Abstract
Although vaccination is historically one of the most successful strategies for the prevention of infectious diseases, development of vaccines for cancer and many chronic infections, such as HIV, malaria, and tuberculosis, has remained a challenge. Strong and long-lasting antigen-specific T cell responses are critical for therapy of these diseases. A major challenge in achieving a robust CD8+ T cell response is the requirement of spatio-temporal orchestration of antigen cross-presentation in antigen-presenting cells with innate stimulation. Here, we discuss the development of nanoparticle vaccine (nanovaccine) that modulates the innate immune system and enhances adaptive immunity with reduced toxicity. We address how nanovaccines can integrate multiple functions, such as lymph node targeting, antigen presentation, and stimulation of innate immunity, to achieve a robust T cell response for immunotherapy.
Graphical abstract
1. Introduction
Vaccines represent one of the greatest medical achievements of modern civilization, and have had a major impact on public health. The first generation of vaccines contains inactivated or attenuated microbes, such as viruses or bacteria. These prophylactic vaccines can induce life-long antibody responses to prevent disease from future exposure. Although these prophylactic vaccines have successfully eliminated or greatly reduced the burden of former epidemics, such as smallpox, poliomyelitis, tetanus, diphtheria and rubella, they do not work well in some patients and have the risk of reversion to virulence [1]. Furthermore, the future impact of vaccination should not only defend against infectious diseases, but also induce immune responses to treat ongoing diseases, such as cancer or chronic infections like HIV, malaria, and tuberculosis. Therapeutic vaccines must overcome pathogen-mediated evasion of the immune response and are likely to require induction of specific cytotoxic T-lymphocyte (CTL; activated CD8+ T cell) responses against pathogens that have already established [2,3]. In the case of cancer, chimeric antigen receptor (CAR) T cell therapy has shown the effectiveness of T cells in killing tumor cells [4], and recently several checkpoint inhibitors (anti-CTLA-4, anti-PD-1 and anti-PD-L1) have been approved, which target the suppression of T cells and enhance anti-tumor T cell response in cancer patients [5]. However, most tumors exhibit low immunogenicity, and a majority of patients fail to generate adequate cancer-specific CTLs and therefore cannot benefit from immune checkpoint therapies, which only remove the inhibition of T cell functions. Therefore, there is an unmet need to develop safe strategies that boost anti-tumor immunity to synergize with immune checkpoint therapy.
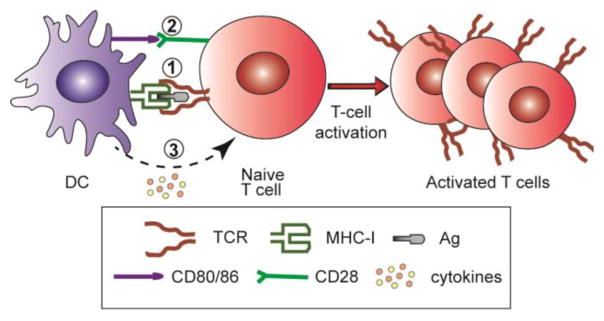
Spatio-temporal orchestration (STO) is essential to produce an antigen-specific CTL response (Fig. 1) [6]. (1) Efficient antigen (Ag) delivery to lymphoid organs (e.g., peripheral lymph nodes), cytosolic delivery and cross presentation by the major histocompatibility complex (MHC) molecule in the dendritic cells (DCs) are important. (2) Induction of co-stimulatory molecules (e.g., CD80/CD86) on DCs is critical for T cell activation. Lack of co-stimulation can lead to immune resistance or T cell apoptosis. (3) Cytokine release also plays a critical role in the differentiation of T cells. For example, type-I interferons stimulate the differentiation of naïve CD4+ T cells into Th1 subtype, whereas IL-4 leads to Th2 subtype. For cancer immunotherapy, Th1 and CD8+ CTL responses are desirable [6,7].
Figure 1.
T cell activation by antigen presenting cells (e.g., dendritic cells). Orchestration of (1) antigen presentation by MHC molecule to the T-cell receptor, (2) CD80/86 co-stimulation, and (3) cytokine signals is necessary to achieve antigen-specific T cell activation.
Nanoparticle vaccines (nanovaccines) are miniscule particulates (20–100 nm) that target the body’s immune system to activate the host’s immune response against diseases. Nanovaccines have unique characteristics that can improve vaccine efficiency and modulate the immune response in vivo [8,9]. Using different materials and manufacturing conditions, researchers can precisely control the size, shape, surface charge, hydrophobicity and loading density of antigens and adjuvants. The incorporation of antigens or adjuvants can be achieved by conjugation of these components to the surface or core of nanoparticles, or by encapsulation within vesicles or micelles. In this review, we will discuss how the unique features of nanoparticles affect their antigen-presenting cell (APC) targeting, antigen presentation, and how the nanoparticle incorporates different vaccine components to achieve enhanced T cell response. We acknowledge that more parameters, such as shape, rigidity, biodegradability and so on, are all important characteristics that affect the efficacy of nanovaccines. The effect of these parameters has been extensively described elsewhere, and will not be the focus of this review [10–14].
2. Antigen delivery and presentation
2.1 Major materials and effect
A vaccine that contains only some components of a pathogen is called a subunit vaccine. Subunit vaccines can eliminate the risk of reversion and reduce the possibility of autoimmune and allergic responses compared to inactivated or attenuated pathogen vaccines. However, despite advantages in safety, subunit vaccines have shown weakness in immune stimulation. Nanoparticles are an excellent platform for subunit vaccines, as they can extend the antigen release and circulation time, as well as target antigens to APCs, enhancing the efficacy of these vaccines. Various materials have been used to create synthetic nanoparticles for use in immunotherapy. This section will outline the major classes of materials and the advantages and weaknesses of each.
Many polymer-based nanoparticles have been investigated for their potential efficacy in immunotherapy, and polylactide-co-glycolide (PLGA) copolymer has been the most widely studied. PLGA is biodegradable, as its ester linkages are cleaved in vivo to produce two monomers, lactic and glycolic acid, which can be easily metabolized. By adjusting the ratio and positioning of the two monomers or conjugating to other molecules, properties such as size, solubility, and stability can be varied. PLGA is considered safe by the Food and Drug Administration (FDA) for clinical use, indicating its lack of toxicity. PLGA may be coupled to other polymers like polyethylene glycol (PEG) or polyethyleneimine (PEI) to form a block copolymer, which can self-assemble into a polymeric micelle that can encapsulate hydrophobic payloads in aqueous solutions, such as antigens [15] and extend blood circulation time [16]. Antigen-loaded polymer-based nanoparticles of various compositions have shown efficacy in increasing T cell responses, compared to the antigen alone [17].
Liposomes are another common platform for nanoparticulate vaccines. They are comprised of a phospholipid bilayer, which is easily biodegradable. Like PLGA, many liposome-based delivery methods have been approved by the FDA. Liposomes can be easily modified by altering the specific phospholipids used, or by coating the surface with other molecules like PEG [18,19]. While liposomes are able to encapsulate many types of compounds due to their amphiphilic nature, liposomes can suffer from poor loading efficiency and shelf stability [15,20]. Compared to antigens alone, antigens both conjugated to [21] and encapsulated [22] in liposomes have shown increased proliferation of antigen-specific CTLs.
Inorganic materials, such as carbon nanotubes and colloidal gold, have also been investigated for their potential in nanovaccine design. Both carbon nanotubes and gold nanoparticles conjugated to tumor-derived antigens have been shown in murine models to suppress tumor growth in an antigen-specific manner, compared to a vaccine comprised of the free antigen [23,24]. Both materials are easily functionalizable and are readily ingested by immune cells. However, concerns exist, particularly over solubility, long-term toxicity, and nonbiodegradability [24–26]. More work need to be performed to further evaluate inorganic nanoparticles for vaccine use.
2.2 Particle characteristics
Peripheral lymph nodes are a hub for the adaptive immune system as a primary site for antigen-presenting cells, which are key for the generation of antigen-specific T cells [27]. Targeted delivery of antigens to lymph nodes has been shown to increase the adaptive immune response, and nanoparticles provide a novel method of delivering antigens to lymph nodes [28].
In designing nanovaccines to migrate preferentially to lymph nodes, many factors must be considered. One is surface charge of the nanoparticles. It is generally accepted that cationic nanoparticles exhibit more toxicity in phagocytic cells, particularly due to the formation of reactive oxygen species and damage to cellular membranes [27,29,30]. Interstitial fluid contains negatively charged proteins, so charge repulsion causes anionic nanoparticles to drain more quickly to lymph nodes [31]. Phagocytic cells, like antigen-presenting cells, ingest anionic nanoparticles more readily than cationic nanoparticles [32–35]. Therefore, a negative surface charge appears to be preferable.
Hydrophobic nanoparticles have been shown to induce higher levels of antibody titers than hydrophilic nanoparticles [36]. Seong and Matzinger hypothesized that hydrophobic moieties can serve as danger signals to activate the immune system [37]. However, blood and other bodily fluids are hydrophilic, so hydrophobic nanoparticles may not be soluble and can lead to formation of aggregates at the injection site. Indeed, Rao et al showed a negative correlation between hydrophobicity and nanoparticle uptake and retention by lymph nodes [31]. Thus, amphiphilic nanoparticles, including the use of hydrophilic PEG as a “cloak” for hydrophobic nanoparticles, have become a focus of research.
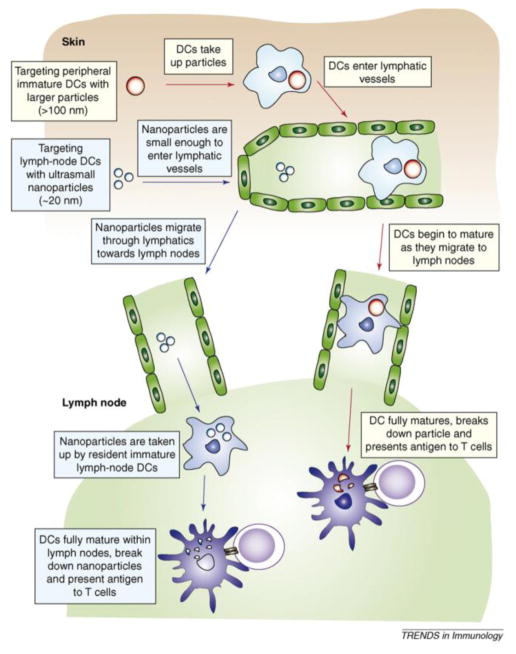
There is an optimal size range for nanoparticles to migrate to the lymph nodes (Fig. 2) [38]. Nanoparticles smaller than 3–5 nm are cleared by the blood and bypass lymph nodes. Larger nanoparticles are drained by the lymphatic system and traffic to lymph nodes via two distinct, size-dependent mechanisms. The first involves antigen-presenting cells at the nanovaccine injection site, which may take up nanoparticles via phagocytosis and then migrate to the lymph nodes. In the second pathway, nanoparticles transport through lymphatic vessels directly to lymph nodes. Manolova et al demonstrated the effect of particle size on the delivery method for nanoparticles to lymph nodes. Nanoparticles larger than 200 nm largely followed the first pathway and were delivered to lymph nodes after 18 hours. Nanoparticles smaller than this radius drained to lymph nodes in the second, dendritic cell-independent mechanism within 2–3 h [39]. As the second pathway is much faster, nanovaccine development has focused on nanoparticles smaller than 200 nm.
Figure 2.
Relationships between particle size and lymph node targeting pathway. Particles less than 100 n m can directly travel to lymph nodes and be taken up by lymph node-resident APCs. Particles bigger than 100 n m are mainly taken up by peripheral APCs, which migrate to lymph nodes to initiate subsequent T cell activation. Reprinted with permission from Ref. 38.
The Hubbell and Swartz groups showed that PEGylated poly(propylene sulfide) nanoparticles smaller than 50 nm had significantly higher uptake and retention by lymph nodes, for up to five days, compared to nanoparticles of 100 nm in diameter. These smaller nanoparticles trafficked to antigen-presenting cells in the lymph nodes with ten times the efficiency of the 100 nm nanoparticles, and were able to induce dendritic cell maturation [40,41].
However, while smaller nanoparticles are known to drain more efficiently to lymph nodes, it is hypothesized that larger nanoparticles are trapped more effectively by lymph nodes, as smaller sizes may cause nanoparticles to bypass lymph nodes. Larger particles are able to be phagocytosed more effectively, increasing their retention in lymph nodes [41]. Thus, there is a delicate balance that must be achieved between these two competing size factors to ensure maximal lymph node trafficking and retention. The optimal size may be compounded by additional variables, such as the flexibility of the nanoparticle material, shape, injection method, and method of uptake (such as phagocytosis or clathrin-mediated endocytosis) [42].
2.3 Cytosolic delivery by nanoparticles
Enhancing cross presentation of antigens by class I major histocompatibility complex (MHC) molecules to activate CTLs is an important aspect of nanovaccine development. MHC-I molecules are typically loaded with cytosol-derived peptides (e.g., processed by proteasomes), so delivery of antigens into the cytosol, is essential. Nanoparticles can be taken up into the cells through endocytosis and are sequestered inside the endosomes and lysosomes. One strategy for cytosolic delivery is the facilitation of endosomal escape to avoid lysosomal degradation of protein antigens. This can occur by multiple mechanisms, such as the formation of pores in the endosomal membrane, the proton sponge effect, and fusion with the endosome membrane [27,43].
An early development in cytosolic delivery of antigens was the vaccine adjuvant ISCOMATRIX™. ISCOMATRIX™ is comprised of a mixture of cholesterol, phospholipids, and a purified extract, ISCOPREP saponin from the bark of the Quillaia saponaria tree, which form a cage-like complex approximately 40–50 nm in diameter. In immature dendritic cells treated with a mixture of ISCOMATRIX™ and a protein antigen, the protein antigen was translocated from lysosomes to the cytosol and cytotoxic T cell activation was observed. Despite these advances, the molecular mechanism of cytosol delivery is not well understood [44]. In a clinical study, patients with resected, NY-ESO-1-expressing melanomas were injected with NY-ESO-1 as well as NY-ESO-1 with ISCOMATRIX™, and were rechallenged several years later with NY-ESO-1 protein. Those who had received ISCOMATRIX™ adjuvant showed higher rates of CD4+ and CD8+ T cell responses, indicating continuing immunity, as well as increased relapse-free survival [45].
Many nanoparticles have taken advantage of the low pH of endosomes and lysosomes as a strategy for inducing endosomal escape. Hu et al designed nanoparticles featuring a hydrophilic shell and a hydrophobic, pH-sensitive core. At low endolysosomal pH, the protonation of the core led to swelling of the nanoparticles and rupture of the endosome membrane via the proton sponge effect, allowing for efficient delivery of antigens into the cytosol of dendritic cells with minimal toxicity. The proton sponge effect is observed with nanoparticles that have a high buffering capacity, as the influx of ions and water into the endosomes leads to high osmotic pressure, and eventually rupture of the organelle [46]. Keller et al have also reported the use of a pH-sensitive polymer micelle nanoparticle for cytosolic delivery of antigens, and demonstrated higher concentrations of antigen-specific cytotoxic T cells compared to controls [47].
Vasdekis et al used light as a trigger for endosome disruption and cargo release into the cytosol. They developed nanoparticles comprised of amphiphilic block copolymers associated with a hydrophobic molecule, ethyl eosin, which acts as a photosensitizer in the presence of light and increases the hydrophobicity of the nanoparticle. Light activation destabilizes the nanoparticle and elicits payload delivery and endosomal escape. MHC I antigen presentation was observed in dendritic cells treated with nanoparticles loaded with a model antigen after light exposure [48].
In addition to harnessing the lower pH of endosomes/lysosomes to enhance cytosolic delivery, other methods of delivery have focused on the reducing environment of the cytosol. Li et al designed cationic, bioreducible alginate -polyethyleneimine (PEI) nanogels loaded with protein antigens. The nanoparticles were thought to escape endosomes via the proton sponge effect, and deliver the antigen efficiently into the cytosol after reduction. Compared to non-bioreducible nanogels, the bioreducible nanogels enhanced CD8+ T cell proliferation and IFN-γ production, indicating that the reducibility of nanoparticles may play a key role in activating the MHC-I pathway [49].
Liposomes that have been modified with Sendai virus proteins are able to fuse with cellular or endosomal membranes to deliver encapsulated molecules directly to the cytosol, but there are concerns over the potential immune responses to the viral proteins [18]. Yuba et al used pH sensitivity instead of viral proteins to induce fusogenicity in liposomes, which were destabilized in low pH environments. These pH-sensitive fusogenic liposomes were loaded with ovalbumin (OVA) antigen, and showed increased numbers of OVA-specific CTLs, tumor growth suppression, and increased survival in vivo in an E.G7-OVA tumor model compared to unmodified liposomes or free OVA antigen [50].
From the above examples, it can be concluded that the first generation of nanovaccines can enhance efficient antigen (Ag) delivery to lymphoid organs (e.g., peripheral lymph nodes), promote cytosolic delivery and cross presentation in the dendritic cells (DCs), and activate T cells, However, without any co-stimulatory molecule expression or cytokine secretion, the vaccine effect is still limited and there is a high likelihood of inducing immune tolerance [51]; additional immune stimulators may be incorporated in nanovaccines to reduce this risk.
3. Immune stimulator co-delivery in nanovaccines
3.1 Cytokines
Cytokines are important modulating agents that can regulate both innate and adaptive immune responses. When cytokines are systemically administered intravenously, harmful side effects, quick degradation and excretion can limit their effects. Treating mice with cytokines can enhance antitumor immune response, but clinical trials using these cytokines have been limited due to patient toxicity [52,53]. To overcome these limitations, many groups have engineered particles, including liposomes or polymer particles, to deliver the cytokines safely. These formulations have been shown to improve the circulation kinetics, enhance the anti-tumor or anti-intracellular microbe efficacy, and lower acute toxicity compared to free cytokines, despite smaller cytokine doses [54–56]. Building on this, several labs have attempted to particulate both cytokines and antigens as a vaccine strategy to induce antigen-specific T cell response.
Interleukin-2 (IL-2) can promote the differentiation of T cells into effector T cells when the naive T cells are exposed to an antigen. Johnston and colleagues encapsulated a model protein antigen, ovalbumin (OVA), and IL-2 together into liposomes, which significantly improved cellular immune responses and tumor protection compared to OVA alone or OVA encapsulated in liposomes [57]. Popescu et al extracted cell-membrane proteins from lymphoma cells and incorporated them together with IL-2 into proteoliposomes [58]. This vaccine elicited T-cell immunity in vivo, as demonstrated by secretion of type I cytokines and tumor protection. Mechanistic study showed that liposomal IL-2 increased both the humoral responses and the cytolytic CD8+ T cells [57,58]. Next, the group formulated IL-2 and a lymphoma-specific antigen into liposomal particles and tested the immunogenicity and toxicity of this cancer vaccine in human patients. In the initial clinical trial, this vaccine generated sustained, tumor-specific T cell response in all ten patients. Six out of ten patients remained in continuous remission after 50 months [59]. In the second clinical trial, one of the eleven patients achieved complete remission for up to 44 months [60]. These results demonstrated that the cytokine-incorporating nanovaccines are well tolerated and are effective in inducing tumor-specific T-cell responses.
Interleukin-12 (IL-12) was shown to promote the development of a Th1 cell phenotype from naive CD4+ T cells in response to antigenic stimulation. IL-12-encapsulated microspheres (IL-12EM) can sustain the release of IL-12 and induce strong Th1 immune responses specific to tuberculosis antigens. Antibody detection showed 128 to 256 fold higher levels of IgG2a, an antibody stimulated by Th1 cells, than the levels in control groups without IL-12, and a 1,024-fold higher level of IgG2a than the Alum-immunized group. In a M. tuberculosis infection model, these microspheres showed a better protection effect than Bacillus Calmette–Guérin (BCG) vaccine, the current gold standard tuberculosis vaccine [61].
Several other cytokines such as granulocyte macrophage colony-stimulating factor (GM-CSF) and interferon alpha (IFN-α) have also been reported to be incorporated into nanovaccines and improve the T cell response and anti-tumor effect [62–65]. Although systemic toxicity and quick clearance still limit the clinical usage of cytokines, strategies using cytokines as adjuvants to treat established diseases in patients show promise and must still be considered.
3.2 TLR agonists
In the past 30 years, many stimulators of the innate immune system have been discovered, which are referred to as pathogen-associated molecular patterns (PAMP) and damage-associated molecular patterns (DAMP) [66]. Recognition of these agents by APCs can potentially induce innate immune responses and effectively stimulate antigen presentation, co-stimulatory molecule expression, and cytokine secretion [67]. It is now generally accepted that dendritic cells (DCs) are the main antigen-presenting cells (APCs) regulating T cell responses. Studies have suggested that direct recognition of PAMPs by DCs is critical for priming an appropriate T cell response; inflammatory mediators such as cytokines can only amplify, but not initiate, adaptive immune responses [68]. Based on the previously described spatio-temporal orchestration, optimal antigen processing and presentation by DCs requires association of appropriate danger signals in the same phagocytosed cargo [69]. Particulate danger signals with antigen can induce DC activation and antigen presentation, which can subsequently enhance adaptive immune response.
On innate immune cells, a family of pattern recognition receptors (PRR) called Toll-like receptors (TLR) recognize several types of PAMPs to initiate a series of innate responses [70,71]. TLRs are expressed on dendritic cells (DC) and other professional APCs, such as macrophages and B cells. Some TLRs are expressed on the cell surface and act as sensors for extracellular PAMPs (e.g., TLR4) [72]. A subset of TLR molecules (TLR3, 7, 8 and 9) are expressed on endosomal membranes and bind nucleic acid-derived molecules, such as double stranded RNA for TLR3 [73], single-stranded RNA of viral and bacterial origin for TLR7 and 8 [74,75], and unmethylated DNA oligonucleotides (ODNs) containing CpG motifs (CpG ODNs) for TLR9 [76]. Besides innate immune stimulators, some TLRs have been shown to bias the Th1 immune response and enhance CD8+ T cell responses [77,78]. Therefore, TLR agonists have been extensively investigated as potential adjuvants. Until now, only monophosphoryl lipid A (MPLA), a TLR4 agonist, and imiquimod, a TLR7 agonist, have been approved by the FDA as adjuvants for some diseases [79,80]. Other TLR agonists might induce strong systemic inflammatory reactions in vivo, leading to potential adverse events [81,82] and autoimmune disease [83]. Particulated TLR agonists could help target adjuvant and antigen to a specific cell type to initiate the immune response, lowering the possibility of systemic toxicity. Geoffrey et al. reported a library of TLR7/8 agonist-conjugated polymers using different linkage groups and TLR7/8 agonist density. They concluded that the polymer particles with high agonist density promoted local retention and APC uptake, and induced the strongest T cell response and at least tenfold less systemic IL-12 production than unimers and free agonist at the same density [84].
Many studies in murine models have demonstrated enhanced humoral and cellular immunity elicited by particulate vaccines co-incorporating antigen and danger signal compounds [84–99]. Nanoparticle vaccine carrying peptide antigens and TLR-7 and -9 ligands have recently been shown to induce memory and effector CD8+ T-cell responses in melanoma patients [100]. Moon et al. developed lipid carriers (interbilayer-crosslinked multilamellar vesicles, or ICMVs, ~240 nm) composed of multiple lipid bilayers around an aqueous core [96]. ICMVs encapsulated protein antigens in the core and TLR-4 agonist MPLA in the vesicle walls, and rapidly released the adjuvant and antigen when they were taken up into the endo-lysosomes of cells. In vivo immunization (10 μg antigens, 0.1 μg MPLA per mouse) achieved a peak 28% tetramer-positive T cells in the CD8+ T-cell population. This response was dependent on the co-delivery of the TLR agonist.
de Titta et al. conjugated both CpG and antigen onto ultra-small polymeric nanoparticles (NPs, ~25–30 nm), which rapidly drained to the LN after intradermal injection. Conjugated nanoparticles (0.05 μM CpG) in vitro demonstrated a 2.5-fold increase in DC cell activation compared to free CpG (1 μM). When administrated in vivo, this nanovaccine showed efficacy at low doses (4 μg CpG per mouse) similar to those elicited by 100 μg free CpG, and induced potent and long-lived cellular immunity [88]. Liu et al. synthesized amphiphiles (amph-vaccines) conjugating an antigen or CpG to a lipophilic albumin-binding tail by a solubility-promoting polar polymer chain. This vaccine (10 μg antigens, 1.24 nmol CpG per mouse) increased the LN accumulation markedly, decreased systemic dissemination, and induced about 30-fold higher T-cell responses compared to free CpG [101]. Radovic-Moreno et al. conjugated both CpG and antigens onto spherical nucleic acids (SNA), which showed 700-fold increase of antibody titer and 400-fold higher cellular responses to model antigens [102]. Kuai et al. designed synthetic high-density lipoprotein (sHDL) nanodiscs, which were conjugated to CpG and antigen peptides (Fig. 3). The nanodiscs (31 nmol antigen peptides, 2.3 nmol CpG per mouse) allowed induction of 50-fold increased T cell response compared to free antigen peptides and CpG. The nanodisc carrier alone, without CpG and antigen peptides, have previously been manufactured for clinical tests and were well tolerated in humans [103].
Figure 3.
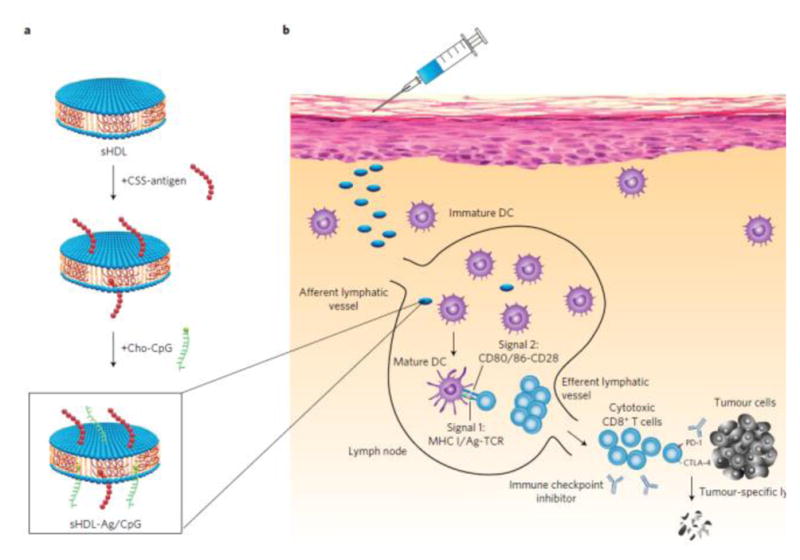
Composition of sHDL nanodisc cancer vaccine. (a) Antigen peptides and adjuvant Cp G are conjugated on nanodiscs. (b) After subcutaneous injection, this nanovaccine can migrate efficiently into draining lymph nodes, and activate tumor-specific T cell response. Reprinted with permission from Ref. 103.
These studies illustrate that particulated co-delivery of antigen and TLR agonist can provide the three signals previously described for T cell initiation, induce a Th1 biased T cell response, and show anti-tumor [86–88] and anti-pathogen effects [84,91,94,104] in mouse models. Although the TLR-9 agonist CpG has been widely tested as an adjuvant for vaccine development that induced strong T cell response in mouse studies, the restricted expression of TLR-9 in pDCs and B cells in humans, compared to expression in all splenic DC subsets in mice, may limit its clinical translation [84,105].
3.3. STING agonists
The STING signaling pathway is emerging as a major TLR-independent mediator of host innate defense. The STING agonist, cyclic dinucleotide (CDN) is either directly released from invading bacteria, or synthesized by the host cyclic GMP-AMP (cGAMP) synthase (cGAS) in response to cytosolic double -stranded DNA (dsDNA) as a danger signal [106–109]. Deng et al reported that irradiated tumor cells in DCs resulted in STING-dependent but not MyD88-dependent type I IFN production and adaptive immune responses [110]. Direct activation of STING by intratumoral injection of STING agonist led to potent immune responses and systemic tumor regression [111]. Listeria monocytogenes vaccine expressing tumor antigens demonstrated a survival advantage in pancreatic cancer patients [112]. This effect may be mediated through production of cyclic -di-AMP by Listeria, which activates STING [113]. More recently, it was shown that intra-muscular injection of cGAMP into tumor bearing mice led to significant inhibition of tumor growth, especially when cGAMP was used in combination with a PD-L1 antibody. Further, it was shown that the anti-tumor effect of the PD-L1 antibody depends on a functional cGAS-STING pathway [114].
The STING pathway has shown several unique characteristics compared to other innate stimulators, such as CpG or poly(I:C). First, although single stranded DNA enriched in CpG sequences can also stimulate type-I IFN production by binding to Toll-like receptor 9, TLR9 is expressed predominantly in plasmacytoid dendritic cells (pDCs). In contrast, the STING pathway is functional in most cell types including all antigen-presenting cells (e.g., pDCs, myeloid DCs or mDCs, and macrophages). Since mDCs (particularly LN -resident CD8+ or tissue-resident CD11b-CD103+ mDCs) have been associated with strong anti-tumor immunity [115,116], this makes STING agonists more effective for innate stimulation. Second, the STING agonists are small molecules (cyclic dinucleotides). The structural simplicity improves stability against conformational changes or enzyme degradation. In contrast, CpG or poly(I:C) have long base sequences and require specific conformation for TLR receptor binding. Despite these advantages, the main challenge for STING agonists may reside in the lack of efficient cytosolic delivery of CDNs and potential autoimmune side effects.
Fu et al. formulated STING agonists with a GM-CSF-producing cellular cancer vaccine, termed STINGVAX. This vaccine induced potent STING-dependent CD4+, CD8+, and T helper 1 (TH1)-biased humoral immunity, and increased antitumor response compared to TLR agonists including MPLA, poly(I:C) and R848. Further analysis indicated marked PD-L1 (programmed death ligand 1) up-regulation, which was associated with tumor-infiltrating CD8+IFNγ+ T cells. When combined with PD-1 (programmed death 1) blockade, STINGVAX induced regression of palpable, poorly immunogenic tumors that did not respond to PD-1 blockade alone [117]. Hanson et al. encapsulated STING agonist cdGMP within PEGylated lipid nanoparticles (NP-cdGMP, ~150 nm) to direct this adjuvant to dLNs [118]. Compared with unformulated CDNs, encapsulation blocked systemic dissemination and markedly enhanced dLN accumulation in murine models. When combined with a poorly immunogenic HIV gp41 peptide antigen (membrane proximal external region), these nanoparticles induced type I IFN in dLNs, a greater, long-lasting expansion of vaccine-specific CD4+ T cells, and B cell response compared with the well-studied TLR agonist monophosphoryl lipid A. Tumor-associated peptide vaccine also induced increased CD8+ T cell responses and enhanced therapeutic antitumor immunity. Miyabe et al. loaded cdGMP into a synthetic, pH-sensitive liposome that has a high fusogenicity. This formulation (~170 nm) facilitated the cytosolic delivery and enhanced antigen-specific CTL effect. In vivo results showed tumor growth inhibition and suppression of lung metastasis [119,120].
The above studies demonstrate that STING or TLR agonists are an effective adjuvant when formulated in a nanoparticle. However, nanovaccines conjugated with antigen or agonist often include low yield covalent chemistry of macromolecules (e.g., conjugation of CpG to lipids) or complex nanostructures (multi-lamellar liposomes), which would be challenging for the QA/QC and CMC (Chemistry, Manufacturing, Controls) in clinical translation. It may also become cost-prohibitive with formidable regulatory barriers by the FDA. Therefore, a simple, robust and easily scalable nanovaccine design that offers antigen-specific CTL response is desirable to realize the potential of nanotechnology for immunotherapy.
4. Examples of nanovaccines with integrated functions
4.1 Immune stimulation with traditional synthetic material
The above studies have all focused on the use of synthetic materials to deliver antigen, immune stimulator or both for vaccine development. Recently, new evidence has shown that synthetic materials themselves can activate innate immune responses without the conventional biological adjuvants.
It is known that FDA-approved adjuvants such as Alum or lipid emulsions (e.g., MF59) provoke a strong Th2 response, but are rather ineffective against pathogens that require Th1-cell-mediated immunity. These adjuvants were originally thought to function as a delivery system by generating depots that trap antigens at the injection site, offering a sustained release in order to continue the stimulation of the immune system. However, recent work has reported that this may not be the case, as antigens have been shown to dissociate very quickly from Alum in interstitial fluid [121]. Studies found that direct interaction between cell-membrane lipids and crystalline compounds such as alum or monosodium urate (a product released by dying cells) can cause receptor aggregation at lipid rafts, high-cholesterol regions of the plasma membrane that are receptor-dense and play a significant role in organization and signal transduction, which leads to recruitment and activation of intracellular kinases [122]. Alum promotes non-phagocytic antigen uptake, leading to endosomal processing of CD4-dependent antigens and then promotes humoral immunity. [123]. Marichal et al reported that Alum-mediated cell death and subsequent host-cell DNA release promote humoral and Th2 cell responses [124].
Polymeric particles like PLGA and PEG-poly(propylene sulphide) have been shown to directly activate the inflammasome in dendritic cells [125]. Chitosan, the deacetylated derivative of chitin, can also activate the inflammasome pathway. However, the adjuvant effect of these nanoparticles is largely independent of this pathway [126,127]. Little evidence thus far has shown that inflammasome activation affects the humoral and cellular immune responses [128,129]. More research efforts are necessary to elucidate the effect of inflammasome activation on immune responses.
4.2 New nanoparticles targeting innate immune system
Several studies have shown that synthetic particles can trigger additional processes in APCs that affect immune responses. Li et al. showed that alumina nanoparticles can deliver conjugated antigens to autophagosomes in dendritic cells (DCs), which might promote antigen cross-presentation and subsequent T cell response [96]. Immunization of mice with these nanoparticles, which are conjugated to either a model tumor antigen or autophagosomes derived from tumor cells resulted in established tumor regression. Reddy et al. designed nanoparticles that displayed external hydroxyl chains, which activated the complement cascade. The ultra-small size (25 nm) targeted particles efficiently to the lymph node-resident dendritic cells, leading to enhanced cellular and humoral responses in vivo [40]. Lizotte et al. reported that in situ vaccination with self-assembling virus-like nanoparticles from Cowpea Mosaic Virus (CPMV) generated potent systemic anti-tumor immunity against poorly immunogenic B16F10 in the skin. Further mechanistic analysis showed that CPMV activated neutrophils, which then increased the frequency of tumor-infiltrating neutrophils [130]. Zanganeh et al showed that an iron oxide nanoparticle, ferumoxytol, can act on tumor-associated immune cells, specifically macrophages, causing them to adapt an anti-tumor “M1” phenotype, and then inhibit tumor growth and metastasis in vivo [131]. Several studies showed the relationship between hydrophobicity of synthetic material and innate immune activation [37,132], but the specific mechanism of action remains unknown.
Recently, data have shown that synthetic polymers alone can activate the STING pathway to trigger innate immune responses, which drives potent cell-mediated immunity. Carroll et al. reported that chitosan can activate dendritic cells by inducing type I interferons (IFNs) and initiate Th1 biased immune response in a type I IFN receptor-dependent manner [127]. Mechanistic studies showed that this cationic polysaccharide induced mitochondrial damage and possible release of mitochondrial DNA into the cell cytosol, which in turn activated the cGAS-STING-type I IFNs pathway. Luo et al. reported a STING-activating nanovaccine (29 nm), by a simple physical mixture of an antigen with a synthetic polymeric nanoparticle, PC7A NP, which generated strong T cell response with low systemic cytokine expression (Fig. 4) [133]. In animal experiments, this nanovaccine effectively suppressed tumor growth and significantly prolonged survival in melanoma, colon cancer, and human papilloma virus -E6/E7 tumor models. Mechanistically, PC7A NP allowed stable antigen loading within a small size confinement that facilitated antigen delivery to the lymph nodes. pH-specific proton sponge effect at early endosomal pH (6.8–7.0) promoted antigen cross-presentation via membrane disruption. This nanovaccine stimulated innate cellular immunity through the STING-type I IFN pathway. Though the mechanism is not yet fully elucidated, PC7A copolymer was able to directly bind the C-terminal domain of STING with a dissociation constant of ~1 μM. Nanovaccines that can move beyond acting as a pure antigen delivery carrier to acting as innate stimulators in antigen-presenting cells, such as these recent examples, will be an exciting direction for future vaccine development.
Figure 4.
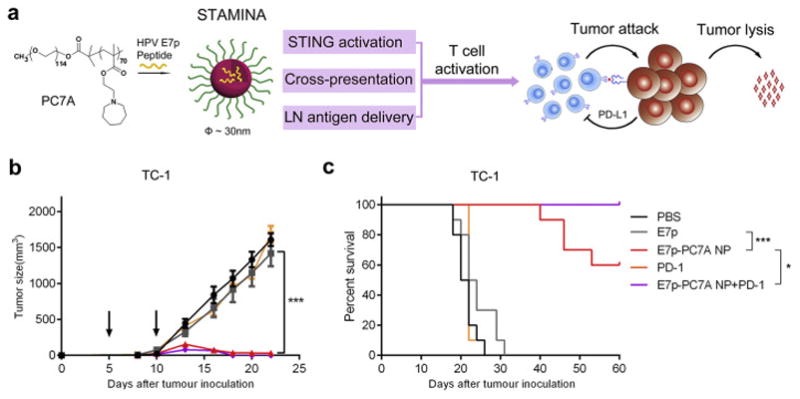
A STING-activating minimalist nanovaccine (STAMINA) inhibits tumor growth and prolongs survival in tumor bearing mice. (a) Schematic of STAMINA to boost tumor-specific T cell immunity. A single polymer, PC7A NP, was able to achieve STING activation, lymph node targeting, and cytosolic delivery of antigen in APCs in one composition with robust T cell production against tumors. In the HPV tumor model, tumor growth inhibition (b) and survival data (c) in C57BL/6 mice showed strong antitumor immunity after tumor inoculation with TC-1 tumor cells. Reprinted with permission from Ref. 132.
5. Combination of nanovaccines with other immunotherapy
Combinations of multiple therapies are widely used in the treatment of challenging illnesses. To fight against cancer or chronic pathogen infections, therapeutic vaccines may need to overcome a variety of suppressive mechanisms, such as immunological ignorance, tolerance, and high rates of mutation in tumor cells or pathogens [134]. Though optimized nanovaccines alone can efficiently stimulate T cell responses, they are not sufficient to maintain the activity and tumor- or tissue- infiltrating ability of those T cells. Nanovaccines should be combined with other immune modulators to reach their full therapeutic potential. Kuai et al. combined nanodisc vaccine with checkpoint inhibitors anti-PD-1 and anti-CTLA-4, which could completely eradicate established tumors [103]. Luo et al. combined STAMINA with anti-PD-1 in a human papilloma virus-E6/E7 tumor model, which showed great synergy with 100% survival over 60 days [133]. Moynihan et al. created a multipronged immunotherapy approach that combined a nanovaccines, cytokine, checkpoint inhibitor, and a tumor-antigen antibody. The nanovaccine induced a strong tumor-specific T cell response, the cytokine IL-2 promoted T cell activation and proliferation, checkpoint inhibitors neutralized tumor suppressive signals, and a tumor antigen antibody enhanced antigen-dependent phagocytosis of APCs and promoted tumor antigen spreading. This combination was able to eradicate large, established melanoma tumors in mice (Fig. 5) [135,136]. In the future, combination of nanovaccines with other immune modulators or checkpoint inhibitors will be a promising direction, mirroring the success of cocktail therapy for HIV in the past 20 years.
Figure 5.
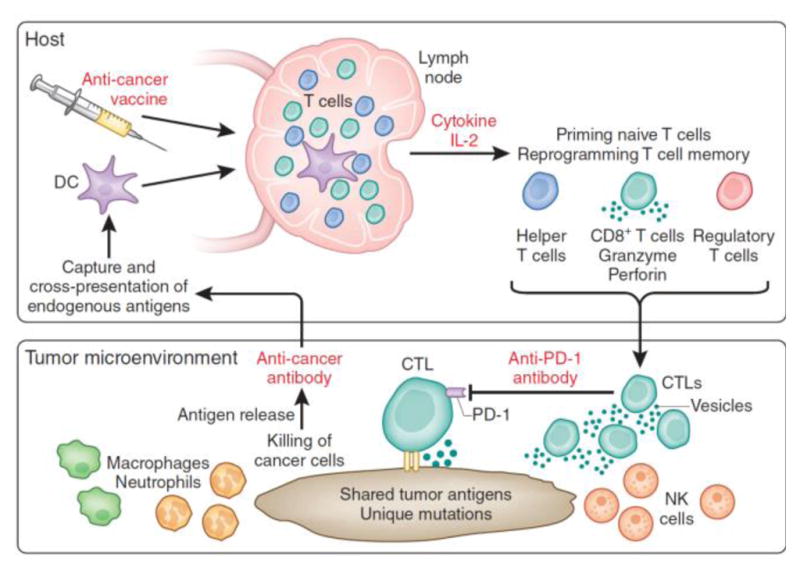
Schematic illustration of a combined anti-tumor approach that included nanovaccine, cytokine, checkpoint inhibitor and a tumor-antigen antibody. Reprinted with permission from Ref.123.
6. Summary and conclusions
T cell activation by nanovaccines is impacted by their size, membrane disruption capability, and ability to stimulate innate immunity. Small size and hydrophilic surface coating (such as with a PEG shell) will aid in lymph node targeting. Albumin hitchhiking or conjugation of a DC receptor-specific ligand will further facilitate targeting of nanovaccines and uptake by APCs. Endosomal escape in response to the pH decrease during subcellular trafficking will enhance the cytosolic delivery of antigens and cross-presentation on MHC-I in DC cells. Loading innate stimulator or stimulating innate immune responses by the particle itself will induce all three signals necessary to bias the Th1 immune response and enhance CD8+ T cell response.
Many questions remain for therapeutic vaccine development. For example, it is critical to determine how to promote a T effector response, but not a T regulatory response, in an immunosuppressive microenvironment. Fine-tuning the balance between the strong T cell responses and auto-immune side effects will also be a key challenge. Finally, the ideal antigens for nanovaccines to differentiate cancer cells from normal tissue must be identified. Recently, several new epitopes have been discovered by new exome-guided technologies and high resolution mass spectrometry [137]. These epitopes, also known as neoantigens, are formed by peptides that are entirely absent from the normal human genome, but arise from mutated proteins in tumor cells. Incorporation of these neoantigens into cationic lipids or nanoparticles can stimulate antigen-specific anti-tumor effects [103,133,138], which is important to overcome the relatively low immunogenicity of these neoantigens.
From the biotechnology standpoint, different formulations provide a broad platform for nanovaccine design. For the antigen, it can be nucleic acids, peptides, or proteins; for the immune stimulator, it can be nucleic acids, small molecules, lipids or the particle itself; for the carrier, it can be natural virus-like particles or synthetic nanoparticles. Much work is still necessary to establish a highly efficient, minimalist nanovaccine with a simplified chemical design. This will be important in reducing the technical barriers for chemistry, manufacturing and quality controls, as well as keeping the production cost low. Until now, few nanovaccines have been tested in clinical trials. One limiting factor is that, although some correlations between human patients and mouse models were shown in checkpoint inhibitor therapy, current animal models such as transplanted tumor models, genetically engineered tumor models, or humanized mouse tumor models do not mimic the natural process of human tumor development, and are not always predictive of outcomes in human patients [139]. This is exemplified by the different responses to cytokines or CpG in mouse and human experiments as we previously mentioned. Another reason limiting the translation of nanovaccines is the relatively short history of synthetic nanoparticles in medicine. More knowledge of the fate of nanoparticles at the cellular and tissue levels is needed to establish a more thorough safety profile to aid in future clinical trials [140]. Approval of nanovaccines by the FDA or other regulatory agencies is key future steps to validate efficacy and safety. Continued progress in immunology and material science, and further collaboration between the two fields, will catalyze the rapid development of future generations of nanovaccines against cancer and infectious diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Germain RN. Vaccines and the future of human immunology. Immunity. 2010;33(4):441–450. doi: 10.1016/j.immuni.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Rappuoli R, Aderem A. A 2020 vision for vaccines against hiv, tuberculosis and malaria. Nature. 2011;473(7348):463–469. doi: 10.1038/nature10124. [DOI] [PubMed] [Google Scholar]
- 3.van der Burg SH, Arens R, Ossendorp F, van Hall T, Melief CJ. Vaccines for established cancer: Overcoming the challenges posed by immune evasion. Nature reviews Cancer. 2016;16(4):219–233. doi: 10.1038/nrc.2016.16. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348(6230):62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 6.Abbas AK, Lichtman AH, Pillai S. Cellular and molecular immunology. Elsevier; 2014. [Google Scholar]
- 7.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: Putting innate immunity to work. Immunity. 2010;33(4):492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith DM, Simon JK, Baker JR., Jr Applications of nanotechnology for immunology. Nature reviews Immunology. 2013;13(8):592–605. doi: 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irvine DJ, Swartz MA, Szeto GL. Engineering synthetic vaccines using cues from natural immunity. Nature materials. 2013;12(11):978–990. doi: 10.1038/nmat3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albanese A, Tang PS, Chan WC. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annual review of biomedical engineering. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 11.Watson DS, Endsley AN, Huang L. Design considerations for liposomal vaccines: Influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine. 2012;30(13):2256–2272. doi: 10.1016/j.vaccine.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Byrne JD, Napier ME, DeSimone JM. More effective nanomedicines through particle design. Small. 2011;7(14):1919–1931. doi: 10.1002/smll.201100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chemical Society reviews. 2012;41(7):2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benne N, van Duijn J, Kuiper J, Jiskoot W, Slutter B. Orchestrating immune responses: How size, shape and rigidity affect the immunogenicity of particulate vaccines. Journal of controlled release: official journal of the Controlled Release Society. 2016;234:124–134. doi: 10.1016/j.jconrel.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 15.Sah H, Thoma LA, Desu HR, Sah E, Wood GC. Concepts and practices used to develop functional plga-based nanoparticulate systems. International journal of nanomedicine. 2013;8:747–765. doi: 10.2147/IJN.S40579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bazile D, Prud’homme C, Bassoullet MT, Marlard M, Spenlehauer G, Veillard M. Stealth me. Peg-pla nanoparticles avoid uptake by the mononuclear phagocytes system. J Pharm Sci. 1995;84(4):493–498. doi: 10.1002/jps.2600840420. [DOI] [PubMed] [Google Scholar]
- 17.Rietscher R, Schroder M, Janke J, Czaplewska J, Gottschaldt M, Scherliess R, Hanefeld A, Schubert US, Schneider M, Knolle PA, Lehr CM. Antigen delivery via hydrophilic peg-b-page-b-plga nanoparticles boosts vaccination induced t cell immunity. European journal of pharmaceutics and biopharmaceutics: official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2016;102:20–31. doi: 10.1016/j.ejpb.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M, Nejati-Koshki K. Liposome: Classification, preparation, and applications. Nanoscale research letters. 2013;8(1):102. doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blume G, Cevc G. Liposomes for the sustained drug release in vivo. Biochimica et biophysica acta. 1990;1029(1):91–97. doi: 10.1016/0005-2736(90)90440-y. [DOI] [PubMed] [Google Scholar]
- 20.Kan P, Tsao CW, Wang AJ, Su WC, Liang HF. A liposomal formulation able to incorporate a high content of paclitaxel and exert promising anticancer effect. Journal of drug delivery. 2011;2011:629234. doi: 10.1155/2011/629234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taneichi M, Ishida H, Kajino K, Ogasawara K, Tanaka Y, Kasai M, Mori M, Nishida M, Yamamura H, Mizuguchi J, Uchida T. Antigen chemically coupled to the surface of liposomes are cross -presented to cd8+ t cells and induce potent antitumor immunity. Journal of immunology. 2006;177(4):2324–2330. doi: 10.4049/jimmunol.177.4.2324. [DOI] [PubMed] [Google Scholar]
- 22.Ignatius R, Mahnke K, Rivera M, Hong K, Isdell F, Steinman RM, Pope M, Stamatatos L. Presentation of proteins encapsulated in sterically stabilized liposomes by dendritic cells initiates cd8(+) t-cell responses in vivo. Blood. 2000;96(10):3505–3513. [PubMed] [Google Scholar]
- 23.Meng J, Duan J, Kong H, Li L, Wang C, Xie S, Chen S, Gu N, Xu H, Yang XD. Carbon nanotubes conjugated to tumor lysate protein enhance the efficacy of an antitumor immunotherapy. Small. 2008;4(9):1364–1370. doi: 10.1002/smll.200701059. [DOI] [PubMed] [Google Scholar]
- 24.Ahn S, Lee IH, Kang S, Kim D, Choi M, Saw PE, Shin EC, Jon S. Gold nanoparticles displaying tumor-associated self-antigens as a potential vaccinefor cancer immunotherapy. Advanced healthcare materials. 2014;3(8):1194–1199. doi: 10.1002/adhm.201300597. [DOI] [PubMed] [Google Scholar]
- 25.Gottardi R, Douradinha B. Carbon nanotubes as a novel tool for vaccination against infectious diseases and cancer. Journal of nanobiotechnology. 2013;11:30. doi: 10.1186/1477-3155-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dykman LA, Khlebtsov NG. Gold nanoparticles in biology and medicine: Recent advances and prospects. Acta naturae. 2011;3(2):34–55. [PMC free article] [PubMed] [Google Scholar]
- 27.Irvine DJ, Hanson MC, Rakhra K, Tokatlian T. Synthetic nanoparticles for vaccines and immunotherapy. Chemical reviews. 2015;115(19):11109–11146. doi: 10.1021/acs.chemrev.5b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeanbart L, Ballester M, de Titta A, Corthesy P, Romero P, Hubbell JA, Swartz MA. Enhancing efficacy of anticancer vaccines by targeted delivery to tumor-draining lymph nodes. Cancer immunology research. 2014;2(5):436–447. doi: 10.1158/2326-6066.CIR-14-0019-T. [DOI] [PubMed] [Google Scholar]
- 29.Luo X, Feng M, Pan S, Wen Y, Zhang W, Wu C. Charge shielding effects on gene delivery of polyethylenimine/DNA complexes: Pegylation and phospholipid coating. J Mater Sci Mater Med. 2012;23(7):1685–1695. doi: 10.1007/s10856-012-4632-4. [DOI] [PubMed] [Google Scholar]
- 30.Ruizendaal L, Bhattacharjee S, Pournazari K, Rosso-Vasic M, de Haan LHJ, Alink GM, Marcelis ATM, Zuilhof H. Synthesis and cytotoxicity of silicon nanoparticles with covalently attached organic monolayers. Nanotoxicology. 2009;3(4):339–347. [Google Scholar]
- 31.Rao DA, Forrest ML, Alani AW, Kwon GS, Robinson JR. Biodegradable plga based nanoparticles for sustained regional lymphatic drug delivery. J Pharm Sci. 2010;99(4):2018–2031. doi: 10.1002/jps.21970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frohlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. International journal of nanomedicine. 2012;7:5577–5591. doi: 10.2147/IJN.S36111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vyas SP, Kannan ME, Jain S, Mishra V, Singh P. Design of liposomal aerosols for improved delivery of rifampicin to alveolar macrophages. International journal of pharmaceutics. 2004;269(1):37–49. doi: 10.1016/j.ijpharm.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 34.Fidler IJ, Raz A, Fogler WE, Kirsh R, Bugelski P, Poste G. Design of liposomes to improve delivery of macrophage-augmenting agents to alveolar macrophages. Cancer Res. 1980;40(12):4460–4466. [PubMed] [Google Scholar]
- 35.Ahsan FL, Rivas IP, Khan MA, Suarez AIT. Targeting to macrophages: Role of physicochemical properties of particulate carriers-liposomes and microspheres-on the phagocytosis by macrophages. Journal of Controlled Release. 2002;79(1–3):29–40. doi: 10.1016/s0168-3659(01)00549-1. [DOI] [PubMed] [Google Scholar]
- 36.Raghuvanshi RS, Katare YK, Lalwani K, Ali MM, Singh O, Panda AK. Improved immune response from biodegradable polymer particles entrapping tetanus toxoid by use of different immunization protocol and adjuvants. International journal of pharmaceutics. 2002;245(1–2):109–121. doi: 10.1016/s0378-5173(02)00342-3. [DOI] [PubMed] [Google Scholar]
- 37.Seong SY, Matzinger P. Hydrophobicity: An ancient damage-associated molecular pattern that initiates innate immune responses. Nature reviews Immunology. 2004;4(6):469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 38.Reddy ST, Swartz MA, Hubbell JA. Targeting dendritic cells with biomaterials: Developing the next generation of vaccines. Trends in immunology. 2006;27(12):573–579. doi: 10.1016/j.it.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. European journal of immunology. 2008;38(5):1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 40.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, Lee LK, Swartz MA, Hubbell JA. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nature biotechnology. 2007;25(10):1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 41.Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. Journal of controlled release: official journal of the Controlled Release Society. 2006;112(1):26–34. doi: 10.1016/j.jconrel.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Yan S, Gu W, Xu ZP. Re-considering how particle size and other properties of antigen-adjuvant complexes impact on the immune responses. Journal of colloid and interface science. 2013;395:1–10. doi: 10.1016/j.jcis.2012.11.061. [DOI] [PubMed] [Google Scholar]
- 43.Varkouhi AK, Scholte M, Storm G, Haisma HJ. Endosomal escape pathways for delivery of biologicals. Journal of controlled release: official journal of the Controlled Release Society. 2011;151(3):220–228. doi: 10.1016/j.jconrel.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Schnurr M, Orban M, Robson NC, Shin A, Braley H, Airey D, Cebon J, Maraskovsky E, Endres S. Iscomatrix adjuvant induces efficient cross-presentation of tumor antigen by dendritic cells via rapid cytosolic antigen delivery and processing via tripeptidyl peptidase ii. Journal of immunology. 2009;182(3):1253–1259. doi: 10.4049/jimmunol.182.3.1253. [DOI] [PubMed] [Google Scholar]
- 45.Nicholaou T, Chen W, Davis ID, Jackson HM, Dimopoulos N, Barrow C, Browning J, Macgregor D, Williams D, Hopkins W, Maraskovsky E, et al. Immunoediting and persistence of antigen-specific immunity in patients who have previously been vaccinated with ny-eso-1 protein formulated in iscomatrix. Cancer immunology, immunotherapy: CII. 2011;60(11):1625–1637. doi: 10.1007/s00262-011-1041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu Y, Litwin T, Nagaraja AR, Kwong B, Katz J, Watson N, Irvine DJ. Cytosolic delivery of membrane-impermeable molecules in dendritic cells using ph-responsive core-shell nanoparticles. Nano letters. 2007;7(10):3056–3064. doi: 10.1021/nl071542i. [DOI] [PubMed] [Google Scholar]
- 47.Keller S, Wilson JT, Patilea GI, Kern HB, Convertine AJ, Stayton PS. Neutral polymer micelle carriers with ph-responsive, endosome-releasing activity modulate antigen trafficking to enhance cd8(+) t cell responses. Journal of controlled release: official journal of the Controlled Release Society. 2014;191:24–33. doi: 10.1016/j.jconrel.2014.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vasdekis AE, Scott EA, O’Neil CP, Psaltis D, Hubbell JA. Precision intracellular delivery based on optofluidic polymersome rupture. ACS nano. 2012;6(9):7850–7857. doi: 10.1021/nn302122h. [DOI] [PubMed] [Google Scholar]
- 49.Li P, Luo Z, Liu P, Gao N, Zhang Y, Pan H, Liu L, Wang C, Cai L, Ma Y. Bioreducible alginate-poly(ethylenimine) nanogels as an antigen-delivery system robustly enhance vaccine-elicited humoral and cellular immune responses. Journal of controlled release: official journal of the Controlled Release Society. 2013;168(3):271–279. doi: 10.1016/j.jconrel.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 50.Yuba E, Harada A, Sakanishi Y, Watarai S, Kono K. A liposome-based antigen delivery system using ph-sensitive fusogenic polymers for cancer immunotherapy. Biomaterials. 2013;34(12):3042–3052. doi: 10.1016/j.biomaterials.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 51.Zhang AH, Rossi RJ, Yoon J, Wang H, Scott DW. Tolerogenic nanoparticles to induce immunologic tolerance: Prevention and reversal of fviii inhibitor formation. Cellular immunology. 2016;301:74–81. doi: 10.1016/j.cellimm.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Klempner MS, Noring R, Mier JW, Atkins MB. An acquired chemotactic defect in neutrophils from patients receiving interleukin-2 immunotherapy. The New England journal of medicine. 1990;322(14):959–965. doi: 10.1056/NEJM199004053221404. [DOI] [PubMed] [Google Scholar]
- 53.Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB, Sosman JA, Dutcher JP, Vogelzang NJ, Ryan JL. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood. 1997;90(7):2541–2548. [PubMed] [Google Scholar]
- 54.Koff WC, Fidler IJ, Showalter SD, Chakrabarty MK, Hampar B, Ceccorulli LM, Kleinerman ES. Human monocytes activated by immunomodulators in liposomes lyse herpesvirus -infected but not normal cells. Science. 1984;224(4652):1007–1009. doi: 10.1126/science.6426057. [DOI] [PubMed] [Google Scholar]
- 55.Hora MS, Rana RK, Nunberg JH, Tice TR, Gilley RM, Hudson ME. Controlled release of interleukin-2 from biodegradable microspheres. Bio/technology. 1990;8(8):755–758. doi: 10.1038/nbt0890-755. [DOI] [PubMed] [Google Scholar]
- 56.Melissen PM, van Vianen W, Bidjai O, van Marion M, Bakker-Woudenberg IA. Free versus liposome-encapsulated muramyl tripeptide phosphatidylethanolamide (mtppe) and interferon-y (ifn-y) in experimental infection with listeria monocytogenes. Biotherapy. 1993;6(2):113–124. doi: 10.1007/BF01877424. [DOI] [PubMed] [Google Scholar]
- 57.Johnston D, Reynolds SR, Bystryn JC. Interleukin-2/liposomes potentiate immune responses to a soluble protein cancer vaccine in mice. Cancer immunology, immunotherapy: CII. 2006;55(4):412–419. doi: 10.1007/s00262-005-0013-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Popescu MC, Robb RJ, Batenjany MM, Boni LT, Neville ME, Pennington RW, Neelapu SS, Kwak LW. A novel proteoliposomal vaccine elicits potent antitumor immunity in mice. Blood. 2007;109(12):5407–5410. doi: 10.1182/blood-2006-08-039446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neelapu SS, Baskar S, Gause BL, Kobrin CB, Watson TM, Frye AR, Pennington R, Harvey L, Jaffe ES, Robb RJ, Popescu MC, et al. Human autologous tumor-specific t-cell responses induced by liposomal delivery of a lymphoma antigen. Clinical cancer research: an official journal of the American Association for Cancer Research. 2004;10(24):8309–8317. doi: 10.1158/1078-0432.CCR-04-1071. [DOI] [PubMed] [Google Scholar]
- 60.Neelapu SS, Gause BL, Harvey L, Lee ST, Frye AR, Horton J, Robb RJ, Popescu MC, Kwak LW. A novel proteoliposomal vaccine induces antitumor immunity against follicular lymphoma. Blood. 2007;109(12):5160–5163. doi: 10.1182/blood-2006-12-063594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ha SJ, Park SH, Kim HJ, Kim SC, Kang HJ, Lee EG, Kwon SG, Kim BM, Lee SH, Kim WB, Sung YC, et al. Enhanced immunogenicity and protective efficacy with the use of interleukin-12-encapsulated microspheres plus as01b in tuberculosis subunit vaccination. Infection and immunity. 2006;74(8):4954–4959. doi: 10.1128/IAI.01781-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ. Infection-mimicking materials to program dendritic cells in situ. Nature materials. 2009;8(2):151–158. doi: 10.1038/nmat2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Killion JJ, Fishbeck R, Bar-Eli M, Chernajovsky Y. Delivery of interferon to intracellular pathways by encapsulation of interferon into multilamellar liposomes is independent of the status of interferon receptors. Cytokine. 1994;6(4):443–449. doi: 10.1016/1043-4666(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 64.Ali OA, Doherty E, Bell WJ, Fradet T, Hudak J, Laliberte MT, Mooney DJ, Emerich DF. Biomaterial-based vaccine induces regression of established intracranial glioma in rats. Pharmaceutical research. 2011;28(5):1074–1080. doi: 10.1007/s11095-010-0361-x. [DOI] [PubMed] [Google Scholar]
- 65.Ali OA, Emerich D, Dranoff G, Mooney DJ. In situ regulation of dc subsets and t cells mediates tumor regression in mice. Science translational medicine. 2009;1(8):8ra19. doi: 10.1126/scitranslmed.3000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Medzhitov R. Approaching the asymptote: 20 years later. Immunity. 2009;30(6):766–775. doi: 10.1016/j.immuni.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 67.Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nature immunology. 2011;12(6):509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sporri R, Reis e Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of cd4+ t cell populations lacking helper function. Nature immunology. 2005;6(2):163–170. doi: 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]
- 69.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440(7085):808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 70.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 71.Schenten D, Medzhitov R. The control of adaptive immune responses by the innate immune system. Advances in immunology. 2011;109:87–124. doi: 10.1016/B978-0-12-387664-5.00003-0. [DOI] [PubMed] [Google Scholar]
- 72.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, et al. Defective lps signaling in c3h/hej and c57bl/10sccr mice: Mutations in tlr4 gene. Science. 1998;282(5396):2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 73.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded rna and activation of nf-kappab by toll-like receptor 3. Nature. 2001;413(6857):732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 74.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of tlr7-mediated recognition of single-stranded rna. Science. 2004;303(5663):1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 75.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded rna via toll-like receptor 7 and 8. Science. 2004;303(5663):1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 76.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 77.Agrawal S, Agrawal A, Doughty B, Gerwitz A, Blenis J, Van Dyke T, Pulendran B. Cutting edge: Different toll-like receptor agonists instruct dendritic cells to induce distinct th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-fos. Journal of immunology. 2003;171(10):4984–4989. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 78.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Miles AP, Saul A, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. Toll-like receptor agonists influence the magnitude and quality of memory t cell responses after prime-boost immunization in nonhuman primates. The Journal of experimental medicine. 2006;203(5):1249–1258. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Basith S, Manavalan B, Lee G, Kim SG, Choi S. Toll-like receptor modulators: A patent review (2006–2010) Expert opinion on therapeutic patents. 2011;21(6):927–944. doi: 10.1517/13543776.2011.569494. [DOI] [PubMed] [Google Scholar]
- 80.Johnson DA. Synthetic tlr4-active glycolipids as vaccine adjuvants and stand-alone immunotherapeutics. Current topics in medicinal chemistry. 2008;8(2):64–79. doi: 10.2174/156802608783378882. [DOI] [PubMed] [Google Scholar]
- 81.Ioannou XP, Griebel P, Mena A, Gomis SM, Godson DL, Mutwiri G, Hecker R, Babiuk LA, van Drunen Littel-van den Hurk S. Safety of cpg oligodeoxynucleotides in veterinary species. Antisense & nucleic acid drug development. 2003;13(3):157–167. doi: 10.1089/108729003768247628. [DOI] [PubMed] [Google Scholar]
- 82.Hafner AM, Corthesy B, Merkle HP. Particulate formulations for the delivery of poly(i:C) as vaccine adjuvant. Advanced drug delivery reviews. 2013;65(10):1386–1399. doi: 10.1016/j.addr.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 83.Marshak-Rothstein A, Rifkin IR. Immunologically active autoantigens: The role of toll-like receptors in the development of chronic inflammatory disease. Annual review of immunology. 2007;25:419–441. doi: 10.1146/annurev.immunol.22.012703.104514. [DOI] [PubMed] [Google Scholar]
- 84.Lynn GM, Laga R, Darrah PA, Ishizuka AS, Balaci AJ, Dulcey AE, Pechar M, Pola R, Gerner MY, Yamamoto A, Buechler CR, et al. In vivo characterization of the physicochemical properties of polymer-linked tlr agonists that enhance vaccine immunogenicity. Nature biotechnology. 2015;33(11):1201–1210. doi: 10.1038/nbt.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Standley SM, Mende I, Goh SL, Kwon YJ, Beaudette TT, Engleman EG, Frechet JM. Incorporation of cpg oligonucleotide ligand into protein-loaded particle vaccines promotes antigen-specific cd8 t-cell immunity. Bioconjugate chemistry. 2007;18(1):77–83. doi: 10.1021/bc060165i. [DOI] [PubMed] [Google Scholar]
- 86.de Jong S, Chikh G, Sekirov L, Raney S, Semple S, Klimuk S, Yuan N, Hope M, Cullis P, Tam Y. Encapsulation in liposomal nanoparticles enhances the immunostimulatory, adjuvant and anti-tumor activity of subcutaneously administered cpg odn. Cancer immunology, immunotherapy: CII. 2007;56(8):1251–1264. doi: 10.1007/s00262-006-0276-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bourquin C, Anz D, Zwiorek K, Lanz AL, Fuchs S, Weigel S, Wurzenberger C, von der Borch P, Golic M, Moder S, Winter G, et al. Targeting cpg oligonucleotides to the lymph node by nanoparticles elicits efficient antitumoral immunity. Journal of immunology. 2008;181(5):2990–2998. doi: 10.4049/jimmunol.181.5.2990. [DOI] [PubMed] [Google Scholar]
- 88.de Titta A, Ballester M, Julier Z, Nembrini C, Jeanbart L, van der Vlies AJ, Swartz MA, Hubbell JA. Nanoparticle conjugation of cpg enhances adjuvancy for cellular immunity and memory recall at low dose. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(49):19902–19907. doi: 10.1073/pnas.1313152110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rozenfeld JH, Silva SR, Raneia PA, Faquim-Mauro E, Carmona-Ribeiro AM. Stable assemblies of cationic bilayer fragments and cpg oligonucleotide with enhanced immunoadjuvant activity in vivo. Journal of controlled release: official journal of the Controlled Release Society. 2012;160(2):367–373. doi: 10.1016/j.jconrel.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 90.Nembrini C, Stano A, Dane KY, Ballester M, van der Vlies AJ, Marsland BJ, Swartz MA, Hubbell JA. Nanoparticle conjugation of antigen enhances cytotoxic t-cell responses in pulmonary vaccination. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(44):E989–997. doi: 10.1073/pnas.1104264108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Orr MT, Beebe EA, Hudson TE, Moon JJ, Fox CB, Reed SG, Coler RN. A dual tlr agonist adjuvant enhances the immunogenicity and protective efficacy of the tuberculosis vaccine antigen id93. PloS one. 2014;9(1):e83884. doi: 10.1371/journal.pone.0083884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ilyinskii PO, Roy CJ, O’Neil CP, Browning EA, Pittet LA, Altreuter DH, Alexis F, Tonti E, Shi J, Basto PA, Iannacone M, et al. Adjuvant-carrying synthetic vaccine particles augment the immune response to encapsulated antigen and exhibit strong local immune activation without inducing systemic cytokine release. Vaccine. 2014;32(24):2882–2895. doi: 10.1016/j.vaccine.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Steers NJ, Peachman KK, McClain S, Alving CR, Rao M. Liposome-encapsulated hiv-1 gag p24 containing lipid a induces effector cd4+ t-cells, memory cd8+ t-cells, and pro-inflammatory cytokines. Vaccine. 2009;27(49):6939–6949. doi: 10.1016/j.vaccine.2009.08.105. [DOI] [PubMed] [Google Scholar]
- 94.Demento SL, Bonafe N, Cui W, Kaech SM, Caplan MJ, Fikrig E, Ledizet M, Fahmy TM. Tlr9-targeted biodegradable nanoparticles as immunization vectors protect against west nile encephalitis. Journal of immunology. 2010;185(5):2989–2997. doi: 10.4049/jimmunol.1000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moon JJ, Suh H, Li AV, Ockenhouse CF, Yadava A, Irvine DJ. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand tfh cells and promote germinal center induction. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(4):1080–1085. doi: 10.1073/pnas.1112648109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moon JJ, Suh H, Bershteyn A, Stephan MT, Liu H, Huang B, Sohail M, Luo S, Um SH, Khant H, Goodwin JT, et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nature materials. 2011;10(3):243–251. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Malyala P, Chesko J, Ugozzoli M, Goodsell A, Zhou F, Vajdy M, O’Hagan DT, Singh M. The potency of the adjuvant, cpg oligos, is enhanced by encapsulation in plg microparticles. J Pharm Sci. 2008;97(3):1155–1164. doi: 10.1002/jps.21065. [DOI] [PubMed] [Google Scholar]
- 98.Tacken PJ, Zeelenberg IS, Cruz LJ, van Hout-Kuijer MA, van de Glind G, Fokkink RG, Lambeck AJ, Figdor CG. Targeted delivery of tlr ligands to human and mouse dendritic cells strongly enhances adjuvanticity. Blood. 2011;118(26):6836–6844. doi: 10.1182/blood-2011-07-367615. [DOI] [PubMed] [Google Scholar]
- 99.Stano A, van der Vlies AJ, Martino MM, Swartz MA, Hubbell JA, Simeoni E. Pps nanoparticles as versatile delivery system to induce systemic and broad mucosal immunity after intranasal administration. Vaccine. 2011;29(4):804–812. doi: 10.1016/j.vaccine.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 100.Goldinger SM, Dummer R, Baumgaertner P, Mihic-Probst D, Schwarz K, Hammann-Haenni A, Willers J, Geldhof C, Prior JO, Kundig TM, Michielin O, et al. Nano-particle vaccination combined with tlr-7 and -9 ligands triggers memory and effector cd8(+) t-cell responses in melanoma patients. European journal of immunology. 2012;42(11):3049–3061. doi: 10.1002/eji.201142361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C, Irvine DJ. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507(7493):519–522. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Radovic-Moreno AF, Chernyak N, Mader CC, Nallagatla S, Kang RS, Hao L, Walker DA, Halo TL, Merkel TJ, Rische CH, Anantatmula S, et al. Immunomodulatory spherical nucleic acids. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(13):3892–3897. doi: 10.1073/pnas.1502850112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A, Moon JJ. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nature materials. 2016 doi: 10.1038/nmat4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470(7335):543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nature immunology. 2004;5(10):987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 106.Diner EJ, Burdette DL, Wilson SC, Monroe KM, Kellenberger CA, Hyodo M, Hayakawa Y, Hammond MC, Vance RE. The innate immune DNA sensor cgas produces a noncanonical cyclic dinucleotide that activates human sting. Cell reports. 2013;3(5):1355–1361. doi: 10.1016/j.celrep.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ablasser A, Goldeck M, Cavlar T, Deimling T, Witte G, Rohl I, Hopfner KP, Ludwig J, Hornung V. Cgas produces a 2′–5′-linked cyclic dinucleotide second messenger that activates sting. Nature. 2013;498(7454):380–384. doi: 10.1038/nature12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gao P, Ascano M, Wu Y, Barchet W, Gaffney BL, Zillinger T, Serganov AA, Liu Y, Jones RA, Hartmann G, Tuschl T, et al. Cyclic [g(2′,5′)pa(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic gmp-amp synthase. Cell. 2013;153(5):1094–1107. doi: 10.1016/j.cell.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic gmp-amp synthase is a cytosolic DNA sensor that activates the type i interferon pathway. Science. 2013;339(6121):786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, Huang X, et al. Sting-dependent cytosolic DNA sensing promotes radiation-induced type i interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41(5):843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, Woo SR, Lemmens E, Banda T, Leong JJ, Metchette K, et al. Direct activation of sting in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell reports. 2015;11(7):1018–1030. doi: 10.1016/j.celrep.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G, Morse M, Zeh H, Cohen D, Fine RL, Onners B, et al. Safety and survival with gvax pancreas prime and listeria monocytogenes-expressing mesothelin (crs-207) boost vaccines for metastatic pancreatic cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33(12):1325–1333. doi: 10.1200/JCO.2014.57.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sauer JD, Sotelo-Troha K, von Moltke J, Monroe KM, Rae CS, Brubaker SW, Hyodo M, Hayakawa Y, Woodward JJ, Portnoy DA, Vance RE. The n-ethyl-n-nitrosourea-induced goldenticket mouse mutant reveals an essential function of sting in the in vivo interferon response to listeria monocytogenes and cyclic dinucleotides. Infection and immunity. 2011;79(2):688–694. doi: 10.1128/IAI.00999-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang H, Hu S, Chen X, Shi H, Chen C, Sun L, Chen ZJ. Cgas is essential for the antitumor effect of immune checkpoint blockade. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(7):1637–1642. doi: 10.1073/pnas.1621363114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, et al. Batf3 deficiency reveals a critical role for cd8alpha+ dendritic cells in cytotoxic t cell immunity. Science. 2008;322(5904):1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: Ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annual review of immunology. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fu J, Kanne DB, Leong M, Glickman LH, McWhirter SM, Lemmens E, Mechette K, Leong JJ, Lauer P, Liu W, Sivick KE, et al. Sting agonist formulated cancer vaccines can cure established tumors resistant to pd-1 blockade. Science translational medicine. 2015;7(283):283ra252. doi: 10.1126/scitranslmed.aaa4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hanson MC, Crespo MP, Abraham W, Moynihan KD, Szeto GL, Chen SH, Melo MB, Mueller S, Irvine DJ. Nanoparticulate sting agonists are potent lymph node-targeted vaccine adjuvants. The Journal of clinical investigation. 2015;125(6):2532–2546. doi: 10.1172/JCI79915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Miyabe H, Hyodo M, Nakamura T, Sato Y, Hayakawa Y, Harashima H. A new adjuvant delivery system ‘cyclic di-gmp/ysk05 liposome’ for cancer immunotherapy. Journal of controlled release: official journal of the Controlled Release Society. 2014;184:20–27. doi: 10.1016/j.jconrel.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 120.Nakamura T, Miyabe H, Hyodo M, Sato Y, Hayakawa Y, Harashima H. Liposomes loaded with a sting pathway ligand, cyclic di-gmp, enhance cancer immunotherapy against metastatic melanoma. Journal of controlled release: official journal of the Controlled Release Society. 2015;216:149–157. doi: 10.1016/j.jconrel.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 121.Quandt D, Rothe K, Baerwald C, Rossol M. Gprc6a mediates alum-induced nlrp3 inflammasome activation but limits th2 type antibody responses. Scientific reports. 2015;5:16719. doi: 10.1038/srep16719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ng G, Sharma K, Ward SM, Desrosiers MD, Stephens LA, Schoel WM, Li T, Lowell CA, Ling CC, Amrein MW, Shi Y. Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and syk kinase activation in dendritic cells. Immunity. 2008;29(5):807–818. doi: 10.1016/j.immuni.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Flach TL, Ng G, Hari A, Desrosiers MD, Zhang P, Ward SM, Seamone ME, Vilaysane A, Mucsi AD, Fong Y, Prenner E, et al. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nature medicine. 2011;17(4):479–487. doi: 10.1038/nm.2306. [DOI] [PubMed] [Google Scholar]
- 124.Marichal T, Ohata K, Bedoret D, Mesnil C, Sabatel C, Kobiyama K, Lekeux P, Coban C, Akira S, Ishii KJ, Bureau F, et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nature medicine. 2011;17(8):996–1002. doi: 10.1038/nm.2403. [DOI] [PubMed] [Google Scholar]
- 125.Ballester M, Nembrini C, Dhar N, de Titta A, de Piano C, Pasquier M, Simeoni E, van der Vlies AJ, McKinney JD, Hubbell JA, Swartz MA. Nanoparticle conjugation and pulmonary delivery enhance the protective efficacy of ag85b and cpg against tuberculosis. Vaccine. 2011;29(40):6959–6966. doi: 10.1016/j.vaccine.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 126.Bueter CL, Lee CK, Rathinam VA, Healy GJ, Taron CH, Specht CA, Levitz SM. Chitosan but not chitin activates the inflammasome by a mechanism dependent upon phagocytosis. The Journal of biological chemistry. 2011;286(41):35447–35455. doi: 10.1074/jbc.M111.274936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Carroll EC, Jin L, Mori A, Munoz-Wolf N, Oleszycka E, Moran HB, Mansouri S, McEntee CP, Lambe E, Agger EM, Andersen P, et al. The vaccine adjuvant chitosan promotes cellular immunity via DNA sensor cgas -sting-dependent induction of type i interferons. Immunity. 2016;44(3):597–608. doi: 10.1016/j.immuni.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sharp FA, Ruane D, Claass B, Creagh E, Harris J, Malyala P, Singh M, O’Hagan DT, Petrilli V, Tschopp J, O’Neill LA, et al. Uptake of particulate vaccine adjuvants by dendritic cells activates the nalp3 inflammasome. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(3):870–875. doi: 10.1073/pnas.0804897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Demento SL, Eisenbarth SC, Foellmer HG, Platt C, Caplan MJ, Mark Saltzman W, Mellman I, Ledizet M, Fikrig E, Flavell RA, Fahmy TM. Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy. Vaccine. 2009;27(23):3013–3021. doi: 10.1016/j.vaccine.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lizotte PH, Wen AM, Sheen MR, Fields J, Rojanasopondist P, Steinmetz NF, Fiering S. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nature nanotechnology. 2016;11(3):295–303. doi: 10.1038/nnano.2015.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, Pajarinen JS, Nejadnik H, Goodman S, Moseley M, Coussens LM, et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nature nanotechnology. 2016;11(11):986–994. doi: 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Petersen LK, Ramer-Tait AE, Broderick SR, Kong CS, Ulery BD, Rajan K, Wannemuehler MJ, Narasimhan B. Activation of innate immune responses in a pathogen-mimicking manner by amphiphilic polyanhydride nanoparticle adjuvants. Biomaterials. 2011;32(28):6815–6822. doi: 10.1016/j.biomaterials.2011.05.063. [DOI] [PubMed] [Google Scholar]
- 133.Luo M, Wang H, Wang Z, Cai H, Lu Z, Li Y, Du M, Huang G, Wang C, Chen X, Porembka MR, Lea J, Frankel AE, Fu Y, Chen ZJ, Gao J. A sting-activating nanovaccine for cancer immunotherapy. Nature nanotechnology. 2017 doi: 10.1038/nnano.2017.52. (Accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Drake CG. Combination immunotherapy approaches. Annals of oncology: official journal of the European Society for Medical Oncology. 2012;23(Suppl 8):viii41–46. doi: 10.1093/annonc/mds262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Moynihan KD, Opel CF, Szeto GL, Tzeng A, Zhu EF, Engreitz JM, Williams RT, Rakhra K, Zhang MH, Rothschilds AM, Kumari S, et al. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nature medicine. 2016;22(12):1402–1410. doi: 10.1038/nm.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Palucka K, Banchereau J. Diversity and collaboration for effective immunotherapy. Nature medicine. 2016;22(12):1390–1391. doi: 10.1038/nm.4249. [DOI] [PubMed] [Google Scholar]
- 137.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 138.Kreiter S, Vormehr M, van de Roemer N, Diken M, Lower M, Diekmann J, Boegel S, Schrors B, Vascotto F, Castle JC, Tadmor AD, et al. Mutant mhc class ii epitopes drive therapeutic immune responses to cancer. Nature. 2015;520(7549):692–696. doi: 10.1038/nature14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Budhu S, Wolchok J, Merghoub T. The importance of animal models in tumor immunity and immunotherapy. Current opinion in genetics & development. 2014;24:46–51. doi: 10.1016/j.gde.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sahdev P, Ochyl LJ, Moon JJ. Biomaterials for nanoparticle vaccine delivery systems. Pharmaceutical research. 2014;31(10):2563–2582. doi: 10.1007/s11095-014-1419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]