Abstract
Background
We hypothesized that packed red blood cell (PRBC) transfusions from older donors would be associated with fewer nosocomial infections among trauma patients.
Methods
We performed a four-year retrospective analysis of 264 consecutive adult trauma patients who received ≥1 PRBC transfusion during admission. The capacity of donor age to predict nosocomial infection was assessed by logistic regression.
Results
Thirty-three percent of all patients developed a nosocomial infection. Donor age was significantly higher among patients with nosocomial infection (40.3 vs. 37.6 years, p = 0.035), and the incidence of infection was directly proportional to donor age. The association between donor age and infection was strongest among recipients age ≥60 years, and was significant on multivariate regression for this cohort (OR 1.07 (95% CI 1.01–1.13), p = 0.024).
Conclusions
Among trauma patients receiving PRBC transfusions, blood from older donors may be associated with increased risk for nosocomial infection.
Keywords: Trauma, transfusion, donor, recipient, age, nosocomial infection
Introduction
Blood loss and anemia are common among patients with traumatic injuries. Severely anemic trauma patients often receive packed red blood cell (PRBC) transfusions to restore hemoglobin levels and oxygen delivery capacity. Unfortunately, PRBC transfusion is also associated with immunomodulation and infectious complications.1–6 Previous studies have investigated the impact of PRBC storage duration and pre-storage leukoreduction on transfusion-related immunomodulation and post-transfusion morbidity and mortality, leading to the adoption of blood bank policies favoring short PRBC storage duration and universal pre-storage leukoreduction.1, 7, 8 However, the effects of blood donor age on post-transfusion morbidity and mortality remain unclear. Multicenter studies investigating relationships between donor age and mortality for diffuse patient populations have reported conflicting results,9, 10 and the effects of blood donor age on nosocomial infection have not been previously reported.
Several pre-clinical studies suggest that the age of a blood donor may affect the immunomodulatory effects of transfused blood. Animal studies have demonstrated that transfusion of aged mice with blood from young mouse donors has significant vascular, muscular, and neurologic effects.11–13 With advanced age, hematopoietic stem cells lose their full proliferative capacity, and their microenvironment is gradually replaced by fat.14 Total myeloid cell output is maintained throughout the aging process, whereas lymphocyte production decreases over time.15, 16 These phenomena may be inconsequential for donated blood that is subjected to pre-storage leukoreduction, which removes nearly all white blood cells, minimizing their physiologic impact and immunosuppressive potential. However, allogenic red blood cells themselves may suppress T-cell receptor expression by an arginase-dependent mechanism, and aging has been associated with decreased erythrocyte arginase production.17–20 Therefore, it is plausible that PRBCs donated from older subjects may be less immunosuppressive than blood from young donors, leading to fewer infections.
The purpose of this study was to assess the effects of PRBC donor age on nosocomial infections among trauma patients, and therefore whether blood donor age should be considered in blood bank policies regarding allocation of PRBC products. We hypothesized that blood from elderly donors would be associated with fewer infectious complications.
Methods
We performed a retrospective analysis of 264 consecutive adult trauma patients who received one or more PRBC transfusions at our level one trauma center from 6/1/2011 – 10/1/2015. Subjects were identified by searching our institutional research database for adult patients (age ≥ 18 years) who received at least one PRBC transfusion. Patients were excluded if they had burn injuries, were transferred from an outside facility, underwent massive transfusion (≥ 10U PRBC within 24 hours), had unmeasured blood loss unrelated to their injury (e.g. postoperative or gastrointestinal bleeding), or death within 48 hours.
Universal pre-storage leukoreduction was performed at our institution for the duration of the study period. Storage duration and donor age were determined for each PRBC unit transfused to each patient in the study population. These variables were collected in cooperation with LifeSouth Community Blood Centers, the private institution that supplied our blood products during the study period. All other data was collected from our institutional research database and by retrospective review of the electronic medical record. Hemorrhagic shock was defined as systolic blood pressure < 90 mmHg or lactic acid ≥ 4 mmol/L on admission. Nosocomial infections were assessed from 48 hours after admission to 30 days after discharge. Six percent of all patients had no post-discharge follow-up, and another six percent had follow-up within 30 days but not beyond 30 days. Urinary tract infection (UTI) was defined as a urine culture with ≥105 pathogenic colony forming units/mL. Pneumonia was defined as a quantitative bronchoalveolar lavage culture with ≥ 104 pathogenic colony forming units/mL or a clinical diagnosis of pneumonia for a non-intubated patient. Bloodstream infection was defined as ≥ 2/4 bottles positive for likely contaminants (Staphylococcus epidermidis, Propionibacterium acnes, Bacillus species, and Corynebacterium species) or ≥ 1/4 bottles positive for all other organisms. Deep and organ/space surgical site infection (SSI) was defined according to CDC criteria.21 Superficial SSIs were not considered due to variability in culture availability and reporting practices.
Statistical analysis was performed with SPSS v23 (IBM, Armonk, NY). Characteristics of the study population were reported as mean (95% confidence interval) or n (%). The difference in blood donor age between patients with and without nosocomial infection was assessed by one-way analysis of variance. Correlations were assessed by Pearson’s r. The effects of donor age on nosocomial infection were analyzed by Fisher’s Exact test and illustrated in a figure created in GraphPad Prism (v6.05, GraphPad Software, La Jolla, CA). Predictors of nosocomial infection were identified on univariate and multivariate logistic regression. Factors were selected for inclusion in the multivariate model if they were predictive of nosocomial infection on univariate analysis and were not collinear to other variables in the model (|r| < 0.20 and p > 0.05). Confidence intervals were set at 95% and significance was set at α = 0.05.
Results
Patient characteristics, management, and outcome parameters are listed in Table 1. The predominant phenotype was a middle aged patient who sustained moderate-severe blunt injury (age 48 years, 89% blunt trauma, Injury Severity Score 25). On average, patients underwent three operations and received five units of PRBCs. The average PRBC storage duration was 21 days; average PRBC donor age was 38 years. One in three patients developed a nosocomial infection, and 9% had multiple infections. Patients with a nosocomial infection had significantly longer ICU length of stay (15.0 (12.7–17.7) vs. 7.4 (6.3–8.6) days, p < 0.001). Inpatient and 180-day mortality were 8% and 11%, respectively. One in ten patients had an unplanned readmission within 30 days, and approximately half of these were related to infectious complications.
Table 1.
Patient characteristics, management, and outcomes.
| Characteristics, management, and outcomes | n = 264 |
|---|---|
| Age (years) | 48 (46–51) |
| Male | 169 (64%) |
| Charlson comorbidity index | 0.6 (0.5–0.8) |
| Penetrating trauma | 29 (11%) |
| Injury Severity Score | 25 (23–26) |
| On admission | |
| Heart rate | 100 (98–103) |
| Systolic blood pressure (mmHg) | 124 (120–128) |
| pH | 7.29 (7.27–7.30) |
| Lactic acid (mmol/L) | 2.9 (2.7–3.2) |
| Hemoglobin (g/dL) | 11.2 (10.9–11.4) |
| Hemorrhagic shock# | 59 (22%) |
| Number of operations during admission | 3.1 (2.8–3.4) |
| Total PRBC transfusions during admission | 5.3 (4.9–5.8) |
| Received a PRBC transfusion within 24h | 154 (58%) |
| PRBC transfusions within 24h | 2.4 (2.1–2.7) |
| Received a PRBC transfusion after 24h | 217 (82%) |
| PRBC transfusions after 24h | 3.0 (2.7–3.3) |
| PRBC storage duration (days) | 21 (20–22) |
| PRBC donor age (years) | 38 (37–40) |
| Patients who had a nosocomial infection | 86 (33%) |
| Urinary tract infection | 41 (16%) |
| Pneumonia | 47 (18%) |
| Bloodstream infection | 16 (6%) |
| Deep or organ/space SSI | 10 (4%) |
| Hospital length of stay (days) | 18 (17–20) |
| Intensive care unit length of stay (days) | 10 (9–12) |
| Inpatient mortality | 20 (8%) |
| Unplanned readmission within 30 days | 26 (10%) |
| Readmission with infection | 12 (5%) |
| Mortality within 180 days | 28 (11%) |
PRBC: packed red blood cell,
systolic blood pressure < 90 mmHg or lactic acid ≥ 4 mmol/L.
Data are presented as mean (95% confidence interval) or n (%).
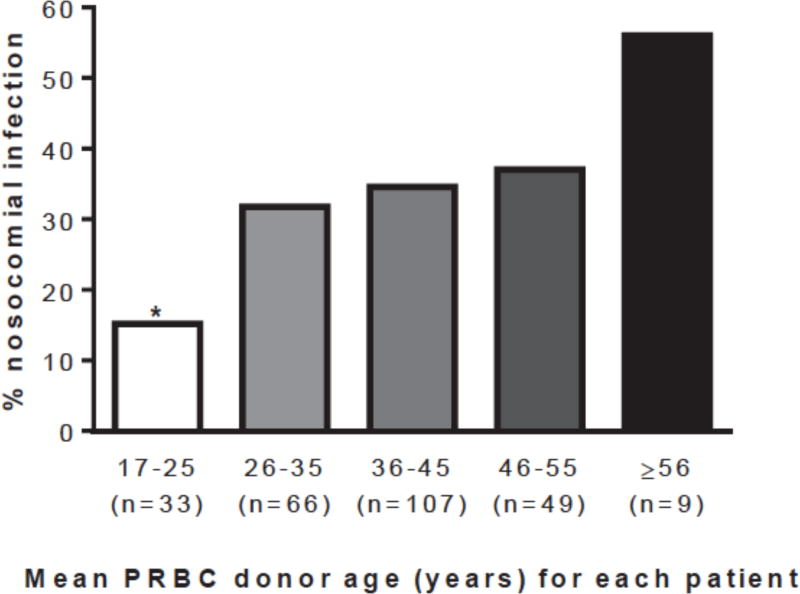
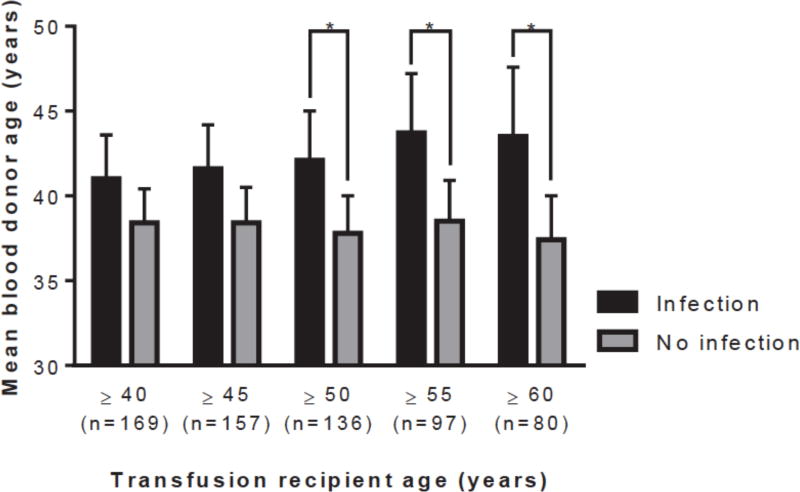
Univariate and multivariate predictors of nosocomial infection are listed in Table 2. Mean and maximum blood donor age were each associated with nosocomial infection. Maximum blood donor age was collinear with the total number of PRBCs transfused (r = 0.55, p < 0.001), whereas mean donor age was not (r = 0.10, p = 0.105); mean donor age was selected for further analysis. The incidence of nosocomial infection increased proportional to blood donor age (Figure 1). Mean donor age was significantly higher among patients who developed a nosocomial infection compared to those that did not (40.3 (38.4–42.2) years vs. 37.6 (36.1–39.1) years, p = 0.035). This association was strongest among older transfusion recipients (Figure 2).
Table 2.
Univariate and multivariate predictors of nosocomial infection.
| Factor | Univariate OR (95% CI) |
p | Multivariate OR (95 CI) |
p |
|---|---|---|---|---|
| Mean PRBC donor age | 1.03 (1.00–1.05) | *0.047 | 1.03 (1.00–1.05) | 0.074 |
| Minimum PRBC donor age | 1.01 (0.99–1.04) | 0.267 | ||
| Maximum PRBC donor age | 1.02 (1.00–1.03) | *0.035 | collinear to total transfusions | |
| Mean PRBC storage duration | 0.99 (0.96–1.03) | 0.693 | ||
| Minimum PRBC storage duration | 0.99 (0.96–1.01) | 0.317 | ||
| Maximum PRBC storage duration | 1.02 (0.99–1.04) | 0.171 | ||
| Total PRBCs transfused | 1.12 (1.04–1.19) | *0.001 | collinear to hemorrhagic shock | |
| within 24 hours | 1.06 (0.97–1.17) | 0.202 | ||
| after 24 hours | 1.18 (1.07–1.30) | *0.001 | 1.17 (1.06–1.29) | *0.003 |
| Injury Severity Score | 1.03 (1.01–1.06) | *0.004 | 1.03 (1.01–1.06) | *0.013 |
| Hemorrhagic shock# | 1.80 (1.09–3.13) | *0.023 | 2.12 (1.13–4.00) | *0.020 |
OR: odds ratio, CI: confidence interval, PRBC: packed red blood cell,
systolic blood pressure < 90 mmHg or lactic acid ≥ 4 mmol/L on admission
Figure 1.
The incidence of nosocomial infection increased in proportion to blood donor age (PRBC: packed red blood cell, *p = 0.028 vs. all other groups combined).
Figure 2.
The difference in blood donor age between recipients with infection versus no infection was greatest among older recipients (PRBC: packed red blood cell, *p < 0.022).
Mean PRBC donor age was entered into a multivariate logistic regression model with three other factors that were associated with nosocomial infection on univariate analysis: hemorrhagic shock, total number of PRBC units transfused > 24 hours after admission, and Injury Severity Score (Table 2). When controlling for these factors and assessing the entire study population, mean donor age was no longer statistically significant (p = 0.074).
Associations between blood donor age and nosocomial infection were stratified by age of the transfusion recipients (Table 3). With increasing age of the transfusion recipient, the correlation between blood donor age and nosocomial infection grew incrementally stronger. The same trend was observed on univariate logistic regression and on multivariate analysis while controlling for hemorrhagic shock, Injury Severity Score, and the number of red cell transfusions administered after 24 hours. Pearson’s correlation, univariate odds ratio, and multivariate odds ratio each became statistically significant at recipient age ≥ 50 and were strongest at age ≥ 60.
Table 3.
The relationship between blood transfusion donor age and nosocomial infection became stronger with increasing age of the patient who received the transfusion. Multivariate odds ratios (OR) were calculated while controlling for three variables associated with nosocomial infection: hemorrhagic shock, Injury Severity Score, and the number of red cell transfusions administered after 24 hours (CI: confidence interval).
| Recipient age (years) |
n | Pearson’s r |
p | Univariate OR (95% CI) |
p | Multivariate OR (95% CI) |
p |
|---|---|---|---|---|---|---|---|
| ≥ 40 | 169 | 0.113 | 0.145 | 1.02 (0.99–1.06) | 0.146 | 1.02 (0.98–1.05) | 0.342 |
| ≥ 45 | 157 | 0.147 | 0.067 | 1.03 (0.99–1.07) | 0.069 | 1.03 (0.99–1.07) | 0.107 |
| ≥ 50 | 136 | 0.190 | 0.027 | 1.04 (1.00–1.08) | 0.030 | 1.04 (1.00–1.08) | 0.049 |
| ≥ 55 | 97 | 0.238 | 0.019 | 1.06 (1.01–1.11) | 0.023 | 1.06 (1.01–1.11) | 0.027 |
| ≥ 60 | 80 | 0.278 | 0.012 | 1.07 (1.01–1.13) | 0.016 | 1.07 (1.01–1.13) | 0.024 |
Discussion
Our data indicate that PRBC transfusions from older donors may be associated with increased risk for nosocomial infections, especially among elderly transfusion recipients. Although the relationship between PRBC donor age and infection became non-significant when controlling for injury severity and transfusion burden, the effect was significant among trauma patients age ≥ 50 years. Although donor age may make a small contribution to transfusion-related morbidity and mortality, this contribution may be clinically significant on a larger scale when applied to blood bank allocation policies. Therefore, this phenomenon warrants further investigation, particularly among elderly transfusion recipients. The observed effect of blood donor age was directly opposed to our hypothesis. Therefore, the mechanisms implicated in generating our hypothesis (fewer immunosuppressive leukocyte products and lower arginase levels in blood from older donors) may have been interpreted and applied erroneously. Delineating the mechanism may be arduous; there are over 250 candidate RBC antigens that may be responsible for the immunosuppressive effects of erythrocytes.22, 23 Bernard et al.24 have demonstrated that leukoreduced RBC suppress T-cell proliferation independent of arginine depletion, and proposed direct cell-cell contact as a component of this poorly understood pathway.
PRBC storage duration was not associated with infectious complications. This may be attributable to universal performance of pre-storage leukoreduction during the study period. Previous work has shown that transfusion of PRBCs stored for more than 14 days increases infectious complications following severe injury.1 However, leukoreduction may abrogate these effects by removing nearly all white blood cells from the donated blood.7, 8 A randomized control trial has shown a non-significant trend toward decreased infectious complications for trauma patients receiving leukoreduced blood within 24 hours of injury.25 The storage duration of transfused PRBCs was similar between groups, and this study may have been underpowered to detect a clinically significant difference in infection rates.25 Widespread adoption of pre-storage leukoreduction and apparent lack of clinical equipoise based upon several additional advantages of leukoreduction (decreased febrile transfusion reactions, HLA alloimmunization among blood cancer patients, and transmission of leukocyte-borne viruses) undermine the feasibility of a large multicenter prospective trial.26–28 However, more recent retrospective and prospective observational data from trauma populations support the conclusion that pre-storage leukoreduction attenuates the effects of prolonged PRBC storage.29, 30
This study was limited by its retrospective design, proclivity to generate false positive results by making multiple comparisons, and small sample size (n = 264). The false-positive rate was limited as much as possible by making comparisons driven by our hypothesis and by controlling potential confounders on multivariate analysis. This study was performed at a single institution, limiting the generalizability of these findings. Our study population was severely ill, and morbidity and mortality were high. One likely explanation is that we selected a high-risk population by including patients who received a transfusion, therefore excluding most patients who had relatively little injury-related blood loss and ICU-related phlebotomy blood loss, and including patients who were at increased risk for transfusion-related morbidity and mortality. In addition, the presence of invasive catheters and intraoperative hypothermia are associated with increased risk for nosocomial infection, but were not described in this study. Future studies should investigate the relationship between PRBC donor age and nosocomial infection in a larger population, focusing on older transfusion recipients.
Conclusions
Among trauma patients receiving PRBC transfusions, blood from older donors may be associated with increased risk for nosocomial infection, especially among older transfusion recipients. The impact of PRBC donor age on immune function and infectious complications warrants further investigation in experimental and clinical settings. Better understanding of the relationships among blood donor age, transfusion-related immunomodulation, and post-transfusion morbidity and mortality may allow for the formation of more effective blood bank procurement and allocation policies.
Acknowledgments
This work was also supported in part by grants R01 GM113945-01 (PAE), R01 GM105893-01A1 (AMM), P50 GM111152-01 (SCB, FAM, PAE, AMM) awarded by the National Institute of General Medical Sciences (NIGMS). TJL was supported by a postgraduate training grant (T32 GM-08721) in burns, trauma and perioperative injury by NIGMS. Research reported in this publication was supported by the National Center for Advancing Translational Sciences under Award Number UL1TR001427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to disclose but do acknowledge the following grant funding.
References
- 1.Offner PJ, Moore EE, Biffl WL, et al. Increased rate of infection associated with transfusion of old blood after severe injury. Arch Surg. 2002;137(6):711–6. doi: 10.1001/archsurg.137.6.711. [DOI] [PubMed] [Google Scholar]
- 2.Opelz G, Vanrenterghem Y, Kirste G, et al. Prospective evaluation of pretransplant blood transfusions in cadaver kidney recipients. Transplantation. 1997;63(7):964–7. doi: 10.1097/00007890-199704150-00010. [DOI] [PubMed] [Google Scholar]
- 3.Bordin JO, Blajchman MA. Immunosuppressive effects of allogeneic blood transfusions: implications for the patient with a malignancy. Hematol Oncol Clin North Am. 1995;9(1):205–18. [PubMed] [Google Scholar]
- 4.Jensen LS, Hokland M, Nielsen HJ. A randomized controlled study of the effect of bedside leucocyte depletion on the immunosuppressive effect of whole blood transfusion in patients undergoing elective colorectal surgery. Br J Surg. 1996;83(7):973–7. doi: 10.1002/bjs.1800830727. [DOI] [PubMed] [Google Scholar]
- 5.Vamvakas EC, Moore SB. Blood transfusion and postoperative septic complications. Transfusion. 1994;34(8):714–27. doi: 10.1046/j.1537-2995.1994.34894353470.x. [DOI] [PubMed] [Google Scholar]
- 6.Chang H, Hall GA, Geerts WH, et al. Allogeneic red blood cell transfusion is an independent risk factor for the development of postoperative bacterial infection. Vox Sang. 2000;78(1):13–8. doi: 10.1159/000031143. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen HJ, Reimert CM, Pedersen AN, et al. Time-dependent, spontaneous release of white cell- and platelet-derived bioactive substances from stored human blood. Transfusion. 1996;36(11–12):960–5. doi: 10.1046/j.1537-2995.1996.36111297091738.x. [DOI] [PubMed] [Google Scholar]
- 8.Bordin JO, Heddle NM, Blajchman MA. Biologic effects of leukocytes present in transfused cellular blood products. Blood. 1994 Sep 15;84(6):1703–21. [PubMed] [Google Scholar]
- 9.Chasse M, Tinmouth A, English SW, et al. Association of Blood Donor Age and Sex With Recipient Survival After Red Blood Cell Transfusion. JAMA Intern Med. 2016;176:1407–14. doi: 10.1001/jamainternmed.2016.3324. [DOI] [PubMed] [Google Scholar]
- 10.Vasan SK, Chiesa F, Rostgaard K, et al. Lack of association between blood donor age and survival of transfused patients. Blood. 2016;127(5):658–61. doi: 10.1182/blood-2015-11-683862. [DOI] [PubMed] [Google Scholar]
- 11.Villeda SA, Plambeck KE, Middeldorp J, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20(6):659–63. doi: 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katsimpardi L, Litterman NK, Schein PA, et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344(6184):630–4. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinha M, Jang YC, Oh J, et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344(6184):649–52. doi: 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pritz T, Weinberger B, Grubeck-Loebenstein B. The aging bone marrow and its impact on immune responses in old age. Immunol Lett. 2014;162(1 Pt B):310–5. doi: 10.1016/j.imlet.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 15.Geiger H, de Haan G, Florian MC. The ageing haematopoietic stem cell compartment. Nat Rev Immunol. 2013;13(5):376–89. doi: 10.1038/nri3433. [DOI] [PubMed] [Google Scholar]
- 16.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5(2):133–9. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 17.Munder M, Schneider H, Luckner C, et al. Suppression of T-cell functions by human granulocyte arginase. Blood. 2006;108(5):1627–34. doi: 10.1182/blood-2006-11-010389. [DOI] [PubMed] [Google Scholar]
- 18.Makarenkova VP, Bansal V, Matta BM, et al. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J Immunol. 2006;176(4):2085–94. doi: 10.4049/jimmunol.176.4.2085. [DOI] [PubMed] [Google Scholar]
- 19.Bernard A, Meier C, Lopez N, et al. Packed red blood cell-associated arginine depletion is mediated by arginase. J Trauma. 2007;63(5):1108–12. doi: 10.1097/TA.0b013e31814b2b17. [DOI] [PubMed] [Google Scholar]
- 20.Smallwood HS, Lopez-Ferrer D, Squier TC. Aging enhances the production of reactive oxygen species and bactericidal activity in peritoneal macrophages by upregulating classical activation pathways. Biochemistry. 2011;50(45):9911–22. doi: 10.1021/bi2011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CDC Surgical Site Infection (SSI) Event. Available at: http://www.cdc.gov/nhsn/PDFs/pscManual/9pscSSIcurrent.pdf.
- 22.Reid ME, Mohandas N. Red blood cell blood group antigens: structure and function. Semin Hematol. 2004;41(2):93–117. doi: 10.1053/j.seminhematol.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Garratty G, Dzik W, Issitt PD, et al. Terminology for blood group antigens and genes-historical origins and guidelines in the new millennium. Transfusion. 2000;40(4):477–89. doi: 10.1046/j.1537-2995.2000.40040477.x. [DOI] [PubMed] [Google Scholar]
- 24.Bernard A, Meier C, Ward M, et al. Packed red blood cells suppress T-cell proliferation through a process involving cell-cell contact. J Trauma. 2010;69(2):320–9. doi: 10.1097/TA.0b013e3181e401f0. [DOI] [PubMed] [Google Scholar]
- 25.Nathens AB, Nester TA, Rubenfeld GD, et al. The effects of leukoreduced blood transfusion on infection risk following injury: a randomized controlled trial. Shock. 2006;26(4):342–7. doi: 10.1097/01.shk.0000228171.32587.a1. [DOI] [PubMed] [Google Scholar]
- 26.Paglino JC, Pomper GJ, Fisch GS, et al. Reduction of febrile but not allergic reactions to RBCs and platelets after conversion to universal prestorage leukoreduction. Transfusion. 2004;44(1):16–24. doi: 10.1046/j.0041-1132.2004.00608.x. [DOI] [PubMed] [Google Scholar]
- 27.Vamvakas EC. Is white blood cell reduction equivalent to antibody screening in preventing transmission of cytomegalovirus by transfusion? A review of the literature and meta-analysis. Transfus Med Rev. 2005;19(3):181–99. doi: 10.1016/j.tmrv.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Trial T. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. The Trial to Reduce Alloimmunization to Platelets Study Group. N Engl J Med. 1997;337(26):1861–9. doi: 10.1056/NEJM199712253372601. [DOI] [PubMed] [Google Scholar]
- 29.Phelan HA, Eastman AL, Aldy K, et al. Prestorage leukoreduction abrogates the detrimental effect of aging on packed red cells transfused after trauma: a prospective cohort study. Am J Surg. 2012;203(2):198–204. doi: 10.1016/j.amjsurg.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phelan HA, Gonzalez RP, Patel HD, et al. Prestorage Leukoreduction Ameliorates the Effects of Aging on Banked Blood. J Trauma. 2010;69(2):330–5. doi: 10.1097/TA.0b013e3181e0b253. [DOI] [PMC free article] [PubMed] [Google Scholar]