Abstract
The aim of this study was to analyze (i) ratios between pro-inflammatory cytokines interleukin 6 (IL-6), interleukin 1 (IL-1), tumour necrosis factor α (TNF-α) and anti-inflammatory cytokine interleukin 10 (IL-10) in patients with acute myocardial infarction (AMI) and stable angina pectoris (ii) as well as correlation between IL-6 and IL-10 in AMI and (iii) correlation between IL-6 and lipoproteins in AMI.
The total of 71 patients were enrolled in this study, 41 of them with AMI (study group) and 30 with stable angina pectoris (control group). The concentrations of cytokines and lipoproteins were measured from blood samples. Pro-inflammatory to anti-inflammatory cytokine ratios were calculated by dividing concentrations of pro-inflammatory cytokines with IL-10. In statistical analyses we used descriptive statistics, normality tests and analysis of correlation.
IL-6: IL-10 ratio is significantly higher in AMI than in stable angina (P < 0,001), TNF-α: IL-10 is also higher in study group but the difference is not significant. We found positive linear correlation between IL-6 and IL-10 (r =0,43; p = 0,015) and negative linear correlation between IL-6 and high density lipoprotein HDL (r = -0,47; p= 0,008) in AMI.
IL-6: IL-10 ratio is higher in AMI than in stable angina. There is linear correlation between IL-6 and IL-10 and IL-6 and HDL in AMI.
Keywords: cytokines, anti-inflammatory, pro-inflammatory, HDL, acute myocardial infarction
INTRODUCTION
Acute myocardial infarction (AMI) is myocardial necrosis due to occlusion (prolonged ischemia) of coronary blood vessels. Over the last decade its immunological aspect is in the focus of interest of cardiologists. Coronary plaque disruption, with consequent platelet aggregation and thrombosis, is the most important mechanism by which atherosclerosis leads to the acute coronary syndromes (ACS) of unstable angina, acute myocardial infarction, and sudden cardiac death (1). There are broad spectrum of alterations involving the plaque surface, including fissuring, erosion, ulceration, and rupture of the plaque surface (2). All of those involve disruption of the endothelium and the underlying connective tissue of the plaque capsule. There are certain types of plaques that are prone to disruption. These “vulnerable plaques” are lipid rich atheroma-tous plaques that have a thin fibrous capsule (3). There is substantial evidence implicating an inflammatory process in the pathogenesis of AMI. Local inflammatory cells can generate and release cytokines that have the potential to activate endothelium, transforming its natural anti-adhesive and anticoagulant properties. Furthermore, pro-inflammatory cytokines may reduce matrix synthesis and increase its degradation, favouring plaque rupture. Finally, cytokines may enhance synthesis of endothelin in endothelial cells and macrophages, resulting in increased smooth muscle cell reactivity to local vasoconstrictors (4). The evidence supporting the hypothesis that inflammation is critical in the pathogen-esis of acute coronary syndromes comes from a variety of sources. The major vascular risk markers high sensitive C-reactive protein (hs-CRP), pro-inflammatory cytokines such as interleukin -1ß (IL-1ß), interleukin- 6 (IL-6), tumour necrosis factor- α (TNF- α), and the anti-inflammatory cytokine interleukin- 10 (IL-10) have been detected in human atherosclerotic plaques (5).
The role of anti-inflammatory cytokine IL-10 is still controversial. The balance between pro-inflammatory and anti-inflammatory cytokines may reflect the intensity of occult plaque inflammation and the vulnerability to rupture (6). Pro-in inflammatory to anti-inflammatory cyto-kine ratios can signal the balance between pro-inflammatory and anti-inflammatory forces (7). The aim of study was to determine the concentrations of pro-inflammatory cytokines IL-6, IL-1ß, TNF-α and hs-CRP, concentration of anti-inflammatory cytokine IL-10 and pro-inflammatory to anti-inflammatory cy-tokine ratios in group of patients with AMI and to compare them with the values obtained in patients with stable angina pectoris. We wanted to explore possible correlation between concentrations of pro-inflammatory cytokine IL-6 and anti-inflammatory cytokine IL-10 and IL-6 and lipoproteins in AMI.
MATERIALS AND METHODS
Patients
The investigation was conducted as a prospective study at Clinic of Internal Medicine in Tuzla. The study included 41 patients with AMI (admitted within 12h after the chest pain onset) and 30 patients with stable angina pectoris who were treated in our institution over the period 01.07.2007-01.11.2007. Myocardial infarction was diagnosed following the criteria: anamnesis of chest pain and/or ECG changes suggestive of infarction or ischemia with an increase in one or more cardiac enzymes levels (measured at the laboratory of our hospital) to at least twice the upper limit of the normal range. Angina pectoris was defined as typical chest pain brought on by exertion and relieved by nitro-glycerine or by rest without increase in cardiac enzymes and positive ergo-metric test. Criteria for exclusion were: active infection, systemic diseases (SLE, rheumatoid arthritis, scleroderma etc.), cancer, loss of consciousness, pregnancy and chronic terminal renal failure. Five patients were excluded on the basis of exclusion criteria (three patients had active urinary infection, the fourth patient had rheumatoid arthritis, the fifth one had pneumonia), so 36 patients with AMI and 30 patients with stable angina pectoris were analyzed. The study was approved by the local ethics committee, and all patients gave written informed consent to participate.
Laboratory analysis
The blood for the analysis was drawn from patients following their admission to Intensive Care Unit. The blood was frozen at -70°C, centrifuged and aliquated at the Department of Immunology and Microbiology in Tuzla. The concentrations of IL-1ß, IL-6, TNF-α, hs-CRP-a and IL-10 were analyzed using quantitative sandwich enzyme immunoassay (ELISA). Cytokine concentrations (IL-1ß, IL-6, TNF and IL-10) were measured from serum manually using high sensitive quantitative sandwich enzyme immunoassay (R&D Systems Inc., Minneapolis, Minnesota) Minimal detectible concentrations of human pro-inflammatory and anti-inflammatory cytokines were as follows: IL-1ß 1 pg/ml, IL-6 0,70 pg/ml, TNF-α 1,6 pg/ml and IL-10 3,9 pg/ml. The intra-assay and inter-assay precision of all the cytokine assays in the study was < 10%. Serum hs-CRP concentrations were measured at the same department using quantitative nephelometric assay (Dade-Beringh II, Max Inc., Germany) with normal values ranging from 0,0 to 3,0 mg/l. Haemogram, enzymes of myocardial necrosis (troponin I, creatine kinase, creatine kinase myo-cardial fraction), electrolytes concentrations, glucose, urea, creatinine, uric acid, lipid profile and other biochemical parameters were carried out by the analytical unit of the Biochemistry Department of our institution using standard methods.
Statistical analysis
SPSS V.15 software (SPSS Inc, Chicago, Illinois, USA), Arcus Quick Stat and Microsoft Excel XP Professional were used for statistical analysis of the results. Variables with asymmetric distribution were summarized as medians and interquartile ranges. Normality tests were used for all variables. In the comparison between patients with AMI and stable angina pectoris, continuous variables with normal distribution were analysed with two-tailed t test while unequally distributed variables were analysed using Mann-Whitney U test. Categorical data and proportions were analysed with χ2 or Fisher’s exact test where appropriate. The ratios of pro-inflammatory cytokine concentrations to anti-inflammatory cytokine IL-10 concentrations were calculated by dividing pro-inflammatory cyto-kine concentrations by IL-10 concentrations and the ratios were compared between the two groups. Regression and correlation analysis were performed, too. Significance threshold was set at two-sided P<0,05.
RESULTS
As we pointed out, 5 patients were excluded because of exclusion criteria, so the study included 36 patients with AMI and 30 patients with stable angina pectoris. Mean age in the study group was 56,26 ±10,62 comparing to 52,24 ± 8,15 in the control group, so the difference between the groups was not statistically significant. Considering sex distribution, there were 8 (22,22%) female patients in the study group and 5/30 (16,67%) females in the control group. λ2 test has not shown statistically significant difference in sex ratios between these two groups (P> 0,05). In study group mean value of total cholesterol was 5, 53 mmol/l, low density lipoprotein (LDL) 3,77 mmol/l and high density lipopro-tein (HDL)= 0,95 mmol/l. Only 16 of patients with AMI, 67% (6/36) were on prior statin therapy. We found statistically significant differences in IL-6 and hs-CRP levels between study and control groups with (P< 0, 0001) in both cases. TNF- α and IL-10 levels were elevated in the study group but the difference was not statistically significant. Table 1 summarize mean values, standard deviations and P values in both groups.
TABLE 1.
Concentrations of IL-6, IL-10, TNF-L, hs-CRP in the study and the control group
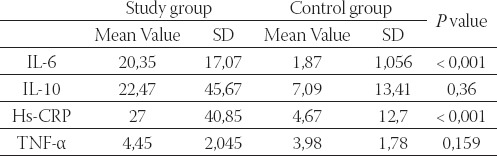
Study group - patients with acute myocardial infarction; control group - patients with stable angina pectoris; SD - standard deviation.
Minimal detectible concentration of IL-1ß in this study was 1 pg/ml. Only 2 patients in each group had detectible concentrations of IL- 1β, so these values were not statistically treated. The ratios of pro-inflammatory cytokine concentrations to anti-inflammatory cytokine IL-10 concentrations were calculated by dividing pro-inflammatory cytokine concentrations by IL-10 concentrations. Using Mann-Whitney U test we found statistically significant difference between IL-6: IL-10 values in study and control groups (P<0, 0001). IL-1: IL-10 ratio was not calculated for the same reason as IL-1. The values were not compared between the study and control group. Although mean TNF-æIL-10 ratio was higher in patients with AMI than in patients with stable angina pectoris the difference between the groups was not statistically significant (Mann-Whitney U test). Table 2. shows medians and interquartile ranges of pro-inflammatory to anti-inflammatory cytokine ratios in the study and the control group, as well as P value.
TABLE 2.
Pro-inflammatory to anti-inflammatory cytokine ratios in the study and the control group
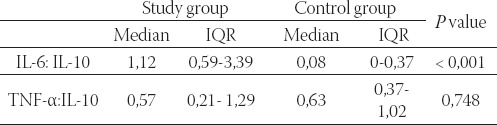
Study group - patients with acute myocardial infarction; control group - patients with stable angina pectoris; IQR - interquartile range. There was strong positive linear correlation between IL-6 and IL-10 in patients with AMI with coefficient of correlation r = 0,432691 and P<0.05 (P=0.015) (Figure 1).
FIGURE 1.
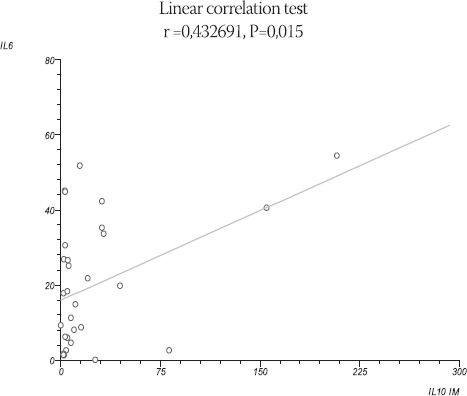
Linear correlation between IL-6 and IL-10 in AMI
We found negative linear correlation between IL-6 and HDL in patients with AMI (r= - 0,47, P= 0,008) with P<0,05. (Figure 2).
FIGURE 2.
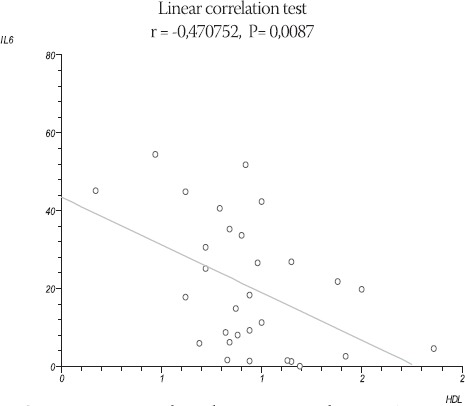
Linear correlation between ΙΙΛ5 and HDL in AMI
DISCUSSION
Coronary atherosclerotic plaque disruption with consequent thrombosis is the major cause of ACS (1, 7,8). Inflammatory mediators, which potentially have a major role in this process, are the “molecular soldiers” of the oxidative modifiers of lipoproteins in vulnerable plaque that have been described as “molecular Trojan horses” (9,10). Previous studies reported increased pro-inflammatory cytokine concentrations and decreased anti-inflammatory cytokine concentrations in patients with ACS (10,11). However, the role of anti-inflammatory cytokines remains unclear and this was in the focus of interest of our investigation. The level of IL-6 is higher in patients with AMI than in patients with stable angina pectoris, and the difference between these two groups in our study is statistically significant (P< 0,001). Few other authors proved the same in their investigations (12,13). The level of TNF-α is higher in patients with AMI than in patients with stable angina pectoris, but the difference between these two groups in our study is not statistically significant (P> 0.05). Other studies reported various TNF-α levels. Some authors found elevated TNF-α in patient with ACS (14,15). However, it has been postulated that the short plasma half life (6-20 min after intravenous injection) of TNF-α may limit its potential clinical utility as a screening tool (7, 11, 16, and 17). The delay between obtaining and studying the samples may have been too long for prognostic usefulness as mentioned in the other TNF-α studies and this may be logical explanation for different reported TNF-α levels (7, 11, and 17). Kilic et al. (10) have reported elevated plasma level of IL-1ß and in that study minimal detectible concentration of IL-1ß was 0,1 pg/ml. Mean value of IL-1 β in patients with acute coronary syndrome without ST elevation (NSTACS) in their study was 0,83 pg/ml. As we have pointed out minimal detectible concentration of IL-1 β in our study was 1 pg/ml and this might be a reason for such result. Concentration of hs-CRP is elevated in first 24 hours of AMI (13, 17-20). We found elevated level of hs-CRP in group of patients with AMI. The difference between the levels of hs-CRP in the study and the control group was statistically significant (P< 0,001).
Few studies reported increased IL-10 in ACS (17,18). On the other hand, there are few investigations that found decreased level of IL-10 in AMI (12,19,20). In these studies low level of IL-10 in AMI is poor prognostic sign. According to the authors, IL-10 is induced in the reperfused myocardium and may modulate the reaction to injury by down-regulating concentrations of pro-inflammatory cytokines TNF-α and IL-6. In isch-emic and reperfused myocardium induction of IL-10 inhibits mRNA for IL-6, but in ischemic myocardium without reperfusion expression of IL-6 is prolonged (21). In other words, increased levels of IL-10 suggest good reperfusion, while low of IL-10 level means ischemic but not reperfused myocardium and is accompanied with increased level of IL-6. We have to mention study by Yip et al. (22) who found that high serum IL-10 level, together with low left ventricular ejection fraction (LVEF), high WBC and unsuccessful reperfusion was suprisely a good prognostic marker independently predictive of increased 30-day mortality.
Similar observations were made by Mälarstig et al. (23) who concluded that elevated IL-10 was an indicator of poor outcome and enhanced systemic inflammation in patients with acute coronary syndrome. As we mentioned earlier, concentration of IL-10 in our study was increased in the group of patients with AMI but the difference was not statistically significant. Further studies have to be done to clear the role of IL-10 level in the prognosis of patients with acute myocardial infarction. In recent years, a new concept emerged of an imbalance between pro-inflammatory and anti-inflammatory factors, in favour of the pro-inflammatory factors, that results in rupture of atherosclerotic plaque (16,20). We found statistically significant difference only in IL-6: IL-10 ratio (P<0,001). Speaking precisely, this ratio was higher in AMI than in stable angina pectoris in our study. According to several authors, IL-6:IL-10 ratio is the most powerful predictor of the development of new coronary event during both the first six months and the one-year follow-up period (7). For the statistical reasons we could not calculate IL-1: IL-10 ratio, so we could not consider its importance in acute coronary syndrome. As we have mentioned earlier, the role of anti-inflammatory cytokines remained unclear despite recent studies and we made it the focus of interest of our investigation. Furthermore, the prognostic value of interleukin IL-10 in patients with acute AMI is also currently unclear. We especially wanted to find out if there was linear correlation between IL-6 and IL-10. Tziakas et al. (24) found positive linear correlation between IL-6 and IL-10. The results of our investigation are concurrent with that finding. Ischemic and reperfused myocardium down-regulates concentration of IL-6 by IL-10 via inhibition of mRNA for IL-6, but in ischemic myocardium without reperfusion expression of IL-6 is prolonged (21). For this reason IL-10 might be useful as marker of myocardial reperfusion and it could be its important clinical role. Analysis of IL-6 and IL-10 correlation in this study suggests more ischemic than re-perfused myocardium. However, further studies have to be done to confirm this hypothesis, but our findings, as well as similar findings by Tziakas et al., are strongly suggestive of IL-10 as marker of myocardial reperfusion. Furthermore, we investigated correlation between li-poproteins and IL-6 in AMI. We found no statistically significant correlation between IL-6 and LDL, but we found strong negative linear correlation between HDL and IL-6 in patients with acute myocardial infarction. This data is interesting since few studies examine correlation between lipoproteins and cytokines in AMI and the available results and conclusions are different. Gomaraschi et al. (25) in their study found that high-density lipoproteins attenuate interleukin-6 production in endothelial cells exposed to pro-inflammatory stimuli and according to them HDL regulates IL-6 concentration via inhibition of mRNA transcription for IL-6 (inhibition of p38 MAP kinas). By inhibiting IL-6 production and lowering plasma IL-6 concentration, HDL may limit pro-atherogenic effects in both acute and chronic inflammatory conditions, of which IL-6 is a key orchestrator (25). Protective role of HDL in AMI is well known in literature, but molecular mechanisms are not explained either. This and similar investigations reiterate the protective role of HDL in AMI.
CONCLUSION
Concentration of pro-inflammatory cytokines (IL-6, TNF-α and hs-CRP) and anti-inflammatory cytokine IL-10 were elevated in AMI patients in comparison with the group with stable angina pectoris, but statistically significant difference was found in IL-6 and hs-CRP level. IL-6: IL-10 ratio is higher in AMI which emphasis the hypothesis of pro-inflammatory to anti-inflammatory cytokine misbalance in AMI. IL-10 may be useful as a marker of myocardial reperfusion in AMI. There is positive linear correlation between IL-6 and IL-10 in acute myocardial infarction and negative linear correlation between HDL and IL-6 and these data emphasise protective role of HDL in AMI.
Limitations of the study
Our study was not a multicentre study and the number of patients in the study was low. As we mentioned above, minimal detectible concentration of IL-1ß was high (1 pg/ml).
Conflicts of interests: none declared List of Abbreviations
hs-CRP - high sensitive-C reactive protein
IL - interleukin
TNF - Tumour necrosis factor
AMI - acute myocardial infarction
HDL - high density lipoprotein
LDL - low density lipoprotein
Acknowledgments
The author wishes to express the gratitude to all the physicians and nurses at Department of Cardiology and Intensive Care Unit, as well as all the employees of Department of Immunology in our hospital.
REFERENCES
- 1.Fuster V, Badimon L, Badimon J, et al. The pathogenesis of coronary artery disease and the acute coronary syndromes. N. Engl. J.Med. 1992;326:242–250. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- 2.Davies M.J, Woolf N, Rowles P, et al. Morphology of the en-dothelium over the atherosclerotic plaques in human coronary arteries. Br. Heart J. 1988;60:459–464. doi: 10.1136/hrt.60.6.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies M.J. Pathophysiology of acute coronary syndromes. Heart. 2000;83:361–366. doi: 10.1136/heart.83.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulvihill N.T, Foley J.B. Inflammation in acute coronary syndromes. Heart. 2002;87:201–204. doi: 10.1136/heart.87.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torzewski M, Rist C, Mortensen R.F, et al. C-reactive protein in the arterial intima: role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2000;20:2094–2099. doi: 10.1161/01.atv.20.9.2094. [DOI] [PubMed] [Google Scholar]
- 6.Kilic T, Ural D, Ural E, et al. Relation between proinflammatory to anti-inflammatory cytokine ratios and long-term prognosis in patients with non-ST elevation acute coronary syndrome. Heart. 2006;92:1041–1046. doi: 10.1136/hrt.2005.080382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corti R, Fuster V, Badimon J.J. Pathogenetic concepts of acute coronary syndromes. J. Am. Coll. Cardiol. 2003;41:7S–14S. doi: 10.1016/s0735-1097(02)02833-4. [DOI] [PubMed] [Google Scholar]
- 8.Hajjar D.P, Haberland M.E. Lipoprotein trafficking in vascular cells: molecular Trojan horses and cellular saboteurs. J. Biol. Chem. 1997;272:22975–22978. doi: 10.1074/jbc.272.37.22975. [DOI] [PubMed] [Google Scholar]
- 9.Paoletti R, Gotto A.M, Jr, Hajjar D.P. Inflammation in atherosclerosis and implications for therapy. Circulation. 2004;109(23 Suppl 1):III20–26. doi: 10.1161/01.CIR.0000131514.71167.2e. [DOI] [PubMed] [Google Scholar]
- 10.Simon A.D, Yazdani S, Wang W, et al. Circulating levels of IL-1beta, a prothrombotic cytokine, are elevated in unstable angina versus stable angina. J. Thromb. Thrombolysis. 2000;9:217–222. doi: 10.1023/a:1018758409934. [DOI] [PubMed] [Google Scholar]
- 11.Smith D.A, Irving S.D, Sheldon E, et al. Serum levels of the an-tiinflammatory cytokine interleukin-10 are decreased in patients with unstable angina. Circulation. 2001;104:746–749. doi: 10.1161/hc3201.094973. [DOI] [PubMed] [Google Scholar]
- 12.Biasucci L.M, Vitelli A, Liuzzo G. Elevated levels of interleukin-6 in unstable angina. Circulation. 1996;94:874–877. doi: 10.1161/01.cir.94.5.874. [DOI] [PubMed] [Google Scholar]
- 13.Dybdahl B, Slordahl S.A, Waage A, et al. Myocardial ischemia and the inflammatory response: release of heat shock protein 70 after myocardial infarction. Heart. 2005;91:299–304. doi: 10.1136/hrt.2003.028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotajima N, Kimura T, Tsugiyasu K, et al. Reciprocal increase of circulating interleukin-10 and interleukin-6 in patients with acute myocardial infarction. Heart. 2001;86:704–705. doi: 10.1136/heart.86.6.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li D, Zhao D. Kinetics of tumor necrosis factor α in plasma and the cardioprotective effects of a monoclonal antibody to tumour necrosis factor α in acute myocardial infarction. Am. Heart. J. 1999;137:1145–1152. doi: 10.1016/s0002-8703(99)70375-3. [DOI] [PubMed] [Google Scholar]
- 16.Waehre T, Halvorsen B, Damas J.K. Inflammatory imbalance between IL-10 and TNF alpha in unstable angina potential plaque stabilizing effects of IL-10. Eur. J. Clin. Invest. 2002;32:803–810. doi: 10.1046/j.1365-2362.2002.01069.x. [DOI] [PubMed] [Google Scholar]
- 17.Blake G.J, Ridker P.M. Novel clinical markers for vascular wall inflammation. Circ. Res. 2001;89:763–771. doi: 10.1161/hh2101.099270. [DOI] [PubMed] [Google Scholar]
- 18.Mizia-Stec K, Gasior Z, Zahorska-Markiewicz B, et al. Serum tumour necrosis factor alpha, interleukin-2 and interleukin-10 activation in stable angina and acute coronary syndromes. Coronary artery disease. 2003;14(6):431–438. doi: 10.1097/00019501-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Shibata M, Endo S, Inada K. Elevated plasma levels of interleu-kin-1 receptor antagonist and interleukin-10 in patients with acute myocardial infarction. J. Interferon Cytokine Res. 1997;17:145–150. doi: 10.1089/jir.1997.17.145. [DOI] [PubMed] [Google Scholar]
- 20.Alam S.E, Nasser S.S, Fernainy K.E. Cytokine imbalance in acute coronary syndrome. Curr. Opin. Pharmacol. 2004;4:166–170. doi: 10.1016/j.coph.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Frangogiannis N.G, Mendoza L.H, Lindsey M.L. IL-10 is induced in the reperfused myocardium and may modulate the reaction to injury. J. Immunol. 2000;165:2798–2808. doi: 10.4049/jimmunol.165.5.2798. [DOI] [PubMed] [Google Scholar]
- 22.Yip H.K, Youseff A.A, Chang L.T, et al. Association of Inter-leukin-10 Level with increased 30-day mortality in patients with ST-segment elevation acute myocardial infarction undergoing primary coronary intervention. Circ. J. 2007;71:1086–1091. doi: 10.1253/circj.71.1086. [DOI] [PubMed] [Google Scholar]
- 23.Mälarstig A, Eriksson P, Hamsten A, et al. Raised interleukin-10 is an indicator of poor outcome and enhanced systemic inflammation in patients with acute coronary syndrome. Heart. 2008;94:724–729. doi: 10.1136/hrt.2007.119271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tziakas N.D, Chalikiasa G.K, Hatzinikolaoua H.I, et al. Anti-inflammatory cytokine profile in acute coronary syndromes: behaviour of interleukin-10 in association with serum metallopro-teinases and proinflammatory cytokines. Int. J. Cardiol. 2004;92:169–175. doi: 10.1016/s0167-5273(03)00084-6. [DOI] [PubMed] [Google Scholar]
- 25.Gomarasci M, Basilico N, Sisto F, et al. High-density lipopro-teins attenuate interleukin-6 production in endothelial cells exposed to pro-inflammatory stimuli. Biochim. Biophys. Acta. 2005;1736(2):136–143. doi: 10.1016/j.bbalip.2005.08.003. [DOI] [PubMed] [Google Scholar]


