Abstract
Fetal exposure to high levels of glucocorticoids programs long-term changes in the physiologic stress response and behaviours. However, it is not known whether effects manifest in subsequent generations of offspring following maternal (MT) or paternal (PT) transmission. We treated pregnant guinea pigs with three courses of saline or synthetic glucocorticoid (sGC) at a clinically relevant dose. Altered cortisol response to stress and behaviours transmitted to juvenile female and male F2 and F3 offspring from both parental lines. Behavioural effects of sGC in F1-F3 PT females associated with altered expression of genes in the prefrontal cortex and hypothalamic paraventricular nucleus (PVN). Exposure to sGC programmed large transgenerational changes in PVN gene expression, including type II diabetes, thermoregulation, and collagen formation gene networks. We demonstrate transgenerational programming to F3 following antenatal sGC. Transmission is sex- and generation-dependent, occurring through both parental lines. Paternal transmission to F3 females strongly implicates epigenetic mechanisms of transmission.
Introduction
Glucocorticoids (GC) are a potent developmental trigger during pregnancy. The late-gestation GC surge promotes rapid maturation of fetal organs, including the lungs and brain1. However, fetal exposure to high levels of GC prior to the surge, either through maternal stress or treatment with synthetic glucocorticoids (sGC), alters the trajectory of development and programs stress-associated behaviours (e.g. locomotor activity, attention, anxiety) through altered gene expression in the medial prefrontal cortex (mPFC) and hypothalamic-pituitary-adrenal (HPA) axis2–5.
Women at risk for preterm birth between 24–34 weeks gestation receive sGC to improve infant respiratory outcomes6. While a single course of sGC is currently the gold-standard therapy, during the 1990’s and early 2000’s multiple course treatment with sGC was common7,8. Antenatal exposure to multiple courses of sGC has been associated with hyperactivity9, impaired attention10,11, and neurodevelopmental impairment12 in young children and animals2. It is imperative that the long-term effects of antenatal exposure to multiple courses of sGC continue to be investigated since the use of a ‘rescue’ (i.e. a second) course of sGC has recently re-introduced the practice of multiple course administration13,14.
While emerging evidence indicates that antenatal exposure to sGC modifies cardiometabolic and endocrine phenotypes across two generations15–19, the effects on HPA activity and stress-related behaviours past the second generation (F2) are not known. Furthermore, only a few studies have investigated programming by antenatal sGC via the paternal line19,20 though there is strong evidence for paternal transmission to F2 and F3 following antenatal exposure to stressors21–24. We investigated whether antenatal exposure to sGC in the guinea pig can affect HPA activity and stress-related behaviours in juvenile offspring across multiple generations. The guinea pig was chosen as a model since they share similar patterns of brain development and placentation with the human, and the long gestation, approximately 69 days, allows for targeting specific phases of development25,26. Additionally, the primary GC in guinea pigs is cortisol (as in humans), as compared to corticosterone in mice and rats. We focused our investigation on juvenile offspring since previous studies have indicated that prenatal exposure to sGC results in particularly strong neuroendocrine and behavioural effects in children and young animals. We hypothesized that prenatal sGC exposure results in modified: 1) HPA responsiveness to stress; 2) locomotor activity, anxiety-like behaviour, and sensorimotor gating (attention); 3) altered transcription of key regulatory genes in the mPFC; and 4) altered patterns of gene expression in the PVN. These effects would manifest across three generations, be sex-specific, and depend on the route of transmission (maternal vs. paternal). We show that; 1) transgenerational inheritance in mammals via the paternal lineage does occur, and 2) the effects of a clinically relevant dose of antenatal sGC can be transmitted over multiple generations. These findings have major implications for the study of developmental health and disease.
Results
Reproduction
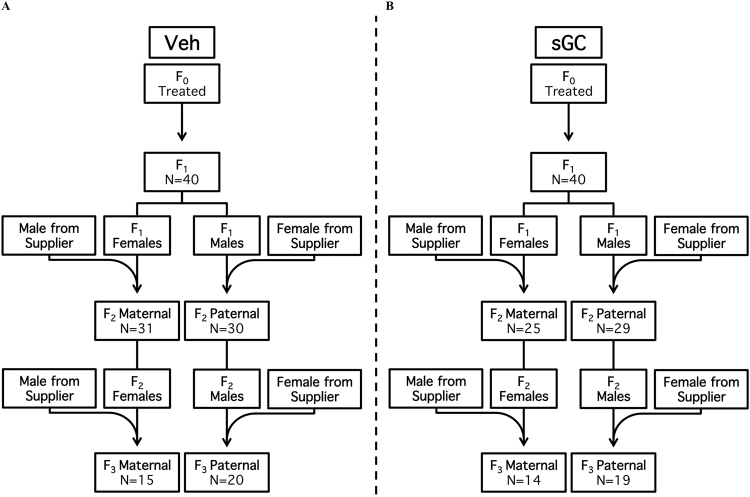
There was no significant effect of sGC treatment on breeding parameters (age at conception-females 110.0 d ± 1.44; males 137.6 d ± 4.5, number of breeding attempts, length of gestation, pregnancy weight gain, litter size and sex ratio) in the F0-F2 pregnancies (data not shown). There was a trend towards exposure to sGC (F0) increasing the length of gestation for the F2 paternal transmission (PT) pregnancy compared to Veh (Veh 68.5 d ± 0.40, sGC 69.6 d ± 0.35; t23 = 2.003; P = 0.053). The breeding paradigm is presented in Fig. 1.
Figure 1.
Breeding scheme for (A) Veh and (B) sGC groups. Maternal (Mat) and paternal (Pat) lines originate from F1 female and male breeders (respectively). N represents the total number of independent litters for each treatment, breeding line and generation.
Growth
F1 & F2
There was no significant effect of sGC treatment on body measures (body length, abdominal circumference, head length and circumference), birth weight, or weight gain in F1 or F2 offspring for either transmission route (data not shown).
F3
Exposure to sGC (F0) increased abdominal circumference compared to Veh in PT males on PND 0 and 20 (PND 0: Veh 8.06 cm ± 0.18, sGC 8.23 cm ± 0.27; PND20: Veh 10.75 cm ± 0.25, sGC 11.33 cm ± 0.22; F(1, 23) = 5.012; P < 0.05). Exposure to sGC also reduced adrenal:body-weight ratio for PT females (Veh 2.00e-4 ± 7.41e-6, sGC 1.73e-4 ± 5.85e-6; t16 = 2.804; P < 0.05). There were no other effects of sGC treatment on growth for either transmission route (data not shown).
HPA Function
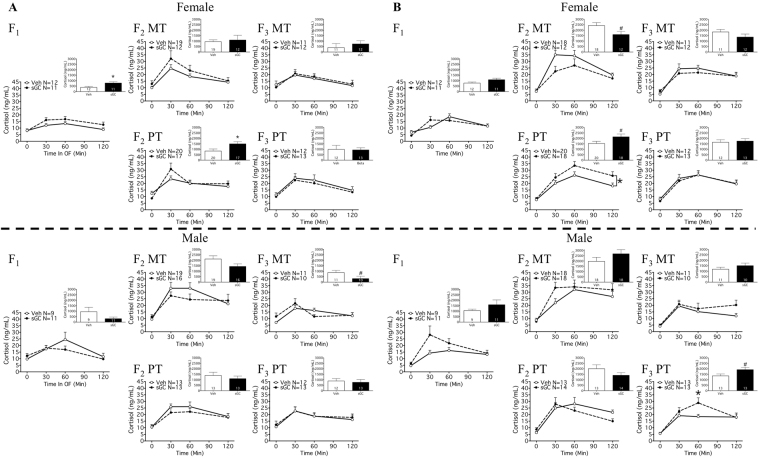
There was an effect of time as a repeated measure at all ages for all groups (Fig. 2A and B; P < 0.05).
Figure 2.
HPA response to open field stress on (A) PND 19 and (B) PND 24 for F1-F3 female and male MT (Maternal Transmission) and PT (Paternal Transmission) offspring. Veh (Vehicle) open symbols, sGC (synthetic glucocorticoid) closed symbols. Insert: salivary cortisol AUCN (net area under the curve). Data are expressed as mean ± SEM. A significant difference between Veh and sGC groups is represented as follows: *P < 0.05; #P < 0.075.
F1
Antenatal treatment with sGC resulted in an increased cortisol response to open field (OF) stress compared with Veh for females on PND 19 (Fig. 2A; AUCN; t20 = 2.596; P < 0.05). There were no effects of treatment with sGC on HPA response to stress for females on PND 24 or for males (Fig. 2A and B).
F2
Exposure to sGC (F0) resulted in an increased cortisol response for PT females on PND 19 (Fig. 2A; AUCN; t35 = 2.348; P < 0.05), and a greater total cortisol response to stress for PT females on PND 24 (Fig. 2B; AUCT: Veh 2441.0 ng/mL ± 194.3, sGC 3143.0 ng/mL ± 247.1; t36 = 2.255; P < 0.05). There was a trend towards exposure to sGC (F0) reducing the cortisol response to stress compared to Veh for maternal transmission (MT) females on PND 24 (Fig. 2B; AUCN; t28 = 2.021; P = 0.053). There were no effects of sGC exposure on HPA response to stress for F2 MT and PT males (Fig. 2A and B).
F3
There was an interaction between treatment and time on the cortisol response to stress for PT males on PND 24 (Fig. 2B; F(3, 72) = 3.383; P < 0.05), and post-hoc analysis revealed increased cortisol at 60 min compared to Veh (Veh 18.48 ng/mL ± 1.74, sGC 28.87 ng/mL ± 3.86; Tukey HSD; P < 0.05). There were trends towards exposure to sGC reducing cortisol response to stress compared to Veh for MT males on PND 19 (Fig. 2A; t19 = 1.906; P = 0.072) and increasing cortisol response to stress for PT males on PND 24 (Fig. 2B: AUCN; t24 = 2.051; P = 0.051). There were no effects of sGC exposure on HPA response to stress for MT or PT females (Fig. 2A and B).
Open Field Behaviour
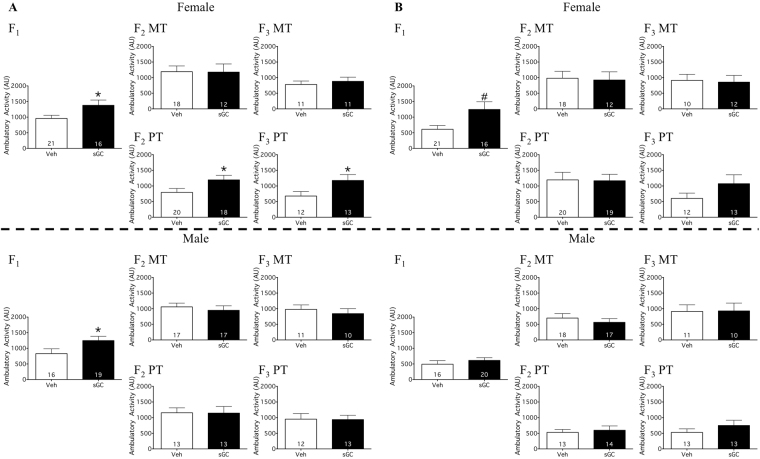
F1
Antenatal treatment with sGC resulted in increased total activity in the OF compared to Veh for females (Fig. 3A; t35 = 2.251; P < 0.05) and males on PND 19 (Fig. 3A; t33 = 2.039; P < 0.05). There was a trend towards sGC treatment increasing total activity compared to Veh for females on PND 24 (Fig. 3B; t35 = 1.958; P = 0.058). There were also trends towards treatment with sGC increasing the distance traveled compared to Veh for males on PND 19 (Veh 833.2 cm ± 158.7, sGC 1233.0 cm ± 143.2; t33 = 1.875; P = 0.070) and females on PND 24 (Veh 582.9 cm ± 117.4, sGC 1213.0 cm ± 243.9; t33 = 1.947; P = 0.058). There were no effects of sGC treatment on total locomotor activity for males on PND 24 (Fig. 3A and B), distance traveled by females on PND 19 and males on PND 24 (data not shown), or on the percent of time that F1 offspring spent at the perimeter of the OF chamber (data not shown).
Figure 3.
Total locomotor activity in the open field on (A) PND 19 and (B) PND 24 for F1-F3 female and male MT (Maternal Transmission) and PT (Paternal Transmission) offspring. Veh (Vehicle) open bars, sGC (synthetic glucocorticoid) closed bars. Data are expressed as mean ± SEM. A significant difference between Veh and sGC groups is represented as follows: *P < 0.05; #P < 0.075.
F2
For PT females, exposure to sGC (F0) resulted in increased total activity (Fig. 3A; t36 = 2.092; P < 0.05), increased distance traveled (Veh 797.3 cm ± 130.0, sGC 1196.0 cm ± 146.6; t36 = 2.042; P < 0.05), and reduced the percent time spent around the perimeter compared to Veh on PND 19 (Veh 96.9% ± 0.92, sGC 88.3% ± 1.66; U = 38; P < 0.05). There were no effects of exposure to sGC on total locomotor activity (Fig. 3A and B), distance traveled (data not shown), or the percent time spent around the perimeter (data not shown) for MT offspring, PT females on PND 24, or PT males.
F3
Exposure to sGC (F0) resulted in increased total locomotor activity compared to Veh for PT females on PND 19 (Fig. 3A; t23 = 2.065; P < 0.05). There was a trend towards sGC increasing the distance traveled compared to Veh for PT females on PND 19 (Veh 668.0 cm ± 145.2, sGC 1173.0 cm ± 196.3; t23 = 2.038; P = 0.053). Exposure to sGC also reduced the percent of time around the perimeter compared to Veh for MT males on PND 19 (Veh 91.84% ± 3.14, sGC 57.0% ± 10.21; U = 22; P < 0.05). There was a trend towards sGC reducing the percent of time around the perimeter compared to Veh for PT females on PND 24 (Veh 96.71% ± 1.51, sGC 92.55% ± 1.38; t23 = 2.036; P = 0.053). There were no effects of exposure to sGC on total locomotor activity (Fig. 3A and B) or distance traveled (data not shown) for MT offspring, PT males, or PT females on PND 24. There were also no effects of exposure to sGC on the percent of time around the perimeter (data not shown) for MT females, MT males on PND 24, PT females on PND 19, or PT males.
Acoustic Startle Response and Prepulse Inhibition
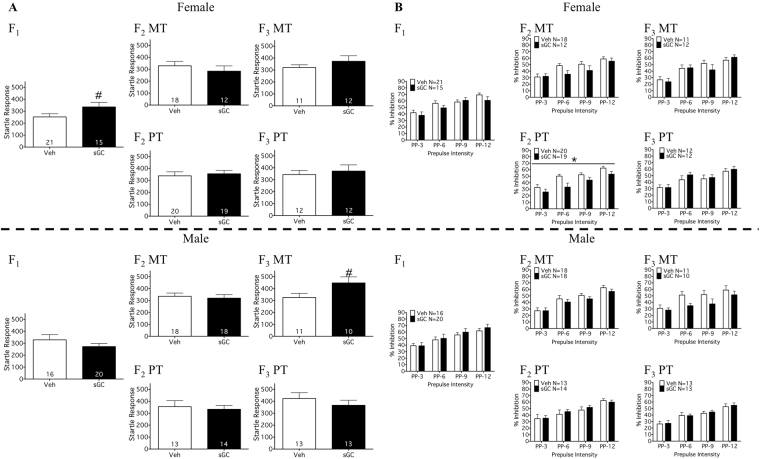
There was an effect of prepulse as a repeated measure at all prepulse intensities in all groups (Fig. 4B; P < 0.05), indicating that percent inhibition increased as a function of prepulse intensity.
Figure 4.
(A) Acoustic startle response (ASR; PND 23) and (B) percent inhibition of the startle response at increasing prepulse intensities (PPI; PND 23) for F1-F3 female and male MT (Maternal Transmission) and PT (Paternal Transmission) offspring. Veh (Vehicle) open bars, sGC (synthetic glucocorticoid) closed bars. Data are expressed as mean ± SEM. A significant difference between Veh and sGC groups is represented as follows: *P < 0.05; #P < 0.075.
F1
There was no significant effect of sGC treatment on the ASR or PPI for F1 offspring (Fig. 4A and B), but there was a trend towards increased ASR compared to Veh for females (Fig. 4A; t34 = 1.864; P = 0.071).
F3 & F3
There were no effects of exposure to sGC on the ASR in F2 or F3 (Fig. 4A), though there was a trend towards increased ASR compared to Veh for F3 MT males (Fig. 4A; t19 = 2.003; P = 0.06). Exposure to sGC resulted in reduced PPI compared to Veh for F2 PT females (Fig. 4B; F(1, 37) = 5.08; P < 0.05). There were no effects of exposure to sGC on PPI for F2 or F3 MT offspring, F2 PT males, or F3 PT offspring (Fig. 4B).
Locomotor Activity: Home Cage
There was an effect of time of day on locomotor activity in all groups (Fig. 5A; P < 0.05).
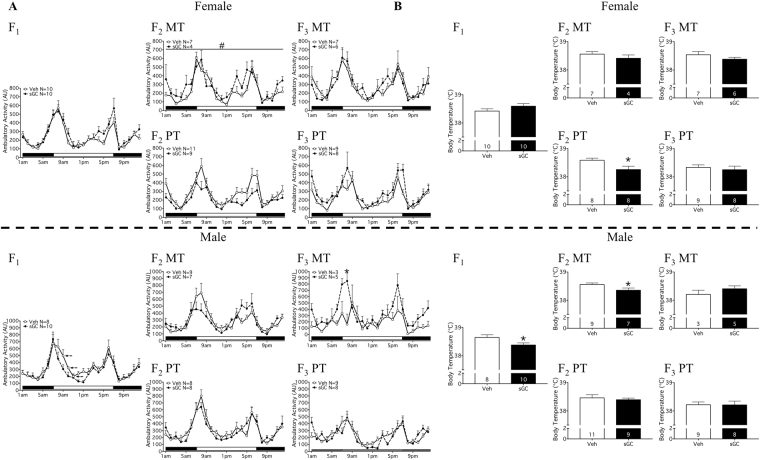
Figure 5.
(A) Locomotor activity and (B) average body temperature over 24-hour period in the animal’s home cage for F1-F3 female and male MT (Maternal Transmission) and PT (Paternal Transmission) offspring. Veh (Vehicle) open symbols/bars, sGC (synthetic glucocorticoid) closed symbols/bars. (A) X-axis: solid bar indicates lights off, open bar indicates lights on. Data are expressed as mean ± SEM. A significant difference between Veh and sGC groups is represented as follows: *P < 0.05; #P = 0.068. A significant 1-hour phase difference between Veh and Beta groups is represented as follows: 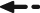 P < 0.05.
P < 0.05.
F1
In males, there was a within treatment effect of time (F(23, 368) = 1.97; P < 0.05), and post-hoc analysis revealed that treatment with sGC resulted in a 1-hour phase advance in the profile of activity compared to Veh for males from 0900 h–1100 h (Fig. 5A; Tukey HSD; P < 0.05 at each time point). There was no effect of sGC treatment on locomotor activity for females (Fig. 5A).
F2
There was no significant effect of exposure to sGC (F0) on locomotor activity for either transmission route (Fig. 5A). There was a trend towards exposure to sGC increasing activity compared to Veh for MT females (Fig. 5A; F(1, 9) = 4.304; P = 0.068).
F3
There was a significant interaction between treatment and time of day on locomotor activity for MT males (Fig. 5A; F(23, 138) = 2.136; P < 0.05), and post-hoc analysis revealed that exposure to sGC increased locomotor activity at 0800 h compared to Veh (Fig. 5A; Tukey HSD; P < 0.05). There were no other effects of exposure to sGC on locomotor activity for either transmission route (Fig. 5A).
Body Temperature
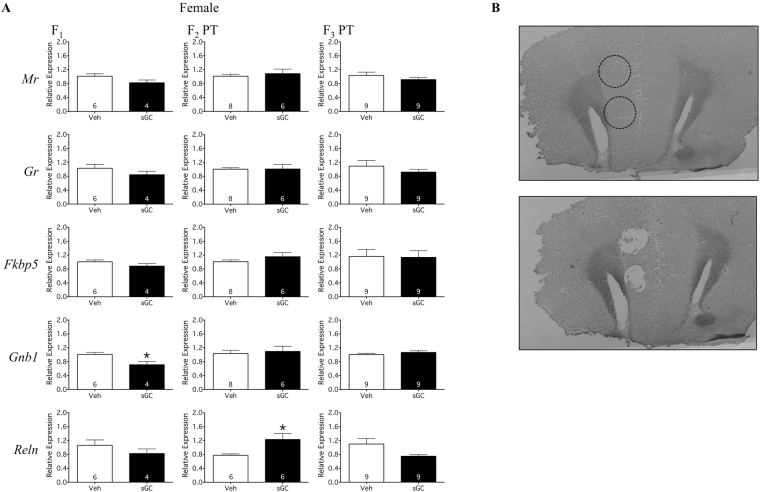
F1
sGC treatment reduced overall body temperature compared to Veh for males (Fig. 5B; t16 = 2.215; P < 0.05). There was no effect of sGC treatment on overall body temperature for females or on rhythmicity in males or females (Fig. 5B).
F2 & F3
Overall body temperature was reduced by exposure to sGC in F2 MT males (Fig. 5B; t14 = 2.173; P < 0.05) and F2 PT females compared to Veh (Fig. 5B; t18 = 2.449; P < 0.05). There were no other effects of exposure to sGC on overall body temperature or rhythmicity in F2 of in F3 (Fig. 5B).
Medial Prefrontal Cortex: Gene Expression
F1
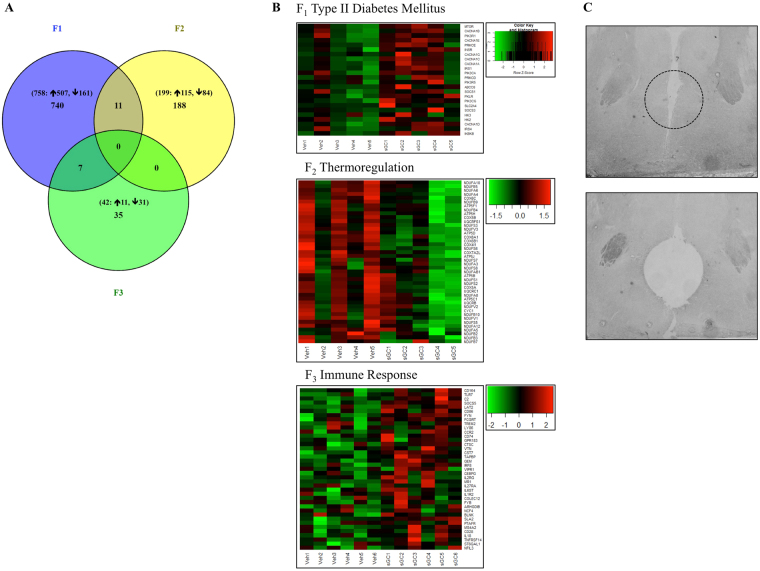
Antenatal treatment with sGC reduced the expression of Gnb1 mRNA in the F1 female mPFC compared to Veh (Fig. 6A; t8 = 2.885; P < 0.05). There was no effect of sGC treatment on the expression of Mr, Gr, Fkbp5, or Reln mRNA (Fig. 6A).
Figure 6.
(A) Gene expression in the mPFC relative to the housekeeping gene Gapdh for F1-F3 female PT (Paternal Transmission) offspring. Veh (Vehicle) open bars, sGC (synthetic glucocorticoid) closed bars. Data are expressed as mean ± SEM. A significant difference between Veh and sGC groups is represented as follows: *P < 0.05. (B) Representative sections of the mPFC before and after tissue punch. Dashed line indicates site of punch.
F2 & F3
In F2, exposure to sGC resulted in increased expression of Reln mRNA in the PT female mPFC compared to Veh (Fig. 6A; t10 = 2.839; P < 0.05). There was no effect of sGC treatment on the expression of Mr, Gr, Fkbp5, or Gnb1 mRNA in F2, or on the expression of any gene in the F3 mPFC (Fig. 6A).
Hypothalamic Paraventricular Nucleus: Gene Expression
F1
758 genes were differentially expressed following antenatal treatment with sGC in F1 females compared to Veh (Fig. 7A; FDR < 0.05); expression was increased in 597 genes and reduced in 161. Gene set enrichment analysis (GSEA) for differential gene expression between the Veh and sGC groups revealed enrichment of 160 gene sets (Supplementary Table 1; NES > 1.6, FDR < 0.25); 72 gene sets were positively enriched (i.e. increased expression with sGC treatment) and 88 were negatively enriched (i.e. decreased expression). 14 gene sets were associated with known behavioural, metabolic, and molecular effects of sGC treatment (Table 1; NES > 1.6, FDR < 0.25), including type II diabetes mellitus (Fig. 7B; NES > 1.6, FDR < 0.25). A custom HPA Activity gene set (Table 3) was not enriched (Table 1; NES = 0.92, FDR = 0.618), although the expression of Nr3c2 (encoding Mr) was significantly increased; no other classic HPA genes were differentially expressed.
Figure 7.
(A) Venn diagram illustrating the number of genes that are significantly differentially expressed in the PVN from F1-F3 female PT (Paternal Transmission) and the number of genes that overlap between generations. Numbers in brackets indicate the total number of differentially expressed genes and the number of genes with increased or decreased expression in the sGC group. (B) Heat maps of significantly enriched gene sets in the PVN for F1-F3 PT female offspring (NES > 1.6, FDR < 0.25). Each row represents one gene, each column represents one animal. Green represents lowly expressed genes and red represents highly expressed genes. (C) Representative images of the PVN before and after tissue punch. Dashed line indicates site of punch.
Table 1.
PVN Gene Sets Associated with Phenotypes.
| Phenotype | Gene Set | Type | Generation | Effect of sGC | Size | NES | P-Value | FDR |
|---|---|---|---|---|---|---|---|---|
| HPA Activity | STRESS_ASSOCIATED_GENES | Custom | F1 | Positive | 18 | 0.92 | 0.618 | 0.618 |
| F2 | Positive | 18 | 1.25 | 0.154 | 0.154 | |||
| F3 | Negative | 18 | 1.06 | 0.409 | 0.409 | |||
| One-Carbon Metabolism and Methylation | S_ADENOSYLMETHIONINE_DEPENDENT_METHYLTRANSFERASE_ACTIVITY | c5 | F1 | Negative | 18 | 1.60 | 0.019 | 0.119 |
| Circadian Rhythm | REACTOME_RORA_ACTIVATES_CIRCADIAN_EXPRESSION | c2 | F1 | Positive | 21 | 1.80 | <0.001 | 0.017 |
| REACTOME_CIRCADIAN_REPRESSION_OF_EXPRESSION_BY_REV_ERBA | c2 | Positive | 19 | 1.79 | 0.001 | 0.018 | ||
| Metabolic Dysfunction | KEGG_TYPE_II_DIABETES_MELLITUS | c2 | F1 | Positive | 36 | 1.71 | 0.001 | 0.031 |
| FEEDING_BEHAVIOR | c5 | F2 | Positive | 16 | 1.74 | 0.016 | 0.124 | |
| DIGESTION | c5 | F3 | Negative | 17 | 1.75 | 0.003 | 0.571 | |
| Thermoregulation | REACTOME_RESPIRATORY_ELECTRON_TRANSPORT_ATP_SYNTHESIS_BY_CHEMIOSMOTIC_COUPLING_AND_HEAT_PRODUCTION_BY_UNCOUPLING_PROTEINS_ | c2 | F1 | Negative | 54 | 3.11 | <0.001 | <0.001 |
| F2 | Negative | 55 | 1.86 | <0.001 | 0.021 | |||
| Collagen and Matrix | REACTOME_COLLAGEN_FORMATION | c2 | F1 | Positive | 34 | 1.87 | <0.001 | 0.006 |
| F3 | Positive | 47 | 1.62 | 0.017 | 0.174 | |||
| EXTRACELLULAR_MATRIX_STRUCTURAL_CONSTITUENT | c5 | F1 | Positive | 19 | 1.92 | <0.001 | 0.005 | |
| REACTOME_EXTRACELLULAR_MATRIX_ORGANIZATION | c2 | F1 | Positive | 44 | 1.78 | 0.001 | 0.017 | |
| Immune Function | IMMUNE_RESPONSE | c5 | F3 | Positive | 107 | 1.89 | <0.001 | 0.162 |
| CELLULAR_DEFENSE_RESPONSE | c5 | F3 | Positive | 21 | 1.87 | 0.002 | 0.116 | |
| KEGG_CYTOKINE_CYTOKINE_RECEPTOR_INTERACTION | c2 | F1 | Positive | 100 | 1.77 | <0.001 | 0.028 | |
| F2 | Negative | 103 | 1.66 | <0.001 | 0.070 | |||
| REACTOME_CELL_SURFACE_INTERACTIONS_AT_THE_VASCULAR_WALL | c2 | F2 | Negative | 54 | 1.62 | 0.005 | 0.125 | |
| F3 | Positive | 57 | 1.89 | <0.001 | 0.017 | |||
| Signaling | REACTOME_PEPTIDE_LIGAND_BINDING_RECEPTORS | c2 | F2 | Positive | 77 | 1.85 | <0.001 | 0.106 |
| F3 | Negative | 79 | 2.07 | <0.001 | 0.001 |
Gene sets that are enriched in the F1-F3 female PT PVN and associate with offspring phenotypes. Type: collections of gene sets; c5 = gene ontology, c2 = curated gene sets from published data sets. Effect of sGC: describes relationship between treatment with sGC and the expression of genes within a gene set, positive-increased expression, negative-decreased expression. Size: number of genes in the gene set. NES: normalized enrichment score used to compare gene sets of different sizes. FDR: false discovery rate, the probability that a gene set with a specific NES is a false positive.
Table 3.
Custom HPA Activity Gene Set: STRESS_ASSOCIATED_GENES.
| Gene | F1 | F2 | F3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Log2 Fold Change | P-Value | FDR | Log2 Fold Change | P-Value | FDR | Log2 Fold Change | P-Value | FDR | |
| Arntl | −0.089 | 0.326 | 0.603 | −0.027 | 0.788 | 0.999 | 0.103 | 0.056 | 0.636 |
| Avp | −1.016 | 0.026 | 0.161 | 0.541 | 0.043 | 0.508 | −0.110 | 0.673 | 0.985 |
| Bdnf | −0.082 | 0.607 | 0.812 | 0.130 | 0.431 | 0.999 | −0.215 | 0.111 | 0.768 |
| Clock | 0.405 | 0.000 | 0.003 | 0.092 | 0.344 | 0.999 | −0.023 | 0.667 | 0.984 |
| Crh | −0.991 | 0.004 | 0.057 | 0.675 | 0.003 | 0.118 | −0.075 | 0.719 | 0.991 |
| Crhr2 | 0.322 | 0.028 | 0.168 | −0.026 | 0.852 | 0.999 | −0.039 | 0.481 | 0.967 |
| Cry1 | 0.045 | 0.671 | 0.849 | 0.011 | 0.924 | 0.999 | −0.037 | 0.624 | 0.979 |
| Cry2 | −0.019 | 0.843 | 0.934 | 0.013 | 0.898 | 0.999 | −0.051 | 0.288 | 0.910 |
| Fkbp5 | 0.433 | 0.071 | 0.276 | 0.115 | 0.555 | 0.999 | 0.081 | 0.501 | 0.971 |
| Gad1 | 0.362 | 0.058 | 0.247 | −0.076 | 0.755 | 0.999 | −0.094 | 0.362 | 0.937 |
| Hsd11b2 | −0.302 | 0.287 | 0.566 | −0.287 | 0.241 | 0.982 | −0.105 | 0.631 | 0.979 |
| Jun | 0.024 | 0.810 | 0.918 | 0.172 | 0.111 | 0.773 | −0.030 | 0.605 | 0.978 |
| Nfkb1 | 0.177 | 0.178 | 0.445 | 0.040 | 0.767 | 0.999 | −0.157 | 0.084 | 0.711 |
| Nr3c1 | 0.070 | 0.518 | 0.756 | 0.059 | 0.550 | 0.999 | 0.093 | 0.024 | 0.490 |
| Nr3c2 | 0.326 | 0.001 | 0.026 | −0.088 | 0.432 | 0.999 | −0.062 | 0.318 | 0.920 |
| Per1 | 0.131 | 0.262 | 0.543 | 0.036 | 0.726 | 0.999 | −0.010 | 0.901 | 0.996 |
| Per2 | 0.293 | 0.025 | 0.156 | 0.059 | 0.622 | 0.999 | 0.013 | 0.884 | 0.996 |
| Trh | −0.823 | 0.002 | 0.041 | 0.264 | 0.163 | 0.883 | −0.139 | 0.602 | 0.978 |
Expression data for individual genes in the HPA activity gene set, STRESS_ASSOCIATED_GENES, in the F1-F3 female PT PVN. Log2 fold-change from Veh. Unadjusted P-value. FDR adjusted.
F2 & F3
Exposure (F0) to sGC resulted in the differential expression of 199 genes in F2 (115 increased, 84 reduced) and 42 genes in F3 (11 increased, 31 reduced; Fig. 7A; FDR < 0.05). 11 genes were differentially expressed in F1 and F2 (Myo1d, Galnt6, Rapgef5, Cdc42ep2, Kcna1, Anln, Galnt15, Pitx2, Cdh19, Trim66, and Col18a1), 7 in F1 and F3 (Ank1, Kiaa1024, Exph5, St8sia1, Kiaa1217, Ccdc109b, and Sptb), and none of the same genes were differentially expressed in F2 and F3 or in all three generations (Fig. 7A). GSEA revealed enrichment of 47 gene sets in F2 (4 positive, 43 negative) and 41 gene sets in F3 (30 positive, 11 negative; Supplementary Data Table 1; NES > 1.6, FDR < 0.25). 5 gene sets in F2 and 6 in F3 were associated with known behavioural, metabolic, and molecular effects of sGC treatment (Table 1; NES > 1.6, FDR < 0.25), including feeding behaviour and immune function (Fig. 7B; NES > 1.6, FDR < 0.25). The custom HPA Activity gene set (Table 3) was not enriched in either F2 or F3 (Table 1; F2 NES = 1.52, FDR = 0.154; F3 NES = 1.06, FDR = 0.409); there was no effect on any individual classic HPA genes.
Discussion
We demonstrate that prenatal treatment with sGC results in transmission of altered HPA response to stress, stress-associated behaviours, and gene expression to F3 via maternal and paternal lineages. These effects are sex- and age-dependent and diminish in advancing generations. The strongest HPA and behavioural effects of sGC treatment occur in female offspring from the paternal line, and F1 and F2 offspring are affected to a greater extent than F3 offspring. Molecular analyses suggest that the mPFC and PVN of female PT animals may be involved in the generation-specific effects of sGC treatment on behaviour. Transmission through the paternal line to the third generation of offspring strongly implicates epigenetic mechanisms in the male germ line as the mode for transgenerational transmission.
Consistent with the effects of sGC in children, we demonstrate an enhanced HPA response to stress in F1 juvenile female offspring27. Furthermore, we demonstrate maternal and paternal transmission of altered HPA activity to F2 and F3 offspring. While the HPA response to stress is reduced following maternal transmission17,18, we demonstrate paternal transmission of increased HPA response to stress to F2 females and F3 males. An interesting pattern emerges whereby exposure to sGC affects the stress response in F2 females and F3 males regardless of parental lineage. Sex- and generation-specific programming have been described following prenatal sGC17 and in other transgenerational studies28. However, such a complex pattern of effects is novel and suggests a role for sex chromosomes and/or imprinted genes. It is possible that sGC affect the expression of imprinted genes from the X-chromosome29 and that this contributes to parent-of-origin30 × offspring effects. In line with this hypothesis, it has been demonstrated that parentally imprinted genes may be involved in mediating the sex-specific effects of high fat diet (increased body size) in F3 female offspring following paternal transmission21.
Prenatal treatment with sGC programs hyperactivity in children and young animals31,32. We demonstrate hyperactivity in F1 females and males on PND 19 following antenatal exposure to sGC, and that this treatment results in increased locomotor behaviour in and open field in F2-F3 females on PND 19 following paternal transmission. While hyperactivity can be disruptive in modern society, there may be evolutionary advantages that contribute to the conservation and transmission of this phenotype (e.g. increased resource acquisition and parental attention33). This is the strongest and most consistent phenotype that we observe and it provides the first evidence for transgenerational transmission (i.e. paternal line to F3) following antenatal sGC treatment. Furthermore, these findings demonstrate that adaptive stress behaviours can be transmitted across generations.
It is important to note that variability in locomotor activity in the open field can impact the HPA response to stress of the open field. Therefore, since HPA activity was measured after the open field test, we cannot separate any effect that the animal’s activity while in the open field may have had on HPA response. While we observed increased locomotor activity in F1 and F2 PT females that coincided with an increased HPA response to stress for these animals, there was no concomitant increase in HPA activity for F1 males or F3 PT females. Furthermore, we have previously demonstrated that prenatal exposure to multiple courses of sGC result in increased locomotor activity in PND 10 female guinea pigs, but there was no effect on HPA response to stress31,34. Thus, while the relationship between stress-induced hyperactivity and increased cortisol production requires further investigation it is clear that prenatal exposure to sGC programs both the HPA and behavioural responses to stress, and that these effects manifest across multiple generations. Offspring were tested twice in the open field to determine the response to the combined stress of maternal separation and the open field (PND 19) and to the open-field alone (PND 24; post-weaning). While it is possible that habituation to repeated testing may have influenced the behavior of the animals in the open field on PND 24, any habituation would have occurred across both control and sGC treated animals, allowing for valid comparisons to be made between groups. Furthermore, the data from the PND 19 clearly demonstrate a robust phenotype of HPA and locomotor hyperactivity in response to stress in F1-F3 female offspring, PT. It is acknowledged that interactions may occur when repeated/multiple behavioural testing occurs in the same animal. However, this is an inherent limitation of this type of study; it is not ethically acceptable to produce a separate cohort for each individual test.
Treatment with sGC does not result in hyperactivity when F1 animals are in the home cage (non-stress environment). However, in F1 males, sGC treatment results in a phase advance of locomotor activity in anticipation of powerful entrainment signals (lights-on and feeding). Similar effects on circadian behaviour have been observed in rats that were exposed to prenatal stress and involve changes in the expression of clock genes35,36. While transgenerational transmission of altered circadian behaviour occurs in F2 and F3, the nature of the effect is different from earlier generations (i.e. change in amplitude of activity rather than a shift in the profile) and appears to diminish by F3.
Contrary to our hypothesis, treatment with sGC does not affect anxiety-like behaviour or attention in juvenile F1 offspring. It may be that testing in older animals37 or using alternative tests would reveal an effect of sGC in our model. Therefore, subsequent studies in older animals need to be undertaken. It is important to note that we assessed passive, automatic attentional processes (gating of irrelevant or unnecessary information), whereas other studies assessed active attention38. Exposure to sGC (F0) reduces anxiety-like behaviour in the open field for juvenile F2 females (PT) and F3 males (MT), and reduces attention in F2 females (PT). However, there is a contradictory trend towards increased anxiety-like behaviour in the acoustic startle chamber for F3 males (MT). Divergence in the response to the acoustic startle, and the open field have been reported in animals that display altered HPA activity39, and is likely due to the tests provoking different forms of anxiety-like behaviour (unavoidable and avoidable40).
Prenatal treatment with sGC reduces body temperature in F1 and F2 males (MT) and F2 females (PT). Similar effects of sGC treatment have been demonstrated in rats and involve impaired thermogenic signaling (thyroid hormone) in the PVN41. In the present study, the hypothermic effects of sGC transmit to the next generation through both parental lines. The fact that sGC reduce overall body temperature without affecting rhythmicity suggests that sGC may affect the magnitude of thermogenic signaling pathways (e.g. production of thyroid hormone) rather than circadian or pulsatile signaling.
Antenatal treatment with sGC does not appear to program an increase in HPA response to stress in F1 and F2 females by altering the expression of HPA regulatory genes in the mPFC or PVN. While this was somewhat surprising, it is important to highlight that the mPFC and PVN were isolated with the animals in a basal state, and future studies need to be undertaken to investigate HPA-related gene expression profiles in the stress-activated state. Alternatively, treatment with sGC may increase HPA responsiveness to stress by increasing the sensitivity of the PVN to stress signals. In support of this possibility, we identify a gene expression signature indicative of reduced inhibitory gamma-aminobutyric acid (GABA) signaling in the F1 PVN following treatment with sGC.
Molecular analyses in the mPFC reveal patterns of gene expression that correspond to the effects of sGC on stress-related behaviours. The increase in open field locomotor activity in F1-F3 female offspring (PT) is associated with reduced Gnb1 expression (a signal transduction gene involved in regulating locomotor activity42,43) only in the F1 mPFC. Additionally, exposure to sGC reduces PPI only in F2 female offspring following paternal transmission, and this is associated with increased Reln (a cortical development gene involved in regulating attention44) expression in the mPFC of these animals. Clearly, further work is required to better define these associations.
GSEA of RNA-seq data in the PVN reveals patterns of gene expression associated with altered homeostasis in F1-F3 females (PT). While thermogenic signaling is reduced in F1 and F2, body temperature is reduced only in F2; it is likely that the depletion of this gene set in F1 has more to do with ATP synthesis than with thermogenesis. Indeed, many of the downregulated genes in the F1 and F2 PVN are integral components in the respiratory electron transport chain, including Ndufs6 (encodes the first enzyme in the electron transport chain) and Cyc1 (encodes part of the complex that transfers electrons to cytochrome c). These data indicate that prenatal exposure to sGC may also impact oxidative phosphorylation in the brain. This is an important finding that requires further investigation since oxidative phosphorylation is integral to the processing capabilities of the brain45. We also observe increased expression of circadian gene sets, including clock genes, in F1 females. These data support the hypothesis that antenatal sGC program circadian behaviour by altering the expression of regulatory clock genes (e.g. Clock and Rora). Maintenance of an intact blood-brain barrier (BBB) at the PVN is paramount to its ability to regulate homeostasis. GSEA reveals enrichment in F1 and F3 for gene networks that regulate BBB competency (e.g. collagen formation and extracellular matrix) following antenatal sGC treatment. Together with our previous study in the fetal guinea pig brain46, these findings demonstrate that prenatal sGC promote BBB competency and provide the first evidence that effects transmit to F3.
Treatment with sGC reduces DNA methylation and increases gene expression in the fetal brain47,48. We also demonstrate increased gene expression in the juvenile F1 PVN, but the effect diminishes in F2 and F3. GSEA reveals reduced expression of a one-carbon metabolism gene set in F1, which suggests that reduced DNA methylation may be involved in the upregulation of gene expression. Interestingly, exposure to sGC (F0) does not affect DNA methylation networks in the F2 or F3 PVN, and the mechanisms underlying the transcriptional effects of sGC in F2 and F3 remain to be determined.
We have also identified molecular signatures associated with risk factors for cardiometabolic disorders2 in the PVN of young F1-F3 female offspring, though we did not assess metabolic phenotypes in the present study. It is not surprising that weight gain or growth are not increased in these female animals since overweight and altered metabolic phenotypes emerge when animals are older or challenged with a high-energy diet49,50. However, we do observe increased growth in F3 male offspring following paternal transmission. These data suggest that sGC result in transgenerational priming of the central circuitry that promotes feeding behaviour and energy storage, this effect is present early in life, and may translate to altered body composition or growth in a sex-dependent manner.
Similar to other instances of transgenerational transmission, exposure to sGC results in complex patterns of effects that emerge and disappear as a function of age, sex, generation, and parental line of transmission51. While the programming effects of sGC generally decrease over time, paternal transmission results in more effects in F2 and F3 offspring when compared to maternal transmission; this implicates different mechanisms of transmission. The most likely mechanism governing maternal transmission is through altered maternal adaptation to pregnancy. Treatment with sGC has been shown to affect maternal HPA activity during the F1 pregnancy in mice20, and such alterations to the pregnancy environment create a ‘metabolic cascade’ that program the fetus to develop along a different trajectory. This cascade creates a self-perpetuating process that alters the next generation’s adaptations to pregnancy and propagates effects across multiple generations52. Since males were not involved in rearing offspring, the most likely mechanism governing paternal transmission to F3 is through the epigenome of sperm and/or seminal fluid. Recent transgenerational studies have provided strong support for the involvement of small non-coding RNAs in paternal transmission53–55. These RNA species are present in sperm/seminal fluid, can recapitulate the programmed phenotype when injected into an embryo, and are present at approximately twice the level on the X-chromosome as compared to autosomes.
Conclusions
This study presents the first evidence that prenatal treatment with sGC results in transgenerational paternal transmission of hyperactivity and altered hypothalamic gene expression through three generations of young offspring. Female offspring appear to be more sensitive than male offspring to the programming effects of sGC, which suggests an interaction between sGC and sex hormones or sex-linked genes. Paternal transmission to F3 strongly implicates epigenetic mechanisms in the process of transmission, and small noncoding RNAs likely play a major role. The mechanisms by which sGC program similar phenotypes in F1-F3 differ between generations, but appear to involve altered transcription of genes in mPFC and PVN. Importantly, neither birth weight nor growth was reduced by sGC. Clearly prenatal treatment with sGC results in very long-term programming effects in the life of an individual, and effects reach into subsequent generations. The findings provide new perspectives on potential factors that may contribute to an individual’s vulnerability to disease development. Given the re-emergence of antenatal treatment with multiple courses of sGC, it is imperative that prospective and follow-up studies of human cohorts continue in order to determine the long-term effects of sGC in humans.
Methods
Animals and Treatments
Twelve week-old female Dunkin-Hartley guinea pigs (F0; Charles River; St Constant, QC, Canada) were singly housed within visual, olfactory and auditory contact of other animals. Food (Teklad Global Guinea Pig Diet # 2040; Envigo) and water were available ad libitum. Animals were maintained in temperature (23 °C) and humidity controlled rooms on a 12-hour light-dark cycle (lights on 0700 h, off 1900 h). F0 females were mated as previously described56. All protocols were approved by the Animal Care Committee at the University of Toronto in accordance with the Canadian Council on Animal Care.
Pregnant F0 females were injected with three courses of saline (Veh; 0.166 ml/kg; N = 40) or clinically relevant dose of sGC betamethasone (sGC; 1 mg/kg; Betaject phosphate-acetate mix - Sabex, Boucherville, QC, Canada; N = 40) on GD 40 & 41, 50 & 51, 60 & 61 as previously described17. Female and male F1-F3 offspring were randomly assigned (via dice roll) to juvenile or breeding streams. Investigator was not blinded to the group allocation. Offspring were weighed every 10 days from birth until postnatal day (PND) 40, and measures of body size were collected at birth and PND 20; food/water intake were not measured. Animals designated for breeding did not undergo any behavioural testing. Litters were weaned on PND 20 (water and Teklad Guinea Pit Diet were available ad libitum) and pair-housed in polycarbonate shoebox cages with an age-, sex-, and treatment-matched partner.
To investigate transgenerational maternal (MT) and paternal (PT) transmission, F1 and F2 female and male animals designated for breeding were mated with naïve animals (males and females, respectively, purchased from Charles River) to create MT and PT lines of F2 and F3 offspring (Fig. 1A and B). F1 and F2 pregnancies were undisturbed other than routine cage maintenance.
HPA Testing
The HPA response to stress of open field (OF) exposure (PND 19 and 24; between 0800 h and 1000 h) was measured through salivary cortisol (animals freely chewed on cotton swabs). Samples were collected at 0 (pre-test in home cage), 30, 60, and 120 min. Saliva was stored at −20 °C until measurement of free cortisol by enzyme linked immunosorbent assay (Salimetrics LLC, State College, PA, USA) as previously described57. Analyses of cortisol for a given test (i.e. OF F1) were run in the same assay. Intra- and interassay coefficients of variance were 8.19% and 11.65%, respectively.
Behavioral Testing
Open Field
Guinea pigs were tested for total locomotor activity, distance traveled, and percent of time spent in the outer zone of the open field (OF; Opto-max animal activity meter; Columbus Instruments, Columbus, OH, USA; clear Plexiglas box, 42.5 × 42.5 × 23 cm) as previously described17,58. Testing occurred for 30 minutes on PND 19 (prior to weaning) and PND 24 (after weaning) between 0800 h and 1000 h, and OF chamber was disinfected (Virox) between animals.
Acoustic Startle/Prepulse Inhibition
Offspring were tested for acoustic startle response (ASR; a measure of unavoidable anxiety-like behaviour) and prepulse inhibition of the startle response (PPI; sensory-motor gating, passive attention) in the SR-Lab Startle Response System (San Diego Instruments, San Diego, CA, USA; clear Plexiglas tube, 20 cm long × 8.89 cm diameter) as previously described59. Testing occurred for 20 min, including 5 min acclimatization, on PND 23 between 1400 h and 1600 h. Startle chamber was disinfected between sessions. Baseline ASR was determined by exposure to 4 pulses at 120 dB following acclimatization. Testing sessions consisted of the following 60 trials presented in a pseudo-randomized order as previously described59.
Activity/Body Temperature
Radiotelemeters (model TA-F40; Data Sciences International, St. Paul, MN, USA) were subcutaneously implanted on PND 33 to monitor locomotor activity and body temperature over a 24-hour period in the animal’s ‘home’ cage. Data were collected (uninterrupted) every 5 min beginning at 0900 h on PND 35, and summed into one-hour bins for analysis.
Prefrontal Cortex and PVN Gene Expression: Quantitative Real-Time PCR
Juvenile guinea pigs were weighed and euthanized in the morning (0900–1100 h) by decapitation at PND 40 (43.6 ± 0.45). The brain was removed, weighed, dissected, and frozen on dry ice.
As strongest behavioural effects of prenatal sGC were observed in the F1-F3 paternal line females, this group underwent subsequent molecular analysis. The right frontal cortex and hypothalamus from F1-F3 paternal line females were cryosectioned at −20 °C. 1.0 mm diameter punches (Harvard Apparatus Inc., Holliston, MA, USA) of the mPFC cingulate cortex area 1 and infralimbic cortex (Fig. 6B), and the anteromedial hypothalamus, containing the entire PVN, (Fig. 7B) were collected. Other hypothalamic regions adjacent to the PVN may be represented in the anteromedial punches. RNA from punches was extracted using AllPrep Universal Kit (Qiagen). Bioanalyzer (RNA 6000 Pico LabChip, Applied Biosystems); determined all RNA samples had RIN ≥ 7. cDNA was made using SensiFAST cDNA synthesis kit (Bioline, London, England). qRT-PCR was run in triplicate (SensiFAST SYBER Hi-ROX 20 μl reaction, Bioline) and quantified by a CFX96 Real-Time System (Bio-Rad). Expression of target mRNA (Table 2) relative to Gapdh housekeeping gene was assessed using the 2−ΔΔct method.
Table 2.
qRT-PCR Primer Pairs.
| Forward | Reverse | |
|---|---|---|
| Mr | AAACGTATCAAGCTCTACTTTAC | CCCATAGTGACATCCTGAG |
| Gr | TGTGAACTTTCCTGGCCGAT | GGTCCCGTTGTTGTTGAGGA |
| Fkbp5 | CTGGCCATGTGCTACCTGAA | TCTGGCGGCTTTATTCTGGG |
| Gnb1 | GCGAGCTTGACCAATTACGG | AGCCTGGAGTCTGTGAGAGAG |
| Reln | GCATTTACATCGGGCAGCAG | CTTCGGGGAGGCATTCAGTT |
| Crh | GCCGCACTGTCTCAGCTC | TTCACCTCTGCCTGCTATTTC |
| Avp | GACAACGCAGTGTCCTCTGT | AACAACCAGGCAACTTCCCA |
| Sdcbp2 | ATGCGTCGTGCAGAGATCAA | CTGCACAAAGATCCCCTGGT |
| Gabrd | CCTACAGGTCGGTGGAGGTA | CGGGCGTAGATGTCAATGGT |
| Acta2 | CGTGTGTGACAATGGTTCCG | TCAGGGTCAGGATTCCCCTT |
| Gapdh | TGTACTGGAGGTCAATGAAGG | GTCGGAGTGAACGGATTTG |
Forward and reverse primer pairs used for qRT-PCR in the mPFC and PVN.
PVN RNA-Sequencing
mRNA libraries were prepared using Illumina TruSeq V2 mRNA enrichment. Samples were sequenced (F1: Veh N = 5, sGC N = 5; F2: Veh N = 5, sGC N = 5; F3: Veh N = 6, sGC N = 6) on Illumina HiSeq. 2500 at 1 × 51 bp by the Donnelly Sequencing Centre. Reads (~45 million reads per sample) were aligned to the Cavia porcellus reference genome (>85% alignment, cavPor3.83) with Tuxedo Suit tools60 accessed through The Galaxy Project61. Subsequent analyses were performed in R (version 3.2.3). Gene read counts were determined with Genomic Alignments (version 1.6.3) as previously described62. Outliers were removed using Cook’s distance with default cut-offs63, and data were normalized by residuals with RUVSeq (version 1.4.0)64. Differential gene expression was assessed using EdgeR’s (version 3.12.1)65,66, general linear model likelihood ratio test, and FDR-corrected P < 0.05 was considered significant. Genotype permutations (1000) were computed in GSEA to determine gene set enrichment. Gene sets with FDR ≤ 0.25, P ≤ 0.01, and NES ≥ 1.6 met significance thresholds. Differential expression for 7 candidate genes assessed by qRT-PCR validated the RNA-seq data as there was a strong correlation between RNA-seq and qRT-PCR results (Supplementary Fig. 1; R2 = 0.9763; P = 2.69e−05).
Samples for F3 were sequenced in 2 batches. The first batch was composed of 6 Veh and 4 sGC. In the second batch, 2 Veh samples were re-sequenced with 2 additional sGC samples at 1 × 58 bp with sequencing depth of >65 million reads per sample and alignment of >85%. Batch effects were corrected using ComBat from R package sva (version 3.18.0)67 after which technical replicates were removed and data (Veh: N = 6, sGC: N = 6) were normalized by residuals with RUVSeq (version 1.4.0)64. Differential gene expression was assessed as described above.
Data Availability
Data is available at Gene Expression Omnibus (GEO) repository, accession number GSE85822. Computer code is available upon request.
Statistical Analyses
Analyses of all data (except RNA-Seq) were conducted using STATISTICA 12 (StatSoft, Inc., Tulsa, OK, USA). Investigator was blinded to the group allocation at the time of analysis, and un-blinded after completing analyses. All data are expressed as mean ± SEM; significance is defined as P < 0.05, trend is defined as 0.05 ≤ P < 0.075. Data from same-sex littermates were meaned to prevent litter bias. Sample sizes (N) correspond to independent litters, and not to the total number of offspring across all litters. Power analyses based on previous studies determined N ≥ 8 sufficient to account for inter-litter variability and detect effects in the tests performed. For RNA-sequencing, Scotty web tool68 determined that 4–6 samples per treatment (N = 4–6) with 40–60 million reads per sample allows for detection of 90–96% of genes with ≥1.5 fold change in expression with 80% power at P < 0.05. Following our hypotheses, separate analyses were conducted for sex (female and male), breeding line (MT and PT), and generation (F1, F2, and F3). Outliers were identified using the Rout method in Prism with the Q set to 1%69. Missing data points were imputed using the MD Imputation in STATISTICA 12 package70. Data from individual animals were excluded if no data were collected for a specific test or if >50% of data points were missing for a specific test. Cortisol area under the curve (AUC) was computed using the trapezoid rule with y = 0 as baseline for total AUC (AUCT) and y = the value at time 0 as the baseline for net AUC (negative and positive areas were summed; AUCN) as previously described17. Two-tailed student’s t-tests were used to analyze the total activity in the OF (Activity Units; AU), distance traveled during first 5 minutes in OF (cm), percent of time spent in outer zone during first 5 minutes in OF, AUCT and AUCN (ng/mL), acoustic startle response, 24-hour average body temperature (°C), organ:body-weight ratio, and fold-change in gene expression from Veh. Data sets with unequal variances between treatment groups were first log-transformed, and if the variances were still unequal a non-parametric Mann Whitney test was used. Two-way ANOVA with repeated measures and Tukey HSD post-hoc tests were used to analyze body measures (treatment × postnatal age; cm), weight gain (treatment × postnatal age; g), cortisol response to OF (treatment × time; ng/ml), PPI (treatment × prepulse intensity), and home-cage activity (treatment × time of day; AU).
Electronic supplementary material
Acknowledgements
We are grateful to Dr. B.Cox for providing advice on bioinformatic analysis. This work was supported by grants from the Canadian Institutes for Health Research (CIHR; MOP-126166). VGM received funding from the Queen Elizabeth II Graduate Scholarships in Science and Technology and the Ontario Graduate Scholarship. AC received a scholarship from Brain Canada and Kids Brain Health Network. The funding source had no involvement in the final design of the study, in the collection, analysis, and interpretation of data, in the writing of the manuscript, or in the decision to submit the article for publication.
Author Contributions
Each of the authors has contributed to the production of the manuscript, have consented to having their names on the manuscript and approve the final version of the manuscript. Individual contributions are as follows: V.G.M., A.K., and S.G.M. conceived and designed the experiments. V.G.M. and A.K. performed the experiments. V.G.M. and A.C. analyzed the data. A.K., M.S., and S.G.M. provided advice on the data analysis and the manuscript. V.G.M., A.C., and S.G.M. wrote the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Vasilis G. Moisiadis and Andrea Constantinof contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-11635-w
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fowden AL, Forhead AJ. Glucocorticoids as regulatory signals during intrauterine development. Exp Physiol. 2015;100:1477–1487. doi: 10.1113/EP085212. [DOI] [PubMed] [Google Scholar]
- 2.Moisiadis, V. G. & Matthews, S. G. Glucocorticoids and fetal programming part 1: outcomes. Nat Rev Endocrinol (2014). [DOI] [PubMed]
- 3.Petit B, Boissy A, Zanella A, Chaillou E, et al. Stress during pregnancy alters dendritic spine density and gene expression in the brain of new-born lambs. Behav Brain Res. 2015;291:155–163. doi: 10.1016/j.bbr.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 4.McKlveen JM, Myers B, Herman JP. The medial prefrontal cortex: coordinator of autonomic, neuroendocrine and behavioural responses to stress. J Neuroendocrinol. 2015;27:446–456. doi: 10.1111/jne.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Wang ZZ, Zuo W, Zhang S, et al. Effects of chronic mild stress on behavioral and neurobiological parameters - Role of glucocorticoid. Horm Behav. 2015;78:150–159. doi: 10.1016/j.yhbeh.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Roberts, D. & Dalziel, S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev CD004454 (2006). [DOI] [PubMed]
- 7.Brocklehurst P, Gates S, McKenzie-McHarg K, Alfirevic Z, Chamberlain G. Are we prescribing multiple courses of antenatal corticosteroids? A survey of practice in the UK. Br J Obstet Gynaecol. 1999;106:977–979. doi: 10.1111/j.1471-0528.1999.tb08440.x. [DOI] [PubMed] [Google Scholar]
- 8.Quinlivan JA, Evans SF, Dunlop SA, Beazley LD, Newnham JP. Use of corticosteroids by Australian obstetricians–a survey of clinical practice. Aust N Z J Obstet Gynaecol. 1998;38:1–7. doi: 10.1111/j.1479-828X.1998.tb02947.x. [DOI] [PubMed] [Google Scholar]
- 9.French NP, Hagan R, Evans SF, Mullan A, Newnham JP. Repeated antenatal corticosteroids: effects on cerebral palsy and childhood behavior. Am J Obstet Gynecol. 2004;190:588–595. doi: 10.1016/j.ajog.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Crowther CA, Doyle LW, Haslam RR, Hiller JE, et al. Outcomes at 2 years of age after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357:1179–1189. doi: 10.1056/NEJMoa071152. [DOI] [PubMed] [Google Scholar]
- 11.Murphy KE, Hannah ME, Willan AR, Hewson SA, et al. Multiple courses of antenatal corticosteroids for preterm birth (MACS): a randomised controlled trial. Lancet. 2008;372:2143–2151. doi: 10.1016/S0140-6736(08)61929-7. [DOI] [PubMed] [Google Scholar]
- 12.Asztalos E, Willan A, Murphy K, Matthews S, et al. Association between gestational age at birth, antenatal corticosteroids, and outcomes at 5 years: multiple courses of antenatal corticosteroids for preterm birth study at 5 years of age (MACS-5) BMC Pregnancy Childbirth. 2014;14:272. doi: 10.1186/1471-2393-14-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jordan, B. K., Schilling, D. & McEvoy, C. T. Pulmonary Function at Hospital Discharge in Preterm Infants Randomized to a Single Rescue Course of Antenatal Steroids. J Pediatr181, 62–66.e1 (2017). [DOI] [PMC free article] [PubMed]
- 14.Surbek D, Drack G, Irion O, Nelle M, et al. Antenatal corticosteroids for fetal lung maturation in threatened preterm delivery: indications and administration. Arch Gynecol Obstet. 2012;286:277–281. doi: 10.1007/s00404-012-2339-x. [DOI] [PubMed] [Google Scholar]
- 15.Long, N. M., Shasa, D. R., Ford, S. P. & Nathanielsz, P. W. Growth and insulin dynamics in two generations of female offspring of mothers receiving a single course of synthetic glucocorticoids. Am J Obstet Gynecol207, 203.e1–203.e8 (2012). [DOI] [PMC free article] [PubMed]
- 16.Buchwald U, Teupser D, Kuehnel F, Grohmann J, et al. Prenatal stress programs lipid metabolism enhancing cardiovascular risk in the female F1, F2, and F3 generation in the primate model common marmoset (Callithrix jacchus) J Med Primatol. 2012;41:231–240. doi: 10.1111/j.1600-0684.2012.00551.x. [DOI] [PubMed] [Google Scholar]
- 17.Iqbal M, Moisiadis VG, Kostaki A, Matthews SG. Transgenerational effects of prenatal synthetic glucocorticoids on hypothalamic-pituitary-adrenal function. Endocrinology. 2012;153:3295–3307. doi: 10.1210/en.2012-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long, N. M., Ford, S. P. & Nathanielsz, P. W. Multigenerational effects of fetal dexamethasone exposure on the hypothalamic-pituitary-adrenal axis of first- and second-generation female offspring. Am J Obstet Gynecol208, 217.e1–217.e8 (2013). [DOI] [PMC free article] [PubMed]
- 19.Drake AJ, Walker BR, Seckl JR. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R34–R38. doi: 10.1152/ajpregu.00106.2004. [DOI] [PubMed] [Google Scholar]
- 20.Vaughan, O. R., Phillips, H. M., Everden, A. J., Sferruzzi-Perri, A. N. & Fowden, A. L. Dexamethasone treatment of pregnant F0 mice leads to parent of origin-specific changes in placental function of the F2 generation. Reprod Fertil Dev (2015). [DOI] [PubMed]
- 21.Dunn GA, Bale TL. Maternal high-fat diet effects on third-generation female body size via the paternal lineage. Endocrinology. 2011;152:2228–2236. doi: 10.1210/en.2010-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dias BG, Ressler KJ. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat Neurosci. 2014;17:89–96. doi: 10.1038/nn.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber-Stadlbauer, U., Richetto, J., Labouesse, M. A., Bohacek, J. et al. Transgenerational transmission and modification of pathological traits induced by prenatal immune activation. Mol Psychiatry (2016). [DOI] [PubMed]
- 24.Bygren LO, Kaati G, Edvinsson S. Longevity determined by paternal ancestors’ nutrition during their slow growth period. Acta Biotheor. 2001;49:53–59. doi: 10.1023/A:1010241825519. [DOI] [PubMed] [Google Scholar]
- 25.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 26.Leiser R, Kaufmann P. Placental structure: in a comparative aspect. Exp Clin Endocrinol. 1994;102:122–134. doi: 10.1055/s-0029-1211275. [DOI] [PubMed] [Google Scholar]
- 27.Alexander N, Rosenlöcher F, Stalder T, Linke J, et al. Impact of antenatal synthetic glucocorticoid exposure on endocrine stress reactivity in term-born children. J Clin Endocrinol Metab. 2012;97:3538–3544. doi: 10.1210/jc.2012-1970. [DOI] [PubMed] [Google Scholar]
- 28.Dunn GA, Morgan CP, Bale TL. Sex-specificity in transgenerational epigenetic programming. Horm Behav. 2011;59:290–295. doi: 10.1016/j.yhbeh.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Naumova AK, Leppert M, Barker DF, Morgan K, Sapienza C. Parental origin-dependent, male offspring-specific transmission-ratio distortion at loci on the human X chromosome. Am J Hum Genet. 1998;62:1493–1499. doi: 10.1086/301860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skuse DH, James RS, Bishop DV, Coppin B, et al. Evidence from Turner’s syndrome of an imprinted X-linked locus affecting cognitive function. Nature. 1997;387:705–708. doi: 10.1038/42706. [DOI] [PubMed] [Google Scholar]
- 31.Owen D, Matthews SG. Repeated maternal glucocorticoid treatment affects activity and hippocampal NMDA receptor expression in juvenile guinea pigs. J Physiol. 2007;578:249–257. doi: 10.1113/jphysiol.2006.122887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hauser J, Dettling-Artho A, Pilloud S, Maier C, et al. Effects of prenatal dexamethasone treatment on postnatal physical, endocrine, and social development in the common marmoset monkey. Endocrinology. 2007;148:1813–1822. doi: 10.1210/en.2006-1306. [DOI] [PubMed] [Google Scholar]
- 33.Glover V. Annual Research Review: Prenatal stress and the origins of psychopathology: an evolutionary perspective. Journal of Child Psychology and Psychiatry. 2011;52:356–367. doi: 10.1111/j.1469-7610.2011.02371.x. [DOI] [PubMed] [Google Scholar]
- 34.Owen D, Matthews SG. Prenatal glucocorticoid exposure alters hypothalamic-pituitary-adrenal function in juvenile guinea pigs. J Neuroendocrinol. 2007;19:172–180. doi: 10.1111/j.1365-2826.2006.01517.x. [DOI] [PubMed] [Google Scholar]
- 35.Maccari S, Darnaudery M, Van Reeth O. Hormonal and behavioural abnormalities induced by stress in utero: an animal model for depression. Stress. 2001;4:169–181. doi: 10.3109/10253890109035016. [DOI] [PubMed] [Google Scholar]
- 36.Koyanagi S, Okazawa S, Kuramoto Y, Ushijima K, et al. Chronic treatment with prednisolone represses the circadian oscillation of clock gene expression in mouse peripheral tissues. Mol Endocrinol. 2006;20:573–583. doi: 10.1210/me.2005-0165. [DOI] [PubMed] [Google Scholar]
- 37.Nagano M, Ozawa H, Suzuki H. Prenatal dexamethasone exposure affects anxiety-like behaviour and neuroendocrine systems in an age-dependent manner. Neurosci Res. 2008;60:364–371. doi: 10.1016/j.neures.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez JS, Zürcher NR, Keenan KE, Bartlett TQ, et al. Prenatal betamethasone exposure has sex specific effects in reversal learning and attention in juvenile baboons. Am J Obstet Gynecol. 2011;204(545):e1–545.10. doi: 10.1016/j.ajog.2011.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rochford J, Beaulieu S, Rousse I, Glowa JR, Barden N. Behavioral reactivity to aversive stimuli in a transgenic mouse model of impaired glucocorticoid (type II) receptor function: effects of diazepam and FG-7142. Psychopharmacology (Berl) 1997;132:145–152. doi: 10.1007/s002130050330. [DOI] [PubMed] [Google Scholar]
- 40.Duvoisin RM, Villasana L, Pfankuch T, Raber J. Sex-dependent cognitive phenotype of mice lacking mGluR8. Behav Brain Res. 2010;209:21–26. doi: 10.1016/j.bbr.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carbone DL, Zuloaga DG, Lacagnina AF, McGivern RF, Handa RJ. Exposure to dexamethasone during late gestation causes female-specific decreases in core body temperature and prepro-thyrotropin-releasing hormone expression in the paraventricular nucleus of the hypothalamus in rats. Physiol Behav. 2012;108:6–12. doi: 10.1016/j.physbeh.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mychasiuk R, Ilnytskyy S, Kovalchuk O, Kolb B, Gibb R. Intensity matters: brain, behaviour and the epigenome of prenatally stressed rats. Neuroscience. 2011;180:105–110. doi: 10.1016/j.neuroscience.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 43.Mychasiuk R, Gibb R, Kolb B. Prenatal stress produces sexually dimorphic and regionally specific changes in gene expression in hippocampus and frontal cortex of developing rat offspring: Supplement. Dev Neurosci. 2011;33:531–538. doi: 10.1159/000335524. [DOI] [PubMed] [Google Scholar]
- 44.Schroeder A, Buret L, Hill RA, van den Buuse M. Gene-environment interaction of reelin and stress in cognitive behaviours in mice: Implications for schizophrenia. Behav Brain Res. 2015;287:304–314. doi: 10.1016/j.bbr.2015.03.063. [DOI] [PubMed] [Google Scholar]
- 45.Hall CN, Klein-Flügge MC, Howarth C, Attwell D. Oxidative phosphorylation, not glycolysis, powers presynaptic and postsynaptic mechanisms underlying brain information processing. J Neurosci. 2012;32:8940–8951. doi: 10.1523/JNEUROSCI.0026-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baello S, Iqbal M, Kearney S, Kuthiala S, et al. Glucocorticoids modify effects of TGF-β1 on multidrug resistance in the fetal blood-brain barrier. Growth Factors. 2016;34:33–41. doi: 10.3109/08977194.2016.1162163. [DOI] [PubMed] [Google Scholar]
- 47.Crudo A, Petropoulos S, Suderman M, Moisiadis VG, et al. Effects of antenatal synthetic glucocorticoid on glucocorticoid receptor binding, DNA methylation, and genome-wide mRNA levels in the fetal male hippocampus. Endocrinology. 2013;154:4170–4181. doi: 10.1210/en.2013-1484. [DOI] [PubMed] [Google Scholar]
- 48.Crudo A, Suderman M, Moisiadis VG, Petropoulos S, et al. Glucocorticoid programming of the fetal male hippocampal epigenome. Endocrinology. 2013;154:1168–1180. doi: 10.1210/en.2012-1980. [DOI] [PubMed] [Google Scholar]
- 49.Sheen JM, Hsieh CS, Tain YL, Li SW, et al. Programming Effects of Prenatal Glucocorticoid Exposure with a Postnatal High-Fat Diet in Diabetes Mellitus. Int J Mol Sci. 2016;17:533. doi: 10.3390/ijms17040533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Long, N. M., Smith, D. T., Ford, S. P. & Nathanielsz, P. W. Elevated glucocorticoids during ovine pregnancy increase appetite and produce glucose dysregulation and adiposity in their granddaughters in response to ad libitum feeding at 1 year of age. Am J Obstet Gynecol209, 353.e1–353.e9 (2013). [DOI] [PMC free article] [PubMed]
- 51.Bale TL. Epigenetic and transgenerational reprogramming of brain development. Nat Rev Neurosci. 2015;16:332–344. doi: 10.1038/nrn3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pembrey M, Saffery R, Bygren LO. & Network in Epigenetic Epidemiology Human transgenerational responses to early-life experience: potential impact on development, health and biomedical research. J Med Genet. 2014;51:563–572. doi: 10.1136/jmedgenet-2014-102577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci. 2013;33:9003–9012. doi: 10.1523/JNEUROSCI.0914-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodgers AB, Morgan CP, Leu NA, Bale TL. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci USA. 2015;112:13699–13704. doi: 10.1073/pnas.1508347112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gapp K, Jawaid A, Sarkies P, Bohacek J, et al. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci. 2014;17:667–669. doi: 10.1038/nn.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dean F, Matthews SG. Maternal dexamethasone treatment in late gestation alters glucocorticoid and mineralocorticoid receptor mRNA in the fetal guinea pig brain. Brain Res. 1999;846:253–259. doi: 10.1016/S0006-8993(99)02064-8. [DOI] [PubMed] [Google Scholar]
- 57.Kapoor A, Matthews SG. Prenatal stress modifies behavior and hypothalamic-pituitary-adrenal function in female guinea pig offspring: effects of timing of prenatal stress and stage of reproductive cycle. Endocrinology. 2008;149:6406–6415. doi: 10.1210/en.2008-0347. [DOI] [PubMed] [Google Scholar]
- 58.Kapoor A, Matthews SG. Short periods of prenatal stress affect growth, behaviour and hypothalamo-pituitary-adrenal axis activity in male guinea pig offspring. J Physiol. 2005;566:967–977. doi: 10.1113/jphysiol.2005.090191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Emack J, Matthews SG. Effects of chronic maternal stress on hypothalamo-pituitary-adrenal (HPA) function and behavior: no reversal by environmental enrichment. Horm Behav. 2011;60:589–598. doi: 10.1016/j.yhbeh.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 60.Trapnell C, Roberts A, Goff L, Pertea G, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Afgan E, Baker D, van den Beek M, Blankenberg D, et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2016 update. Nucleic Acids Res. 2016;44:W3–W10. doi: 10.1093/nar/gkw343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lawrence M, Huber W, Pagès H, Aboyoun P, et al. Software for computing and annotating genomic ranges. PLoS Comput Biol. 2013;9:e1003118. doi: 10.1371/journal.pcbi.1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq. 2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Risso D, Ngai J, Speed TP, Dudoit S. Normalization of RNA-seq data using factor analysis of control genes or samples. Nat Biotechnol. 2014;32:896–902. doi: 10.1038/nbt.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robinson MD, McCarthy DJ, Smyth G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou X, Lindsay H, Robinson MD. Robustly detecting differential expression in RNA sequencing data using observation weights. Nucleic Acids Res. 2014;42:e91. doi: 10.1093/nar/gku310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28:882–883. doi: 10.1093/bioinformatics/bts034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Busby MA, Stewart C, Miller CA, Grzeda KR, Marth GT. Scotty: a web tool for designing RNA-Seq experiments to measure differential gene expression. Bioinformatics. 2013;29:656–657. doi: 10.1093/bioinformatics/btt015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Motulsky HJ, Brown RE. Detecting outliers when fitting data with nonlinear regression – a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics. 2006;7:123. doi: 10.1186/1471-2105-7-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schwender H. Imputing missing genotypes with weighted k nearest neighbors. J Toxicol Environ Health A. 2012;75:438–446. doi: 10.1080/15287394.2012.674910. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available at Gene Expression Omnibus (GEO) repository, accession number GSE85822. Computer code is available upon request.