Abstract
A rat HIRI model was constructed and treated with an intraperitoneal injection of agomir-miR-494 or agomir-NC (negative control) for 7 days after the surgery. The pathophysiological changes in sham-operated rats, HIRI, HIRI + agomir-miR-494, and HIRI + agomir-NC were compared. The effect of miR-494 was also assessed in an H2O2-induced apoptosis model. Hepatic AML12 cells were transfected with mimics NC or miR-494 mimics, followed by 6-h H2O2 treatment. Cell proliferation and apoptosis were detected by CCK8 assay and flow cytometry, respectively. Further, the miR-494 target gene was identified by luciferase reporter assay, and verified both in vitro and in vivo experiments. The activity of AKT pathway was further analyzed in vivo by Western blot. HIRI + agomir-miR-494 rats exhibited significantly higher miR-494 expression, lower serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and glutamate dehydrogenase (GLDH) level, lower hepatic MDA, TOA, and OSI, alleviated hepatic necrosis, reduced hepatocyte apoptosis, and decreased expression of apoptosis-related proteins, when compared with HIRI + agomir-NC rats (P<0.05 or 0.01). After H2O2 treatment, AML-12 cells transfected with miR-494 mimics had significantly higher proliferation and lower apoptosis rate compared with mimics NC group (P<0.01). PTEN was identified as an miR-494 target gene. PTEN expression was significantly down-regulated in AML12 cells transfected with miR-494 mimics, and was up-regulated by treatment of miR-494 inhibitor (P<0.01). Moreover, HIRI + agomir-miR-494 rats exhibited significantly lower PTEN expression, and higher p-AKT, p-mTOR, and p-p70S6K levels compared with HIRI + agomir-NC rats. Therefore, miR-494 protected rats against hepatic ischemia/reperfusion (I/R) injury through down-regulating its downstream target gene PTEN, leading to the activation of PI3K/AKT signaling pathway.
Keywords: apoptosis, AML12 cells, hepatic ischemia/reperfusion injury, MicroRNA-494, PI3K/Akt
Introduction
Hepatic ischemia/reperfusion (I/R) injury is an inevitable complication occurring during surgical procedures such as partial hepatectomy and liver transplantation [1,2]. During hepatic I/R injury, high levels of reactive oxygen species (ROS) are produced, leading to acute inflammatory responses and hepatocyte apoptosis, which ultimately result in liver dysfunction and even liver failure [3–8].
miRNAs are a class of small non-coding RNAs (21–25 nts) that suppress gene expression by binding to the 3′-UTR of the target mRNA [9], and therefore are associated with a variety of biological processes, such as cell differentiation, proliferation and apoptosis, as well as the occurrence and development of diseases [10]. Several studies have suggested the important roles of miRNAs in I/R injury, such as miR-122, miR-124, miR-146a, miR-223, miR-370 [11–15]. Differentially expressed miR-494 has been reported in rats with cerebral I/R injury [16]. miR-494 alleviates the I/R-induced myocardium injury in a mouse model through activating the AKT signaling pathway [17]. Overexpression of miR-494 exerts protective effects against hypoxia/ischemia-induced apoptosis in human hepatic L02 cells via the modulation of PI3K/Akt pathway [18]. However, the potential in vivo role of miR-494 in hepatic I/R injury remains unknown. Therefore, the current study was undertaken to investigate the effect and relevant molecular mechanism of miR-494 in response to I/R-induced hepatic injury in a rat model. We further validated our findings in hepatic AML12 cells treated with H2O2-induced stress. Our study may provide new insights into the development of novel therapeutic strategies for hepatic I/R injury.
Materials and methods
Construction of a rat HI/RI model
Male Sprague–Dawley (SD) rats weighing 180–220 g were purchased from the Slac Laboratory Animals (Shanghai, China) and housed in standard conditions with free access to water and food before this experiment. SD rats were randomly divided into sham, HIRI, HIRI + agomir-NC (negative control), and HIRI + agomir-miR-494 groups (n=6 in each group). Rats in HIRI group were anesthetized by intraperitoneal injection of 3 ml/kg chloral hydrate. Partial (70%) hepatic ischemia was introduced by clamping the artery and portal venous blood supply to the middle and left liver lobes with atraumatic vascular clamps. After 60-min ischemia, the clamp was removed and the liver was reperfused for 6 h. The sham group underwent abdominal surgery without liver I/R. Rats in HIRI + agomir-miR-494 were given an intraperitoneal injection of 20 μl of 500 pmol agomir-miR-494 (GenePharma Biotech, Shanghai, China)/day for 7 days prior to ischemia. Rats in HIRI + agomir-NC were given equal amount of agomir-NC. Rats were immediately killed after the surgery, and blood and left liver samples were collected. The present study was approved by the Research Ethics Committee and performed in strict accordance with the institutional animal care instructions.
Liver enzyme assay
Indexes of liver injury including serum activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and glutamate dehydrogenase (GLDH) were determined using commercial kits (Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer’s instructions.
Quantitative reverse-transcription PCR
Liver tissue was homogenized on ice. Total RNA was extracted using the TRIzol reagent (Invitrogen, Shanghai, China), and reverse-transcribed into cDNA using SuperScript II (Invitrogen). Real-time PCR was then performed using SYBR Green PCR Master Mix (TaKaRa, Qingdao, China) in an ABI 7500 System (Applied Biosystems, Foster City, CA, U.S.A.). The following primers were synthesized by Invitrogen and used in the PCR: miR-494-F, 5′-TGGTGATGGGATTTGAAACATACACGGGAAAC-3′, miR-494-R, 5′-AGATAGACGG-TGTCGCTGTTGAAGTCAG-3′; U6-F, 5′-GCTTCGGCAGCACATATACTAAAAT-3′, U6-R, 5′-CGCTTCACGAATTTGCGTGTCAT-3′; PTEN-F, 5′-TGGAAAGGGACGAACTGGTG-3′, PTEN-R, 5′-CATAGCGCCTCTGACTGGGA-3′. The experiment was repeated three times. Data were analyzed using the 2−ΔCt method. The relative expression of miR-494 was calculated using small nuclear RNA U6 (snU6) as the internal control.
Histological examination
Liver samples were fixed in 4% buffered formalin, dehydrated in graded ethanol (70, 80, 90, and 100%), embedded in paraffin, and cut into 4-μm sections. Tissue sections were then dewaxed with xylene, rehydrated in graded ethanol (100, 90, 80, and 70%), and stained with Hematoxylin and Eosin (HE). The pathological changes including inflammatory infiltration and hepatic cell necrosis in five randomly selected visual fields was observed.
TUNEL apoptosis assay
Paraffin sections were dewaxed and hepatic cell apoptosis was detected by terminal deoxynucleotidyl transferase mediated dUTP nick-end labeling (TUNEL) assay using the TUNEL assay kit (Roche, Shanghai, China) according to the manufacturer’s protocol. Nuclei were labeled by DAPI staining (Biohao Biotech, Shanghai, China). Sections were then observed under a confocal laser microscope (Olympus, Japan). The percentage of apoptotic cells was calculated by counting TUNEL-positive cells in five randomly selected fields on each slide.
Western blot
Total protein was extracted from harvested liver tissues or cells using lysis buffer (Beyotime, Shanghai, China) and quantitated using BCA Protein Assay Kit (Beyotime) following the manufacturer’s instructions. Protein samples were then separated by 10% SDS/PAGE and transferred on to PVDF membranes (GE Healthcare, Amersham, U.K.). Membranes were blocked with 5% skim milk and incubated with appropriate dilutions of primary antibodies overnight at 4°C as follows: rabbit anti-rat Bax, cleaved caspase-3, cleaved PARP, PTEN, AKT, p-AKT, mTOR, p-mTOR, p70S6K, p-p70S6K, and β-actin. Membranes were then washed with PBST and incubated with goat anti-rabbit HRP–conjugated IgG at room temperature for 1 h. Immunoreactivity was detected using the ECL detection system (GE Healthcare). All antibodies were purchased from Abcam (Cambridge, MA, U.S.A.) or Cell Signaling Technology (Danvers, MA, U.S.A.). β-actin was used as the internal control.
DNA fragmentation assay
The cytoplasmic histone-associated DNA fragmentation in different groups was determined using an ELISA Kit (Roche, Indianapolis, IN, U.S.A.). The experiment was performed in triplicate.
Prediction of miR-494 targets
Both PicTar (http://pictar.mdc-berlin.de) and TargetScan version 6.2 (http://www.target-scanorg/index.html) were used to identify potential downstream targets of miR-494. Genes that were predicted by both databases were considered as potential targets. PTEN, one of the potential targets was selected for further analysis.
Luciferase reporter assay
The PTEN gene was analyzed using the online tool (http://www.targetscan.org) to predict the 3′-UTR sequence for miR-494. The oligonucleotide sequences (3′-UTR of PTEN wild and mutated type) were cloned into the site of a firefly luciferase reporter vector pMIR (Promega, WI, U.S.A.). HEK293T cells at 70% confluence were cotransfected with 500 ng of pMIR-PTEN-wt/pMIR-PTEN-mut and 50 nM of miR-494 mimics/mimics C,G(enePharma Biotech) using a Lipofectamine 3000 transfection kit (Invitrogen). After 24 h, the luciferase activity was measured using a dual luciferase reporter assay system (Promega) following the manufacturer’s instructions. The relative luciferase activity (RLA) was calculated as the ratio of firefly luciferase activity (Ff) to Renilla luciferase activity (Rn).
Cell culture and transfection
Murine hepatic cell line AML12 cells (ATCC, Manassas, VA, U.S.A.) were cultured in DMEM/Ham’s F12 medium supplemented with 10% FBS, 100 u/ml penicillin, 100 μg/ml streptomycin, 5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml selenium, and 40 ng/ml dexamethasone. AML12 cells at 70% confluence in six-well plates were transfected with 50 nM of miR-494 mimics, miR-494 inhibitor, mimics NC, or inhibitor NC (GenePharma Biotech) using Lipofectamine 3000 transfection agent. After 48 h, total RNA and protein were extracted, and PTEN mRNA and protein expression was analyzed by quantitative reverse-transcription PCR (qRT-PCR) and Western blot as described above.
H2O2-induced oxidative stress
AML12 cells at 70% confluence were treated with H2O2 (0, 25, 50, 100, 200, 300, and 400 μM) for 6 h. Cells were collected and miR-494 level was measured by qRT-PCR as described above.
CCK8 detection of cell viability
AML12 cells at 70% confluence were transfected with 50 nM of miR-494 mimics or mimics NC. At 24, 36, and 48 h after transfection, cells were treated with 200 μM H2O2 for 6 h. CCK8 (Beyotime, 10 μl) was added to the cells, and the viability of the cells was measured at 450 nm using a microplate reader.
Annexin V-FITC apoptosis detection
Transfected AML12 cells were treated with 200 μM H2O2 for 6 h. Cells were then collected and resuspended in 500 μl of binding buffer. Annexin V-FITC (5 μl) was added and cell apoptosis was detected by flow cytometry within 30 min.
Detection of apoptosis-related proteins
Transfected AML12 cells were treated with 200 μM H2O2 for 6 h. The expression of cle-PARP, cle-caspase-3, AKT, and p-AKT was determined by Western blot as described above.
Immunohistochemical analysis of apoptosis in liver tissue
Paraffin-embedded sections were dewaxed with xylene, and dehydrated with ethanol. Slices were processed to remove endogenous peroxidase, followed by antigen retrieval and serum blocking. The sections were incubated successively with rabbit anti-rat cleaved caspase-3, biotin-labeled secondary IgG, and horseradish peroxidase labeled streptavidin. Yellow or brown stained cells were positive for cleaved caspase-3.
Detection of AKT signaling activity
Liver tissues collected from sham, HIRI, HIRI + agomir-NC, and HIRI + agomir-miR-494 groups (n=6) was homogenized. The expression of PTEN, AKT, p-AKT, mTOR, p-mTOR, p70S6K, and p-p70S6K were analyzed by Western blot analysis as described above.
Statistical analysis
All experiments were performed in triplicate. Data were expressed as mean ± S.D. All statistical analyses were performed using SPSS 16.0 software (IBM SPSS, Chicago, IL, U.S.A.). Differences between two groups were determined by the Student’s t test. Differences amongst groups were compared using one-way ANOVA followed by post-hoc Tukey HSD test. P<0.05 was considered as statistically significant.
Results
I/R led to injury of rat liver through induction of oxidative stress and apoptosis
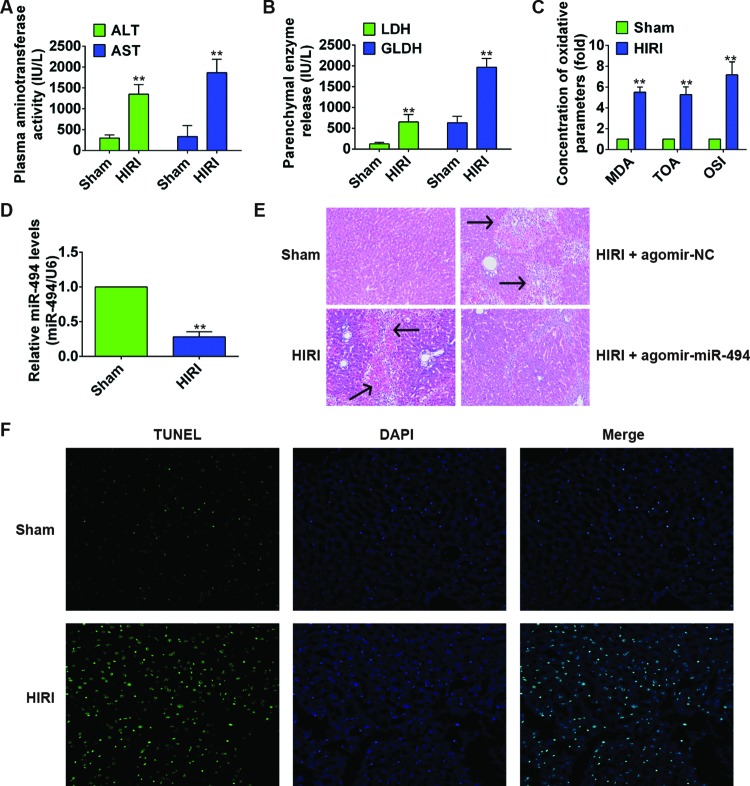
To investigate the potential role of miR-494 in liver I/R injury, we constructed a rat HIRI model. As shown in Figure 1A,B, after the liver I/R surgery, serum level of liver injury indexes including ALT, AST, LDH, and GLDH enzymes was significantly increased compared with sham surgery group (all P<0.01), revealing that I/R induced liver injuries. Moreover, the concentration of oxidative parameters MDA, TOA, and OSI was markedly increased in HIRI group (Figure 1C). HE staining revealed loss of cell integrity, large areas of hepatocyte necrosis, and inflammatory infiltration in the livers in HIRI group (Figure 1E). We further evaluated the extent of apoptosis in livers by TUNEL assay, and found that the number of TUNEL-positive cells in HIRI group was notably higher compared with sham surgery group (Figure 1F). These pathophysiological changes suggested the successful construction of HIRI model in the present study. We then examined the expression levels of miR-494. As shown in Figure 1D, miR-494 in HIRI group was significantly down-regulated at least two fold when compared with sham surgery group (P=0.007).
Figure 1. miR-494 protected against liver I/R injury in SD rats.
SD rats (n=6 rats per group) were sham-operated or subjected to 60-min liver ischemia followed by 6-h reperfusion. HIRI rats were treated with agomir-miR-494 or agomir-NC. (A,B) Serum level of ALT and AST, LDH, and GLDH was determined using commercial kits. (C) Concentration of liver oxidative parameters MDA, TOA, and OSI. (D) Relative miR-494 level in liver samples from different groups was measured by qRT-PCR. miR-494 expression was normalized to U6. (E) HE staining of liver samples. Arrow indicates hepatocyte injury. (F) TUNEL staining of hepatocellular apoptosis. All experiments were performed in triplicate, and data were represented as mean ± S.D. *, P<0.05 and **, P<0.01 compared with sham surgery group.
miR-494 attenuated hepatic I/R injury in rats
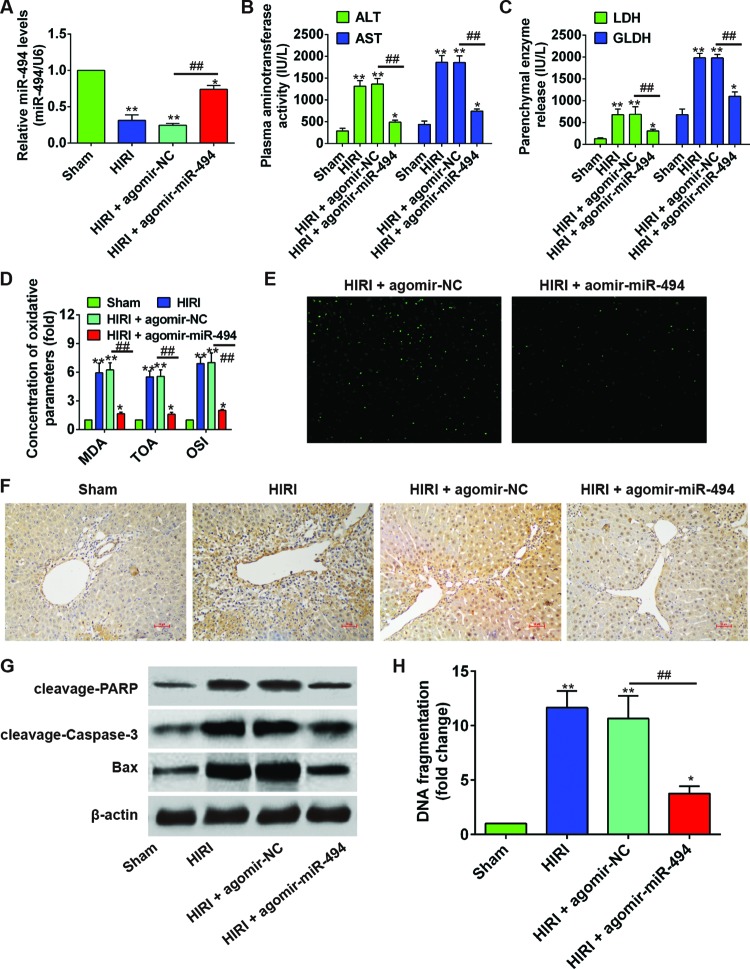
In order to analyze the regulatory role of miR-494 in I/R injury, we treated the HIRI rats with an intraperitoneal injection of agomir-miR-494 or agomir-NC. As shown in Figure 2A, although the liver miR-494 concentration in HIRI + agomir-miR-494 group was significantly lower than that in sham surgery group (P=0.046), it was substantially increased compared with HIRI + agomir-NC (P=0.004). Serum ALT, AST, LDH, and GLDH levels in HIRI + agomir-miR-494 group were significantly decreased, as compared with HIRI + agomir-NC group (all P<0.01, Figure 2B,C). Likewise, oxidative parameters of the liver including MDA, TOA, and OSI was significantly reduced compared with agomir-NC treated group (all P<0.01, Figure 2D). Moreover, liver histology from HIRI + agomir-miR-494 rats revealed obvious reduction in I/R-induced hepatocellular necrosis and improvement of cell integrity (Figure 1E). IHF demonstrated a markedly lower number of TUNEL-positive cells in HIRI + agomir-miR-494 group (Figure 2E). Immunohistochemical (IHC) results demonstrated a substantially higher expression of cleaved caspase-3 in HIRI and HIRI + agomir-NC groups compared with sham group (Figure 2F). In contrast, the cleaved caspase-3 expression in HIRI + agomir-miR-494 group was only slightly higher compared with sham group. Consistently, the hepatic levels of cleavage PARP, cleavage Caspase-3, and Bax in HIRI + agomir-miR-494 group was clearly lower compared with agomir-NC group (Figure 2G). DNA fragment was significantly reduced in agomir-miR-494 treated rats (P=0.001, Figure 2H). All together, these results suggested that miR-494 effectively alleviated the I/R injuries in rat liver via inhibiting the cell apoptosis.
Figure 2. miR-494 protected against liver I/R injury in SD rats.
SD rats (n=6 rats per group) were sham operated or subjected to 60-min liver ischemia followed by 6-h reperfusion. HIRI rats were treated with agomir-miR-494 or agomir-NC. (A) Relative miR-494 level in liver samples from difference groups was measured by qRT-PCR. miR-494 expression was normalized to U6. (B,C) Serum level of ALT, AST, LDH, and GLDH was determined using commercial kits. (D) Concentration of liver oxidative parameters MDA, TOA, and OSI. (E) TUNEL staining of hepatocellular apoptosis. (F) IHC detection of cleaved caspase-3 in different groups. (G) Hepatic expression of apoptosis-related proteins cleavage PARP, cleavage Caspase-3, Bax were determined by Western blot using β-actin as an internal control. (H) DNA fragmentation assay was performed. All experiments were performed in triplicate, and data were represented as mean ± S.D. *, P<0.05 and **, P<0.01 compared with sham surgery group; ##, P<0.01 compared with agomir-NC group.
miR-494 decreased H2O2-induced apoptosis of hepatic AML12 cells by activating AKT
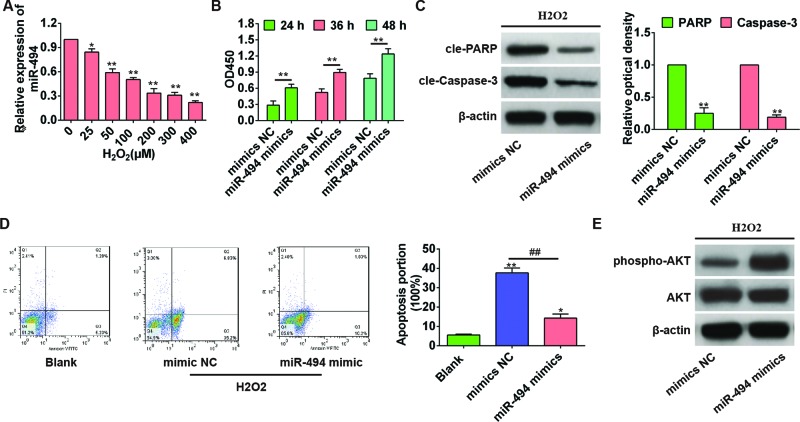
In order to simulate hepatic I/R injury, we further established an in vitro H2O2-induced apoptosis model. AML-12 cells were treated with H2O2 (0–400 µM) for 6 h, and miR-494 mRNA expression was decreased in a dose-dependent manner when compared with control cells without H2O2 treatment (all P<0.05 or 0.01, Figure 3A). Further, AML-12 cells were transfected with miR-494 mimics or mimics NC, and treated with 200 µM H2O2 for 6 h at all indicated time points. CCK8 assay showed that miR-494 mimics significantly improved cell viability compared with mimics NC group (all P<0.01, Figure 3B). As shown in Figure 3C,D, the apoptosis rate of miR-494 mimics was significantly lower than that in mimics NC group (P=0.002), and the expression of apoptosis-related proteins cle-PARP and cle-Caspase-3 was also significantly reduced (P=0.014 and 0.038, respectively), suggesting that miR-494 decreased H2O2-induced apoptosis of hepatic AML12 cells. To elucidate the potential mechanism, we further examined the intracellular level of activated AKT, and found that p-AKT expression in miR-494 mimics was obviously higher compared with mimics NC group (Figure 3E), indicating that the protective effect of miR-494 against H2O2-induced cell apoptosis was mediated through the activation of AKT.
Figure 3. miR-494 reduced the H2O2-induced apoptosis of hepatic AML12 cells by activating AKT in vitro.
(A) Hepatic AML12 cells were treated with H2O2 (0–400 µM) for 6 h, and the level of miR-494 was evaluated by qRT-PCR using U6 as an internal control. *, P<0.05 and **, P<0.01 compared with control cells without H2O2 treatment. (B) AML12 hepatocytes were transfected with mimics NC or miR-494 mimics for 24, 36, and 48 h, and then treated with 200 µM H2O2. Cell viability was measured by CCK8 assay. **, P<0.01 compared with mimics NC group. (C) Apoptosis-associated protein was detected by Western blot. **, P<0.01 compared with mimics NC group. (D) Cell apoptosis rate was detected by flow cytometry (annexin V-FITC staining). *, P<0.05 and **, P<0.01 compared with blank control group. ##, P<0.01 compared with mimics NC group. (E) The activation of AKT was assessed by Western blot. All experiments were performed in triplicate, and data were represented as mean ± S.D.
PTEN is a downstream target gene of miR-494
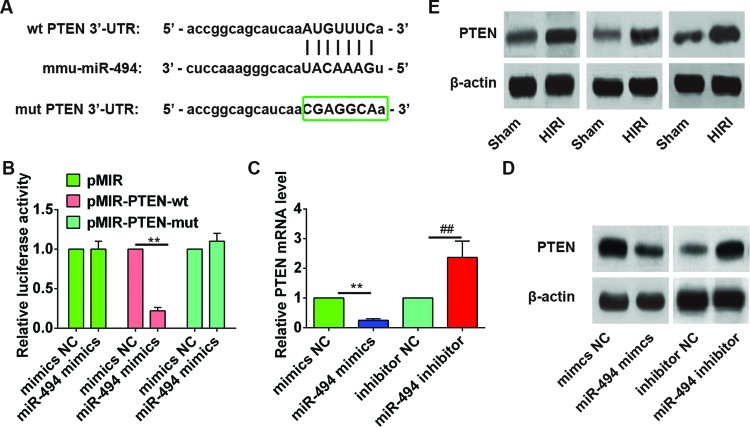
To identify the downstream target gene of miR-494, bioinformatics analyses (PicTar and TargetScan) were performed. PTEN was identified as one of the candidate targets of miR-494 (Figure 4A). To validate this prediction, the 3′-UTR region of PTEN was then cloned into a luciferase system. It was found that miR-494 mimics significantly suppressed the luciferase activity of the 3′-UTR wild-type of PTEN (P=0.006), but not that of the pMIR-PTEN-mut or pMIR group, when compared with the mimics NC group (Figure 4B). Further, the expression of PTEN mRNA in AML12 cells treated with miR-494 mimics or miR-494 inhibitor was compared by qRT-PCR. As shown in Figure 4C, PTEN mRNA expression was significantly reduced by miR-494 mimics (P=0.008) and enhanced by miR-494 inhibitor (P=0.000). Consistently, PTEN protein expression was also obviously down-regulated after miR-494 mimics transfection and up-regulated after miR-494 inhibitor transfection (Figure 4D). Moreover, Western blot revealed that the HIRI rats with lower hepatic miR-494 expression had obviously higher PTEN level in the liver when compared with sham-operated rats, indicating a negative association between miR-494 level and PTEN expression (Figure 4E). All these findings confirmed PTEN as the downstream target gene of miR-494.
Figure 4. PTEN is a downstream target gene of miR-494.
(A) miR-494-binding sequences in the 3′-UTR of PTEN and mutated sequence of PTEN 3′-UTR. (B) AML12 hepatocytes were cotransfected with PTEN 3′-UTR luciferase constructs (pMIR, pMIR-PTEN-wt or pMIR-PTEN-mut) and mimics NC or miR-494 mimics for 48 h. Cells were then collected and luciferase activity was determined. The level of firefly luciferase activity was normalized to Renilla luciferase activity. miR-494 suppressed the luciferase activities of the construct containing the 3′-UTR of PTEN. **, P<0.01 compared with mimics NC. (C,D) AML12 cells were transfected with mimics NC, miR-494 mimics, inhibitor NC, and miR-494 inhibitor for 48 h. The expression of PTEN mRNA and protein was detected by qRT-PCR and Western blot, respectively. All experiments were performed in triplicate, and data were represented as mean ± S.D. **, P<0.01 compared with mimics NC. ##, P<0.01 compared with mimics NC group. (E) Representative images of Western blot analysis comparing PTEN expression in liver samples in sham surgery and HIRI groups (n=6).
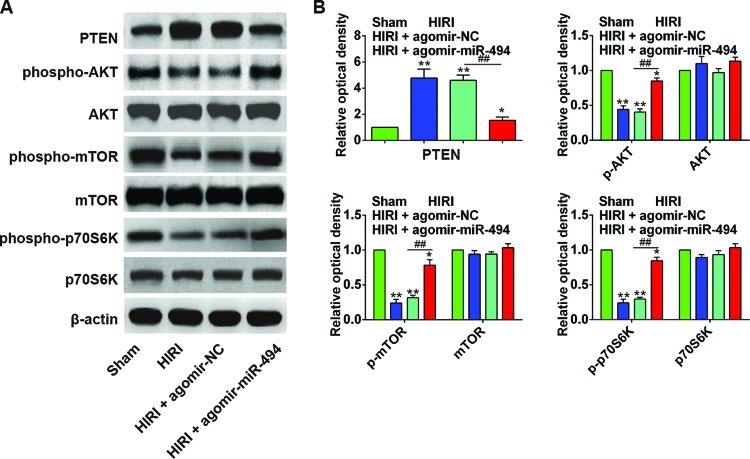
The protective effect of miR-494 in HIRI rats is associated with PI3K/AKT signaling pathway
To further explore the molecular mechanism underlining the protective effect of miR-494 in HIRI rats, we examined the expression of proteins in rat liver, including PTEN, p-AKT, AKT, and the downstream effectors of PI3K/AKT pathway (p-mTOR, mTOR, p-p70S6K, and p70S6K). As shown in Figure 5A,B, the HIRI rats had significantly increased PTEN level in the liver, but decreased p-AKT, p-mTOR, and p-p70S6K compared with sham group (all P<0.01). After agomir-miR-494 treatment, the PTEN level in rat liver was significantly inhibited, and p-AKT, p-mTOR, and p-p70S6K were increased compared with agomir-NC group (P<0.05 or 0.01), indicating that miR-494 protected rat liver against I/R injury through activating the PI3K/AKT signaling pathway.
Figure 5. miR-494 protected liver I/R injury in rats through activating PTEN/PI3K/AKT signaling pathway.
(A,B) Western blot analysis comparing PTEN, p-AKT, AKT, p-mTOR, mTOR, p-p70S6K, and p70S6K expression in sham surgery, HIRI, HIRI + agomir-NC, HIRI + agomir-miR-494 groups (n=6); *, P<0.05 and **, P<0.01 compared with sham surgery group. ##, P<0.01 compared with agomir-NC group.
Discussion
Hepatic I/R injury is one of the major problems during liver surgery, and it greatly affects the surgical outcome(s). Liver I/R injury triggers a rapid release of ROS, leading to hepatocyte injury and apoptosis [19,20]. In the present study, we successfully constructed a rat model of liver I/R injury as indicated by the elevated serum levels of ALT, AST, LDH, and GLDH enzymes, enhanced concentration of hepatic oxidative parameters MDA, TOA, and OSI, extensive hepatocyte necrosis, and increased rate of hepatocyte apoptosis. Differentiated expression of several miRNAs in hepatic I/R injury has been previously reported. For instance, miR-223 was strongly up-regulated in hepatic IRI, whereas miR-122 and miR-146a were markedly down-regulated [11–13]. In the present study, we found that miR-494 in HIRI group was significantly down-regulated by at least two fold when compared with sham surgery group. To analyze the regulatory role of miR-494 in hepatic I/R injury, we treated the HIRI rats with an intraperitoneal injection of agomir-miR-494 or agomir-NC, and compared the pathophysiological changes in the liver in the two groups. It was found that agomir-miR-494 significantly decreased serum ALT, AST, LDH, and GLDH levels, liver MDA, TOA, and OSI concentration, hepatocyte apoptosis rate, liver expression of apoptosis-associated proteins (cleaved PARP, cleaved caspase-3, and Bax), hepatocyte necrosis and DNA fragment, when compared with agomir-NC rats. These results suggested that miR-494 effectively alleviated the liver I/R injuries.
The generation of ROS is an essential factor leading to hepatic I/R injury [21,22]. Therefore, cell models with H2O2-induced oxidative stress have been commonly used to simulate the hepatic I/R injury [14]. In the present study, we further established an in vitro oxidative stress model by H2O2 treatment, and found that the expression of miR-494 in hepatic AML12 cells was dose-dependent decrease. We also showed that transfection of miR-494 mimics significantly improved the proliferation, and reduced apoptosis rate of H2O2-treated AML12 cells, when compared with mimics NC transfection, indicating that miR-494 exerted protective effects against H2O2-induced oxidative stress. The PI3K/AKT/mTOR pathway is an important intracellular signaling pathway in the regulation of cell cycle [23]. Studies have shown that the activation of AKT pathway can significantly reduce H2O2-induced cell apoptosis [24–26]. In our study, we found that miR-494 up-regulated the phosphorylation level of AKT in AML12 cells. Moreover, the activity of apoptosis-associated proteins PARP and caspase-3 was decreased after transfection of miR-494 mimics, suggesting that miR-494 reduced the H2O2-induced apoptosis of AML12 cells via activating the AKT pathway.
To locate the target gene of miR-494, we searched the miRNA target prediction databases PicTar and TargetScan. PTEN was identified as one of the candidate targets of miR-494. Subsequent luciferase reporter assay also confirmed that miR-494 specifically targetted 3′-UTR of PTEN gene. Subsequent in vitro cell experiments showed that PTEN mRNA and protein expression were markedly down-regulated after miR-494 mimics transfection and up-regulated after miR-494 inhibitor transfection, confirming PTEN as a downstream target of miR-494. As a natural inhibitor of the PI3K/AKT pathway, PTEN inhibits the phosphorylation of AKT, and plays an important role in the regulation of cell apoptosis [27,28]. Previous studies have shown that miR-494 can specifically target PTEN, leading to the activation of the PI3K/AKT pathway during various pathophysiologic processes including cell apoptosis, tumor metastasis, and angiogenesis [18,29,30]. The cardioprotective effects of miR-494 against I/R-induced myocardium injury are also dependent on Akt activation [17]. We therefore hypothesized that miR-494 might inhibit the expression of PTEN, which activates the PI3K/AKT pathway and reduces the H/R-induced hepatic apoptosis. To test this hypothesis, we analyzed the activity of PTEN and PI3K/AKT pathway, and found that the HIRI rats had significantly increased PTEN level in the liver, but decreased p-AKT, p-mTOR, and p-p70S6K compared with sham group. After agomir-miR-494 treatment, the PTEN level in rat liver was significantly inhibited, and p-AKT, p-mTOR, and p-p70S6K was increased compared with agomir-NC group (P<0.05 or 0.01). Taken together, miR-494 protected rat liver against I/R injury through activating the PI3K/AKT signaling pathway.
In conclusion, our study showed that miR-494 protected rats against hepatic I/R injury through down-regulating its downstream target gene PTEN, leading to the activation of PI3K/AKT signaling pathway. Our finding may contribute to the development of novel therapies for hepatic H/R injuries.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- DMEM
Dulbecco's modified Eagle medium
- GLDH
glutamate dehydrogenase
- HE
hematoxylin and eosin
- I/R
ischemia/reperfusion
- LDH
lactate dehydrogenase
- mut
mutant type
- NC
negative control
- qRT-PCR
quantitative reverse-transcription PCR
- RLA
relative luciferase activity
- ROS
reactive oxygen species
- SD
Sprague–Dawley
- TUNEL
terminal deoxynucleotidyl transferase mediated dUTP nick-end labeling
- wt
wild type
Author contribution
S.S. conceived and designed the entire study. S.S., D.L., and X.L. performed the experiments. J.L. and F.P. analysed the data. C.F. and B.L. performed literature research and statistical analysis. S.S. and D.L. drafted the paper. S.S. guided the whole study. All the authors read and agreed with the final version of this manuscript.
Funding
The authors confirm that there are no sources of funding to be acknowledged.
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Banga N.R., Homer-Vanniasinkam S., Graham A., Al-Mukhtar A., White S.A. and Prasad K.R. (2005) Ischaemic preconditioning in transplantation and major resection of the liver. Br. J. Surg. 92, 528–538 [DOI] [PubMed] [Google Scholar]
- 2.Theodoraki K., Arkadopoulos N., Nastos C., Vassiliou I., Karmaniolou I. and Smyrniotis V. (2012) Small liver remnants are more vulnerable to ischemia/reperfusion injury after extended hepatectomies: a case-control study. World J. Surg. 36, 2895–2900 [DOI] [PubMed] [Google Scholar]
- 3.Lentsch A.B., Kato A., Yoshidome H., McMasters K.M. and Edwards M.J. (2000) Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology (Baltimore, Md) 32, 169–173 [DOI] [PubMed] [Google Scholar]
- 4.Jaeschke H. (2003) Role of reactive oxygen species in hepatic ischemia-reperfusion injury and preconditioning. J. Invest. Surg. 16, 127–140 [PubMed] [Google Scholar]
- 5.Kupiec-Weglinski J.W. and Busuttil R.W. (2005) Ischemia and reperfusion injury in liver transplantation. Transplant. Proc. 37, 1653–1656 [DOI] [PubMed] [Google Scholar]
- 6.Jaeschke H. (2006) Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G1083–G1088 [DOI] [PubMed] [Google Scholar]
- 7.Urakami H., Abe Y. and Grisham M.B. (2007) Role of reactive metabolites of oxygen and nitrogen in partial liver transplantation: lessons learned from reduced-size liver ischaemia and reperfusion injury. Clin. Exp. Pharmacol. Physiol. 34, 912–919 [DOI] [PubMed] [Google Scholar]
- 8.Hines I.N. and Grisham M.B. (2011) Divergent roles of superoxide and nitric oxide in liver ischemia and reperfusion injury. J. Clin. Biochem. Nutr. 48, 50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartel D.P. (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 [DOI] [PubMed] [Google Scholar]
- 10.Shivdasani R.A. (2006) MicroRNAs: regulators of gene expression and cell differentiation. Blood 108, 3646–3653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu C.H., Xu C.F. and Li Y.M. (2009) Association of microRNA-223 expression with hepatic ischemia/reperfusion injury in mice. Dig. Dis. Sci. 54, 2362–2366 [DOI] [PubMed] [Google Scholar]
- 12.Chen Q., Kong L., Xu X., Geng Q., Tang W. and Jiang W. (2013) Down-regulation of microRNA-146a in the early stage of liver ischemia-reperfusion injury. Transplant. Proc. 45, 492–496 [DOI] [PubMed] [Google Scholar]
- 13.Farid W.R., Pan Q., van der Meer A.J., de Ruiter P.E., Ramakrishnaiah V., de Jonge J. et al. (2012) Hepatocyte-derived microRNAs as serum biomarkers of hepatic injury and rejection after liver transplantation. Liver Transpl. 18, 290–297 [DOI] [PubMed] [Google Scholar]
- 14.Li X., Yi S., Deng Y., Cheng J., Wu X., Liu W. et al. (2014) MiR-124 protects human hepatic L02 cells from H2O2-induced apoptosis by targeting Rab38 gene. Biochem. Biophys. Res. Commun. 450, 148–153 [DOI] [PubMed] [Google Scholar]
- 15.Li L., Li G., Yu C., Shen Z., Xu C., Feng Z. et al. (2015) A role of microRNA-370 in hepatic ischaemia-reperfusion injury by targeting transforming growth factor-beta receptor II. Liver Int. 35, 1124–1132 [DOI] [PubMed] [Google Scholar]
- 16.Zhai F., Zhang X., Guan Y., Yang X., Li Y., Song G. et al. (2012) Expression profiles of microRNAs after focal cerebral ischemia/reperfusion injury in rats. Neural Regen. Res. 7, 917–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X., Zhang X., Ren X.P., Chen J., Liu H., Yang J. et al. (2010) MicroRNA-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion-induced cardiac injury. Circulation 122, 1308–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun G., Zhou Y., Li H., Guo Y., Shan J., Xia M. et al. (2013) Over-expression of microRNA-494 up-regulates hypoxia-inducible factor-1 alpha expression via PI3K/Akt pathway and protects against hypoxia-induced apoptosis. J. Biomed. Sci. 20, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montalvo-Jave E.E., Escalante-Tattersfield T., Ortega-Salgado J.A., Pina E. and Geller D.A. (2008) Factors in the pathophysiology of the liver ischemia-reperfusion injury. J. Surg. Res. 147, 153–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klune J.R. and Tsung A. (2010) Molecular biology of liver ischemia/reperfusion injury: established mechanisms and recent advancements. Surg. Clin. North Am. 90, 665–677 [DOI] [PubMed] [Google Scholar]
- 21.Jaeschke H. (2000) Reactive oxygen and mechanisms of inflammatory liver injury. Journal of Gastroenterol. Hepatol. 15, 718–724 [DOI] [PubMed] [Google Scholar]
- 22.Zhang W., Wang M., Xie H.Y., Zhou L., Meng X.Q., Shi J. et al. (2007) Role of reactive oxygen species in mediating hepatic ischemia-reperfusion injury and its therapeutic applications in liver transplantation. Transplant. Proc. 39, 1332–1337 [DOI] [PubMed] [Google Scholar]
- 23.Yang S.X., Polley E. and Lipkowitz S. (2016) New insights on PI3K/AKT pathway alterations and clinical outcomes in breast cancer. Cancer Treat. Rev. 45, 87–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang P., Peairs J.J., Tano R. and Jaffe G.J. (2006) Oxidant-mediated Akt activation in human RPE cells. Invest. Ophthalmol. Vis. Sci. 47, 4598–4606 [DOI] [PubMed] [Google Scholar]
- 25.Byeon S.H., Lee S.C., Choi S.H., Lee H.K., Lee J.H., Chu Y.K. et al. (2010) Vascular endothelial growth factor as an autocrine survival factor for retinal pigment epithelial cells under oxidative stress via the VEGF-R2/PI3K/Akt. Invest. Ophthalmol. Vis. Sci. 51, 1190–1197 [DOI] [PubMed] [Google Scholar]
- 26.Li H., Wang B., Zhu C., Feng Y., Wang S., Shahzad M. et al. (2013) 17β-estradiol impedes Bax-involved mitochondrial apoptosis of retinal nerve cells induced by oxidative damage via the phosphatidylinositol 3-kinase/Akt signal pathway. J. Mol. Neurosci. 50, 482–493 [DOI] [PubMed] [Google Scholar]
- 27.Yang H., Kong W., He L., Zhao J.J., O’Donnell J.D., Wang J. et al. (2008) MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 68, 425–433 [DOI] [PubMed] [Google Scholar]
- 28.Sayed D., He M., Hong C., Gao S., Rane S., Yang Z. et al. (2010) MicroRNA-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of Fas ligand. J. Biol. Chem. 285, 20281–20290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y., Lai L., Chen Q., Song Y., Xu S., Ma F. et al. (2012) MicroRNA-494 is required for the accumulation and functions of tumor-expanded myeloid-derived suppressor cells via targeting of PTEN. J. Immunol. 188, 5500–5510 [DOI] [PubMed] [Google Scholar]
- 30.Liu L., Jiang Y., Zhang H., Greenlee A.R. and Han Z. (2010) Overexpressed miR-494 down-regulates PTEN gene expression in cells transformed by anti-benzo(a)pyrene-trans-7,8-dihydrodiol-9,10-epoxide. Life Sci. 86, 192–198 [DOI] [PubMed] [Google Scholar]