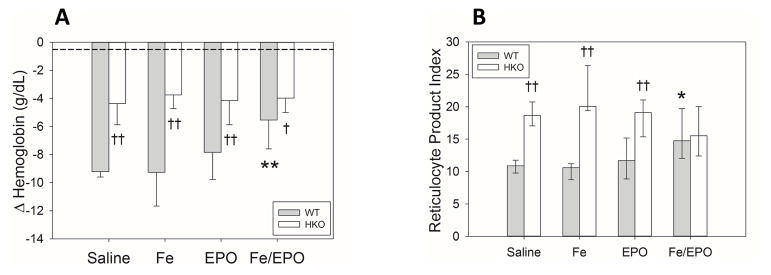
Delayed combination Fe+EPO treatment increases erythropoiesis in acutely inflamed WT mice, but cannot surpass the protective effect of hepcidin deletion
As acute inflammation has been shown to blunt erythropoiesis through multiple mechanisms involving inflammatory cytokines and hepcidin (25), we hypothesized that BA-injected mice could achieve the erythropoietic benefit of Fe and EPO treatments if given at a later time point when inflammation is less intense. In this set of experiments, both WT and HKO mice were injected with BA on day 0, underwent Fe and/or EPO injections on days 7&8, and were analyzed on day 14 (Supplemental Digital Content - Figure 1B). ΔHgb measurements showed that in BA-injected WT mice, only treatment with both Fe and EPO significantly lessened their hemoglobin drops compared to saline-treated mice (Figure 4A) (mean ΔHgb in WT saline group −9.2 g/dL vs. Fe-only −9.3 g/dL vs. EPO-only −7.8 g/dL vs. Fe/EPO −5.5 g/dL; p<0.001 for Fe/EPO vs. saline). The HKO mice in all treatment groups had less severe anemia than the WT mice, again demonstrating the erythropoietic advantage of having increased iron availability secondary to hepcidin deletion. Even the WT mice that benefited significantly from Fe+EPO therapy had larger hemoglobin drops than the HKO mice with the same treatment (mean ΔHgb WT Fe/EPO −5.5 g/dL vs. HKO Fe/EPO −4.0 g/dL; p<0.05). Interestingly, HKO mice had no significant improvement in their ΔHgbs with any treatment regimen as compared to the saline-treated HKO group. Thus, the protective effect of hepcidin deletion on the development of AI is superior to even the delayed Fe+EPO therapy.
Figure 4. Delayed treatment with both Fe and EPO increases hemoglobin and reticulocytosis in inflamed WT mice.
(A) Δ Hemoglobin. (B) Reticulocyte product index. The dashed lines represent the mean values from the uninflamed control WT mice of Figure 1. Treatment groups for each genotype included 7–10 evaluable male mice. Means ± SD (Panel A) or medians ± 75th/25th percentile (Panel B) are shown; *p<0.05 and **p<0.001 compared to saline group, †p<0.05 and ††p<0.001 compared to genotype counterpart, all by Holm-Sidak method.
RPI measurements also showed that within the WT groups, only Fe/EPO-treated WT mice had increased reticulocytosis as compared to the saline WT mice (Figure 4B) (median RPI in WT saline group 10.9 vs. Fe-only 10.6 vs. EPO-only 11.7 vs. Fe/EPO 14.8; p<0.05 for Fe/EPO vs. saline), confirming the increased erythropoiesis of inflamed WT mice receiving delayed combination Fe+EPO therapy. The HKO mice had increased RPI as compared to their WT counterparts in all treatment groups, except for the Fe/EPO group where they were similar (median RPI HKO Fe/EPO 15.5 vs. WT Fe/EPO 14.8).
These results show that in contrast to the ineffectiveness of early Fe+EPO therapy in inflamed WT mice, delayed Fe+EPO therapy did increase their erythropoiesis and ΔHgb. This benefit of delayed Fe+EPO therapy likely occurs as a result of EPO-induced hepcidin suppression with concomitant iron supplementation. Although our terminal serum hepcidin measurements did not find a difference among the various treatment groups at the analyzed time point (Supplemental Digital Content - Figure 2), the hepcidin differences likely occurred earlier in the timeline after EPO therapy (26).
Delayed combination Fe+EPO treatment increases iron utilization in HKO mice
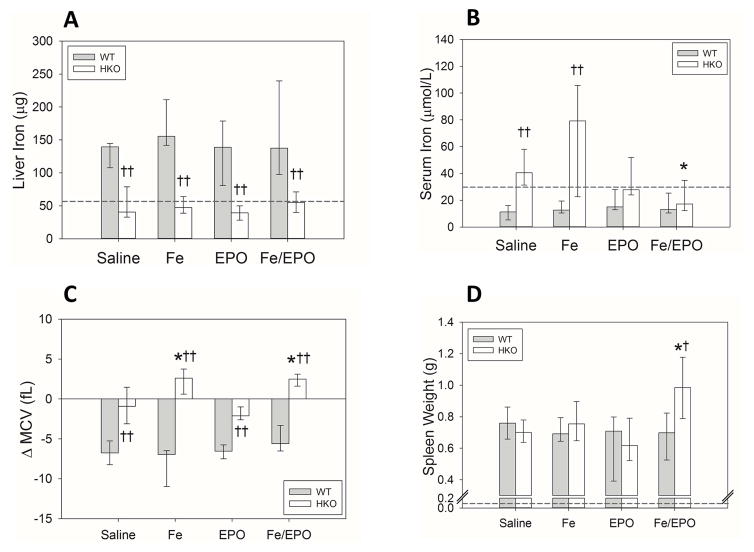
Multiple iron parameters were again measured in the WT and HKO mice that had received delayed Fe and/or EPO treatments after BA injection. The HKO mice had consistently lower liver iron levels than the WT mice in all treatment groups, confirming that the HKO mice were adequately iron-depleted by their iron-deficient diet (Figure 5A). Despite iron supplementation, the BA-injected WT mice did not show increases in iron stores. Because of hepcidin-mediated iron dysregulation, these inflamed mice already have markedly elevated baseline liver iron levels as compared to uninflamed mice and would likely require higher doses of iron supplementation to achieve a detectable increase in iron storage levels.
Figure 5. Iron status and utilization in inflamed WT and HKO mice undergoing delayed treatment with Fe and/or EPO.
(A) Liver iron. (B) Serum iron. (C) Δ MCV. (D) Spleen weights. The dashed lines represent the mean values from the uninflamed control WT mice of Figure 1. Treatment groups for each genotype included 7–10 evaluable male mice. Medians ± 75th/25th percentile are shown; *p<0.05 and **p<0.001 compared to saline group, †p<0.05 and ††p<0.001 compared to genotype counterpart, all by Holm-Sidak method.
Serum iron measurements illustrated the effects of the various delayed treatments on circulating iron (Figure 5B). As expected, the inflamed WT mice consistently had hypoferremia secondary to hepcidin-mediated iron restriction, and iron supplementation was insufficient to reverse this. The saline and Fe-treated HKO mice had increased serum iron levels, as compared to WT mice in the same treatment groups, secondary to the effects of hepcidin deletion on the efflux of iron from intracellular storage into circulation. Interestingly, EPO-treated and Fe/EPO-treated HKO mice lowered their serum iron to levels comparable to those of their WT counterparts (median serum iron HKO saline 40.5 μmol/L vs. HKO Fe/EPO 17.2 μmol/L; p<0.05). We postulate that this decrease in serum iron is secondary to EPO-stimulated erythropoiesis and iron utilization that has not yet manifested in an increase in hemoglobin. ΔMCVs were increased in HKO mice compared to WT mice in all treatment groups (Figure 5C), again reflecting the attenuation of circulating iron restriction that occurs with hepcidin deletion. Within the WT mouse groups, ΔMCV values were similar in all treatment groups. Within HKO mice, ΔMCVs were increased in the Fe-treated groups (median ΔMCV in HKO saline group −0.9 fL vs. Fe-only 2.6 fL vs. EPO-only −2.1 fL vs. Fe/EPO 2.5 fL; p<0.05 for each Fe-treatment group vs. saline). As expected, hepcidin deletion allows a significant reversal of iron restriction in the context of iron supplementation.
Spleen weights were measured as a marker of extramedullary erythropoiesis in the inflamed WT and HKO mice in the various delayed treatment groups (Figure 5D). WT mice showed no significant differences regardless of the treatment, despite the increased ΔHgb noted in the Fe/EPO treatment group. This may be a limitation of having measured only one time point after the treatment. The HKO mice did have increased spleen weights with delayed Fe+EPO therapy (median HKO saline spleen weight 0.70 g vs. HKO Fe/EPO spleen weight 0.98 g; p<0.05). This increase in spleen weight is consistent with the observed decrease in serum iron in these same mice, indicating an increase in iron utilization and extramedullary erythropoiesis that has not yet resulted in an increase in hemoglobin at this time point.
DISCUSSION
The pathogenesis of ICU anemia is multifactorial, including hepcidin-mediated iron restriction, impaired proliferation of erythroid precursors, and shortened erythrocyte lifespan (1, 2), and may be further exacerbated by blood loss and iron deficiency. AI is also characterized by reduced erythropoiesis that can occur in the context of either decreased EPO levels and/or impaired erythroid responsiveness to adequate levels of circulating EPO (25). Multiple cytokines have been reported to have a suppressive effect on the proliferation of erythroid progenitor cells, including tumor necrosis factor α, IL-1, and interferon-γ (IFN-γ) (27–29). Notably, one recent study found that murine transgenic overexpression of IFN-γ stimulates monocytic differentiation at the expense of erythroid differentiation (30), possibly allowing faster production of leukocytes during times of infection. AI is also associated with a shortened erythrocyte lifespan which has been largely attributed to cytokine effects on macrophage activation and erythrophagocytosis. In mouse models, IL-4 and IFN-γ specifically have been shown to activate macrophages for erythrophagocytosis (30, 31).
We report a systematic study of the effects of supplemental Fe and/or EPO on the development of AI in our previously characterized mouse model of anemia of sepsis (15). The BA-injected mice were treated with Fe, EPO, or Fe+EPO at either early or late time points after the inflammatory stimulus. We had previously illustrated that these acutely inflamed mice had elevated SAA-1 levels that peaked on day 1, and an early elevation in hepcidin followed by a steep decline after 7 days as erythropoietic recovery started. In order to elucidate the effects of acute inflammation and hepcidin-mediated iron dysregulation on the responsiveness to Fe and EPO therapy, we administered the therapies immediately after inflammatory stimulus (days 1&2) or a week later (days 7&8) when acute inflammation had subsided. Consistent with the known effects of inflammation and iron dysregulation on erythropoiesis, early therapy with Fe and/or EPO did not result in any attenuation of anemia in BA-injected WT or HKO mice (compared to their own saline control group). The unchanged RPI in all of the treatment groups of both genotypes confirmed the lack of erythropoietic response to the supplemental Fe/EPO. Also of note, hepcidin deletion appeared to have a protective effect in the development of AI. While the HKO mice did decrease hemoglobin with acute inflammation, the decrease was milder by 2.5 g/dL as compared to their WT counterparts. Thus, although early supplemental Fe/EPO therapies are not effective in the context of acute inflammation, early neutralization of hepcidin could be beneficial.
Late treatment of inflamed WT mice with combination Fe/EPO therapy yielded a significant attenuation of the hemoglobin drop by 3.7 g/dL as compared to untreated mice. Of note, no benefit was seen in the Fe-only or EPO-only treatment groups. We hypothesize that EPO administration ameliorated the development of AI by both providing a direct stimulus for erythroid production, as well as decreasing hepcidin levels. The recently described hormone erythroferrone (ERFE) has been shown to be a powerful mediator of hepcidin suppression during stress erythropoiesis (26). By reducing hepcidin levels with EPO and providing supplemental iron, we were able to reverse hepcidin-mediated iron dysregulation while providing the necessary iron for hemoglobin synthesis. These Fe/EPO treated mice also had a 26% increase in RPI compared to controls, confirming the increased erythropoiesis in this treatment group. Interestingly, while the HKO mice consistently had lower hemoglobin drops in all treatment groups as compared to WT mice, they had no demonstrable benefit from any therapy. Thus, complete hepcidin ablation appears to be more effective in attenuating the development of AI than combination Fe+EPO therapy. The removal of hepcidin-mediated iron dysregulation in these inflamed mice allows for increases in circulating and available iron for hemoglobin production and erythropoiesis.
Our study emphasizes the significant role of hepcidin in the development of ICU anemia. Multiple experimental therapies targeting the hepcidin-ferroportin axis are in development for the treatment of inflammatory anemias. Two drugs that target IL-6-induced hepcidin expression are tocilizumab, an IL-6 receptor antibody that reduces hepcidin and improves anemia in rheumatoid arthritis (25), and siltuximab, an IL-6 antibody recently approved by the FDA for its use in lowering hepcidin and improving anemia in patients with multicentric Castleman’s Disase (32). Two example inhibitors of hepcidin production are a BMP type I receptor inhibitor (LDN-193189) and a soluble hemojuvelin-Fc fusion protein, both of which have been shown to improve anemia in murine models of AI (25). Perhaps the most relevant therapeutic option for ICU anemia would be those that target hepcidin directly, including hepcidin antibodies, anticalins, Spiegelmers, and antisense oligonucleotides. The hepcidin Spiegelmer lexaptepid (NOX-H94) improved LPS-induced hypoferremia in humans (33) and is currently in Phase 2 clinical trials. A neutralizing hepcidin antibody (12B9m) has been shown to improve iron availability and erythrocyte hemoglobinization in mice, and allow more efficient hemoglobinization in monkeys (34). A hepcidin anticalin (PRS-080) has completed a phase I clinical trial that demonstrated marked reduction of plasma hepcidin levels followed by elevation of serum iron levels (35).
Our paper illustrates the significant role of hepcidin in the development of acute and severe anemia, as well as the importance of appropriately timing the currently available therapeutic interventions. While our study serves as an important proof of concept for the role of hepcidin in ICU anemia, further investigation is necessary to determine the applicability of the developing antihepcidin therapies in the treatment of ICU anemia.
Supplementary Material
Acknowledgments
FINANCIAL SUPPORT:
NIH NHLBI 5K08-HL127293 (AK)
NIH NIDDK R01-DK090554 (TG and EN)
Institutional UCLA Department of Medicine startup funds (AK)
Footnotes
FINANCIAL CONFLICTS OF INTEREST:
T.G. and E.N. are consultants and stockholders of Intrinsic LifeSciences, Merganser Biotech, and Silarus Therapeutics.
References
- 1.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352(10):1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 2.Adamson JW. The anemia of inflammation/malignancy: mechanisms and management. Hematology Am Soc Hematol Educ Program. 2008:159–165. doi: 10.1182/asheducation-2008.1.159. [DOI] [PubMed] [Google Scholar]
- 3.Ganz T. Iron in innate immunity: starve the invaders. Curr Opin Immunol. 2009;21(1):63–67. doi: 10.1016/j.coi.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276(11):7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 5.Lesbordes-Brion JC, Viatte L, Bennoun M, Lou DQ, Ramey G, Houbron C, Hamard G, Kahn A, Vaulont S. Targeted disruption of the hepcidin 1 gene results in severe hemochromatosis. Blood. 2006;108(4):1402–1405. doi: 10.1182/blood-2006-02-003376. [DOI] [PubMed] [Google Scholar]
- 6.Nicolas G, Bennoun M, Porteu A, Mativet S, Beaumont C, Grandchamp B, Sirito M, Sawadogo M, Kahn A, Vaulont S. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci U S A. 2002;99(7):4596–4601. doi: 10.1073/pnas.072632499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayeur C, Lohmeyer LK, Leyton P, Kao SM, Pappas AE, Kolodziej SA, Spagnolli E, Yu B, Galdos RL, Yu PB, Peterson RT, Bloch DB, Bloch KD, Steinbicker AU. The type I BMP receptor Alk3 is required for the induction of hepatic hepcidin gene expression by interleukin-6. Blood. 2014;123(14):2261–2268. doi: 10.1182/blood-2013-02-480095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, Meier-Hellmann A, Nollet G, Peres-Bota D Investigators ABC. Anemia and blood transfusion in critically ill patients. Jama. 2002;288(12):1499–1507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 9.Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Abraham E, MacIntyre NR, Shabot MM, Duh MS, Shapiro MJ. The CRIT Study: Anemia and blood transfusion in the critically ill--current clinical practice in the United States. Crit Care Med. 2004;32(1):39–52. doi: 10.1097/01.CCM.0000104112.34142.79. [DOI] [PubMed] [Google Scholar]
- 10.Thomas J, Jensen L, Nahirniak S, Gibney RT. Anemia and blood transfusion practices in the critically ill: a prospective cohort review. Heart Lung. 2010;39(3):217–225. doi: 10.1016/j.hrtlng.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Khamiees M, Raju P, DeGirolamo A, Amoateng-Adjepong Y, Manthous CA. Predictors of extubation outcome in patients who have successfully completed a spontaneous breathing trial. Chest. 2001;120(4):1262–1270. doi: 10.1378/chest.120.4.1262. [DOI] [PubMed] [Google Scholar]
- 12.Pieracci FM, Henderson P, Rodney JR, Holena DN, Genisca A, Ip I, Benkert S, Hydo LJ, Eachempati SR, Shou J, Barie PS. Randomized, double-blind, placebo-controlled trial of effects of enteral iron supplementation on anemia and risk of infection during surgical critical illness. Surg Infect (Larchmt) 2009;10(1):9–19. doi: 10.1089/sur.2008.043. [DOI] [PubMed] [Google Scholar]
- 13.Pieracci FM, Stovall RT, Jaouen B, Rodil M, Cappa A, Burlew CC, Holena DN, Maier R, Berry S, Jurkovich J, Moore EE. A multicenter, randomized clinical trial of IV iron supplementation for anemia of traumatic critical illness*. Crit Care Med. 2014;42(9):2048–2057. doi: 10.1097/CCM.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 14.Corwin HL, Gettinger A, Fabian TC, May A, Pearl RG, Heard S, An R, Bowers PJ, Burton P, Klausner MA, Corwin MJ Group EPOCCT. Efficacy and safety of epoetin alfa in critically ill patients. N Engl J Med. 2007;357(10):965–976. doi: 10.1056/NEJMoa071533. [DOI] [PubMed] [Google Scholar]
- 15.Kim A, Fung E, Parikh SG, Valore EV, Gabayan V, Nemeth E, Ganz T. A mouse model of anemia of inflammation: complex pathogenesis with partial dependence on hepcidin. Blood. 2014;123(8):1129–1136. doi: 10.1182/blood-2013-08-521419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Courselaud B, Troadec MB, Fruchon S, Ilyin G, Borot N, Leroyer P, Coppin H, Brissot P, Roth MP, Loreal O. Strain and gender modulate hepatic hepcidin 1 and 2 mRNA expression in mice. Blood cells, molecules & diseases. 2004;32(2):283–289. doi: 10.1016/j.bcmd.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113(9):1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramos E, Kautz L, Rodriguez R, Hansen M, Gabayan V, Ginzburg Y, Roth MP, Nemeth E, Ganz T. Evidence for distinct pathways of hepcidin regulation by acute and chronic iron loading in mice. Hepatology. 2011;53(4):1333–1341. doi: 10.1002/hep.24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramos E, Ruchala P, Goodnough JB, Kautz L, Preza GC, Nemeth E, Ganz T. Minihepcidins prevent iron overload in a hepcidin-deficient mouse model of severe hemochromatosis. Blood. 2012;120(18):3829–3836. doi: 10.1182/blood-2012-07-440743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasu BJ, Cooke KS, Arvedson TL, Plewa C, Ellison AR, Sheng J, Winters A, Juan T, Li H, Begley CG, Molineux G. Antihepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation-induced anemia. Blood. 2010;115(17):3616–3624. doi: 10.1182/blood-2009-09-245977. [DOI] [PubMed] [Google Scholar]
- 21.Wong SS, Qutishat AS, Lange J, Gornet TG, Buja LM. Detection of iron-deficiency anemia in hospitalized patients by zinc protoporphyrin. Clin Chim Acta. 1996;244(1):91–101. doi: 10.1016/0009-8981(95)06200-9. [DOI] [PubMed] [Google Scholar]
- 22.Zhou XJ, Laszik Z, Wang XQ, Silva FG, Vaziri ND. Association of renal injury with increased oxygen free radical activity and altered nitric oxide metabolism in chronic experimental hemosiderosis. Lab Invest. 2000;80(12):1905–1914. doi: 10.1038/labinvest.3780200. [DOI] [PubMed] [Google Scholar]
- 23.Avni T, Bieber A, Steinmetz T, Leibovici L, Gafter-Gvili A. Treatment of anemia in inflammatory bowel disease--systematic review and meta-analysis. PLoS One. 2013;8(12):e75540. doi: 10.1371/journal.pone.0075540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108(12):3730–3735. doi: 10.1182/blood-2006-06-028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nemeth E, Ganz T. Anemia of inflammation. Hematology/oncology clinics of North America. 2014;28(4):671–681. vi. doi: 10.1016/j.hoc.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46(7):678–684. doi: 10.1038/ng.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang CQ, Udupa KB, Lipschitz DA. Interferon-gamma exerts its negative regulatory effect primarily on the earliest stages of murine erythroid progenitor cell development. Journal of cellular physiology. 1995;162(1):134–138. doi: 10.1002/jcp.1041620116. [DOI] [PubMed] [Google Scholar]
- 28.Means RT, Jr, Krantz SB. Inhibition of human erythroid colony-forming units by tumor necrosis factor requires beta interferon. J Clin Invest. 1993;91(2):416–419. doi: 10.1172/JCI116216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Means RT, Jr, Dessypris EN, Krantz SB. Inhibition of human erythroid colony-forming units by interleukin-1 is mediated by gamma interferon. Journal of cellular physiology. 1992;150(1):59–64. doi: 10.1002/jcp.1041500109. [DOI] [PubMed] [Google Scholar]
- 30.Libregts SF, Gutierrez L, de Bruin AM, Wensveen FM, Papadopoulos P, van Ijcken W, Ozgur Z, Philipsen S, Nolte MA. Chronic IFN-gamma production in mice induces anemia by reducing erythrocyte life span and inhibiting erythropoiesis through an IRF-1/PU.1 axis. Blood. 2011;118(9):2578–2588. doi: 10.1182/blood-2010-10-315218. [DOI] [PubMed] [Google Scholar]
- 31.Milner JD, Orekov T, Ward JM, Cheng L, Torres-Velez F, Junttila I, Sun G, Buller M, Morris SC, Finkelman FD, Paul WE. Sustained IL-4 exposure leads to a novel pathway for hemophagocytosis, inflammation, and tissue macrophage accumulation. Blood. 2010;116(14):2476–2483. doi: 10.1182/blood-2009-11-255174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casper C, Chaturvedi S, Munshi N, Wong R, Qi M, Schaffer M, Bandekar R, Hall B, van de Velde H, Vermeulen J, Reddy M, van Rhee F. Analysis of Inflammatory and Anemia-Related Biomarkers in a Randomized, Double-Blind, Placebo-Controlled Study of Siltuximab (Anti-IL6 Monoclonal Antibody) in Patients With Multicentric Castleman Disease. Clin Cancer Res. 2015;21(19):4294–4304. doi: 10.1158/1078-0432.CCR-15-0134. [DOI] [PubMed] [Google Scholar]
- 33.van Eijk LT, John AS, Schwoebel F, Summo L, Vauleon S, Zollner S, Laarakkers CM, Kox M, van der Hoeven JG, Swinkels DW, Riecke K, Pickkers P. Effect of the antihepcidin Spiegelmer lexaptepid on inflammation-induced decrease in serum iron in humans. Blood. 2014;124(17):2643–2646. doi: 10.1182/blood-2014-03-559484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooke KS, Hinkle B, Salimi-Moosavi H, Foltz I, King C, Rathanaswami P, Winters A, Steavenson S, Begley CG, Molineux G, Sasu BJ. A fully human anti-hepcidin antibody modulates iron metabolism in both mice and nonhuman primates. Blood. 2013;122(17):3054–3061. doi: 10.1182/blood-2013-06-505792. [DOI] [PubMed] [Google Scholar]
- 35.Ulrich Moebius WF, Fenzl Edgar, van Swelm Rachel, Swinkels Dorine W, Hohlbaum Andreas. A Phase I Study Investigating the Safety, Tolerability, Pharmacokinetics and Pharmacodynamic Activity of the Hepcidin Antagonist PRS-080#022. Results from a Randomized, Placebo Controlled, Double-Blind Study Following Single Administration to Healthy Subjects. Blood. 2015;126(23):536. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.