Abstract
Background/Objectives
There is a recognized role of dopaminergic systems in mobility among patients with Parkinson's disease, yet, this association is not well studied in community-dwelling aging adults. Catechol-O-methyltransferase (COMT) regulates dopamine availability, particularly in the pre-frontal cortex. The COMT Val158Met polymorphism leads to higher (Val/Val), lower (Met/Met), or intermediate (Met/Val) dopamine availability. The objectives were to determine the association between COMT genotype and six-meter walk time and determine if these associations were quadratic in nature, similar to previously reported U-shaped associations of dopamine with gait and cognition.
Design
Prospective cohort study.
Setting
Health, Aging and Body Composition Study.
Participants
Black (n=850) and white (n=1,352) men and women, 73.5±2.85 years of age at baseline.
Measurement
Mixed models assessed the association between the COMT genotype and six-meter walk time, cross-sectionally and longitudinally over ten years. Models were assessed unstratified, and stratified by race due to different allele distributions among white and black participants.
Results
There was a significant U-shaped association between COMT genotype and six-meter walk time: those with Val/Val and Met/Met genotypes slowed more over 10 years (0.22 sec per visit±0.02 and 0.23 sec per visit ±0.02, respectively), compared to those with the Met/Val genotype (0.20 sec per visit ±0.02, p=0.005). Stratified results showed a significant relationship in black participants (p=0.01), but not in white participants (p=0.15)
Conclusion
These findings indicate a role for dopaminergic regulation of gait speed in community-dwelling older adults and supports pre-frontal cortex involvement in gait performance. Future work should investigate the molecular integrity of dopaminergic networks and gait changes over time, and structural changes in the brain with COMT and gait decline among older adults.
Keywords: Aging, Catechol-O-methyltransferase, Dopamine, Mobility Disability, Physical Function
INTRODUCTION
Dopaminergic systems have been identified as a contributor to gait speed and mobility deficits in Parkinson's disease1, 2, but not among community-dwelling older adults free of neurologic disease. However, there is a documented relationship between higher dopamine and better attention and executive function3, 4, both related to mobility.5–7 Furthermore, dopamine release in the brain modulates connections to the pre-frontal cortex (PFC)8, which may enhance gait performance9, 10 due to its involvement in attention and executive control processes. Additionally, declines in dopamine and mobility are both associated with increased age and aging brain changes.11, 12 Some PET studies demonstrate associations between low striatal dopamine and increased slip perturbation and falls, worse balance and slower gait in small samples of older adults.13–16 One limitation is actual dopamine levels, especially within the brain, are not often available in epidemiological studies.
Catechol-O-methyltransferase (COMT) is an dopamine-regulating enzyme in the brain, particularly the PFC. The COMT val158met polymorphism determines levels of the COMT enzyme and consequently, dopamine levels within the brain.1 In the absence of actual dopamine levels, the COMT val158met polymorphism provides an excellent surrogate. The Met allele, associated with lower COMT, results in slower clearance and higher tonic dopamine levels, particularly in the PFC.1, 17 Conversely, the Val allele has faster clearance and lower tonic dopamine levels.1, 17 The val158met polymorphism has previously been associated with attention, executive function18–20, and gait speed in one cross-sectional study of non-demented older adults.21 Results indicated the Met/Val genotype, compared to the Met/Met genotype, was associated with faster gait speed, due to a U-shaped dopamine-gait association.21 A similarly shaped association was also reported between dopamine and cognition: those with intermediate levels of dopamine performed better than those with high or low levels.21–23 More studies are needed to investigate the longitudinal association between the COMT val158met polymorphism to see if the relationship persists in other populations and over time, and if the quadratic relationship remains.
The objectives of this study were to determine the association between the COMT genotype and six-m walk time, cross-sectionally and longitudinally, and determine if these associations were quadratic in nature. Another objective was to determine if associations were robust to adjustments for demographic and health-related factors that may affect the association between dopamine and gait changes.
METHODS
Study population
Community-dwelling white and black older adults were enrolled in the Health, Aging and Body Composition (Health ABC) prospective cohort study beginning in 1997. At baseline, 3,075 adults were 70 to 79 years old, and lived in Memphis, TN or Pittsburgh, PA. Participants were recruited from a random sample of Medicare-eligible adults within designated zip codes, and eligible if they reported no difficulties performing activities of daily living, walking a quarter mile, or climbing 10 steps without resting. Follow-up visits were conducted yearly, with more detailed performance based measures collected at visits 4, 6 and 10. This study was approved by institutional review boards of participating institutions. All participants provided written informed consent.
Analytic cohort
We defined our analytic sample as participants who had baseline six-meter walk time data, completed a six-meter walk in at least one other clinic visit, and had complete data on all covariates of interest (n=2,202 of the original 3,075). Participants were excluded due to missing COMT (n=220), baseline six-meter walk time (n=27), or a second measure of walk time or covariate of interest (n=626). All 2,202 participants contributed to all analyses; n=2,202 had baseline six-meter walk time data, n=1,786 had visit 4, n=1,578 had visit 6, and n=1,080 had visit 10.
COMT genotype
COMT was measured from genomic DNA extracted from EDTA anticoagulated whole blood by standard methods (Gentra Systems, Minneapolis, MN), and PCR-based COMT genotyping, as previously described.24
Six-meter walk time
Time to walk six-meters, a modification of the short walk used in many epidemiological studies, was assessed at Years 1, 4, 6 and 10. Participants were asked to walk a six-meters, on a marked, obstacle-free course in a long corridor, at least 122 cm wide, at usual pace. Participants started behind the start line, and were instructed to walk through the finish line at their usual pace. Time was measured from the first footfall over the starting line to the first footfall across the finish line, and measured to the nearest 0.01 second. Participants completed two trials at each visit. and the fastest time recorded.
Modified Physiologic Index
The Modified Physiologic Index (MPI) was designed to utilize several widely available components to assess a range of physiologic systems, including: vasculature, lungs, kidneys, brain and glucose metabolism, and act as one measure of multi-system comorbidity to predict mortality and disability beyond age.25 It has previously been associated with gait speed at baseline and over time26, and was chosen as a primary covariate to represent comorbidity. Contributing components included: blood pressure to assess vasculature; spirometry measuring forced vital capacity (FVC) to assess lungs; the Digit Symbol Substitution Test (DSST) to assess brain health; serum fasting glucose to assess glucose metabolism; and serum cystatin-C to assess kidney function.25 Details on the creation of the MPI were previously described.25 Briefly, it is a continuous measure, grouped into categories of: 0–2 (healthiest), 3–4, 5–6, and 7–10 (unhealthiest).25
Covariates
At baseline, demographic data including self-reported age, race, sex and education were recorded. European Ancestry admixture was measured in Health ABC for genetic analyses.27, 28 Admixture is approximated using STRUCTURE, and was previously described.29 Baseline height and weight were used to calculate body mass index (BMI, kg/m2). Knee extensor strength was measured concentrically at 60° per second using an isokinetic dynamometer (Kin-Com 125 AP Dynamometer, Harrison, TN). Quadriceps strength was calculated as the mean maximal torque (Nm) from the 3 best trials.30 Sex-specific median cutoffs (men: 132.27 Nm; women: 81.84 Nm) classified weak versus strong quadriceps strength.30 Self-reported knee pain in either knee on most days or for ≥1 month in the past year was recorded. Physical activity was estimated as kcal/kg/week spent walking and climbing stairs, using a questionnaire.31
Statistical analyses
An a priori decision to conduct all analyses unstratified, then stratified by race, was based on previous literature showing strong race-specific differences in the distribution of COMT24, evidenced by the distribution in our sample, and strong differences in six-meter walk time by race (t-test statistic = −27.27, p<0.001) and European ancestry (F-test statistic=11.81, p=0.0001). The decision to report unstratified results was based a previous cross-sectional study, so comparisons could be made more easily.21 Chi-square tests, analysis of variance tests (ANOVAs) and independent samples t-tests were used to examine the cross-sectional associations of demographic characteristics by COMT genotype at baseline. Pairwise comparisons, and overall tests were conducted, consistent with prior work.21
Mixed models assessed cross-sectional and longitudinal associations between COMT genotype and six-m walk time, and predicted estimated mean (standard error) six-meter walk time at baseline and over time, by COMT genotype. In unstratified analyses, Model 1 was adjusted for race and European ancestry, which were kept in all subsequent models, additionally adjusted for: the MPI (Model 2), age, sex and BMI (Model 3), knee pain and weak quadriceps strength (Model 4).Race-stratified analyses made similar adjustments, with the exception of race and European ancestry admixture as covariates.
COMT genotypes are presented and compared as predicted baseline mean (SE) six-meter walk times and annualized slopes of change. Model checks to ensure fit were rigorous and included assessing the interaction of all covariates with time, and all two-way interactions; only significant interactions were included in final models. The quadratic association between COMT and six-m walk time at baseline and over time was assessed with an interaction of COMT*COMT and COMT*COMT*Time. Linear and quadratic terms were included in all models, and p-values for each are reported. All analyses were conducted with SAS, version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Of the2,202 participants in this analytic sample,850 (38.6%) were black, 1,139 (51.7%) female, and average age at baseline was 73.5 years (±2.85). There were 422 (19.2%) with the Met/Met, 1,073 (48.7%) Met/Val, and 707 (32.1%) Val/Val genotype. Participants were followed for a maximum of 10 years, with an average of 7.85 years (±1.99) and 7.90 years (±2.08), for black and white participants, respectively. Participants excluded from analyses were more likely to be black (49.37% vs. 38.60%, p<0.001), and for those with available data were less likely to be in the healthiest MPI category (11.6% vs. 19.2%, p<0.05), but did not differ on other characteristics, including mobility disability at baseline. Participants in our analytic sample were less likely to have died during follow-up (38% versus 55%, p<0.0001). Participants who died were more likely to be black (52.4% versus 47.6%, p<0.0001).
Breakdown of COMT genotype by race is as follows: for black participants, 386 (45.4%) had the Val/Val, 380 (44.7%) the Met/Val, and 84 (9.9%) the Met/Met genotype; for white participants, 321 (23.7%) had the Val/Val, 693 (51.3%) the Met/Val, and 338 (25.0%) the Met/Met genotype. The genotype distribution was consistent with Hardy-Weinberg equilibrium in black (χ2 = 0.13, p=0.72) and white (χ2 = 0.45, p=0.50) participants.
Those who were homozygous for the Met allele were significantly more likely to be white, compared to those with a Val/Val genotype (80.1% vs. 45.4%, p<0.001) and compared to those with a Met/Val genotype (80.1% vs. 64.6%, p<0.001). Those with a Met/Met genotype also had a significantly lower BMI (27.2 vs. 28.0, p=0.01), , and were less likely to have a MPI score of 7–10, indicating better health status (13.3% vs. 20.9%, p=0.0002), when compared to those with a Val/Val genotype (Table 1). Those with a Met/Met genotype (5.0 sec ± 1.0) also had a significantly slower baseline six m walk time when compared to those with a Val/Val genotype (5.3 sec ± 1.2, p<0.0001) or a Met/Val genotype (5.2 sec ±1.0, p=0.02) in unstratified analyses (Table 1). There were no significant differences in sex, age, quadriceps strength or knee pain, by genotype. There was less heterogeneity by genotype for these characteristics when stratified by race (Table 1).
Table 1.
Baseline characteristics of 2,202 participants by COMT genotype, stratified by race.
| Characteristic N (%) or Mean (SD) |
Val/Val | Pairwise* p-value |
Met/Val | Pairwise* p-value |
Met/Met | Overall p-value |
|---|---|---|---|---|---|---|
| Unstratified (White and Black Older Adults)
| ||||||
| n=707 | n=1,073 | n=422 | ||||
| Black race | 386 (54.6%) | <0.001 | 380 (35.4%) | <0.001 | 84 (19.9%) | <0.001 |
| Female sex | 360 (50.9%) | 0.45 | 574 (53.5%) | 0.09 | 205 (49.6%) | 0.20 |
| Age | 73.7 (2.9) | 0.34 | 73.5 (2.8) | 0.88 | 73.5 (2.9) | 0.36 |
| BMI | 28.0 (4.8) | 0.01 | 27.0 (4.7) | 0.30 | 27.2 (4.3) | <0.001 |
| Weak quadriceps | 349 (49.4%) | 0.90 | 544 (50.7%) | 0.74 | 210 (49.8%) | 0.85 |
| Any knee pain | 228 (32.3%) | 0.94 | 339 (31.6%) | 0.74 | 137 (32.5%) | 0.93 |
| Six-m walk time (sec) | 5.3 (1.2) | <0.001 | 5.2 (1.0) | 0.02 | 5.0 (1.0) | <0.001 |
| MPI score 7–10 | 148 (20.9%) | 0.0002 | 135 (12.6%) | 0.74 | 56 (13.3%) | <0.001 |
|
| ||||||
| White Older Adults | ||||||
| n=321 | n=693 | n=338 | ||||
| Female sex | 138 (43.0%) | 0.37 | 352 (50.8%) | 0.19 | 157 (46.5%) | 0.06 |
| Age | 73.88 (2.80) | 0.24 | 73.56 (2.79) | 0.75 | 73.62 (2.91) | 0.24 |
| BMI | 26.62 (4.22) | 0.88 | 26.35 (4.27) | 0.23 | 26.66 (3.70) | 0.44 |
| Weak quadriceps | 179 (55.8%) | 0.38 | 380 (54.8%) | 0.46 | 177 (52.4%) | 0.65 |
| Any knee pain | 84 (26.2%) | 0.37 | 213 (30.7%) | 0.64 | 99 (29.3%) | 0.33 |
| Six-m walk time (sec) | 4.89 (0.84) | 0.96 | 4.97 (0.91) | 0.21 | 4.89 (3.70) | 0.29 |
| MPI score 7–10 | 30 (9.3%) | 0.90 | 48 (6.9%) | 0.14 | 30 (8.9%) | 0.13 |
| Black Older Adults | ||||||
| n=386 | n=380 | n=84 | ||||
| Female sex | 164 (42.5%) | 0.95 | 158 (41.6%) | 0.83 | 36 (42.9%) | 0.96 |
| Age | 73.48 (2.92) | 0.16 | 73.31 (2.83) | 0.35 | 72.99 (2.78) | 0.32 |
| BMI | 29.07 (4.93) | 0.56 | 28.06 (5.28) | 0.03 | 29.46 (5.82) | 0.01 |
| Weak quad strength | 170 (44.0%) | 0.43 | 164 (43.2%) | 0.52 | 33 (39.3%) | 0.73 |
| Any knee pain | 144 (37.3%) | 0.18 | 126 (33.2%) | 0.04 | 38 (45.2%) | 0.10 |
| Six-m walk time (sec) | 5.71 (1.24) | 0.63 | 5.57 (1.02) | 0.57 | 5.64 (1.05) | 0.23 |
| MPI score 7–10 | 27 (7.0%) | Ref | 31 (8.2%) | Ref | 11 (13.1%) | Ref |
Pairwise p-values calculated with the Met/Met group serving as the reference group.
At baseline, in mixed models, there was no significant relationship with COMT and six-m walk time in unstratified (Table 2), or stratified analyses (Table 3). Among the entire analytic sample, over ten years, those with a Val/Val genotype increased in time to walk six-m by an average of 0.22 sec per visit (±0.02), a Met/Val genotype increased by 0.20 sec per visit (±0.02), and a Met/Met genotype increased by 0.23 sec per visit (±0.02); this relationship was significant, quadratic in nature, and (p=0.005, Table 2) remained significant after adjustment for all covariates.
Table 2.
Unstratified, race- and European ancestry-adjusted* predicted mean (SE) six-meter walk time by COMT genotype among 2,202 white and black older adults.
| Mean Seconds (SE) | p-value for linear trend |
p-value for quadratic trend |
|||
|---|---|---|---|---|---|
| Val/Val | Met/Val | Met/Met | |||
| Model 1 | |||||
| Baseline | 5.11 (0.22) | 5.11 (0.22) | 5.09 (0.22) | 0.93 | 0.76 |
| Change per visit (slope) | 0.22 (0.02) | 0.20 (0.02) | 0.23 (0.02) | 0.02 | 0.005 |
| Model 2 | |||||
| Baseline | 4.95 (0.21) | 4.96 (0.21) | 4.93 (0.22) | 0.82 | 0.53 |
| Change per visit (slope) | 0.24 (0.03) | 0.22 (0.02) | 0.25 (0.03) | 0.04 | 0.01 |
| Model 3 | |||||
| Baseline | 4.98 (0.21) | 5.00 (0.21) | 4.97 (0.21) | 0.91 | 0.67 |
| Change per visit (slope) | 0.22 (0.02) | 0.21 (0.02) | 0.24 (0.02) | 0.06 | 0.02 |
| Model 4 | |||||
| Baseline | 4.86 (0.20) | 4.87 (0.20) | 4.85 (0.21) | 0.91 | 0.68 |
| Change per visit (slope) | 0.23 (0.02) | 0.22 (0.02) | 0.24 (0.02) | 0.07 | 0.02 |
All models are adjusted for European ancestry admixture and race, including the unadjusted model due to previous studies showing racial differences in distribution of COMT genotype. Model 1: adjusted only for race and European ancestry admixture; Model 2: adjusted for modified physiologic index, European ancestry admixture and race; Model 3: adjusted for European ancestry admixture, race, age, sex and body mass index (BMI); Model 4: adjusted for European ancestry admixture, race, age, sex, BMI, joint pain and weak quadriceps strength.
Table 3.
Predicted mean (SE) six-meter walk time by COMT genotype among 2,202 white and black older adults, stratified by race.
| Mean Seconds (SE) | p-value for linear trend |
p-value for quadratic trend |
|||
|---|---|---|---|---|---|
| Val/Val | Met/Val | Met/Met | |||
| White older adults (n=1,352) | |||||
| Model 1 | |||||
| Baseline | 4.87 (0.05) | 4.94 (0.03) | 4.89 (0.05) | 0.38 | 0.17 |
| Change per visit (slope) | 0.12 (0.01) | 0.12 (0.01) | 0.14 (0.01) | 0.20 | 0.15 |
| Model 2 | |||||
| Baseline | 4.94 (0.05) | 5.03 (0.04) | 4.95 (0.05) | 0.22 | 0.08 |
| Change per visit (slope) | 0.15 (0.02) | 0.14 (0.02) | 0.16 (0.02) | 0.25 | 0.17 |
| Model 3 | |||||
| Baseline | 4.70 (0.05) | 4.76 (0.04) | 4.71 (0.04) | 0.41 | 0.19 |
| Change per visit (slope) | 0.14 (0.01) | 0.14 (0.01) | 0.15 (0.01) | 0.31 | 0.29 |
| Model 4 | |||||
| Baseline | 4.55 (0.06) | 4.61 (0.05) | 4.57 (0.05) | 0.44 | 0.21 |
| Change per visit (slope) | 0.14 (0.01) | 0.14 (0.01) | 0.15 (0.01) | 0.29 | 0.24 |
| Black older adults (n=850) | |||||
| Model 1 | |||||
| Baseline | 5.68 (0.06) | 5.59 (0.06) | 5.65 (0.12) | 0.52 | 0.38 |
| Change per visit (slope) | 0.18 (0.01) | 0.14 (0.01) | 0.21 (0.03) | 0.02 | 0.01 |
| Model 2 | |||||
| Baseline | 5.60 (0.06) | 5.53 (0.06) | 5.61 (0.12) | 0.62 | 0.38 |
| Change per visit (slope) | 0.23 (0.02) | 0.19 (0.02) | 0.27 (0.03) | 0.05 | 0.01 |
| Model 3 | |||||
| Baseline | 5.45 (0.07) | 5.39 (0.07) | 5.44 (0.13) | 0.76 | 0.54 |
| Change per visit (slope) | 0.19 (0.01) | 0.16 (0.01) | 0.23 (0.03) | 0.04 | 0.01 |
| Model 4 | |||||
| Baseline | 5.26 (0.08) | 5.21 (0.08) | 5.26 (0.14) | 0.79 | 0.56 |
| Change per visit (slope) | 0.19 (0.01) | 0.16 (0.01) | 0.23 (0.03) | 0.10 | 0.03 |
Model 1: unadjusted; Model 2: adjusted for modified physiologic index; Model 3: adjusted for age, sex and body mass index (BMI); Model 4: adjusted for age, sex, BMI, joint pain and weak quadriceps strength.
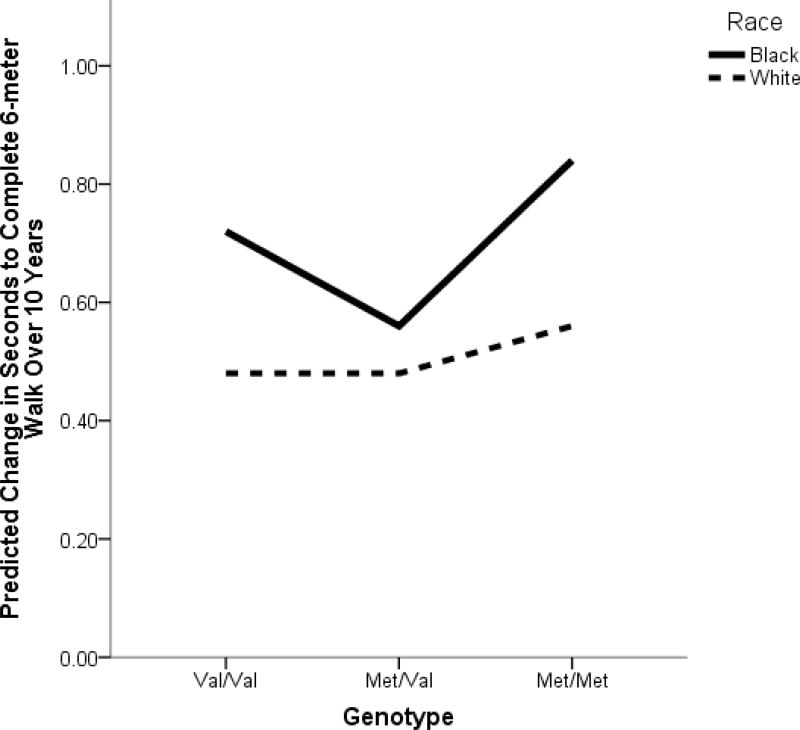
In stratified analyses, there was no significant relationship between COMT and six-m walk time over ten years among white older adults (Table 3). However, among black older adults, over ten years, those with a Val/Val genotype slowed by an average of 0.18 (±0.01) sec per visit, a Met/Val genotype slowed by 0.14 (±0.01) sec per visit, and a Met/Met genotype slowed by an average of 0.21 (±0.03) sec per visit. This relationship was statistically significant, quadratic in nature (p=0.01, Table 3, Figure 1), and remained statistically significant after adjustment for all covariates (Table 3).
Figure 1.
Change in seconds to complete the 6-meter walk over 10 years, for black and white participants, by COMT genotype.
DISCUSSION
To our knowledge, this is the first longitudinal study examining the association between COMT and gait speed in community-dwelling older adults. In both unstratified and race-stratified analyses, those with homozygous genotypes experienced a greater gait slowing speed over ten years, compared to those with a Met/Val genotype.
Our cross-sectional results differ slightly from a previous cross-sectional study reporting those with the Met/Val genotype were faster than those with the Met/Met genotype, but found no differences between the Val/Val and Met/Met genotypes.21 We found a significant cross-sectional association in univariate analyses, but it diminished after adjustment for race and European ancestry, which we believe explains the discrepancy, as the previous study did not report distribution of COMT by race/ethnicity, nor adjust their models for race/ethnicity.21 Participants in the prior study were also about five years older, on average, than participants in our study, so significant cross-sectional results may reflect longitudinal differences we see by genotype.
There are several potential mechanisms underlying an association between COMT and gait speed declines. COMT regulates dopamine levels primarily in the PFC, and affects attention and executive function3, 4 both directly related to mobility and gait speed.5–7 As dopamine levels decline with age, and dopamine release modulates connections to the PFC, less efficient PFC function may result in slower gait.9, 10 Related, genetic polymorphisms influencing dopamine, including COMT, can change functionality and plasticity in the dopaminergic system32–34, negatively impacting executive control 35–37, and could cause mobility declines. In this study, we see a stronger association among black participants, in spite of a larger sample size among whites . This could be due to black older adults being more vulnerable to gait declines over time.38 We believe vulnerability to gait speed declines over time also explains the lack of significant cross-sectional results; over ten years, all participants, who were relatively healthy at baseline, become more vulnerable to gait changes and thus, we see meaningful declines and differences.
We found a U-shaped association consistent with others showing homozygous groups are performing worse than heterozygotes21, 23. It is hypothesized the U-shaped association demonstrates an interaction with gene and environment, specifically, COMT and the aging brain, such that those who benefit from high dopamine in early age may not maintain these benefits as they age and vice versa. For example, Met carriers, shown to have increased executive function and working memory, especially in young adults20, 39, 40, have associated reduced gray matter PFC volume as older adults, related to poorer cognitive performance.41 This idea would be supported by an association between COMT and maintained gait speed among those with loss of structural integrity. Another potential explanation is dopaminergic systems have effects on two different cognitive processes: sustained attention on executively demanding tasks versus cognitive flexibility for transient tasks, which are each influenced differently by COMT.21, 40 This theory, summarized by the tonic phases dopamine hypothesis1, briefly states there are two ways dopamine is released: one in phasic, short bursts in response to an environmental, cognitive or motor task (i.e. cognitive flexibility tasks), and one in a consistent, low-level background manner (i.e. sustained attention tasks). The quadratic associations exemplify this theory because Val allele carriers have higher enzymatic activity, reducing the constant background levels of dopamine, and creating a low threshold for phasic dopamine bursts among COMT Val carriers, making carriers of this allele worse at sustained attention tasks, but better at cognitive flexibility tasks. Conversely Met allele carriers have higher background dopamine, due to lower enzymatic activity, and require higher phasic dopamine to exert an effect, thus performing better on sustained attention, but worse on cognitive flexibility tasks.21, 40 More work is needed to determine the underlying cause of this U-shaped relationship, and why it persists across multiple outcomes. While we don't expect a single gene variant to have a large effect on a complex behavior, such as gait speed, and our results reflect this, as the effect sizes are small, these findings do suggest the dopaminergic system, specifically COMT, is important for mobility in older ages.
This study had several strengths, including the measurement of six-meter walk time at fourpoints over ten years. We had a large sample size providing ample analytical power for this mixed model approach. Measurement of numerous comorbidities and demographic data allowed us to investigate a large number of covariates and confounders, including the MPI, previously established as a predictor of morbidity and functional disability in healthy older adults25. Finally, the differences found are believed to be small, but clinically meaningful based on previous research.42, 43 To explain, after converting the changes from six-m walk time to gait speed changes per year, the results indicate black participants with a Met/Met genotype slow by 0.04 m/sec more than those with the Met/Val genotype, and black participants with the Val/Val genotype slow by 0.02 m/sec more than those with the Met/Val genotype. While these differences are small, gait speed changes as small as 0.04 m/sec to 0.06 m/sec per year have previously been reported to acquire clinical significance.43 As expressed earlier, because we are only looking at one gene and how it predicts a multi-faceted process, these small effects are in line with what we would expect from a single gene and warrant further investigation of dopaminergic pathways on gait. There are also several weaknesses that should be considered when interpreting these results. Adults were all well-functioning and community-dwelling at baseline, represented persons who survived a minimum of three years post-baseline, and on average much longer, and were significantly less likely to die over ten years, so results may not be generalizable to all older adults – for example, nursing home populations. To investigate how missing data affected our results, we performed a sensitivity analysis to include all previously excluded participants who were missing health or demographic covariates, except for race and European ancestry, as we felt we had to keep those adjustments. Results and conclusions from this sensitivity analysis of 2,855 participants did not significantly change (data not shown). We were limited to examining only COMT, which regulates tonic dopamine levels in the brain, but examining PET imaging of molecular integrity of dopaminergic systems within the brain, would be a useful next step in this research.
We found a significant U-shaped association between COMT and gait speed over ten years. Those with homozygous COMT genotypes were more likely to experience gait decline over ten years, compared to those with a Met/Val genotype. Future studies should investigate potential underlying mechanisms of the association between COMT and gait speed decline, including PET imaging, and MRI studies to examine structural changes that are associated with COMT genotype and gait speed. Imaging studies may further elucidate the dynamic nature of the relationship, and help us gain a better understanding of the shape of this relationship and if it is due to dual functionality of dopamine - important for both sustained attention and cognitive flexibility. Replicating results in independent samples with genotyping arrays, and including more dopamine-related genes would be useful.
Acknowledgments
Funding Sources: This research was supported by National Institute on Aging (NIA) contracts #N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050; NINR grant R01-NR012459. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging.
Sponsor's Role: The sponsor's had no role in the study of manuscript writing.
Footnotes
Conflict of Interest: None.
Author Contributions: All the authors meet the criteria for authorship stated in Uniform Requirements for Manuscripts Submitted to Biomedical Journals
References
- 1.Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neurpsychiatric phenotypes. Neuropscyhopharmacology. 2004;29:1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- 2.Narayanan NS, Rodintzky RL, Uc EY. Prefrontal dopamine signaling and cognitive symptoms of Parkinson's disease. Rev Neurosci. 2013;24:267–278. doi: 10.1515/revneuro-2013-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol. 2002;67:53–83. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 4.Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci. 2001;21:3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desjardins-Crepeau L, Berryman N, Vu TT, et al. Physical functioning is associated with processing speed and executive functions in community-dwelling older adults. J Gerontol B Psychol Sci Soc Sci. 2014;69:837–844. doi: 10.1093/geronb/gbu036. [DOI] [PubMed] [Google Scholar]
- 6.Inzitari M, Newman AB, Yaffe K, et al. Gait speed predicts decline in attention and psychomotor speed in older adults: the health aging and body composition study. Neuroepidemiology. 2007;29:156–162. doi: 10.1159/000111577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosso AL, Studenski SA, Chen WG, et al. Aging, the central nervous system, and mobility. J Gerontol A Biol Sci Med Sci. 2013;68:1379–1386. doi: 10.1093/gerona/glt089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 9.Wrightson JG, Twomey R, Ross EZ, Smeeton NJ. The effect of transcranial direct current stimulation on task processing and prioritisation during dual-task gait. Exp Brain Res. 2015;233:1575–1583. doi: 10.1007/s00221-015-4232-x. [DOI] [PubMed] [Google Scholar]
- 10.Zhou J, Hao Y, Wang Y, et al. Transcranial direct current stimulation reduces the cost of performing a cognitive task on gait and postural control. Eur J Neurosci. 2014;39:1343–1348. doi: 10.1111/ejn.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Backman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neuroscience and biobehavioral reviews. 2006;30:791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT, Jr, Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc. 2005;53:649–654. doi: 10.1111/j.1532-5415.2005.53214.x. [DOI] [PubMed] [Google Scholar]
- 13.Bohnen NI, Muller ML, Kuwabara H, Cham R, Constantine GM, Studenski SA. Age-associated striatal dopaminergic denervation and falls in community-dwelling subjects. J Rehabil Res Dev. 2009;46:1045–1052. doi: 10.1682/jrrd.2009.03.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cham R, Perera S, Studenski SA, Bohnen NI. Striatal dopamine denervation and sensory integration for balance in middle-aged and older adults. Gait Posture. 2007;26:516–525. doi: 10.1016/j.gaitpost.2006.11.204. [DOI] [PubMed] [Google Scholar]
- 15.Cham R, Perera S, Studenski SA, Bohnen NI. Age-related striatal dopaminergic denervation and severity of a slip perturbation. J Gerontol A Biol Sci Med Sci. 2011;66:980–985. doi: 10.1093/gerona/glr060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cham R, Studenski SA, Perera S, Bohnen NI. Striatal dopaminergic denervation and gait in healthy adults. Exp Brain Res. 2008;185:391–398. doi: 10.1007/s00221-007-1161-3. [DOI] [PubMed] [Google Scholar]
- 17.Blasi G, Mattay VS, Bertolino A, et al. Effect of the Catechol-O-Methyltransferase valsuperscript 1-sup-5-sup-8met genotype on attentional control. Journal of Neuroscience. 2005;25:5038–5045. doi: 10.1523/JNEUROSCI.0476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Frias CM, Annerbrink K, Westberg L, Eriksson E, Adolfsson R, Nilsson LG. Catechol O-methyltransferase Val158Met polymorphism is associated with cognitive performance in nondemented adults. J Cogn Neurosci. 2005;17:1018–1025. doi: 10.1162/0898929054475136. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg TE, Weinberger DR. Genes and the parsing of cognitive processes. Trends Cogn Sci. 2004;8:325–335. doi: 10.1016/j.tics.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Malhotra AK, Kestler LJ, Mazzanti C, Bates JA, Goldberg T, Goldman D. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am J Psychiatry. 2002;159:652–654. doi: 10.1176/appi.ajp.159.4.652. [DOI] [PubMed] [Google Scholar]
- 21.Holtzer R, Ozelius L, Xue X, Wang T, Lipton RB, Verghese J. Differential effects of COMT on gait and executive control in aging. Neurobiol Aging. 2010;31:523–538. doi: 10.1016/j.neurobiolaging.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li SC, Sikstrom S. Integrative neurocomputation perspectives on cognitive aging, neuromodulation, and representation. Neurosci Biobehav Rev. 2002;26:795–808. doi: 10.1016/s0149-7634(02)00066-0. [DOI] [PubMed] [Google Scholar]
- 23.Witte AV, Floel A. Effects of COMT polymorphisms on brain function and behavior in health and disease. Brain Res Bull. 2012;88:418–428. doi: 10.1016/j.brainresbull.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Palmatier MA, Kang AM, Kidd KK. Global variation in the frequencies of functionally different catechol-O-methyltransferase alleles. Biol Psychiatry. 1999;46:557–567. doi: 10.1016/s0006-3223(99)00098-0. [DOI] [PubMed] [Google Scholar]
- 25.Sanders JL, Boudreau RM, Penninx BW, et al. Association of a Modified Physiologic Index with mortality and incident disability: the Health, Aging, and Body Composition study. J Gerontol A Biol Sci Med Sci. 2012;67:1439–1446. doi: 10.1093/gerona/gls123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosso AL, Sanders JL, Arnold AM, et al. Multisystem physiologic impairments and changes in gait speed of older adults. J Gerontol A Biol Sci Med Sci. 2015;70:319–324. doi: 10.1093/gerona/glu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parra EJ, Marcini A, Akey J, et al. Estimating African American admixture proportions by use of population-specific alleles. Am J Hum Genet. 1998;63:1839–1851. doi: 10.1086/302148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reich D, Patterson N, Ramesh V, et al. Admixture mapping of an allele affecting interleukin 6 soluble receptor and interleukin 6 levels. Am J Hum Genet. 2007;80:716–726. doi: 10.1086/513206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newman AB, Haggerty CL, Goodpaster B, et al. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51:323–330. doi: 10.1046/j.1532-5415.2003.51105.x. [DOI] [PubMed] [Google Scholar]
- 31.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Bertolino A, Blasi G, Latorre V, et al. Additive effects of genetic variation in dopamine regulating genes on working memory cortical activity in human brain. J Neurosci. 2006;26:3918–3922. doi: 10.1523/JNEUROSCI.4975-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the DAT. Proc Natl Acad Sci U S A. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lachman HM, Papolos DF, Saito T, Yu Y-M, Szumlanski CL, Weinshilboum RM. Human COMT pharmacogenetics: Description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Savitz J, Solms M, Ramesar R. The molecular genetics of cognition: dopamine, COMT and BDNF. Genes Brain Behav. 2006;5:311–328. doi: 10.1111/j.1601-183X.2005.00163.x. [DOI] [PubMed] [Google Scholar]
- 36.Raz N, Rodrigue KM, Kennedy KM, Land S. Genetic and vascular modifiers of age-sensitive cognitive skills: effects of COMT, BDNF, ApoE, and hypertension. Neuropsychology. 2009;23:105–116. doi: 10.1037/a0013487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Witte AV, Floel A. Effects of COMT polymorphisms on brain function and behavior in health and disease. Brain Res Bull. 2012;88:418–428. doi: 10.1016/j.brainresbull.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 38.White DK, Neogi T, Nevitt MC, et al. Trajectories of gait speed predict mortality in well-functioning older adults: the Health, Aging and Body Composition study. J Gerontol A Biol Sci Med Sci. 2013;68:456–464. doi: 10.1093/gerona/gls197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egan MF, Goldberg TE, Kolachana BS, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindenberger U, Nagel IE, Chicherio C, Li SC, Heekeren HR, Backman L. Age-related decline in brain resources modulates genetic effects on cognitive functioning. Front Neurosci. 2008;2:234–244. doi: 10.3389/neuro.01.039.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu X, Mill J, Zhou K, Brookes K, Chen CK, Asherson P. Family-based association study between brain-derived neurotrophic factor gene polymorphisms and attention deficit hyperactivity disorder in UK and Taiwanese samples. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:83–86. doi: 10.1002/ajmg.b.30406. [DOI] [PubMed] [Google Scholar]
- 42.Brach JS, Perera S, Studenski S, Katz M, Hall C, Verghese J. Meaningful change in measures of gait variability in older adults. Gait Posture. 2010;31:175–179. doi: 10.1016/j.gaitpost.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]