Abstract
Introduction
The modified frailty index (mFI) is an important method to risk-stratify surgical patients and has been validated for general surgery and selected surgical subspecialties. However, there are currently no data assessing the efficacy of the mFI to predict acute morbidity and mortality in patients undergoing surgery for retroperitoneal sarcoma (RPS).
Methods
Using the American College of Surgeons’ National Surgical Quality Improvement Program from 2007 to 2012, we performed a retrospective analysis of patients with a diagnosis of primary malignant retroperitoneal neoplasm who underwent surgical resection. The mFI was calculated according to standard published methods. Univariate and multivariate statistical analyses including χ2 and logistic regression were utilized to identify predictors of 30-day overall morbidity, 30-day severe morbidity (Clavien III/IV), and 30-day mortality.
Results
We identified 846 patients with the diagnosis of primary malignant retroperitoneal neoplasm who underwent surgical resection. The distribution mFI scores was 0 (48.5%) or 1 (36.3%), with only 4.5% of patients presenting with a score ≥3. Rates of 30-day overall morbidity, serious morbidity, and mortality were 22.6%, 12.9%, and 1.2%, respectively. Only selected mFI scores were associated with serious morbidity and overall morbidity on multivariate analysis (p<0.05), and mFI did not predict 30-day mortality (p>0.05).
Conclusion
Our data demonstrate that the majority of patients undergoing RPS resections have few, if any, comorbidities. The mFI was a limited predictor of overall and serious complications and was not a significant predictor of mortality. Better discriminators of preoperative risk stratification may be needed for this patient population.
Keywords: retroperitoneal sarcoma, morbidity, mortality, frailty
Introduction
Retroperitoneal sarcomas (RPS) are a rare group of heterogeneous malignancies which together comprise approximately 10–15% of all soft tissue sarcomas (STS) [1, 2]. Although there are published data on predictors of long term oncological outcome for RPS such as tumor histology, tumor grade, and extent of resection, there is currently no established way to risk stratify patients preoperatively who are candidates for RPS resection [3–6]. However, preoperative risk assessment is critical given the inherent risks of surgical intervention, especially for procedures which entail significant morbidity and mortality like RPS resection.
To date, frailty, defined as decreased physiological reserve and increased vulnerability to stressors has been helpful in predicting the risk of complications and mortality in surgical procedures for various malignancies and benign disease such as pancreatic, hepatic, and lung cancers, and vascular disease, paraesophageal hernia repairs, and emergent intra-abdominal operations [7–14]. The modified frailty index (mFI) utilizes 11 variables from the American College of Surgeons National Surgical Quality Improvement Project (ACS-NSQIP) to quantify frailty [9, 12, 15]. Previously, the mFI score has been used to identify patients at risk for 30-day morbidity and mortality in multiple studies including patients undergoing lobectomies, pancreatic resections and gynecologic oncologic resections [7, 9, 10]. However, there are currently no published data on the use of the mFI to predict acute morbidity and mortality for patients undergoing RPS resection. The goal of this study was to analyze mFI as a predictor of the risk of morbidity and mortality in patients undergoing RPS resection.
Methods
We performed a retrospective review of patients undergoing surgical resection of RPS. We queried the ACS-NSQIP database from the years 2007 to 2012. Years beyond 2012 were not included as they were missing several key mFI variables including history of pneumonia, myocardial infarction (MI), percutaneous coronary intervention (PCI), cardiac surgery, angina, peripheral vascular disease (PVD), transient ischemic attack (TIA), cerebrovascular accident (CVA) and impaired sensorium.
Patients were selected who had (1) undergone a RPS resection based on Current Procedural Terminology (CPT) codes (49200, 49201, 49203, 49204, & 49205) and (2) a primary diagnosis of malignant retroperitoneal neoplasm based on International Classification of Disease, 9th Revision (ICD-9) codes (158.0, 158.8, 159.9 & 235.4). Patients with one or more missing components of the mFI were excluded (n=221). Overall, we identified 846 patients who met these specific inclusion and exclusion criteria for data analysis.
Our main predictor variable of interest was the modified frailty index (mFI). Per this scoring system, all of the patients were given frailty scores (1 point per condition) based on the following variables: impaired functional status (partially and totally dependent), history of CVA with deficits, history of CVA without deficits or TIA, impaired sensorium, history of PVD, history of chronic obstructive pulmonary disease (COPD) or pneumonia, history of PCI, cardiac surgery or angina, history of MI, history of congestive heart failure (CHF), history of hypertension (HTN) and history of diabetes mellitus (DM). Consistent with the study by Augustin et al., we did not divide the total score by 11, but kept a whole integer score for data analysis [7]. As few patients had a score of >3 (5 patients with a score of 4 and 1 patient with a score of 5), we combined patients who had a score of ≥ 3 into one category for data analysis.
We assessed patient demographics and operative characteristics including age, gender, ethnicity, body mass index (BMI), preoperative laboratory values (albumin and hematocrit), preoperative chemotherapy, and radiotherapy, and American Society of Anesthesiologist (ASA) physical status classification. Operative variables included wound classification, tumor size, and whether or not multivisceral resection was performed. Multivisceral resection was defined as concomitant adjacent organ resection during the same operation, including gastric, hepatic, pancreatic, small and large bowel, adrenal, renal, bladder, gallbladder and uterine resections based on relevant CPT codes [16].
Our primary outcome measures included 30-day serious morbidity, 30-day overall morbidity and 30-day mortality. Serious morbidity was defined as the occurrence ≥1 Clavien III/IV complication (i.e. any complication that required further invasive procedures, led to lasting disability or was life-threatening requiring intensive care unit (ICU) level of care). Examples of Clavien III/IV complications included organ space infection, fascial dehiscence, pulmonary embolism requiring ICU transfer, respiratory failure requiring reintubation, prolonged intubation, acute renal failure requiring dialysis, reoperation, graft or flap failure requiring further procedures, stroke, coma, myocardial infarction, cardiac arrest or systemic shock.[17, 18].
Overall morbidity was defined as sustaining any ACS-NSQIP complication including the following: superficial or deep wound infection, organ space infection, fascial dehiscence, pneumonia, reintubation, prolonged intubation, pulmonary embolism, progressive renal insufficiency, acute renal failure requiring dialysis, urinary tract infection, stroke, coma, peripheral nerve injury, cardiac arrest, myocardial infarction, graft/prosthesis/flap failure, deep vein thrombosis, reoperation, sepsis, and septic shock. 30-day mortality was defined as death before or at 30 days after the principal operation. Since all patient information was de-identified, we were exempt from the University of California, Davis Institutional Review Board approval.
Statistical Analysis
We first performed univariate analysis using Fisher’s exact test and chi-squared analysis for categorical variables, student’s independent t-test for normally distributed continuous variables (i.e. age and preoperative hematocrit) and the Wilcoxon rank-sum test for non-normally distributed continuous variables (i.e. BMI and albumin) to assess for potential predictors to be used in the multivariate analysis. Patient and operative characteristics with a p value ≤0.10 on univariate analyses were included in the multivariate model. Multivariate logistic regression was used to determine predictors of 30-day serious morbidity, 30-day overall morbidity and 30-day mortality. Patient and operative characteristics included in the multivariate model included: age, gender, wound classification, ASA classification, multivisceral resection, preoperative hematocrit, albumin and mFI. Multiple imputation was performed to address missing values for preoperative albumin (n=210, 24.8%) and hematocrit (n=26, 3.1%). Statistical significance was defined as a p value <0.05. All analyses were performed using IBM SPSS software (Version 22; IBM Corp, Armonk NY) and SAS version 9.4 (SAS Institute Inc).
Results
Patient Preoperative and Operative Characteristics
A total of 846 patients met criteria for undergoing RPS resections with complete data. Table 1 depicts the patient demographics and operative characteristics. The average age was 59 years (±14), 51.7% (n=437) of the patients were female, and 78.9% (n=667) were Caucasian. The average BMI was 28.7 (±6.9). The vast majority of the patients (97.4%, n=824) had independent functional status. The majority of the patients had retroperitoneal sarcomas that were >10 cm in size (n=442, 52.3%), and 43.4% (n=367) underwent a multivisceral resection.
Table 1.
Patient Demographics and Perioperative Characteristics
| Variable | Mean (±SD) or N (%) |
|---|---|
|
| |
| Age | 59 (±14) |
|
| |
| Female gender | 437 (51.7%) |
|
| |
| Ethnicity | |
| Caucasian | 667 (78.9%) |
| Black | 88 (10.4%) |
| Hispanic | 43 (5.2%) |
| Asian | 22 (2.6%) |
| American Indian/Alaskan Native | 3 (0.4%) |
| Unknown | 65 (7.7%) |
|
| |
| BMI | 28.7 (±6.9) |
| Underweight (BMI<18.5) | 22 (2.6%) |
| Obese (BMI≥30.0) | 288 (34.0%) |
|
| |
| Independent Functional Status | 824 (97.4%) |
|
| |
| Preoperative Chemotherapy (within 30 days) | 39 (4.6%) |
|
| |
| Preoperative Radiotherapy (within 90 days) | 64 (7.6%) |
|
| |
| Preoperative Labs | |
| Albumin (g/dL) | 3.8 (±0.7) |
| Hematocrit (%) | 37.7 (±5.2) |
|
| |
| ASA Classification | |
| ASA 1 | 19 (2.3%) |
| ASA 2 | 283 (33.5%) |
| ASA 3 | 512 (60.5%) |
| ASA 4 | 32 (3.8%) |
|
| |
| Wound Classification | |
| 1-Clean | 331 (39.1%) |
| 2-Clean/Contaminated | 481 (56.9%) |
| 3-Contaminated/Dirty | 24 (2.8%) |
| 4-Infected | 10 (1.2%) |
|
| |
| Modified Frailty Index Score | |
| 0 | 410 (48.5%) |
| 1 | 307 (36.3%) |
| 2 | 91 (10.8%) |
| ≥3 | 38 (4.5%) |
|
| |
| Tumor size | |
| ≥5.0 cm | 158 (18.7%) |
| 5.1 to 10.0 cm | 129 (15.3%) |
| >10.0 cm | 442 (52.3%) |
| Unknown | 117 (11.0%) |
|
| |
| Multivisceral Resection | 367 (43.4%) |
|
| |
| 30-day Overall Morbidity | 191 (22.6%) |
|
| |
| 30-day Serious Morbidity | 109 (12.9%) |
|
| |
| 30-day Mortality | 11 (1.2%) |
The preponderance of patients (48.5%, n=410) were not frail with a mFI of 0, and an additional 36.3% (n=307) of patients had an mFI score of 1. In contrast, only 10.8% (n=91) had an mFI score of 2, and 4.5 % (n=38) an mFI score of ≥3. Figure 1 depicts the distribution of the mFI components stratified by score. Hypertension and diabetes were the most common components of the frailty score for all patients.
Figure 1.
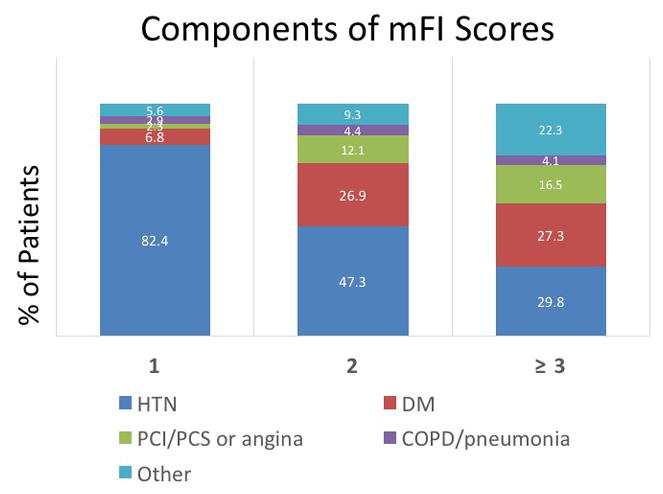
The prevalence of comorbidities by individual modified frailty index score.
30-Day Overall Morbidity, Serious Morbidity, and Mortality
Overall, the rate of 30-day morbidity was 22.6% (n=191), and rates of 30-day serious morbidity and mortality were 12.9% (n=109) and 1.2% (n=11), respectively. Univariate analysis of patient and operative characteristics showed that rates of 30-day overall morbidity were greater among males (26.0% vs. 19.5%, p=0.02), those with multivisceral resections (30.3% vs. 16.7%, p<0.0001), higher wound and ASA classification scores (p<0.0001 both), and lower mean hematocrit and albumin levels (p<0.05 both). Rates of 30-day morbidity were not significantly different for patients who had undergone preoperative chemotherapy (20.5% vs. 22.7%, p=0.85) or radiotherapy (21.9% vs. 22.5%, p=0.91).
Univariate analysis also showed that rates of 30-day serious morbidity were greater among males (17.9% vs. 8.2%, p<0.0001), those who underwent multivisceral resections (18.0% vs. 9.0%, p=0.0001), higher wound classification scores (p=0.01), and those who had lower preoperative albumin levels (p=0.04). Again, rates of 30-day serious morbidity were not significantly different among patients who underwent preoperative chemotherapy (7.7% vs. 13.1%, p=0.46) or radiotherapy (9.4% vs. 13.1%, p=0.56). With respect to 30-day mortality, univariate analysis demonstrated increased rates of 30-day mortality among males (2.2% vs 0.2%, p=0.0009), patients with impaired functional status (18.2% vs. 0.7%, p<0.0001), and lower mean hematocrit and albumin levels (p<0.01).
There were very few statistically significant differences in rates of our primary outcomes by mFI scores on our multivariate logistic regression model controlling for age, gender, wound and ASA classification and multivisceral resection (Table 2). Patients with an mFI score of 1 had a statistically significant increase rate of overall morbidity compared to non-frail patients (OR 1.56, 95%CI 1.05–2.32, p=0.03) shown in Figure 2A. Patients who had an mFI score of ≥3 had a statistically significant increased rate of serious complications compared to non-frail patients (OR 2.93, 95%CI 1.22–7.03, p=0.02) (Figure 2B). The modified frailty index was not a significant predictor of 30-day mortality on univariate and multivariate analysis (Figure 2C).
Table 2.
Multivariate analysis for Overall morbidity, Serious Morbidity and Mortality
| 30-Day Overall Morbidity OR (95%CI) |
P value | 30-Day Serious Morbidity OR (95%CI) |
P value | 30-Day Mortality OR (95%CI) |
P value | |
|---|---|---|---|---|---|---|
|
| ||||||
| Age | 0.99 (0.98–1.01) | 0.26 | 1.00 (0.98–1.01) | 0.62 | 1.01 (0.95–1.07) | 0.76 |
|
| ||||||
| Gender | 0.77 (0.54–1.09) | 0.14 | 0.44 (male) (0.28–0.69) | <0.001 | 0.11 (0.01–0.93) | 0.04 |
|
| ||||||
| Wound Classification | 1.46 (1.09–1.95) | 0.01 | 1.38 (0.97–1.97) | 0.08 | 1.21 (0.53–2.78) | 0.65 |
|
| ||||||
| ASA Classification | 1.50 (1.09–2.08) | 0.01 | 1.17 (0.78–1.75) | 0.44 | 3.14 (0.83–11.89) | 0.09 |
|
| ||||||
| Multivisceral Resection | 1.83 (1.29–2.60) | 0.001 | 1.86 (1.19–2.89) | 0.006 | 0.52 (0.13–2.02) | 0.34 |
|
| ||||||
| Preoperative Hematocrit (%) | 1.00 (0.96–1.05) | 0.90 | 0.99 (0.94–1.04) | 0.67 | 0.97 (0.84–1.13) | 0.72 |
|
| ||||||
| Preoperative Albumin (g/dL) | 0.78 (0.56–1.09) | 0.15 | 0.87 (0.58–1.31) | 0.50 | 0.40 (0.14–1.19) | 0.10 |
|
| ||||||
| mFI | ||||||
| 1 | 1.56 (1.05–2.32) | 0.03 | 1.50 (0.91–2.48) | 0.11 | 1.40 (0.29–6.73) | 0.67 |
| 2 | 1.42 (0.78–2.60) | 0.26 | 1.34 (0.62–2.91) | 0.46 | 0.56 (0.05–6.83) | 0.65 |
| 3 | 1.83 (0.83–4.04) | 0.14 | 2.93 (1.22–7.03) | 0.02 | 0.89 (0.07–11.29) | 0.93 |
All odds ratios are a comparison to nonfrail patients (modified frailty index = 0). Patients with mFI of 4 or 5 were analyzed with mFI ≥3 score given low prevalence of these patients.
CI: confidence interval; ASA: American Society of Anesthesiologists; mFI: modified Frailty Index.
Figure 2.
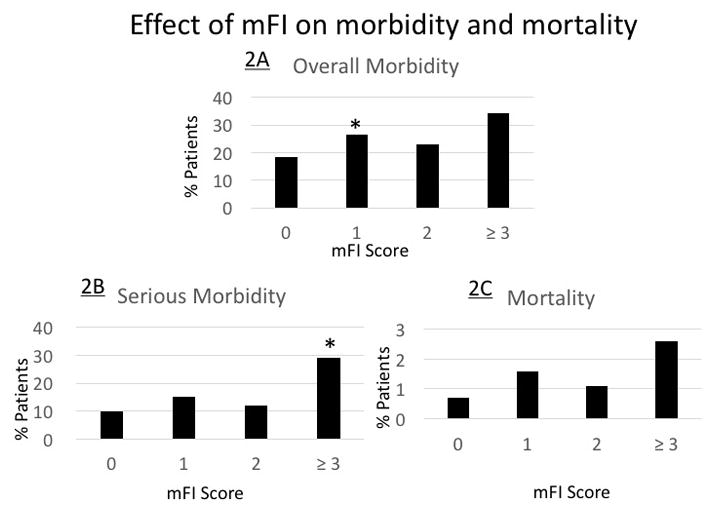
The effect of modified frailty index score on overall morbidity (2A), serious morbidity (2B), and 30-day mortality (2C)
* P <.05 is considered statistically significant.
Table 2 depicts the multivariate analysis for 30-day overall morbidity, serious morbidity and mortality. Significant predictors of 30-day overall morbidity included wound classification (OR 1.46, 95%CI 1.09–1.95, p=0.01), ASA classification (OR 1.50, 95%CI 1.09–2.08, p=0.01), multivisceral resection (OR 1.83, 95%CI 1.29–2.60, p=0.001) and a mFI score of 1. Significant predictors of 30-day serious morbidity were male gender (OR 0.44, 95%CI 0.28–0.69, p<0.001), multivisceral resection (1.86, 95%CI 1.19–2.89, p-=0.006) and a mFI score of ≥3 compared to nonfrail patients. Male gender was the only significant predictor of mortality (OR 0.11, 95%CI 0.01–0.93, p=0.04).
Table 3 depicts the multivariate analysis of the components of the mFI and the primary outcomes of this study. Hypertension was the only mFI component to be statistically significant for 30-day overall morbidity (OR 1.53, 95%CI 1.05–2.22, p=0.03) and serious morbidity (OR 1.60, 95%CI 1.00–2.56, p=0.049). Impaired functional status was the only statistically significant component of the mFI which was associated with an increased risk of mortality (OR 44.17, 95%CI 5.65–345.20, p<0.0001).
Table 3.
Multivariate analysis of the modified frailty index components and primary outcomes.
| 30-Day Overall Morbidity OR (95%CI) |
P value | 30-Day Serious Morbidity OR (95%CI) |
P value | Mortality OR (95%CI) |
P value | |
|---|---|---|---|---|---|---|
| Impaired Functional Status | 1.51 (0.58–3.96) | 0.40 | 1.26 (0.38–4.21) | 0.71 | 44.17 (5.65–345.20) | <0.0001 |
| Diabetes | 0.99 (0.58–1.69) | 0.98 | 0.93 (0.48–1.79) | 0.82 | OR N/A | 1.00 |
| Hypertension | 1.53 (1.05–2.22) | 0.03 | 1.60 (1.00–2.56) | 0.049 | 1.24 (0.26–5.79) | 0.79 |
| CHF | OR N/A | 1.00 | OR N/A | 1.00 | OR N/A | 1.00 |
| History of PCI, PCS, or Angina | 1.09 (.53–2.22) | 0.82 | 1.26 (0.56–2.84) | 0.57 | 0.30 (0.02–5.66) | 0.43 |
| COPD or Pneumonia | 1.32 (0.50–3.46) | 0.57 | 1.28 (0.39–4.18) | 0.68 | OR N/A | 1.00 |
| PVD or Rest pain | 1.13 (0.09–13.45) | 0.93 | 1.93 (0.14–26.83) | 0.63 | OR N/A | 1.00 |
| Impaired Sensorium | OR N/A | 1.00 | OR N/A | 1.00 | OR N/A | 1.00 |
| TIA or CVA without deficits | 0.95 (0.31–2.87) | 0.93 | 1.66 (0.49–5.64) | 0.42 | OR NA | 1.00 |
| CVA with deficits | 0.88 (0.22–3.47) | 0.85 | 1.74 (0.43–7.12) | 0.44 | OR N/A | 1.00 |
CHF: congestive heart failure; PCI: percutaneous cardiac intervention; PCS: previous cardiac surgery; COPD: chronic obstructive pulmonary disease; PVD: peripheral vascular disease. TIA: transient ischemic attack; CVA: cerebrovascular accident;
Model also consists of the following variables: age, gender, wound and ASA classification, multivisceral resection, albumin and hematocrit.
No patients had a myocardial infarction within 6 months of the principal operation.
Discussion
Retroperitoneal sarcomas are rare sarcomas that account for about 10–15% of all soft tissue sarcomas and have an annual incidence of 0.3 to 0.4 new cases per 100,000 people, which has been a stable incidence rate for the past 30 years [19, 20]. Although numerous studies have evaluated oncologic outcomes and predictors of long term prognosis in RPS, relatively few studies have assessed preoperative risk stratification and patient selection for surgical intervention. To date, previous publications of the surgical management of RPS have focused on the role of contiguous organ resection and pain, lower limb impairment, and impaired renal function [16, 21]. However, these studies have not significantly addressed the impact of physiological reserve and/or frailty on surgical outcomes, and this topic is increasingly important as our population ages with a concomitant growing incidence of cancer.
Predictive models which identify patients at risk for increased morbidity and mortality have been established, including the ‘phenotypic model’ that involves a questionnaire about weight loss and physical mobility as well as using the cumulative deficit index made up of 48 different signs and symptoms of the elderly [22, 23]. The cumulative deficit model was simplified to the modified frailty index and has been applied to various different surgical procedures for malignancy [9, 10, 12, 24, 25]. Although RPS resection, in conjunction with multivisceral resections, have been studied in the elderly, studies evaluating the predictive power of the mFI for patients undergoing RPS resections have not been performed to date [2, 16, 17].
Our study demonstrates that with increasing mFI, there is an increased risk of both overall and serious morbidity, although the association of mFI with these endpoints was not robust since only limited mFI scores correlated with worse overall and serious morbidity outcomes. In addition, there was no association of mFI score with 30- day mortality, further reinforcing the limited predictive power of this index in the study population. With our aging population in whom the role of surgical management of solid tumors remains strong, careful preoperative risk assessment, including frailty and risk for perioperative complications, is an important aspect of the pre-operative evaluation process.
Risk stratification prior to morbid operations has important implications for helping to choose the right patient population and identifying those patients who are at increased risk for life-threatening complications with increased hospital stay, discharge to facilities, increased rates of re-admission and death [7, 10, 26, 27]. As such, a validated tool to reliably evaluate surgical risk among patients undergoing RPS resection would likely contribute to a personalized approach to surgical decision-making. However, unlike studies demonstrating a high concordance for the mFI in predicting risk of acute surgical morbidity in other tumor types, the mFI appears to have limited predictive power in RPS.
We observed a relatively higher rate of non-frail patients in comparison to previous studies analyzing frailty as a predictor of post-surgical morbidity and mortality following resection of solid tumors. For instance, Hodari et al. evaluatedthe mFI scores in patients undergoing esophagectomy and found that 37.9% (n=795) of the 2,095 patients had an mFI score of 0. This study showed that increasing mFI scores had increasing mortality rates ranging from 1.8 to 23.1 % which is higher than our overall mortality rate of 1.2%. [11]. Another study by Tsiouris evaluated patients undergoing lobectomy for lung cancer. In this series,26% (n=504) of 1940 patients had an mFI score of 0. These authors observed, increasing mortality rates (ranging from 1 to 5.6% as mFI increased) with a mortality rate of 2.7% for the entire cohort, suggesting a higher overall mortality rate than the 1.2% mortality rate observed in our study [9]. Similar trends were demonstrated in a study by Augustin et al. assessing patients undergoing pancreatic resections. In this report,38.4% of 13,020 patients (n=4,996) had an mFI of 0 with mortality rates increasing from a lower limit of 1.2 in non-frail patients to an upper limit of18.4% at the highest mFI scores [7]. A study by Louwers et al. used the frailty index to analyze predictors of morbidity and mortality in patients undergoing liver resections. Overall, the authors observed a mortality rate of 2.3 % which was higher than our series [25]. Morever, our mortality rate is lower than these other studies and this rate seems to be comparable to another retroperitoneal sarcoma outcomes study by Smith et al. which found an overall mortality rate of 1.4% [28].
Our patient demographics compared to other series show a younger population with an average age of 59 years old, compared to these other studies with average age of 63 – 67. However, overall, the other demographic factors in our study were similar to other NSQIP studies evaluating the mFI as a predictor of postoperative complications. For example, among patients undergoing pulmonary lobectomy 51.3% of patients were female (51.7% in our study) and the majority of patients were Caucasian (93.8% versus 78.9% in our study), the majority of patients had independent functional status (97.5% versus 97.4% in our study), and the majority of patients were ASA Class 3 (70.2% versus 60.5% in our study) [9].
A potential explanation for our findings is that most of the patients in this cohort undergoing RPS resection were non-frail with 48.5% having an mFI score of 0 and only 6 patients having scores of 4 or 5. Consequently, our study may have been subject to type II error since the power to detect small but significant differences was admittedly low with such a low event rate. Another potential limitation of our study is the increasing proportion of missing data related to components of the mFI over the later years of the study, specifically after 2012 [29]. Because of this, we excluded 221 patients due to missing variables, including no patients after 2012. Although our impression is that patients undergoing RPS resection after 2012 are unlikely to have different patient characteristics or incidence of comorbidities as those operated on before 2012, it is possible that a larger sample size, as noted above, may have changed the results of our study. Yet, despite these limitations, we find that the mFI appears to have limited value for predicting adverse events following RPS resection, including overall morbidity, serious morbidity, and 30-day mortality.
Conclusion
In conclusion, our study shows that patients who undergo RPS resections are non-frail with few comorbidities. Given the skewed distribution towards low mFI scores, the mFI appears to have limited predictive power for risk stratification in patients with retroperitoneal sarcomas. Since the mFI is an accumulated deficits model (based on the number of pre-defined comorbid conditions), it may not directly assess underlying physical function and performance status, especially in a somewhat younger patient population where comorbid conditions tend to be less prevalent. Further studies directly comparing the mFI to other frailty indices (such as psoas muscle mass or grip strength) may shed light on the sensitivity and specificity of various risk stratification indices in RPS.
Acknowledgments
The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Footnotes
Presented in part at the 12th Annual Academic Surgical Congress (ASC), Las Vegas, NV, February 6–9, 2017
Author Contributions
J.S. Park – conception and design, analysis and interpretation of data, writing the manuscript
S.B. Bateni – analysis and interpretation of data, critical revision of the article
R.J. Bold – critical revision of the article
A.R. Kirane- critical revision of the article
D.J. Canter- critical revision of the article, conception and design
RJ. Canter– conception and design, analysis and interpretation of data, critical revision of the manuscript
Disclosure:
Funding: This project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1TR001860 and by the Agency for Health Care Research and Quality, through grant number T32HS 022236. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Anaya DA, Lev DC, Pollock RE. The role of surgical margin status in retroperitoneal sarcoma. J Surg Oncol. 2008;98(8):607–610. doi: 10.1002/jso.21031. [DOI] [PubMed] [Google Scholar]
- 2.Smith HG, Thomas JM, Smith MJ, Hayes AJ, Strauss DC. Multivisceral resection of retroperitoneal sarcomas in the elderly. Eur J Cancer. 2016;69:119–126. doi: 10.1016/j.ejca.2016.09.037. [DOI] [PubMed] [Google Scholar]
- 3.Gronchi A. Extended surgery for retroperitoneal sarcoma: the key to maximizing the potential for cure and survival. Pro Oncology (Williston Park) 2013;27(7):640, 642. [PubMed] [Google Scholar]
- 4.Doepker MP, Hanna KH, Thompson ZJ, Binitie OT, Letson DG, Gonzalez RJ. Recurrence and survival analysis of resected soft tissue sarcomas of pelvic retroperitoneal structures. J Surg Oncol. 2016;113(1):103–107. doi: 10.1002/jso.24090. [DOI] [PubMed] [Google Scholar]
- 5.Anaya DA, Lahat G, Wang X, Xiao L, Tuvin D, Pisters PW, Lev DC, Pollock RE. Establishing prognosis in retroperitoneal sarcoma: a new histology-based paradigm. Ann Surg Oncol. 2009;16(3):667–675. doi: 10.1245/s10434-008-0250-2. [DOI] [PubMed] [Google Scholar]
- 6.Ferrario T, Karakousis CP. Retroperitoneal sarcomas: grade and survival. Arch Surg. 2003;138(3):248–251. doi: 10.1001/archsurg.138.3.248. [DOI] [PubMed] [Google Scholar]
- 7.Augustin T, Burstein MD, Schneider EB, Morris-Stiff G, Wey J, Chalikonda S, Walsh RM. Frailty predicts risk of life-threatening complications and mortality after pancreatic resections. Surgery. 2016;160(4):987–996. doi: 10.1016/j.surg.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Chimukangara M, Frelich MJ, Bosler ME, Rein LE, Szabo A, Gould JC. The impact of frailty on outcomes of paraesophageal hernia repair. J Surg Res. 2016;202(2):259–266. doi: 10.1016/j.jss.2016.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsiouris A, Hammoud ZT, Velanovich V, Hodari A, Borgi J, Rubinfeld I. A modified frailty index to assess morbidity and mortality after lobectomy. J Surg Res. 2013;183(1):40–46. doi: 10.1016/j.jss.2012.11.059. [DOI] [PubMed] [Google Scholar]
- 10.Uppal S, Igwe E, Rice LW, Spencer RJ, Rose SL. Frailty index predicts severe complications in gynecologic oncology patients. Gynecol Oncol. 2015;137(1):98–101. doi: 10.1016/j.ygyno.2015.01.532. [DOI] [PubMed] [Google Scholar]
- 11.Hodari A, Hammoud ZT, Borgi JF, Tsiouris A, Rubinfeld IS. Assessment of morbidity and mortality after esophagectomy using a modified frailty index. Ann Thorac Surg. 2013;96(4):1240–1245. doi: 10.1016/j.athoracsur.2013.05.051. [DOI] [PubMed] [Google Scholar]
- 12.Karam J, Tsiouris A, Shepard A, Velanovich V, Rubinfeld I. Simplified frailty index to predict adverse outcomes and mortality in vascular surgery patients. Ann Vasc Surg. 2013;27(7):904–908. doi: 10.1016/j.avsg.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Farhat JS, Velanovich V, Falvo AJ, Horst HM, Swartz A, Patton JH, Jr, Rubinfeld IS. Are the frail destined to fail? Frailty index as predictor of surgical morbidity and mortality in the elderly. J Trauma Acute Care Surg. 2012;72(6):1526–1530. doi: 10.1097/TA.0b013e3182542fab. discussion 1530–1521. [DOI] [PubMed] [Google Scholar]
- 14.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obeid NM, Azuh O, Reddy S, Webb S, Reickert C, Velanovich V, Horst HM, Rubinfeld I. Predictors of critical care-related complications in colectomy patients using the National Surgical Quality Improvement Program: exploring frailty and aggressive laparoscopic approaches. J Trauma Acute Care Surg. 2012;72(4):878–883. doi: 10.1097/TA.0b013e31824d0f70. [DOI] [PubMed] [Google Scholar]
- 16.Tseng WH, Martinez SR, Tamurian RM, Chen SL, Bold RJ, Canter RJ. Contiguous organ resection is safe in patients with retroperitoneal sarcoma: An ACS-NSQIP analysis. J Surg Oncol. 2011;103(5):390–394. doi: 10.1002/jso.21849. [DOI] [PubMed] [Google Scholar]
- 17.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webb S, Rubinfeld I, Velanovich V, Horst HM, Reickert C. Using National Surgical Quality Improvement Program (NSQIP) data for risk adjustment to compare Clavien 4 and 5 complications in open and laparoscopic colectomy. Surg Endosc. 2012;26(3):732–737. doi: 10.1007/s00464-011-1944-2. [DOI] [PubMed] [Google Scholar]
- 19.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 20.Canter RJ. Surgical approach for soft tissue sarcoma: standard of care and future approaches. Curr Opin Oncol. 2015;27(4):343–348. doi: 10.1097/CCO.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 21.Callegaro D, Miceli R, Brunelli C, Colombo C, Sanfilippo R, Radaelli S, Casali PG, Caraceni A, Gronchi A, Fiore M. Long-term morbidity after multivisceral resection for retroperitoneal sarcoma. Br J Surg. 2015;102(9):1079–1087. doi: 10.1002/bjs.9829. [DOI] [PubMed] [Google Scholar]
- 22.Kulminski AM, Ukraintseva SV, Kulminskaya IV, Arbeev KG, Land K, Yashin AI. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc. 2008;56(5):898–903. doi: 10.1111/j.1532-5415.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 24.Velanovich V, Antoine H, Swartz A, Peters D, Rubinfeld I. Accumulating deficits model of frailty and postoperative mortality and morbidity: its application to a national database. J Surg Res. 2013;183(1):104–110. doi: 10.1016/j.jss.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Louwers L, Schnickel G, Rubinfeld I. Use of a simplified frailty index to predict Clavien 4 complications and mortality after hepatectomy: analysis of the National Surgical Quality Improvement Project database. Am J Surg. 2016;211(6):1071–1076. doi: 10.1016/j.amjsurg.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Bateni SB, Meyers FJ, Bold RJ, Canter RJ. Increased Rates of Prolonged Length of Stay, Readmissions, and Discharge to Care Facilities among Postoperative Patients with Disseminated Malignancy: Implications for Clinical Practice. PLoS One. 2016;11(10):e0165315. doi: 10.1371/journal.pone.0165315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li JL, Henderson MA, Revenig LM, Sweeney JF, Kooby DA, Maithel SK, Master VA, Ogan K. Frailty and one-year mortality in major intra-abdominal operations. J Surg Res. 2016;203(2):507–512. e501. doi: 10.1016/j.jss.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Smith HG, Panchalingam D, Hannay JA, Smith MJ, Thomas JM, Hayes AJ, Strauss DC. Outcome following resection of retroperitoneal sarcoma. Br J Surg. 2015;102(13):1698–1709. doi: 10.1002/bjs.9934. [DOI] [PubMed] [Google Scholar]
- 29.Gani F, Canner JK, Pawlik TM. Use of the Modified Frailty Index in the American College of Surgeons National Surgical Improvement Program Database: Highlighting the Problem of Missing Data. JAMA Surg. 2016 doi: 10.1001/jamasurg.2016.3479. [DOI] [PubMed] [Google Scholar]


