Abstract
Wheat powdery mildew (PM), caused by Blumeria graminis f. sp. tritici, is a major fungal disease of wheat worldwide. It can cause considerable yield losses when epidemics occur. Use of genetic resistance is the most effective approach to control the disease. To determine the genomic regions responsible for PM resistance in a set of U.S. winter wheat and identify DNA markers in these regions, we conducted a genome-wide association study on a set of 185 U.S. winter wheat accessions using single nucleotide polymorphism (SNP) markers from 90 K wheat SNP arrays. We identified significant SNP markers linked to nine quantitative trait loci (QTLs) and simple sequence repeats (SSR) markers linked to three QTLs for PM resistance. Most of the QTLs in the US winter wheat population have been reported previously, but some such as these on chromosomes 1A, 6A and 1B have not been reported previously, and are likely new QTLs for PM resistance in U.S. winter wheat. The germplasm with immunity to PM are good sources of resistance for PM resistance breeding and the markers closely linked to the QTLs can be used in marker-assisted selection to improve wheat PM resistance after further validation.
Introduction
Wheat (Triticum aestivum L.) is one of the staple food crops and serves nearly 35% of the world populations1. Powdery mildew (PM), caused by Blumeria graminis f. sp. tritici, is one of the most destructive wheat diseases worldwide2,3. PM occurs in wheat fields where high moisture is available4,5 and its epidemics can cause a significant grain yield reduction up to 34%6–8. As high demand for wheat yield increase to meet world-growing population, PM is becoming more severe because denser plant canopy of modern cultivars under high nitrogen fertilizer supply favors PM development. Use of resistant cultivars is the most economical environmental friendly approach to reduce the yield loss due to the disease9. However, rapid development of new races of the pathogen can quickly overcome the host resistance and result in PM outbursts10.
Wheat PM resistance is conditioned by both race specific and non-race specific resistance genes. To date, 55 resistant genes have been formally designated (Pm1-Pm55) and mapped to various chromosomes11,12. Pm1a was the first PM resistance gene identified on the long arm of chromosome 7A from a Canadian wheat cultivar Axminster13–17. Since then, other genes have been identified and tightly linked DNA markers have been reported. Some PM resistance genes, such as Pm2 on 5DS13,14, Pm3 on 1AS14,18,19, Pm18 on 7A13, were identified from bread wheat, whereas others were introgressed into wheat from wheat close relatives, including Pm4a on 2AL from T. monococcum, Pm4b on 2AL from T. carthlicum 14, Pm12 on 6BS from Aegilops speltoides 20, Pm13 on 3B and 3D from Ae. longissima 21, and Pm25 on 1A from T. monococcum 22. Some Pm genes were identified from non-progenitor species of wheat, including Pm7, Pm8, Pm17, Pm20 and PmCn17 from Secale cereal, Pm21 from Haynaldia villosa, and Pm40 and Pm43 from Thinopyrum intermedium 23–30. Most of these genes have been used in breeding for PM resistance in wheat. However, new PM pathogen races may easily overcome the resistance conferred by these major genes31. Therefore, it is very important to continuously explore new resistance genes to diversify resistance sources against rapidly evolving pathogen races.
Molecular markers have been successfully used to tag genes or quantitative trait loci (QTLs) and to estimate their effects32–35. Traditionally, bi-parental mapping populations are used to determine the locations of resistance genes or QTLs in one or two cultivars36–40. However, markers linked to a QTL identified from a specific mapping population may not be useful for marker-assisted breeding in other breeding populations41. More recently, association mapping (AM) has been used for identification and dissection of disease resistance genes or QTLs in many plant species42. Unlike bi-parental QTL mapping, AM does not require development of populations, and can quickly assemble a population by collecting a set of diversity germplasm43. Tightly linked markers to QTLs identified from AM study have high potential to be used for selection of the identified genes or QTLs in breeding.
Population structure displayed a systematic difference in allele frequencies between subpopulations44. The unequal distribution of alleles among the subpopulations may result in an increase in false association42,45. Population structure (Q) and genetic relatedness (Kingship) can be integrated together into a statistical model for association analysis to reduce such false association46,47.
In this study, we used the AM approach to study PM resistance in a set of elite breeding lines from U.S. winter wheat breeding programs. The objectives of this study were to 1) determine the genes or QTLs for PM resistance in a panel of US winter wheat germplasm, and 2) identify SNP and SSR markers associated with the QTLs or genes for marker-assisted selection.
Results
Powdery mildew resistance in the association mapping population
The PM scores of the 185 wheat accessions in the association mapping population ranged from 0 to 90%. In seedling stage, about 18% accessions were resistant when <20% severity was classified resistant; whereas in adult plant stage, 43% and 66% of accessions, respectively, were resistant in the two greenhouse experiments and 71% of the tested accessions showed resistance in the field experiment (Supplemental Table S1, Fig. 1). Thirty-three lines including 20 soft wheat lines and 13 hard wheat lines showed immune response in adult plant stage for all three experiments and they are good sources of resistance to PM. About 50 lines showed trace PM at adult stage in one of the three experiments. The results suggest that more tested accessions, especially soft winter wheat, had adult plant resistance than seedling resistance. For adult plant resistance, more resistant accessions were observed in the 2013 field and the 2011 greenhouse experiments than the 2010 greenhouse experiments. The correlation coefficients of PM scores were all significant among the four experiments and was the highest between 2010 and 2011 greenhouse experiments (r = 0.762) and the lowest between 2013 field and 2013 greenhouse (r = 0.466) experiments, suggesting that some adult plant resistance genes expressed in the field experiment might be different from these expressed in seedling stage in the greenhouse experiments.
Figure 1.
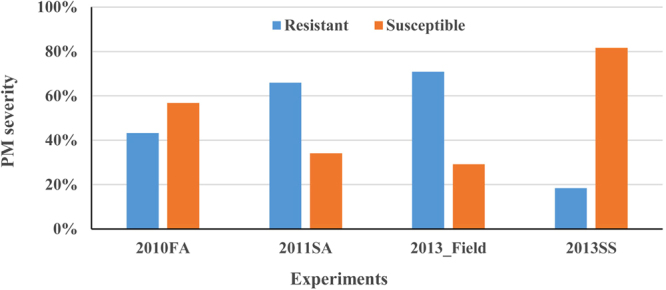
Percentage of powdery mildew resistant and susceptible accessions in the association mapping population evaluated at adult plant stage in fall 2010 (2010FA) and spring 2011 (2011SA) greenhouse experiments and field 2013 experiment (2013_Field), and at seedling stage in spring 2013 greenhouse experiment (2013SS).
Population structure
Structure analysis indicated that the population could be divided into three groups (Fig. 2). Group I (102 accessions) and Group II (23 accessions) are mainly hard winter wheat, whereas Group III (60 accessions) is mainly soft winter wheat. One quarter of accessions in the Group I are hard white winter wheat (HWWW) and others are hard red winter wheat (HRWW). Group II consists of all HRWW with most of accessions having Jagger (13 accessions) in their pedigrees. Principal coordinate analysis (PCoA) and a similarity matrix heat-map (Supplementary Fig. S1) confirmed the three groups derived from structure analysis (Fig. 3).
Figure 2.
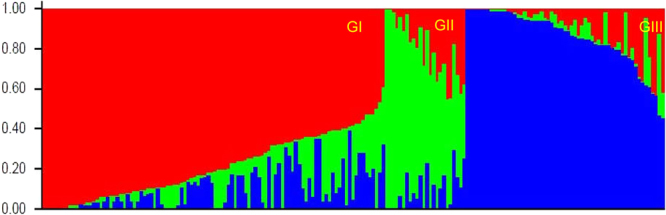
Structure analysis devided the population of 185 U.S. wheat accessions into three groups (GI, GII and GIII).
Figure 3.
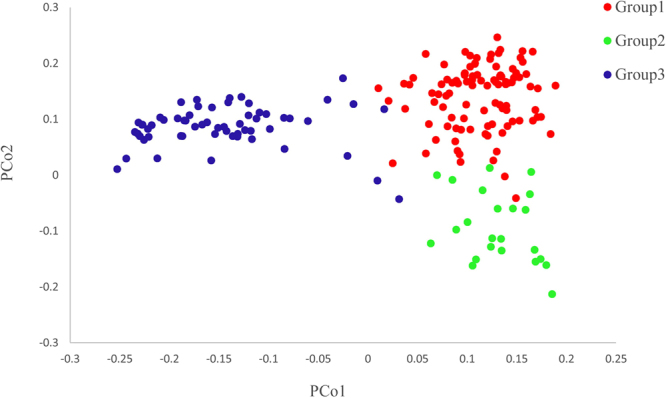
Principal coordinate analysis (PCoA) separated the population of 185 U.S. wheat accessions into three groups that correspond to the three groups derived from structure analysis.
AMOVA showed that individuals within groups accounted for 89% of the genetic variation, whereas only 11% was explained by the variation among the groups (Table 1).
Table 1.
Analysis of molecular variance on the association mapping population of 185 winter wheat accessions using SNP data.
| Sources | df | SS | MS | Est. var. | % | P value |
|---|---|---|---|---|---|---|
| Among pops | 2 | 1939.733 | 969.866 | 15.826 | 11% | 0.001 |
| Within pops | 182 | 22579.348 | 124.062 | 124.062 | 89% | |
| Total | 184 | 24519.081 | 139.889 | 100% |
Markers significantly associate with PM resistance
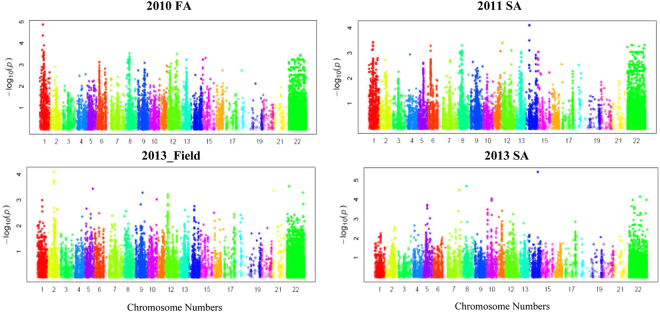
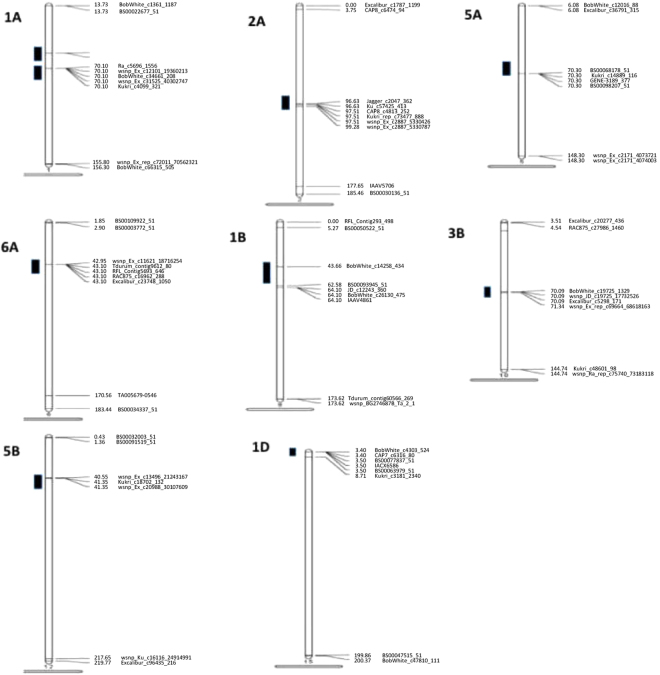
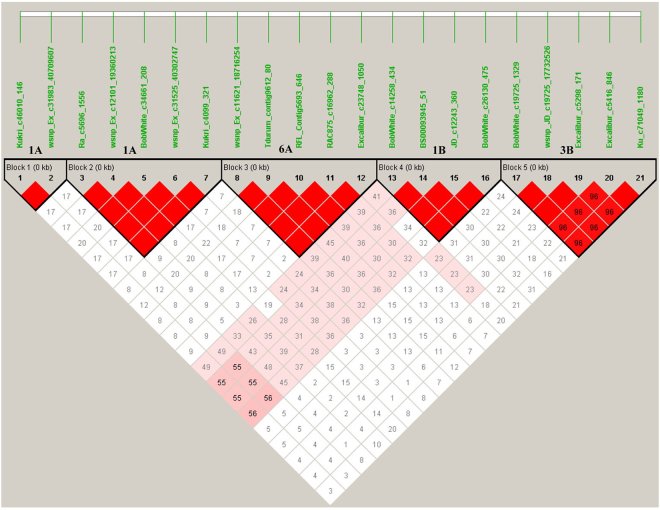
The optimal model was selected based on the observed P-values and the expected P-values for each trait (Q–Q plots). The MLM model including population structure (Q) and kingship (K) showed a better fit than GLM, and thus was selected for further association analysis (Supplementary Fig. S2). A total of 37 SNPs showed significant associations with PM resistance (Table 2, Fig. 4) and were mapped to nine regions on eight chromosomes (Fig. 5). SNPs on three chromosomes (6A, 1B and 1D) were significantly associated with PM resistance in at least two experiments. Other five chromosome regions showed a significant association with PM resistance in only one experiment. On chromosome 6A, three SNPs (wsnp_Ex_c11621_18716254, RFL_Contig5693_646, RAC875_c16962_288) were significant in the 2010 greenhouse experiment and two SNPs (Tdurum_contig9612_80, Excalibur_c23748_1050) were significant in the 2011 greenhouse experiment. The five SNPs were located within 1 cM (Table 2) and mapped in the same LD group (Fig. 6), and thus they are tightly linked markers and most likely associated with the same gene that showed a significant effect on PM resistance in the two experiments. Four SNPs on chromosome 1B were significant in the 2010 and 2011 greenhouse experiments. One of them was mapped in the chromosome position 43.66 cM and other three were mapped in about 19 cM away on the reference map (Table 2, Fig. 5)48. LD analysis indicated they were in the same LD (Fig. 6) and likely the same QTL for PM resistance. Three SNPs that were mapped within a 5 cM region on the chromosome 1D were also significantly associated with PM resistance in the 2010 and 2011 greenhouse experiments (Table 2), and they are more likely linked to the same QTL on the chromosome 1D.
Table 2.
Significant SNP markers associated with wheat powdery mildew resistance evaluated in the greenhouse experiments of fall 2010 (2010FA) and spring 2011 (2011SA) and the field experiment of 2013 (2013_Field) for adult plant resistance, spring 2013 greenhouse for seedling resistance (2013SS).
| Marker name | Chromosome | Position* (cM) | 2010FA | 2011SA | 2013_Field | 2013SS | Resistance allele | Sensitive allele | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P value | R Squared | P value | R Squared | P value | R Squared | P value | R Squared | |||||
| Kukri_c46010_146 | 1A | 56.39 | 4.31E-05 | 0.09 | — | — | — | — | — | — | C | T |
| wsnp_Ex_c31983_40709607 | 1A | 56.39 | 4.31E-05 | 0.09 | — | — | — | — | — | — | C | T |
| Ra_c5696_1556 | 1A | 70.1 | 2.85E-04 | 0.07 | — | — | — | — | — | — | T | C |
| wsnp_Ex_c12101_19360213 | 1A | 70.1 | 3.71E-04 | 0.06 | — | — | — | — | — | — | T | C |
| BobWhite_c34661_208 | 1A | 70.1 | 4.81E-04 | 0.06 | — | — | — | — | — | — | G | T |
| wsnp_Ex_c31525_40302747 | 1A | 70.1 | 5.90E-04 | 0.06 | — | — | — | — | — | — | T | C |
| Kukri_c4099_321 | 1A | 70.1 | 5.90E-04 | 0.06 | — | — | — | — | — | — | C | T |
| Jagger_c2047_362 | 2A | 96.63 | — | — | — | — | 1.76E-04 | 0.07 | — | — | G | A |
| Ku_c57425_413 | 2A | 96.63 | — | — | — | — | 1.76E-04 | 0.07 | — | — | G | A |
| CAP8_c4813_252 | 2A | 97.51 | — | — | — | — | 7.72E-05 | 0.07 | — | — | A | C |
| Kukri_rep_c73477_888 | 2A | 97.51 | — | — | — | – | 1.81E-04 | 0.06 | — | — | T | C |
| wsnp_Ex_c2887_5330426 | 2A | 97.51 | — | — | — | — | 1.81E-04 | 0.06 | — | — | T | C |
| wsnp_Ex_c2887_5330787 | 2A | 99.28 | — | — | — | — | 2.29E-04 | 0.06 | — | — | T | C |
| BS00068178_51 | 5A | 70.3 | — | — | — | — | — | — | 1.91E-04 | 0.08 | T | C |
| Kukri_c14889_116 | 5A | 70.3 | — | — | — | — | — | — | 1.91E-04 | 0.08 | T | C |
| GENE-3189_377 | 5A | 70.3 | — | — | — | — | — | — | 2.73E-04 | 0.07 | C | T |
| BS00098207_51 | 5A | 70.3 | — | — | — | — | — | — | 2.73E-04 | 0.07 | T | C |
| wsnp_Ex_c11621_18716254 | 6A | 42.95 | 7.32E-04 | 0.06 | — | — | — | — | — | — | T | C |
| Tdurum_contig9612_80 | 6A | 43.1 | — | — | 5.12E-04 | 0.05 | — | — | — | — | T | C |
| RFL_Contig5693_646 | 6A | 43.1 | 7.32E-04 | 0.06 | — | — | — | — | — | — | A | G |
| RAC875_c16962_288 | 6A | 43.1 | 7.32E-04 | 0.06 | — | — | — | — | — | — | C | T |
| Excalibur_c23748_1050 | 6A | 43.1 | — | — | 7.92E-04 | 0.05 | — | — | — | — | A | G |
| BobWhite_c14258_434 | 1B | 43.66 | 5.67E-04 | 0.06 | 9.12E-04 | 0.05 | — | — | — | — | A | C |
| BS00093945_51 | 1B | 62.58 | 5.39E-04 | 0.06 | — | — | — | — | — | — | A | G |
| JD_c12243_360 | 1B | 64.1 | 4.47E-04 | 0.06 | 8.45E-04 | 0.05 | — | — | — | — | C | T |
| BobWhite_c26130_475 | 1B | 64.1 | 5.67E-04 | 0.06 | 9.12E-04 | 0.05 | — | — | — | — | C | T |
| BobWhite_c19725_1329 | 3B | 70.09 | — | — | — | — | — | — | 8.87E-05 | 0.09 | G | A |
| wsnp_JD_c19725_17732526 | 3B | 70.09 | — | — | — | — | — | — | 9.96E-05 | 0.09 | A | G |
| Excalibur_c5298_171 | 3B | 70.09 | — | — | — | — | — | — | 1.14E-04 | 0.08 | C | T |
| Excalibur_c5416_846 | 22 | - | — | — | — | — | — | — | 6.18E-04 | 0.07 | A | T |
| Ku_c71049_1180 | 22 | - | — | — | — | — | — | — | 6.13E-04 | 0.07 | T | C |
| wsnp_Ex_c13496_21243167 | 5B | 40.55 | — | — | — | — | 7.03E-04 | 0.05 | — | — | A | G |
| Kukri_c18702_132 | 5B | 41.35 | — | — | — | — | 5.89E-04 | 0.05 | — | — | A | G |
| wsnp_Ex_c20988_30107609 | 5B | 41.35 | — | — | — | — | 8.10E-04 | 0.05 | — | — | G | A |
| BS00077837_51 | 1D | 3.5 | 5.67E-04 | 0.06 | 9.12E-04 | 0.05 | — | — | — | — | A | G |
| IACX6586 | 1D | 3.5 | 5.67E-04 | 0.06 | 9.12E-04 | 0.05 | — | — | — | — | T | C |
| Kukri_c3181_2340 | 1D | 8.71 | 5.67E-04 | 0.06 | 9.12E-04 | 0.05 | — | — | — | — | T | G |
Note: “—” denotes not significant. *The position of markers on wheat consensus SNP map.
Figure 4.
Manhattan plots for SNPs associated with powdery mildew resistance in wheat.
Figure 5.
Linkage maps to show chromosomal locations of the significant QTLs for powdery mildew resistance from association mapping using 185 winter wheat accessions.
Figure 6.
Linkage Disequilibrium (LD) of some significant SNP markers.
In five other chromosome regions that were significantly associated with PM resistance in a single experiment (Table 2), two SNPs, Kukri_c46010_146 and wsnp_Ex_c31983_40709607, which were mapped at 56.39 cM on chromosome 1A (Table 2), were significant in the 2010 greenhouse experiment. Five other significant SNPs (Ra_c5696_1556, wsnp_Ex_c12101_19360213, BobWhite_c34661_208, wsnp_Ex_c31525_40302747, and Kukri_c4099_321) were mapped at 14 cM away on the same chromosome (Table 2). The two sets of markers showed no LD and more likely associated with two different QTLs for PM resistance (Fig. 6). Six SNPs were mapped within a 3 cM region on the chromosome 2A and all significant in 2013 field experiment (R 2 = 0.073, P < 0.000077), suggesting one PM resistance QTL is more likely in the region. Three SNPs mapped within a 1 cM region on 5B were also significant in 2013 field experiment (R 2 = 0.078, P < 0.0003). Four SNPs on chromosome 5A (P < 0.0003) and five SNPs on chromosome 3B (P < 0.0007) were significant in the 2013 greenhouse experiment. The four SNPs on 5A were mapped together, suggesting that one QTL for seedling resistance may link to these SNPs (Table 2). SNPs on 3B either were mapped together or shared the same LD, suggesting they more likely link to a single gene for seedling resistance on the chromosome 3B (Table 2, Fig. 6).
Among 457 SSR markers from all 21 chromosomes screened, three were significant for PM resistance. Xscm0009 on chromosome 1A was significantly associated with PM resistance in 2010 greenhouse experiments (R 2 = 0.08, P < 0.0007, Table 3). Two other markers, Xcfd9-2 on 3D and Xcfd95 on 6D, were significant in the 2013 field experiment.
Table 3.
Significant SSR markers associated with wheat powdery mildew resistance evaluated in the greenhouse experiments of fall 2010 (2010FA) and spring 2011 (2011SA) and the field experiment of 2013 (2013_Field) for adult plant resistance, spring 2013 greenhouse for seedling resistance (2013SS).
| Test Methods | Marker name | Chromosome | 2010FA | 2011SA | 2013_Field | 2013SS | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| P value | R Squared | P value | R Squared | P value | R Squared | P value | R Squared | |||
| MLM | Xscm0009 | 1 A | 7.09E-04 | 0.08 | — | — | — | — | — | — |
| Xcfd9-2 | 3D | — | — | — | — | 5.92E-04 | 0.09 | — | — | |
| Xcfd95 | 6D | — | – | — | — | 3.78E-04 | 0.07 | — | — | |
Note: “–” denotes not significant.
The adult plant PM resistance level of each line is highly correlated with number of QTLs in the lines (r = 0.6083). These QTLs showed obvious additive effects. When a line carried four or more QTLs, it usually showed immune response to PM infection.
Discussion
Population structure may result in false associations between traits and markers if it is not properly treated during analysis49. In this study, both structure analysis and PCoA stratified the AM panel of 185 accessions into three groups and AMOVA also indicated significant population differentiation (P < 0.001), demonstrating the presence of obvious structure in the population. Wheat cultivars from NRPN were mostly clustered to Group I and wheat cultivars from RGON and SRPN were mostly clustered to Group I and Group II, whereas accessions in USSRWWN and UESRWWN showed higher PM resistance and clustered into Group III. The result indicated that PM resistance was partly influenced by the geographic distribution.
A significantly higher level of variation was detected within a population (89%) than among populations (11%) by AMOVA, indicating that a high level of genetic diversity, but not genetic divergence, was observed in US winter wheat, therefore, breeding selection has played a significant role in maintaining genetic diversity in the breeding populations.
Using the AM panel in this study, we identified SNP markers linked to nine QTLs for PM resistance and SSR markers linked to three QTLs for PM resistance on the chromosomes 1A, 2A, 5A, 6A, 1B, 3B, 5B, 1D, 3D and 6D. Among them, SNPs closely linked to the three genes on the chromosomes 6A, 1B, 1D were significant in adult plant stage in two experiments, suggesting these genes may have stable adult plant resistance to multiple races presented in both environments.
When significant QTLs identified in the current study were compared with the QTLs reported previously, we found that several QTLs have been mapped to the similar positions where PM resistance genes were reported previously. The seven SNPs that were significantly linked to PM resistance QTL in the 2010 field experiment were mapped to two locations of the chromosome 1A at 15 cM apart, and LD analysis suggested that they were linked to two different genes. Pm3 has been previously reported on the chromosome 1A50–54; Pm 17 on chromosome 1RS.1AL was mapped about 1.5 cM away from the IAG95-CA (RFLP)/CT355 (AFLP) markers55; and Pm25 on chromosome 1A was mapped at about 12.8 cM from a RAPD marker OPA0495022. Five SNP-associated QTLs on the chromosome 1A are more than 40 cM away from Pm3, therefore neither of them are Pm3. However, this QTL could be the same or a closely linked gene to QPm.caas-1A between SSR marker Xbarc148 and Xwmc55056 according to the marker sequence position in the W7984 reference map (Supplemental Table S2). The SSR marker, Xscm0009 was associated with PM resistance in the greenhouse experiments of 2010. The banding pattern of Xscm0009 showed that the marker is in 1A/1 R translocation, which suggests the linked gene is most likely Pm17. For other two SNPs on chromosome 1A, known linked genes can not be found based on available information, and they may link to a novel gene. However further research is needed to determine its identity.
Pm4a, Pm4b, Pm4c (or Pm23), Pm4d, PmDR147, PmPS5A and PmLK906 were identified on the chromosome 2A in previous reports14,57–60,61,62. Among them, Pm4a was about 1.5 cM away from the marker Xbcd29214 and QPm.inra-2A is linked to the SSR marker Xgwm275 that is also in the vicinity of Pm4a 63. In this study, the QTL on 2 A was mapped to a chromosome region near QPm.inra-2A according to the marker sequence position in the W7984 reference map (Supplemental Table S2) therefore it may be the same QTL as QPm.inra-2A.
Pm2026 was mapped to the distal portion of chromosome 5 AL and flanked by SSR markers Xcfd39 and Xgwm12664. The QTLs QPm.sfr-5A.1, QPm.ttu-5A, QPm.sfr-5A.2, QPm.sfr-5A.3 and QPm.nuls-5A were also reported on the chromosome 5A65–67. The QTLs QPm.sfr-5A.1, QPm.sfr-5A.2, QPm.sfr-5A.3 were linked to RFLP markers66. QPm.ttu-5A and QPm.nuls-5A were mapped in the marker intervals Xgwm186-Xgwm415 and Xgwm617b-Xwmc327, respectively65,67. In the current study, the 5A QTL is likely different from the QTLs QPm.ttu-5A, QPm.nuls-5A and Pm2026 based on its marker position in the W7984 reference (Supplemental Table S2). Whether the 5A QTL is the one of the previously reported genes remains to be determined due to lack of common markers among the QTLs reported in different studies.
One QTL was identified on chromosome 3B in this study. This QTL is likely PmHNK, a gene was previously mapped at 3.8 cM away from the SSR marker Xwmc29168 according to the W7984 reference. The map locations of SNPs, Excalibur_c5416_846 and Ku_c71049_1180, are not available, but a strong LD between the two SNPs and the markers mapped on the chromosome 3B located the two SNPs on the same chromosome (Fig. 6).
Pm30, Pm36, PmAS846 and MIVE29 were all mapped on chromosome 5B in previous studies. Liu et al.69 mapped Pm30 on the chromosome 5B about 6 cM from the SSR marker Xgwm159/460. Three significant SNPs (wsnp_Ex_c13496_21243167, Kukri_c18702_132, wsnp_Ex_c20988_30107609) mapped on the chromosome 5B in the current study are close to Pm30 based on the W7984 reference map (Supplemental Table S2), therefore, they are likely the same gene.
Pm10, Pm22, Pm24a and Pm24b were previously reported on chromosomes 1D37,70–72. Tightly linked markers were identified for Pm24a 37 and Pm24b 72. Other QTLs have also been reported in this chromosome including QPm.inra-1D.1 and QPm.sfr-1D 63,66. The markers flanking Pm24a were Xgwm789/Xgwm603 and Xbarc229 at 2.4 and 3.6 cM to the gene, respectively. Pm24b was flanked by Xgwm337 and Xbarc229 at the genetic distances of 3.7 and 1.0 cM, respectively. Here, we identified three significant SNPs linked to a gene on chromosome 1D (Table 2, Fig. 5), which is 2.2 cM away from SSR marker Xbarc229 based on W7984 reference map, and thus the QTL identified in this study is more likely Pm24.
Among these nine PM resistance QTLs/genes identified by SNP markers in our study, some are located in the chromosome locations where PM resistance genes have not been reported before, and thus they are likely novel genes for PM resistance (Table 2). Resistance genes Pm21, Pm31, PmY39-2 and MIRE were mapped on chromosome 6A73–76. However, the QTL on 6A identified in the current study does not link to any of the genes and is more likely a new QTL for PM resistance.
Pm8, Pm32 and Pm39 were previously reported on chromosomes 1B28,67,77. However, Pm8 and Pm32 have not been mapped to date. Pm39 67 was closely linked to SSR marker Xwmc719, but it is about 56.9 cM away from the 1B QTL identified in this study (Table 2, Fig. 5) according to the W7984 reference map. Several other QTLs have been reported in 1B including QPm.sfr-1B, QPm.ttu-1B, QPm.vt-1B, QPm.vt-1BL, QPm.vt-1B, QPm.osu-1B 65,66,78–80, but we can not determine the relationship between the newly identified QTL from this study and previous reported ones due to lack of common markers between these studies.
The SSR markers, Xcfd9-2 on chromosome 3D and Xcfd95 on chromosome 6D, also showed significant associations with PM resistance. Pm45 was mapped on chromosome 6D flanked by Xcfd80, Xmag6139 and Xmag614081. The QTL detected in this study can be mapped near Pm45 based on the W7984 reference sequence. The QTL detected on 3D is close to QPm.inra-3D that was flanked by Xcfd152 and Xgwm70763, hence, they may be the same QTL.
In this study, soft wheat carries at least two resistance QTLs that were identified by SNP markers, and showed a higher level of resistance than hard wheat (Supplemental Table 1). For adult plant, a high level correlation between number of QTLs and PM resistance was observed (r = 0.6083). The PM resistance QTLs showed obvious additive effects. The result indicated that four or more QTLs together produce immune response in adult plant. Therefore, pyramiding four or more of the QTLs using marker-assisted selection can obtain PM immune wheat cultivars.
Our study demonstrates that most of US winter wheat germplasm, especially soft winter wheat, have high levels of PM resistance and carry several QTLs for adult plant resistance to PM. Association mapping effectively identified these genomic regions associated with PM resistance and associated markers linked to these QTLs. They showed obvious additive effect on adult plant PM resistance. The accessions carrying multiple resistance QTLs could be excellent sources of PM resistance for US winter wheat breeding because they are either elite breeding lines or locally adapted cultivars. Future work will be to develop bi-parental populations of the accessions to validate the resistance loci identified in this study and develop user-friendly markers that can be used to accelerate the incorporation of these resistance QTLs into new cultivars.
Materials and Methods
Plant materials
A set of 185 elite breeding lines and cultivars were selected from the 2008 U.S Southern (SRPN) and Northern (NRPN) Hard Winter Wheat Regional Performance Nurseries, Regional Germplasm Observation Nurseries (RGON), U.S. Uniform Eastern Soft Red Winter Wheat Nursery (UESRWWN), Uniform Southern Soft Red Winter Wheat Nursery (USSRWWN), and elite breeding lines from Oklahoma State University by removal of sibling lines (Supplemental Table S1). Among these accessions, 130 are hard winter wheat and 55 are soft winter wheat.
DNA extraction and marker analysis
Leaf tissue at the two-leaf stage was collected into 1.1-ml deep-well plates and dried for 2 d in a freeze-dryer (Thermo Fisher Scientific Inc., Waltham, MA, USA) for DNA isolation. The plates containing dried tissue and a 3.2-mm stainless steel bead in each well were shaken at 25 times per sec for 5 min in a Mixer Mill (Retsch GmbH, Germany). Genomic DNA was extracted using the cetyl trimethyl ammonium bromide method and SSR markers were analyzed using an M13-tailed primer as described by Li et al.82. All PCR products were separated on an ABI PRISM 3730 DNA Analyzer (Applied Biosystems, Foster City, CA, USA), and marker data were scored using GeneMarker version 1.6 (Soft Genetics LLC, State College, PA, USA) and manually checked twice for accuracy.
Wheat accessions were genotyped using the wheat 90 K SNP assay developed by Illumina Inc. (San Diego, CA, USA). The assay was designed under the protocols of the International Wheat SNP Consortium83 and conducted at the USDA Small Grains Genotyping Laboratory in Fargo, ND. SNP genotypes were called using GenomeStudio v2011.1 software (Illumina Inc.). SNPs with minor allele frequency less than 5% or with missing data more than 15% were removed. A total of 21,600 SNPs were scored and used for association analysis.
Disease evaluation
PM was evaluated in both greenhouse and field experiments at Kansas State University, Manhattan, KS from 2010 to 2013. In the greenhouse experiments, five plants were transplanted into 12.5 × 12.5 cm Tara pots (Hummert International, Topeka, KS) after seven weeks of vernalization at 6 °C. The greenhouse temperatures were set at 22 ± 5 °C during a day with supplemental light of 12 h, and at 17 ± 2 °C during a night. The cultivar, Wesley, was used as the susceptible control in all trials. Field experiments were carried out in Rocky Ford Wheat Disease Nursery in Manhattan, KS. Thirty seeds per accession were sown in a 1.3-m row using a randomized complete block design with two replicates. Naturally occurred B. graminis f. sp. tritici was used as inoculum for both field and greenhouse experiments.
PM severity was visually estimated as overall percentage of infected leaf area two weeks after anthesis when disease reached maximum levels following Chen and Xu10. In each experiment, two replications were evaluated for each accession and a mean value of two replications was used for association analysis. Resistance classification followed Liu et al. 31, with some modification. In brief, plants without PM symptom (0) or low PM coverage (≤20%) were rated as resistant, whereas plants with >20% PM coverage were rated as susceptible.
Association analysis
The population structure (Q matrix) was analyzed using the program STRUCTURE version 2.284, with a burn-in length of 10,000 and a total of 10,000 Markov chain Monte Carlo iterations for each k. Ten independent runs were carried out for each k value. The maximum likelihood of each k value, the variance among 10 runs, and the pedigree information of each line were weighted to determine the optimal number of groups. The relative kinship (K) matrix was calculated using SPAGeDi85,86. Components of genetic variances among and within groups were estimated by analysis of molecular variance (AMOVA) with 1000 permutations using GenAlEx 6.50187.
Association analysis was carried out using both generalized linear model (GLM) and mixed linear model (MLM). GLM includes Q matrix for fixed effects, whereas MLM includes both a Q matrix for fixed effects and a kinship matrix for random effects. The observed P values and expected P values for each trait (Q-Q plot) were used for model comparison to select the best model88–90. Association analysis between SSR and PM severity was conducted using TASSEL 2.147,91–93. Associations between SNP markers and PM severity were determined using the Genome Association and Prediction Integrated Tool (GAPIT)94, an R package for genome wide association study (GWAS) and genome prediction (http://www.r-project.org). A threshold of p < 0.001 was set up to claim significant associations between SSR or SNP markers and the traits. The genetic positions (cM) of SNP markers on chromosomes were determined based on the 2015 wheat consensus map48. The marker-trait associations were cross-referenced against all reported QTLs in the literature and the GrainGenes database (https://wheat.pw.usda.gov/GG3/)95. Sequences that harbored significant SNPs were further blasted against the W7984 reference sequences to estimate their putative chromosome positions.
To estimate linkage between significant SNPs, Haploview 4.2 (http://www.broad.mit.edu/mpg/haploview/) was used to calculate the linkage disequilibrium (LD) among all significant markers96. Markers in close vicinity with strong LDs were considered to represent the same gene.
Electronic supplementary material
Acknowledgements
This is contribution number 17–J from the Kansas Agricultural Experiment Station. This study was funded by the National Research Initiative Competitive Grants 2011-68002-30029 and 2017-67007-25939, 2017-67007-25929 from the USDA National Institute of Food and Agriculture. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. USDA is an equal opportunity provider and employer.
Author Contributions
G.B. conceived and designed this study, N.L. and M.L. performed the experiments, N.L., G.B., M.L., X.X. and W.Z. analyzed the data, N.L. and G.B. wrote the paper. All authors revised the manuscript and approved the final version of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-11230-z
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Paux E, et al. A physical map of the 1-gigabase bread wheat chromosome 3B. Science. 2008;322:101–104. doi: 10.1126/science.1161847. [DOI] [PubMed] [Google Scholar]
- 2.Mandal MDN, Fu Y, Zhang S, Ji W. Proteomic Analysis of the Defense Response of Wheat to the Powdery Mildew Fungus, Blumeria graminis f. sp. tritici. Protein J. 2015;33:513–524. doi: 10.1007/s10930-014-9583-9. [DOI] [PubMed] [Google Scholar]
- 3.Mwale VM, Tang X, Chilembwe E. Molecular detection of disease resistance genes to powdery mildew (Blumeria graminis f. sp. tritici) in wheat (Triticum aestivum) cultivars. Afr. J. Biotechnol. 2017;16:22–31. doi: 10.5897/AJB2016.15720. [DOI] [Google Scholar]
- 4.Bennett F. Resistance to powdery mildew in wheat: a review of its use in agriculture and breeding programmes. Plant Pathol. 1984;33:279–300. doi: 10.1111/j.1365-3059.1984.tb01324.x. [DOI] [Google Scholar]
- 5.Shao HB, Liang ZS, Shao MA. Changes of anti-oxidative enzymes and MDA content under soil water deficits among 10 wheat (Triticum aestivum L.) genotypes at maturation stage. Colloid. Surf. B. 2005;45:7–13. doi: 10.1016/j.colsurfb.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Griffey CA, Das MK, Stromberg EL. Effectiveness of adult-plant resistance in reducing grain yield loss to powdery mildew in winter wheat. Plant Dis. 1993;77:618–622. doi: 10.1094/PD-77-0618. [DOI] [Google Scholar]
- 7.Johnson JW, Baenziger PS, Yamazaki WT, Smith RT. Effects of Powdery Mildew on Yield and Quality of Isogenic Lines of ‘Chancellor’ Wheat. Crop Sci. 1979;19:349–352. doi: 10.2135/cropsci1979.0011183X001900030018x. [DOI] [Google Scholar]
- 8.Leath S, Bowen KL. Effects of powdery mildew, triadimenol seed treatment, and triadimefon foliar sprays on yield of winter wheat in North Carolina. Phytopathology. 1989;79:152–155. doi: 10.1094/Phyto-79-152. [DOI] [Google Scholar]
- 9.Uloth MB, You MP, Barbetti MJ. Cultivar resistance offers the first opportunity for effective management of the emerging powdery mildew (Erysiphe cruciferarum) threat to oilseed brassicas in Australia. Crop Pasture Sci. 2016;67:1179–1187. doi: 10.1071/CP16182. [DOI] [Google Scholar]
- 10.Chen L. F. & Xu J. Y. Agricultural Phytopathology. China Agriculture Press, China, 141–147 (2001).
- 11.McIntosh R. A. et al. Catalogue of Gene Symbols for Wheat. In: KOMUGI-integrated wheat science database at http://www.shigen.nig.ac.jp/wheat/komugi/genes/download.jsp: Accessed 4 April 2014 (2013).
- 12.Xu H, et al. Molecular tagging of a new broad-spectrum powdery mildew resistance allele Pm2c in Chinese wheat landrace Niaomai. Theor. Appl. Genet. 2015;128:2077–2084. doi: 10.1007/s00122-015-2568-z. [DOI] [PubMed] [Google Scholar]
- 13.Hartl L, Weiss H, Stephan U, Zeller FJ, Jahoor A. Molecular identification of powdery mildew resistance genes in common wheat (Triticum aestivum L.) Theor. Appl. Genet. 1995;90:601–606. doi: 10.1007/BF00222121. [DOI] [PubMed] [Google Scholar]
- 14.Ma ZQ, Sorrells ME, Tanksley SD. RFLP markers linked to powdery mildew resistance genes Pm1, Pm2, Pm3, and Pm4 in wheat. Genome. 1994;37:871–875. doi: 10.1139/g94-123. [DOI] [PubMed] [Google Scholar]
- 15.Neu C, Stein N, Keller B. Genetic mapping of the Lr20-Pm1 resistance locus reveals suppressed recombination on chromosome arm 7AL in hexaploid wheat. Genome. 2002;45:737–744. doi: 10.1139/g02-040. [DOI] [PubMed] [Google Scholar]
- 16.Sears ER, Briggle LW. Mapping the Gene Pml for Resistance to Erysiphe graminis f. sp. tritici on Chromosome 7A of Wheat. Crop Sci. 1969;9:96–97. doi: 10.2135/cropsci1969.0011183X000900010033x. [DOI] [Google Scholar]
- 17.Zhang Q, Liu Y, Tao L, Gao J, Wang H. Identification of RAPD markers linked to powdery mildew resistance gene Pm23 in wheat. Acta Bot. Boreali-occident. Sin. 2003;23:1882–1888. [Google Scholar]
- 18.Hartl L, Weiss H, Zeller FJ, Jahoor A. Use of RFLP markers for the identification of alleles of the Pm3 locus conferring powdery mildew resistance in wheat (Triticum aestivum L.) Theor. Appl. Genet. 1993;86:959–963. doi: 10.1007/BF00211048. [DOI] [PubMed] [Google Scholar]
- 19.Sourdille P, et al. Location of Pm3, a powdery mildew resistance allele in wheat, by using a monosomic analysis and by identifying associated molecular markers. Euphytica. 1999;110:193–198. doi: 10.1023/A:1003713219799. [DOI] [Google Scholar]
- 20.Jia J, et al. RFLP-based maps of the homoeologous group-6 chromosomes of wheat and their application in the tagging of Pm12, a powdery mildew resistance gene transferred from Aegilops speltoides to wheat. Theor. Appl. Genet. 1996;92:559–565. doi: 10.1007/BF00224558. [DOI] [PubMed] [Google Scholar]
- 21.Donini P, Koebner RMD, Ceoloni C. Cytogenetic and molecular mapping of the wheat-Aegilops longissima chromatin breakpoints in powdery mildew-resistant introgression lines. Theor. Appl. Genet. 1995;91:738–743. doi: 10.1007/BF00220952. [DOI] [PubMed] [Google Scholar]
- 22.Shi AN, Leath S, Murphy JP. A major gene for powdery mildew resistance transferred to common wheat from wild einkorn wheat. Phytopathology. 1998;88:144–147. doi: 10.1094/PHYTO.1998.88.2.144. [DOI] [PubMed] [Google Scholar]
- 23.Chen PD, Qi LL, Zhou B, Zhang SZ, Liu DJ. Development and molecular cytogenetic analysis of wheat-Haynaldia villosa 6VS/6AL translocation lines specifying resistance to powdery mildew. Theor. Appl. Genet. 1995;91:1125–1128. doi: 10.1007/BF00223930. [DOI] [PubMed] [Google Scholar]
- 24.Friebe B, Heun M, Tuleen N, Zeller FJ, Gill BS. Cytogenetically Monitored Transfer of Powdery Mildew Resistance from Rye into Wheat. Crop Sci. 1994;34:621–625. doi: 10.2135/cropsci1994.0011183X003400030003x. [DOI] [Google Scholar]
- 25.Friebe B, Jiang J, Raupp WJ, Mcintosh RA, Gill BS. Characterization of wheat-alien translocations conferring resistance to diseases and pests: current status. Euphytica. 1996;91:59–87. doi: 10.1007/BF00035277. [DOI] [Google Scholar]
- 26.He R, et al. Inheritance and mapping of powdery mildew resistance gene Pm43 introgressed from Thinopyrumintermedium into wheat. Theor. Appl. Genet. 2009;118:1173–1180. doi: 10.1007/s00122-009-0971-z. [DOI] [PubMed] [Google Scholar]
- 27.Heun M, Friebe B, Bushuk W. Chromosomal location of the powdery mildew resistance gene of Amigo wheat. Phytopathology. 1990;80:1129–1133. doi: 10.1094/Phyto-80-1129. [DOI] [Google Scholar]
- 28.Hsam SLK, Zeller FJ. Evidence of allelism between genes Pm8 and Pm17 and chromosomal location of powdery mildew and leaf rust resistance genes in the common wheat cultivar’Amigo’. Plant Breed. 1997;116:119–122. doi: 10.1111/j.1439-0523.1997.tb02164.x. [DOI] [Google Scholar]
- 29.Luo PG, et al. Characterization and chromosomal location of Pm40 in common wheat: a new gene for resistance to powdery mildew derived from Elytrigia intermedium. Theor. Appl. Genet. 2009;118:1059–1064. doi: 10.1007/s00122-009-0962-0. [DOI] [PubMed] [Google Scholar]
- 30.Ma P, et al. Molecular mapping of a new powdery mildew resistance gene Pm2b in Chinese breeding line KM2939. Theor. Appl. Genet. 2015;128:613–622. doi: 10.1007/s00122-015-2457-5. [DOI] [PubMed] [Google Scholar]
- 31.Liu N, et al. Virulence Structure of Blumeria graminis f. sp. tritici and Its Genetic Diversity by ISSR and SRAP Profiling Analyses. PloS One. 2015;10:e0130881. doi: 10.1371/journal.pone.0130881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hao Y, et al. Molecular characterization of a new powdery mildew resistance gene Pm54 in soft red winter wheat. Theor. Appl. Genet. 2015;128:465–476. doi: 10.1007/s00122-014-2445-1. [DOI] [PubMed] [Google Scholar]
- 33.Kollers S, et al. Whole genome association mapping of Fusarium head blight resistance in European winter wheat (Triticum aestivum L.) PloS One. 2013;8:e57500. doi: 10.1371/journal.pone.0057500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohler V, Bauer C, Schweizer G, Kempf H, Hartl L. Pm50: a new powdery mildew resistance gene in common wheat derived from cultivated emmer. J. Appl. Genet. 2013;54:259–263. doi: 10.1007/s13353-013-0158-9. [DOI] [PubMed] [Google Scholar]
- 35.Petersen S, et al. Mapping of powdery mildew resistance gene Pm53 introgressed from Aegilops speltoides into soft red winter wheat. Theor. Appl. Genet. 2015;128:303–312. doi: 10.1007/s00122-014-2430-8. [DOI] [PubMed] [Google Scholar]
- 36.Hua W, et al. Identification and genetic mapping of pm42, a new recessive wheat powdery mildew resistance gene derived from wild emmer (Triticum turgidum var. dicoccoides) Theor. Appl. Genet. 2009;119:223–230. doi: 10.1007/s00122-009-1031-4. [DOI] [PubMed] [Google Scholar]
- 37.Huang X, Hsam SLK, Zeller FJ, Wenzel G, Mohler V. Molecular mapping of the wheat powdery mildew resistance gene Pm24 and marker validation for molecular breeding. Theor. Appl. Genet. 2000;101:407–414. doi: 10.1007/s001220051497. [DOI] [Google Scholar]
- 38.Letta T, et al. Searching for novel sources of field resistance to Ug99 and Ethiopian stem rust races in durum wheat via association mapping. Theor. Appl. Genet. 2013;126:1237–1256. doi: 10.1007/s00122-013-2050-8. [DOI] [PubMed] [Google Scholar]
- 39.Mater Y, et al. Linkage mapping of powdery mildew and greenbug resistance genes on recombinant 1RS from’Amigo’and’Kavkaz’wheat-rye translocations of chromosome 1RS.1AL. Genome. 2004;47:292–298. doi: 10.1139/g03-101. [DOI] [PubMed] [Google Scholar]
- 40.Miranda LM, Murphy JP, Marshall D, Leath S. Pm34: a new powdery mildew resistance gene transferred from Aegilops tauschii Coss.to common wheat (Triticum aestivum L.) Theor. App. Genet. 2006;113:1497–1504. doi: 10.1007/s00122-006-0397-9. [DOI] [PubMed] [Google Scholar]
- 41.Goudemand E, et al. Association mapping and meta-analysis: two complementary approaches for the detection of reliable Septoria tritici blotch quantitative resistance in bread wheat (Triticum aestivum L.) Mol. Breed. 2013;32:563–584. doi: 10.1007/s11032-013-9890-4. [DOI] [Google Scholar]
- 42.Joukhadar R, El-Bouhssini M, Jighly A, Ogbonnaya FC. Genome-wide association mapping for five major pest resistances in wheat. Mol. Breed. 2013;32:943–960. doi: 10.1007/s11032-013-9924-y. [DOI] [Google Scholar]
- 43.Buckler ES, Thornsberry JM. Plant molecular diversity and applications to genomics. Cur. Opin. in Plant Biol. 2002;5:107–111. doi: 10.1016/S1369-5266(02)00238-8. [DOI] [PubMed] [Google Scholar]
- 44.Yu J, Buckler ES. Genetic association mapping and genome organization of maize. Cur. Opin. in Biotech. 2006;17:155–160. doi: 10.1016/j.copbio.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 45.Knowler WC, Williams RC, Pettitt DJ, Steinberg AG. Gm3; 5; 13; 14 and type 2 diabetes mellitus: an association in American Indians with genetic admixture. Am. J. Hum. Genet. 1988;43:520–526. [PMC free article] [PubMed] [Google Scholar]
- 46.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu J, et al. A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat. Genet. 2006;38:203–208. doi: 10.1038/ng1702. [DOI] [PubMed] [Google Scholar]
- 48.Wang S, et al. Characterization of polyploid wheat genomic diversity using a high-density 90000 single nucleotide polymorphism array. Plant Biotech. J. 2014;12:787–796. doi: 10.1111/pbi.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao H. H., Dekkers J. C. M. & Fernando R. L. Power and precision of regression-based linkage disequilibrium mapping of QTL. Proceedings of the 8th World Congress on Genetics Applied to Livestock Production, Belo Horizonte, Minas Gerais, Brazil, 13–18 August, 2006. CD-ROM Publication, 20–21 (2006).
- 50.Briggle LW. Three Loci in Wheat Involving Resistance to Erysiphe graminis f. sp. tritici. Crop Sci. 1966;6:461–465. doi: 10.2135/cropsci1966.0011183X000600050021x. [DOI] [Google Scholar]
- 51.Briggle LW, Sears ER. Linkage of Resistance to Erysiphe graminis f sp. tritici (Pm3) and Hairy Glume (Hg) on Chromosome 1A of Wheat. Crop Sci. 1966;6:559–562. doi: 10.2135/cropsci1966.0011183X000600060017x. [DOI] [Google Scholar]
- 52.Yahiaoui N, Srichumpa P, Dudler R, Keller B. Genome analysis at different ploidy levels allows cloning of the powdery mildew resistance gene Pm3b from hexaploid wheat. Plant J. 2004;37:528–538. doi: 10.1046/j.1365-313X.2003.01977.x. [DOI] [PubMed] [Google Scholar]
- 53.Zeller F. J. & Hasm S. L. K. Progress in breeding for resistance to powdery mildew in common wheat (Triticum aestivum L.). In: Slinkard AE (ed) Proceedings of the 9th International Wheat Genetics Symposium. University of Saskatchewan, Saskatoon, Canada, 178–180 (1998).
- 54.Zeller FJ, Lutz J, Stephan U. Chromosome location of genes for resistance to powdery mildew in common wheat (Triticum aestivum L.) 1. Mlk and other alleles at the Pm3 locus. Euphytica. 1993;68:223–229. doi: 10.1007/BF00029876. [DOI] [Google Scholar]
- 55.Hsam SLK, Mohler V, Hartl L, Wenzel G, Zeller FJ. Mapping of powdery mildew and leaf rust resistance genes on the wheat-rye translocated chromosome T1BL·1RS using molecular and biochemical markers. Plant Breed. 2000;119:87–89. doi: 10.1046/j.1439-0523.2000.00444.x. [DOI] [Google Scholar]
- 56.Lan C, et al. Quantitative trait loci mapping for adult-plant resistance to powdery mildew in Chinese wheat cultivar Bainong 64. Phytopathology. 2009;99:1121–1126. doi: 10.1094/PHYTO-99-10-1121. [DOI] [PubMed] [Google Scholar]
- 57.Hao Y, et al. Pm23: a new allele of Pm4 located on chromosome 2AL in wheat. Theor. Appl. Genet. 2008;117:1205–1212. doi: 10.1007/s00122-008-0827-y. [DOI] [PubMed] [Google Scholar]
- 58.Schmolke M, Mohler V, Hartl L, Zeller FJ, Hsam SLK. A new powdery mildew resistance allele at the Pm4 wheat locus transferred from einkorn (Triticum monococcum) Mol. Breed. 2012;29:449–456. doi: 10.1007/s11032-011-9561-2. [DOI] [Google Scholar]
- 59.Yi YJ, et al. Development of molecular markers linked to the wheat powdery mildew resistance gene Pm4b and marker validation for molecular breeding. Plant Breed. 2008;127:116–120. doi: 10.1111/j.1439-0523.2007.01443.x. [DOI] [Google Scholar]
- 60.Zhu ZD, Kong XY, Zhou RH, Jia JZ. Identification and Microsatellite Markers of a Resistance Gene to Powdery Mildew in Common Wheat Introgressed from Triticum durum. Acta Bot. Sin. 2004;46:867–872. [Google Scholar]
- 61.Ronghua Zhou et al. Development of wheat near-isogenic lines for powdery mildew resistance. Theoretical and Applied Genetics110(4), 640–648 (2005). [DOI] [PubMed]
- 62.Nia, J. S., et al. Shen, Chromosome location and microsatellite markers linked to a powdery mildew resistance gene in wheat line Lankao 90(6). Plant Breeding127(4), 346–349 (2008).
- 63.Bougot Y, et al. A major QTL effect controlling resistance to powdery mildew in winter wheat at the adult plant stage. Plant Breed. 2006;125:550–556. doi: 10.1111/j.1439-0523.2006.01308.x. [DOI] [Google Scholar]
- 64.Xu H, et al. Identification and mapping of pm2026: a recessive powdery mildew resistance gene in an einkorn (Triticum monococcum L.) accession. Theor. Appl. Genet. 2008;117:471–477. doi: 10.1007/s00122-008-0791-6. [DOI] [PubMed] [Google Scholar]
- 65.Jakobson I, Peusha H, Timofejeva L, Järve K. Adult plant and seedling resistance to powdery mildew in a Triticum aestivum × Triticum militinae hybrid line. Theor. Appl. Genet. 2006;112:760–769. doi: 10.1007/s00122-005-0181-2. [DOI] [PubMed] [Google Scholar]
- 66.Keller M, et al. Quantitative trait loci for resistance against powdery mildew in a segregating wheat × spelt population. Theor. Appl. Genet. 1999;98:903–912. doi: 10.1007/s001220051149. [DOI] [Google Scholar]
- 67.Lillemo M, et al. The adult plant rust resistance loci Lr34/Yr18 and Lr46/Yr29 are important determinants of partial resistance to powdery mildew in bread wheat line Saar. Theor. Appl. Genet. 2008;116:1155–1166. doi: 10.1007/s00122-008-0743-1. [DOI] [PubMed] [Google Scholar]
- 68.Xu WG, et al. Molecular mapping of powdery mildew resistance gene PmHNK in winter wheat (Triticum aestivum L.) cultivar Zhoumai 22. Mol. Breed. 2010;26:31–38. doi: 10.1007/s11032-009-9374-8. [DOI] [Google Scholar]
- 69.Liu Z, Sun Q, Ni Z, Nevo E, Yang T. Molecular characterization of a novel powdery mildew resistance gene Pm30 in wheat originating from wild emmer. Euphytica. 2002;123:21–29. doi: 10.1023/A:1014471113511. [DOI] [Google Scholar]
- 70.Peusha H, Hsam SLK, Zeller FJ. Chromosomal location of powdery mildew resistance genes in common wheat (Triticum aestivum L. em. Thell.) 3. Gene Pm22 in cultivar Virest. Euphytica. 1996;91:149–152. [Google Scholar]
- 71.Tosa Y, Tsujimoto H, Ogura H. A gene involved in the resistance of wheat to wheatgrass powdery mildew fungus. Genome. 1987;29:850–852. doi: 10.1139/g87-145. [DOI] [Google Scholar]
- 72.Xue F, et al. Molecular mapping of a powdery mildew resistance gene in common wheat landrace Baihulu and its allelism with Pm24. Theor. Appl. Genet. 2012;125:1425–1432. doi: 10.1007/s00122-012-1923-6. [DOI] [PubMed] [Google Scholar]
- 73.Liu SL, Wang CY, Wang QY, Ji WQ. SSR Analysis of Powdery Mildew Resistance Gene in a New Germ-plasm N9628-2 of Triticum aestivum L. Acta Agron. Sin. 2008;34:84–88. [Google Scholar]
- 74.Qi L, Cao M, Chen P, Li W, Liu D. Identification, mapping, and application of polymorphic DNA associated with resistance gene Pm21 of wheat. Genome. 1996;39:191–197. doi: 10.1139/g96-025. [DOI] [PubMed] [Google Scholar]
- 75.Xie CJ, Sun QX, Ni ZT, Nevo E, Fahima T. Chromosomal location of a Triticum dicoccoides-derived powdery mildew resistance gene in common wheat by using microsatellite markers. Theor. Appl. Genet. 2003;106:341–345. doi: 10.1007/s00122-002-1022-1. [DOI] [PubMed] [Google Scholar]
- 76.Chantret N, et al. Location and mapping of the powdery mildew resistance gene MlRE and detection of a resistance QTL by bulked segregant analysis (BSA) with microsatellites in wheat. Theor. Appl. Genet. 2000;100:1217–1224. doi: 10.1007/s001220051427. [DOI] [Google Scholar]
- 77.Hsam SLK, Lapochkina IF, Zeller FJ. Chromosomal location of genes for resistance to powdery mildew in common wheat (Triticum aestivum L. em Thell.). 8. Gene Pm32 in a wheat-Aegilops speltoides translocation line. Euphytica. 2003;133:367–370. doi: 10.1023/A:1025738513638. [DOI] [Google Scholar]
- 78.Chen Y, Hunger RM, Carver BF, Zhang H, Yan L. Genetic characterization of powdery mildew resistance in US hard winter wheat. Mol. Breed. 2009;24:141–152. doi: 10.1007/s11032-009-9279-6. [DOI] [Google Scholar]
- 79.Liu S, Griffey CA, Maroof MAS. Identification of molecular markers associated with adult plant resistance to powdery mildew in common wheat cultivar Massey. Crop Sci. 2001;41:1268–1275. doi: 10.2135/cropsci2001.4141268x. [DOI] [Google Scholar]
- 80.Tucker DM, et al. Confirmation of three quantitative trait loci conferring adult plant resistance to powdery mildew in two winter wheat populations. Euphytica. 2007;155:1–13. doi: 10.1007/s10681-006-9295-0. [DOI] [Google Scholar]
- 81.Ma H., et al. Identification and mapping of a new powdery mildew resistance gene on chromosome 6D of common wheat. Theor. Appl. Genet.123, 1099 (2011). [DOI] [PubMed]
- 82.Li C, Chen M, Chao S, Yu J, Bai G. Identification of a novel gene, H34, in wheat using recombinant inbred lines and single nucleotide polymorphism markers. Theor. Appl. Genet. 2013;126:2065–2071. doi: 10.1007/s00122-013-2118-5. [DOI] [PubMed] [Google Scholar]
- 83.C. R. Cavanagh et al. Genome-wide comparative diversity uncovers multiple targets of selection for improvement in hexaploid wheat landraces and cultivars. Proceedings of the National Academy of Sciences110(20), 8057–8062 (2013). [DOI] [PMC free article] [PubMed]
- 84.Pritchard J. K., Wen X. & Falush D. Structure, version 2.2. Software documentation Department of Human Genetics, University of Chicago, Illinois, USA (2007).
- 85.Hardy OJ, Vekemans X. SPAGeDi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol. Ecol. Notes. 2002;2:618–620. doi: 10.1046/j.1471-8286.2002.00305.x. [DOI] [Google Scholar]
- 86.Ritland K. A marker-based method for inferences about quantitative inheritance in natural populations. Evolution. 1996;50:1062–1073. doi: 10.1111/j.1558-5646.1996.tb02347.x. [DOI] [PubMed] [Google Scholar]
- 87.Peakall R, Smouse PE. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics. 2012;28:2537–2539. doi: 10.1093/bioinformatics/bts460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Edae EA, Byrne PF, Haley SD, Lopes MS, Reynolds MP. Genome-wide association mapping of yield and yield components of spring wheat under contrasting moisture regimes. Theor. Appl. Genet. 2014;127:791–807. doi: 10.1007/s00122-013-2257-8. [DOI] [PubMed] [Google Scholar]
- 89.Sukumaran S, et al. Association Mapping for Grain Quality in a Diverse Sorghum Collection. Plant. Genome. 2012;5:126–135. [Google Scholar]
- 90.Valluru R., Reynolds M. P., Davies W. J. & Sukumaran S. Phenotypic and genome-wide association analysis of spike ethylene in diverse wheat genotypes under heat stress. New Phytologist: doi: 10.1111/nph.14367 (2016). [DOI] [PubMed]
- 91.Henderson CR. Best linear unbiased estimation and prediction under a selection model. Biometrics. 1975;31:423–447. doi: 10.2307/2529430. [DOI] [PubMed] [Google Scholar]
- 92.Bradbury PJ, et al. TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics. 2007;23:2633–2635. doi: 10.1093/bioinformatics/btm308. [DOI] [PubMed] [Google Scholar]
- 93.Pritchard JK, Stephens M, Rosenberg NA, Donnelly P. Association mapping in structured populations. Am. J. Hum. Genet. 2000;67:170–181. doi: 10.1086/302959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lipka AE, et al. GAPIT: genome association and prediction integrated tool. Bioinformatics. 2012;28:2397–2399. doi: 10.1093/bioinformatics/bts444. [DOI] [PubMed] [Google Scholar]
- 95.Anonymous. GrainGene. http://wheat.pw.usda.gov/cgi-bin/GG3/browse.cgi?class=marker (2010).
- 96.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.