Abstract
Background
Recently introduced cumulative lab-based frailty indices (FIs) have focused on health-related issues as outcomes, but provide little information on FI concomitants. Addressing this issue, we explore association with intrinsic (personal), and extrinsic (social, environmental) characteristics.
Design
Cross-sectional/longitudinal study
Setting
The 3rd and 4th waves of the community representative, five-county, 10-year Duke Established Populations for Epidemiological Studies of the Elderly (D-EPESE) study, a health service-rich area.
Participants
Cognitively intact third wave survivors (N=1,740), who provided blood samples for standard laboratory work.
Measurements
Biomarkers (N=28), to develop a cumulative deficit laboratory-test-based FI (Duke-FI), derived from standard laboratory tests: SMAC-24 chemistry panel, HDL cholesterol panel, and CBC. Scales assessing intrinsic characteristics (personal locus of control, life satisfaction, self-esteem, depressive symptomatology), and measures of extrinsic characteristics (support received from and provided to family and friends, stressful life events, neighborhood disadvantage).
Results
The newly developed Duke-FI had content, construct, concurrent and predictive validity. In addition to sex, race, and income, the Duke-FI was associated at the intrinsic level with locus of control, self-esteem, life satisfaction and depressive symptomatology (each P<0.001); and at the extrinsic level with provision (P<0.001) and marginally (P<0.10) with receipt of instrumental help, with social stressors (P<0.03), and with neighborhood disadvantage (P<.001) in unadjusted analysis; the latter association was fully explained by race.
Conclusion
Here, intrinsic (personality) characteristics, and personally close extrinsic characteristics (contacts with family/friends, personal stressors), are associated with laboratory-test-based frailty, as is neighborhood disadvantage. However, in this accessible, health service-rich environment, the latter association is fully explained by race, suggesting that intervention to reduce frailty in residents of such an environment should pay particular attention to characteristics that immediately affect the individual.
Keywords: lab-test-based frailty index, neighborhood disadvantage, personality, race/ethnicity
Introduction
There is no universally accepted definition of frailty, although it is “considered to be a physiological loss of reserve capacity and resistance to stressors”.1 Neither is there agreement on what should be assessed, with some measures focused solely on physical health, while others take a much broader view.2–4
While the number of measures of frailty is continually increasing,5–7 two approaches to assessing frailty predominate: the three-level phenotype approach based on physiological measures,8 identifies frailty as the presence of three or more of weight loss, tiredness, low physical activity, slow gait speed, and low grip strength (one or two problems indicates prefrailty, no problems indicates a robust state). In contrast, the cumulative deficit approach,9–12 seeks information on as broad a range of health-associated items as is available (health conditions, functional and cognitive status, physical performance, physiological measures, etc.). Score is the proportion of selected items impaired, allowing assessment along a continuum from minimal impairment (robust), to maximal impairment (frail).
Recently, this cumulative deficit approach has been applied to cellular level biomarkers, which have been shown to predict clinical outcomes.6,9,13,14 Cellular biomarkers have the advantage of providing information on a substantial array of body systems even before there are clinical manifestations,15 while avoiding possibly erroneous self-report, or confounding outcomes with determinants, as in the original clinically-based cumulative deficit approach.
To date, most studies of frailty have been cross-sectional, and have examined the association of frailty with demographic characteristics and health status,16 or with adverse outcomes (falls, functional decline, hospitalization, institutionalization, death).8,9,17–19 Other characteristics known to affect health status have been less examined. The psychological resilience literature has emphasized that in addition to personal characteristics that affect outcomes “…individuals are embedded in social systems…” that may have an impact also.20 Such social systems range from family support and the stressors that personal circumstances can occasion, to characteristics of the neighborhood and beyond.3
While some studies have added extrinsic (social, environmental) characteristics to frailty indexes, or reported on associations with these,2,4,21 none has as yet reported on the associations of intrinsic and extrinsic factors with a laboratory-test-based cumulative impairment frailty index. We address this gap. Using a newly developed cumulative impairment laboratory-test-based frailty index (the Duke-FI, described here), we examine association with selected aspects of intrinsic characteristics (personal locus of control, life satisfaction, self-esteem, depressive symptomatology), and extrinsic characteristics (bi-directional family/friend support, stressful life events; and the neighborhood environment). We anticipate a gradient of association – strongest with intrinsic characteristics, weakest with neighborhood characteristics.
Methods
Sample
The sample for the current study comes from the 3rd in-person wave (1991/92) of the 10-year (1986–1996), community-representative Duke-EPESE study of persons age 65–105 (response rate 80%, N=4,162, 54% African-American, 45% white, <1% other race), living in five adjacent Piedmont-area counties (one urban, four rural) in North Carolina. The study focused on self-reported health, change in health status, and health service use; functional status (also measured at wave 8); cognitive status; and social resources. Data were gathered annually over the first seven waves, with a final wave three years later; in-person at waves 1, 4, 7 (baseline for the current study), and 8, by telephone at waves 2, 3, 5, and 6.22
At wave 7 (age 71 and over), blood was drawn for standard laboratory tests from sample members who lived within range, personally gave consent for the blood draw, and who were able to provide blood samples (N = 1,742). Duke University Medical Center institutional review board approved this study. Written consent was obtained from all participants.
Core information relevant to the current study
Using structured questionnaires, information was gathered in person in the home, and four years later. The information used to assess the validity of the Duke-FI (see on-line supplement and below), included: Demographic characteristics (age, sex, race, education [truncated at 17 years], income); health status: self-rated health (1 = excellent, 4 = poor), and functional status (5-item basic activities of daily living (ADL): bathe, dress self, bed to chair transfer, use toilet, feed self;23 items summed to indicate number of problems, range 0–5). All information gathered at time of blood draw. Survival status and time to death through December 2006 (14 years after blood draw), was obtained from the National Death Index.24
Cumulative deficit lab-based frailty index development (also see on-line supplement) Blood drawing procedures and laboratory analyses
Blood was drawn for SMAC-24 chemistry panel, HDL cholesterol panel, and CBC analysis by trained phlebotomists using standard procedures, who followed universal precautions for handling blood and body fluids.
Supplementary Table S1 describes the 28 resulting biomarkers, the biological problems they assess, and individual psychometrics. For each sample member we determined the number of biomarkers for which there was information. Fifty people lacked information on between 1 and 6 biomarkers, of whom 17 lacked information on six biomarkers (21.4% of the biomarkers available). Since an FI can legitimately be constructed if 20% of the desired markers are absent,12 a standard often relaxed, all these persons were retained. Two people lacked information on 20 or more biomarkers, and were dropped, reducing the sample to 1,740 persons.
Intrinsic and extrinsic characteristics used to assess association with the Duke-FI (gathered at time of blood draw)
Intrinsic characteristics, i.e., personality, was assessed by locus of control (7 dichotomous items, scored to indicate lack of internal control, range 0–7);25 life satisfaction (13-item scale (3=agree, 2=not sure, 1=disagree; range 0–39),26 items re-scored as necessary, higher scores indicating poorer life satisfaction); self-esteem (10 dichotomously scored items, the five positively phrased items were reverse-coded, higher score indicating lower self-esteem);27 and depressive symptomatology (assessed by revised Center for Epidemiological Studies Depression Scale (CES-D), with items dichotomized (range 0–20).22,28
Extrinsic characteristics were represented by social support, stress and neighborhood status. Social support was assessed by services received from family/friends (range 0–12), and a reciprocal set of services (but adding child care) that the subject provided (range 0–13). Services were divided into instrumental (e.g., receives help with money, transportation, range 0–8 for services received, 0–9 for services provided), and emotional (e.g., receives advice, companionship, range 0–4). Social stress was indicated by number of negative life events in the last year (e.g., death of spouse, range 0–14).
Neighborhood, assessed in terms of disadvantage (1990 census data)
Census tract disadvantage, determined according to criteria proposed by Sampson and colleagues,29 included six characteristics – percentage: Black, families with below poverty-level income, households on public assistance, female-headed households, under age 18, and unemployed male civilians age 16 years and older. Principal components analysis of Duke-EPESE data indicated that, comparable to Sampson et al.,29 factor 1 had an Eigenvalue of 4.23, and explained 70.5% of the variance; five variables had loadings of 0.81–0.92, the sixth a loading of 0.59. This measure was normed on all US census tracts (mean=0, standard deviation=1), and the resulting standardized values applied to the 84 Duke-EPESE tracts, linked to previously geocoded addresses.30
The mean z-score for neighborhood disadvantage was 0.53 (standard deviation (SD) 0.83), indicating that 16%–17% more of the census tracts were disadvantaged compared to the US as a whole. Z-scores for neighborhood disadvantage tracts ranged from −1.03 (indicating that neighborhood disadvantage was ~1 SD, or ~30% less disadvantaged than the mean) to 4.45 (high neighborhood disadvantage).
Statistical analysis
Descriptive statistics characterized the sample, biomarker and Duke-FI data. To ensure that the Duke-FI was a measure of frailty and met standards,12 age was regressed on all biomarkers, as was time to death (with demographic characteristics controlled). These findings also indicated content and construct validity of the Duke-FI. Concurrent validity was determined by association with self-rated health and Katz ADL,23 using demographically adjusted ordered logit regression. Criterion (predictive) validity was ascertained by Kaplan-Meier survival curves for Duke-FI, trichotomised as robust (0–<0.1), prefrail (0.1–<0.2), and frail (≥0.20), cutpoints comparable to those used by other investigators,14 and by Cox regression, adjusted for demographic characteristics, to examine time to death.
Multivariable regression was used to examine association of demographic characteristics with Duke-FI, and, with demographic characteristics adjusted, to determine the association of Duke-FI with personality characteristics, family/friend support, and social stress.
Demographically adjusted ordered logit regression analyses were also used to compare the association of neighborhood disadvantage with Katz ADL23 and self-rated health, and to examine the effect on Duke-FI when neighborhood disadvantage was also included in the model. Cox regression was used to identify the association with mortality of Duke-FI and neighborhood disadvantage, both individually and in combination.
Multilevel regression models (hierarchical linear models, which addressed the multilevel nesting of respondents within census tracts) were used to determine the association of Duke-FI with neighborhood disadvantage without demographic adjustment (Model 1), fully adjusted for all demographic characteristics (Model 2), and adjusted for individual demographic characteristics (Models 3–7) to isolate individual effects. Analyses were performed using Stata 13.0.
Results
(See Supplementary Table S2.) The mean age of the sample was 78 years, two thirds were women, just over half were African-American; education (mean of 9 years) and income (mean $11,500 in 1986 dollars) were low. Health was rated excellent or good by 58%. Mean number of basic ADL problems was 0.3 (out of 5 activities).
Intrinsic (personality) characteristics: on average, participants were more likely to report external control than internal control, indicated fair satisfaction in life, low self-esteem, with 8.7% of the sample scoring above the cutoff value of 9 for depressive symptomatology.
Extrinsic characteristics: sample members received more help (emotional plus instrumental) than they provided, and averaged 1.7 stressful events in the previous year. On average they had lived 25 years at their current address, but 14.5% moved in the following four years. A quarter had died within four years of the blood draw, and 87% within 14 years.
Development of a human cumulative deficit lab-test-based frailty index (Duke-FI)
Supplementary Table S1 gives the psychometric characteristics of the 28 biomarkers constituting the Duke-FI. Regression of age on the biomarkers, and demographically adjusted prediction of death within 14 years, found an association of P <.10 for all but 5 of the 28 biomarkers (mean corpuscular volume, potassium, total protein, bilirubin, and carbon dioxide). Since these are known to be associated with health status, all biomarkers were retained. Duke-FI score (number of biomarkers out-of-range divided by total number of biomarkers for which there was information), ranged from 0.00–0.46 (practical range is considered to be 0.00–0.70). Supplementary Figure S1 displays the range of scores.
Validity of the Duke-FI
Content and construct validity were ascertained through determination of association with age and time to death (see Statistical analysis), and also with other demographic characteristics (Table 1, top section). Concurrent validity (demographically adjusted association with self-rated health and Katz ADL23), indicated that increased frailty was associated with poorer self-rated health, and increased basic ADL problems (Table 2). Criterion (predictive) validity indicated that higher frailty score was associated with shorter time to death (Table 2, Figure 1). Survival curves for Duke-FI, trichotomised as robust (0–<0.1, N=830, 37.4%), prefrail (0.1–<0.2, N=609, 51.0%) and frail (≥0.20, N=201, 11.6%), indicated that 50% of the frail were likely to die within five years, compared to approximately eight years for the robust (Figure 1).
Table 1.
Association of demographic characteristics with Duke-Frailty Index (N = 1740), and with these demographic characteristics controlled, association of personality characteristics, social environment, and social stressors with Duke-Frailty Index (multivariable regression)
| Duke Frailty Index | |||
|---|---|---|---|
| b | se | p | |
| Demographic characteristics | |||
| Age | 0.005 | 0.003 | 0.080 |
| Male (vs female) | 0.134 | 0.032 | 0.000 |
| African American (vsWhite) | 0.238 | 0.032 | 0.000 |
| Education (0 – 17 years) | −0.008 | 0.004 | 0.040 |
| Income $5000 – $22000a | −0.085 | 0.034 | 0.058 |
| Income >$22000 | −0.218 | 0.073 | 0.018 |
| Personality | |||
| Locus of control | −0.0039 | 0.001 | 0.000 |
| Self-esteem | −0.0067 | 0.0016 | 0.000 |
| Life satisfaction | 0.0021 | 0.0004 | 0.000 |
| Depressive symptomatology | 0.0016 | 0.0005 | 0.001 |
| Social environment | |||
| Gives help (total) | −0.0018 | 0.0006 | 0.002 |
| Gets help (total) | 0.001 | 0.0006 | 0.099 |
| Gives emotional help | −0.0017 | 0.0018 | 0.349 |
| Gets emotional help | 0.0018 | 0.0016 | 0.279 |
| Gives instrumental help | −0.0026 | 0.0008 | 0.001 |
| Gets instrumental help | 0.0013 | 0.0008 | 0.099 |
| Negative life events (stress) | |||
| Number of negative life events | 0.0033 | 0.0015 | 0.028 |
Reference = Income <$5,000
Table 2.
Individual and combined association of Duke-FI and neighborhood disadvantage with basic ADL, self-rated health (multilevel ordered logit models), and time to death (Cox regression), adjusted for age, sex, race, education, and income.
| Katz Basic ADL | ||
|---|---|---|
| Duke Frailty Index ORa (95% CIb) P value | Neighborhood disadvantage OR (95% CI) P value | |
| Only Duke Frailty Index entered | 5.05 (3.13–6.96) 0.001 | -------------------------------- |
| Only neighborhood disadvantage entered | ------------------------------- | 0.04 (−0.14–0.22) 0.940 |
| Duke Frailty Index and neighborhood disadvantage entered simultaneously | 5.04 (3.12–6.95) 0.001 | 0.03 (−0.15–0.21) 0.900 |
| Self-rated health | ||
| Only Duke Frailty Index entered | 4.25 (2.89–5.60) 0.001 | -------------------------------- |
| Only neighborhood disadvantage entered | ------------------------------- | 0.07 (−0.04–0.18) 0.154 |
| Duke Frailty Index and neighborhood disadvantage entered simultaneously | 4.23 (2.87–5.58) 0.001 | 0.06 (−0.05–0.18) 0.179 |
| Time to death | ||
| Only Duke Frailty Index entered | 2.92 (2.13–3.70) 0.001 | -------------------------------- |
| Only neighborhood disadvantage entered | -------------------------------- | 0.04 (−0.03–0.10) 0.921 |
| Duke Frailty Index and neighborhood disadvantage entered simultaneously | 2.92 (2.13–3.70) 0.001) | 0.04 (−0.03–0.10) 0.941 |
OR = Odds Ratio;
CI = Confidence Interval
Figure 1.
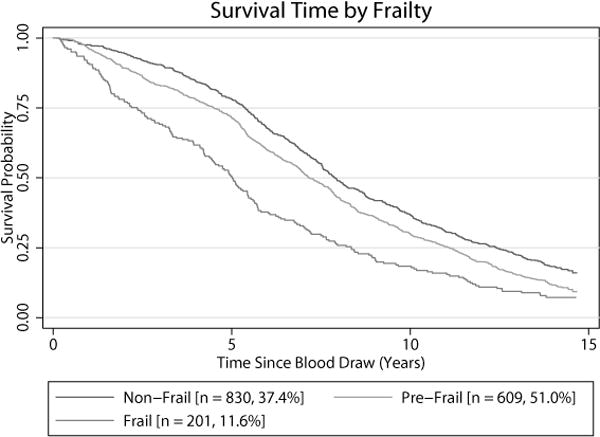
Kaplan-Meier curves for time to death by trichotomized frailty (score 0 – <0.1 = non-frail [robust], score 0.1 – <0.2 = pre-frail, score ≥0.20 = frail)
Association of Duke FI with demographic characteristics
As anticipated, in adjusted analyses, score on the Duke-FI was higher for men, those with lower income, and for African Americans; a modest increase was found for higher age and fewer years of education (Table 1).
Association of Duke-FI with intrinsic and extrinsic characteristics
Higher frailty score was associated with less satisfaction in life, and more depressive symptomatology, but also with greater self-esteem, and internal locus of control (Table 1). Regarding the social environment, higher frailty score was associated with providing less help to family and friends, but marginally with greater receipt of help (instrumental rather than emotional, which was at close to maximum level); and with increased number of stressors (Table 1).
Neighborhood disadvantage
In unadjusted analyses (Table 3, Model 1), neighborhood disadvantage was significantly associated with Duke-FI score. Simultaneous entry of all demographic characteristics fully explained the association (Table 3, Model 2). Examination found this to be attributable solely to race (Model 5; with only race included, the statistically significant association between Duke-FI score and neighborhood disadvantage was lost). Further, demographically-adjusted analyses (Table 2), indicated that neighborhood disadvantage was not associated with basic ADL, self-rated health, or time to death, although Duke-FI maintained its association with these characteristics, even with neighborhood disadvantage in the model. With the possible exception of depression score, disadvantaged neighborhoods were associated only, and minimally, with personality characteristics, and with provision and receipt of instrumental help (Supplementary Table S3).
Table 3.
Association of neighborhood disadvantage with Duke-FI score when individual demographic characteristics are entered (left hand section), and association of neighborhood disadvantage with Duke-FI score after simultaneous entry of all demographic characteristics (right hand section)
| Unadjusted association of neighborhood disadvantage with Duke-FI (model 1), association of neighborhood disadvantage after adjustment only for age (model 2), only for gender (model 3), only for race (model 4), only for education (model 5), only for income (model 6) | Association of neighborhood disadvantage and of each demographic characteristic after simultaneous entry of all characteristics. | ||||||
|---|---|---|---|---|---|---|---|
| Model | b | se | p | b | se | p | |
| 1 | 0.008 | 0.002 | 0.001 | Neighborhood disadvantage | 0.001 | 0.002 | 0.635 |
| 2 | 0.008 | 0.002 | 0.001 | Age | 0.001 | 0.001 | 0.090 |
| 3 | 0.008 | 0.002 | 0.001 | Gender: Male (vs female) | 0.014 | 0.004 | 0.001 |
| 4 | 0.002 | 0.002 | 0.427 | Race: African American (vs White) | 0.021 | 0.004 | 0.001 |
| 5 | 0.006 | 0.002 | 0.002 | Education (0–17 years) | −0.001 | 0.001 | 0.055 |
| 6 | 0.006 | 0.002 | 0.030 | Income ($0–maximum) | ______ | ______ | _____ |
| Income <$5,000 | Reference | ||||||
| Income $5,000–$22,000 | −0.007 | 0.004 | 0.058 | ||||
| Income >$22,000 | −0.018 | 0.008 | 0.019 | ||||
Because of limited association, we did not pursue the possibility that the characteristics examined might mediate between frailty and neighborhood disadvantage.
Discussion
Using standard blood lab measures, we have developed a validated cumulative deficit lab-based frailty index, the Duke-FI, for persons over 70 years of age. Guidelines for variable inclusion12 virtually assure association of such an index with age and mortality. Validity is, however, confirmed by association with other demographic characteristics, self-rated health, basic ADL, and time to death.
Most inquiry to date has focused on association of physical frailty measures with, or prediction of, specific health-related issues,16,17,19 and, with a few exceptions,2,4,21 largely ignored intrinsic and extrinsic factors found to influence health, including personality characteristics, the personal social environment, and the broader neighborhood environment.19,31–35 We have expanded enquiry to focus on the association of laboratory-test-based frailty with these issues.
We found, as anticipated, that association with intrinsic and extrinsic characteristics varies as a function of closeness to the individual. Specifically, we found a marked association of Duke-FI with various measures of personality, a lesser association with measures of the immediate social environment, and no significant association with neighborhood disadvantage.
While those who were more frail expressed lower satisfaction in life and greater depressive symptomatology, they also reported higher internal locus of control and higher self-esteem. Since these data are cross-sectional, we cannot distinguish cause from effect, but it suggests that those who are frail nevertheless have strengths on which to build.
Frailty did not affect receipt or provision of emotional services (near maximum in both directions). The more frail provided fewer instrumental services overall to friends and family (as might be expected), but received only marginally more services. This may reflect lack of recognition by family and friends of early manifestations of frailty, or reduced resources for persons living in areas of neighborhood disadvantage. Higher social stress was associated with frailty; it is not possible to determine whether frailty preceded the events, or was subsequent to, and possibly attributable to the event.
In demographically adjusted analyses, neighborhood disadvantage was not associated with baseline ADL, self-rated health, mortality, personality characteristics (except for increased depressive symptomatology), or the social environment (except for lower provision and receipt of instrumental services). Neighborhood disadvantage significantly predicted higher Duke-FI in unadjusted analyses, but this association was fully explained by race. Other studies that have also examined the association of neighborhood effects on residents have also shown the over-riding importance of individuals’ demographic characteristics.36–39
We considered various possible explanations for these findings. Neighborhood effects may be dampened if participants enjoy better health than the sample from which they are drawn, possibly the case here. Compared to the total sample, of those participating in the blood draw, 48% (compared to 37%) were free of basic ADL impairments both at baseline and four years later, 27% (compared to 31%) died during the next four years, and participants with higher frailty scores were more likely to move in the following four years (suggesting that frailer persons may have moved before the current study).
When race and disadvantage are so closely related in a model, the explanation is often structural confounding (an artifact of high levels of segregation).40 While this was not found here, the spatial scale used in the current study (census tracts in which there are definite pockets of segregation, rather than more intimate areas), may be an issue.
Where race is concerned, it may be difficult here to distinguish between neighborhood characteristics and personal characteristics. While neighborhood disadvantage may have a significant effect for some matters (e.g., crowding, violence), and for some people (e.g., those unable to move freely), it may have an attenuated effect when needed resources are provided and used at a broader geographic level, and when access is facilitated. While the five counties from which the sample was drawn included several medically underserved regions, the total area was richly endowed with nationally and internationally acclaimed medical facilities to which area residents had ready access through health insurance (which nearly all had held for a minimum of six years), and designated transportation, thus likely reducing effects of neighborhood disadvantage on health.
Limitations
This study is primarily cross-sectional. The study sample is likely to be more robust than a representative sample their age. Examination of the social environment was restricted because of data lack, and distinction between family and friends as service providers, although important, was not feasible.32 Because information is absent in the current data, additional inflammatory and endocrine markers, recognized as indicative of frailty, could not be included.
Advantages
The Duke-FI can be readily replicated in any medical setting since it draws on standard laboratory blood analyses. It relies on objective information and is not subject to personal recall, dependent on physician (or self) diagnosis, or to reporting bias, and does not confound underlying conditions with outcomes. Determination of score is rapid, and cut-points are clear. Identification of persons in whom frailty is developing may be feasible before problems are openly manifested.15 An understanding of basic underlying biological processes may be facilitated, and hence guide interventions.41
We have expanded information on frailty by examining its association with intrinsic (personal) and extrinsic (social, environmental) characteristics. We found that intrinsic characteristics were closely associated with frailty, as, to a lesser extent, were extrinsic social ties. In controlled analyses, there was no association with neighborhood disadvantage, possibly because of better health of the sample, and ready access to health services. While personality characteristics and social ties should be considered in interventions to reduce frailty, neighborhood characteristics should not be ignored.
Supplementary Material
Supplementary Material S1. Blood drawing procedures and laboratory analyses
Supplementary Table S1. Descriptive characteristics of the Duke EPESE sample for current study
Supplementary Table S2. Association of personality characteristics, social resources, and social stressors with neighborhood disadvantage, adjusted for demographic characteristics (age, sex, race, education, income)
Supplementary Figure S1. Duke-FI distribution of scores
Supplementary Figure S2a. Duke-FI mean scores (95% CI) by age
Supplementary Figure S2b. Duke-FI mean scores (95% CI) by age by gender
Acknowledgments
Funding
This work was supported by National Institute on Aging at the National Institutes of Health (contract Number N01-AG12102, grant numbers R01 AG12765, P30 AG028716).
Footnotes
Conflict of interest – None
Author contributions
K.E. King, G.G. Fillenbaum and H.J. Cohen contributed to the concept and design. Data were available under the original contract and grants that funded the Duke EPESE study (see Funding). H.J. Cohen was responsible for acquisition of laboratory-test-based data, and for selecting the laboratory-test-based variables and providing information on them. K.E. King performed all statistical analyses. All authors interpreted the data and were involved in preparation of the manuscript.
Sponsor’s role – The sponsor had no role in this study
References
- 1.De Vries NM, Staal JB, van Ravensburg CD, et al. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10:104–114. doi: 10.1016/j.arr.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 2.De Witte N, Gobbens R, De Donder L, et al. Validation of the Comprehensive Frailty Assessment Instrument against the Tilburg Frailty Indicator. Eur Geriatr Med. 2013;4:248–254. [Google Scholar]
- 3.Gobbens RJJ, Luijkx KG, Wijnen-Sponselee MT, Schols JMGA. Towards an integral conceptual model of frailty. J Nutr Health Aging. 2010;14:175–181. doi: 10.1007/s12603-010-0045-6. [DOI] [PubMed] [Google Scholar]
- 4.Woo J, Goggins W, Sham A, Ho SC. Social determinants of frailty. Gerontology. 2005;51:402–408. doi: 10.1159/000088705. [DOI] [PubMed] [Google Scholar]
- 5.Bouillon K, Kivimaki M, Hamer M, et al. Measures of frailty in population-based studies: an overview. BMC Geriatrics. 2013;21:13, 64. doi: 10.1186/1471-2318-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erusalimsky JD, Grillari J, Grune T, et al. FRAILOMIC Consortium In search of ‘omics’-based biomarkers to predict risk of frailty and its consequences in older individuals: The FRAILOMIC Initiative. Gerontology. 2016;62:182–190. doi: 10.1159/000435853. [DOI] [PubMed] [Google Scholar]
- 7.Sternberg SA, Wershof Schwartz A, Karunananthan S, et al. The identification of frailty: a systematic literature review. J Am Geriatr Soc. 2011;59:2129–2138. doi: 10.1111/j.1532-5415.2011.03597.x. [DOI] [PubMed] [Google Scholar]
- 8.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Bio Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 9.Mitnitski A, Collerton J, Martin-Ruiz C, et al. Age-related frailty and its association with biological markers of ageing. BMC Med. 2015;13:161. doi: 10.1186/s12916-015-0400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Bio Sci Med Sci. 2007;62:722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 11.Rockwood K, Hogan DB, MacKnight C. Conceptualisation and measurement of frailty in elderly people. Drugs Aging. 2000;17:295–302. doi: 10.2165/00002512-200017040-00005. [DOI] [PubMed] [Google Scholar]
- 12.Searle D, Mitnitski A, Gahbauer EA, et al. A standard procedure for creating a frailty index. BMC Geriatrics. 2008;8:24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collerton J, Martin-Ruiz C, Davies K, et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85+ Study. Mech Ageing Dev. 2012;133:456–466. doi: 10.1016/j.mad.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Howlett SE, Rockwood MRH, Mitnitski A, et al. Standard laboratory tests to identify older adults at increased risk of death. BMC Med. 2014;12:171. doi: 10.1186/s12916-014-0171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howlett SE, Rockwood K. New horizons in frailty: ageing and the deficit-scaling problem. Age Ageing. 2013;4:416–423. doi: 10.1093/ageing/aft059. [DOI] [PubMed] [Google Scholar]
- 16.Mello AdeC, Engstrom EM, Alves LC. Health-related and socio-demographic factors associated with frailty in the elderly: a systematic literature review. Cadernos de Saúde Pública. 2014;30:1143–1168. doi: 10.1590/0102-311x00148213. [DOI] [PubMed] [Google Scholar]
- 17.Bandeen-Roche K, Sep CL, Huang J, et al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70:1427–1434. doi: 10.1093/gerona/glv133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rockwood K, Howlett SE, MacKnight C, et al. Prevalence attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian Study of Health and Aging. J Gerontol A Bio Sci Med Sci. 2004;59:1310–1317. doi: 10.1093/gerona/59.12.1310. [DOI] [PubMed] [Google Scholar]
- 19.Shor E, Roelfs DJ. Social contact frequency and all-cause mortality: A meta-analysis and meta-regression. Soc Sci Med. 2015;128:76–86. doi: 10.1016/j.socscimed.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Southwick SM, Sippel L, Krystal J, et al. Why are some individuals more resilient than others: the role of social support. World Psychiatry. 2016;15:77–79. doi: 10.1002/wps.20282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Kempen JAL, Schers HJ, Melis RJF, et al. Construct validity and reliability of a two-step tool for the identification of frail older people in primary care. J Clin Epidemiol. 2014;67:176–183. doi: 10.1016/j.jclinepi.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Blazer DG, Burchett B, Service C, et al. The association of age and depression among the elderly: an epidemiologic exploration. J Gerontol A Bio Sci Med Sci. 1991;46:M210–M215. doi: 10.1093/geronj/46.6.m210. [DOI] [PubMed] [Google Scholar]
- 23.Katz S, Downs T, Cash H, et al. Progress in the development of an index of ADL. Gerontologist. 1970;10:20–27. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 24.Doody MM, Hayes HM, Bilgrad R. Comparability of National Death Index Plus and standard procedures for determining cause of death in epidemiologic studies. Ann Epidemiol. 2001;11:46–50. doi: 10.1016/s1047-2797(00)00177-0. [DOI] [PubMed] [Google Scholar]
- 25.Pearlin L, Menaghan EG, Lieberman MA, Mullan JT. The stress process. J Health Soc Behav. 1981;22:337–356. [PubMed] [Google Scholar]
- 26.Wood V, Wylie ML, Sheafor B. An analysis of a short self-report measure of life satisfaction: correlation with rater judgments. J Gerontol. 1969;24:465–469. doi: 10.1093/geronj/24.4.465. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg M. The dissonant religious context and emotional disturbance. Am J Sociol. 1962;68:1–10. [Google Scholar]
- 28.Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 29.Sampson RJ, Raudenbush SW, Earls F. Neighborhoods and violent crime: a multilevel study of collective efficacy. Science. 1997;277:918–924. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- 30.Purser JL, Kuchibhatla MN, Miranda ML, et al. Geographical segregation and chronic inflammation in older adults: effects on interleukin-6. Biomedical Journal. 2008;2:335–348. doi: 10.2217/17520363.2.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: A meta-analytic review. PLoS Medicine. 2010;7:7 e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mendes de Leon CF, Glass TA, Beckett LA, et al. Social networks and disability transitions across eight intervals of yearly data in the New Haven EPESE. J Gerontol B Psych Sci Soc Sci. 1999;54:S162–172. doi: 10.1093/geronb/54b.3.s162. [DOI] [PubMed] [Google Scholar]
- 33.Dohrenwend BS, Dohrenwend BP. Stressful life events and their contexts. New York: Prodist; 1981. [Google Scholar]
- 34.King KE, Morenoff JD, House JS. Neighborhood context and social disparities in cumulative biological risk factors. Psychosom Med. 2011;73:572–579. doi: 10.1097/PSY.0b013e318227b062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lang IA, Hubbard RE, Andrew MK, et al. Neighborhood deprivation, individual socioeconomic status, and frailty in older adults. J Am Geriatr Soc. 2009;57:1776–1780. doi: 10.1111/j.1532-5415.2009.02480.x. [DOI] [PubMed] [Google Scholar]
- 36.Armstrong JJ, Andrew MK, Mitnitski A, et al. Social vulnerability and survival across levels of frailty in the Honolulu-Asia Aging Study. Age Ageing. 2015;44:709–712. doi: 10.1093/ageing/afv016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marshall A, Nazroo J, Tampubolon G, et al. Cohort differences in the levels and trajectories of frailty among older people in England. J Epidemiol Community Health. 2015;69:316–321. doi: 10.1136/jech-2014-204655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glass TA, Balfour JL. Neighborhood aging and functional limitations In Kawachi I, Berkman LF eds Neighborhoods and health. Oxford: Oxford University Press; 2003. [Google Scholar]
- 39.Hybels CF, Blazer DG, Pieper CF, et al. Sociodemographic characteristics of the neighborhood and depressive symptoms in older adults: using multilevel modeling in geriatric psychiatry. Am J Geriatr Psychiat. 2006;14:498–506. doi: 10.1097/01.JGP.0000194649.49784.29. [DOI] [PubMed] [Google Scholar]
- 40.Marcus AF, Echeverria SE, Holland BK, et al. The joint contribution of neighborhood poverty and social integration to mortality risk in the United States. Ann Epidemiol. 2016;26:261–266. doi: 10.1016/j.annepidem.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Mõttus R, Gale CR, Starr JM, et al. ‘On the street where you live’: Neighbourhood deprivation and quality of life among community-dwelling older people in Edinburgh, Scotland. Soc Sci Med. 2012;74:1368–1374. doi: 10.1016/j.socscimed.2011.12.050. [DOI] [PubMed] [Google Scholar]
- 42.Oakes JM. Commentary: Advancing neighbourhood-effects research–selection, inferential support, and structural confounding. Int J Epidemiol. 2006;35:643–647. doi: 10.1093/ije/dyl054. [DOI] [PubMed] [Google Scholar]
- 43.Li Q, Wang S, Milot E, et al. Homeostatic dysregulation proceeds in parallel in multiple physiological systems. Aging Cell. 2015;14:1103–1112. doi: 10.1111/acel.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material S1. Blood drawing procedures and laboratory analyses
Supplementary Table S1. Descriptive characteristics of the Duke EPESE sample for current study
Supplementary Table S2. Association of personality characteristics, social resources, and social stressors with neighborhood disadvantage, adjusted for demographic characteristics (age, sex, race, education, income)
Supplementary Figure S1. Duke-FI distribution of scores
Supplementary Figure S2a. Duke-FI mean scores (95% CI) by age
Supplementary Figure S2b. Duke-FI mean scores (95% CI) by age by gender


