Abstract
Autistic disorder is a severe neurodevelopment disorder characterized by a triad of impairments in reciprocal social interaction, verbal and nonverbal communication, and a pattern of repetitive stereotyped activities, behaviours and interests. There are strong lines of evidence to suggest that the immune system plays an important role in the pathogenesis of autistic disorder. The aim of this study was to analyze quantitative plasma concentration of immunoglobulin classes, and subclasses in autistic patients and their families. The investigation was performed retrospectively in 50 persons with autistic disorder in the Republic of Macedonia. Infantile autistic disorder was diagnosed by DSM-IV and ICD-10 criteria. Plasma immunoglobulin classes (IgM, IgA, and IgG) and subclasses (IgG1, IgG2, IgG3, and IgG4) were determined using Nephelometer Analyzer BN-100. Multiple comparisons for the IgA variable have shown statistically significant differences between three pairs: male autistic from the fathers (p = 0,001), female autistic from the mothers (p = 0,008), as well as healthy sisters from the fathers (p = 0,011). Statistically significant differences found between three groups regarding autistic disorder (person with autistic disorder, father/mother of a person with autistic disorder, and brother/sister) independent of sex belongs to IgA, IgG2, and IgG3 variables. Multiple comparisons for the IgA variable have shown statistically significant differences between children with autistic disorder from the fathers and mothers (p < 0,001), and healthy brothers and sisters from the fathers and mothers (p < 0,001). Comparison between healthy children and children with autistic disorder from the same family should be tested for immunoglobulin classes and subclasses in order to avoid differences between generations.
Keywords: autistic disorder, immunoglobulin classes, immunoglobulin subclasses, family analysis
INTRODUCTION
Autistic disorder is a severe neurodevelopment disorder characterized by a triad of impairments in reciprocal social interaction, verbal and nonverbal communication, and a pattern of repetitive stereotyped activities, behaviours and interests (1). A number of factors have been implicated in the patho-genesis of autistic disorder including genetic (2), environmental (3), and immunological factors (4). There are strong lines of evidence to suggest that the immune system plays an important role in the pathogenesis of autistic disorder. These include changes in lymphocyte subsets (5), alteration in serum concentration of im-munoglobulin classes and subclasses (6,7) and cytokine production (8), presence of auto antibodies to neural antigens (9), increased frequency of the C4b null allele (10), and linkage with some immune response genes (11). Abnormal immunoglobulin (low IgA, increased IgE), decreased natural killer (NK) cells and other T-cell abnormalities may reflect “disregulation” of the immune system in persons with autistic disorder. These findings may also occur in parallel to changes in the developing CNS, and both may have the same aetiologies that underlie autistic disorder (9, 12-14). The immune abnormalities would therefore give the appearance of being causative (15). Individuals with autistic disorder had significantly lower serum concentrations of cortisol (p < 10-6), and significantly higher concentrations of ACTH (p = 0,002) than control age-and sex-matched subjects. The observed hormonal changes may indicate dysfunction of the hypothalamo-pituitary-ad- renal axis in individuals with autistic disorder (16). We investigated plasma concentration of IgA, IgM, IgG classes, and IgG1, IgG2, IgG3, and IgG4 subclasses in children with autism and found that children with autism have increased plasma concentration of im-munoglobulines (17). In addition, we investigated specific IgA, IgG, and IgE antibodies to food antigens in 35 participants with autistic disorder and 21 of their siblings in the Republic of Macedonia and found increased levels for some of the food antigens (18). The aim of our study was to analyze quantitative plasma concentration of immunoglobulin classes, and subclasses in autistic patients and their families to confirm the differences found in previous studies between the affected and the healthy control groups. The main point of our study was to examine whether there was a correlation in plasma concentration of immunoglobulin classes, and subclasses between autistic patients and their parents, brothers, and sisters, because no such analysis of autistic patients and their family members has been made before.
MATERIAL AND METHODS
Subjects
The investigation was performed retrospectively in 50 persons with autistic disorder registered by the social institutions of the Republic of Macedonia. Infantile autistic disorder was diagnosed based on DSM-IV (1) and ICD-10 (19) criteria. The sample included 30 autistic patients (22 male and 8 female patients 10,14 ± 5,81 years of age). We also analyzed all family members who consented to participate during the continual checkups of their children. There were 30 mothers and 26 fathers aged between 25 and 70 years; and 15 sisters and 7 brothers aged between 3 and 30 years. In most families the autistic child was the only child, although there were some families with two autistic children. The diagnosis of autistic disorder in children-patients and their siblings was reached in all cases by a specialist assessment of a senior pediatrician and with contributions from specialist in mental health care where appropriate.
Immunoglobulin determination
Blood for immunogenetic investigations was taken from the subjects after obtaining their informed consent. Ten millilitres of venous blood was drawn from each donor by a standard venipuncture using vacutainer. At the time of blood drawing, none of autistic children were receiving any prescription medication or antipsychotic drug. The test requests were properly documented and kept in a confidential file. All persons gave their informed consent and allowed inclusion of their data in the manuscript without disclosure of their identity in the publication. Blood samples were separated by centrifugation, and stored at - 20°C until the determination. Serum immunoglobulin classes (IgA, IgG, and IgM) and subclasses (IgG1, IgG2, IgG3, and IgG4) were determined immunonephelometricallly by an automated Nephelometer Analyzer BN-100 (Dade-Behring, Vienna, Austria) as anonymous samples using reagents and methods supplied by the analyzer’s manufacturer.
Statistics
The analysis included descriptive statistics (means ± standard deviations), multivariate analysis of variance (MANOVA) with Tukey HSD post hoc test for multiple comparisons and discriminant analysis for eight groups of examinees (20,21). Interclass correlation coefficients were calculated for paired familial data (22). P-values lower than 0,05 were considered statistically significant.
RESULTS
We analyzed all immunoglobulin classes and subclasses and presented the results as mean ± SD separately for male (Table 1), and female participants (Table 2).
TABLE 1.
Plasma concentration of immunoglobulin classes and subclasses (mean ± SD) in male persons with autistic disorder, their fathers, and brothers
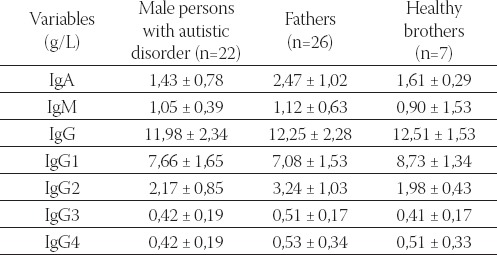
TABLE 2.
Plasma concentration of immunoglobulin classes and subclasses (mean ± SD) in female persons with autistic disorder, their mothers, and sisters
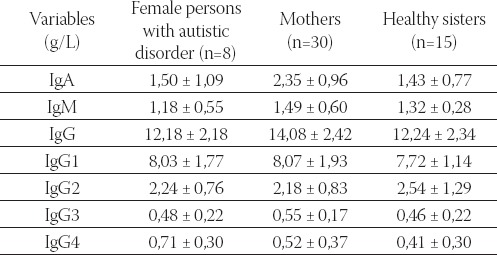
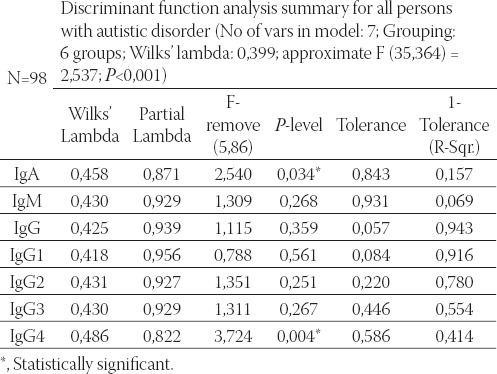
Seven variables (IgA, IgM, IgG, IgG1, IgG2, IgG3, and IgG4) entered into factorial multivariate analysis of variance (MANOVA) with two factors, sex (male/ female), and three groups regarding autistic disorder (person with autistic disorder, father/mother of a person with autistic disorder, and brother/sister), giving altogether six groups. Post hoc multiple comparisons with Tukey HSD test were made to reveal statistically significant pairs of groups for the IgA variable. We can see statistically significant differences between three pairs: male autistic from the fathers (p = 0,001), male autistic from the mothers (p = 0,008), as well as healthy sisters from the fathers (p = 0,011) (Table 3). The results of multivariate discriminate analysis for six examined groups (1= male autistic; 2=female autistic; 3=fathers; 4=mothers; 5=healthy brothers; 6=healthy sisters) and seven variables revealed that the model is significant only for the variables IgA (p=0,034) and IgG4 (p=0,004) (Table 4).
TABLE 3.
Multivariate analysis of variance (MANOVA) for 6 variables, factorial model for sex (male/female), and group membership regarding autistic disorder (person with autistic disorder, father/mother of a person with autistic disorder, and brother/sister)
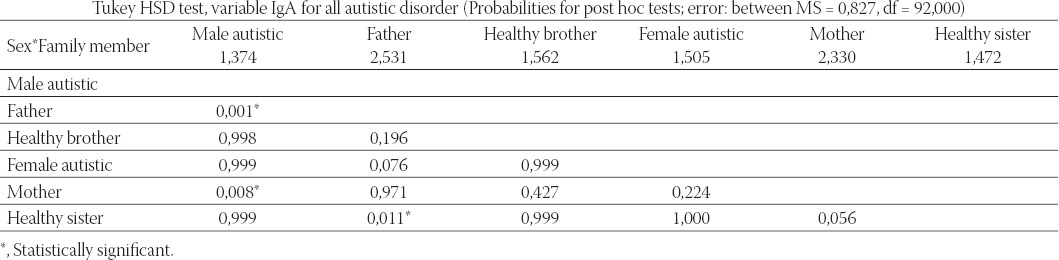
TABLE 4.
Discriminant function analysis summary for all persons with autistic disorder
Structure matrix gives the correlation between original variables and discriminant functions (variables were sorted by ascending absolute correlation between each variable and any discriminant function). In the last two rows are given Eigenvalues, and cumulative proportion of variance. Standardized canonical discriminant function coefficients are given in Table 5. Graphical presentation of group centroids on discriminant functions 1 and 2 is shown in Figure 1. Discriminant function 1 (horizontal) clearly separated mothers and fathers (left) from children (right), whereas function 2 (vertical) separated fathers and autistic male (negative coordinates of group centroids) from the rest of the participants (positive coordinates).
TABLE 5.
Standardized canonical discriminant function coefficients, enabling transformation of standardized values of original variables into scores on discriminant functions
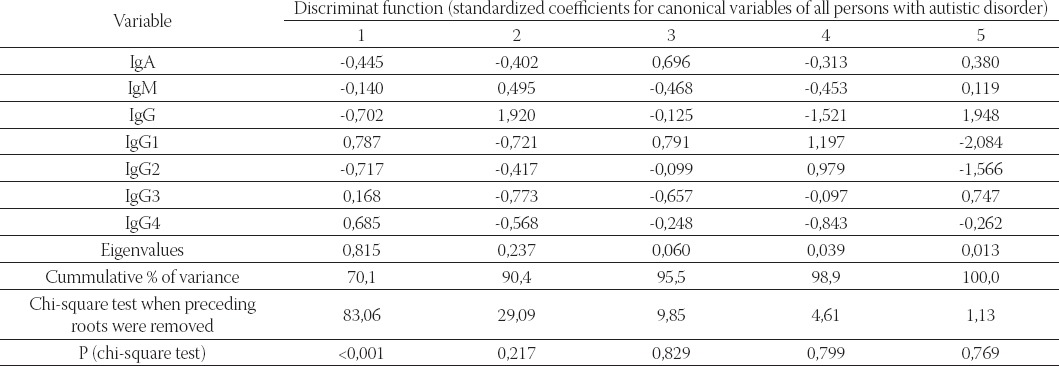
FIGURE 1.
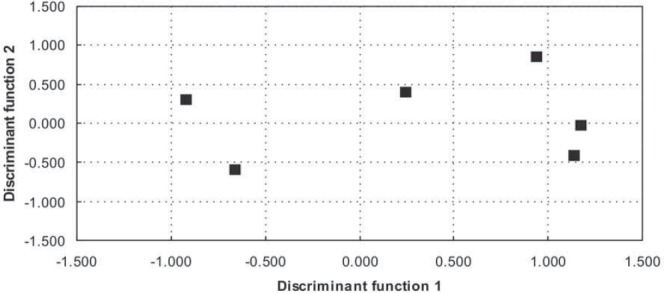
Graphical presentation of group centroids on discriminant functions 1 and 2. Discriminant function 1 (horizontal) clearly separated mothers and fathers (left) from children (right), whereas function 2 (vertical) separated fathers and autistic male (negative coordinates of group centroids) from the rest of the participants (positive coordinates)
Graphical presentation of group centroids on discriminant functions 3 and 4 is shown in Figure 2. Discriminant function 3 (horizontal) clearly separated sisters, mothers, and autistic male (left) from brothers, fathers, and autistic female (right), whereas function 4 (vertical) separated autistic male, mothers, and autistic female (negative coordinates of group centroids) from sisters, brothers, and fathers (positive coordinates).
FIGURE 2.
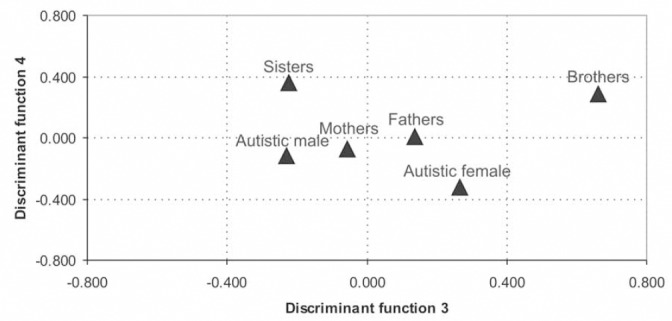
Graphical presentation of group centroids on discriminant functions 3 and 4. Discriminant function 3 (horizontal) clearly separated sisters, mothers, and autistic male (left) from brothers, fathers, and autistic female (right), whereas function 4 (vertical) separated autistic male, mothers, and autistic female (negative coordinates of group centroids) from sisters, brothers, and fathers (positive coordinates)
DISCUSSION
Our study showed that statistically significant differences found with factorial multivariate analysis of variance with two factors, sex (male/female), and three groups regarding autistic disorder (person with autistic disorder, father/mother of a person with autistic disorder, and brother/sister) belong to IgA, IgM, and IgG2 variables. Post hoc multiple comparisons with Tukey HSD reveal statistically significant pairs of groups for the IgA variable. We found statistically significant differences between three pairs: male autistic from the fathers, female autistic from the mothers, as well as healthy sisters from the fathers. The results should be interpreted very carefully. We can interpret that IgA, IgM, and IgG2 are connected somehow with the autistic disorder in the male and female persons, but we cannot interpret connection of healthy sisters with the fathers. There is also possibility that found differences are rather between generations (older and younger generations), and/or due to the small sizes of the groups (there are only 8 female with autistic disorder, and only 7 healthy brothers). Discriminant functions at group centroids showed that we did not find any significant difference between healthy children and children with autistic disorder, as well as with their parents (fathers, and mothers). It could be simply because the immunoglobulin classes and subclasses are significantly dependent of age (and sex), that in this case differences between generations are dominant (differences between parents and children), and differences in the sex are on the second place (the second discriminant function is not significant).
The analysis of three groups regarding autistic disorder, independent of sex, have shown that statistically significant differences with factorial multivariate analysis of variance belongs to IgA, IgG2, and IgG3 variables. Post hoc multiple comparisons with Tukey HSD test reveal statistically significant differences between children with autistic disorder from the fathers and mothers, and healthy brothers and sisters from the fathers and mothers. Again, we found statistically significant differences between generation, independent of sex (parents:children), but we did not find statistically significant differences between the healthy and children with autistic disorder (20 healthy brothers and sisters, and 28 children with autistic disorder). This investigation is the first family analysis of immu-noglobulin classes and subclasses in children with autistic disorder. There are few papers in the literature dealing with immunoglobulin classes and subclasses in patients with autistic disorder. Decreased serum IgA (23), higher plasma concentration of IgM (17), higher plasma concentration of IgG (7,17), increased plasma concentration of IgG1 in autistic males and autistic females (17), increased IgG2 (7), increased IgG4 (7,17), and increased total IgE (18) were reported (Table 6). There is potential that such aberrant immune activity during vulnerable and critical periods of neurodevelop-ment could participate in the generation of neurological dysfunction characteristic of autistic disorder (24).
TABLE 6.
Studies of immunoglobulin classes and subclasses in children with autistic disorder
Circulating autoantibodies against various neuronal antigens have been found at a higher frequency in ASD groups compared to controls. Some of the targets include serotonin receptors (25), myelin basic protein (MBP) (26,27), glial fibrillary acidic protein (GFAP), neuron-axon filament protein (NAFP) and neurofilament proteins (28), neuron-specific antigens (29), antibrain endothelial cell proteins and neurotrophic factors (9), cerebellar neurofilaments (30), and nerve growth factor (31). Serum autoantibodies to human brain, identified by ELISA and Western immunoblotting, were evaluated in children with autistic disorder, non-autistic siblings and controls. More autistic subjects than controls had bands at 100 kDa in caudate, putamen and prefrontal cortex as well as larger peak heights of bands at 73 kDa in the cerebellum and cingulate gyrus. Both autistic disorder subjects and their matched non-autistic siblings had denser bands (peak height and/or area under the curve) at 73 kDa in the cerebellum and cingulate gyrus than did controls. Results suggest that children with autistic disorder and their siblings exhibit differences compared to controls in autoimmune reactivity to specific epitopes located in distinct brain regions (32). Specific IgA, IgG, and IgE antibodies to food antigens in 35 participants with autistic disorder and 21 of their siblings in the Republic of Macedonia were examined. Statistically significant higher plasma concentration of IgA antibodies against alpha-lactalbumin, beta-lactoglobulin, casein, and gliadin were found in the children with autistic disorder. Plasma concentrations of IgG antibodies against alpha-lactalbumin, beta-lactoglobulin, and casein in participants with autistic disorder were significantly higher. IgE-specific antibodies (alpha-lactalbumin, beta- lactoglobulin, casein, and gluten), as well as plasma concentration of total IgE, also were statistically significantly higher in the participants with autistic disorder. Gender differences were found for select IgA, IgG, and IgE (but not for total IgE) food-specific antibodies (kU/L) in the participants with autistic disorder and their siblings (18).
CONCLUSION
We investigated retrospectively 50 persons with autistic disorder from the Republic of Macedonia. Infantile autistic disorder was diagnosed by DSM-IV and ICD-10 criteria. Plasma immunoglobulin classes (IgM, IgA, and IgG) and subclasses (IgG1, IgG2, IgG3, and IgG4) were determined using Nephelometer Analyzer BN-100. The most important findings were found for multiple comparisons for the IgA variable between three pairs: male autistic from the fathers, female autistic from the mothers, as well as healthy sisters from the fathers. We also found statistically significant differences between three groups regarding autistic disorder (person with autistic disorder, father/mother of a person with autistic disorder, and brother/sister) independent of sex belongs to IgA, IgG2, and IgG3 variables. Multiple comparisons for the IgA variable also have shown statistically significant differences between children with autistic disorder from the fathers and mothers, and healthy brothers and sisters from the fathers and mothers. In the conclusion, we could say that comparison between healthy children and children with autistic disorder from the same family should be tested for immunoglobulin classes and subclasses, because these children are from the similar generations.
Acknowledgement
This work was part of the project “Serum Immunoglobulins and Specific Food Allergens in the Persons with Autism in Republic of Macedonia” sponsored by the Ministry of Sciences and Education of Republic of Macedonia (Grant No. 40221400/0). Special thanks go to Elena Cvetkovska for her valuable help.
REFERENCES
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: 1994. [Google Scholar]
- 2.Korvatska E, Van de Water J, Anders T.F, Gershwin M.E. Genetic and mmunologic considerations in autism. Neurobiol. Dis. 2002;9(2):107–25. doi: 10.1006/nbdi.2002.0479. Review. Erratum in: Neurobiol Dis. 2002;10(1):69. [DOI] [PubMed] [Google Scholar]
- 3.Vojdani A, Pangborn J.B, Vojdani E, Cooper E.L. Infections, toxic chemicals and dietary peptides binding to lymphocyte receptors and tissue enzymes are major instigators of autoimmunity in autism. Int. J. Immunopathol. Pharmacol. 2003;16(3):189–199. doi: 10.1177/039463200301600302. [DOI] [PubMed] [Google Scholar]
- 4.Warren R.P, Singh V.K, Averett R.E, Odell J.D, Maciulis A, Burger R.A, Daniels W.W, Warren W.L. Immunogenetic studies in autism and related disorders. Mol Chem Neuropathol. 1996;28(1-3):77–81. doi: 10.1007/BF02815207. [DOI] [PubMed] [Google Scholar]
- 5.Fiumara A, Sciotto A, Barone R, D’Asero G, Munda S, Parano E, Pavone L. Peripheral lymphocyte subsets and other immune aspects in Rett syndrome. Pediatr Neurol. 1999;21(3):619–621. doi: 10.1016/s0887-8994(99)00053-3. [DOI] [PubMed] [Google Scholar]
- 6.Zimmerman A.W, Potter N.T, Stakkestad A, Frye V.H. Serum immunoglobulins and autoimmune profiles in children with autism. Ann Neurol. 1995;38:528. [Google Scholar]
- 7.Croonenberghs J, Wauters A, Devreese K, Verkerk R, Scharpe S, Bosmans E, Egyed B, Deboutte D, Maes M. Increased serum albumin, gamma globulin, immunoglobulin IgG, and IgG2 and IgG4 in autism. Psychol Med. 2002;32(8):1457–1463. doi: 10.1017/s0033291702006037. [DOI] [PubMed] [Google Scholar]
- 8.Jyonouchi H, Sun S, Itokazu N. Innate immunity associated with inflammatory responses and cytokine production against common dietary proteins in patients with autism spectrum disorder. Neuropsychobiology. 2002;46(2):76–84. doi: 10.1159/000065416. [DOI] [PubMed] [Google Scholar]
- 9.Singh V.K, Warren R, Averett R, Ghaziuddin M. Circulating autoantibodies to neuronal and glial filament proteins in autism. Pediatr Neurol. 1997;17(1):88–90. doi: 10.1016/s0887-8994(97)00045-3. [DOI] [PubMed] [Google Scholar]
- 10.Warren R.P, Yonk J, Burger R.W, Odell D, Warren W.L. DR-positive T cells in autism: association with decreased plasma levels of the complement C4B protein. Neuropsychobiology. 1995;31(2):53–57. doi: 10.1159/000119172. [DOI] [PubMed] [Google Scholar]
- 11.Gupta S. Immunological treatments for autism. J Autism Dev Disord. 2000;30(5):475–479. doi: 10.1023/a:1005568027292. [DOI] [PubMed] [Google Scholar]
- 12.Plyoplys A.V, Greaves A, Yoshida W. Anti-CNS antibodies in childhood neurologic diseases. Neuropsychiatrics. 1989;20:93–102. doi: 10.1055/s-2008-1071273. [DOI] [PubMed] [Google Scholar]
- 13.Connolly A.M, Chez M.G, Pestronk A, Arnold S.T, Mehta S, Deuel R.K. Serum autoantibodies to brain in Landau-Kleffner variant, autism, and other neurologic disorders. J Pediatr. 1999;134(5):607–613. doi: 10.1016/s0022-3476(99)70248-9. [DOI] [PubMed] [Google Scholar]
- 14.Singh V.K, Lin S.X, Yang V.C. Serological association of measles virus and human herpesvirus-6 with brain autoantibodies in autism. Clin. Immunol. Immunopathol. 1998;89(1):105–108. doi: 10.1006/clin.1998.4588. [DOI] [PubMed] [Google Scholar]
- 15.Zimmerman A. Commentary: immunological treatments for autism: in search of reasons for promising approaches. J. Autism. Dev. Disord. 2000;30(5):481–484. doi: 10.1023/a:1005520111362. [DOI] [PubMed] [Google Scholar]
- 16.Curin J.M, Terzic J, Petkovic Z.B, Zekan L, Terzic I.M, Susnjara I.M. Lower cortisol and higher ACTH levels in individuals with autism. J. Autism. Dev. Disord. 2003;33(4):443–448. doi: 10.1023/a:1025019030121. [DOI] [PubMed] [Google Scholar]
- 17.Trajkovski V, Ajdinski L, Spiroski M. Plasma concentration of immunoglobulin classes and subclasses in children with autism in the Republic of Macedonia: retrospective study. Croat. Med J. 2004;45(6):746–749. [PubMed] [Google Scholar]
- 18.Trajkovski V, Petlichkovski A, Efinska-Mladenovska O, Trajkov D, Arsov T, Strezova A, Ajdinski L, Spiroski M. Higher plasma concentration of food-specific antibodies in persons with autistic disorder in comparison to their siblings. Focus on Autism and Other Developmental Disabilities. 2008;23:176–185. [Google Scholar]
- 19.World Health Organization. The ICD-10 Classification of Mental and Behavioral Health Organization. 1992 [Google Scholar]
- 20.SAS Institute. The SAS System for Windows [computer program]. Version 8.02. Cary (NC): SAS Institute Inc; 2001. [Google Scholar]
- 21.StatSoft, Inc. Statistica [computer program]. Version 6.0. Tulsa (OK): StatSoft; 2001. [Google Scholar]
- 22.Rosner B, Donner A, Hennekens C.H. Significance testing of in-terclass correlations from familial data. Biometrics. 1979;35:461–471. [Google Scholar]
- 23.Warren R.P, Odell J.D, Warren W.L, Burger R.A, Maciulis A, Daniels W.W, Torres A.R. Brief Report: Immunoglobulin A Deficiency in a Subset of Autistic Subjects. J. Autism. Dev. Disord. 1997;27(2):187–192. doi: 10.1023/a:1025895925178. [DOI] [PubMed] [Google Scholar]
- 24.Ashwood P, Wills S, Van de Water J. The immune response in autism: a new frontier for autism research. J. Leukoc Biol. 2006;80(1):1–15. doi: 10.1189/jlb.1205707. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg J, Anderson G.M, Zwaigenbaum L, Hall G.B, Nahmias C, Thompson A, Szatmari P. Cortical serotonin type-2 receptor density in parents of children with autism spectrum disorders. J. Autism. Dev. Disord. 2009;39(1):97–104. doi: 10.1007/s10803-008-0604-4. [DOI] [PubMed] [Google Scholar]
- 26.Singh V.K, Warren R.P, Odell J.D, Warren W.L, Cole P. Antibodies to myelin basic protein in children with autistic behavior. Brain. Behav. Immun. 1993;7(1):97–103. doi: 10.1006/brbi.1993.1010. [DOI] [PubMed] [Google Scholar]
- 27.Libbey J.E, Coon H.H, Kirkman N.J, Sweeten T.L, Miller J.N, Stevenson E.K, Lainhart J.E, McMahon W.M, Fujinami R.S. Are there enhanced MBP autoantibodies in autism? J. Autism Dev. Disord. 2008;38(2):324–332. doi: 10.1007/s10803-007-0400-6. [DOI] [PubMed] [Google Scholar]
- 28.Connolly A.M, Chez M, Streif EM, Keeling R.M, Golumbek P.T, Kwon J.M, Riviello J.J, Robinson R.G, Neuman R.J, Deuel R.M. Brain-derived neurotrophic factor and autoantibodies to neural antigens in sera of children with autistic spectrum disorders, Landau-Kleffner syndrome, and epilepsy. Biol. Psychiatry. 2006;59(4):354–363. doi: 10.1016/j.biopsych.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Vojdani A, Campbell A.W, Anyanwu E, Kashanian A, Bock K, Vojdani E. Antibodies to neuron-specific antigens in children with autism: possible cross-reaction with encephalitogenic proteins from milk, Chlamydia pneumoniae and Streptococcus group A. J. Neuroimmunol. 2002;129(1-2):168–177. doi: 10.1016/s0165-5728(02)00180-7. [DOI] [PubMed] [Google Scholar]
- 30.Jellinger K, Armstrong D, Zoghbi H.Y, Percy A.K. Neuropathol-ogy of Rett syndrome. Acta Neuropathol. 1988;76(2):142–158. doi: 10.1007/BF00688098. [DOI] [PubMed] [Google Scholar]
- 31.Kliushnik T.P, Lideman R.R. [Autoimmune mechanisms in the genesis of developmental abnormalities of the nervous system] Vestn. Ross. Akad Med. Nauk. 2001;(7):32–34. [PubMed] [Google Scholar]
- 32.Singer H.S, Morris C.M, Williams P.N, Yoon D.Y, Hong J.J, Zimmerman A.W. Antibrain antibodies in children with autism and their unaffected siblings. J. Neuroimmunol. 2006;178(1-2):149–155. doi: 10.1016/j.jneuroim.2006.05.025. [DOI] [PubMed] [Google Scholar]


