Abstract
AIM
To investigate the effects of herb-partitioned moxibustion (HPM) on phosphorylation of mitogen-activated extracellular signal-regulated kinase (MEK)1, extracellular signal-regulated kinase (ERK)1/2 and cAMP response element binding protein (CREB) in spinal cord of rats with chronic inflammatory visceral pain (CIVP), and to explore the central mechanism of HPM in treating CIVP.
METHODS
Male Sprague-Dawley rats were randomized into normal, model, HPM, sham-HPM, MEK-inhibitor and dimethyl sulfoxide (DMSO) groups. The CIVP model was established using an enema mixture of trinitrobenzene sulfonic acid and ethanol. HPM was applied at bilateral Tianshu (ST25) and Qihai (CV6) acupoints in the HPM group, while in the sham-HPM group, moxa cones and herb cakes were only placed on the same points but not ignited. The MEK-inhibitor and DMSO groups received L5-L6 intrathecal injection of U0126 and 30% DMSO, respectively. Abdominal withdrawal reflex (AWR), mechanical withdrawal threshold (MWT) and thermal withdrawal latency (TWL) were applied for the assessment of pain behavior. The colonic tissue was observed under an optical microscope after hematoxylin-eosin staining. Expression of phosphor (p)MEK1, pERK1/2 and pCREB in rat spinal cord was detected using Western blotting. The levels of MEK, ERK and CREB mRNA in rat spinal cord were detected using real-time polymerase chain reaction.
RESULTS
Compared with the normal group, the AWR scores were increased significantly (P < 0.01) and the MWT and TWL scores were decreased significantly (P < 0.05) in the model, sham-HPM and DMSO groups. Compared with the model group, the AWR scores were decreased significantly (P < 0.01) and the MWT and TWL scores were increased significantly in the HPM and MEK-inhibitor groups (P < 0.05). Compared with the sham-HPM and DMSO groups, the AWR scores were decreased significantly (P < 0.01) and the MWT and TWL scores were increased significantly (P < 0.05) in the HPM and MEK-inhibitor groups. Compared with the normal group, the expression of pMEK1, pERK1/2 and pCREB proteins and the levels of MEK, ERK and CREB mRNA in rat spinal cord were increased significantly in the model, sham-HPM and DMSO groups (P < 0.01 or < 0.05). Compared with the model group, the expression of pMEK1, pERK1/2 and pCREB proteins and the levels of MEK, ERK and CREB mRNA in rat spinal cord were reduced significantly in the HPM and MEK-inhibitor groups (P < 0.01 or < 0.05). Compared with the sham-HPM and DMSO groups, expression of pMEK1, pERK1/2 and pCREB proteins and the levels of MEK, ERK and CREB mRNA in rat spinal cord were reduced significantly in the HPM and MEK-inhibitor groups (P < 0.01 or < 0.05).
CONCLUSION
HPM down-regulates protein phosphorylation of MEK1, ERK1/2 and CREB, and mRNA expression of MEK, ERK and CREB, inhibiting activation of the MEK/ERK/CREB signaling pathway in the spinal cord of CIVP rats, which is possibly a critical central mechanism of the analgesic effect of HPM.
Keywords: Herb-partitioned moxibustion, Chronic inflammatory visceral pain, Pain behavior, Analgesia, MEK, Extracellular signal-regulated kinase, cAMP response element binding protein, Signaling pathway
Core tip: Chronic inflammatory visceral pain (CIVP) (abdominal pain) is one of the major symptoms of inflammatory bowel disease. Herb-partitioned moxibustion (HPM) at bilateral Tianshu (ST25) and Qihai (CV6) acupoints was effective in enhancing the pain threshold and easing pain in CIVP rats. Levels of phosphorylated protein and mRNA expression of mitogen-activated extracellular signal-regulated kinase 1, extracellular signal-regulated kinase and cAMP response element binding protein were abnormally increased in the spinal cord of CIVP rats. HPM effectively down-regulated the abnormally increased expression, which is possibly one of the analgesic mechanisms of HPM.
INTRODUCTION
Chronic inflammatory visceral pain (CIVP) is a complex process involving a variety of neurotransmitters, cytokines, and signaling pathways. The extracellular signal-regulated kinase (ERK) signaling pathway is a major branch of the mitogen-activated protein kinase (MAPK) pathway and has been thoroughly explored. The ERK pathway is responsible for the regulation of pain and inflammation[1]. It has been shown that electroacupuncture (EA) can enhance the pain threshold of rats with inflammatory pain in the hind paw and that this effect is mediated via regulation of the ERK signaling pathway[2].
Mitogen-activated extracellular signal-regulated kinase (MEK)/ERK/CREB is part of the ERK pathway. MEK resides in the upstream region of the ERK pathway, and induces ERK1/2 through phosphorylating its threonine and tyrosine. Activated ERK1/2 regulates phosphorylation of various proteins such as cAMP-response element binding protein (CREB) and transcription factors (TFs), subsequently influencing multiple biological functions[3]. The MEK/ERK/CREB signaling pathway plays an important role in modulating the transmission and maintenance of pain signals[4,5].
Our previous studies have demonstrated that herb-partitioned moxibustion (HPM) reduces chronic inflammatory pain in rat models of trinitrobenzene sulfonic (TNBS)/ethanol-induced ulcerative colitis (UC)[6,7]. However, the questions remain whether the MEK/ERK/CREB signaling pathway is involved in CIVP and whether HPM exerts its analgesic effect via regulation of this pathway. We used Western blotting and real-time polymerase chain reaction (PCR) to observe the effects of HPM on protein phosphorylation and mRNA expression of MEK, ERK and CREB in the spinal cord of rats with TNBS/ethanol-induced CIVP, to discover the analgesic mechanism of HPM from the perspective of the MEK/ERK/CREB signaling pathway.
MATERIALS AND METHODS
Animals
Fifty-four healthy adult male Sprague-Dawley rats, weighing 150 ± 20 g, were provided by Shanghai Sippr-BK Laboratory Animal Co. Ltd. [license no: SCXK(Hu)2013-0016]. The rats were housed in a room with a 12/12-h light/dark cycle (08:00-20:00 light; 20:00-08:00 dark). The feeding room and behavior detection room were both at 20 ± 1 °C with a relative humidity of 50%. After 1 wk of adaptive feeding, the rats all presented with normal behavior for ingestion and drinking and were included in the investigation. The experiment was conducted following the Guide for the Care and Use of Laboratory Animals, and the protocol was approved by the Committee on Use of Human and Animal Subjects in Teaching and Research, Shanghai University of Traditional Chinese Medicine.
Using a completely randomized design, the 54 rats were randomized into normal, model, HPM, sham-HPM, MEK-inhibitor and DMSO groups, with 9 rats each. A TNBS/ethanol-induced CIVP rat model was replicated in the six groups, except for the normal group[8,9]. Prior to modeling, the rats were fasted for 24 h but allowed free access to water. CIVP was induced with 5% (w/v) TNBS and 50% ethanol in a 2:1 ratio. The rats were weighed and then anesthetized with 2% pentobarbital sodium intraperitoneally at 30 mg/kg. The TNBS enema solution was administered at 3 mL/kg with the needle inserted into anus by 6-8 cm. After enema administration, the tails of the rats were lifted to keep the rats upside down for 1 min. The enema was performed once every 7 d, for four times in total.
After modeling, the rats were subjected to pain behavior tests and one rat from each group was killed to collect colon tissue for hematoxylin-eosin (HE) staining to verify successful induction of the model; after which, the intervention began.
Main reagents and instruments
The following reagents and instruments were used: 5% TNBS (Sigma, St Louis, MO, United States); phospho (p)MEK antibody (Cell Signaling Technology, Danvers, MA, United States); pERK antibody (Cell Signaling Technology); pCREB antibody (Cell Signaling Technology); MEK inhibitor U0126 (Calbiochem, Darmstadt, Germany); DMSO (Santa Cruz Biotechnology, Dallas, TX, United States); horseradish peroxidase goat anti-rabbit IgG (Beyotime, Shanghai, China); Von Frey filaments (Stoelting Co, Wood Dale, IL, United States); BME2410A thermal stimulator (Institute of Medical Biology, Chinese Academy of Medical Sciences, Beijing, China); electrophoresis system: Mini-PROTEAN 3 cell (Bio-Rad, Hercules, CA, United States); imaging system: ChemiDocTM (Bio-Rad, Hercules, CA, United States); PCR primers ABI 7300 (Thermo Fisher Scientific, Waltham, MA, United States).
Pain behavior tests
Abdominal withdrawal reflex (AWR) was measured based on Al-Chaer’s method[10] by offering a series of colorectal distending (CRD) stimuli: 20, 40, 60 and 80 mmHg. Each rat was measured three times; each stimulus lasted 20 s, with an interval of 5 min, and the average value of the three measurements was considered as the final record.
Mechanical withdrawal threshold (MWT) was measured using a Von Frey filament (gradient folding force: 2.0, 4.0, 6.0, 8.0 and 15.0 g) to poke perpendicularly the center of the hind paw, lasting ≤ 4 s[11]. It was considered positive when the rat lifted or licked the hind paw. The test started with 2 g; each force was applied five times, with an interval of 30 s between measurements. The maximum force was 15 g, as was the maximum MWT value. The 50% positive response threshold was calculated using an up-down method.
According to Hargreaves’ method[12], we examined the thermal withdrawal latency (TWL) by radiating the hind paw with a thermal stimulator. The duration from the beginning of radiation to the withdrawal reaction was designated the TWL. The stimulation was controlled at the same intensity and automatically cut off at 20.1 s to prevent burns. Each rat was examined five times, with an interval of 3 min. The maximum and minimum values were excluded and the mean of the middle three values was taken as the final record.
Intervention
Rats in the normal and model groups were only taken out and fixed at the same time as those in the HPM group but did not receive any treatment. Rats in the HPM group received HPM treatment at bilateral Tianshu (ST25) and Qihai (CV6) points[13-15], with two moxa cones for each point at each session (about 10 min); one session was performed every day, for a total of seven sessions. The herb cakes were mainly made of Aconiti praeparata powder and yellow wine. The moxa cones were made using a specific mold, and each moxa cone weighed 90 mg. HPM treatment was performed as shown in Figure 1.
Figure 1.
Herb-partitioned moxibustion treatment.
In the sham-HPM group[16,17], when the rats were fixed in position, the moxa cones were placed on the same acupoints for 10 min but not ignited. The control procedure was also conducted once every day, for seven times in total. Rats in the MEK-inhibitor group received intrathecal injection at L5-L6 of U0126 which was dissolved in 30% DMSO solution[18] (1 μg/μL), 10 μL each time, once every day, for 7 consecutive days. Rats in the DMSO group received intrathecal injection of 30% DMSO at the same location, 10 μL each time, once per day, for 7 consecutive days.
Sample collection and processing
Pain behavior was retested on the second day of the last intervention session. The rats were killed for collection of colon and spinal cord tissue. After weighing and anesthetic overdose, the abdomen was dissected along the median line and 5 cm of colon was rapidly removed. After cutting longitudinally and rinsing, the colonic tissues were fixed in 4% formaldehyde solution. The samples were then dehydrated in an alcohol series, made transparent, and embedded for subsequent use. The spinal cord from L6 to S2 was collected and kept at -80 °C.
Pathological observation of colonic tissue
The colonic samples were stained with HE to observe the pathological alterations under an optical microscope.
Western blotting of pMEK1, pERK1/2 and pCREB proteins in spinal cord
The spinal cord tissues were removed from the -80 °C freezer, weighed and mixed with lysate solution in a proportion of 100 mg:1 mL. The sample was centrifuged at 4 °C and 12000 r/min for 15 min, and the supernatant obtained contained total protein. The bicinchoninic acid method was used for the determination of total protein content. Electrophoresis was performed with each well containing 50 μg total protein.
The resolved protein sample was transferred to a nitrocellulose membrane using a wet transfer printing method. Primary antibody (1:1000) was incubated overnight at 4 °C, followed by secondary antibody (1:1000) for 2 h at room temperature. After 2-min incubation with electrochemiluminescence, the samples were analyzed using a Bio-Rad imaging system. The gray values of target protein and internal reference protein bands were read, and the ratio between the gray value of the target protein band and that of the internal reference protein band was taken as the relative expression level.
Detection of MEK, ERK and CREB mRNAs by real-time PCR
TRIzol reagent (Invitrogen, Carlsbad, CA, United States) was used to acquire total RNA from the tissues. A total RNA equivalent was used to reverse transcribe cDNA using a Thermo Scientific PCR kit (United States). ABI Prism7300 SDS software was used to analyze the mRNA expression level of the target gene. The relative mRNA expression of a target gene was calculated as 2-ΔΔCT × 100%; ΔCT = CT value of the target gene - CT value of the internal reference (GAPDH). The sequences of primers were as follows: GAPDH forward 5’-GGAGAAACCTGCCAAGTATG-3’, reverse 5’-GACAACCTGGTCCTCAGTGT-3’; MEK forward 5’-AGAGCGTGCAGACCTCAAGCT-3’, reverse 5’-AGCCGCAGGGTTCTGCATA-3’; ERK forward 5’-ATCAACACCACCTGCGACCTT-3’, reverse 5’-TGGCCACATACTCGGTCAGAA-3’; CREB forward 5’-CCACTCAGCCGGGTCTACCAT-3’, reverse 5’-CCAGAGGCAGCTTGAACAACA-3’.
Statistical analysis
SPSS version 19.0 software was used for data analysis. Measurement data in normal distribution were expressed as mean ± SD; measurement data in non-normal distribution were expressed as median (P25, P75). One-way analysis of variance was used to analyze the inter-group differences if the data followed a normal distribution, least significant difference (LSD) for those with homogeneous variances, and Games-Howell for those with heterogeneous variances. A rank-sum test was adopted if the data did not follow a normal distribution. P < 0.05 indicated statistical significance.
RESULTS
Comparison of AWR scores
Compared with the normal group, the AWR scores were increased significantly in the model, sham-HPM and DMSO groups under 20, 40, 60 and 80 mmHg distension (P < 0.01). Compared with the model group, the AWR scores were decreased significantly in the HPM and MEK-inhibitor groups after intervention under the four levels of distension (P < 0.01). Compared with the sham-HPM group, the AWR scores were decreased significantly in the HPM and MEK-inhibitor groups after intervention under the four levels of distension (P < 0.01). Compared with the DMSO group, the AWR scores decreased significantly in the HPM and MEK-inhibitor groups after intervention (P < 0.01). The results indicated that HPM and U0126 reduced the AWR scores and enhanced the pain threshold in the CIVP rats (Figure 2).
Figure 2.

Comparison of the abdominal withdrawal reflex scores after treatment. A: AWR scores under 20 mmHg CRD stimulation; B: AWR scores under 40 mmHg CRD stimulation; C: AWR scores under 60 mmHg CRD stimulation; D: AWR scores under 80 mmHg CRD stimulation. bP < 0.01 vs normal group; dP < 0.01 vs model group; fP < 0.01 vs sham-HPM group; hP < 0.01 vs DMSO group. AWR: Abdominal withdrawal reflex; CRD: Colorectal distending; NG: Normal group; MG: Model group; SHPMG: Sham-HPM group; MIG: MEK-inhibitor group; DG: Dimethyl sulfoxide group; HPM: Herb-partitioned moxibustion.
Comparison of MWT and TWL scores
Compared with the normal group, the MWT and TWL scores were decreased significantly in the model, sham-HPM and DMSO groups (P < 0.05). Compared with the model group, the MWT and TWL scores were increased significantly in the HPM and MEK-inhibitor after intervention (P < 0.05). Compared with the sham-HPM group, the MWT and TWL scores were increased significantly in the HPM and MEK-inhibitor groups after intervention (P < 0.05). Compared with the DMSO group, the MWT and TWL scores were increased significantly in the HPM and MEK-inhibitor groups after intervention (P < 0.05). This indicated that HPM and MEK inhibitor enhanced the MWT and TWL scores, and the pain threshold of the CIVP rats (Figure 3).
Figure 3.
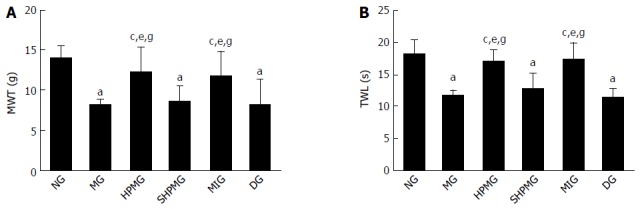
Comparison of the mechanical withdrawal threshold and thermal withdrawal latency scores. A: MWT test used the Von Frey filament; B: TWL test followed Hargreaves. aP < 0.05 vs normal group; cP < 0.05 vs model group; eP < 0.05 vs sham-HPM group; gP < 0.05 vs DMSO group. MWT: Mechanical withdrawal threshold; TWL: Thermal withdrawal latency; NG: Normal group; MG: Model group; SHPMG: Sham-HPM group; MIG: MEK-inhibitor group; DG: Dimethyl sulfoxide group; HPM: Herb-partitioned moxibustion.
Histomorphological observation of colonic tissue
In the normal group, colonic epithelium had the following: a clear structure; mucosa that was complete; arrangement of glands that was regular; and an occasional minor infiltration of inflammatory cells in the lamina propria, without hyperemia, edema or ulceration. In the model group, colonic tissue had an indistinct structure, substantially impaired mucosa, ulcerations, a large amount of inflammatory cell infiltration in the mucosa and submucosa and proliferation of lymph cells, accompanied by hyperemia, edema, damaged glands and indistinct muscular layer. In the HPM group, the mucosal epithelium was generally complete; the arrangement of glands was substantially regular; there was also a large amount of inflammatory cell infiltration in the mucosa and submucosa, accompanied by edema. In the sham-HPM group, the colonic tissue had an indistinct structure, impaired mucosa, a large amount of inflammatory cell infiltration and deficient glands, accompanied by severe hyperemia and edema, but the muscular layer was generally complete. In the MEK-inhibitor group, the colonic tissue had generally complete mucosal epithelium, regular arrangement of glands, obvious infiltration of inflammatory cells (mainly eosinophils and lymph cells) and mild edema. In the DMSO group, the colonic tissue had an indistinct structure, partially impaired mucosal epithelium, ulcerations, obvious hyperemia, edema, inflammatory cell infiltration in the submucosa, damaged glands and indistinct muscular layer (Figure 4).
Figure 4.
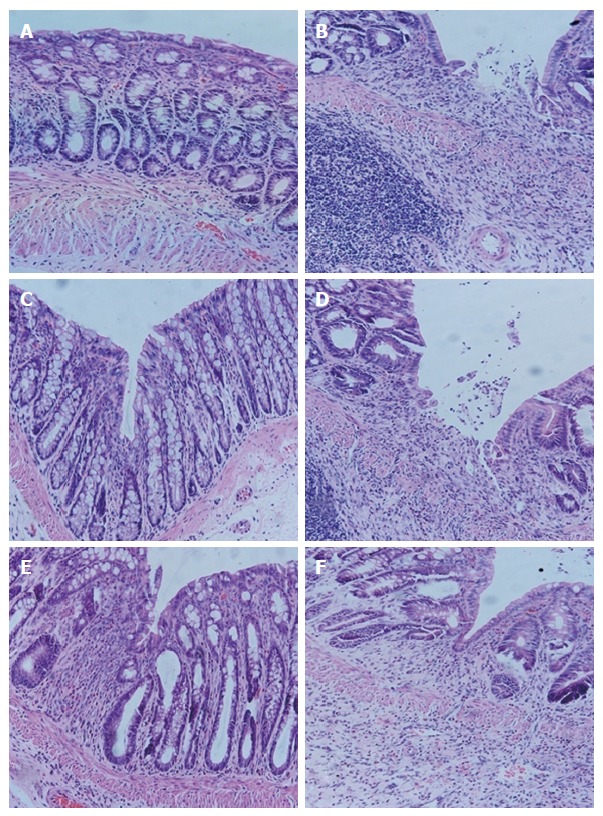
Hematoxylin-eosin staining of colonic mucosa. A: Normal group; B: Model group; C: HPM group; D: Sham-HPM group; E: MEK-inhibitor group; F: DMSO group (HE staining, × 200 magnification). HE: Hematoxylin-eosin; HPM: Herb-partitioned moxibustion; MEK: Mitogen-activated extracellular signal-regulated kinase; NG: Normal group; MG: Model group; SHPMG: Sham-HPM group; MIG: MEK-inhibitor group; DG: Dimethyl sulfoxide group.
Comparison of pMEK1, pERK1/2 and pCREB expression in rat spinal cord
Compared with the normal group, expression of pMEK1, pERK1/2 and pCREB was increased significantly in the model, sham-HPM and DMSO groups (P < 0.01 or < 0.05). Compared with the model group, expression of pMEK1, pERK1/2 and pCREB was reduced significantly after intervention in HPM and MEK-inhibitor groups (P < 0.01 or < 0.05). Compared with the sham-HPM group, expression of pMEK1, pERK1/2 and pCREB was decreased significantly after intervention in the HPM and MEK-inhibitor groups (P < 0.01 or < 0.05). Compared with the DMSO group, expression of pMEK1, pERK1/2 and pCREB was reduced significantly after intervention in the HPM and MEK-inhibitor groups (P < 0.01 or < 0.05) (Figure 5). The results suggested that HPM and U0126 down-regulated expression of pMEK1, pERK1/2 and pCREB, and inhibited activation of the MEK/ERK/CREB signaling pathway.
Figure 5.
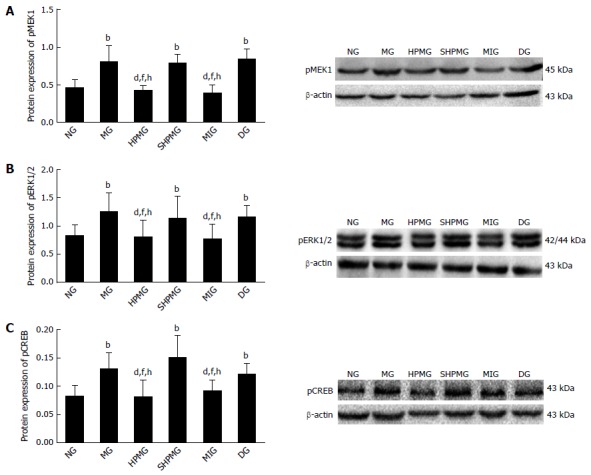
Comparison of expression of phospo-mitogen-activated extracellular signal-regulated kinase 1, phosphor-extracellular signal-regulated kinase 1/2 and cAMP response element binding protein in rat spinal cord. A: Protein expression of pMEK1; B: Protein expression of pERK1/2; C: Protein expression of pCREB. bP < 0.01 vs normal group; dP < 0.01 vs model group; fP < 0.01 vs sham-HPM group; hP < 0.01 vs DMSO group. pCREB: cAMP response element binding protein; DMSO: Dimethyl sulfoxide; pERK: Phosphor-extracellular signal-regulated kinase; HPM: Herb-partitioned moxibustion; pMEK1: Phospo-mitogen-activated extracellular signal-regulated kinase 1; NG: Normal group; MG: Model group; SHPMG: Sham-HPM group; MIG: MEK-inhibitor group; DG: Dimethyl sulfoxide group.
Comparison of mRNA expression of MEK, ERK and CREB in rat spinal cord
Compared with the normal group, expression of MEK, ERK and CREB mRNA was increased significantly in the model, sham-HPM and DMSO groups (P < 0.01). Compared with the model group, expression of MEK, ERK and CREB mRNA was decreased significantly in the HPM and MEK-inhibitor groups after intervention (P < 0.01). Compared with the sham-HPM group, expression of MEK, ERK and CREB mRNA was decreased significantly in the HPM and MEK-inhibitor groups after intervention (P < 0.01). Compared with the DMSO group, expression of MEK, ERK and CREB mRNA was reduced significantly in the HPM and MEK-inhibitor groups after intervention (P < 0.01) (Figure 6). This indicated that HPM and U0126 down-regulated expression of MEK, ERK and CREB mRNA, and inhibited activation of the MEK/ERK/CREB signaling pathway.
Figure 6.
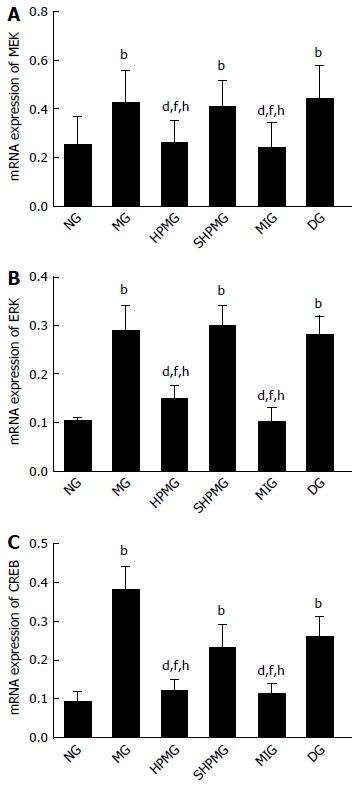
Comparison of expression of mitogen-activated extracellular signal-regulated kinase, extracellular signal-regulated kinase and cAMP response element binding protein mRNAs in rat spinal cord. A: mRNA expression of MEK; B: mRNA expression of ERK; C: mRNA expression of CREB. bP < 0.01 vs normal group; dP < 0.01 vs model group; fP < 0.01 vs sham-HPM group; hP < 0.01 vs DMSO group. CREB: cAMP response element binding protein; DMSO: Dimethyl sulfoxide; ERK: Extracellular signal-regulated kinase; HPM: Herb-partitioned moxibustion; MEK: Mitogen-activated extracellular signal-regulated kinase; NG: Normal group; MG: Model group; SHPMG: Sham-HPM group; MIG: MEK-inhibitor group; DG: Dimethyl sulfoxide group.
DISCUSSION
CIVP is a common manifestation of inflammatory bowel diseases. Its chronic expansion, unpredictable location and unclear pathogenesis all make it harder to evaluate compared to somatic pain. So far, the measurement of CIVP is generally based on behavioral response, such as visceral pain index, AWR, abdominal contraction record and application of analgesics[19], which may only partially reflect the situation of CIVP. It has been shown by clinical and experimental studies that CIVP involves both somatic (mainly mechanical and thermal hyperalgesia, as shown by animal experiments)[6,20] and visceral pain. This is possibly caused by central sensitization triggered by transmission of peripheral nociceptive stimulation into the spinal cord, or by joint action of visceral afferent and limb efferent fibers in the center[8]. Our previous experiment on a rat model of UC showed that HPM at Tianshu (ST25) and Qihai (CV6) was effective in easing CIVP[6], which has made it a significant project to reveal the analgesic mechanism of HPM.
Currently, neural plasticity is considered one of the key factors in the development of CIVP[21]. Peripheral injury or inflammation induces secretion of large amounts of inflammatory mediators, leading to sensitization of peripheral receptors, central nervous system plasticity, and reorganization and reconstruction of the central neurons involved in pain transmission. This long-term neural plasticity can occur simultaneously in the spinal cord and brain, presenting as allodynia (decreased pain threshold, pain induced by painless stimulators) and hyperalgesia (enlarged pain receptive field, enhanced response to nociceptive stimulations, and prolonged pain duration). The MAPK signaling pathway is widely distributed in various types of cells and transmits extracellular signals from the cell surface to the nucleus through cascade activation and activation of numerous proteinases, nucleoproteins and TFs. This produces a critical regulatory effect on pain, inflammation and tumors that is associated with cell proliferation and differentiation[22].
The ERK signaling pathway is a classic MAPK signaling pathway[23]. By following Ras-Raf-MEK-ERK, ERK plays a key role in signal transduction from cell membrane receptors to the nucleus. This shows that activation of ERK in the spinal cord dorsal horn neurons is significant in producing and maintaining central sensitization[24]. This indicates that the ERK pathway is involved in regulation of nociceptive signals in the spinal cord, and early intervention at the spinal cord level should be effective in blocking pain transmission[25]. The application of specific ERK inhibitor further highlights the signal transduction mechanism in chronic visceral pain.
U0126 effectively and exclusively blocks activation of the ERK pathway and enhances the pain threshold[26]. When the ERK pathway is blocked by U0126, the inflammatory mediators produced are also inhibited[27], which means that U0126 has an anti-inflammatory function. Hence, we selected U0126 for injection into the UC CIVP rat model as a positive control for the HPM group, to observe how HPM intervenes in the ERK pathway in CIVP, by detecting the critical proteins upstream and downstream of the ERK pathway.
When receiving MEK upstream signals, pERK migrates to the nucleus, inducing activation of a series of TFs such as CREB, Elk, activator protein-1, c-myc and c-fos[28]. Therefore, activation of MEK and precise transmission of signals to the ERK pathway are critical to the biological function of cells. When receiving nociceptive stimulation, the phosphorylation of ME34921R and ERK in spinal cord dorsal horn neurons is increased[29], suggesting that nociceptive stimulation can provoke the central ERK signaling pathway. U0126 inhibits activation of MEK and ERK, as well as the downstream functional factors, and thus can regulate inflammation and pain[30].
The downstream factors of the ERK pathway include multiple protein kinases and TFs, and CREB is one of the most important. As a type of endonuclear TF, when phosphorylated, CREB can modulate various pain-related target genes, for example, c-fos, neurokinin-1 and brain-derived neurotrophic factor, through which, it can influence the integration and regulation of pain signals. The whole process is of major significance in adjusting pain signal transduction and central sensitization[31]. ERK and CREB are also closely related in the chronic pain depression model[4,32]. Since CREB is a downstream factor of the ERK pathway, inhibition of pERK leads to decreased expression of pCREB, suggesting that pain can be controlled by inhibiting ERK and expression of its downstream functional factor CREB.
Phosphorylation is the basis of the transcription function of CREB, and pERK can migrate from the cytoplasm to the nucleus and phosphorylate CREB to play its role in modulating the target genes. Peripheral nociception triggers the expression of pCREB in the spinal cord dorsal horn of rat models of chronic pain, while intrathecal injection of CREB inhibitor significantly inhibits pain induced by nociception[33]. This indicates that CREB plays an important role in central sensitization and long-term neural plasticity, which is a potential mechanism by which activated ERK participates in central sensitization and development of chronic pain[34].
By adopting the rat model of Crohn’s disease CIVP induced by TNBS/ethanol enema, we observed the effect of HPM on the phosphorylated protein and mRNA expression of MEK1, ERK1/2 and CREB in the spinal cord. CIVP rats had both visceral and somatic pain, manifested by increased AWR, decreased pain threshold, and reduced MWT and TWL. The expression of phosphorylated protein and mRNA of MEK, ERK and CREB was increased significantly in the models, showing that the MEK/ERK/CREB signaling pathway in the spinal cord is activated in CIVP rat models. After application of MEK inhibitor, expression of phosphorylated protein and mRNA of MEK1, ERK1/2 and CREB was decreased significantly, and the pain behavior also showed significant improvement, suggesting a close relationship between the MEK/ERK/CREB pathway in the spinal cord and CIVP. After HPM at Tianshu (ST25) and Qihai (CV6), expression of phosphorylated protein and mRNA of MEK1, ERK1/2 and CREB was also decreased significantly, indicating that HPM can inhibit activation of the MEK/ERK/CREB pathway in the spinal cord of CIVP rats and improve pain behavior. In summary, the MEK/ERK/CREB signaling pathway is closely associated with the development of CIVP. HPM can alleviate the pain in CIVP rats, and this action is related to inhibition of the MEK/ERK/CREB pathway in the spinal cord (Figure 7).
Figure 7.

Flow chart of the study design and treatment. AWR: Abdominal withdrawal reflex: CREB: cAMP response element binding protein; ERK: Extracellular signal-regulated kinase; DG: DMSO group; HE: Hematoxylin-eosin; HPM: Herb-partitioned moxibustion; MEK: Mitogen-activated extracellular signal-regulated kinase; MIG: MEK-inhibitor group; MG: Model group; MWT: Mechanical withdrawal threshold; NG: Normal group; SD: Sprague-Dawley; SHPMG: Sham-HPM group; TWL: Thermal withdrawal latency.
We suppose that HPM exerts its analgesic effect through down-regulating phosphorylation and mRNA expression of MEK1, ERK1/2 and CREB in the spinal cord of CIVP rats, and subsequently inhibiting activation of the MEK/ERK/CREB signaling pathway. However, this study only focused on the MEK/ERK/CREB pathway in the spinal cord to discover the analgesic mechanism of HPM, and it is unclear whether other MAPK pathways, such as p38 MAPK and C-Jun N-terminal kinase, are involved in the signal transmission in CIVP. Pain has a complex pathogenesis, and a variety of non-neural cells, for example, glial cells and stem cells, also play an important role in the development of inflammation and pain[35]. The relationship between these cells and the MEK/ERK/CREB signaling pathway, and the questions in which cells this signaling pathway is activated, and how they bind, require further studies.
COMMENTS
Background
Recent studies suggest that central sensitization is one of the critical factors causing chronic inflammatory visceral pain (CIVP), and the extracellular signal-regulated kinase (ERK) signaling pathway is involved in transduction of pain signals. Moxibustion can effectively mitigate CIVP, but its potential mechanism is still unknown.
Research frontiers
The pathogenesis of CIVP is complicated, involving multiple neurotransmitters, cytokines and signaling pathways. ERK signaling pathway is closely related to the development of pain and inflammation. Moxibustion can successfully mitigate CIVP, and studies on the ERK signaling pathway will help reveal its mechanism of action.
Innovations and breakthroughs
This study discovered that the spinal cord MEK/ERK/CREB signaling pathway was activated in CIVP rats. Herb-partitioned moxibustion (HPM) exerted its analgesic effect by down-regulating protein phosphorylation and mRNA expression of mitogen-activated extracellular signal-regulated kinase MEK, ERK1/2 and cAMP response element binding protein (CREB), and inhibiting activation of the MEK/ERK/CREB signaling pathway in the spinal cord of CIVP rats.
Applications
To provide experimental evidence for HPM in treating CIVP, and the results may reveal one of the mechanisms of the analgesic effects of HPM in treating CIVP.
Terminology
HPM is an external therapy in traditional Chinese medicine that reduces chronic inflammatory pain.
Peer-review
The authors aimed to investigate the effect of HPM on the phosphorylation of MEK1, ERK1/2 and CREB in spinal cord of rats with CIVP, and to explore the central mechanism of HPM in treating CIVP. This is a great, exciting and very tough study relying on establishing a rat model.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Shanghai University of Traditional Chinese Medicine Institutional Review Board.
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of Shanghai University of Traditional Chinese Medicine.
Conflict-of-interest statement: The authors declare that there is no conflict of interest regarding the publication of this paper.
Data sharing statement: No additional data are available.
Peer-review started: February 28, 2017
First decision: June 22, 2017
Article in press: August 8, 2017
P- Reviewer: Yuksel I S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Zhang FF
Contributor Information
Zhi-Yuan Li, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China.
Yan Huang, Huangpu Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai 200010, China.
Yan-Ting Yang, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China.
Dan Zhang, Shanghai Research Institute of Acupuncture and Meridian, Shanghai 200030, China.
Yan Zhao, Yunnan University of Traditional Chinese Medicine, Kunming 650500, Yunnan Province, China.
Jue Hong, Shanghai Research Institute of Acupuncture and Meridian, Shanghai 200030, China.
Jie Liu, Shanghai Research Institute of Acupuncture and Meridian, Shanghai 200030, China.
Li-Jie Wu, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China.
Cui-Hong Zhang, Shanghai Research Institute of Acupuncture and Meridian, Shanghai 200030, China.
Huan-Gan Wu, Shanghai Research Institute of Acupuncture and Meridian, Shanghai 200030, China.
Ji Zhang, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China.
Xiao-Peng Ma, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China; Shanghai Research Institute of Acupuncture and Meridian, Shanghai 200030, China. pengpengma@163.com.
References
- 1.Cao H, Zhang YQ. ERK/MAPK signal pathway and pain information process. Zhongguo Tengtong Yixue Zazhi. 2011;17:370–373. [Google Scholar]
- 2.Fang JQ, Fang JF, Liang Y, Du JY, Qiu YJ, Liu J. [Immediate analgesic effect of electroacupuncture and its regulation mechanism via spinal p-ERK1/2] Zhongguo Zhen Jiu. 2012;32:1007–1011. [PubMed] [Google Scholar]
- 3.Hao PQ, An S, Yang Y, Liu Y, Guo XX, Xu TR. The research progress on MEK kinases and their inhibitors. Zhongguo Xibao Shengwuxue Xuebao. 2015;37:1425–1431. [Google Scholar]
- 4.Xu L, Qiu T, Zhang LJ, Zhang WJ. Expression of ERK, CREB and BDNF in spinal cord dorsal horn of rat model of chronic compression injury-induced neuropathic pain. Zhejiang Yixue. 2016;38:1341–1344. [Google Scholar]
- 5.Wang JY. Analysis on interrelation between cumulative electroacupuncture-induced analgesic effect and neuronal plasticity and MAPK/ERK signal pathway in hippocampus. Beijing: Zhongguo Zhongyi Kexueyuan, 2013 [Google Scholar]
- 6.Huang Y, Yang YT, Liu XX, Zhao Y, Feng XM, Zhang D, Wu HG, Zhu Y, Huang WY, Ma XP. Effect of herbal-partitioned moxibustion at Tianshu (ST 25) and Qihai (CV 6) on pain-related behavior and emotion in rats with chronic inflammatory visceral pain. J Acupunct Tuina Sci. 2015;13:1–8. [Google Scholar]
- 7.Li ZY, Huang Y, Yang YT, Zhang D, Wu HG, Shi Z, Jia YF, Zhang CH, Liu J, Hong J, et al. Impacts of herb-partitioned moxibustion on hypothalamic SP, 5-HT and c-Fos in rats with chronic inflammatory visceral pain. Shijie Kexue Jishu. 2016;18:395–401. [Google Scholar]
- 8.Zhou Q, Price DD, Caudle RM, Verne GN. Visceral and somatic hypersensitivity in TNBS-induced colitis in rats. Dig Dis Sci. 2008;53:429–435. doi: 10.1007/s10620-007-9881-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- 10.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 11.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 12.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 13.Guo Y, Fang JQ. Experimental acupuncture and moxibustion. Publisher: Zhongguo Zhongyiyao Chubanshe, 2012; 415 [Google Scholar]
- 14.Wang HJ, Ji LX. About the location of Shenque (CV 8) acupoint in rats. Zhenci Yanjiu. 2007;32:312. [Google Scholar]
- 15.Li CR, Hua XB, Zhou HL, Song DL, Hu YL. Invention of the acupoints atlas of guinea pig. Shanghai Zhenjiu Zazhi. 1992;11:28–30. [Google Scholar]
- 16.Huang MF, Yu CD. Detection of reliability of placebo control acupuncture and moxibustion methods. Shiyong Zhenjiuxue. 2004;24:65–67. [Google Scholar]
- 17.Yu Qiang. Choice of blind and placebo methods in evaluating the efficacy of acupuncture. Zhongxiyi Jiehe Xinnaoxue Guanbing Zazhi. 2003;1:196–198. [Google Scholar]
- 18.Yang F, Sun W, Yang Y, Wang Y, Li CL, Fu H, Wang XL, Yang F, He T, Chen J. SDF1-CXCR4 signaling contributes to persistent pain and hypersensitivity via regulating excitability of primary nociceptive neurons: involvement of ERK-dependent Nav1.8 up-regulation. J Neuroinflammation. 2015;12:219. doi: 10.1186/s12974-015-0441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Y, Zhu Y, Huang WY, Zhao CY, Ma XP. Advances in experimental studies of acupuncture-moxibustion treatment for intestinal disease-induced visceral pain. Shanghai Zhenjiu Zazhi. 2014;33:1073–1078. [Google Scholar]
- 20.Tian XL, Wang YF, Chen SL, Mo JZ, Chen WH, Cao ZJ. Intestinal inflammation induces visceral and somatic hypersensitivity. Weichangbing Xue. 2012;17:399–403. [Google Scholar]
- 21.Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis. 2001;8:1–10. doi: 10.1006/nbdi.2000.0360. [DOI] [PubMed] [Google Scholar]
- 22.Yu WC, Huang GY, Zhang MM. Central sensitization of MAPK pathway and pathological pain. Zhongxiyi Jiehe Yanjiu. 2011;3:249–253. [Google Scholar]
- 23.Ji RR. Mitogen-activated protein kinases as potential targets for pain killers. Curr Opin Investig Drugs. 2004;5:71–75. [PubMed] [Google Scholar]
- 24.Chen NF, Chen WF, Sung CS, Lu CH, Chen CL, Hung HC, Feng CW, Chen CH, Tsui KH, Kuo HM, et al. Contributions of p38 and ERK to the antinociceptive effects of TGF-β1 in chronic constriction injury-induced neuropathic rats. J Headache Pain. 2016;17:72. doi: 10.1186/s10194-016-0665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han M, Huang RY, Du YM, Zhao ZQ, Zhang YQ. Early intervention of ERK activation in the spinal cord can block initiation of peripheral nerve injury-induced neuropathic pain in rats. Shengli Xuebao. 2011;63:106–114. [PubMed] [Google Scholar]
- 26.Luo F, Yin PP, Li Z. Analgesic effect of intra-amygdala infusion of U0126 on fentanyl-induced hyperalgesia in rats. Linchuang Mazuixue Zazhi. 2016;32:794–797. [Google Scholar]
- 27.Albano GD, Bonanno A, Cavalieri L, Ingrassia E, Di Sano C, Siena L, Riccobono L, Gagliardo R, Profita M. Effect of High, Medium, and Low Molecular Weight Hyaluronan on Inflammation and Oxidative Stress in an In Vitro Model of Human Nasal Epithelial Cells. Mediators Inflamm. 2016;2016:8727289. doi: 10.1155/2016/8727289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cruz CD, Neto FL, Castro-Lopes J, McMahon SB, Cruz F. Inhibition of ERK phosphorylation decreases nociceptive behaviour in monoarthritic rats. Pain. 2005;116:411–419. doi: 10.1016/j.pain.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 29.Hu XD, Liu YN, Zhang ZY, Ma ZA, Suo ZW, Yang X. Spinophilin-Targeted Protein Phosphatase-1 Alleviated Inflammatory Pain by Negative Control of MEK/ERK Signaling in Spinal Cord Dorsal Horn of Rats. J Neurosci. 2015;35:13989–14001. doi: 10.1523/JNEUROSCI.2293-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang JQ, Fang JF, Liang Y, Du JY. Electroacupuncture mediates extracellular signal-regulated kinase 1/2 pathways in the spinal cord of rats with inflammatory pain. BMC Complement Altern Med. 2014;14:285. doi: 10.1186/1472-6882-14-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao FR, Cao DY, Zhao Y. cAMP response element bound protein pain modulation. Shengli Kexue Jinzhan. 2006;37:125–128. [PubMed] [Google Scholar]
- 32.Xu MM. Effects of acupuncture on ERK1/2-CREB-BDNF signal pathway in prefrontal cortex of depression model rats. Beijing: Beijing Zhongyiyao Daxue, 2016 [Google Scholar]
- 33.Song XS, Yang TW, Du B, Tong DM, Du BD, Cao JL, Zeng YM. Effects of intrathecal U0126 on expression of phosphorylated cAMP response element binding protein in spinal cord dorsal horn in a rat model of neuropathic pain. Chin J Anesthesiol. 2005;25:183–186. [Google Scholar]
- 34.Zan HS, Ji XJ, Dong P. Classification and research progress of visceral pain model. Zhongguo Linchuang Jiepouxue Zazhi. 2009;27:195. [Google Scholar]
- 35.Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science. 2016;354:572–577. doi: 10.1126/science.aaf8924. [DOI] [PMC free article] [PubMed] [Google Scholar]


