Abstract
Extracellular matrix composition and stiffness are known to be critical determinants of cell behavior, modulating processes including differentiation, traction generation, and migration. Recent studies have demonstrated that the ECM composition can modulate how cells migrate in response to gradients in environmental stiffness, altering a cell’s ability to undergo durotaxis. These observations were limited to single varieties of extracellular matrix, and typically cells are exposed to environments containing complex mixtures of extracellular matrix proteins. Here, we investigate migration of NIH 3T3 fibroblasts on mechanical gradients coated with one or more type of extracellular matrix protein. Our results show that NIH 3T3 fibroblasts exhibit durotaxis on fibronectin-coated mechanical gradients but not on those coated with laminin, demonstrating that extracellular matrix type can act as a regulator of cell response to mechanical gradients. Interestingly, NIH 3T3 fibroblasts were also observed to migrate randomly on gradients coated with a mixture of both fibronectin and laminin, suggesting that there may be a complex interplay in the cellular response to mechanical gradients in the presence of multiple extracellular matrix signals. These findings indicate that specific composition of available adhesion ligands is a critical determinant of a cell’s migratory response to mechanical gradients.
Keywords: Cell Migration, Durotaxis, Substrate Stiffness, Fibronectin, Laminin
1. Introduction
Extracellular matrix stiffness has increasingly come to be seen as an important regulator of cell behavior, driven by observations of numerous cell functions modulated by substrate stiffness and by observations of the prevalence of tissue stiffness changes in disease. Physiological stiffness is known to vary over many orders of magnitude, from as low as tens to hundreds of pascals in brain and adipose tissue to megapascals and gigapascals in tendon and in bone, respectively, with the majority of soft tissues falling in the stiffness range of hundreds of pascals to tens of kilopascals [1,2]. Additionally, matrix stiffness is known to change in both development and disease, likely leading to changes in cell behavior. For instance, in pulmonary fibrosis, increasing stiffness of lung tissue is believed to contribute to increased myofibroblast proliferation [3–5]. Given the importance of stiffness in these processes, a substantial effort has been made to understand how changes in stiffness modify cell behavior in vitro. Stiffness has been demonstrated to alter cell adhesion and proliferation rates [6], cell-cell attachment [7], sensitivity of cells to soluble factors [8,9], differentiation of cells into various lineages [10–12], magnitude of traction forces applied to substrates [13–15], and rates of cell migration [16,17]. In addition to responding to changes in the absolute stiffness of their environment, cells have been observed to respond to gradients in environmental stiffness. Durotaxis, a process by which cells migrate preferentially from regions of lower to higher substrate stiffness, has been documented in a variety of cell types on mechanical gradients spanning a wide range of absolute and relative substrate stiffnesses [18,19].
Differences in the stiffness of tissue matrices are accompanied by differences in extracellular matrix composition, and the type of extracellular matrix in a given tissue will also function as a major determinant of the behavior of cells in that tissue [20]. The interplay between mechanical stiffness and matrix composition in normal and pathological physiology is only now becoming appreciated. Recent studies in which tissue stiffness was mapped by AFM indentation have identified heterogeneities that indicate the presence of mechanical stiffness gradients in both healthy and diseased tissues. These measurements indicate the presence of a wide range of absolute stiffnesses and gradient strengths in vivo [21–26]. Importantly, such stiffness gradients have been demonstrated to accompany changes in extracellular matrix composition in a number of diseases. For instance, in lung fibrosis, local increases in lung parenchymal tissue stiffness are accompanied by an increase in collagen I concentration [4], and in breast cancer an increase in stiffness from the tumor core to the periphery is associated with increased levels of collagen I and laminin [24]. In atherosclerosis, a disease characterized by the thickening of the intimal region of the arterial wall, changes in the mechanics and composition of the intimal matrix occur in conjunction with accumulation of smooth muscle and inflammatory cells [27–29]. Stiffness mapping experiments have shown that plaque stiffness is spatially heterogeneous, and that these changes can be histologically related to extracellular matrix composition of the plaque [26,30]. Given the increasing number of examples for which changes in extracellular matrix composition in disease are coupled to changes in mechanical properties of diseased tissue, there is a need for in vitro studies that systematically explore how the cellular response to stiffness is altered by extracellular matrix composition.
Extracellular matrix composition has been demonstrated to modulate in vitro responses to substrate stiffness in behaviors such as cell adhesion, spreading, differentiation, junction formation, traction force generation, and matrix production [31–36]. These studies suggest that many observed responses to changes in stiffness will be subject to the type of extracellular matrix available for cells to adhere to. Thus, it will be important to assess whether migratory responses of cells to mechanical gradients are also regulated by extracellular matrix composition. We recently reported an experimental system to generate polyacrylamide gels with highly reproducible linear mechanical gradients in substrate stiffness and used it to explore whether migration of vascular smooth muscle cells on mechanical gradients was extracellular matrix type-dependent [37]. However, the effect of combinations of extracellular matrix on the cellular response to mechanical gradients has yet to be explored. To address this, we have utilized mechanical gradient hydrogels coated with different extracellular matrix types to study migration of NIH 3T3 fibroblasts. Cells were cultured on mechanical gradient hydrogels with an 18.6 kPa/mm gradient between 1 kPa and 25 kPa low and high stiffness regions coated with fibronectin, laminin, and a 50:50 ratio of fibronectin and laminin by mass. We observed durotaxis behavior on fibronectin, as has been previously reported, and observed random migration on laminin and mixed-matrix gradients. Our results illustrate that matrix-type may act as a regulator of a cell’s ability to respond to gradients in environmental mechanics, and the lack of observable durotaxis on mixed-matrix gradients suggests that the presence of laminin could act to inhibit the durotactic response usually seen in response to fibronectin-coated gradients.
2. Materials and Methods
2.1. Gradient gel fabrication and surface functionalization
Polyacrylamide gels featuring gradients in mechanical compliance between uniform stiffness control regions were prepared as previously described [37]. Briefly, gradient generator slides were prepared by micropatterning a hydrophobic silane boundary around an adhesive, hydrophilic silane region of defined geometry using maskless lithography [38].The micropattern features a “dumbbell-shaped” geometry, with large reservoir regions for low- and high-stiffness polyacrylamide gel solutions connected by a narrow gradient mixing region. A patterned slide and a bare, sacrificial glass slide were sandwiched around a pair of 250µm teflon spacers to form a gradient generator device into which polyacrylamide gel solutions could be injected for controlled mixing. Low- and high-stiffness pre-gel solutions were prepared with 10% acrylamide monomer (Biorad), 0.1% (low stiffness) or 0.5% (high stiffness) N,N’-methylenebisacrylamide (Biorad), amine-reactive cross-linker NHS-ester acrylic acid (Sigma), and I2959 photoinitiator (Irgacure) in PBS adjusted to pH 6.0 with hydrochloric acid. The two pre-gel solutions were injected into either side the gradient generator device, meeting in the center of the gradient mixing region. The pre-gel solutions were allowed to diffuse across the mixing region for 10 minutes in order to establish a linear gradient in bis-acrylamide cross-linker concentration, at which point the devices were irradiated with ultraviolet light for 240 seconds to initiated polymerization of the gel solutions. Sacrificial slides were removed, and the resulting polyacrylamide gradient gels were stored in PBS adjusted to pH 6.0. The resulting gradient gels featured 1mm gradient regions with an 18.6 kPa/mm gradient between 1 and 25 kPa low- and high-stiffness control regions [37].
Polyacrylamide gradient gels were functionalized with extracellular matrix proteins by incubation in concentrated ECM solutions. Covalent attachment to the gel surface was facilitated by reaction with NHS-ester groups incorporated into the polyacrylamide backbone by inclusion of NHS-ester acrylic acid in the pre-gel solutions. Polyacrylamide gels were incubated in a solution of fibronectin (Millipore), laminin-1 (Sigma), or a 50:50 mixture by mass of fibronectin and laminin at 5µg/cm2 surface concentration in PBS adjusted to pH 8.0 for two hours at room temperature. After incubation, gels were rinsed three times with PBS then stored overnight at 4°C in 1M glycine in PBS to ensure there would be no unreacted NHS-ester groups remaining. Gels were rinsed three times with PBS then stored in PBS prior to use.
2.2. Cell culture and reagents
NIH/3T3 fibroblasts (ATCC) were cultured in high glucose Dulbecco’s modified Eagle’s medium (Gibco) supplemented with L-glutamine (Gibco) and 10% bovine calf serum (Hyclone). Cells were cultured on plasma-treated tissue culture dishes (Corning) and passaged with 0.05% trypsin-EDTA (Gibco).
2.3. Cell migration on gradient gels
NIH/3T3 fibroblasts were seeded on gradient gels at a density of 1500/cm2 and were allowed to attach for 3 hours prior to imaging. 3 or more gels were used for each experimental condition in independent trials. For soluble laminin treatment experiments, cells were suspended at 106/mL concentration in culture media and incubated with 50 µg/mL laminin for ten minutes prior to seeding. Cells adhered to the low stiffness, high stiffness, and gradient stiffness regions of each gel were imaged in 20 minute intervals over a period of 18 hours using an inverted optical microscope (Zeiss) equipped with a motorized stage (Ludl Electronic Products) and a custom-built incubator to control temperature, CO2, and humidity.
2.4. Quantification of cell migration parameters
Cell centroid positions were recorded in ImageJ at each time point. Migration tracks were assembled as vector displacements between the centroid positions recorded from sequential slices and were analyzed using a custom R script. For each cell tracked, X-displacement was calculated as the vector component of displacement in the direction of increasing substrate stiffness, and the durotactic index was calculated as the X-displacement divided by the cumulative path length. Statistical analysis of cell migration data was performed using one-factor or two-factor ANOVA as needed and Tukey’s honest significant difference test for post-hoc analysis where appropriate.
3. Results and Discussions
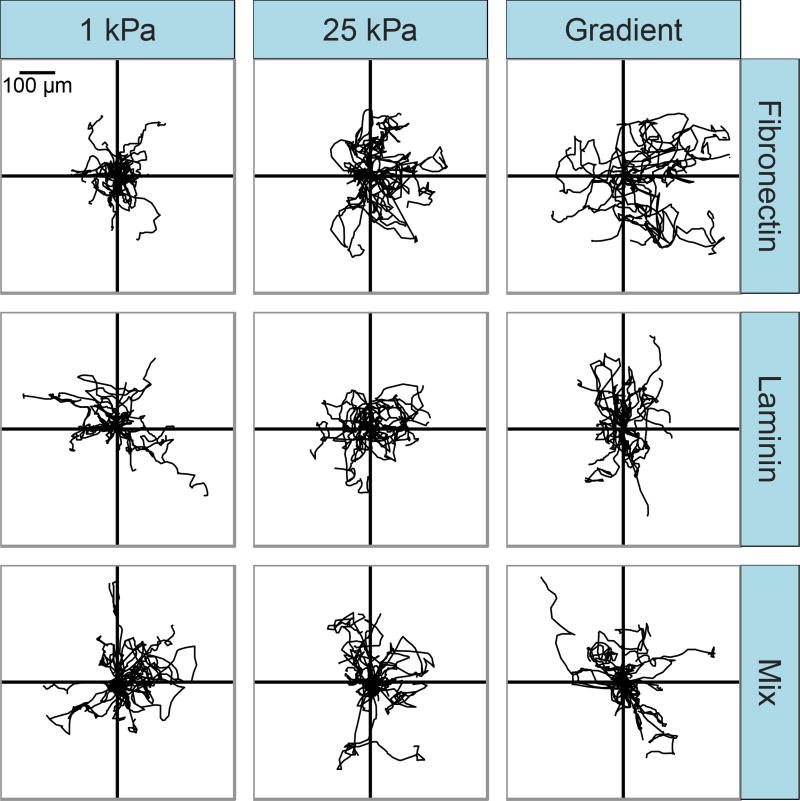
We sought to investigate whether previously observed responses of NIH 3T3 fibroblasts to gradients in mechanical stiffness were dependent on extracellular matrix type and how cells migrated on mechanical gradients in the presence of multiple matrix molecules. We previously observed that bovine vascular smooth muscle cells would undergo durotaxis on gradient gels coated with fibronectin, but would migrate randomly on laminin [37]. Here, we asked whether the same behavior could be observed in another cell type previously reported to exhibit durotaxis, and investigated effects of a mixture of both fibronectin and laminin on the migratory response to mechanical gradients. The effect of extracellular matrix composition on migration of NIH 3T3 fibroblasts on mechanical gradient gels and uniform stiffness control gels was assessed qualitatively by plotting the trajectory of each cell tracked relative to a common origin. A random subset of cell tracks from each experimental condition is shown in Fig. 1. Visual inspection of cell tracks suggest random migration with low persistence times occurs across all control conditions, with similar migration velocities yielding similar displacements from a common origin. Similar cell track profiles are also observed for cells on fibronectin-, laminin-, and mix-coated gradients. Cells were able to attach and migrate at similar rates across all experimental conditions, suggesting that the extracellular matrix concentration used to coat the substrates was sufficient to observe similar migratory behavior in cells on both fibronectin and laminin. Across all protein coating conditions, there was a small but not statistically significant increase in migration velocity with substrate stiffness. Thus, changes in migration velocity with extracellular matrix type or stiffness are not sufficient to explain differences in cell migration in response to mechanical gradients across different extracellular matrix coatings.
Figure 1.
Representative migration tracks for NIH 3T3 fibroblasts migrating on mechanical gradient and uniform stiffness gels coated with fibronectin, laminin, or a 50:50 mix. Cell centroid position was tracked at 20-minute intervals for 18 hours, n=15 randomly selected cells per condition.
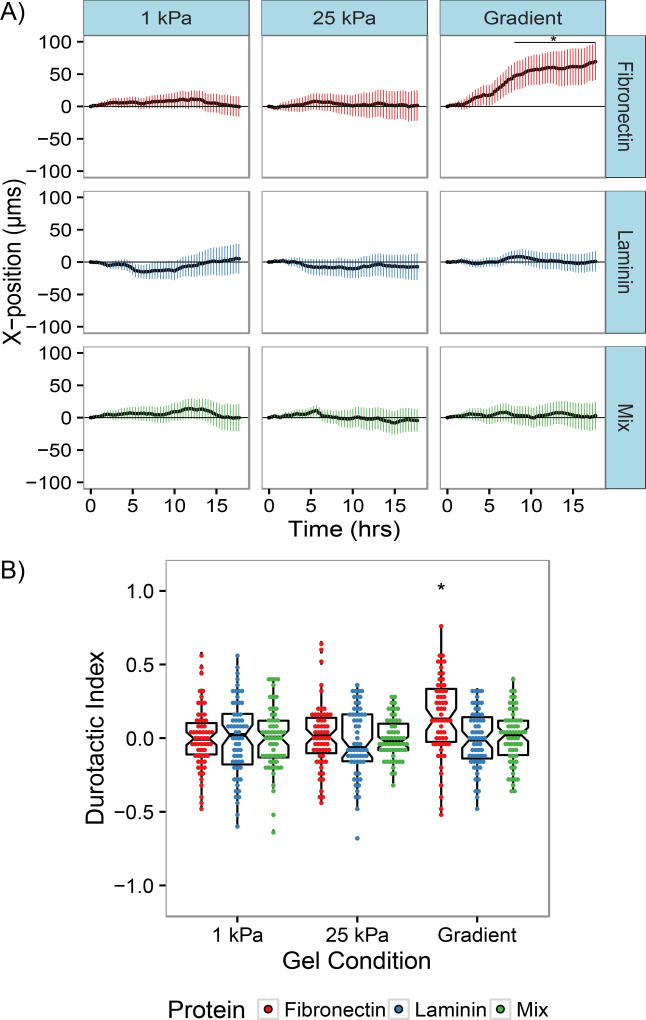
In order to quantitatively assess whether the type of extracellular matrix available for cell attachment altered the migratory response to gradients in mechanical stiffness, the average X-direction trajectory was calculated from cell migration tracks as a function of time (Fig. 2A). NIH 3T3s on uniform stiffness control gels experienced an average X-directional displacement that fluctuated around zero as time increased for all protein conditions. Cells migrating on mechanical gradient gels coated with fibronectin were found to experience an average X-displacement of 69.4 µm after 18 hours, consistent with previous reports indicating 3T3 fibroblasts will undergo durotaxis on mechanical gradients [39,40]. In contrast, cells on laminin-coated gradients were found to migrate to an average X-displacement of 1 µm after 18 hours. This suggests cells on laminin-coated gradients did not experience a bias in migration direction from the presence of a mechanical gradient, which agrees with results from our previous study of vascular smooth muscle cells. Interestingly, cells migrating on gradients coated with a mixture of fibronectin and laminin were found to migrate to average X-displacements of 3.01µm after 18 hours. Statistical testing of the X-displacement values by two-factor ANOVA and Tukey’s honest significant difference test found that the X-displacements on fibronectin-coated gradients were statistically different from the X-displacements observed for all other samples. Additionally, there was no significant difference between displacements on laminin-coated gradients, mixed-coated gradients, and any of the control uniform stiffness controls.
Figure 2.
NIH 3T3 fibroblast response to mechanical gradients is altered by extracellular matrix composition. A) Average X-displacements a function of time of NIH 3T3 fibroblasts migrating on polyacrylamide gels coated with fibronectin, laminin, or a 50:50 mix. Values are presented as mean ± the 95% confidence interval. B) Durotactic index distributions of NIH 3T3 fibroblasts migrating on substrates coated with fibronectin, laminin, or a 50:50 mix. Box plots overlaid on each distribution denote the median and quartile values for each distribution, with notches for the 95% confidence interval of the median. N=60 cells from n>3 gels for each condition; *, P < 0.05 relative to all other conditions.
To further quantify migration of cells in the direction of increasing substrate stiffness, a durotactic index was calculated by dividing the X-displacement of each cell by the contour length of its migration track. The distributions of such durotactic indices for NIH 3T3 fibroblasts on fibronectin, laminin, and mixed protein gradient gels are plotted in Fig. 2B. For both cells on uniform stiffness control gels and laminin- and mixed protein- coated gradients, the mean durotactic index is approximately zero, indicative of nonbiased migration, whereas for the population of cells migrating on fibronectin-coated gradients, the distribution of durotactic indices is significantly shifted to a mean of 0.14 (p<0.005), demonstrating a bias towards stiffer regions of the gradient gel.
These results indicate that the ability of cells to respond to gradients in substrate stiffness may be extracellular-matrix type dependent. The observation of durotaxis occurring on fibronectin-coated mechanical gradients but not on laminin-coated mechanical gradients agrees with our previous report of extracellular-matrix type dependence of vascular smooth muscle cell behavior. Although mechanisms leading to these different responses to mechanical gradients based on ECM type have not been studied, it has long been appreciated that different integrin subtypes interact with different extracellular matrix proteins. Different integrin-ligand pairs can vary substantially in bond strength and adhesion dynamics [41–45], and different integrin types have been shown to support different cytoskeletal structures and responses to substrate stiffness [46,47]. Thus, it is possible that different extracellular matrix proteins, which would be expected to bind to different sets of integrins, would allow for varied sensitivity to substrate stiffness in mechanosensing processes, potentially leading to an apparent response to mechanical gradients on one ECM protein but not on another.
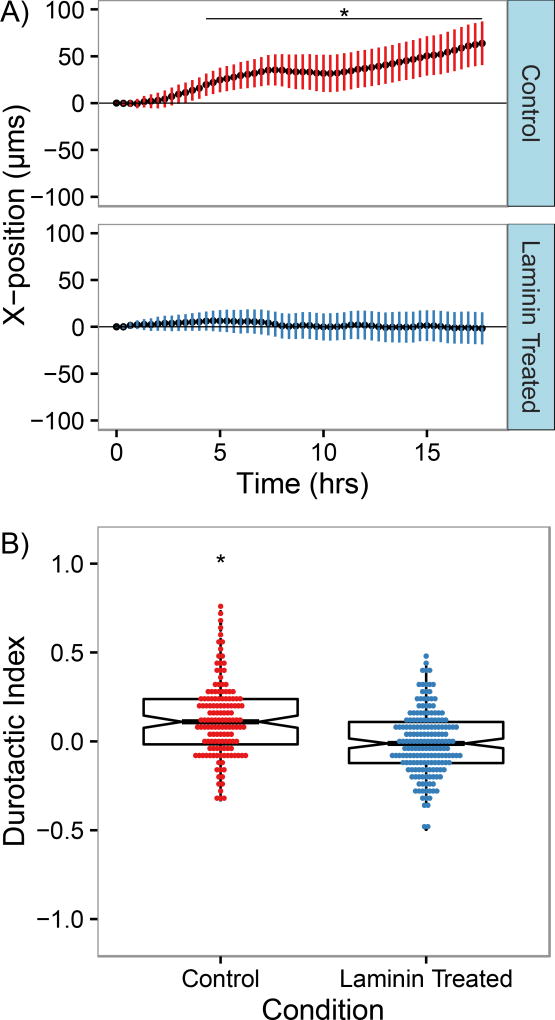
The lack of directed migration observed on gradients coated with a mixture of fibronectin and laminin suggests that while 3T3s are capable of attaching to and migrating on both fibronectin and laminin independently and migrating at comparable rates, they do not respond to the presence of fibronectin on gradient substrates when laminin is present the same way they would if fibronectin by itself is available. While further studies are needed to elucidate the mechanisms leading to this outcome, one possible explanation for this behavior is crosstalk between laminin and fibronectin binding integrins. A wide variety of crosstalk pathways have been identified between different integrin pairs, including potential-talk pathways between fibronectin and laminin binding integrins [48–50]. As a preliminary investigation into whether crosstalk between laminin-binding and fibronectin-binding integrins could lead to a lack of durotaxis on mixed-matrix gradients in spite of the presence of fibronectin, we analyzed migration of cells on fibronectin-coated mechanical gradients after treatment with soluble laminin prior to seeding. In order to reduce the likelihood of laminin attaching to the substrate surface during seeding, a highly concentrated cell solution was incubated with a small amount of soluble laminin, then was diluted substantially for cell seeding such that the maximum possible surface concentration of laminin were it to absorb to the gel surface was 50 times less than that of fibronectin. As had been previously observed, untreated NIH 3T3 fibroblasts seeded on fibronectin-coated gradients underwent biased migration in the direction of increasing stiffness (Fig. 3A). However, cells that had been treated with laminin prior to seeding appeared to migrate more randomly, with average displacements similar to those observed for untreated cells migrating on laminin- or mixed protein-coated gradients. The mean durotactic index for untreated cells was found to be 0.13, demonstrating that biased migration was occurring in the population, while for cells treated with soluble laminin, the average tactic index was −0.01, suggesting that random migration was occurring (Fig. 3B). While this data does not implicate any specific crosstalk mechanism in altering the cellular response to fibronectin-coated mechanical gradients, it does suggest that binding of laminin integrins could have downstream effects leading changes in how cells sense or respond to mechanical gradients when attached via fibronectin.
Figure 3.
Fibroblast migration on fibronectin-coated gradient hydrogels is altered by exposure to soluble laminin. A) Average X-displacement s a function of time of NIH 3T3 fibroblasts with and without soluble laminin treatment migrating on gradient polyacrylamide gels coated with fibronectin. Values are presented as mean ± the 95% confidence interval. B) Durotactic index distributions of NIH 3T3 fibroblasts with and without soluble laminin treatment migrating on gradient polyacrylamide gels coated with fibronectin. Box plots overlaid on each distribution denote the median and quartile values for each distribution, with notches for the 95% confidence interval of the median. N=130 cells from n>3 gels for each condition; *, P < 0.05 relative to laminin treated condition.
The lack of observable durotaxis on gradient gels coated with a mixture of fibronectin and laminin and loss of durotaxis in cells treated with soluble laminin before seeding on fibronectin suggest that the mechanism by which NIH 3T3s respond to stiffness gradients coated with multiple extracellular matrix protein types bound by different integrins is not a simple interpolation between responses to individual matrix coatings, but rather may involve additional regulatory elements. However, it is difficult to identify a distinct mechanism to explain this result. It is possible differences in integrin expression levels, binding strength, and turnover rates could result in different levels of sensitivity to the presence of different ECM proteins. Furthermore, crosstalk pathways could potentially alter a cell’s migratory behavior by modulating the activity of different integrin-ligand pairs. Thus, studies should be designed to investigate whether such crosstalk pathways could be responsible for the lack of observed durotaxis in migrating fibroblasts on mixed fibronectin- and laminin-coated gradients. However, it is not trivial to experimentally identify and target crosstalk pathway(s) as a mechanism to explain these results, as both fibronectin and laminin are known to interact with multiple integrin types expressed in normal cells. Thus, in order for more targeted experiments to be done, cells with defined integrin expression profiles could be used. While the data presented here using soluble laminin to induce changes in migratory behavior cannot implicate any specific set of integrins or crosstalk mechanisms, the results do suggest that the presence of laminin, even when not utilized for binding to the substrate surface, can alter the ability of cells to detect and/or respond to mechanical gradients through binding to fibronectin.
In addition to prospective studies evaluating the possible role of integrin crosstalk in regulating cellular responses to mechanical gradients, there are a variety of other avenues of investigation that could be explored. First, it would be important to assess whether migration on laminin-coated gradients is random independent of gradient strength. Given previous work from our lab reporting that the strength of durotaxis observed in VSMCs on collagen-coated gradients is gradient strength-dependent, it is possible that a higher gradient strength than that utilized in this study could be necessary to induce durotaxis on laminin [51]. Additionally, it is possible that the behavior could only be observed in a different absolute stiffness range than that studied here. Second, while we chose to evaluate responses to fibronectin and laminin because of previous studies suggesting that these proteins could differentially regulate cell behaviors [37,52], they are hardly the only relevant components of extracellular matrix. Durotaxis has also previously been studied on type-1 collagen-coated gradients, but to our knowledge, other matrix proteins, including elastin and other collagen and laminin subtypes, and combinations of matrix proteins have not been evaluated. Additionally, it would be interesting to assess whether the observed responses to fibronectin and laminin are also observed in a wider variety of cell types, particularly for cells such as neurons that have been observed to turn towards regions of reduced stiffness when migrating in vitro [53]. Experiments could also be performed to assess whether the responses to laminin and fibronectin are concentration dependent, particularly for the case of mixed-protein gradients, where only a 50:50 ratio by mass was explored. The collective results of these suggested studies could generate a library of cell responses to mechanical gradients with different extracellular matrix availabilities to aid in designing complex engineered tissues, for which control over positioning and migration of one or more cell types may be desirable.
Highlights.
Fibroblasts undergo durotaxis on gradients coated with fibronectin but not laminin.
Migration on gradients coated with a mixture of fibronectin and laminin is random.
Soluble laminin prevents durotaxis on fibronectin-coated mechanical gradients.
ECM type is a key determinant of a cell’s response to mechanical gradients.
Acknowledgments
This work was supported by NIH Grants R01 HL072900 and R01 HL124280 (to J.Y.W.); NIH Predoctoral Training Grant NIGMS 5T32 GM008764 (to C.D.H.); and a Boston University Lutchen fellowship (to S.G.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levental I, Georges PC, Janmey PA. Soft biological materials and their impact on cell function. Soft Matter. 2006;3:299–306. doi: 10.1039/b610522j. [DOI] [PubMed] [Google Scholar]
- 2.Zysset PK, Edward Guo X, Edward Hoffler C, Moore KE, Goldstein SA. Elastic modulus and hardness of cortical and trabecular bone lamellae measured by nanoindentation in the human femur. J. Biomech. 1999;32:1005–1012. doi: 10.1016/s0021-9290(99)00111-6. [DOI] [PubMed] [Google Scholar]
- 3.Hinz B. Mechanical Aspects of Lung Fibrosis: A Spotlight on the Myofibroblast. Proc. Am. Thorac. Soc. 2012;9:137–147. doi: 10.1513/pats.201202-017AW. [DOI] [PubMed] [Google Scholar]
- 4.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J. Cell Biol. 2010;190:693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marinković A, Liu F, Tschumperlin DJ. Matrices of physiologic stiffness potently inactivate idiopathic pulmonary fibrosis fibroblasts. Am. J. Respir. Cell Mol. Biol. 2013;48:422–430. doi: 10.1165/rcmb.2012-0335OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown XQ, Ookawa K, Wong JY. Evaluation of polydimethylsiloxane scaffolds with physiologically-relevant elastic moduli: interplay of substrate mechanics and surface chemistry effects on vascular smooth muscle cell response. Biomaterials. 2005;26:3123–3129. doi: 10.1016/j.biomaterials.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Guo W, Frey MT, Burnham NA, Wang Y. Substrate rigidity regulates the formation and maintenance of tissues. Biophys. J. 2006;90:2213–20. doi: 10.1529/biophysj.105.070144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown XQ, Bartolak-Suki E, Williams C, Walker ML, Weaver VM, Wong JY. Effect of substrate stiffness and PDGF on the behavior of vascular smooth muscle cells: implications for atherosclerosis. J. Cell. Physiol. 2010;225:115–22. doi: 10.1002/jcp.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park JS, Chu JS, Tsou AD, Diop R, Wang A, Li S. The Effect of Matrix Stiffness on the Differentiation of Mesenchymal Stem Cells in Response to TGF-β. Biomaterials. 2011;32:3921–3930. doi: 10.1016/j.biomaterials.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 11.Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5:17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans ND, Minelli C, Gentleman E, LaPointe V, Patankar SN, Kallivretaki M, Chen X, Roberts CJ, Stevens MM. Substrate stiffness affects early differentiation events in embryonic stem cells. Eur. Cells Mater. 2009;18:1–14. doi: 10.22203/ecm.v018a01. [DOI] [PubMed] [Google Scholar]
- 13.Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc. Natl. Acad. Sci. U. S. A. 2003;100:1484–9. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong HJ, Polte TR, Alsberg E, Mooney DJ. FRET measurements of cell-traction forces and nano-scale clustering of adhesion ligands varied by substrate stiffness. Proc. Natl. Acad. Sci. U. S. A. 2005;102:4300–4305. doi: 10.1073/pnas.0405873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Califano JP, Reinhart-King CA. Substrate Stiffness and Cell Area Predict Cellular Traction in Single Cells and Cells in Contact. Cell. Mol. Bioeng. 2010;3:68–75. doi: 10.1007/s12195-010-0102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelham RJ, Wang YL. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. U. S. A. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peyton SR, Putnam AJ. Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. J. Cell. Physiol. 2005;204:198–209. doi: 10.1002/jcp.20274. [DOI] [PubMed] [Google Scholar]
- 18.Lo CM, Wang HB, Dembo M, Wang Y-L. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000;79:144–52. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roca-Cusachs P, Sunyer R, Trepat X. Mechanical guidance of cell migration: lessons from chemotaxis. Curr. Opin. Cell Biol. 2013;25:543–549. doi: 10.1016/j.ceb.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J. Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berry MF, Engler AJ, Woo YJ, Pirolli TJ, T L, Jayasankar V, Morine KJ, Gardner TJ, Discher DE, Sweeney HL, Bish LT, F M, J T. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am. J. Physiol. Hear. Circ. Physiol. 2006;290:H2196–2203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- 22.Liu F, Tschumperlin DJ. Micro-mechanical characterization of lung tissue using atomic force microscopy. J. Vis. Exp. 2011:2911. doi: 10.3791/2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez JI, Kang I, You W-K, McDonald DM, Weaver VM. In situ force mapping of mammary gland transformation. Integr. Biol. (Camb) 2011;3:910–921. doi: 10.1039/c1ib00043h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plodinec M, Loparic M, a Monnier C, Obermann EC, Zanetti-Dallenbach R, Oertle P, Hyotyla JT, Aebi U, Bentires-Alj M, Lim RYH, Schoenenberger C-A. The nanomechanical signature of breast cancer. Nat. Nanotechnol. 2012;7:757–65. doi: 10.1038/nnano.2012.167. [DOI] [PubMed] [Google Scholar]
- 25.Darling EM, Wilusz RE, Bolognesi MP, Zauscher S, Guilak F. Spatial mapping of the biomechanical properties of the pericellular matrix of articular cartilage measured in situ via atomic force microscopy. Biophys. J. 2010;98:2848–2856. doi: 10.1016/j.bpj.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tracqui P, Broisat A, Toczek J, Mesnier N, Ohayon J, Riou L. Mapping elasticity moduli of atherosclerotic plaque in situ via atomic force microscopy. J. Struct. Biol. 2011;174:115–23. doi: 10.1016/j.jsb.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Akyildiz AC, Speelman L, Gijsen FJH. Mechanical properties of human atherosclerotic intima tissue. J. Biomech. 2014;47:773–783. doi: 10.1016/j.jbiomech.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 28.Raines EW. The extracellular matrix can regulate vascular cell migration, proliferation, and survival: relationships to vascular disease. Int. J. Exp. Pathol. 2000;81:173–82. doi: 10.1046/j.1365-2613.2000.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doran AC, Meller N, McNamara CA. Role of Smooth Muscle Cells in the Initiation and Early Progression of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2008;28:812–819. doi: 10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebenstein DM, Coughlin D, Chapman J, Li C, Pruitt LA. Nanomechanical properties of calcification, fibrous tissue, and hematoma from atherosclerotic plaques. J. Biomed. Mater. Res. Part A. 2009;91:1028–1037. doi: 10.1002/jbm.a.32321. [DOI] [PubMed] [Google Scholar]
- 31.Gilchrist CL, Darling EM, Chen J, Setton LA. Extracellular matrix ligand and stiffness modulate immature nucleus pulposus cell-cell interactions. PLoS One. 2011;6:e27170. doi: 10.1371/journal.pone.0027170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rowlands A, George P, Cooper-White J. Directing osteogenic and myogenic differentiation of MSCs: interplay of stiffness and adhesive ligand presentation. Am. J. Physiol. Cell Physiol. 2008;295:C1037–1044. doi: 10.1152/ajpcell.67.2008. [DOI] [PubMed] [Google Scholar]
- 33.Sazonova OV, Isenberg BC, Herrmann J, Lee KL, Purwada A, Valentine AD, Buczek-Thomas JA, Wong JY, Nugent MA. Extracellular matrix presentation modulates vascular smooth muscle cell mechanotransduction. Matrix Biol. 2015;41:36–43. doi: 10.1016/j.matbio.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Calve S, Simon H-G. Biochemical and mechanical environment cooperatively regulate skeletal muscle regeneration. FASEB J. 2012;26:2538–2545. doi: 10.1096/fj.11-200162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaudhuri O, Koshy ST, Branco da Cunha C, Shin J-W, Verbeke CS, Allison KH, Mooney DJ. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat. Mater. 2014;13:970–8. doi: 10.1038/nmat4009. [DOI] [PubMed] [Google Scholar]
- 36.Gershlak JR, Resnikoff JIN, Sullivan KE, Williams C, Wang RM, Black LD. Mesenchymal stem cells ability to generate traction stress in response to substrate stiffness is modulated by the changing extracellular matrix composition of the heart during development. Biochem. Biophys. Res. Commun. 2013;439:161–6. doi: 10.1016/j.bbrc.2013.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hartman CD, Isenberg BC, Chua SG, Wong JY. Vascular smooth muscle cell durotaxis depends on extracellular matrix composition. Proc. Natl. Acad. Sci. U. S. A. 2016;113:11190–11195. doi: 10.1073/pnas.1611324113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Itoga K, Kobayashi J, Tsuda Y, Yamato M, Okano T. Second-generation maskless photolithography device for surface micropatterning and microfluidic channel fabrication. Anal. Chem. 2008;80:1323–7. doi: 10.1021/ac702208d. [DOI] [PubMed] [Google Scholar]
- 39.Gray D, Tien J, Chen CS. Repositioning of cells by mechanotaxis on surfaces with micropatterned Young’s modulus. J. Biomed. Mater. Res. Part A. 2003;66:605–614. doi: 10.1002/jbm.a.10585. [DOI] [PubMed] [Google Scholar]
- 40.Kawano T, Kidoaki S. Elasticity boundary conditions required for cell mechanotaxis on microelastically-patterned gels. Biomaterials. 2011;32:2725–33. doi: 10.1016/j.biomaterials.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Sheetz MP, Felsenfeld DP, Galbraith CG. Cell migration: regulation of force on extracellular-integrin complexes. Trends Cell Biol. 1998;8:51–54. doi: 10.1016/s0962-8924(98)80005-6. [DOI] [PubMed] [Google Scholar]
- 42.Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–40. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 43.Huttenlocher A, Horwitz AR. Integrins in cell migration. Cold Spring Harb. Perspect. Biol. 2011;3:a005074. doi: 10.1101/cshperspect.a005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- 45.Keselowsky BG, Collard DM, García AJ. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J. Biomed. Mater. Res. Part A. 2003;66:247–259. doi: 10.1002/jbm.a.10537. [DOI] [PubMed] [Google Scholar]
- 46.Schiller HB, Hermann M-R, Polleux J, Vignaud T, Zanivan S, Friedel CC, Sun Z, Raducanu A, Gottschalk K-E, Théry M, Mann M, Fässler R. beta1- and alphav-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nat. Cell Biol. 2013;15:625–636. doi: 10.1038/ncb2747. [DOI] [PubMed] [Google Scholar]
- 47.Elosegui-Artola A, Bazellières E, Allen M, Andreu I, Oria R, Sunyer R, Gomm JJ, Marshall JF, Jones JL, Trepat X, Roca-Cusachs P. Rigidity sensing and adaptation through regulation of integrin types. Nat. Mater. 2014;13:631–637. doi: 10.1038/nmat3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwartz MA, Ginsberg MH. Networks and crosstalk: integrin signalling spreads. Nat. Cell Biol. 2002;4:E65–8. doi: 10.1038/ncb0402-e65. [DOI] [PubMed] [Google Scholar]
- 49.Huveneers S, Danen EHJ. Adhesion signaling - crosstalk between integrins, Src and Rho. J. Cell Sci. 2009;122:1059–69. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez AM, Claiborne J, Jones JCR. Integrin cross-talk in endothelial cells is regulated by protein kinase A and protein phosphatase 1. J. Biol. Chem. 2008;283:31849–31860. doi: 10.1074/jbc.M801345200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isenberg BC, DiMilla PA, Walker M, Kim S, Wong JY. Vascular Smooth Muscle Cell Durotaxis Depends on Substrate Stiffness Gradient Strength. Biophys. J. 2009;97:1313–1322. doi: 10.1016/j.bpj.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sazonova OV, Lee KL, Isenberg BC, Rich CB, Nugent MA, Wong JY. Cell-cell interactions mediate the response of vascular smooth muscle cells to substrate stiffness. Biophys. J. 2011;101:622–630. doi: 10.1016/j.bpj.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koser DE, Thompson AJ, Foster SK, Dwivedy A, Pillai EK, Sheridan GK, Svoboda H, Viana M, Costa L. da F, Guck J, Holt CE, Franze K. Mechanosensing is critical for axon growth in the developing brain. Nat. Neurosci. 2016;19:1592–1598. doi: 10.1038/nn.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]