Abstract
AIM
To investigate whether Dihydromyricetin (DHM) inhibits cell proliferation and promotes apoptosis by downregulating Notch1 expression.
METHODS
The correlation between Notch1 and Hes1 (a Notch1 target molecule) expression in hepatoma samples was confirmed by qRT-PCR. In addition, MTT assays, flow cytometry and TUNEL analysis showed that DHM possessed strong anti-tumor properties, evidenced not only by reduced cell proliferation but also by enhanced apoptosis in QGY7701 and HepG2 hepatocellular carcinoma (HCC) cells. The expressions of Notch1, Hes1, Bcl-2 and Bax were determined by Western blot.
RESULTS
Among the tested samples (n = 64), the expression levels of Notch1 (75% of patients) and Hes1 (79.7% of patients) mRNA in tumor tissues were higher than in the normal liver tissues. There was a negative correlation between the expression of Notch1 and the degree of differentiation and positively correlated with the Alpha Fetal Protein concentration. The viability of HCC cells treated with DHM was significantly inhibited in a dose and time-dependent manner. Apoptosis was induced in HepG2 and QGY7701 cell lines following 24 h of DHM treatment. After treatment with DHM, the protein expression of Notch1 was downregulated, the apoptosis-related protein Bax was upregulated and Bcl2 was downregulated. Notch1 siRNA further enhanced the anti-tumor properties of DHM.
CONCLUSION
Notch1 is involved in the development of HCC and DHM inhibits cell proliferation and promotes apoptosis by down-regulating the expression of Notch1.
Keywords: Dihydromyricetin, Apoptosis, Hepatocellular carcinoma, Notch1
Core tip: The novel findings of this study are that Notch1 is highly expressed in tumor cells with higher degrees of malignancy and proliferative activity, Notch1 is involved in the development of hepatocellular carcinoma (HCC) and may serve as a potential diagnostic marker for HCC. Dihydromyricetin can strongly induce apoptosis of the hepatic cancer cell lines QGY7701 and HepG2 by downregulating the expression of Notch1.
INTRODUCTION
Hepatocellular carcinoma (HCC) ranks sixth and third worldwide in tumor incidence and mortality, respectively[1]. Despite dramatic improvements in surgical treatment and chemotherapy, the one-year survival rate of patients diagnosed with HCC is not high[2,3]. In addition, traditional chemotherapy drugs have obvious side effects. Therefore, seeking an effective drug treatment that reduces the mortality of patients with HCC is of great significance.
Dihydromyricetin (DHM, C15H12O8, PubChem CID: 161557) is a component of wild woody vines of the genus Vitis, which is a flavonoid compound[4]. DHM was reported to have antioxidant[5,6], anti-inflammatory[7], anti-hypertensive[8], hypoglycemic[9], hepatoprotective[10,11], and anti-carcinogenic effects[12]. Previous studies have reported that DHM can inhibit the invasion and metastasis of HCC cells[13]. Combined application of DHM and nedaplatin induced apoptosis of HCC cells through the p53/Bcl-2 pathway, and enhanced sensitivity to nedaplatin chemotherapy[14]. DHM has great potential as a treatment option for liver cancer.
The Notch signaling pathway plays an important role in the fate of many cells and is crucial for the biological development of embryos. The Notch pathway is composed of a transmembrane family of receptors (Notch 1-4), their ligands (Jagged 1-2, Delta-like 1-3), and downstream target genes in the HES and HEY protein families[15]. Notch receptors bind to ligands in the membranes of adjacent cells, causing the intracellular segment of the Notch receptor to release, and the receptor then travels to the nucleus, resulting in activation of Notch target genes, thus successfully mediating cell growth and development[16].
Studies of Notch and liver diseases have been conducted in recent years. Notch3 is highly expressed in HCC, can promote the self-renewal of HCC cells and plays a significant role in maintaining stem cells by downregulating β-catenin and upregulating the expression of Nanog[17]. High expression of Notch1 and Notch4 is closely associated with poor prognosis of patients with HCC[18]. Suppression of the Notch signaling pathway averts cholestatic liver fibrosis by lessening the differentiation of hepatic progenitor cells into cholangiocytes[19]. Inhibition of Notch1 expression promoted pancreatic cancer and prostate cancer cell apoptosis[20,21], but whether downregulation of Notch1 induces apoptosis in hepatoma cells has rarely been reported. Moreover, the anti-tumor activity of DHM and its regulation of the Notch pathway are still unclear.
In the present study, we demonstrated that aberrant Notch1 expression was involved in tumor progression. DHM could downregulate the expression of Notch1 to induce apoptosis in HepG2 and QGY7701 HCC cells and restrain their proliferation. Inhibition of Notch1 signaling may serve as a novel target for HCC treatment.
MATERIALS AND METHODS
Reagents and chemicals
DHM (Sigma, United States) was solubilized in 100% dimethyl sulfoxide to prepare a primary solution of 50 mmol/L and stored at -20 °C. Rabbit anti-human primary antibodies against Notch1, Hes1, Bcl-2 and Bax were obtained from Cell Signaling Technology (Beverly, MA, the United States of America). Notch1 siRNA was obtained from Santa Cruz Biotechnology (the United States of America). FITC Annexin V staining Kit was brought from BD Biosciences (the United States of America). MTT [3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] was obtained from Sigma-Aldrich Biotechnology (United States).
Clinical specimens
All patients were diagnosed with pathological primary HCC. Tissue samples were obtained from these patients during hepatectomy surgery. HCC was confirmed by pathology. All tissue samples were frozen in liquid nitrogen. This study was approved by the Medical Ethics Committee of the Affiliated Hospital of Guangdong Medical University.
Cell lines and cell culture
The cells (SK-Hep-1, QGY7703, MHCC97L, HepG2, QGY7701 and HL7702) were maintained in DMEM supplemented with 10% fetal bovine serum and antibiotics (penicillin and streptomycin) in a humidified 5% CO2 incubator. Upon reaching 80% confluence, the cells were treated with various concentrations of DHM (0, 5, 25, 50, 100 or 150 μmol/L) for 12 h, 24 h and 36 h and DMSO treatment served as a control.
Cell inhibition and cytotoxicity assay
The cells were placed into 96-well plates and treated with different concentrations of DHM. DMSO treatment served as a control. Twenty μL of MTT (5 mg/mL) solution was transferred to each well, and then the plate was incubated at 37 °C for 4 h. Next, the cell supernatants were removed, and 150 μL of DMSO was added to each well to solubilize the formed crystals. The optical density values were measured at 570 nm with a spectrophotometer (PerkinElmer, the United States of America).
Apoptosis assay
The HepG2 and QGY7701 cells were seeded in the 6-well plates. Following overnight growth, the cells were pretreated with 50, 100, or 150 μmol/L DHM and incubated for 24 h. Following careful collection and suspension in binding buffer, the HepG2 and QGY7701 cells were incubated with 5 mL of annexin V-FITC and 5 mL of PI for 15 min in darkness prior to flow cytometric analysis.
Western blot
The tissues protein samples were prepared with Fastprep-24 Sample Preparation equipment (MP, United States). The cells treated with DHM were harvested and lysed in lysis buffer to assess the expression of Notch1, Hes1, Bcl-2 and Bax. Equal amounts of protein samples were loaded per well. Membranes containing protein blots were incubated in blocking buffer (5% non-fat milk) for 1 h at room temperature, and then the membranes were incubated with the primary antibodies (1:1000 dilution) at 4 °C overnight. Following a wash with TBST, membranes were incubated with goat anti-rabbit secondary antibodies for 1 h. Detection was performed using an Odyssey Infrared Imaging System (LI-COR Biosciences Inc., Lincoln, NE, the United States of America).
qRT-PCR analysis
Frozen tissues were used to isolate messenger RNA (mRNA) using TRIzol reagent (Invitrogen, Guangzhou, China), according to the manufacturer’s protocol. Reverse transcription was performed with 1 μg of RNA using the PrimeScript RT Reagent kit with a gDNA Eraser kit (Takara Bio, Inc, Otsu, Japan). The primer sequences used were as follows: Notch1 forward, 5’-CACCCATGACCACTACCCAGTT-3’ and reverse, 5’-CCTCGGACCAATCAGAGATGTT-3’; Hes1 forward, 5’-AGATCTATGCCAGCTGATAAT-3’and reverse, 5’-AAGCTTCACTTAATACAGCT-3’, and 18s forward, 5’-CGGCGACGACCCATTCGAAC-3’ and reverse, 5’-GAATCGAACCCTGATTCCCCGTC-3’. Data were evaluated using the comparative count method and normalized to the corresponding 18S value.
TUNEL assay
In situ cell death detection kit-POD (Roche, Switzerland) was used to detect the late-stage apoptosis. Cells were seeded in 96well plates and treated with various concentrations of DHM. Cells were fixed with 4% paraformaldehyde, and then counterstained with 4’,6diamidino2phenylindole for 5 min at room temperature in the dark and observed under a fluorescence microscope (Olympus IX70, Japan) to detect the apoptotic cells.
siRNA transfection
QGY7701 and HepG2 cells were plated into 6-well plates and transfection procedures were performed according to the manufacturer’s protocol. Three different Notch1 siRNA sequences were tested in both QGY7701 and HepG2 cell lines for screening the efficacy of Notch1 knockdown and selecting the best performing siRNA transcript. Notch1 siRNA-2 showed the best degree of knockdown and was used in subsequent experiments. Before each experiment, we transiently transfected cells using an Notch1 siRNA gene knockdown kit (JIMA Biotechnology Company, Shanghai, China) and the negative control siRNA.
Colony formation assay
Cells in each logarithmic growth phase were detached from culture flasks using 0.25% trypsin. Then, the cells were percussed into a single cell suspension, and 1000 cells were cultured in each well of a 6-well plate, and humidity was saturated for 1 wk. The cells were stained with crystal violet. Clones were directly counted by the naked eye or counted using a microscope, and data were analyzed using ImageJ software.
Statistical analysis
The data were obtained from at least three independent experiments, and all results are presented as the mean ± SD. The results were evaluated using Student’s t test, Pearson correlation analyses were used to examine the correlation of two parameters. The significance levels were defined as P < 0.05.
RESULTS
Notch1 expression is activated in HCC tissues and reflects differentiation properties
The expression of Notch1 protein by Western blots and found that Notch1 accumulated predominantly in HCC tissues (Figure 1A). In addition, the expression of Notch1 and Hes1 genes in HCC and normal liver tissue samples was analyzed by qRT-PCR (Figure 1B and C). Among the tested samples (n = 64), the expression levels of Notch1 (75% of patients) and Hes1 (79.7% of patients) mRNA in tumor tissues were higher than in the normal liver tissues (Figure 1D). A positive correlation between Notch1 and Hes1 expression suggests that high expression of Notch1 in tumors may result in an upregulation of the target gene Hes1 (Figure 1E). To clarify the role of Notch1 in tumorigenesis and tumor differentiation, we analyzed the association among Notch1 expression, AFP concentration, and the degree of tumor differentiation. There was a negative correlation between the expression of Notch1 and the degree of differentiation and a positive correlation with the AFP concentration (Figure 1F and G). The results showed that Notch1 was highly expressed in tumor cells with higher degrees of malignancy and proliferative activity. Based on the results above, Notch1 was involved in the development and progression of HCC.
Figure 1.
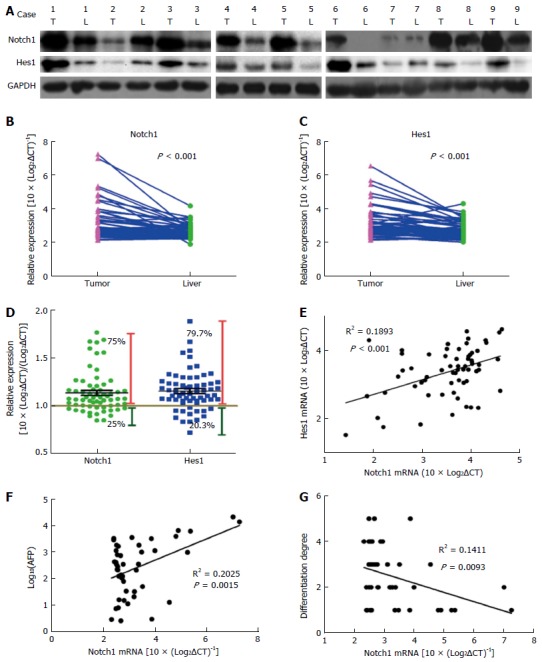
Notch1 expression is activated in hepatocellular carcinoma tissues and reflects differentiation properties. Protein levels were detected by Western blot analysis. A: Notch1 and Hes1 proteins were obviously abundant in most of the tumor tissues; B and C: Gene expression levels were measured with qRT-PCR. Notch1 and Hes1 genes were expressed at higher levels in tumor tissues compared with normal liver tissues; D: Notch1 and Hes1 gene expression levels were higher in 75% and 79.7% of the hepatocellular carcinoma patients, respectively; E: A Pearson correlation analysis revealed a correlation between Notch1 and Hes1 expression (r2 = 0.1893, P < 0.001); E and G: Notch1 is positively correlated with the AFP level (r2 = 0.2025, P = 0.0015) and negatively correlated with the degree of differentiation (r2 = 0.1411, P = 0.0093).
DHM inhibits the proliferation of HCC cells
We also screened the Notch1 protein expression profiles in several hepatoma cell lines. Notch1 exhibited higher expression levels in hepatoma cells compared with the immortalized normal liver cell line HL7702 (Figure 2B). Among the hepatoma cell lines, we found that QGY7701 and HepG2 cells highly express Notch1. Therefore, we chose these two lines for the next experiment. MTT assays showed that the proliferation of DHM-treated QGY7701 and HepG2 cells was inhibited. After 12 h, 24 h and 36 h, the viability of HCC cells treated with DHM was significantly inhibited in a dose- and time-dependent manner (Figure 2C and D). As shown in Figure 2, the proliferation of cells was significantly inhibited after treatment with 150 μmol/L and 50 μmol/L DHM for 12 h (P < 0.01), and the cell viability of both QGY7701 and HepG2 cells treated with 50 μmol/L DHM for 48 h and 25 μmol/L DHM for 24 h was significantly inhibited (P < 0.001). The microscopy suggested that the proliferation of the two cell lines was inhibited and partial apoptosis occurred compared with the control group (Figure 2E). A colony formation assay was utilized to further determine the proliferative ability of cells treated with various concentrations of DHM, and similarly, DHM suppressed colony-forming capability in a dose-dependent manner (Figure 2F-H).
Figure 2.
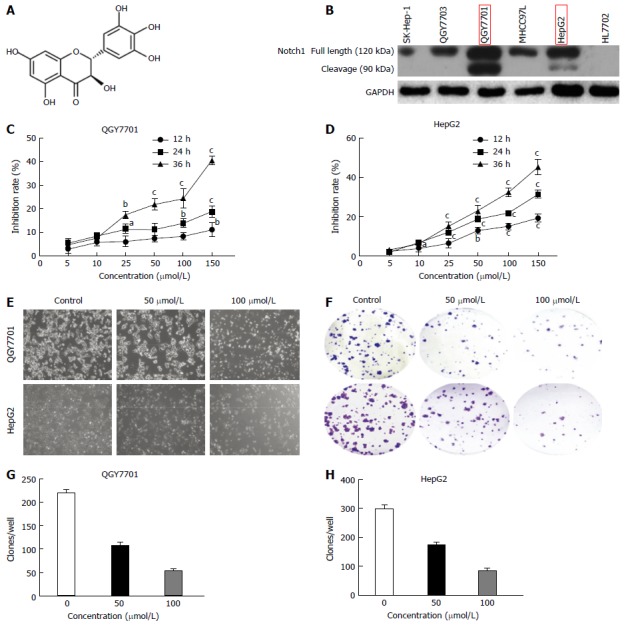
Dihydromyricetin inhibits the proliferation of QGY7701 and HepG2 hepatocellular carcinoma cells. A: Chemical structure of DHM; B: The Notch1 expression profiles of several hepatocellular carcinoma (HCC) cell lines were screened by Western blotting; C and D: MTT assays were used to analyze the rate of cell inhibition (means ± SD) of QGY7701 and HepG2 cells treated with dihydromyricetin (DHM) at different concentrations (5, 10, 25, 50, 100 and 150 μmol/L) at 12 h, 24 h and 36 h; E: The proliferation of QGY7701 and HepG2 cells treated with different concentrations of DHM (0, 50, or 100 μmol/L) for 24 h was observed under microscope (× 200 magnification); F: Colony formation capability assay with different concentrations of DHM in HCC QGY7701 and HepG2 cells; G and H: The clones were quantified, and the data are presented as a statistical figure; Each experiment was repeated at least three times. aP < 0.05, bP < 0.01 and cP < 0.001, vs 0 μmol/L (control) group.
DHM induces apoptosis in HCC cells
Flow cytometry revealed that apoptosis was induced in HepG2 and QGY7701 cell lines following 24 h of DHM treatment (Figure 3A-C). A TUNEL assay was utilized to detect latestage apoptosis of the cell lines treated with various concentrations of DHM for 24 h. In the fluorescence images, there were cells in late-stage apoptosis in both the HepG2 and QGY7701 cell lines (Figure 3D-F).
Figure 3.
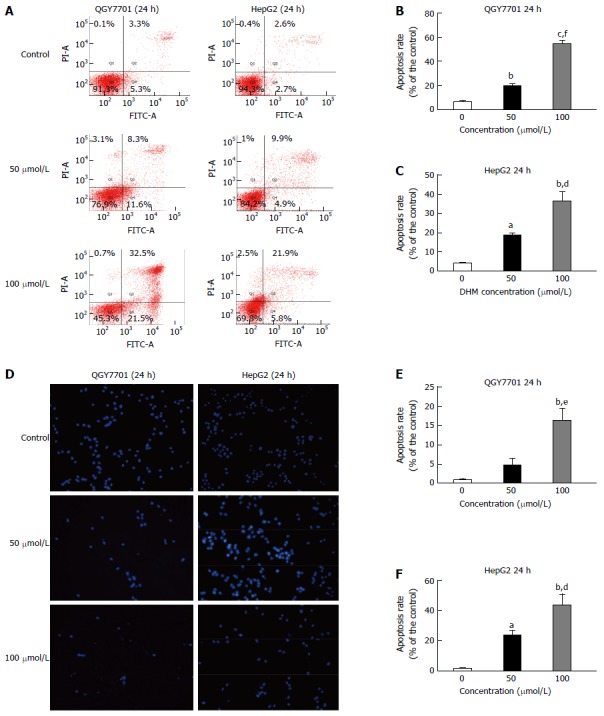
Dihydromyricetin induces apoptosis in QGY7701 and HepG2 hepatocellular carcinoma cells. A: Apoptosis of QGY7701 and HepG2 cells treated with DHM at different concentrations (0, 50, or 100 μmol/L) for 24 h was analyzed by flow cytometry; B and C: Apoptosis was quantified and presented as a statistical figure; D: A TUNEL assay was utilized to examine the apoptosis of QGY7701 and HepG2 cells treated with different concentrations (0, 50, or 100 μmol/L) of DHM for 24 h; E and F: Apoptosis was quantified and presented as a statistical figure; Each experiment was repeated more than three times; aP < 0.05, bP < 0.01, cP < 0.001 vs 0 μmol/L (control) group; eP < 0.05, dP < 0.01, fP < 0.001 vs 50 μmol/L group.
Effects of DHM treatment on proteins in the Notch1 pathway and on apoptosis-related proteins in HCC cells
In this study, Notch1, Hes1 and apoptosis-related proteins were detected by Western blotting. The results demonstrated that DHM downregulated the expression of Notch1 and Hes1 in QGY7701 and HepG2 cells. Meanwhile, Bax was upregulated, and Bcl-2 was downregulated. The results suggested that down-regulation of Notch1 activated the mitochondrial apoptotic pathway (Figure 4A and C). It is known that a lower ratio of Bcl-2/Bax leads to apoptosis, and our data showed that the ratio of Bcl-2/Bax was reduced (Figure 4B and D).
Figure 4.
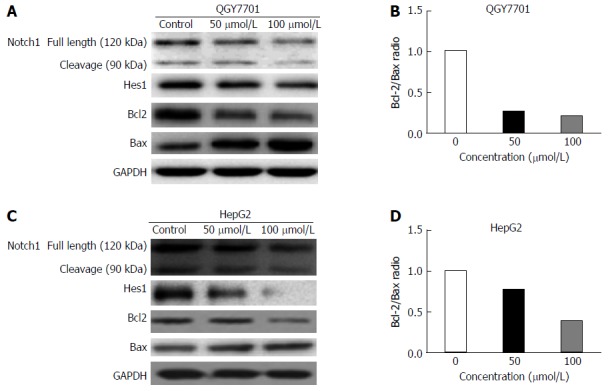
Effects of dihydromyricetin treatment on proteins in the Notch1 pathway and on apoptosis-related proteins in hepatocellular carcinoma cells. A and C: The levels of the Notch1, Hes1, Bcl-2, and Bax proteins in QGY7701 and HepG2 cells treated with DHM at different concentrations (0, 50, or 100 μmol/L) were analyzed by western blot; B and D: The Bcl-2/Bax protein ratios were measured by optical density analysis with ImageJ software.
DHM induces apoptosis in HCC cells by downregulating the Notch1 pathway
To further elucidate whether the inhibition of proliferation and the promotion of apoptosis of QGY7701 and HepG2 cells were caused by the down-regulation of Notch1 following DHM treatment, siRNA was used to silence Notch1 expression, and Notch1-silenced cells were treated with DHM. The knockout efficiency of the three different Notch1 siRNA sequences is shown in Figure 5A. The expression levels of Notch1, Hes1 and the apoptotic proteins Bcl-2 and Bax were analyzed by Western blot. Compared with the control group, Bax was upregulated, and Hes1 and Bcl-2 were downregulated after the depletion of Notch1 via siRNA. The results were consistent with the DHM 100 μmol/L treatment group. However, the effect of the Notch1-siRNA and DHM 100 μmol/L combination on protein expression was more obvious (Figure 5B and D). As expected, the ratio of Bcl2/Bax decreased the most in the Notch1-siRNA + DHM 100 μmol/L group (Figure 5C and E). In summary, DHM exerted anti-tumor activity by downregulating the expression of Notch1.
Figure 5.
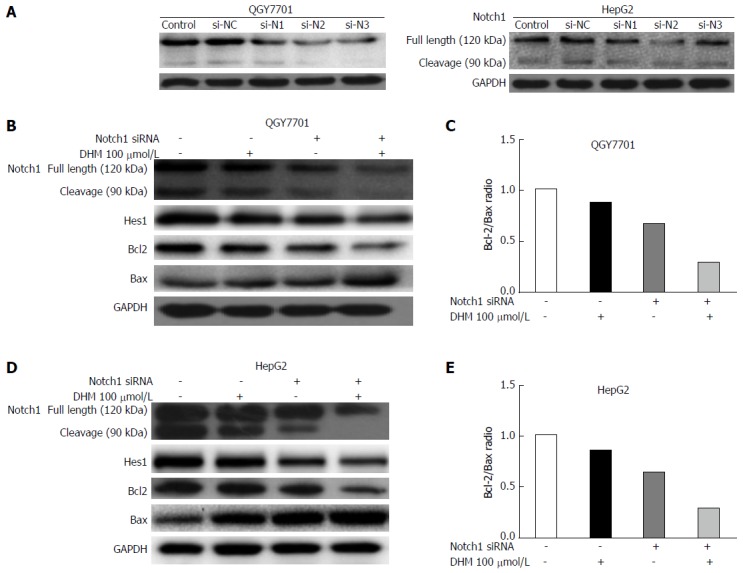
Dihydromyricetin induced apoptosis in hepatocarcinoma cells by downregulating the Notch1 pathway. A: Knockdown efficiency of 3 different Notch1 siRNA sequences was tested in both QGY7701 and HepG2 cell lines by Western blot; B and D: The effects of different treatment methods (Notch1-siRNA, dihydromyricetin 100 μmol/L, and combined treatment) on Notch1, Hes1, Bcl-2, and Bax protein levels as determined by Western blot; C and E: The Bcl-2/Bax protein ratios were measured by optical density analysis with ImageJ software. si-NC: si-negative control; si-N1: si-Notch1-1; si-N2: si-Notch1-2; si-N3: si-Notch1-3.
DISCUSSION
As a multifunctional protein, Notch1 plays a role in regulating cell differentiation, development, proliferation and survival in various contexts[15,16]. Abnormal expression of Notch1 often leads to tumors. For example, in pancreatic cancer, colon cancer, non-small cell lung cancer, cervical cancer and other tumors, Notch1 expression is increased, and Notch1 participates in the promotion of cell proliferation, inhibition of apoptosis and cellular differentiation[22-24]. Overexpression of Hes1 has also been reported in the development of HCC[17]. Western blotting and qRT-PCR analysis of clinical HCC samples indicated that Notch1 and Hes1 were highly expressed in tumor samples, and the expression rates of Notch1 and Hes1 accounted for 75% and 79.7% of the total data, respectively. The above results show that Notch1-mediated activation of Notch signaling is a hallmark of HCC. This further explains the correlation among Notch1 expression, AFP (a clinical diagnostic marker of HCC) expression, and the degree of differentiation. The expression of Notch1 was positively correlated with AFP expression and negatively correlated with the degree of differentiation. This indicated that Notch1 is highly expressed in tumor cells with higher degrees of malignancy and proliferative activity. Experienced clinicians frequently find that tumors with higher growth activity seem to be more susceptible to chemotherapeutic treatment. These facts suggest that Notch1 is involved in the development of HCC and Notch1 may be a potential diagnostic indicator of HCC and target for chemotherapeutic treatment.
The inhibition of cell proliferation and promotion of apoptosis are common targets by many chemotherapeutic agents[25]. DHM inhibits the proliferation of diverse types of cells, including osteosarcoma, melanoma, leukemia and gastric cancer cells[26-29]. Many molecules and signaling pathways are involved in the anti-tumor effect of DHM, including the Chk1/Chk2/Cdc25C pathway[29], the NF-κB and MAPK signaling pathways[7], and the transforming growth factor-β pathway[30]. Nevertheless, the effect of DHM on liver cancer and the specific underlying mechanism remain unclear. We found that DHM treatment of the QGY7701 and HepG2 hepatoma cell lines led to cell proliferation inhibition in a time- and dose-dependent manner. The colony-forming capability of QGY7701 and HepG2 cells treated with different concentrations of DHM was inhibited. Cell images demonstrated the inhibition of cell proliferation and induction of apoptosis in cells treated with high concentrations of DHM. Flow cytometry and TUNEL assays further verified the occurrence of significant apoptosis in QGY7701 and HepG2 cells treated with DHM. In view of the high expression of Notch1 in HCC samples, we examined the effect of various concentrations of DHM on the expression of Notch1, and found that Notch1 and Hes1 were downregulated. Bcl-2 and Bax proteins play major roles in the regulation of mitochondria-mediated apoptosis, which is a common apoptosis pathway. The Bcl-2 (pro-apoptotic protein) and Bax (anti-apoptotic protein) ratio can further reflect the anti-apoptotic tendencies. The ratio of anti-apoptotic to pro-apoptotic molecules, such as the Bcl-2/Bax ratio, indicates the threshold sensitivity of cells to the induction of apoptosis via the intrinsic (mitochondria-mediated) pathway[31,32]. The data showed that Bcl-2 and Bax were downregulated in DHM-treated QGY7701 and HepG2 cells, and the Bcl-2/Bax ratio was also decreased. Additionally, we knocked out the expression of Notch1 and treated these Notch1-deficient cells with DHM. The results showed that the effects of Notch1 silencing and DHM treatment were not different. However, when used in combination, Bcl-2 was downregulated, Bax was upregulated, and the reduction in the ratio of Bcl-2/Bax was more significant. These results show that the anti-tumor capacity of DHM in the QGY7701 and HepG2 hepatocarcinoma cell lines is partially exerted through inhibition of the Notch1 pathway.
In summary, our experiments confirmed that Notch1 is involved in the development of HCC and may serve as a potential diagnostic marker for HCC and an indicator of malignancy. DHM inhibits cell proliferation and promotes apoptosis by down-regulating the expression of Notch1, indicating that it can be used as a candidate drug for the treatment of HCC.
COMMENTS
Background
Hepatocellular carcinoma (HCC) is a highly malignant type of solid tumor which is a serious threat to human health. It is urgent to develop novel chemotherapeutic drugs and therapeutic targets for the treatment of HCC.
Research frontiers
Notch-1 was detected to be overexpressed in HCC; Notch1 is involved in the development and progression of HCC. Dihydromyricetin (DHM) has numerous pharmacological activities, such as antiinflammatory, antioxidation and anticarcinogenic effects. However, whether DHM can induce hepatoma cell apoptosis by Notch1 is not yet known.
Innovations and breakthroughs
This is the first study to demonstrate that DHM inhibits cell proliferation and promotes apoptosis by down-regulating the expression of Notch1.
Applications
DHM may be used a potential effective candidate agent for the treatment of HCC.
Peer-review
This article is well written. The authors investigate whether DHM inhibits cell proliferation and promotes apoptosis by downregulating Notch1 expression. These results show that the anti-tumor activity of DHM in the QGY7701 and HepG2 HCC cell lines is partially exerted through inhibition of the Notch1 pathway.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
Institutional review board statement: This study was approved by the Medical Ethics Committee of the Affiliated Hospital of Guangdong Medical University, Zhanjiang, China.
Informed consent statement: All patients have signed an informed consent form to participate in the study.
Conflict-of-interest statement: The authors deny any conflict of interest.
Data sharing statement: No additional data are available.
Peer-review started: May 8, 2017
First decision: June 23, 2017
Article in press: August 8, 2017
P- Reviewer: Hernanda PY, Sazci A, Sirin G S- Editor: Qi Y L- Editor: Ma JY E- Editor: Zhang FF
Contributor Information
Cai-Jie Lu, Laboratory of Hepatobiliary Surgery, Affiliated Hospital of Guangdong Medical University, Zhanjiang 524001, Guangdong Province, China.
Yi-Feng He, Laboratory of Hepatobiliary Surgery, Affiliated Hospital of Guangdong Medical University, Zhanjiang 524001, Guangdong Province, China.
Wei-Zhuang Yuan, the First Clinical Medical College, Southern Medical University, Guangzhou 510000, Guangdong Province, China.
Li-Jun Xiang, Laboratory of Hepatobiliary Surgery, Affiliated Hospital of Guangdong Medical University, Zhanjiang 524001, Guangdong Province, China.
Jian Zhang, Laboratory of Hepatobiliary Surgery, Affiliated Hospital of Guangdong Medical University, Zhanjiang 524001, Guangdong Province, China.
Yan-Rui Liang, Department of Gastrointestinal Surgery. The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou 510000, Guangdong Province, China.
Juan Duan, Laboratory of Hepatobiliary Surgery, Affiliated Hospital of Guangdong Medical University, Zhanjiang 524001, Guangdong Province, China.
Yun-He He, TCM-Integrated Hospital, Southern Medical University, Guangzhou 510000, Guangdong Province, China.
Ming-Yi Li, Laboratory of Hepatobiliary Surgery, Affiliated Hospital of Guangdong Medical University, Zhanjiang 524001, Guangdong Province, China. limingyi62@163.com.
References
- 1.Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, Wolfe C, Hamadeh RR, Moore A, Werdecker A, Gessner BD, Te Ao B, McMahon B, Karimkhani C, Yu C, Cooke GS, Schwebel DC, Carpenter DO, Pereira DM, Nash D, Kazi DS, De Leo D, Plass D, Ukwaja KN, Thurston GD, Yun Jin K, Simard EP, Mills E, Park EK, Catalá-López F, deVeber G, Gotay C, Khan G, Hosgood HD 3rd, Santos IS, Leasher JL, Singh J, Leigh J, Jonas JB, Sanabria J, Beardsley J, Jacobsen KH, Takahashi K, Franklin RC, Ronfani L, Montico M, Naldi L, Tonelli M, Geleijnse J, Petzold M, Shrime MG, Younis M, Yonemoto N, Breitborde N, Yip P, Pourmalek F, Lotufo PA, Esteghamati A, Hankey GJ, Ali R, Lunevicius R, Malekzadeh R, Dellavalle R, Weintraub R, Lucas R, Hay R, Rojas-Rueda D, Westerman R, Sepanlou SG, Nolte S, Patten S, Weichenthal S, Abera SF, Fereshtehnejad SM, Shiue I, Driscoll T, Vasankari T, Alsharif U, Rahimi-Movaghar V, Vlassov VV, Marcenes WS, Mekonnen W, Melaku YA, Yano Y, Artaman A, Campos I, MacLachlan J, Mueller U, Kim D, Trillini M, Eshrati B, Williams HC, Shibuya K, Dandona R, Murthy K, Cowie B, Amare AT, Antonio CA, Castañeda-Orjuela C, van Gool CH, Violante F, Oh IH, Deribe K, Soreide K, Knibbs L, Kereselidze M, Green M, Cardenas R, Roy N, Tillmann T, Li Y, Krueger H, Monasta L, Dey S, Sheikhbahaei S, Hafezi-Nejad N, Kumar GA, Sreeramareddy CT, Dandona L, Wang H, Vollset SE, Mokdad A, Salomon JA, Lozano R, Vos T, Forouzanfar M, Lopez A, Murray C, Naghavi M. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1:505–527. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morise Z, Kawabe N, Tomishige H, Nagata H, Kawase J, Arakawa S, Yoshida R, Isetani M. Recent advances in the surgical treatment of hepatocellular carcinoma. World J Gastroenterol. 2014;20:14381–14392. doi: 10.3748/wjg.v20.i39.14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan M, Li T, Ahmad Khan MK, Rasul A, Nawaz F, Sun M, Zheng Y, Ma T. Alantolactone induces apoptosis in HepG2 cells through GSH depletion, inhibition of STAT3 activation, and mitochondrial dysfunction. Biomed Res Int. 2013;2013:719858. doi: 10.1155/2013/719858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woo HJ, Kang HK, Nguyen TT, Kim GE, Kim YM, Park JS, Kim D, Cha J, Moon YH, Nam SH, et al. Synthesis and characterization of ampelopsin glucosides using dextransucrase from Leuconostoc mesenteroides B-1299CB4: glucosylation enhancing physicochemical properties. Enzyme Microb Technol. 2012;51:311–318. doi: 10.1016/j.enzmictec.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Liao W, Ning Z, Ma L, Yin X, Wei Q, Yuan E, Yang J, Ren J. Recrystallization of dihydromyricetin from Ampelopsis grossedentata and its anti-oxidant activity evaluation. Rejuvenation Res. 2014;17:422–429. doi: 10.1089/rej.2014.1555. [DOI] [PubMed] [Google Scholar]
- 6.Hou X, Tong Q, Wang W, Xiong W, Shi C, Fang J. Dihydromyricetin protects endothelial cells from hydrogen peroxide-induced oxidative stress damage by regulating mitochondrial pathways. Life Sci. 2015;130:38–46. doi: 10.1016/j.lfs.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Hou XL, Tong Q, Wang WQ, Shi CY, Xiong W, Chen J, Liu X, Fang JG. Suppression of Inflammatory Responses by Dihydromyricetin, a Flavonoid from Ampelopsis grossedentata, via Inhibiting the Activation of NF-κB and MAPK Signaling Pathways. J Nat Prod. 2015;78:1689–1696. doi: 10.1021/acs.jnatprod.5b00275. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Ma JN, Ma CL, Qi Z, Ma CM. Simultaneous quantification of ten constituents of Xanthoceras sorbifolia Bunge using UHPLC-MS methods and evaluation of their radical scavenging, DNA scission protective, and α-glucosidase inhibitory activities. Chin J Nat Med. 2015;13:873–880. doi: 10.1016/S1875-5364(15)30092-3. [DOI] [PubMed] [Google Scholar]
- 9.Zhong ZX, Qin JP, Zhou GF, Chen XF. [Experimental studies of hypoglycemic action on total flavone of Ampelopsis grossedentata from Guangxi] Zhongguo Zhongyao Zazhi. 2002;27:687–689. [PubMed] [Google Scholar]
- 10.Xie J, Liu J, Chen TM, Lan Q, Zhang QY, Liu B, Dai D, Zhang WD, Hu LP, Zhu RZ. Dihydromyricetin alleviates carbon tetrachloride-induced acute liver injury via JNK-dependent mechanism in mice. World J Gastroenterol. 2015;21:5473–5481. doi: 10.3748/wjg.v21.i18.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen S, Zhao X, Wan J, Ran L, Qin Y, Wang X, Gao Y, Shu F, Zhang Y, Liu P, et al. Dihydromyricetin improves glucose and lipid metabolism and exerts anti-inflammatory effects in nonalcoholic fatty liver disease: A randomized controlled trial. Pharmacol Res. 2015;99:74–81. doi: 10.1016/j.phrs.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Q, Liu J, Liu B, Xia J, Chen N, Chen X, Cao Y, Zhang C, Lu C, Li M, et al. Dihydromyricetin promotes hepatocellular carcinoma regression via a p53 activation-dependent mechanism. Sci Rep. 2014;4:4628. doi: 10.1038/srep04628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang QY, Li R, Zeng GF, Liu B, Liu J, Shu Y, Liu ZK, Qiu ZD, Wang DJ, Miao HL, et al. Dihydromyricetin inhibits migration and invasion of hepatoma cells through regulation of MMP-9 expression. World J Gastroenterol. 2014;20:10082–10093. doi: 10.3748/wjg.v20.i29.10082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang L, Zhang Q, Ren H, Ma S, Lu C, Liu B, Liu J, Liang J, Li M, Zhu R. Dihydromyricetin Enhances the Chemo-Sensitivity of Nedaplatin via Regulation of the p53/Bcl-2 Pathway in Hepatocellular Carcinoma Cells. PLoS One. 2015;10:e0124994. doi: 10.1371/journal.pone.0124994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 16.Matsuno K, Diederich RJ, Go MJ, Blaumueller CM, Artavanis-Tsakonas S. Deltex acts as a positive regulator of Notch signaling through interactions with the Notch ankyrin repeats. Development. 1995;121:2633–2644. doi: 10.1242/dev.121.8.2633. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q, Lu C, Fang T, Wang Y, Hu W, Qiao J, Liu B, Liu J, Chen N, Li M, et al. Notch3 functions as a regulator of cell self-renewal by interacting with the β-catenin pathway in hepatocellular carcinoma. Oncotarget. 2015;6:3669–3679. doi: 10.18632/oncotarget.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahn S, Hyeon J, Park CK. Notch1 and Notch4 are markers for poor prognosis of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2013;12:286–294. doi: 10.1016/s1499-3872(13)60046-6. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Du G, Xu Y, Li X, Fan W, Chen J, Liu C, Chen G, Liu C, Zern MA, et al. Inhibition of notch signaling pathway prevents cholestatic liver fibrosis by decreasing the differentiation of hepatic progenitor cells into cholangiocytes. Lab Invest. 2016;96:350–360. doi: 10.1038/labinvest.2015.149. [DOI] [PubMed] [Google Scholar]
- 20.Kunnimalaiyaan S, Gamblin TC, Kunnimalaiyaan M. Glycogen synthase kinase-3 inhibitor AR-A014418 suppresses pancreatic cancer cell growth via inhibition of GSK-3-mediated Notch1 expression. HPB (Oxford) 2015;17:770–776. doi: 10.1111/hpb.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Li Y, Banerjee S, Kong D, Ahmad A, Nogueira V, Hay N, Sarkar FH. Down-regulation of Notch-1 and Jagged-1 inhibits prostate cancer cell growth, migration and invasion, and induces apoptosis via inactivation of Akt, mTOR, and NF-kappaB signaling pathways. J Cell Biochem. 2010;109:726–736. doi: 10.1002/jcb.22451. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Li B, Ji ZZ, Zheng PS. Notch1 regulates the growth of human colon cancers. Cancer. 2010;116:5207–5218. doi: 10.1002/cncr.25449. [DOI] [PubMed] [Google Scholar]
- 23.Yuan X, Wu H, Xu H, Han N, Chu Q, Yu S, Chen Y, Wu K. Meta-analysis reveals the correlation of Notch signaling with non-small cell lung cancer progression and prognosis. Sci Rep. 2015;5:10338. doi: 10.1038/srep10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franko-Tobin LG, Mackey LV, Huang W, Song X, Jin B, Luo J, Morris LM, Liu M, Fuselier JA, Coy DH, et al. Notch1-mediated tumor suppression in cervical cancer with the involvement of SST signaling and its application in enhanced SSTR-targeted therapeutics. Oncologist. 2012;17:220–232. doi: 10.1634/theoncologist.2011-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen KC, Willmore WG, Tayabali AF. Cadmium telluride quantum dots cause oxidative stress leading to extrinsic and intrinsic apoptosis in hepatocellular carcinoma HepG2 cells. Toxicology. 2013;306:114–123. doi: 10.1016/j.tox.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Z, Yin JQ, Wu MS, Song G, Xie XB, Zou C, Tang Q, Wu Y, Lu J, Wang Y, et al. Dihydromyricetin activates AMP-activated protein kinase and P38(MAPK) exerting antitumor potential in osteosarcoma. Cancer Prev Res (Phila) 2014;7:927–938. doi: 10.1158/1940-6207.CAPR-14-0067. [DOI] [PubMed] [Google Scholar]
- 27.Zeng G, Liu J, Chen H, Liu B, Zhang Q, Li M, Zhu R. Dihydromyricetin induces cell cycle arrest and apoptosis in melanoma SK-MEL-28 cells. Oncol Rep. 2014;31:2713–2719. doi: 10.3892/or.2014.3160. [DOI] [PubMed] [Google Scholar]
- 28.Ji FJ, Tian XF, Liu XW, Fu LB, Wu YY, Fang XD, Jin HY. Dihydromyricetin induces cell apoptosis via a p53-related pathway in AGS human gastric cancer cells. Genet Mol Res. 2015;14:15564–15571. doi: 10.4238/2015.December.1.7. [DOI] [PubMed] [Google Scholar]
- 29.Huang H, Hu M, Zhao R, Li P, Li M. Dihydromyricetin suppresses the proliferation of hepatocellular carcinoma cells by inducing G2/M arrest through the Chk1/Chk2/Cdc25C pathway. Oncol Rep. 2013;30:2467–2475. doi: 10.3892/or.2013.2705. [DOI] [PubMed] [Google Scholar]
- 30.Liu B, Zhou W, Chen X, Xu F, Chen Y, Liu J, Zhang Q, Bao S, Chen N, Li M, et al. Dihydromyricetin induces mouse hepatoma Hepal-6 cell apoptosis via the transforming growth factor-β pathway. Mol Med Rep. 2015;11:1609–1614. doi: 10.3892/mmr.2014.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bagci EZ, Vodovotz Y, Billiar TR, Ermentrout GB, Bahar I. Bistability in apoptosis: roles of bax, bcl-2, and mitochondrial permeability transition pores. Biophys J. 2006;90:1546–1559. doi: 10.1529/biophysj.105.068122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang L, Wang P, Wang H, Li Q, Teng H, Liu Z, Yang W, Hou L, Zou X. Fucoidan derived from Undaria pinnatifida induces apoptosis in human hepatocellular carcinoma SMMC-7721 cells via the ROS-mediated mitochondrial pathway. Mar Drugs. 2013;11:1961–1976. doi: 10.3390/md11061961. [DOI] [PMC free article] [PubMed] [Google Scholar]


