Abstract
One of the largest therapeutic problem during the continuous treatment of the patients with Hemophilia A and B, are viral infections as Hepatitis B and C, and HIV, and the other infective diseases, which can be transmitted by the transfusion of blood products.
The aim of this study is to analyze the complications of the hemophiliacs in Kosovo which have been treated with fresh frozen plasma, cryoprecipitate and concentrated products of FVIII and FIX. We have tested 75 patients with hemophilia A or B and there were used enzyme immunoassay test-Elisa method for the following: anti-HCV HBsAg, HIV and TPHA.
The serological data showed that HCV infection was positive in 29 cases or 38,7%, whereas infection with HBV and HIV were present in a smaller percentage of the patients (2,7% HBV and 1,4% for HIV). HCV infection was present only in 9,5% of the cases of the age group under 18 years. Infected hemophiliacs with one or two infective agents were found in 34,7%, respectively 4%. Infection with T. pallidum was present at none of the examined patients with hemophilia. HCV infection was higher in severe forms of hemophilia B (44,4%), compared with severe form of hemophilia A (30%).
Based on our results, despite the infrequent application of FVIII and FIX concentrates, and other anti hemophilic preparations used in treating hemophilia patients, the number of infected hemophiliacs with blood-transmittable infectious agents was substantially high, especially with hepatitis C virus.
Keywords: hepatitis C Virus (HCV), hepatitis virus B (HBV), Human immune deficiency Virus (HIV), Hemophilia A and Hemophilia B
INTRODUCTION
Transfusion-transmitted infections (TTI) are serious complications at Hemophilia patients treated by factor VIII and IX concentrates (1,2). Multitransfused hemophiliacs with antihemophilic products are endangered of acquiring viral hepatitis (3). These infections occurred more often before 1985 (4). From these infections we must mention viral hepatitis A (HAV) (5), viral hepatitis B (HBV) (6), viral hepatitis C (HCV) (7), viral hepatitis G (HGV) (8) etc. The cause of manifestation of these infections is on the fact that concentrating coagulation factors are prepared of plasma from thousand of blood donors that didn’t undergo viral inactivation (9,10,11). Although the majority of infected patients do not suffer acute symptoms and clear the infection spontaneously, the remaining portion (<50%) become chronic carriers of virus (12).
The latter may develop into chronic active hepatitis and finally progress to liver cirrhosis; especially in HBV and HCV infections (13,14,15). About 80% of adult hemophiliacs develop antibodies against surface antigen of hepatitis B virus (HBsAg), while 10% among this group become chronic carriers (6). HBsAg positive hemophiliacs are very often accompanied with delta hepatitis infection, which causes severe active hepatitis, cirrhosis and hepatocellular carcinoma that appears six times more often in haemophiliacs than in other population (16,17,18). In 77% of aged patients with Hemophilia A and 42% with Hemophilia B (19) were found HIV antibodies as well as with middle aged patients, but with lower incidence that is in correlation with the used amount of FVIII concentrates. Some data suggesting that 80% of patients treated with Factor VIII concentrates have HIV antibodies (20), compared to 14% of patients treated with cryoprecipitate (12). After 1985, the risk of acquiring HIV infection through antihemophilic products is increasingly rare because of the new used methods of factor VIII concentrates preparation and laboratory tests for blood donors’ examination (21,22). At many HIV positive hemophiliacs AIDS is developed. According to literature data, during period 1976-1991 HIV infection was the main death cause of hemophiliacs, compared to bleeding with only 5% (23,24). FVIII concentrates available on the market are considered safe in the sense of not transmitting infectious agents, especially to recombinant factor concentrates (25,26,27). Due to the fact that haemophilia patients received multiple transfusions of FFP, cryoprecipitate, the factor VIII and IX concentrates prepared from pool plasma of thousands of donations, there exists a risk of acquiring TTI. So, this paper is focused on the above mentioned complications of the patients with hemophilia associated with transfusion of blood components or derivatives.
MATERIAL AND METHODS
Study Subjects
Our cohort comprises 75 patients with Hemophilia A and B, with average age 24,7 years, with SD 15,3 (age range 3 to 61 years); 48 (64%) with haemophilia A and average age 23,5 years, and 27 (36%) with hemophilia B and average age 23,5 years. From the total number of individuals studied, only 30 (40%) are below age 18 years.
Anamnesis
The study group have included all hemophiliacs in Kosovo, diagnosed at NBTCK. They are all males due to X-linked recessive inheritance pattern for hemophilia A and B. Anamnesis data suggest that most of patients were treated occasionally during bleeding episodes (or prophylactically prior to dental extraction or surgery.
Analysis in detail
All the patients with hemophilia A and B were tested for known blood-transmittable disease agents. The tests were performed on blood samples drawn with special sealed tubes (VACUETTE) containing Sodium citricum (3, 8 %) at 1:9 ratios. After centrifugation, plasma has been separated and stored at -30°C until the tests were performed. The tests were carried using ELISA immu-noassay method using the sophisticated automated platform “TECAN” (Mini Swift). The test and companies were as follows: HIV1/2: Ag/Ab Combination (Abbot-MurexHIV), this reagent detected antigens of virus tip 1 (HIV-1, HIV-1 group O) and also anti-HIV-2. HBV Surface antigen: HBsAg (ver.3, Ab- bot-Murex) with this reagent was detected superficial antigen of hepatitis B virus. HCV: anti-HCV (ver.4 Abbot-Murex) this reagent detected the antibodies against hepatitis C virus. T.pallidum: ICE*Syphilis (Abbot-Murex), very sensitive reagents which detected antibodies against T. pallidum.
Diagnosis
Diagnosis was determinated on the following infectious agents: HIV, HBV, HCV and T. pallidum. Most of the patients showed no clinical picture of blood-transmittable disease.
Therapy
Generally, hemophiliacs were treated with fresh frozen plasma (FFP) and cryoprecipitate, and only occasionally with DDAVP, FVIII and FIX concentrates. None of them had received recombinant products, like rFVIIa. Patients with clinical picture of infection, after being diagnosed were referred to the clinic of infectious diseases for further treatment.
Statistical analysis
Study results were stored in Instat 2 statistical software. Arithmetic average, standard deviation, minimal and maximal values, and P value (student t-test) of ages in both groups of Haemophilia A and B were used for statistical data pro- cesing. All the results are presented in data tables.
RESULTS
In Table 1. are presented data for the TTI in patients with hemophilia A and B, which shows that infection with HCV is found in a large group of patients, in 29 patients or in 38, 7% of cases. But, infection with hepatitis virus B (HBV) and HIV virus (HIV) is found only in small number of cases, in 2,7% (HBV) respectively 1,4% (HIV).
TABLE 1.
Viral infectious complications at patients with Hemophilia A and B
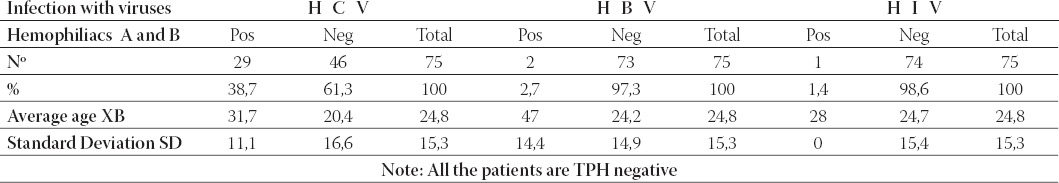
There were no patients with T. pallidum infection. In Table 2, the major percentage of the infected patients (45%) with HCV is in the mild form of hemophilia A. In the moderate and severe form of hemophilia A infection with HCV was lower (30% respectively 25%). From the total number of patients with hemophilia A (48), 41,7% are infected with hepatitis C virus. At the mild form of hemophilia A it is found a case infected with hepatitis B virus and another case with HIV virus.
TABLE 2.
Viral infectious complications in patients with different forms of hemophilia A
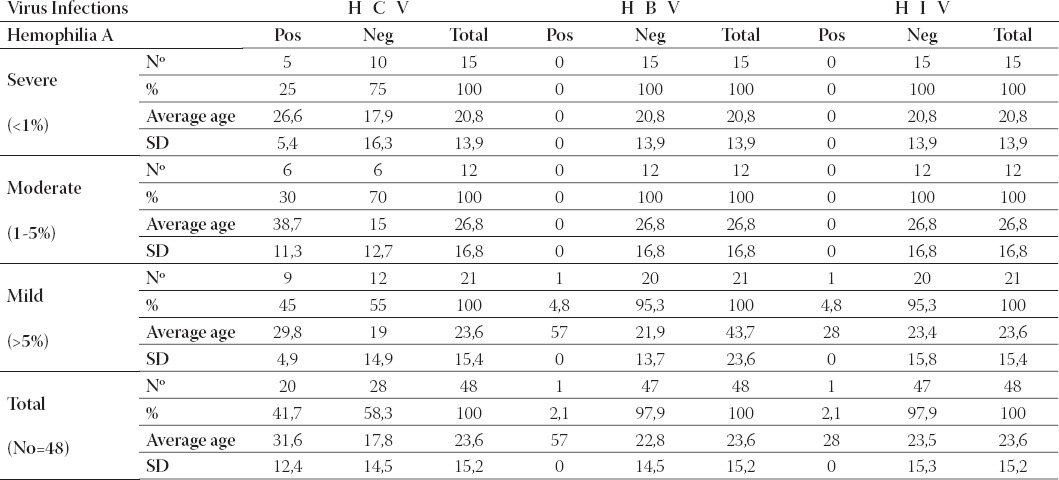
From the total number of the patients (Table 3.) with hemophilia B (27), 33,3% were infected with HCV. From the infected patients, the majority of them (44,4%) have the severe form of hemophilia B, 22,2 % moderate form and 33,4% the mild form. One patient with moderate form of hemophilia B was diagnosed with HBV infection, but patients with hemophilia B were not infected with HIV virus.
TABLE 3.
Viral infectious complications in patients with different forms of hemophilia B
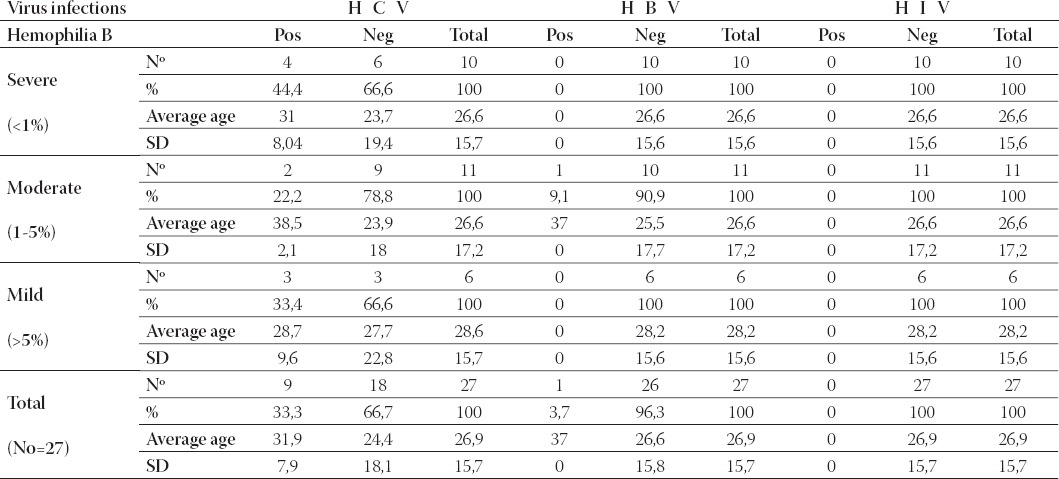
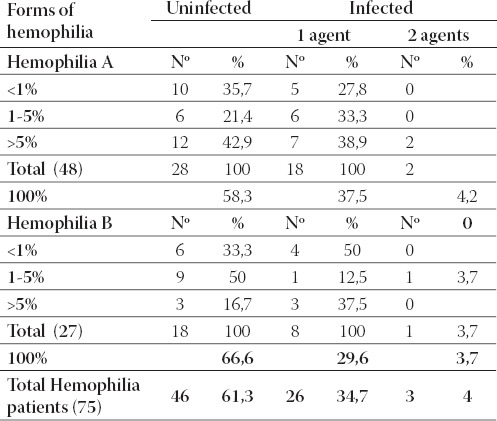
Patients with hemophilia A, 60,4% of them were not infected, while infected with one or two infective agents were found 35,4%, respectively 4,2% (Table 4.). An approximate number of infected patients (66,6%) was found and in cases of hemophilia B, while infected with one or two infective agents were 29,6%, respectively 3,7%.
TABLE 4.
Presentation of uninfected and infected hemophiliacs with one or two infective agents.
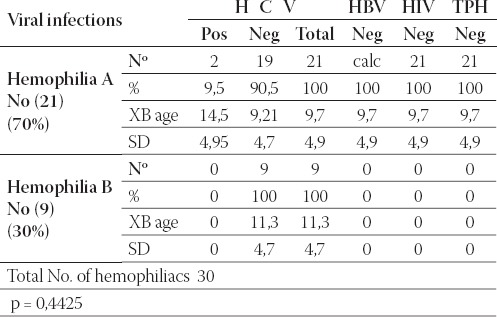
In Table 5. it can be noticed that only 2 (9,5%) hemophilia A patients within this subgroup were infected with HCV. Other blood-transmittable infectious agents were not found within this subgroup. Age was not significantly different between hemophilia A (9,7 years) and B(11,3 years) (P=0,4425).
TABLE 5.
Viral infectious complications at boys with hemophilia < or = 18 years of age.
DISCUSSION
After the application of factor VIII concentrates there was a significant reduction in the morbidity and mortality from bleeding in hemophiliacs. However, the use of replacement therapy wasn’t without significant complications especially the development of antibodies against factor VIII and TTI (19). The use of clotting factor concentrates manufactured from a large pool of plasma that didn’t undergo viral attenuation increased the risk of TTI (14) such as HBV, HCV, and HIV with subsequent rise in morbidity and mortality rate up to five fold (8). While before 1960 bleeding was the main lethal complication in haemophilia patients, during the 1980s the leading cause of mortality were infectious complications with HBV, HCV and HIV (28). The aim of this study was to investigate the infectious complications at hemophilia patients in Kosovo, who have been enrolled for a long-term treatment with anti hemophilic preparations such as FFP, cryoprecipi- tate, and occasionally FVIII and FIX concentrates in small doses. The administration of these preparations was mostly carried during spontaneous or traumatic episodes of bleeding, or on patients undergoing surgery. Our study results revealed that out of 75 patients with Hemophilia A or B, 38,7% were seropositive for HCV, while conversely, seropositivity for HBV and HIV was significantly lower; 2,7% and 1,4%, respectively. Other publications (18, 29, 30, 31, 32, 33, 34, 35, 36, 37) have given higher HIV infection percentage (1,7~44%). Higher prevalence of HIV infection was noted especially in hemophilia patients treated before 1985. HIV sero-positivity prevalence in such patients was up to 80% (4). The results for hepatitis C were similar to results published by Goedert et al., whose studies conducted in a dozen of Hemophilia centres during the period 2001-2003 found HCV seroprevalence in 30% of hemophiliacs (38). Other studies suggest higher prevalence ranging 43,9%~84% (33, 35, 39, 40, 41, 42). The prevalence at patients treated before 1985 when blood screening for HCV first began is the highest, ranging from 92% to 100% (13,43). The prevalence of hemophilia patients infected with HBV (2,7%) is at the lower level of the range published by other authors (0,3%-8,86%) (18,30). None of the subjects in this study resulted seropositive for T. pallidum, while the other authors reported an incidence of 4,9% (20).
Hepatitis C prevalence is closely associated with the frequency of exogenous FVIII and FIX concentrates administered. Therefore, an increase in the number of doses directly increases the residual risk of acquiring an infective agent (34). Such statement was confirmed by Makris et al. on a study done in England; 154 hemophilia patients were taking different amounts of FVIII and FIX concentrates; they confirmed that anti-HCV prevalence was dependent on the number of doses. For example, HCV was found to be in 76% of patients treated with above 10.000 UI of factor concentrates, while HCV infection at patients who took less than 10 000 UI was 46%. Non-virus-inactivated Clotting Factor Concentrates had the probability of containing HCV up to 80%, while preparations undergoing such procedure have the probability of 20%. The presence of viral agents in plasma antihemophilic products depends on whether donations are from volunteer or paid donors. The presence of HCV in plasma products prepared from paid donations may be up to 64% (3). Due to such infectious complications in hemophilia patients, alternative pathways through recombinant clotting factor manufacturing took place. The safety and efficacy of recombinant therapy was confirmed on a study involving 93 hemophiliacs, treated with recombinant clotting factors (26). TTI can be transmitted through cryoprecipitate despite donor screening due to the fact that cryo-precipitate didn’t undergo viral inactivation. Studies from several authors confirmed that 23,3% of hemophiliacs contracted HIV, 58,9% HCV, and with lesser extend HBV counting 5,5% (44). In reference to incidence between hemophilia A and B patients, it was found that out of 48 individuals with hemophilia A, 41,7% were seropositive on HCV. Coinfection with HIV and HBV was found on a single case of hemophilia A patients. Other authors published data suggesting higher rates of HIV infection in hemophilia A patients (69,4%) (45). Within hemophilic boys < or =18 years of age, HCV infection was present on 9,5%. None of the individuals within this subgroup was seropositive for HIV or HBV, while other authors published higher rates of TTI; HBV 3,7%: HIV 11,1% and HCV 35,2% (9) Other studies found dissimilar HBV seroprevalence (6,4%) (6), or HCV (6%) (46). Our data corresponds with Kavakli et al. (47) which found no HIV infection in boys with Hemophilia. Although our results found co-infection only in 3 individuals (4%), other author’s studies found a higher rate of HIV-HCV coinfection ranging 18,8%-19,2% (36,48). Another group of authors suggest that the rate of coinfection with HIV and HCV among hemophiliacs ranges between 50-80 % (14,41), although on specific occasions coinfection rate was up to 99% (23).
CONCLUSION
Based on our results, despite the insufficient application of FVIII and FIX concentrates, and other antihemophilic preparations used in the treatment of hemophilia patients, the number of infected hemophiliacs infected with blood-transmit- table infectious agents is substantially high, especially with hepatitis C virus, found in 38,7% of cases. In spite of that, HBV and HIV infection was very low (2,7% and 1,4 %, respectively), while T. pallidum infection was not found.
Improvements in transfusion-transmitted disease risk reduction through nucleic acid amplification assays (PCR), implementation of efficient viral inactivation techniques and the use of recombinant factor concentrates would significantly reduce the infectious complications hemophiliacs face today.
Acknowledgments
This study was supported by the National Blood Transfusion Centre of Kosovo in Prishtina.
REFERENCES
- 1.Goedert J.J, Eyster M.E, Lederman M.M, et al. End-stage liver disease in persons with hemophilia and transfusion-associated infections. Blood. 2002;100(5):1584, 1589. [PubMed] [Google Scholar]
- 2.Sharara A.I, Hunt M.Ch, Hamilton J.D. Hepatitis C. Ann. Intern. Med. 1996;125(8):658–668. doi: 10.7326/0003-4819-125-8-199610150-00006. [DOI] [PubMed] [Google Scholar]
- 3.Makris M, Garson J.A, Ring C.J.A, et al. Hepatitis C viral RNA in Clotting Factor Concentrates and the Development of Hepatitis in Recipients. Blood. 1993;81(7):1898–1902. [PubMed] [Google Scholar]
- 4.Yee T.T, Griffioen A, Sabin C.A, et al. The natyral history of HCV in a cohort of haemophilic patients infected between 1961 and 1985. Gut. 2000;(47):845, 851. doi: 10.1136/gut.47.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mannucci P.M, Gdovin S, Gringeri A, et al. Transmission of hepatitis A to patients with haemophilia by factor VIII Concentrates treated with organic Solvent and detergent to inactivate Viruses. Ann. Intern. Med. 1994;120(1):1–7. doi: 10.7326/0003-4819-120-1-199401010-00001. [DOI] [PubMed] [Google Scholar]
- 6.Rumi M.G, Colombo M, Romeo R, et al. Serum hepatitis B Virus DNA detects cryptic hepatits B virus infections in multitrans-fused hemophilic patients. Blood. 1990;75(8):1654–1658. [PubMed] [Google Scholar]
- 7.Brettler D.B, Alter H.J, Dienstag J.L, et al. Prevalence of hepatitis C virus antibody in a cohort haemophilia patients. Blood. 1990;76(1):254–256. [PubMed] [Google Scholar]
- 8.Giangrande P.L.F. Hepatitis in Haemophilia. Review. Br. J. Hae-matol. 1998;103:1–9. doi: 10.1046/j.1365-2141.1998.00855.x. [DOI] [PubMed] [Google Scholar]
- 9.Blanchette V.S, Vorstman E, Shore A, et al. Hepatitis C infection in children with Hemophilia A and B. Blood. 1991;78(2):285–289. [PubMed] [Google Scholar]
- 10.Franchini M, Rossetti G, Tagliaferri A, et al. The natyral history of chronic hepatitis C in a cohort of HIV-negative Italian patients with hereditary bleeding disorders. Blood. 2001;98(6):1836, 1841. doi: 10.1182/blood.v98.6.1836. [DOI] [PubMed] [Google Scholar]
- 11.Tong M.J, El-Farra N.S, Reikes A.R, et al. Clinical Outcomes after Transfusion-Associated Hepatitis. N. Engl. J. Med. 1995;332(22):1463, 1466. doi: 10.1056/NEJM199506013322202. [DOI] [PubMed] [Google Scholar]
- 12.Roberts H.R, Hoffman M. Hemophilia A and Hemophilia B. In: Beutler E, Lichtman M.A, Coller B.S, editors. Willams Hematology. New York: Mc Graw-Hill; 2000. pp. 1639–1658. [Google Scholar]
- 13.Laursen A.L, Scheibel E, Ingerslev J, et al. Alpha interferon therapy in Danish haemophiliac patients with chronic hepatitis C: results of a randomized controlled open label study comparing two different maintenance regimens following standard interferon-alpha-2b treatment. Haemophilia. 1998;4:25–32. doi: 10.1046/j.1365-2516.1998.00141.x. [DOI] [PubMed] [Google Scholar]
- 14.Rumi M.G, Santagostino E, Morfini M, et al. A multicenter Controlled Randomized Open Trial of Interferon alfa-2b Treatment of Anti-Human Immunodeficiency Virus-Negative Hemo-philic Patients With Chronic Hepatitis C. Blood. 1997;89(10):3529–3533. [PubMed] [Google Scholar]
- 15.Santagostino E, Rumi G.M, Rivi M, et al. Sustained suppression of hepatitis C virus by interferon and ribavirin in hemophilic patients not responding to interferon monotherapy. Blood. 2002;99(3):1089–1091. doi: 10.1182/blood.v99.3.1089. [DOI] [PubMed] [Google Scholar]
- 16.Posthouwer D, Wolters V.M, Fischer K, et al. Hepatitis C infection in children with haemophilia: a pilot study. Haemophilia. 2004;10:722–726. doi: 10.1111/j.1365-2516.2004.01038.x. [DOI] [PubMed] [Google Scholar]
- 17.Rumi M.G, De Filippi F, Santagostino E, et al. Hepatitis C in Haemophilia: lights and shadows. Haemophilia. 2004;10(4):211–215. doi: 10.1111/j.1365-2516.2004.00980.x. [DOI] [PubMed] [Google Scholar]
- 18.Tradati F, Colombo M, Manucci P.M, et al. A Prospective Multicenter Study of Hepatocellular Carcinoma in Italian Hemophiliacs With Chronic Hepatitis C. Blood. 1998;91(4):1173–1177. [PubMed] [Google Scholar]
- 19.Kasper C.K. Hereditary plasma clotting factor disorders and their management. Haemophilia. 2000;6(1):13–27. doi: 10.1046/j.1365-2516.2000.00042.x. [DOI] [PubMed] [Google Scholar]
- 20.Carneiro-Proietti A.B.F, Lima-Martins M.V.C, Passos V.M.A, et al. Presence of human immunodeficiency virus (HIV) and T-lymphotropic virus type I and II(HTLV-I/II) in a haemophiliac population in Belo Horizonte, Brazil, and correlation with additional serological results. Haemophilia. 1998;4:47–50. doi: 10.1046/j.1365-2516.1998.00128.x. [DOI] [PubMed] [Google Scholar]
- 21.Evan Sadler J, Davie E.W. Hemophilia A, Hemophilia B, and von Willebrand Disease. In: Stamatoyannopoulos G, Majerus P.W, Perlmutter R.M, editors. The Molecular Basis of Blood Diseases. Philadelphia: W.B Saunders Company; 2001. p. 680.p. 697. [Google Scholar]
- 22.Montoro J.B, Oliveras J, Ignacio Lorenzo J, et al. An association Between Clotting Factor Concentrates Use and Mortality in Human Immunodeficiency Virus-Infected Hemophilic Patients. Blood. 1995;86(6):2213–2219. [PubMed] [Google Scholar]
- 23.Astermark J, Johnsson H, Stigendal L, et al. HIV-disease progression in Swedish haemophiliacs and the influence of replacement therapy. Haemophilia. 1997;3:277–282. doi: 10.1046/j.1365-2516.1997.00122.x. [DOI] [PubMed] [Google Scholar]
- 24.Mannucci P.M. Hemophilia and related bleeding disorders. A story of dismay and success. Hematology. 2002:1–7. doi: 10.1182/asheducation-2002.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Bray G.L, Gomperts E.D, Courter S, et al. A multicenter study of recombinant factor VIII (Recombinate): safety, efficacy, and inhibitor risk in previously untreated patients with hemophilia A. Blood. 1994;83:2428–2435. [PubMed] [Google Scholar]
- 26.Lusher J.M, Arkin S, Abildgaard C.F, et al. Recombinant factor VIII for the treatment of previously untreated patients with hemophilia A. Safety, efficacy, and development of inhibitors. N. Engl. J. Med. 1993;328(7):453–459. doi: 10.1056/NEJM199302183280701. [DOI] [PubMed] [Google Scholar]
- 27.Scharrer I. Recombinant factor VIIa for patients with inhibitors to factor VIII or IX or factor VII deficiency. Haemophilia. 1999;5(4):253–259. doi: 10.1046/j.1365-2516.1999.00319.x. [DOI] [PubMed] [Google Scholar]
- 28.Triemstra M, Rosendaal F.R, Smit C, et al. Mortality in patients with hemophilia. Changes in a Dutch population from 1986 to 1992 and 1973 to 1986. Ann. Intern. Med. 1995;123(11):823–830. doi: 10.7326/0003-4819-123-11-199512010-00002. [DOI] [PubMed] [Google Scholar]
- 29.Valentino I.A, Ewenstein B, Navickis R.J, et al. Central venous access devices in haemophilia. Haemophilia. 2004;10:134–146. doi: 10.1046/j.1365-2516.2003.00840.x. [DOI] [PubMed] [Google Scholar]
- 30.Chuansumrit A, Krasaesub S, Angchaisuksiri P, et al. Survival analysis of patients with haemophilia at the Intrnational Haemophilia Training Centre, Bangkok, Thailand. Haemophilia. 2004;10:542–549. doi: 10.1111/j.1365-2516.2004.00928.x. [DOI] [PubMed] [Google Scholar]
- 31.Ghosh K, Shetty S, Kulkarni B, et al. Development of inhibitors in patients with haemophilia from India. Haemophilia. 2001;7:273–278. doi: 10.1046/j.1365-2516.2001.00505.x. [DOI] [PubMed] [Google Scholar]
- 32.Evensen S.A, Ulstrup J, Skaug K, et al. HIV infection in Norwegian haemophiliacs: the prevalence of antibodies against HIV in haemophiliacs treated with lyophilize cryoprecipitate from volunteer donors. Eur J Haematol. 1987;39(1):44–48. doi: 10.1111/j.1600-0609.1987.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 33.Yamada-Osaki M, Sumazaki R, Kajiwara Y, et al. Natural course of HGV infection in haemophiliacs. Br. J. Haematol. 1988;102:616–621. doi: 10.1046/j.1365-2141.1998.00793.x. [DOI] [PubMed] [Google Scholar]
- 34.Ludlam C.A, Lee R.J, Prescott R.J, et al. Haemophilia care in central Scotland 1980-94. I. Demographic characteristics, hospital admission and causes of death. Haemophilia. 2000;6:494–503. doi: 10.1046/j.1365-2516.2000.00405.x. [DOI] [PubMed] [Google Scholar]
- 35.Bauduer F, Degioanni A, Ducout L, et al. Distribution of haemophilia in the French Basque Country. Haemophilia. 2002;8:735–739. doi: 10.1046/j.1365-2516.2002.00659.x. [DOI] [PubMed] [Google Scholar]
- 36.Yokozaki Sh, Takamatsu J, Nakano I, et al. Immunologic dynamics in hemophiliac patients infected with hepatitis C virus and human immunodeficiency virus: influence of antiretroviral therapy. Blood. 2000;96(13):4293–4299. [PubMed] [Google Scholar]
- 37.Globe D.R, Curtis R.G, Koerper M.A. Utilization of care in haemophilia: a resource-based method for coast analysis from the Haemophilia Utilization Group Study (HUGS) Haemophilia. 2004;10(1):63–70. doi: 10.1111/j.1355-0691.2004.00881.x. [DOI] [PubMed] [Google Scholar]
- 38.Goedert J.J, Brown D.L, Hoots K, et al. Human immunodeficiency and hepatits virus infections and their associated conditions and treatments among people with haemophilia. Haemophilia. 2004;10(4):205–210. doi: 10.1111/j.1365-2516.2004.00997.x. [DOI] [PubMed] [Google Scholar]
- 39.Brettler D.B, Alter H.J, Dienstag J.L, et al. Prevalence of hepatitis C virus antibody in a cohort of hemophilia patients. Blood. 1990;76(1):254–256. [PubMed] [Google Scholar]
- 40.Maisonneuve P, Laurian Y, Guerois C, et al. Antibody to hepatitis C (anti C 100-3) in French hemophiliacs. Nouv. Rev. Fr. Hematol. 1991;33(3):263–266. [PubMed] [Google Scholar]
- 41.Makris M, Preston F.E, Triger D.R, et al. Hepatitis C antibody and chronic liver disease in haemophilia. Lancet. 1990;12((335)8698):1117, 1119. doi: 10.1016/0140-6736(90)91124-s. [DOI] [PubMed] [Google Scholar]
- 42.Mauser-Bunschoten E.P, Damen M, Reesink H.W, et al. Formation of Antibodies to Factor VIII in Patients with Hemophilia A Who Are Treated with Interferon for Chronic Hepatitis C. Ann Intern. Med. 1996;125(4):297–299. doi: 10.7326/0003-4819-125-4-199608150-00007. [DOI] [PubMed] [Google Scholar]
- 43.Foster P.R, Mcintosh R.V, Macleod A.J. Hepatitis C and haemophilia. BMJ. 1995;311(7007):754–755. doi: 10.1136/bmj.311.7007.754c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinez-Murillo C, Quintana S, Ambriz R, et al. An economic model of Haemophilia in Mexico. Haemophilia. 2004;10:9–17. doi: 10.1046/j.1365-2516.2003.00811.x. [DOI] [PubMed] [Google Scholar]
- 45.Peerlinck K, Rosendaal F.R, Vermylen J. Incidence of inhibitor development in a Group of Young Hemophilia A patients treated exclusively with Lyophilized cryoprecipitate. Blood. 1993;81(12):3332–3335. [PubMed] [Google Scholar]
- 46.Gringeri A, Von Mackensen S, Auerswald G, et al. Health status and health-related quality of life of children with haemophilia from six West European countries. Haemophilia. 2004;10(1):2633. doi: 10.1111/j.1355-0691.2004.00876.x. [DOI] [PubMed] [Google Scholar]
- 47.Kavakli K, Gringeri A, Bader R, et al. Inhibitor development and substitution therapy in a developing country: Turkey. Haemophilia. 1998;4:106–108. doi: 10.1046/j.1365-2516.1998.00171.x. [DOI] [PubMed] [Google Scholar]
- 48.Takayama S, Taki M, Meguro T, et al. Virological Characteristics of HCV infection in Japanese haemophiliacs. Haemophilia. 1997;3:131–136. doi: 10.1046/j.1365-2516.1997.00097.x. [DOI] [PubMed] [Google Scholar]


