Abstract
AIM
To investigate the clinical significance of preoperative systemic immune-inflammation index (SII) in patients with colorectal cancer (CRC).
METHODS
A retrospective analysis of 1383 cases with CRC was performed following radical surgery. SII was calculated with the formula SII = (P × N)/L, where P, N, and L refer to peripheral platelet, neutrophil, and lymphocyte counts, respectively. The clinicopathological features and follow-up data were evaluated to compare SII with other systemic inflammation-based prognostic indices such as the neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) in patients with CRC.
RESULTS
The optimal cut-off point for SII was defined as 340. The overall survival (OS) and disease-free survival (DFS) were better in patients with low NLR, PLR, and SII (P < 0.05). The SII was an independent predictor of OS and DFS in multivariate analysis. The area under the receiver-operating characteristics (ROC) curve for SII (0.707) was larger than those for NLR (0.602) and PLR (0.566). In contrast to NLR and PLR, SII could effectively discriminate between the TNM subgroups.
CONCLUSION
SII is a more powerful tool for predicting survival outcome in patients with CRC. It might assist the identification of high-risk patients among patients with the same TNM stage.
Keywords: Colorectal cancer, Systemic immune-inflammation index, Neutrophil-lymphocyte ratio, Platelet-lymphocyte ratio, Prognosis
Core tip: A preoperative systemic immune-inflammation index based on peripheral lymphocyte, neutrophil, and platelet counts was established, and better prognostic predictive abilities for overall survival and recurrence were found when compared with neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in patients with colorectal cancer. This index might assist the identification of high-risk patients among patients with the same TNM stage in clinical practice.
INTRODUCTION
Colorectal cancer (CRC), the third most frequently diagnosed cancer in men, and the second in women, is the third most common cause of cancer-related mortality worldwide[1]. The incidence of CRC in the United States has decreased owing to the improvements in cancer screening and the removal of precancerous adenomas[2]. However, an increase in the incidence of CRC was observed in many developing countries[3]. Owing to the absence of early symptoms and a hesitation in performing colonoscopy, a considerable number of CRC patients are diagnosed at an advanced stage, with an unfavorable overall survival (OS)[4]. Currently, the TNM staging system for CRC is the most commonly used predictor of OS and recurrence. However, prognostic heterogeneity was observed among patients with the same TNM stage[5], which causes confusion among clinicians when making therapeutic choices. Hence, more potential biomarkers should be included in clinical practice to improve prognostic prediction.
The interplay between systemic inflammation and the local immune response was recognized as the seventh hallmark of cancer, and it has been demonstrated to be involved in the initiation, development, and progression of several types of malignancies[6,7]. Cancer-related inflammation encompasses tumor-derived and host-derived cytokines, immune cells, and small inflammatory protein mediators[8,9], and is determined by the levels of serum leukocytes, neutrophils, lymphocytes, platelets, and acute-phase proteins such as C-reactive protein. Recently, the combinations of these systemic inflammation parameters, including neutrophil-lymphocyte ratio (NLR)[10] and platelet-lymphocyte ratio (PLR)[11], were reported as prognostic factors in some malignant solid tumors, including CRC. However, Hu et al[12] reported that systemic immune-inflammation index (SII), an integrated indicator based on peripheral lymphocyte, neutrophil, and platelet counts, was a powerful prognostic marker for patients with hepatocellular carcinoma. However, the SII for CRC has not been reported to date, and little is known about its prognostic value for CRC.
The aim of the present study was to investigate and compare the clinical significance and prognostic value of NLR, PLR, and SII in patients with CRC who underwent radical surgery.
MATERIALS AND METHODS
Ethics statement
The present study was approved by the ethics committee of the First Affiliated Hospital of Sun Yat-sen University. The requirement for informed consent was waived owing to the retrospective nature of this study.
Patients
We retrospectively analyzed the patients with primary CRC who underwent radical surgery at the Department of Gastrointestinal Surgery, the First Affiliated Hospital of Sun Yat-sen University between January 1994 and December 2010. 5-fluorouracil (5-FU)-based adjuvant chemotherapy was administered to stage III/IV patients and high-risk stage II patients. The inclusion criteria for patient enrollment were as follows: (1) primary colorectal adenocarcinoma confirmed by histopathology; (2) patients who underwent radical surgery; and (3) the availability of complete peripheral blood counts and follow-up data. The exclusion criteria were as follows: (1) clinical evidence of infection; (2) the presence of hematological system diseases; (3) previous treatment with neoadjuvant chemotherapy or radiochemotherapy; (4) bowel obstruction or enterobrosis resulting in emergency surgery; (5) concurrent cancers or CRC recurrence; and (6) the use of anti-inflammatory or immunosuppressive medicines. Finally, 1383 cases were enrolled in the present study.
Data collection
The following variables were analyzed: demographics (age and sex), clinicopathological features (tumor location, tumor size, histological type, and tumor stage), and treatment with chemotherapy. We defined cecum carcinoma, ascending colon cancer, and right-half transverse colon as right-sided CRC, whereas the rest were classified as left-sided CRCs. The well and moderately differentiated adenocarcinomas were histologically categorized as the well-differentiated type, and the poorly differentiated adenocarcinomas included poorly differentiated adenocarcinoma, mucinous adenocarcinoma, signet ring cell cancer, and undifferentiated cancer. Tumor staging was performed according to the 7th edition of the Union for International Cancer Control-American Joint Committee on cancer classification for CRC.
Preoperative blood sampling was performed to measure the neutrophil, lymphocyte, and platelet levels for the calculation of the NLR, PLR, and SII indices. NLR and PLR were defined as the total number of neutrophils or platelets divided by the total number of lymphocytes. SII was calculated with the formula SII = (P × N)/L, where P, N, and L refer to peripheral platelet, neutrophil, and lymphocyte counts, respectively.
Follow-up
Patients were followed every 3 mo in the first 2 years following surgery, every 6 mo in years 3-5, and annually thereafter. As previously described[13], clinical history was taken, physical examination was performed, peripheral tumor biomarker levels were measured, and chest radiography, abdominal and pelvic computed tomography or ultrasonography, and colonoscopy were performed in the follow-up period according to the NCCN Clinical Practice Guidelines in Oncology. The OS and disease-free survival (DFS) were defined as the interval between surgery and time of death or the time from the last follow-up to the time of first confirmed recurrence, respectively.
Statistical analysis
Data was analyzed using the SPSS statistical software for Windows, version 17 (SPSS Inc., Chicago, United States). Receiver operating characteristic (ROC) curve analysis was performed to analyze the area under the ROC curve (AUC), and the Youden Index was used to identify the optimal cut-off values for NLR, PLR, and SII. Pearson χ2 test or Fisher exact test were performed to compare the different categorical variable groups. Survival analysis was performed using the Kaplan-Meier method and the Log-Rank test was used to compare the survival differences. Univariate and multivariate analyses were performed with the Cox proportional hazards regression model. A P value < 0.05 was considered statistically significant.
RESULTS
ROC analysis
Using cancer-specific death as the end point, ROC analysis was performed to identify the optimal cut-off point with the highest sensitivity and specificity, which was 2.7 for NLR, 210 for PLR, and 340 for SII (sensitivity and specificity: 0.414 and 0.750 for NLR, 0.425 and 0.708 for PLR, and 0.857 and 0.524 for SII, respectively). For each immune-inflammation index, patients were divided into two groups for further analysis [NLR ≤ 2.7 (low) and NLR > 2.7 (high); PLR ≤ 210 (low) and PLR > 210 (high); SII ≤ 340 (low) and SII > 340 (high)].
Baseline characteristics of patients
In total, 1383 cases were enrolled in the present study. Patients in the high NLR group were more elderly compared to the low NLR group (> 60 years old: 54.7% vs 46.8%, respectively); however, associations between age and the levels of PLR and SII were not identified. Moreover, there were significant sex distribution differences in the three groups. In addition, cases in the high NLR and PLR groups were more likely to have left-sided CRC; however, the tumor location did not differ significantly between the high and low SII groups. High levels of NLR, PLR, and SII correlated with poor histological differentiation, larger tumor size, advanced T stage, N stage, M stage, TNM stage, and chemotherapy. The associations of NLR, PLR, and SII with clinicopathological parameters are demonstrated in Table 1.
Table 1.
Baseline patient characteristics based on neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and systemic immune-inflammation index n (%)
| Cases |
NLR |
P value |
PLR |
P value |
SII |
P value | ||||
| ≤ 2.7 | > 2.7 | ≤ 210 | > 210 | ≤ 340 | > 340 | |||||
| Age (yr) | 0.005 | 0.904 | 0.063 | |||||||
| ≤ 60 | 698 (50.5) | 480 (53.2) | 218 (45.3) | 437 (50.3) | 261 (50.7) | 233 (54.2) | 465 (48.8) | |||
| > 60 | 685 (49.5) | 422 (46.8) | 263 (54.7) | 431 (49.7) | 254 (49.7) | 197 (45.8) | 488 (51.2) | |||
| Gender | 0.067 | 0.422 | 0.927 | |||||||
| Male | 808 (58.4) | 511 (56.7) | 297 (61.7) | 500 (57.6) | 308 (59.8) | 252 (58.6) | 536 (58.3) | |||
| Female | 575 (41.6) | 391 (43.3) | 184 (38.3) | 368 (42.4) | 207 (40.2) | 178 (41.4) | 397 (41.7) | |||
| Tumor location | < 0.001 | 0.008 | 0.060 | |||||||
| Right-sided | 324 (23.4) | 185 (20.5) | 139 (28.9) | 183 (21.1) | 141 (27.4) | 87 (20.2) | 237 (24.9) | |||
| Left-sided | 1059 (76.6) | 717 (79.5) | 342 (71.1) | 685 (78.9) | 374 (72.3) | 343 (79.8) | 716 (75.1) | |||
| Histological type | < 0.001 | 0.020 | < 0.001 | |||||||
| Well-differentiated | 1126 (81.4) | 761 (84.4) | 365 (75.9) | 723 (83.3) | 403 (78.3) | 376 (87.4) | 750 (78.7) | |||
| Poorly differentiated | 257 (18.6) | 141 (15.6) | 116 (24.1) | 145 (16.7) | 112 (21.7) | 54 (12.6) | 203 (21.3) | |||
| Tumor size | < 0.001 | < 0.001 | 0.003 | |||||||
| ≤ 5 cm | 936 (67.7) | 660 (73.2) | 276 (57.4) | 617 (71.1) | 319 (61.9) | 315 (73.3) | 621 (65.2) | |||
| > 5 cm | 447 (32.3) | 242 (26.8) | 205 (42.6) | 251 (28.9) | 196 (38.1) | 115 (26.7) | 332 (34.8) | |||
| T stage | < 0.001 | < 0.001 | < 0.001 | |||||||
| T1 | 67 (4.8) | 45 (5.0) | 22 (4.6) | 54 (4.9) | 13 (4.8) | 38 (8.8) | 29 (3.0) | |||
| T2 | 256 (18.5) | 197 (21.8) | 59 (12.3) | 170 (19.9) | 86 (16.7) | 104 (24.2) | 152 (15.9) | |||
| T3 | 748 (54.1) | 507 (56.2) | 241 (50.1) | 475 (54.7) | 273 (53.0) | 232 (54.0) | 516 (54.1) | |||
| T4 | 312 (22.6) | 153 (17.0) | 159 (33.1) | 169 (19.5) | 143 (27.8) | 56 (13.0) | 256 (26.9) | |||
| N stage | < 0.001 | 0.021 | < 0.001 | |||||||
| N0 | 487 (35.2) | 353 (39.1) | 134 (27.9) | 325 (37.4) | 162 (31.5) | 196 (45.6) | 291 (30.5) | |||
| N1 | 416 (30.1) | 277 (30.7) | 139 (28.9) | 261 (30.1) | 155 (30.1) | 121 (28.1) | 295 (31.0) | |||
| N2 | 308 (22.3) | 188 (20.8) | 120 (24.9) | 190 (21.9) | 118 (22.9) | 77 (17.9) | 231 (24.2) | |||
| N3 | 172 (12.4) | 84 (9.3) | 88 (18.3) | 92 (10.6) | 80 (15.5) | 36 (8.4) | 136 (14.3) | |||
| M stage | < 0.001 | < 0.001 | < 0.001 | |||||||
| M0 | 1115 (80.6) | 774 (85.8) | 341 (70.9) | 726 (83.6) | 389 (75.5) | 386 (89.8) | 729 (76.5) | |||
| M1 | 268 (19.4) | 128 (14.2) | 140 (29.1) | 142 (16.4) | 126 (24.5) | 44 (10.2) | 224 (23.5) | |||
| TNM stage | < 0.001 | 0.001 | < 0.001 | |||||||
| I | 187 (13.5) | 141 (15.6) | 46 (9.6) | 133 (15.3) | 54 (10.5) | 92 (21.4) | 95 (10.0) | |||
| II | 515 (37.2) | 368 (40.8) | 147 (30.6) | 335 (38.6) | 180 (35.0) | 171 (39.8) | 344 (36.1) | |||
| III | 413 (29.9) | 265 (29.4) | 148 (30.8) | 258 (29.7) | 155 (30.1) | 123 (28.6) | 290 (30.4) | |||
| IV | 268 (19.4) | 128 (14.2) | 140 (29.1) | 142 (16.4) | 126 (24.5) | 44 (10.2) | 224 (23.5) | |||
| Chemotherapy | < 0.001 | < 0.001 | < 0.001 | |||||||
| No | 684 (49.5) | 488 (54.1) | 196 (40.7) | 472 (54.4) | 212 (41.2) | 243 (56.5) | 441 (46.3) | |||
| Yes | 699 (50.5) | 414 (45.9) | 285 (59.3) | 396 (45.6) | 303 (58.8) | 187 (43.5) | 512 (53.7) | |||
NLR: Neutrophil-lymphocyte ratio; PLR: Platelet-lymphocyte ratio; SII: Systemic immune-inflammation index.
Prognostic value of NLR, PLR, and SII
In the present study, the 5-, 10-, 15-, and 20-year OS rates were 61.2%, 45.1%, 35.6%, and 28.1%, respectively. The patients in the high NLR, PLR, and SII groups showed poorer OS compared to patients in the low NLR, PLR, and SII groups, respectively (Figure 1A-C). In order to identify the prognostic parameters for OS, 13 variables were included in the univariate Cox regression analysis, which showed that the NLR, PLR, SII, age, histological type, tumor invasion, lymph node involvement, distant metastasis, TNM stage, and chemotherapy were the variables that had significant impact on OS. After the exclusion of variables that showed no impact on the OS in univariate analysis, Cox multivariate regression analysis was performed, which identified SII (95%CI: 2.616-3.824), PLR (95%CI: 1.123-1.492), age (95%CI: 1.355-1.798), distant metastasis (95%CI: 1.512-2.517), and TNM stage (95%CI: 1.191-1.518) as the independent prognostic factors of OS (Table 2).
Figure 1.
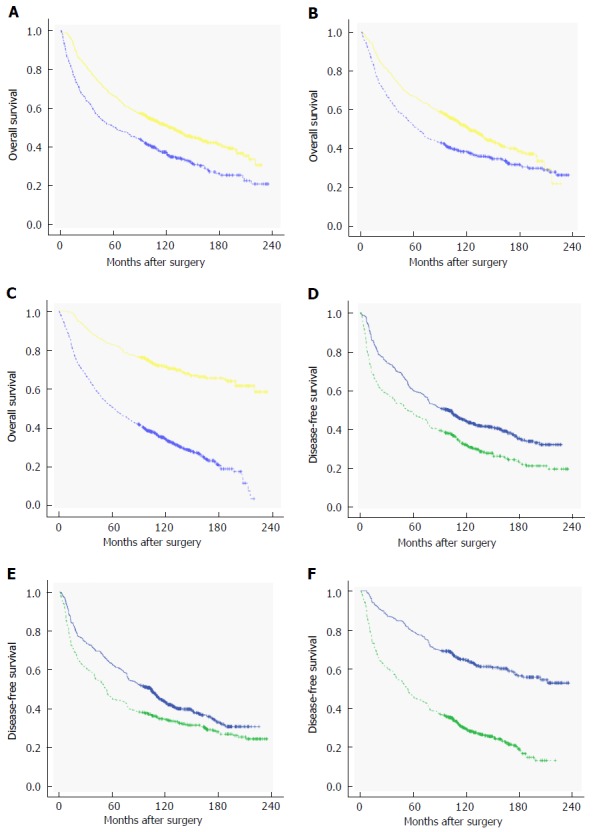
Kaplan-Meier curves of overall survival and disease-free survival of colorectal cancer patients based on neutrophil-lymphocyte ratio (A and D), platelet-lymphocyte ratio (B and E), and systemic immune-inflammation index (C and F).
Table 2.
Univariate and multivariate Cox regression analysis of the associations between clinical parameters and overall survival in patients with colorectal cancer
|
Univariate analysis |
Multivariate analysis |
|||||||
| χ2 value | HR | 95%CI | P value | χ2 value | HR | 95%CI | P value | |
| Age | 22.965 | 1.405 | 1.223-1.614 | < 0.001 | 38.131 | 1.561 | 1.355-1.798 | < 0.001 |
| Gender | 0.017 | - | - | 0.896 | ||||
| Tumor location | 0.269 | - | - | 0.604 | ||||
| Histological type | 22.178 | 1.493 | 1.264-1.764 | < 0.001 | ||||
| Tumor size | 1.166 | - | - | 0.280 | ||||
| T stage | 71.702 | 1.498 | 1.364-1.645 | < 0.001 | ||||
| N stage | 113.905 | 1.432 | 1.340-1.529 | < 0.001 | ||||
| M stage | 239.937 | 3.415 | 2.923-3.989 | < 0.001 | 26.390 | 1.951 | 1.512-2.517 | < 0.001 |
| TNM stage | 207.252 | 1.785 | 1.650-1.932 | < 0.001 | 22.971 | 1.345 | 1.191-1.518 | < 0.001 |
| NLR status | 40.824 | 1.582 | 1.374-1.821 | < 0.001 | ||||
| PLR status | 23.604 | 1.416 | 1.231-1.649 | < 0.001 | 12.618 | 1.294 | 1.123-1.492 | < 0.001 |
| SII status | 173.330 | 3.529 | 2.925-4.258 | < 0.001 | 141.427 | 3.163 | 2.616-3.824 | < 0.001 |
| Chemotherapy | 76.931 | 1.894 | 1.642-2.184 | < 0.001 | ||||
NLR: Neutrophil-lymphocyte ratio; PLR: Platelet-lymphocyte ratio.
Similarly, the 5-, 10-, 15-, and 20-year DFS rates were 56.5%, 39.1%, 31.3%, and 26.5%, respectively. The DFS rates were lower in patients with high NLR, PLR, and SII compared to those of the patients with low NLR, PLR, and SII, respectively (Figure 1D-F). The DFS data for the three patient groups are demonstrated in Table 3. Univariate Cox proportional hazards regression analysis revealed that age, histological type, tumor invasion, lymph node involvement, distant metastasis, TNM stage, chemotherapy, NLR, PLR, and SII had statistically significant associations with DFS. In addition, multivariate analysis indicated that SII was a significant independent prognostic parameter for DFS, whereas PLR and NLR were not (Table 3).
Table 3.
Univariate and multivariate Cox regression analysis of the associations between clinical parameters and PFS in patients with colorectal cancer
|
Univariate analysis |
Multivariate analysis |
|||||||
| χ2 value | HR | 95%CI | P value | χ2 value | HR | 95%CI | P value | |
| Age | 21.774 | 1.373 | 1.202-1.569 | < 0.001 | 34.479 | 1.499 | 1.310-1.716 | < 0.001 |
| Gender | 0.144 | - | - | 0.705 | ||||
| Tumor location | 0.509 | - | - | 0.476 | ||||
| Histological type | 20.091 | 1.447 | 1.231-1.701 | < 0.001 | ||||
| Tumor size | 1.095 | - | - | 0.295 | ||||
| T stage | 59.847 | 1.419 | 1.299-1.550 | < 0.001 | ||||
| N stage | 86.718 | 1.354 | 1.270-1.443 | < 0.001 | ||||
| M stage | 221.926 | 3.172 | 2.725-3.692 | < 0.001 | 31.580 | 2.024 | 1.583-2.589 | < 0.001 |
| TNM stage | 179.589 | 1.673 | 1.552-1.804 | < 0.001 | 15.414 | 1.255 | 1.121-1.406 | < 0.001 |
| NLR status | 36.441 | 1.518 | 1.325-1.738 | < 0.001 | ||||
| PLR status | 20.826 | 1.369 | 1.196-1.567 | < 0.001 | ||||
| SII status | 159.123 | 2.988 | 2.521-3.542 | < 0.001 | 129.495 | 2.717 | 2.287-3.228 | < 0.001 |
| Chemotherapy | 61.159 | 1.716 | 1.499-1.965 | < 0.001 | ||||
NLR: Neutrophil-lymphocyte ratio; PLR: Platelet-lymphocyte ratio.
The AUCs of the NLR, PLR, and SII for OS were 0.602, 0.566, and 0.707, respectively (Figure 2A) and the AUCs for DFS were 0.597, 0.558, and 0.701, respectively (Figure 2B). Hence, among the immune-inflammation indices analyzed in the present study, SII was the best predictor of long-term survival and recurrence in cases with CRC.
Figure 2.
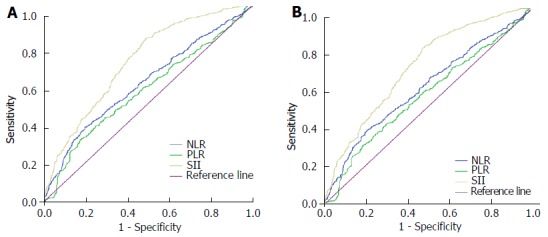
Receiver operating curve analysis of overall survival (A) and disease-free survival (B). NLR: Neutrophil-lymphocyte ratio; PLR: Platelet-lymphocyte ratio; SII: Systemic immune-inflammation index.
Prognostic value of NLR, PLR, and SII stratified according to TNM stage
Further subgroup analyses were performed to investigate the prognostic value of SII, NLR, and PLR in patients with CRC who were stratified according to the TNM stage. The results of the analyses showed that only SII was able to distinguish the OS and DFS for each TNM stage (Figure 3A-F). On the other hand, NLR could identify the survival differences between TNM stages II-IV, while PLR could only detect the prognostic differences of stage II-III cancers (Figure 4A-F for NLR and Figure 5A-F for PLR). Hence, the results indicated that only SII had prognostic significance for the CRC cases stratified according to TNM stage.
Figure 3.
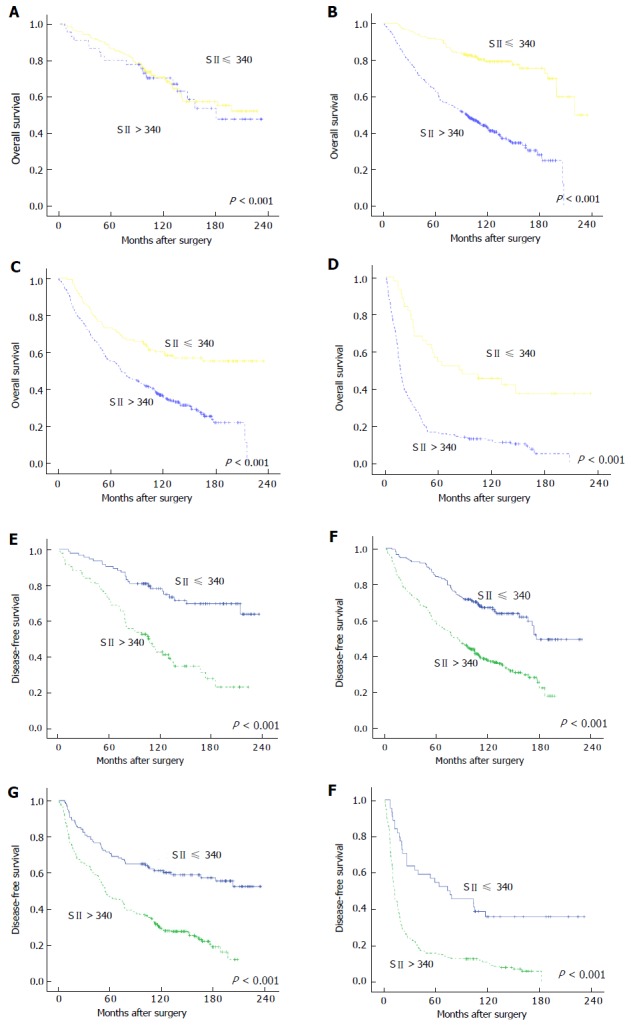
Kaplan-Meier curves of overall survival and disease-free survival of colorectal cancer patients based on different levels of systemic immune-inflammation index. A-D: OS for cases with stages I, II, III, and IV CRC, respectively; E and F: DFS for cases with stages I, II, III, and IV CRC, respectively. OS: Overall survival; DFS: Disease-free survival; CRC: Colorectal cancer.
Figure 4.
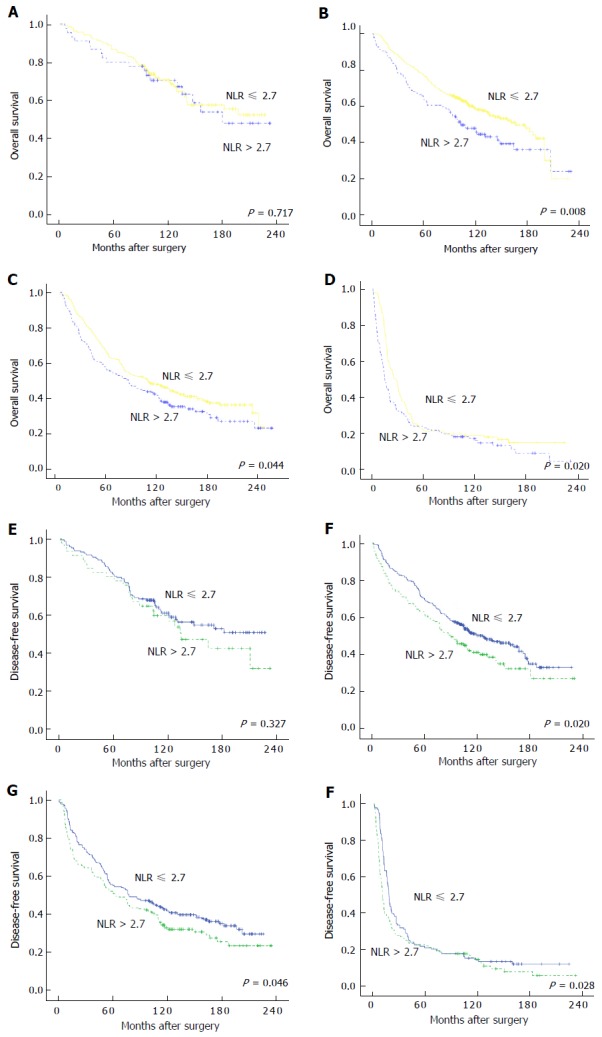
Kaplan-Meier curves of overall survival and disease-free survival of colorectal cancer patients based on different levels of neutrophil-lymphocyte ratio. A-D: OS for cases with stages I, II, III, and IV CRC, respectively; E and F: DFS for cases with stages I, II, III, and IV CRC, respectively. OS: Overall survival; DFS: Disease-free survival; CRC: Colorectal cancer; NLR: Neutrophil-lymphocyte ratio.
Figure 5.
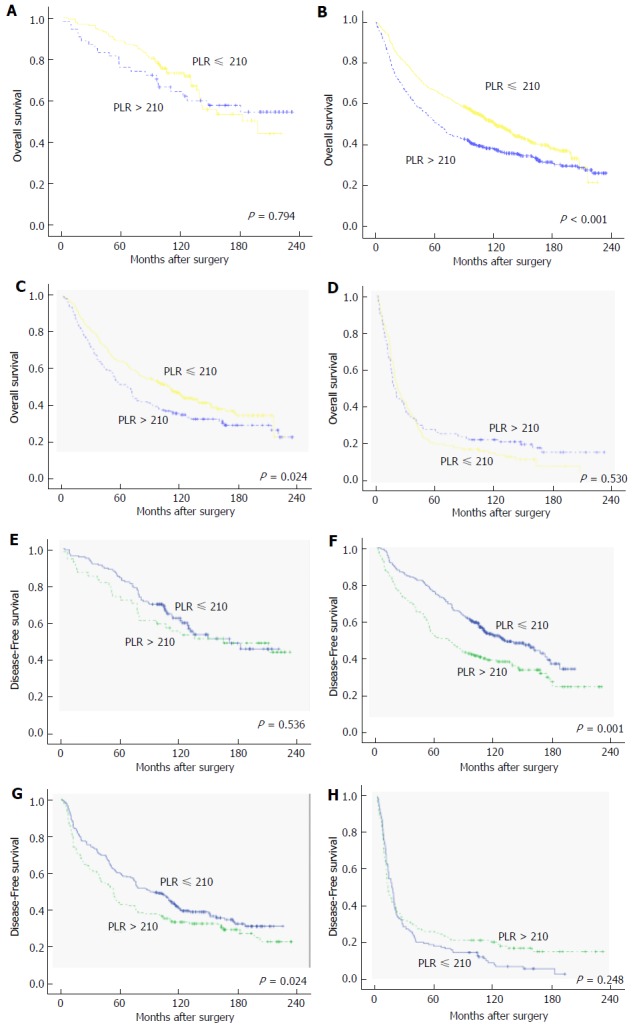
Kaplan-Meier curves of overall survival and disease-free survival of colorectal cancer patients based on different levels of platelet-lymphocyte ratio. A-D: OS for cases with stages I, II, III, and IV CRC, respectively; E-F: DFS for cases with stages I, II, III, and IV CRC, respectively. OS: Overall survival; DFS: Disease-free survival; CRC: Colorectal cancer; PLR: Platelet-lymphocyte ratio.
DISCUSSION
In the present study, we established an immune-inflammation-based prognostic index (SII) based on peripheral neutrophil, platelet, and lymphocyte counts and demonstrated that elevated SII was correlated with poor OS and recurrence in patients with CRC. In addition, SII was a superior prognostic factor for survival outcome compared to NLR and PLR.
It was recognized that inflammatory-based indices were associated with poor tumor behavior and survival outcome in various malignant solid tumors, including CRC. Several combinations such as NLR[14], PLR[15], prognostic nutritional index[16], Glasgow Prognostic score[17], and lymphocyte monocyte ratio (LMR)[18] showed positive correlations between elevated inflammation-based factors and poor survival outcome in patients with CRC. To our knowledge, this was the first report investigating the prognostic value of SII in patients with CRC after radical surgery. Using an integrated index based on peripheral neutrophil, platelet, and lymphocyte counts, Hu et al[12] found that patients having elevated preoperative SII were usually diagnosed with thrombocytosis, neutropenia, or lymphopenia. They believed that a better understanding of the roles of neutrophils, platelets, and lymphocytes in cancer development and progression would help clarify the association between SII and its clinical impact. Neutrophils do not only alter the tumor microenvironment via the extrinsic pathway, but they also secrete some inflammatory mediators to promote tumor cell proliferation, invasion, metastasis to lymph nodes or distant organs, and cellular senescence via the intrinsic pathway[19,20]. Accumulating experimental and clinical evidence showed that platelet activation could act as chemoattractants for cancer cells, induce the formation of optimized conditions for metastatic foci, promote the epithelial to mesenchymal transition in tumor cells, and increase the level of circulating tumor cells[21,22]. Lymphopenia was commonly accompanied by leukocytosis and thrombocytosis, which might help tumor cells to escape immune surveillance and prevent damage from the autoimmune response by cytotoxic T cells[23]. There was a good and a bad inflammatory reaction. In other words if the inflammation was based on the production of simply growth factors, the inflammatory reaction has a negative effect. But if the inflammatory reaction consists on neutralizing antibodies produced by activated lymph nodes, this reaction can have a positive effect. Thus, a high SII level reflected alterations in the cancer microenvironment that favor cancer initiation, progression, and metastasis.
The present study revealed interesting associations between inflammation-based indices and clinicopathological features. Consistent with the clinicopathological features associated with NLR and PLR, which are the most common indices, SII was also associated with poor histological differentiation, larger tumor size, more advanced T stage, N stage, M stage, and TNM stage, validating the above hypothesis that the elevated inflammatory response might promote tumor proliferation, progression, and metastasis.
As a simple, convenient, easily obtained, cheap, and non-invasive marker, SII was first described by Hu et al[12] in hepatocellular carcinoma. They concluded that preoperative SII might be related to circulating tumor cells and act as a powerful prognostic predictor in patients with hepatocellular carcinoma. Consistent with the results of previous studies, Yang et al[24] also reported that elevated SII with a cut-off value of 300 was negatively associated with OS in HBV-related hepatocellular carcinoma[25]. Moreover, SII was reported as a predictor of metastatic CRC in patients who received first-line chemotherapy with bevacizumab[26]. To our knowledge, the present study was the first to investigate the prognostic value of SII in CRC. Confirmed by the Kaplan-Meier analysis using the log-rank method, all the inflammation-based indices were significantly associated with OS and recurrence. However, SII was identified in Cox multivariate analysis to be a superior predictor of OS and DFS compared to other inflammation-based prognostic indices. The discriminative abilities of these three indices were further evaluated and compared; based on the AUC values obtained from ROC curves, SII was the most effective predictor of long-term survival outcome compared to NLR and PLR. The potential explanation of a better prognostic value might be that SII was more comprehensive in reflecting the status of inflammatory and immune response than the other factors.
Pathological TNM staging is presently the gold standard for predicting survival outcome and the treatment choice. However, because TNM staging was performed postoperatively, survival prediction before surgery and decision of further treatment strategies became difficult. Moreover, TNM stage can only reflect the biological behavior of the tumor. To our knowledge, prognosis was not only associated with the clinicopathological characteristics of the tumor, but also with the host inflammatory response[27]. SII is based on peripheral neutrophil, platelet, and lymphocyte counts, and it reflects the status of the tumor microenvironment and the preoperative host inflammatory response, serving as a complementary to the TNM stage for predicting OS. Our findings demonstrated that preoperative SII had powerful prognostic discriminative abilities in terms of each TNM subgroup compared to NLR and PLR. Therefore, using a combination of parameters that reflect both the tumor characteristics and the host systemic inflammatory status might be important for accurately predicting survival outcome in patients with CRC.
The present study had a few limitations. First, it was a retrospective, single-center study. Therefore, a large-scale prospective validation study is required to validate the results of the present study. Second, only the patients who received radical surgery were enrolled and thus, the results of the present study are not applicable in incurable patients or in those for whom the treatment was terminated because of various reasons.
In conclusion, this was the first study to demonstrate that preoperative SII is a simple and powerful prognostic indicator of OS and DFS in patients with CRC. SII might be used along with the TNM staging for individualized treatment in future clinical practice. A larger prospective study is warranted for the validation of the preliminary results obtained in the present study.
COMMENTS
Background
Recently, there were many papers describing mathematical formulas, such as neutrophil-lymphocyte ratio and platelet-lymphocyte ratio, which were considered important prognostic factors for colorectal cancer (CRC). In this article, the authors want to investigate the clinical value of systemic immune-inflammation index (SII), an integrated indicator based on peripheral lymphocyte, neutrophil, and platelet counts.
Research frontiers
The interplay between systemic inflammation and the local immune response was recognized as the seventh hallmark of cancer, and it has been demonstrated to be involved in the initiation, development, and progression of several types of malignancies. Inflammation can produce several cytokines and growth factors which can facilitate the occurrence and development of cancer. We established a systemic inflammation parameter, SII and found that SII had better prognostic predictive abilities for overall survival and recurrence of CRC in this study.
Innovations and breakthroughs
Preoperative SII based on peripheral lymphocyte, neutrophil, and platelet counts was established, and no study investigated the clinical value of SII in CRC before. We found that SII had better prognostic predicting abilities for overall survival and recurrence when compared with neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in patients with CRC. It might assist the identification of high-risk patients among patients with the same TNM stage in clinical practice.
Applications
Patients with high SII showed aggressive tumor biological behavior, poor overall survival and early tumor recurrence. Hence, SII may give help to identify the high-risk patients among patients with the same TNM stage in clinical practice.
Terminology
SII is based on the peripheral lymphocyte, neutrophil, and platelet counts, and is calculated using the formula SII = (P × N)/L, where P, N, and L refer to the preoperative peripheral platelet, neutrophil, and lymphocyte counts, respectively.
Peer-review
This is an interesting study. Obvious conclusions were drawn from a well-known phenomenon: Correlation between inflammation and cancer development and progression. I think the paper should be published to address an important point: To cure or prevent inflammation can prevent cancer formation and progression. The result may give help to clinical doctors.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The present study was approved by the ethics committee of the First Affiliated Hospital of Sun Yat-sen University.
Informed consent statement: The requirement for informed consent was waived owing to the retrospective nature of this study.
Conflict-of-interest statement: The authors have no conflict of interest related to the manuscript.
Data sharing statement: The original anonymous dataset is available on request from the corresponding author at chenchqi@mail.sysu.edu.cn.
Peer-review started: March 29, 2017
First decision: March 16, 2017
Article in press: July 4, 2017
P- Reviewer: Negoi I, Sterpetti AV S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Xu XR
Contributor Information
Jian-Hui Chen, Gastrointestinal Surgery Center, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China; Gastric Cancer Center, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China.
Er-Tao Zhai, Gastrointestinal Surgery Center, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China; Gastric Cancer Center, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China.
Yu-Jie Yuan, Gastrointestinal Surgery Center, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China; Gastric Cancer Center, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China.
Kai-Ming Wu, Gastrointestinal Surgery Center, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China; Gastric Cancer Center, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China.
Jian-Bo Xu, Gastrointestinal Surgery Center, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China; Gastric Cancer Center, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China.
Jian-Jun Peng, Gastrointestinal Surgery Center, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China; Gastric Cancer Center, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China.
Chuang-Qi Chen, Gastrointestinal Surgery Center, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China; Gastric Cancer Center, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China. chenchqi@mail.sysu.edu.cn.
Yu-Long He, Gastrointestinal Surgery Center, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China; Gastric Cancer Center, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China.
Shi-Rong Cai, Gastrointestinal Surgery Center, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China; Gastric Cancer Center, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 4.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–1502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 5.Fan XJ, Wan XB, Fu XH, Wu PH, Chen DK, Wang PN, Jiang L, Wang DH, Chen ZT, Huang Y, et al. Phosphorylated p38, a negative prognostic biomarker, complements TNM staging prognostication in colorectal cancer. Tumour Biol. 2014;35:10487–10495. doi: 10.1007/s13277-014-2320-3. [DOI] [PubMed] [Google Scholar]
- 6.Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493–e503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 7.Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015;12:584–596. doi: 10.1038/nrclinonc.2015.105. [DOI] [PubMed] [Google Scholar]
- 8.West NR, McCuaig S, Franchini F, Powrie F. Emerging cytokine networks in colorectal cancer. Nat Rev Immunol. 2015;15:615–629. doi: 10.1038/nri3896. [DOI] [PubMed] [Google Scholar]
- 9.Shalapour S, Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J Clin Invest. 2015;125:3347–3355. doi: 10.1172/JCI80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Jia H, Yu W, Xu Y, Li X, Li Q, Cai S. Nomograms for predicting prognostic value of inflammatory biomarkers in colorectal cancer patients after radical resection. Int J Cancer. 2016;139:220–231. doi: 10.1002/ijc.30071. [DOI] [PubMed] [Google Scholar]
- 11.Zou ZY, Liu HL, Ning N, Li SY, DU XH, Li R. Clinical significance of pre-operative neutrophil lymphocyte ratio and platelet lymphocyte ratio as prognostic factors for patients with colorectal cancer. Oncol Lett. 2016;11:2241–2248. doi: 10.3892/ol.2016.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, Zhang X, Wang WM, Qiu SJ, Zhou J, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212–6222. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- 13.Jian-Hui C, Iskandar EA, Cai ShI, Chen CQ, Wu H, Xu JB, He YL. Significance of Onodera’s prognostic nutritional index in patients with colorectal cancer: a large cohort study in a single Chinese institution. Tumour Biol. 2016;37:3277–3283. doi: 10.1007/s13277-015-4008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pine JK, Morris E, Hutchins GG, West NP, Jayne DG, Quirke P, Prasad KR. Systemic neutrophil-to-lymphocyte ratio in colorectal cancer: the relationship to patient survival, tumour biology and local lymphocytic response to tumour. Br J Cancer. 2015;113:204–211. doi: 10.1038/bjc.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ying HQ, Deng QW, He BS, Pan YQ, Wang F, Sun HL, Chen J, Liu X, Wang SK. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol. 2014;31:305. doi: 10.1007/s12032-014-0305-0. [DOI] [PubMed] [Google Scholar]
- 16.Tokunaga R, Sakamoto Y, Nakagawa S, Miyamoto Y, Yoshida N, Oki E, Watanabe M, Baba H. Prognostic Nutritional Index Predicts Severe Complications, Recurrence, and Poor Prognosis in Patients With Colorectal Cancer Undergoing Primary Tumor Resection. Dis Colon Rectum. 2015;58:1048–1057. doi: 10.1097/DCR.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 17.Lin MS, Huang JX, Yu H. Prognostic significance of Glasgow prognostic score in patients with stage II colorectal cancer. Int J Clin Exp Med. 2015;8:19138–19143. [PMC free article] [PubMed] [Google Scholar]
- 18.Shibutani M, Maeda K, Nagahara H, Ohtani H, Sakurai K, Yamazoe S, Kimura K, Toyokawa T, Amano R, Tanaka H, et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with metastatic colorectal cancer. World J Gastroenterol. 2015;21:9966–9973. doi: 10.3748/wjg.v21.i34.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moses K, Brandau S. Human neutrophils: Their role in cancer and relation to myeloid-derived suppressor cells. Semin Immunol. 2016;28:187–196. doi: 10.1016/j.smim.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Felix K, Gaida MM. Neutrophil-Derived Proteases in the Microenvironment of Pancreatic Cancer -Active Players in Tumor Progression. Int J Biol Sci. 2016;12:302–313. doi: 10.7150/ijbs.14996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orellana R, Kato S, Erices R, Bravo ML, Gonzalez P, Oliva B, Cubillos S, Valdivia A, Ibañez C, Brañes J, et al. Platelets enhance tissue factor protein and metastasis initiating cell markers, and act as chemoattractants increasing the migration of ovarian cancer cells. BMC Cancer. 2015;15:290. doi: 10.1186/s12885-015-1304-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coupland LA, Parish CR. Platelets, selectins, and the control of tumor metastasis. Semin Oncol. 2014;41:422–434. doi: 10.1053/j.seminoncol.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Quigley DA, Kristensen V. Predicting prognosis and therapeutic response from interactions between lymphocytes and tumor cells. Mol Oncol. 2015;9:2054–2062. doi: 10.1016/j.molonc.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Z, Zhang J, Lu Y, Xu Q, Tang B, Wang Q, Zhang W, Chen S, Lu L, Chen X. Aspartate aminotransferase-lymphocyte ratio index and systemic immune-inflammation index predict overall survival in HBV-related hepatocellular carcinoma patients after transcatheter arterial chemoembolization. Oncotarget. 2015;6:43090–43098. doi: 10.18632/oncotarget.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong X, Cui B, Wang M, Yang Z, Wang L, Xu Q. Systemic Immune-inflammation Index, Based on Platelet Counts and Neutrophil-Lymphocyte Ratio, Is Useful for Predicting Prognosis in Small Cell Lung Cancer. Tohoku J Exp Med. 2015;236:297–304. doi: 10.1620/tjem.236.297. [DOI] [PubMed] [Google Scholar]
- 26.Passardi A, Scarpi E, Cavanna L, Dall’Agata M, Tassinari D, Leo S, Bernardini I, Gelsomino F, Tamberi S, Brandes AA, et al. Inflammatory indexes as predictors of prognosis and bevacizumab efficacy in patients with metastatic colorectal cancer. Oncotarget. 2016;7:33210–33219. doi: 10.18632/oncotarget.8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karn T, Pusztai L, Rody A, Holtrich U, Becker S. The Influence of Host Factors on the Prognosis of Breast Cancer: Stroma and Immune Cell Components as Cancer Biomarkers. Curr Cancer Drug Targets. 2015;15:652–664. doi: 10.2174/156800961508151001101209. [DOI] [PubMed] [Google Scholar]


