Abstract
A beneficial impact of the Human Pegivirus (HPgV) – formerly called GB virus C (GBV-C) – on HIV disease progression has been reported previously. One possible mechanism by which HPgV inhibits HIV replication is an alteration of the cytokine/chemokine milieu. Their expression has not been specifically evaluated in women despite their influence on disease progression and the possibility of gender-based differences in expression. Moreover, the impact of HPgV genotype on cytokine/chemokine expression is unknown.
Sera levels of IL-2, IL-4, IL-7, IL-8, IL-10, IL-12p70, IL-13, IFNγ, TNFα, IP-10, MIP-1α, MIP-1β, and TGF-β1 were quantified in 150 HIV-positive women based on HPgV RNA status. Cytokines/chemokines with detection rates of at least 50% included IL-2, IL-4, IL-8, IL-10, IL-12p70, IFNγ, TNFα, IP-10, MIP-1α, MIP-1β, and TGF-β1. Absolute values were significantly higher for HPgV positive compared to HPgV negative women for IL-7, IL-13, IL-12p70, and IFNγ. Absolute values were significantly lower for HPgV positive women for IL-4, IL-8, TGF-β1, and IP-10. IFNγ values were higher for HPgV genotype 2 than for genotype 1 (p = 0.036).
Further study of cytokine/chemokine regulation by HPgV may ultimately lead to the development of novel therapeutic agents to treat HIV infection and/or the design of vaccine strategies that mimic the ‘protective’ effects of HPgV replication.
Keywords: Cytokine, chemokine, GB virus C (GBV-C), human pegivirus (HPgV), HIV, women
Introduction
Human Pegivirus (HPgV), formerly known as hepatitis G virus/GB virus C (GBV-C), is a single-stranded RNA virus classified within the newly assigned Pegivirus genus of the Flaviviridae [Smith DB et al., 2016]. The viral genome consists of a single open reading frame (ORF) of ~3000 amino acids that encodes for multiple structural and non-structural genes. Most investigations have failed to demonstrate any pathogenic consequence of HPgV replication on human disease (reviewed in [Bhattarai N and Stapleton, 2012]), although it may be weakly associated with non-Hodgkin lymphoma [Chang CM et al., 2014]. HPgV is transmitted efficiently via intravenous and sexual routes, and the prevalence varies widely based on geographic location and the population studied. While male-to-male sex is an effective mode of transmission, HPgV is transmitted heterosexually and perinatally also [Berzsenyi MD et al., 2005a; Stapleton, 2003].
The prevalence of HPgV RNA ranges from 14 to 45% in HIV-positive persons [Berzsenyi MD et al., 2005b]. Several groups have reported beneficial effects of HPgV viremia on HIV disease [Heringlake S et al., 1998; Lefrere JJ et al., 1999; Tillmann et al., 2001; Williams CF et al., 2004; Xiang J et al., 2001]. For example, Tillman et al. reported longer AIDS-free survival, higher CD4 cell counts, and lower plasma HIV viral loads in individuals with HPgV RNA, as well as an inverse correlation between the HPgV and HIV viral loads [Tillmann et al., 2001]. Xiang et al. further demonstrated prolonged survival among HIV-positive persons co-infected with HPgV [Xiang J et al., 2001]. Bjorkman et al. reported that clearance of HPgV replication during follow-up was associated with the poorest survival [Bjorkman P et al., 2004], a finding that was confirmed in other studies [Van der Bij AK et al., 2005; Williams CF et al., 2004]. Nevertheless, prolonged survival in HPgV/HIV co-infected persons has not been reported by all studies [Birk et al., 2002; Bjorkman P et al., 2004; Quiros-Roldan et al., 2002; Toyoda H et al., 1998; Van der Bij AK et al., 2005]. Due to the potential importance of persistence of HPgV viremia, a meta-analysis [Zhang W et al., 2006] was conducted of prospective studies of HIV-positive persons with HPgV RNA. There was no conclusive evidence for an association between survival and HPgV infection early in HIV disease; however, there was a significant reduction in mortality when HPgV infection was present later in HIV disease. Clearance of HPgV viremia is associated with accelerated HIV disease compared to non-clearance [Birk et al., 2002; Bjorkman P et al., 2004; Williams CF et al., 2004].
At the population level, multiple HPgV genotypes exist [Feng Y et al., 2011; Muerhoff AS et al., 2006], leading some to suggest that they impact HIV disease [Berzsenyi MD et al., 2005b; Kaye S et al., 2005; Muerhoff AS et al., 2003]. Muerhoff et al. reported lower CD4 cell counts in HIV co-infected patients infected with HPgV 2a than in patients with subtype 2b [Muerhoff AS et al., 2003]. We subsequently found that during HIV/HCV/ HPgV triple infection, HPgV genotype 2 was associated with higher CD4 cell counts compared to HPgV genotype 1 [Schwarze-Zander C et al., 2006]. While similar findings were reported in Brazil [Alcalde R et al., 2010], no difference in CD4 cell counts was observed in Australia based on HPgV genotype [Berzsenyi MD et al., 2009]. HPgV viral load may also differ by genotype. For example, among HIV/HPgV co-infected individuals in Brazil, HPgV RNA levels were highest for genotype 1, followed by genotype 2a, and then 2b [Giret MT et al., 2011].
Characterizing the mechanisms by which HPgV inhibits HIV replication may lead to novel therapeutic treatments for HIV disease. Several potential pathways by which HPgV influences HIV replication and pathogenesis include altered chemokine expression and downregulation of chemokine co-receptors [Chang Q et al., 2007; Nattermann et al., 2003; Xiang et al., 2004]. Additionally, HPgV viremia is associated with stable T-helper 1 cytokine levels compared to HIV-infected individuals without HPgV in whom Th1 cytokines decreased and Th2 cytokines increased over time [Nunnari G et al., 2003]. Numerous studies support the importance of the Th1/2 response to HIV infection [Spellberg B and Jr., 2001]. However, a separate study found no significant differences in plasma CCL5, CXCL12/SDF-1, IL-7, or TNFα in the presence/absence of HPgV RNA [Gimenez-Barcons et al., 2005]. More recent studies also suggest that HPgV activates the endogenous interferon system, thereby resulting in partial control of HIV replication [Capobianchi MR et al., 2006; Chang Q et al., 2007; Lalle E et al., 2008; Xiang J et al., 2005]. While cytokines/chemokines have not been specifically evaluated in HIV/ HPgV co-infected women, gender differences in cytokine/chemokine expression exist and may influence disease progression of other hepatitis viruses. Only a single US study and 3 international studies have evaluated the influence of HPgV on cytokine or chemokine expression in the serum or plasma [Gimenez-Barcons et al., 2005; Lanteri MC et al., 2015; Mostafa HM et al., 2007; Nunnari G et al., 2003; Rodríguez AK et al., 2014]; none specifically focused on women or evaluated the potential impact of HPgV genotype. Thus, the current study evaluated the impact of HPgV on cytokine/chemokine expression in women with HIV infection.
Materials and Methods
Study population and sample selection
The HIV Epidemiologic Research (HER) Study was a prospective natural history study of HIV infection conducted in US women from 1993 through 2000 [Smith et al., 1997]. 871 HIV-infected women and 438 demographically similar, uninfected women were recruited from Baltimore, Maryland; Bronx, New York; Providence, Rhode Island; and Detroit, Michigan. Women were assessed at 6-month intervals for up to 14 study visits. Women with a clinical diagnosis of AIDS or any AIDS-defining opportunistic infections were ineligible for enrollment. ~30% of HIV-infected women received monotherapy or dual antiretroviral therapy at study entry; none received HAART [Mayer et al., 2003]. The HER Study protocol was approved by the institutional review boards at the Centers for Disease Control and Prevention, as well as participating medical centers. All participants provided informed consent prior to clinical data and sample collection.
Detection of HPgV RNA and determination of HPgV genotype
HPgV RNA was detected and genotypes evaluated as described previously [Blackard JT et al., 2014]. Briefly, viral RNA was extracted from serum with the QIAmp Viral RNA Mini Kit (QIAGEN, Valencia, CA), and HPgV RNA was detected by nested RT-PCR amplification of the 5’ untranslated region (UTR). Genotype was determined by population-based sequencing of nucleotides 107–362 of the 5’UTR and compared to GenBank reference sequences. Sequences were aligned using Clustal X 2.1 with GenBank accession numbers U59540 and U59543 (genotype 1A); U59549 and U59555 (genotype 1B); HGU59518, HGU59519, HGU59522, HGU59527, and D90600 (genotype 2A); HGU59534, HGU59535, U59529, U59533, and U63715 (genotype 2B); U59538 and U59539 (genotype 3), AB018667 and AB021287 (genotype 4), AY949771 and AF092894 (genotype 5); AB003292 and AF177619 (genotype 6), and HQ331233, HQ331234, and HQ331235 (genotype 7). Additional phylogenetic inference was performed using a Bayesian Markov chain Monte Carlo (MCMC) approach executed in the Bayesian Evolutionary Analysis by Sampling Trees (BEAST) v1.7.5 [Drummond AJ et al., 2012] with an uncorrelated log-normal relaxed molecular clock, a generalized time reversible (GTR) model, and nucleotide site heterogeneity estimated using a gamma distribution. The BEAST MCMC analysis was run for a chain length of 200,000,000. All effective sample sizes were >100 indicating sufficient sampling. The maximum clade credibility tree was selected from the posterior tree distribution after a 10% burn-in using TreeAnnotator v1.7.5.
Multiplex immunoassay
The current analysis included available serum samples chosen at random from 70 women co-infected with HIV and HPgV (study visits from April 1993 to January 1995) and 80 HIV-positive women who were HPgV RNA negative (study visits from April 1993 to October 1994) based on our previous study of HPgV in this cohort [Blackard JT et al., 2014]. These 150 women did not differ at baseline from the remaining HIV-positive women in the HERS cohort with respect to race; however, they were older (median age 36.9 years versus 34.7 years; p < 0.001), had a higher median CD4 count (431.2 versus 367.6; p = 0.012), and lower log viral load (3.09 versus 3.20; p = 0.023). MILLIPLEX multiplex biomarker panels utilizing the Luminex xMAP technology were used to determine cytokine levels from patient serum. These included kits MPXHCYTO-70K-12 to quantify IL-2, IL-4, IL-7, IL-8, IL-10, IL-12p70, IL-13, IFNγ, TNFα, IP-10, MIP-1α, and MIP-1β (minimal detectable concentrations of 0.1 to 4.5 pg/mL) and TGFB-64K-01 to quantify TGF-β1 (minimal detectable concentration of 10 pg/mL).
Statistical analysis
Wilcoxon rank-sum tests were utilized to compare baseline values for age, CD4 cell count, and HIV viral load for women included in the current analysis to the remaining HIV-positive women, whereas the chi-square test was used for the baseline comparison of race. Categorical demographic and clinical factors were compared by HPgV RNA status (positive / negative) using either the chi-square test or Fisher’s exact test if any expected cell count was less than 5. Age approximated a normal distribution and was compared by HPgV RNA status using the t-test. Cytokine/chemokine values (expressed in pg/mL) were not normally distributed and were log-transformed prior to evaluation by HPgV RNA status and genotype (genotype 1 / genotype 2) using separate Wilcoxon rank-sum tests. The value of undetectable cytokines/chemokines was set to half of the lowest detectable value for that cytokine/chemokine. In Figure 1, the values at the bottom of each cytokine/chemokine denote the relative proportion of undetectable data. P values less than 0.05 were considered statistically significant for all tests, although p values less than 0.10 are also indicated.
Figure 1.
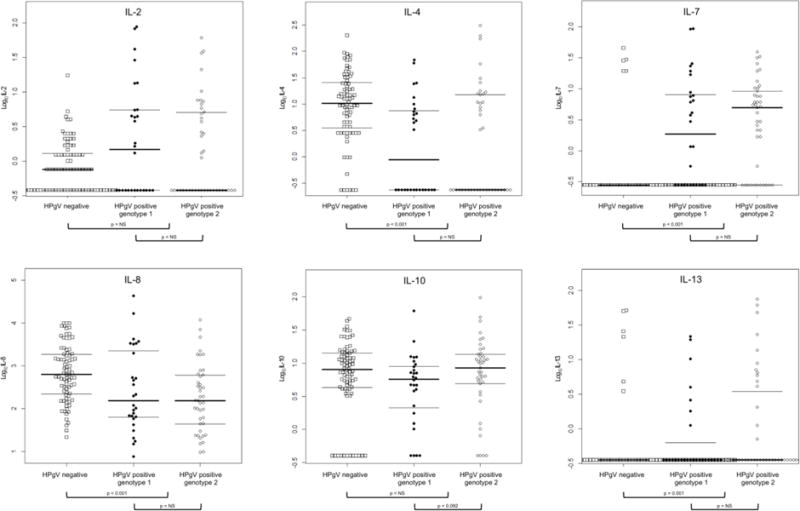
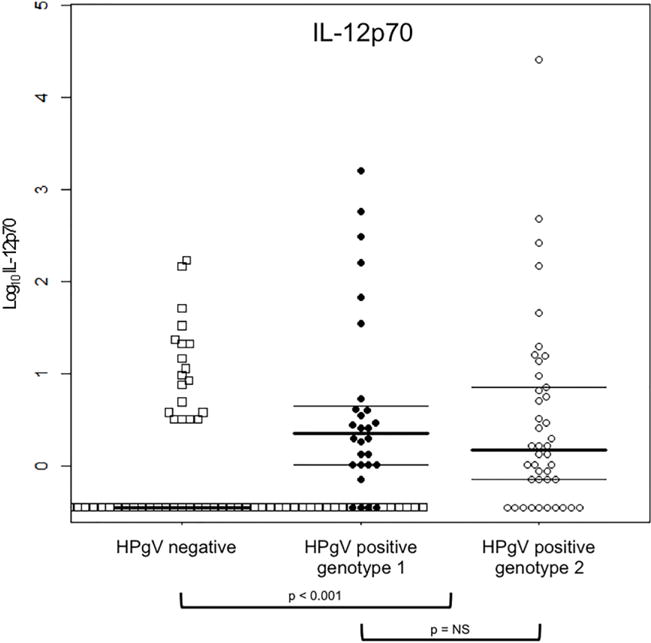
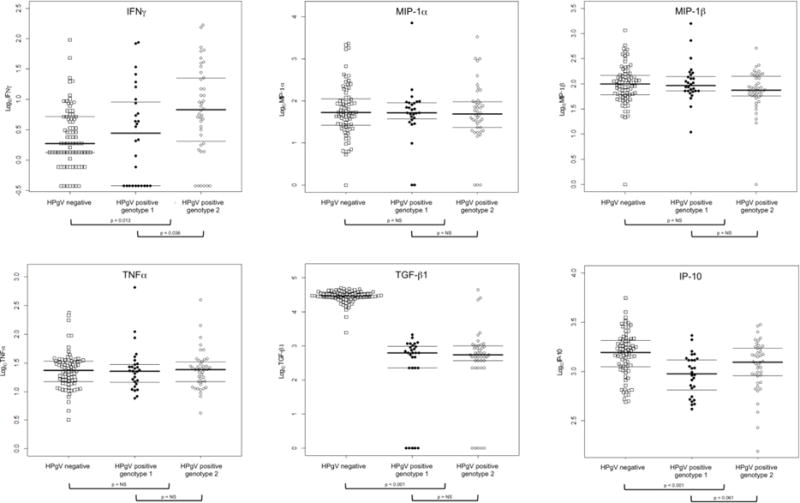
Log-transformed cytokine/chemokine levels (in pg/mL) in serum are shown based on HPgV status (squares for HPgV negative or circles for HPgV positive) and genotype (closed circles for genotype 1 or open circles for genotype 2). Thick horizontal bars denote the median value for each group, while thin horizontal bars denote the 25th and 75 percentile. The values at the bottom of each cytokine/chemokine denote the relative proportion of undetectable data. Only p values < 0.10 are shown; all others are labeled ‘NS’ (not shown)
Results
Characterization of patient population
We previously reported a 20.8% prevalence of HPgV RNA in the HERS cohort with most infections due to genotype 1 (44.3%) or genotype 2 (51.4%) [Blackard JT et al., 2014]. Patient demographics for the 150 women included in the current analysis are provided in Table 1. The 70 HIV-positive, HPgV-positive women were not different from the 80 HIV-positive, HPgV-negative women with respect to sexual or drug use behaviors, alcohol use, all-cause mortality, or HBV surface antigen (HBsAg) positivity; however, HPgV-positive women were younger (median age 34.7 years versus 39.3 years; p < 0.001) and more likely to be white or Hispanic and less likely to be black (p = 0.025) compared to HPgV-negative women. In this analysis, 28 women had HPgV genotype 1, while 42 had HPgV genotype 2 (data not shown).
Table 1.
Demographic and clinical data for the 150 HIV-infected women from the HERS cohort included in the current analysis based on HPgV RNA status.
| Characteristic | HPgV RNA negative (N = 80) |
HPgV RNA positive (N = 70) |
P value |
|---|---|---|---|
| Mean age in years (SD) | 39.3 (6.1) | 34.7 (5.4) | <0.001 |
| Race (%) | 0.025 | ||
| Black | 63 (78.8%) | 40 (57.1%) | |
| White | 9 (11.3%) | 16 (22.9%) | |
| Hispanic | 7 (8.7%) | 13 (18.6%) | |
| Native American/Asian/Other | 1 (1.2%) | 1 (1.4%) | |
| Study site | <0.001 | ||
| NY | 17 (21.3) | 14 (20.0) | |
| MI | 35 (43.7) | 12 (17.1) | |
| MD | 28 (35.0) | 24 (34.3) | |
| RI | 0 | 20 (28.6) | |
| Sexual and drug use behaviors | |||
| IDU since 1985 (%) | 75 (93.8%) | 61 (87.1%) | 0.165 |
| IDU in previous 6 months (%) | 29 (36.3%) | 30 (42.9%) | 0.409 |
| ≥5 sex partners in past 6 months (%) | 19 (23.8%) | 18 (25.7%) | 0.781 |
| Ever had sex with male IDU (%) | 70 (87.5%) | 61 (88.4%) | 0.866 |
| Ever had sex with partner known/suspected HIV+ (%) | 43 (53.8%) | 43 (62.3%) | 0.291 |
| Ever had sex for money or drugs (%) | 35 (43.8%) | 34 (48.6%) | 0.555 |
| Male sex partners in previous 6 months (%) | 0.652 | ||
| 0 | 27 (33.7%) | 20 (28.6%) | |
| 1–10 | 51 (63.8%) | 49 (70.0%) | |
| >10 | 2 (2.5%) | 1 (1.4%) | |
| Currently using hormonal contraceptives (%) | 1 (1.3%) | 3 (4.3%) | 0.340 |
| Currently using condoms (%) [102 sexually active] | 34 (64.2%) | 37 (74.0%) | 0.281 |
| Ever been pregnant (%) | 74 (92.5%) | 67 (95.7%) | 0.504 |
| Currently pregnant (%) | 1 (1.3%) | 0 | 1.0 |
| Currently using alcohol (%) | 49 (61.3%) | 40 (57.1%) | 0.609 |
| CD4 count (Median, IQR) | 447 (286–623) | 421 (311–577) | 0.531 |
| Log viral load (SD) | 3.0 (0.86) | 3.2 (0.80) | 0.105 |
| Non-HAART antiretroviral therapy* | 24 (30.0%) | 24 (34.3%) | 0.575 |
| All-cause mortality (%) | 0.598 | ||
| 1993 – 1996 | 12 (15.0%) | 7 (10.0%) | |
| 1997 – 2000 | 10 (12.5%) | 11 (15.7%) | |
| HBsAg positive (%) | 2 (3.2%) | 3 (5.4%) | 0.665 |
P-values for potential associations between HPgV status and categorical characteristics were determined by chi-square test or by Fisher’s exact test if any expected cell count was <5. For continuous characteristics, p-values were determined by t tests if normally distributed and by Wilcoxon rank-sum test if not normally distributed.
SD – standard deviation; IDU – injection drug use; HBsAg – Hepatitis B virus surface antigen; NS – not significant
None of the women were on HAART at HERS enrollment.
Serum cytokine/chemokine expression by HPgV RNA status
Detection rates varied significantly by cytokine/chemokine (Table 2). Cytokines/chemokines with detection rates of at least 50% included IL-2 (55%), IL-4 (72%), IL-8 (100%), IL-10 (87%), IL-12p70 (50%), IFNγ (85%), TNFα (100%), IP-10 (100%), MIP-1α (95%), MIP-1β (98.7%), and TGF-β1 (93.3). As shown in Figure 1, absolute values were significantly higher for HPgV positive compared to HPgV negative women for IL-7 (p < 0.001), IL-13 (p = 0.001), IL-12p70 (p < 0.001), and IFNγ (p = 0.013). Absolute values were significantly lower for HPgV positive compared to negative women for IL-4 (p < 0.001), IL-8 (p < 0.001), TGF-β1 (p < 0.001), and IP-10 (p < 0.001). There were no significant differences between these two groups for IL-2, IL-10, MIP-1α, MIP-1β, or TNFα.
Table 2.
Detection rates and interquartile ranges for chemokines/cytokines based on HPgV RNA status and HPGV genotype.
| Cytokine/chemokine | Detection rate (%) | HPgV RNA negative | HPgV RNA positive | HPgV RNA positive versus negative | HPgV genotype 1 | HPgV genotype 2 | HPgV genotype 1 versus genotype 2 |
|---|---|---|---|---|---|---|---|
| N = 80 | N = 70 | P value | N = 28 | N = 42 | P value | ||
| IL-2 | 55% | 0.8 (0–1.4) | 0.6 (0–5.5) | 0.138 | 1.5 (0–5.5) | 0 (0–5.1) | 0.513 |
| IL-4 | 72% | 10.4 (3.3–26.6) | 0 (0–10.9) | <0.001 | 1.7 (0–7.7) | 0 (0–15.3) | 0.702 |
| IL-7 | 35% | 0 (0–0) | 3.7 (0–8.7) | <0.001 | 2.1 (0–8.2) | 5.0 (0–9.1) | 0.494 |
| IL-8 | 100% | 630 (218–1892) | 155 (45.6–648) | <0.001 | 159 (61.5–2609) | 155 (44.2–626) | 0.615 |
| IL-10 | 87% | 8.0 (4.2–14.2) | 7.0 (3.7–12.2) | 0.403 | 5.8 (2.0–9.2) | 8.5 (5.0–13.6) | 0.092 |
| IL-12p70 | 50% | 0 (0–0) | 1.7 (0.7–6.6) | <0.001 | 2.3 (1.0–4.8) | 1.5 (0.7–7.2) | 0.341 |
| IL-13 | 18% | 0 (0–0) | 0 (0–1.8) | 0.001 | 0 (0–1.1) | 0 (0–4.1) | 0.433 |
| IFNγ | 85% | 1.9 (1.3–5.2) | 5.1 (1.2–16.0) | 0.013 | 2.9 (0–9.4) | 6.8 (1.9–22.8) | 0.036 |
| TNFα | 100% | 23.3 (14.6–34.0) | 23.9 (14.9–30.3) | 0.799 | 22.9 (14.2–29.9) | 26.2 (14.9–33.7) | 0.787 |
| IP-10 | 100% | 1561 (1100–2063) | 1105 (721–1505) | <0.001 | 950 (616–1323) | 1240 (898–1724) | 0.061 |
| MIP-1α | 95% | 53.0 (26.4–113.9) | 52.2 (29.2–93.1) | 0.758 | 52.7 (35.8–90.2) | 48.9 (23.3–98.6) | 0.749 |
| MIP-1β | 98.7% | 99.7 (60.1–148.0) | 82.4 (63.01–146) | 0.414 | 92.3 (72.1–144) | 75.5 (57.7–146) | 0.164 |
| TGF-β1 | 93.3% | 29644 (25714–36105) | 628 (228–1036) | <0.001 | 628 (228–993) | 556 (372–1036) | 0.957 |
Statistical evaluation was based on log-transformed values since the values were not normally distributed; however, to demonstrate the range of cytokine/chemokine measures, the absolute values are shown.
Serum cytokine/chemokine expression by HPgV genotype
IFNγ values were higher for HPgV genotype 2 than for genotype 1 (p = 0.036). Although not statisitically signficant at p < 0.05, IL-10 (p = 0.092) and IP-10 (p = 0.061) values tended to be higher for HPgV genotype 2 than for genotype 1. There were no significant differences between HPgV genotype 1 versus genotype 2 for IL-2, IL-4, IL-7, IL-8, IL-12p70, IL-13, TNFα, MIP-1α, MIP-1β, or TGF-β1.
Discussion
Characterizing the mechanisms underlying the ability of HPgV to inhibit HIV replication may lead to novel therapeutic treatments for HIV disease. In vitro infection of PBMCs with HPgV suggests that reduced HIV replication is related to the induction of chemokines and that this inhibitory effect can be reduced by antibodies to RANTES, MIP-1a, or MIP-1β [Xiang et al., 2004]. The HPgV E2 glycoprotein also induces a dose-dependent release of RANTES and down regulation of CCR5 chemokine receptor expression, thereby blocking HIV entry [Nattermann et al., 2003]. Furthermore, the HPgV NS5A protein decreases CXCR4 receptor expression, upregulates the chemokine SDF-1, and inhibits HIV replication [Chang Q et al., 2007]. Similarly, we have reported that HPgV alters CCR5 and CXCR4 surface expression on CD4+ T cells [Schwarze-Zander C et al., 2010].
A small number of studies have evaluated different cytokines and chemokines in the context of HPgV infection, although no standard set has been included in all studies. For instance, Lanteri et al. described an anti-inflammatory effect of HPgV in HIV-positive individuals, an overall downregulation of immune pathways and functions following HPgV detection, and increased cell death in cell types that support HIV replication [Lanteri MC et al., 2015]. The cytokines evaluated in the current study – IL-2, IL-4, IL-7, IL-8, IL-10, IL-12p70, IL-13, IFNγ, TNFα, IP-10, MIP-1α, MIP-1β, and TGF-β1 – are involved in promoting antiviral activity and viral clearance, inhibition of antiviral activity, the development of viral persistence, chemotaxis and/or fibrogenesis. Our findings show that HPgV infection was associated with higher levels of IL-7, IL-13, IL-12p70, and IFNγ but lower levels of IL-4, IL-8, TGF-β1, and IP-10. HPgV infection was not significantly associated with IL-2, IL-10, MIP-1α, MIP-1β, or TNFα levels. These results are largely consistent with those from other studies conducted among HIV-positive individuals in Spain, Venezuela, and Italy [Gimenez-Barcons et al., 2005; Nunnari G et al., 2003; Rodríguez AK et al., 2014], although discrepancies were noted. For instance, Gimenez-Barcons et al. reported no difference in IL-7 based on HPgV RNA status [Gimenez-Barcons et al., 2005], while we observed increased IL-7 in HPgV positive women, and Rodriguez et al. reported lower TNFα levels in individuals with HPgV infection [Rodríguez AK et al., 2014], while we observed no impact of HPgV infection on TNFα levels in women. Nunnari et al. reported no difference in IL-4 or IL-12 expression [Nunnari G et al., 2003], while we found higher IL-12 levels and lower IL-4 levels in women who were HPgV positive. The single US study of cytokine/chemokine expression during HPgV co-infection was conducted in HIV-positive blood transfusion recipients, of whom 44 of 60 (73.3%) were male, and there were no significant differences in cytokine/chemokine levels in persons with or without HPgV [Lanteri MC et al., 2015]. Divergent results amongst these studies and ours may reflect the distinct at-risk populations studied. Moreover, the sample size, cross-sectional study design, and unique population characteristics may limit comparability to other published studies. Additionally, the exact dates of HIV and/or HPgV infection are unknown, although this information is rarely known, particularly for HPgV, which is not evaluated in most routine clinical settings. The present study was considered exploratory in nature; therefore, the Bonferroni correction for multiple comparisons was not applied. Nonetheless, application of this correction would have changed the significance for only chemokine – IFNγ – from significant to non-significant for both HPgV status and genotype comparisons.
Our study focused on HPgV in women given that viral infections are frequently understudied in females and the potential for gender-specific differences in cytokine expression (reviewed in [Ruggieri A et al., 2010]). This study was limited by the relatively modest sample size and the restricted circulation of HPgV genotypes other than 1 and 2 in the US; thus, the results may not be generalizable to all individuals at risk or infected with HIV and/or HPgV. Another potential study limitation includes the dilution of circulating cytokines compared to the tissue/cell microenvironment in which they are produced and where viral replication occurs, thereby limiting systemic detection of certain cytokines/chemokines. Despite this, our study utlized the gold-standard method for quantification of cytokines/chemokines and is the first to describe the influence of HPgV infection and genotype on cytokine or chemokine expression among women. These data may ultimately lead to the development of novel therapeutic agents to treat HIV infection and/or the design of vaccine strategies that simulate the ‘protective’ effects of HPgV replication.
Acknowledgments
The authors would like to thank the HER Study staff and participants. The HER Study group consists of Robert S. Klein, MD, Ellie Schoenbaum, MD, Julia Arnsten, MD, MPH, Robert D. Burk, MD, Chee Jen Chang, PhD, Penelope Demas, PhD, and Andrea Howard, MD, MSc, from Montefiore Medical Center and the Albert Einstein College of Medicine; Jack Sobel, MD, Suzanne Ohmit, DrPH, William Brown PhD, Michael Long PhD, Wayne Lancaster PhD, and Jose Vazquez, MD, from the Wayne State University School of Medicine; Anne Rompalo, MD, David Vlahov, PhD and David Celentano, PhD, from the Johns Hopkins University School of Medicine; Charles Carpenter, MD, Kenneth Mayer, MD, Susan Cu-Uvin, MD, Timothy Flanigan, MD, Joseph Hogan, ScD, Valerie Stone, MD, Karen Tashima, MD, and Josiah Rich, MD from the Brown University School of Medicine; Ann Duerr, MD, PhD, Lytt I. Gardner, PhD, Chad Heilig, PhD, Scott D. Holmberg, MD, Caroline C. King, PhD, Denise J. Jamieson, MD, MPH, Janet S. Moore, PhD, Ruby M. Phelps, BS, Dawn K. Smith, MD, MPH and Dora Warren, PhD from the Centers for Disease Control and Prevention; and Katherine Davenny, MPH from the National Institute of Drug Abuse. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. We would like to thank Alyssa Sproles for performing the LINCOplex multiplex assays. This work was funded by the National Institute on Allergy and Infectious Diseases (award AI081564 to JTB).
References
- Alcalde R, Nishiya A, Casseb J, Inocêncio L, Fonseca LA, Duarte A. Prevalence and distribution of the GBV-C/HGV among HIV-1-infected patients under anti-retroviral therapy. Virus Research. 2010;151(2):148–152. doi: 10.1016/j.virusres.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Berzsenyi MD, Bowden DS, Bailey MJ, White C, Coghlan P, Dudley FJ, Roberts S. Male to male sex is associated with a high prevalence of exposure to GB virus C. Journal of Clinical Virology. 2005a;33(3):243–246. doi: 10.1016/j.jcv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Berzsenyi MD, Bowden DS, Roberts S. GB virus C: insights into co-infection. Journal of Clinical Virology. 2005b;33(4):257–266. doi: 10.1016/j.jcv.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Berzsenyi MD, Bowden DS, Roberts SK, Revill P. GB virus C genotype 2 predominance in a hepatitis C virus/HIV infected population associated with reduced liver disease. Journal of Gastroenterology and Hepatology. 2009;24(8):1407–1410. doi: 10.1111/j.1440-1746.2009.05920.x. [DOI] [PubMed] [Google Scholar]
- Bhattarai N, Stapleton J. GB virus C: the good boy virus? Trends in Microbiology. 2012;20(3):124–130. doi: 10.1016/j.tim.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk M, Lindback S, Lidman C. No influence of GB virus C replication on the prognosis in a cohort of HIV-1-infected patients. AIDS. 2002;16(18):2482–2485. doi: 10.1097/00002030-200212060-00017. [DOI] [PubMed] [Google Scholar]
- Bjorkman P, Flamholc L, Naucler A, Molnegren V, Wallmark E, Widell A. GB virus C during the natural course of HIV-1 infection: viremia at diagnosis does not predict mortality. AIDS. 2004;18(6):877–886. doi: 10.1097/00002030-200404090-00005. [DOI] [PubMed] [Google Scholar]
- Blackard JT, Ma G, Welge JA, King CC, Taylor LE, Mayer KH, Klein RS, Celentano DD, Sobel JD, Jamieson DJ, Gardner L. GB virus C (GBV-C) infection in hepatitis C virus (HCV) seropositive women with or at risk for HIV infection. PLoS One. 2014;9(12):e114467. doi: 10.1371/journal.pone.0114467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capobianchi MR, Lalle E, Martini F, Poccia F, D’Offizi G, Antonucci G, Abbate I, Dianzani F. Influence of GBV-C infection on the endogenous activation of the IFN system in HIV-1 co-infected patients. Cellular and Molecular Biology (Noisy-le-grand) 2006;52(1):3–8. [PubMed] [Google Scholar]
- Chang CM, Stapleton JT, Klinzman D, McLinden JH, Purdue MP, Katki HA, Engels E. GBV-C infection and risk of NHL among U.S. adults. Cancer Research. 2014;74(19):5553–5560. doi: 10.1158/0008-5472.CAN-14-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, McLinden JH, Stapleton JT, Sathar MA, Xiang J. Expression of GB virus C NS5A protein from genotypes 1, 2, 3 and 5 and a 30 aa NS5A fragment inhibit human immunodeficiency virus type 1 replication in a CD4+ T-lymphocyte cell line. Journal of General Virology. 2007;88(12):3341–3346. doi: 10.1099/vir.0.83198-0. [DOI] [PubMed] [Google Scholar]
- Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular Biology and Evolution. 2012;29(8):1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Zhao W, Feng Y, Dai J, Li Z, Zhang X, Liu L, Bai J, Zhang H, Lu L, Xia X. A novel genotype of GB virus C: its identification and predominance among injecting drug users in Yunnan, China. PLoS One. 2011;6(10):e21151. doi: 10.1371/journal.pone.0021151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Barcons M, Ribera M, Llano A, Clotet B, Este J, Martinez M. Analysis of chemokine and cytokine expression in patients with HIV and GB virus type C coinfection. Clinical Infectious Diseases. 2005;40:1342–1349. doi: 10.1086/429320. [DOI] [PubMed] [Google Scholar]
- Giret MT, Miraglia JL, Sucupira MC, Nishiya A, Levi JE, Diaz RS, Sabino EC, Kallas E. Prevalence, incidence density, and genotype distribution of GB virus C infection in a cohort of recently HIV-1-infected subjects in Sao Paulo, Brazil. PLoS One. 2011;6(4):e18407. doi: 10.1371/journal.pone.0018407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heringlake S, Ockenga J, Tillmann HL, Trautwein C, Meissner D, Stoll M, Hunt J, Jou C, Solomon N, Schmidt RE, Manns M. GB virus C/hepatitis G virus infection: a favorable prognostic factor in human immunodeficiency virus-infected patients? Journal of Infectious Diseases. 1998;177(6):1723–1726. doi: 10.1086/517431. [DOI] [PubMed] [Google Scholar]
- Kaye S, Howard M, Alabi A, Hansmann A, Whittle H, Shim van der Loeff M. No observed effect of GB virus C coinfection on disease progression in a cohort of African woman infected with HIV-1 or HIV-2. Clinical Infectious Diseases. 2005;40(6):876–878. doi: 10.1086/428123. [DOI] [PubMed] [Google Scholar]
- Lalle E, Sacchi A, Abbate I, Vitale A, Martini F, Offizi G, Antonucci G, Castilletti C, Poccia F, Capobianchi M. Activation of interferon response genes and of plasmacytoid dendritic cells in HIV-1 positive subjects with GB virus C co-infection. International Journal of Immunopathology and Pharmacology. 2008;21(1):161–171. doi: 10.1177/039463200802100118. [DOI] [PubMed] [Google Scholar]
- Lanteri MC, Vahidnia F, Tan S, Stapleton JT, Norris PJ, Heitman J, Deng X, Keating SM, Brambilla D, Busch MP, Custer B, NHLBI REDS III Study Downregulation of cytokines and chemokines by GB virus C after transmission via blood transfusion in HIV-positive blood recipients. Journal of Infectious Diseases. 2015;211(10):1585–1596. doi: 10.1093/infdis/jiu660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrere JJ, Roudot-Thoraval F, Morand-Joubert L, Petit JC, Lerable J, Thauvin M, Mariotti M. Carriage of GB virus C/hepatitis G virus RNA is associated with a slower immunologic, virologic, and clinical progression of human immunodeficiency virus disease in coinfected persons. Journal of infectious Diseases. 1999;179(4):783–789. doi: 10.1086/314671. [DOI] [PubMed] [Google Scholar]
- Mayer K, Hogan J, Smith D, Klein R, Schuman P, Margolick J, Korkontzelou C, Farzedegan H, Vlahov D, Carpenter C, for the HIV Epidemiology Research (HERS) Study Group Clinical and immunologic progression in HIV-infected US women before and after the introduction of highly active antiretroviral therapy. Journal of Acquired Immune Deficiency Syndromes. 2003;33(5):614–624. doi: 10.1097/00126334-200308150-00011. [DOI] [PubMed] [Google Scholar]
- Mostafa HM, Ali-Akbar P, Zahra S, Minoo M, Sedigheh A, Mahnaz A, Shahram S, Mahin N, Mina M. Soluble CD26 and CD30 plasma levels in HIV infected patients with and without GB virus type C coinfection. Pakistani Journal of Biological Sciences. 2007;10(12):2014–2019. doi: 10.3923/pjbs.2007.2014.2019. [DOI] [PubMed] [Google Scholar]
- Muerhoff AS, Dawson GJ, Desai S. A previously unrecognized sixth genotype of GB virus C revealed by analysis of 5′-untranslated region sequences. Journal of Medical Virology. 2006;78(1):105–111. doi: 10.1002/jmv.20510. [DOI] [PubMed] [Google Scholar]
- Muerhoff AS, Tillmann HL, Manns MP, Dawson GJ, Desai S. GB virus C genotype determination in GB virus-C/HIV co-infected individuals. Journal of Medical Virology. 2003;70(1):141–149. doi: 10.1002/jmv.10375. [DOI] [PubMed] [Google Scholar]
- Nattermann J, Nischalke HD, Kupfer B, Rockstroh J, Hess L, Sauerbruch T, Spengler U. Regulation of CC chemokine receptor 5 in hepatitis G virus infection. AIDS. 2003;17(10):1457–1462. doi: 10.1097/00002030-200307040-00006. [DOI] [PubMed] [Google Scholar]
- Nunnari G, Nigro L, Palermo F, Attanasio M, Berger A, Doerr HW, Pomerantz RJ, Cacopardo B. Slower progression of HIV-1 infection in persons with GB virus C co-infection correlates with an intact T-helper 1 cytokine profile. Annals of Internal Medicine. 2003;139(1):26–30. doi: 10.7326/0003-4819-139-1-200307010-00009. [DOI] [PubMed] [Google Scholar]
- Quiros-Roldan E, Maroto MC, Torti C, Moretti F, Casari S, Pan A, Carosi G. No evidence of beneficial effect of GB virus type C infection on the course of HIV infection. AIDS. 2002;16(10):1430–1431. doi: 10.1097/00002030-200207050-00019. [DOI] [PubMed] [Google Scholar]
- Rodríguez AK, Garzaro DJ, Loureiro CL, Gutiérrez CR, Ameli G, Jaspe RC, Porto L, Monsalve F, Pozada Á, Vázquez L, Quiñones-Mateu ME, Pujol FH, Rangel H. HIV-1 and GBV-C co-infection in Venezuela. Journal of Infection in Developing Countries. 2014;8(7):863–868. doi: 10.3855/jidc.3830. [DOI] [PubMed] [Google Scholar]
- Ruggieri A, Barbati C, Malorni W. Cellular and molecular mechanisms involved in hepatocellular carcinoma gender disparity. International Journal of Cancer. 2010;127(3):499–504. doi: 10.1002/ijc.25298. [DOI] [PubMed] [Google Scholar]
- Schwarze-Zander C, Blackard JT, Zheng H, Addo MM, Lin W, Robbins GK, Sherman KE, Zdunek D, Hess G, Chung R, for the AIDS Clinical Trial Group A5071 Study Team GB virus-C (GBV-C) infection in hepatitis C virus (HCV)/HIV co-infected patients receiving HCV treatment: importance of the GBV-C genotype. Journal of Infectious Diseases. 2006;194(4):410–419. doi: 10.1086/505713. [DOI] [PubMed] [Google Scholar]
- Schwarze-Zander C, Neibecker M, Othman S, Tural C, Clotet B, Blackard JT, Kupfer B, Luechters G, Chung RT, Rockstroh JK, Spengler U. GB virus C coinfection in advanced HIV type-1 disease is associated with low CCR5 and CXCR4 surface expression on CD4(+) T-cells. Antiviral Therapy. 2010;15(5):745–752. doi: 10.3851/IMP1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D, Warrne D, Vlahov D, Schuman P, Stein M, Greenberg B, Holmberg S, for the Human Immunodeficiency Virus Epidemiology Research Study Group Design and baseline participant characteristics of the Human Immunodeficiency Virus Epidemiology Research (HER) Study: a prospective cohort of human immunodeficiency virus infection in US women. American Journal of Epidemiology. 1997;146(6):459–469. doi: 10.1093/oxfordjournals.aje.a009299. [DOI] [PubMed] [Google Scholar]
- Smith DB, Becher P, Bukh J, Gould EA, Meyers G, Monath T, Muerhoff AS, Pletnev A, Rico-Hesse R, Stapleton JT, Simmonds P. Proposed update to the taxonom of the genera Hepaciviru and Pegivirus within the Flaviviridae family. Journal of General Virology. 2016;97:2894–2907. doi: 10.1099/jgv.0.000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellberg B, JE Type 1/Type 2 immunity in infectious diseases. Clinical Infectious Diseases. 2001;32(1):76–102. doi: 10.1086/317537. [DOI] [PubMed] [Google Scholar]
- Stapleton J. GB virus type C/Hepatitis G virus. Seminars in Liver Disease. 2003;23(2):137–148. doi: 10.1055/s-2003-39943. [DOI] [PubMed] [Google Scholar]
- Tillmann H, Heiken H, Knapik-Botor A, Heringlake S, Ockenga J, Wilber J, Goergen B, Detmer J, McMorrow M, Stoll M, Schmidt R, Manns M. Infection with GB virus C and reduced mortality among HIV-infected patients. New England Journal of Medicine. 2001;345(10):715–724. doi: 10.1056/NEJMoa010398. [DOI] [PubMed] [Google Scholar]
- Toyoda H, Fukuda Y, Hayakawa T, Takamatsu J, Saito H. Effect of GB virus C/hepatitis G virus coinfection on the course of HIV infection in hemophilia patients in Japan. Journal of Acquired Immune Deficiency Syndromes. 1998;17(3):209–213. doi: 10.1097/00042560-199803010-00004. [DOI] [PubMed] [Google Scholar]
- Van der Bij AK, Kloosterboer N, Prins M, Boeser-Nunnink B, Geskus RB, Lange JM, Coutinho RA, Schuitemaker H. GB virus C coinfection and HIV-1 disease progression: the Amsterdam cohort study. Journal of Infectious Diseases. 2005;191:678–685. doi: 10.1086/427559. [DOI] [PubMed] [Google Scholar]
- Williams CF, Klinzman D, Yamashita TE, Xiang J, Polgreen PM, Rinaldo C, Liu C, Phair J, Margolick JB, Zdunek D, Hess G, Stapleton J. Persistent GB virus C infection and survival in HIV-infected men. New England Journal of Medicine. 2004;350(10):981–990. doi: 10.1056/NEJMoa030107. [DOI] [PubMed] [Google Scholar]
- Xiang J, Martinez-Smith C, Gale M, Chang Q, Labrecque DR, Schmidt WN, Stapleton J. GB virus type C NS5A sequence polymorphisms: association with interferon susceptibility and inhibition of PKR-mediated eIF2alpha phosphorylation. Journal of Interferon & Cytokine Research. 2005;25(5):261–270. doi: 10.1089/jir.2005.25.261. [DOI] [PubMed] [Google Scholar]
- Xiang J, Wunschmann S, Diekema DJ, Klinzman D, Patrick KD, George SL, Stapleton J. Effect of coinfection with GB virus C on survival among patients with HIV infection. New England Journal of Medicine. 2001;345(10):707–714. doi: 10.1056/NEJMoa003364. [DOI] [PubMed] [Google Scholar]
- Xiang J, George SL, Wunschmann S, Chang Q, Klinzman D, Stapleton JT. Inhibition of HIV-1 replication by GB virus C infection through increases in RANTES, MIP-1alpha, MIP-1beta, and SDF-1. Lancet. 2004;363(9426):2040–2046. doi: 10.1016/S0140-6736(04)16453-2. [DOI] [PubMed] [Google Scholar]
- Zhang W, Chaloner K, Tillmann HL, Williams CF, Stapleton J. Effect of early and late GB virus C viraemia on survival of HIV-infected individuals: a meta-analysis. HIV Medicine. 2006;7(3):173–180. doi: 10.1111/j.1468-1293.2006.00366.x. [DOI] [PubMed] [Google Scholar]


