Abstract
Background
There is a serious public health need for better understanding of alcohol use disorder disease mechanisms and for improved treatments. At this writing, only three drugs are approved by the Food and Drug Administration as medications to treat alcohol use disorders – disulfiram, naltrexone, and acamprosate. Binge drinking is a form of abusive alcohol drinking defined by the NIAAA as a drinking to blood alcohol levels (BALs) > 0.08% during a period of approximately 2 hr. To model genetic risk for binge-like drinking, we have used selective breeding to create a unique animal model, High Drinking in the Dark (HDID) mice. Behavioral characterization of HDID mice has revealed that HDID mice exhibit behavioral impairment after drinking, withdrawal after a single binge-drinking session, and escalate their intake in response to induction of successive cycles of dependence. Notably, HDID mice do not exhibit altered tastant preference or alcohol clearance rates. We therefore asked whether drugs of known clinical relevance could modulate binge-like ethanol drinking in HDID mice, reasoning that this characterization of HDID responses should inform future use of this genetic animal model for screening and development of novel potential therapeutics.
Methods
We tested the efficacy of acamprosate and naltrexone to reduce binge-like drinking in HDID mice. Additionally, we tested the GABAB receptor agonist, baclofen, based on recent pre-clinical and clinical studies demonstrating that it reduces alcohol drinking. We elected not to include disulfiram due to its more limited clinical usage. Mice were tested after acute doses of drugs in the limited-access Drinking in the Dark (DID) paradigm.
Results
HDID mice were sensitive to the effects of acamprosate and baclofen, but not naltrexone. Both drugs reduced binge-like drinking. However, naltrexone failed to reduce drinking in HDID mice. Thus, HDID mice may represent a useful model for screening novel compounds.
Keywords: Genetic animal model, Drinking in the Dark, Binge, Pharmacotherapy, Mouse
1. Introduction
Preclinical studies are essential for efficient screening of novel therapeutic drugs to treat alcohol use disorders (AUD). The need for new drugs remains acute, as fewer than 15% of AUD individuals receive any treatment at all, and only three compounds are approved by the Food and Drug Administration for this purpose (https://pubs.niaaa.nih.gov/publications/AA81/AA81.htmpatients). The most recently approved is acamprosate, in 2004. Disulfiram, naltrexone, acamprosate and several other compounds have proven clinically useful in alcohol-dependent populations in some studies; however, all have limitations (e.g., side effects, compliance), and additional compounds are still needed 1. Most current preclinical tests use rats or mice, and the majority of those use some form of preference drinking to gauge the animal’s tendency to overindulge. In such studies, solutions of 10–20% alcohol in tap water are typically offered as a choice versus plain tap water, with access offered 24 hours a day. Using the existing rodent models based on preference drinking, many drugs reduce drinking 2. One limitation of these studies is that many compounds initially shown to be effective in rodent preference models have not proven to be effective in subsequent human clinical trials 3.
Most high-drinking rodent models have targeted genetic influences on risk and have used long-term directional selective breeding for alcohol preference. Rat lines thus selected drink 5–7 g/kg/day 4; mouse lines drink between 14 and 23 g/kg/day, in part due to their higher elimination rate 5. However, even after long-term selection for many generations, these animals generally only reach BALs > 0.08% under certain circumstances, mostly because when alcohol is continuously available, they drink in bouts spaced throughout the night (and day) and when water is also available, they also ingest water 6.
A binge has been defined by the NIAAA as a period of temporally-focused drinking that leads to a BAL > 0.08% 7. Binge drinking is a risk for development of an AUD, and most AUD individuals binge drink. Following earlier work in the area 8, we developed an assay for binge-like drinking (drinking in the dark - DID) where mice consume enough alcohol to reach intoxicating BALs 9. We subsequently selectively bred HDID-1 and HDID-2 mouse lines for high BALs after a 4 hr DID session; these mice drink to intoxicating BALs and average 0.18% and 0.16%, respectively 10;11. Behavioral characterization of HDID mice has revealed that HDID mice exhibit behavioral impairment after drinking, withdrawal after a single binge-drinking session, and escalate their intake in response to induction of successive cycles of dependence and withdrawal 11;12. Notably, HDID mice do not exhibit altered tastant preference or alcohol clearance rates 13;14. One clear limitation of the DID model is that ethanol is not offered as a choice vs water, and when it is, ethanol intake and BALs are somewhat lower 11;14.
In our current studies, we are exploring the idea that HDID mice may be an excellent candidate subject population for testing novel drugs. Comprehensive reviews of studies attempting to reduce rat preference drinking in the several selectively-bred, high-preferring rat lines describe results for many compounds 2;4. Reviews of studies with the DID limited access drinking model have also shown that many peripherally administered drugs can reduce drinking 15;16. However, a limitation of these DID studies is that they have been conducted nearly exclusively in C57BL/6J inbred mice. C57BL/6J mice have long been known to drink more alcohol than other inbred strains 17, but they represent a single genotype: that is, all same-sex animals have 2 copies of the same gene-specific allele for every gene, making them in some sense like a set of clones. This inbred state may limit the generalizability of findings to other mouse inbred strains and genetically segregating genotypes 18;19. Each human, on the other hand, is genetically unique (with the exception of identical twins). Also, mice of the C57BL/6J genotype do not drink as much in the DID test as the selected line HDID-1 mice and reach significantly lower BALs 10. We reasoned that the specific genetic and phenotypic characteristics of the HDID mice might provide a means to detect drugs that affected binge-like drinking, and/or a specific group of treatment-seeking individuals.
2. Materials and Methods
2.1 Animals and husbandry
All animals were bred and maintained in the Portland VA Medical Center Veterinary Medical Unit in standard polycarbonate cages (19 × 31 × 13 cm) on Bed-o’cobs® bedding (Andersons, Maumee, OH, USA) with stainless steel wire bar tops with a recess for chow. Cages were changed once weekly. Animals were bred and maintained on a reverse 12 hr:12 hr light:dark schedule with lights off at 09:30AM at a room temperature of 21 ± 1°C. Purina 5LOD chow (PMI Nutrition International, Brentwood, MO, USA) was available at all times. Until the beginning of the drinking tests, mice were maintained in groups of 2–5 females or 2–4 males. Mice were than habituated to single housing conditions for at least 1 week prior to the start of testing. All HDID-1 mice were from selected generations S26-S36 and were between the ages of 53–119 days old at the start of testing (see individual experiments for specific sex, generation and age ranges). All procedures were approved by the local Institutional Animal Care and Use Committee and were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals.
HDID-1 mice were developed from a genetically heterogeneous stock (HS/Npt), which was itself created from an 8-way cross of standard inbred strains (including C57BL/6J), using within-family selection for 5 generations and individual selection thereafter. Maintenance of 15–20 families and avoiding common-grandparent matings had led to a cumulative inbreeding coefficient of about 0.3 by S26 11;20. Thus, most of the HDID-1 genome remains segregating.
2.2 Drugs
Acamprosate calcium (Tocris, Minneapolis MN), R(+)-baclofen hydrochloride (Sigma-Aldrich, St. Louis MO) and naltrexone HCl (Sigma-Aldrich) were dissolved in normal saline, which served as the vehicle. Drugs or vehicle were administered by intraperitoneal injection at a volume of 0.1 ml/10 g body weight. For the DID tests, ethanol (200 proof, Decon Labs, King of Prussia, PA) was dissolved in tap water (20% v/v). In some experiments, saccharin sodium salt hydrate (Sigma-Aldrich) was dissolved in tap water to a concentration of 9.2 mM and offered as a drinking solution. Because of the more limited clinical use of disulfiram, we elected not to test this compound 21.
2.3 Drinking in the Dark tests
The standard 4-day DID procedure was used for Experiment 1 9. Mice were weighed at least once a week. Three hours after lights off, the water bottle on each cage was replaced with a single 10 ml pipette tube containing 20% ethanol. On Days 1–3, ethanol was offered for two hours. After 2 hr, the drinking tube was replaced with the standard water bottle. On Day 4, a drug or saline was administered 2½ hour after lights off. Thirty minutes after injection, ethanol was again offered as a single tube (20%), and fluid levels were read at 2 hr and at 4 hr, and then removed. Immediately thereafter, a 20 µl blood sample was taken from the peri-orbital sinus. Blood samples were processed and analyzed to determine blood alcohol levels using gas chromatography 22 or 23. For Experiments 2–4, a variant of the DID test lasting only 2 days was used (where mice were offered 2 hr access on day 1 and 4 hr access on day 2). For discussion of other procedural details about different versions of the DID test, see 24.
2.3.1 Experiment 1. Acute test with 3 drugs
Days 1–3 were 2 hr DID tests with 20% ethanol, and Day 4 repeated the test but allowed tubes to remain for 4 hr. Male and female HDID-1 mice from Selected Generations S30-S31 were 55–119 days old at the start of the study and were distributed approximately equally across treatment groups. On day 4, mice were administered vehicle (15 males, 17 females) or drug (n = 17–18, 7–10/sex/drug) 30 min before ethanol drinking tubes were offered. Drug treatment (and dose) was R-baclofen (10 mg/kg), acamprosate (300 mg/kg), or naltrexone (10 mg/kg). In this initial test, we decided to test the highest effective doses of these clinically relevant compounds. Testing with additional doses was performed in subsequent experiments.
2.3.2 Experiment 2. Dose-response for acamprosate
In this study, mice were subjected to 3 weeks of DID testing, with 2 d of ethanol DID during the first week, 2 days of water DID during the second week, and 2 d of saccharin DID during the third week. Male and female HDID-1 mice from Selected Generations S35-S36 were 71–88 days old at the start. Groups of 23–24 mice were given injections of saline, acamprosate (150 mg/kg) or acamprosate (300 mg/kg) 30 min before ethanol drinking tubes were offered on Day 2. During Days 5–7, all mice had free access to their normal water bottles. In the second week, another 2-day DID study was performed. Mice were given the same injections as they received during the first week of DID (on Day 2, during the ethanol DID), but this time only offered water during the second DID day. During the third week, the same treatment and injections were given, but saccharin (9.2 mM) was offered. No periorbital sinus samples for assessing BALs were drawn after weeks 2 and 3.
2.3.3 Experiment 3. Dose-response for R-baclofen
This 2-day DID study resembled Experiment 2 during weeks 1 and 2. Male and female HDID-1 mice from Selected Generations S33-S35 were 60–102 days old at the start. One mouse died for unknown reasons during the study, and three others were excluded for low initial body weight; these exclusions were unrelated to treatment condition. Groups of 10–14 mice/treatment/sex were given injections of saline, R-baclofen (5 mg/kg) or R-baclofen (10 mg/kg) immediately before ethanol drinking tubes were offered on Day 2. In the second week, the 2-day DID study was repeated. Mice this time were offered a single water tube during the DID test and were given the same injections on Day 2 that they had been given before ethanol. Because of the apparent short duration of the baclofen effect in Experiment 1 (see Results), Day 2 DID sessions were limited to 2 hr in this experiment. As these animals were designated for use in a different study, we did not continue to offer saccharin during a third week.
2.3.4 Experiment 4. Dose-response for naltrexone
This study resembled Week 1 of Experiment 2. However, only male HDID-1 mice (S26-S27, 59–96 days old) were available, and we gave groups of 9–11 mice either saline, 1.0, 4.0, or 8.0 mg/kg naltrexone before Day 2 testing for ethanol DID. One mouse died for unknown reasons before treatment. A leaky tube compromised Day 2 intake data for another mouse, but did not affect its BAL. Naltrexone did not attenuate intake (see Results), so we did not continue to tests with water or saccharin.
2.4 Statistical analyses
We employed one-way or factorial between-subjects ANOVAs as appropriate for each experiment. Where we saw no significant sex differences as main effects or interactions, we performed lower-order ANOVAs on data collapsed across sexes. Post-hoc analyses used the Tukey HSD method. To insure that treatment groups did not differ meaningfully before drug treatment, we analyzed data for the early day(s) of each experiment. Since we generally found no significant differences across treatment groups, we present these data, but not their statistical analyses. Experiment 1 employed a larger, common control group for 3 drugs, so we employed Dunnett’s test to ask only whether each drug group differed from controls. In Experiments 2 and 3, where animals were tested in subsequent weeks with a fluid other than ethanol, drug vs vehicle groups were the same as during the ethanol DID test in Week 1, but we analyzed each week’s data separately. The principal dependent variables of interest were g/kg ethanol intake on Day 2 after 2 hr or total g/kg intake after 4 hr, and BAL at the end of the test (2 or 4 hr). Intakes for other fluids were analyzed as ml/20 g body weight.
3. Results
3.1 Experiment 1: Acute tests with 3 drugs
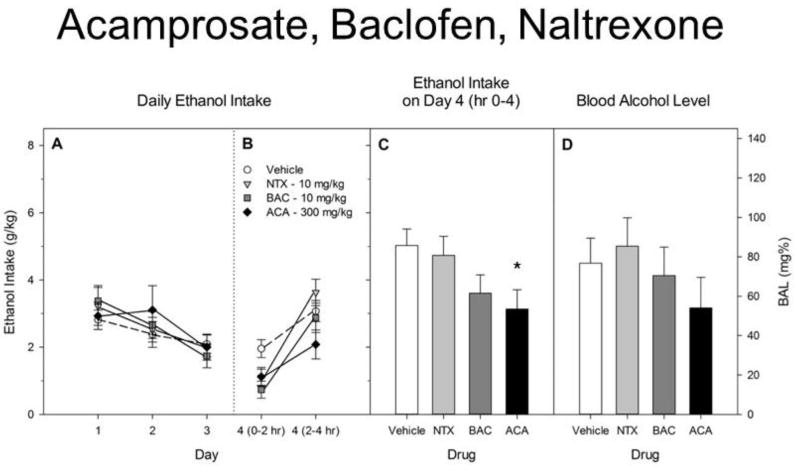
Results are shown in Figure 1. Ethanol intake did not differ significantly among groups during the 3 days before drug administration. Preliminary analyses of Day 4 intake of ethanol during the first 2 hr revealed significant effects of Drug [F(3,75) = 6.8, p < 0.01], but no Sex or Sex X Drug interaction [both F< 1.0]. We therefore pooled the data for the sexes and compared each drug group’s mean with that of the vehicle-treated group using a one way ANOVA followed by Dunnett’s post-hoc test. After 2 hr, baclofen had significantly reduced drinking (p < 0.01), and acamprosate and naltrexone tended to reduce intake (0.05 < p < 0.10). For total intake across 4 hr, there was also a significant effect of Drug [F(3,75) = 2.9, p < 0.05] and a trend toward a main effect of Sex [F(1,76) = 3.9, p = 0.05], but no significant Sex X Drug interaction [F(3,76) = 2.1, p > 0.10]. For total intake after 4 hr, only acamprosate significantly reduced intake (p < 0.05). For BAL at 4 hr, none of the three drugs significantly reduced BAL versus the vehicle-treated group [F < 1].
Figure 1.
A. Ethanol intake during 2 hr DID tests on Days 1–3. Drug groups are shown but did not receive any injections until Day 4. B. Ethanol intake during the first 2 hr and second 2 hr on Day 4. Vehicle, acamprosate (ACA), R-baclofen (BAC) or naltrexone (NTX) was administered ip ½ hr before the DID test. C. Total ethanol intake by each group during the 4 hr DID test on Day 4. Acamposate significantly reduced drinking. D. Blood alcohol level immediately after the Day 4 test.
All groups represent mean ± SE combined data from approximately equal numbers of female and male mice. *p < 0.05.
3.2 Experiment 2. Acamprosate
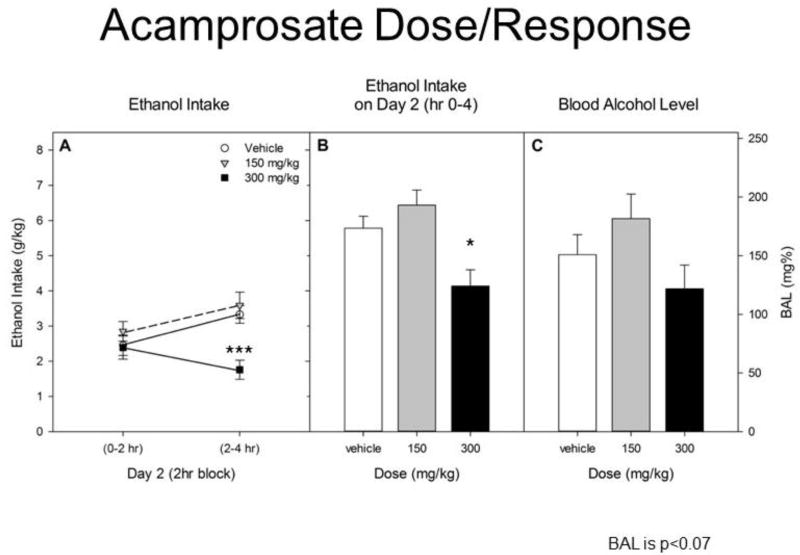
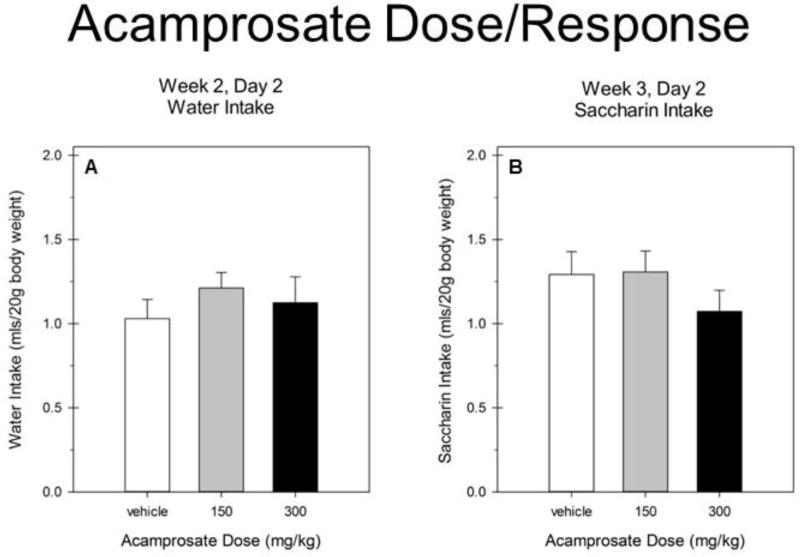
Repeated ethanol intake data across 2-hr blocks of drinking on Day 2 showed a significant main of dose [F(2,63) = 8.7, p < 0.001] and a dose X block interaction [F(2,63) = 3.7, p < 0.05]. No significant effects of sex were observed [all Fs(1–2,63) ≤ 2.14, ps > 0.14]. We therefore report analyses of data collapsed on sex in Figure 2. Separate ANOVAs for each 2hr block showed that 300 mg/kg acamprosate only reduced intake during the second 2 hrs [F(2,66) = 11.4, p ≤ 0.0001]. Total ethanol intake on Day 2 was significantly reduced by acamprosate [F(2,66) = 8.7, p < 0.001] at the 300 mg/kg dose (vs vehicle; Tukey’s HSD p < 0.05). Analyses of BAL revealed a trend toward a significant effect of drug treatment [F(2,66) = 2.8, p = 0.07]. No significant effects of sex were observed in water or saccharin intake (all F(1–2,63) ≤ 2.09, p > 0.13]. We therefore report analyses of data collapsed on sex in Figure 3. Acamprosate did not reduce water or saccharin intake [Fs ≤ 1].
Figure 2.
A. Ethanol intake during the first 2 hr and second 2 hr on Day 2. Vehicle, acamprosate 150 mg/kg or acamprosate 300 mg/kg was administered ip ½ hr before the DID test. B. Total ethanol intake by each group during the 4 hr DID test on Day 2.
Acamposate (300 mg/kg) significantly reduced drinking vs vehicle. C. Blood alcohol level immediately after the Day 2 test.
All groups represent mean ± SE combined data from approximately equal numbers of female and male mice. *p < 0.05. ***p < 0.001.
Figure 3.
A. Water intake during the 4 hr DID test on Day 2 in Week 2. B. Saccharin intake during the 4 hr DID test on Day 2 in Week 3.
All groups represent mean ± SE combined data from approximately equal numbers of female and male mice.
3.3 Experiment 3. Baclofen
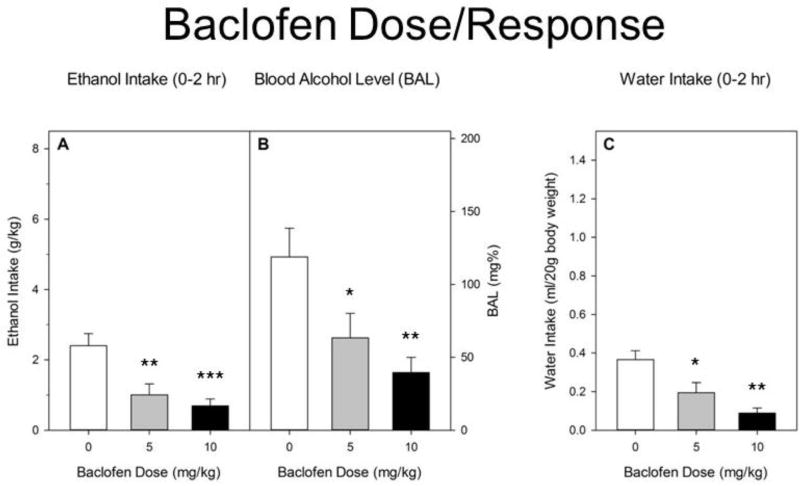
Absent any significant effects of sex (all Fs ≤ 1), we show results of analyses after pooling data across sexes (Figure 4). On Day 2, baclofen significantly reduced ethanol intake [F(2,66) = 9.7, p < 0.001]. Groups given 5 (p < 0.01) or 10 (p < 0.001) mg/kg baclofen differed significantly from controls, but not from each other. Significant reductions in BAL [F(2,66) = 6.4, p < 0.01] paralleled the reductions in intake levels. Both the 10 mg/kg (p < 0.01) and 5 mg/kg (p < 0.05) doses reduced BAL vs vehicle treatment. Baclofen also significantly reduced water intake during the Week 2 test [F(2,65) = 10.8, p < 0.001] at both 5 mg/kg (p < 0.05) and 10 mg/kg (p < 0.001).
Figure 4.
A. Ethanol intake during 2 hr DID test on Day 2 in Week 1. R-baclofen or saline was administered ip immediately before the DID test. Baclofen significantly reduced g/kg ethanol intake at both doses. B. Blood alcohol level immediately after the Day 2 test. Significant reductions in BAL paralleled the reductions in intake levels. C. Baclofen at both doses also significantly reduced water intake during Day 2 of the Week 2 test. All groups represent mean ± SEM. Data combined from approximately equal numbers of female and male mice. *p < 0.05. **p < 0.01. ***p < 0.001.
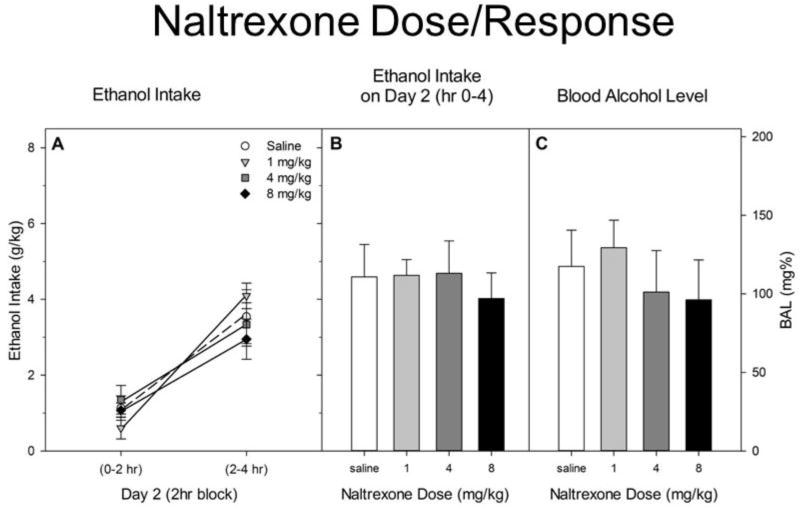
3.4 Experiment 4. Naltrexone
Results are shown in Figure 5. Drinking was significantly lower during the first 2 hr block than during the second [F(1,36) = 85.8, p < 0.001]. However, there was neither a main (F < 1) nor interactive [F(3,36) = 2.0, p > 0.10] effect of naltrexone treatment on drinking. Neither was there a drug effect on BAL (F < 1).
Figure 5.
A. Ethanol intake during hrs 0–2 and 2–4 of the DID test on Day 2. Naltrexone or saline was administered ip ½ hr before the DID test. B. Total ethanol intake by each group during the 4 hr DID test on Day 2. C. Blood alcohol levels immediately after the Day 2 test.
Discussion
We tested three drugs with some degree of known clinical efficacy for their effects on binge-like alcohol drinking in HDID-1 mice. For each drug, we assessed both ethanol intake and the resulting BAL in two experiments. Two drugs, acamprosate and baclofen, reduced drinking dose-dependently, while naltrexone had no effect at doses up to 10 mg/kg.
Acamprosate showed clear efficacy in reducing intake at the 300 mg/kg dose (Figures 1 & 2). It also showed specificity in that this dose did not reduce either water or saccharin intake during subsequent tests (Figure 3). This suggests that the reduced ethanol intake was related to ethanol’s pharmacological and/or behavioral effects rather than through more general mechanisms such as reduced caloric drive, motivation for fluids, taste alteration, or general malaise 19. However, the reductions in ethanol intake did not result in significantly lower BALs at the end of the drinking sessions. Baclofen also yielded promising results, with dose-dependently reduced ethanol intake, in this case accompanied by significantly reduced BALs (Figure 4). Both 5 and 10 mg/kg doses led to BALs below the NIAAA binge threshold. However, baclofen also significantly reduced water intake, with efficacy seemingly about equal to its effects on ethanol drinking. This suggests that the effects may not relate specifically to ethanol’s pharmacology. Finally, naltrexone was apparently without effect on ethanol drinking (Figures 1 & 5) in these mice in this paradigm.
Acamprosate [calcium-bis (N-acetylhomotaurinate)] is an FDA approved treatment for reducing craving and relapse in alcoholics 25;26. Although the exact mechanism of action is unclear, several studies have shown that acamprosate can act as an NMDA modulator and may restore balance to alcohol-induced perturbations in excitatory and inhibitory neurotransmission 25;27. Recent studies have investigated the role of calcium in acamprosate’s actions, yet convergent evidence remains elusive 28;29. Although acamprosate has been studied extensively, there are few published studies of its effects on drinking to intoxication in a DID-like paradigm. Single injections of acamprosate (300 and 400 mg/kg) were reported to reduce ethanol intake when given immediately before 2hr 1-bottle DID tests to male C57BL/6J mice. The 400 mg/kg dose reduced ethanol intake 37% vs vehicle, produced a 42% reduction when water was offered, but did not affect intake of 10% sucrose. The 300 mg/kg dose in that study significantly reduced ethanol intake by 20% without affecting either water or sucrose drinking 30. In our 2 studies, the 300 mg/kg dose (also given ip, but 30 minutes before the 2 hr 1-bottle DID test) significantly reduced ethanol intake by 36% and (non-significantly) BAL by 31% (Figure 1) in Experiment 1; the relative reductions in Experiment 2 were 28% and 20% (Figure 2). While this dose did not affect water intake, it non-significantly reduced saccharin intake by 15% in our Experiment 2 (Figure 3). Given the differences in experimental design and subject genotype (and sex) we find these outcomes to be reasonably consonant. Another study offered male C57BL/6 mice 2 hr limited access to a single bottle of 10% ethanol at unstated clock time for several weeks, and administered two, 50 or 100 mg/kg doses of acamprosate daily for the last 10 days, 12 hr before and 30 min before the ethanol access. Acamprosate dose-dependently reduced intake by the last two-day injection block, by 17% and 35%, respectively. All animals had first been exposed to forced access to 7% ethanol as their only fluid for one week before the tests, and all mice were also given 0.25 mg/kg injections of naltrexone daily accompanying the acamprosate 31. In a 2-bottle choice version of the 2 hr access DID test, male C57BL/6J mice showed no reductions in intake of 15% ethanol following repeated daily injections of 400 mg/kg acamprosate. Mice had previously been acclimated to the ethanol choice for 14 days, and lack of response was found regardless of whether or not the mice had been exposed to chronic unpredictable stress 32.
Repeated injections of acamprosate (300 mg/kg) have been reported to reduce drinking and preference in continuous-access ethanol preference studies in male mPer2 null mutant and wild-type mice on a mixed 129 X C57BL/6 background by reducing both frequency and size of drinking bouts 33. This group also showed similar reductions in intake and preference after microinjections of acamprosate into ventral tegmental area, pedunculopontine area or nucleus accumbens, but not hippocampus 34. Repeated ip injections of acamprosate (300 mg/kg, 15 minutes prior to ethanol access) reduced ethanol intake in a 4 day DID test by 30% in female Clockd19 mutant and wild-type mice on a BALB/c background (Ozburn et al., 2013). These studies elaborated on an earlier report 35. Various studies with rats, mostly employing chronic choice drinking paradigms [e.g.36;37;28] showing positive effects of acamprosate on alcohol drinking have been reviewed 2.
Baclofen, a GABAB receptor agonist, is an FDA approved drug for the treatment of spasticity. Recent studies have revealed that baclofen may prove useful for reducing relapse-like drinking in animal models, and reduce alcohol withdrawal symptoms, drinking, and craving in alcohol dependent individuals [reviewed in 38]. Baclofen has also been reported to affect ethanol drinking in DID procedures using C57BL/6J mice. In a 1 hr single bottle test, one injection of 10 mg/kg baclofen (but not higher or lower doses from 5–20 mg/kg) increased drinking and the resulting BAL 39. However, multiple studies with different test parameters and subject populations including both mice and rats have shown that baclofen could either increase or decrease ethanol intake [for reviews, see 2;40]. Reasoning that enantiomer-specific effects could have contributed to this variability, Steve Boehm’s group showed that a peripheral injection of 10 mg/kg of the R(+) enantiomer deceased DID drinking and BAL in C57BL/6J male mice while the same dose of the S(-) enantiomer modestly increased ethanol intake without affecting BAL. R(+) baclofen also decreased saccharin drinking. The bidirectional effects on drinking were also seen during the three hour window after injection in High Alcohol Preference (HAP1) male and female mice previously exposed to a substantial period of free-access 2-bottle choice (ethanol vs water). Again, R(+) reductions but not S(-) increases were also seen in saccharin drinking 40. Another experiment from this group with male C57BL/6J mice showed that intra-nucleus accumbens shell infusions of the R(+) enantiomer decreased, while the S(-) enantiomer increased ethanol intake 41. Our results with R(+) baclofen in mice are thus in agreement with those of the Boehm group, including the generalization that baclofen reduces intake of a sweet solution (in their case) and water (in ours) in addition to ethanol. They also seem consistent with results that employed a different model of binge-like drinking in C57BL/6J mice, the Scheduled High Access to alcohol Consumption (SHAC) procedure 42.
Naltrexone, a competitive opioid receptor antagonist and toll-like receptor 4 antagonist, is an FDA approved treatment for opioid dependence, as well as alcoholism. The mechanism of action of naltrexone for the treatment of alcoholism is not well understood; however there is evidence that endogenous opioids are important for the positive reinforcing effects of alcohol and that naltrexone reduces the positive reinforcing effects of alcohol 43–46. Perhaps the most surprising of our results was the lack of any effect of naltrexone at doses up to 10 mg/kg (Figures 1 & 5). Several rat papers reporting naltrexone effects over the years have found reduced drinking using operant and choice drinking procedures using various rat genotypes [reviewed in 2]. A recent study reported reduced ethanol intake in the DID procedure with male C57BL/6J mice after single injections of 3 or 10 mg/kg naltrexone, but not doses of 0.3 or 1 mg/kg. Naltrexone also dose-dependently reduced BALs 47. Also using the DID procedure with male C57BL/6J mice, Justin Rhodes’ group had found that single doses of 1 or 2 mg/kg naltrexone significantly reduced ethanol intake by about 25% without reducing water or sucrose intake 30. They also reported similar reductions after 4 mg/kg and more pronounced reductions with 8 and 16 mg/kg. In contrast, we found no significant response in HDID-1 mice to doses of 1, 4, 8 or 10 mg/kg in the two experiments we report here. Besides the difference in subject genotypes, the experiments of the Rhodes group used relatively large groups of mice (typically n = 24) in a more complex, within-subjects experimental design where each animal received 4, 2-day DID tests after saline and one of three naltrexone doses. Results of multiple experiments with different dose combinations were then combined for an omnibus statistical analysis which may have been more sensitive for detecting group (i.e., drug vs vehicle) differences than our simpler, between-subjects approach [30 and Rhodes, personal communication].
Using a different binge drinking procedure (SHAC), genetically heterogeneous mouse lines (WSC-1 and WSC-2) were reported to show reduced intake of 5% ethanol during 30 min periods of access after 0.6 mg/kg naltrexone, and nearly total cessation of ethanol intake after 1.2 mg/kg. Neither dose affected water intake during the rest of the daily fluid access period 42. The SHAC procedure starts with restriction of total daily access to fluids 48, but this is gradually reduced in duration over the many days preceding the drug tests and by the time of testing, the animals are not physiologically fluid deprived 49. While blood alcohol levels were not determined, saline-treated mice ingested sufficient ethanol to reach expected BALs in excess of 100 mg%, and BALs following naltrexone were clearly reduced to below 50 mg% 48. Another study has reported that a 10 mg/kg subcutaneous dose of naltrexone could reduce post-abstinence ethanol drinking in C57BL/6NCRL and DBA/2J mice 50.
Nonetheless, we saw no hint of efficacy in our studies, and we believe that the failure of HDID-1 mice to respond to naltrexone is not due to these methodological differences. Rather, we propose that the HDID-1 genotype renders them insensitive to naltrexone effects on binge-like DID. That they responded to baclofen and acamprosate shows that they are not generally insensitive to drug effects on DID. We have also tested numerous other compounds using variants of the DID experiments described here and found both reductions of drinking (e.g., the PDE-4 inhibitor rolipram, the Bruton’s Tyrosine Kinase inhibitor terreic acid, and a mixed dopamine and serotonin agonist, pergolide) and no effects (e.g., several peroxisome proliferator-activated receptor inhibitors) on drinking. Reports of these studies are currently in preparation (Ferguson et al., submitted; Ozburn et al., in preparation). Additional future work will be aimed at determining whether HDID mice exhibit altered sensitivity to compounds selectively targeting specific neurotransmitter systems.
Because the HDID-1 mice were bred specifically to reach high BALs using this DID assay, they have accumulated specific genetic variations that lead to their high binge phenotype. Thus, their specific pattern of sensitivity may be a correlated response to selection that could also reflect the influence of those selected polymorphisms. This could explain why C57BL/6J mice responded to naltrexone while HDID-1 did not, as the genetic composition of C57BL/6J mice represents the accidental accretion of systematic inbreeding, of which one result is high alcohol drinking across nearly all paradigms examined. A strong test of this hypothesis would be to test the HDID-2 line of mice for these three drugs’ effects on DID 51. However, studies comparing striatal gene expression profiles among HDID-1, HDID-2 and the non-selected, genetically heterogeneous stock from which they were selected (HS/Npt) show that the HDID-1 and HDID-2 lines differ substantially genetically from each other as well as from HS/Npt, indicating that directional selection has achieved very similar phenotypic endpoints in the two binge selected lines by exerting influence on different genes as well as some common genes 52. Genomic studies with both HDID-1 and HDID-2 lines and their progenitor HS stock have suggested that the influence of intense selective breeding has predominantly been on the structural coherence of gene co-expression networks, rather than on changing the basal levels of expression of a common set of genes 52. Thus, the transcriptional signatures of the HDID lines may offer opportunities to explore “personalized medicine” approaches to treating specific subjects.
If this drug sensitivity pattern is indeed related to the selection, another possible explanation could be ventured based on putative subjective response to the drugs. It has been proposed that in humans, acamprosate modulates the negative reinforcement associated with alcohol and that naltrexone modulates positive reinforcing effects 46. Both HDID-1 and HDID-2 mice were found to be less sensitive than the HS/Npt stock to the conditioned aversive effects of alcohol (a taste aversion for a novel fluid conditioned by injection of 2 g/kg ethanol). The three genotypes did not differ in sensitivity to the conditioned rewarding effects of ethanol (ethanol conditioned place preference; 53. Thus, we speculate that naltrexone may exert a negative interoceptive state when combined with ingestion of intoxicating doses of ethanol. If acamprosate and baclofen do not do so, this could explain the failure of HDID-1 mice to respond to specifically to naltrexone. Neither naltrexone nor acamprosate reduce drinking or symptoms in all those with AUD. Several hypotheses have been ventured as bases for individuals’ responsiveness. A recent systematic literature review of clinical trials examined several potential mediators of response to naltrexone including a sweet-liking phenotype and craving for alcohol. These authors concluded that a family history positive for alcoholism or presence of the OPRM1 Asn-40Asp polymorphism had strongest support, but that evidence for all proposed mediators remained inconclusive at this point 54. A meta-analysis examining many factors concluded that both naltrexone and acamprosate had efficacy in those who were dependent on alcohol 55.
It remains possible that our results are simply an idiosyncrasy of our specific DID assay. As with any laboratory assay, the DID test offers both advantages and disadvantages, which are discussed elsewhere 14. We would not expect to detect all compounds that prove later to be clinically useful (e.g., naltrexone). We did not examine animals dependent on ethanol, nor those in a state of withdrawal. Thus, a potential therapeutic effective only in already-dependent subjects that worked to reduce alcohol craving would likely not be detected in this screen. We did not treat repeatedly with drugs, so we have no information about the potential for drug tolerance or sensitization. On the positive side, we can screen a novel compound relatively quickly and efficiently. The short duration of the DID test makes it possible to test a compound with a short elimination half-life like baclofen. A positive finding could potentially lead to development of a longer-acting drug with clinical efficacy. We are proceeding to test other drugs, and have thus far found far more negative than positive results, which potentially could discourage progressing to perform some clinical trials that will eventually fail 3. Finally, we believe that the reduction in drinking that results in a non-intoxicating BAL is a major advantage of this test and genetic animal model. We hope that these lines will prove to be a useful addition to the array of laboratory tools to search for novel therapeutics.
Highlights.
HDID mice offer a novel genetic animal model for binge-like alcohol drinking
Drinking in the dark can be used to screen novel pharmacotherapies
Acamprosate and R-baclofen reduce drinking in HDID mice respond to
Naltrexone does not reduce drinking in HDID mice
Acknowledgments
We thank the following individuals for technical assistance: Dove Spector, Tanvi Batish, Snigdha Kanadibhotla, Katherine LeBlanc, Gian Greenberg, and Chelsea Lin
Source of Funding: Supported by the NIAAA [Integrative Neuroscience Initiative on Alcoholism (INIA-Neuroimmune) grant AA013519; NIAAA Center grant AA10760; NIAAA R24 AA020245; NIAAA F31 AA022009; the US Department of Veterans Affairs Grants BX000313 and CDA2 BX002488; and the John R. Andrews Family. Authors have full control of all primary data and agree to allow the journal and any subsequent readers of the published work to review the data if requested.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: All authors report no conflicts of interest.
Reference List
- 1.Testino G, Leone S, Borro P. Treatment of alcohol dependence: recent progress and reduction of consumption. Minerva Med. 2014;105:447–466. [PubMed] [Google Scholar]
- 2.Bell RL, Hauser SR, Liang T, Sari Y, Maldonado-Devincci A, Rodd ZA. Rat animal models for screening medications to treat alcohol use disorders. Neuropharmacology. 2017 doi: 10.1016/j.neuropharm.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egli M. Can experimental paradigms and animal models be used to discover clinically effective medications for alcoholism? Addiction Biology. 2005;10:309–319. doi: 10.1080/13556210500314550. [DOI] [PubMed] [Google Scholar]
- 4.Bell RL, Sable HJ, Colombo G, Hyytia P, Rodd ZA, Lumeng L. Animal models for medications development targeting alcohol abuse using selectively bred rat lines: Neurobiological and pharmacological validity. Pharmacol Biochem Behav. 2012;103:119–155. doi: 10.1016/j.pbb.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oberlin B, Best C, Matson L, Henderson A, Grahame N. Derivation and characterization of replicate high- and low-alcohol preferring lines of mice and a high-drinking crossed HAP line. Behav Genet. 2011;41:288–302. doi: 10.1007/s10519-010-9394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crabbe JC. Use of animal models of alcohol-related behavior. In: Pfefferbaum A, Sullivan EV, editors. Handbook of Clinical Neurology: Alcoholism. New York: Elsevier; 2013. [DOI] [PubMed] [Google Scholar]
- 7.NIAAA Council Approves Definition of Binge Drinking. NIAAA Newsletter. 2004;3 [Google Scholar]
- 8.Sharpe AL, Tsivkovskaia NO, Ryabinin AE. Ataxia and c-Fos expression in mice drinking ethanol in a limited access session. Alcohol Clin Exp Res. 2005;29:1419–1426. doi: 10.1097/01.alc.0000174746.64499.83. [DOI] [PubMed] [Google Scholar]
- 9.Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiology and Behavior. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Crabbe JC, Metten P, Rhodes JS, et al. A line of mice selected for high blood ethanol concentrations shows drinking in the dark to intoxication. Biological Psychiatry. 2009;65:662–670. doi: 10.1016/j.biopsych.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crabbe JC, Metten P, Belknap JK, et al. Progress in a replicated selection for elevated blood ethanol concentrations in HDID mice. Genes Brain Behav. 2014;13:236–246. doi: 10.1111/gbb.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crabbe JC, Metten P, Huang LC, et al. Ethanol withdrawal-associated drinking and drinking in the dark: Common and discrete genetic contributions. Addiction Genetics. 2012;1:3–11. doi: 10.2478/addge-2012-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crabbe JC, Spence SE, Brown LL, Metten P. Alcohol preference drinking in a mouse line selectively bred for high drinking in the dark. Alcohol. 2011;45:427–440. doi: 10.1016/j.alcohol.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barkley-Levenson AM, Crabbe JC. High drinking in the dark mice: a genetic model of drinking to intoxication. Alcohol. 2014;48:217–223. doi: 10.1016/j.alcohol.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crabbe JC, Harris RA, Koob GF. Preclinical studies of alcohol binge drinking. Ann N Y Acad Sci. 2011;1216:24–40. doi: 10.1111/j.1749-6632.2010.05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fritz BM, Boehm SL. Rodent models and mechanisms of voluntary binge-like ethanol consumption: Examples, opportunities, and strategies for preclinical research. Prog Neuropsychopharmacol Biol Psychiatry. 2015 doi: 10.1016/j.pnpbp.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wahlsten D, Bachmanov A, Finn DA, Crabbe JC. Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proceedings of the National Academy of Sciences USA. 2006;103:16364–16369. doi: 10.1073/pnas.0605342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Didion JP, de Villena FP. Deconstructing Mus gemischus: advances in understanding ancestry, structure, and variation in the genome of the laboratory mouse. Mamm Genome. 2013;24:1–20. doi: 10.1007/s00335-012-9441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crabbe JC. The genetic complexity of alcohol drinking in rodents. In: Noronha ABCCCHRACJC, editor. The Neurobiology of Alcohol Dependence. San Diego: Elsevier/Academic Press; 2014. pp. 359–375. [Google Scholar]
- 20.Falconer DS, Mackay TFC. Introduction to Quantitative Genetics. 4. Harlow, England: Longman; 1996. [Google Scholar]
- 21.Crowley P. Long-term drug treatment of patients with alcohol dependence. Aust Prescr. 2015;38:41–43. doi: 10.18773/austprescr.2015.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rustay NR, Crabbe JC. Genetic analysis of rapid tolerance to ethanol's incoordinating effects in mice: Inbred strains and artificial selection. Behav Genet. 2004;34:441–451. doi: 10.1023/B:BEGE.0000023649.60539.dd. [DOI] [PubMed] [Google Scholar]
- 23.Finn DA, Snelling C, Fretwell AM, et al. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12–41) Alcohol Clin Exp Res. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- 24.Thiele TE, Crabbe JC, Boehm SL. "Drinking in the Dark" (DID): a simple mouse model of binge-like alcohol intake. Curr Protoc Neurosci. 2014;68:9. doi: 10.1002/0471142301.ns0949s68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olive MF, Cleva RM, Kalivas PW, Malcolm RJ. Glutamatergic medications for the treatment of drug and behavioral addictions. Pharmacol Biochem Behav. 2012;100:801–810. doi: 10.1016/j.pbb.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yahn SL, Watterson LR, Olive MF. Safety and efficacy of acamprosate for the treatment of alcohol dependence. Subst Abuse. 2013;6:1–12. doi: 10.4137/SART.S9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiefer F, Mann K. Acamprosate: how, where, and for whom does it work? Mechanism of action, treatment targets, and individualized therapy. Curr Pharm Des. 2010;16:2098–2102. doi: 10.2174/138161210791516341. [DOI] [PubMed] [Google Scholar]
- 28.Spanagel R, Vengeliene V, Jandeleit B, et al. Acamprosate produces its anti-relapse effects via calcium. npp. 2014;39:783–791. doi: 10.1038/npp.2013.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mann K, Hoffmann S, Pawlak CR. Does Acamprosate Really Produce its Anti-Relapse Effects via Calcium? No Support from the PREDICT Study in Human Alcoholics. npp. 2016;41:659–660. doi: 10.1038/npp.2015.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamdar NK, Miller SA, Syed YM, Bhayana R, Gupta T, Rhodes JS. Acute effects of naltrexone and GBR 12909 on ethanol drinking-in-the-dark in C57BL/6J mice. Psychopharmacology. 2007;192:207–217. doi: 10.1007/s00213-007-0711-5. [DOI] [PubMed] [Google Scholar]
- 31.Kim SG, Han BD, Park JM, Kim MJ, Stromberg MF. Effect of the combination of naltrexone and acamprosate on alcohol intake in mice. Psychiatry Clin Neurosci. 2004;58:30–36. doi: 10.1111/j.1440-1819.2004.01189.x. [DOI] [PubMed] [Google Scholar]
- 32.Ho AM, Qiu Y, Jia YF, et al. Combined Effects of Acamprosate and Escitalopram on Ethanol Consumption in Mice. Alcohol Clin Exp Res. 2016;40:1531–1539. doi: 10.1111/acer.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brager AJ, Prosser RA, Glass JD. Circadian and acamprosate modulation of elevated ethanol drinking in mPer2 clock gene mutant mice. Chronobiol Int. 2011;28:664–672. doi: 10.3109/07420528.2011.601968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brager A, Prosser RA, Glass JD. Acamprosate-responsive brain sites for suppression of ethanol intake and preference. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1032–R1043. doi: 10.1152/ajpregu.00179.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spanagel R, Pendyala G, Abarca C, et al. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- 36.Olive MF, Nannini MA, Ou CJ, Koenig HN, Hodge CW. Effects of acute acamprosate and homotaurine on ethanol intake and ethanol-stimulated mesolimbic dopamine release. Eur J Pharmacol. 2002;437:55–61. doi: 10.1016/s0014-2999(02)01272-4. [DOI] [PubMed] [Google Scholar]
- 37.Spanagel R, Holter SM, Allingham K, Landgraf R, Zieglgansberger W. Acamprosate and alcohol: I. Effects on alcohol intake following alcohol deprivation in the rat. ejp. 1996;305:39–44. doi: 10.1016/0014-2999(96)00174-4. [DOI] [PubMed] [Google Scholar]
- 38.Agabio R, Colombo G. GABAB receptor ligands for the treatment of alcohol use disorder: preclinical and clinical evidence. Front Neurosci. 2014;8:140. doi: 10.3389/fnins.2014.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore EM, Serio KM, Goldfarb KJ, Stepanovska S, Linsenbardt DN, Boehm SL. GABAergic modulation of binge-like ethanol intake in C57BL/6J mice. Pharmacol Biochem Behav. 2007;88:105–113. doi: 10.1016/j.pbb.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kasten CR, Blasingame SN, Boehm SL. Bidirectional enantioselective effects of the GABAB receptor agonist baclofen in two mouse models of excessive ethanol consumption. Alcohol. 2015;49:37–46. doi: 10.1016/j.alcohol.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kasten CR, Boehm SL. Intra-nucleus accumbens shell injections of R(+)- and S(−)-baclofen bidirectionally alter binge-like ethanol, but not saccharin, intake in C57Bl/6J mice. Behav Brain Res. 2014;272:238–247. doi: 10.1016/j.bbr.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanchuck MA, Yoneyama N, Ford MM, Fretwell AM, Finn DA. Assessment of GABA-B, metabotropic glutamate, and opioid receptor involvement in an animal model of binge drinking. Alcohol. 2011;45:33–44. doi: 10.1016/j.alcohol.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Littleton J, Zieglgansberger W. Pharmacological mechanisms of naltrexone and acamprosate in the prevention of relapse in alcohol dependence. Am J Addict. 2003;12(Suppl 1):S3–11. doi: 10.1111/j.1521-0391.2003.tb00492.x. [DOI] [PubMed] [Google Scholar]
- 44.Heilig M, Thorsell A, Sommer WH, et al. Translating the neuroscience of alcoholism into clinical treatments: from blocking the buzz to curing the blues. Neurosci Biobehav Rev. 2010;35:334–344. doi: 10.1016/j.neubiorev.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heilig M, Goldman D, Berrettini W, O'Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat Rev Neurosci. 2011;12:670–684. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mann K, Vollstadt-Klein S, Reinhard I, et al. Predicting naltrexone response in alcohol-dependent patients: the contribution of functional magnetic resonance imaging. Alcohol Clin Exp Res. 2014;38:2754–2762. doi: 10.1111/acer.12546. [DOI] [PubMed] [Google Scholar]
- 47.Navarro M, Carvajal F, Lerma-Cabrera JM, Cubero I, Picker MJ, Thiele TE. Evidence that Melanocortin Receptor Agonist Melanotan-II Synergistically Augments the Ability of Naltrexone to Blunt Binge-Like Ethanol Intake in Male C57BL/6J Mice. Alcohol Clin Exp Res. 2015;39:1425–1433. doi: 10.1111/acer.12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finn DA, Belknap JK, Cronise K, Yoneyama N, Murillo A, Crabbe JC. A procedure to produce high alcohol intake in mice. Psychopharmacology. 2005;178:471–480. doi: 10.1007/s00213-004-2039-8. [DOI] [PubMed] [Google Scholar]
- 49.Toth LA, Gardiner TW. Food and water restriction protocols: physiological and behavioral considerations. Contemp Top Lab Anim Sci. 2000;39:9–17. [PubMed] [Google Scholar]
- 50.Tomie A, Azogu I, Yu L. Effects of naltrexone on post-abstinence alcohol drinking in C57BL/6NCRL and DBA/2J mice. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:240–247. doi: 10.1016/j.pnpbp.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crabbe JC, Phillips TJ, Kosobud A, Belknap JK. Estimation of genetic correlation: interpretation of experiments using selectively bred and inbred animals. Alcohol Clin Exp Res. 1990;14(2):141–151. doi: 10.1111/j.1530-0277.1990.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 52.Iancu OD, Overbeck D, Darakjian P, et al. High Drinking in the Dark selected lines and brain gene coexpression networks. Alcoholism Clinical and Experimental Research. 2013;37:1295–1303. doi: 10.1111/acer.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barkley-Levenson AM, Cunningham CL, Smitasin PJ, Crabbe JC. Rewarding and aversive effects of ethanol in High Drinking in the Dark selectively bred mice. Addict Biol. 2015;20:80–90. doi: 10.1111/adb.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garbutt JC, Greenblatt AM, West SL, et al. Clinical and biological moderators of response to naltrexone in alcohol dependence: a systematic review of the evidence. Addiction. 2014;109:1274–1284. doi: 10.1111/add.12557. [DOI] [PubMed] [Google Scholar]
- 55.Donoghue K, Elzerbi C, Saunders R, Whittington C, Pilling S, Drummond C. The efficacy of acamprosate and naltrexone in the treatment of alcohol dependence, Europe versus the rest of the world: a meta-analysis. Addiction. 2015;110:920–930. doi: 10.1111/add.12875. [DOI] [PubMed] [Google Scholar]