Abstract
Müller cells are the predominant glial cell type in the retina of vertebrates. They play a wide variety of roles in both the developing and the mature retina that have been widely reported in the literature. However, less attention has been paid to their role in phagocytosis of cell debris under physiological, pathological or experimental conditions. Müller glia have been shown to phagocytose apoptotic cell bodies originated during development of the visual system. They also engulf foreign molecules that are injected into the eye, cone outer segments and injured photoreceptors. Phagocytosis of photoreceptor cell debris in the light‐damaged teleost retina is primarily carried out by Müller cells. Once the microglial cells become activated and migrate to the photoreceptor cell layer, the phagocytic activity of Müller cells progressively decreases, suggesting a possible mechanism of communication between Müller cells and neighbouring microglia and photoreceptors. Additionally, it has been shown that phagocytic Müller cells acquire proliferating activity in the damaged teleost retina, suggesting that engulfment of apoptotic photoreceptor debris might stimulate the Müller glia to proliferate during the regenerative response. These findings highlight Müller glia phagocytosis as an underlying mechanism contributing to degeneration and regeneration under pathological conditions.
Keywords: microglia, müller cells, paracrine factors, phagocytosis, retina
Introduction
Phagocytosis has been considered to be the final stage of programmed cell death (PCD). PCD plays a crucial part during development of the vertebrate visual system (for a review, see Francisco‐Morcillo et al. 2014). However, PCD also occurs in this region of the central nervous system (CNS) under pathological and experimental conditions. Cell debris generated during the degeneration process in the vertebrate retina is mostly removed through heterophagy by a variety of cells, including professional migratory phagocytes and non‐professional stationary phagocytes. Professional phagocytes include cells of the macrophage lineage and microglial cells. Macrophages invade the vertebrate retina during early stages of development (Cuadros et al. 1991; Knabe et al. 2000; Rodríguez‐Gallardo et al. 2005; Santos et al. 2008; Bejarano‐Escobar et al. 2011). Later, microglial precursors enter the retina by different forms of migration, and differentiate into microglial cells (Navascués et al. 1995; Marín‐Teva et al. 1999a,b,c; Santos et al. 2008; Bejarano‐Escobar et al. 2011, 2013). These specialized phagocytes are thought to be the main cell types involved in clearing degenerating cells in the healthy, experimental and pathological retina (Egensperger et al. 1996; Rodríguez‐Gallardo et al. 2005; Santos et al. 2008, 2010; Bailey et al. 2010; Bejarano‐Escobar et al. 2011, 2012b). Retinal photoreceptor degeneration is accompanied by the migration of professional phagocytic cells into the outer nuclear layer (ONL). These phagocytes are derived from resident microglial cells, not from peripheral macrophages (Roque et al. 1996; Bailey et al. 2010; Santos et al. 2010; Bejarano‐Escobar et al. 2012b). During this process, degenerating cells lose the ‘do‐not‐eat‐me' signals and then express soluble ‘find‐me' signals that activate resting microglia that become highly motile, migrating to the lesion area, and phagocytosing cell debris or damaged photoreceptors. The mechanics of phagocytosis is provoked by exposure of ‘eat‐me’ signals on the surface of the injured neurons (for a review, see Ravichandran, 2011). In sum, clearance of cell debris by specialized phagocytes is well characterized.
On the contrary, much less is known about the molecular mechanisms involved in phagocytosis by non‐professional phagocytes in the retinal tissue. Neuroepithelial cells have been described as participating in the removal of degenerating cells in undifferentiated tissue (García‐Porrero & Ojeda, 1979; Martín‐Partido et al. 1988; Francisco‐Morcillo et al. 2004; Mellén et al. 2008; Bejarano‐Escobar et al. 2011, 2013). Moreover, Müller cells, the principal glial cells of the vertebrate retina, are capable of phagocytosing fragments of retinal cells and foreign substances in the differentiating and mature retina (Table 1). It has been described that, under experimental conditions, different signalling molecules emanating from Müller glia and microglia may coordinate phagocytosis of cell debris in the retinal tissue. Phagocytic removal of tissue debris by microglia has an important function in creating an appropriate environment for the stimulation of regeneration in other regions of the CNS (Neumann et al. 2009). Therefore, reciprocal Müller cell–microglia interactions could be the basis of the acute retinal responses to injury and disease, and may be involved in stimulating the regenerative process in the retinal tissue.
Table 1.
Phagocytic activity of Müller cells
| Reference | Species | Technique | Most relevant events described |
|---|---|---|---|
| Fish | |||
| Wagner & Raymond (1991) | Goldfish | Immunohistochemistry and cell culture | Müller cells are phagocytic for latex beads in culture but not in vivo |
| Morris et al. (2005) | Zebrafish | Immunohistochemistry and TUNEL histochemistry in transgenic zebrafish | Müller glia scavenge the cell debris from degenerating photoreceptors |
| Bailey et al. (2010) | Zebrafish | Immunohistochemistry and TUNEL histochemistry in transgenic zebrafish | A subset of Müller glia engulfed apoptotic photoreceptor cell bodies in light‐damaged retinas. The Müller glia proliferative response is linked to phagocytic activity. |
| Bejarano‐Escobar et al. (2012b) | Tench | Immunohistochemistry and TUNEL histochemistry in teleosts | Müller glia engulfed apoptotic photoreceptor cell bodies in light‐damaged retinas during the first hours of intense light treatment. As activated microglial cells invade the ONL, the phagocytic activity of Müller cells progressively decreased |
| Reptiles | |||
| Francisco‐Morcillo et al. (2004) | Reptiles | TUNEL histochemistry | Müller cells phagocytose dying cells during retinal development |
| Birds | |||
| Hughes & LaVelle (1975) | Chick | TEM study | Müller cells can phagocytose degenerating ganglion cells after tectal lesions during embryonic development |
| Hughes & McLoon (1979) | Chick | TEM study | Ganglion cell debris during retinal development is phagocytosed by Müller cells |
| Marín‐Teva et al. (1999c) | Quail | TEM study, immunohistochemistry and TUNEL histochemistry | Müller cells can phagocytose cell debris in any layer of the developing retina |
| Thanos (1999) | Chick | Fluorescent retrograde staining of degenerating ganglion cells. Fluorescent ganglion cell debris is internalized by phagocytic cells | Müller cells perform phagocytosis at early stages of ontogenetic cell death. In more advanced stages, Müller cells are replaced by microglial cells |
| Mammals | |||
| Friedenwald & Chan (1932) | Albino rabbit | TEM study | Müller cell phagocytic activity after the injection of melanin granules into the vitreous body |
| Blanks et al. (1972) | Retinal degenerative (rd) mouse | TEM study | Photoreceptor cell debris is phagocytosed by Müller cells |
| Caley et al. (1972) | Rodless CBA mouse | TEM study | Photoreceptor cell debris is phagocytosed by Müller cells |
| Kuwabara & Weidman (1974) | Rat | TEM study | Cell debris originated during retinal ontogeny is removed by Müller cells |
| Rosenthal & Appleton (1975) | Rabbit | Classical histological staining | Intravitreal copper foreign bodies are deposited in the Muller cell bodies |
| Algvere & Kock (1983) | Rabbit | TEM study | Müller cells penetrated the ILM and removed carbon particles from the vitreous body by endocytosis |
| Long et al. (1986) | Ground squirrel | TEM study | Phagocytosis of the outer segment discs shed from cones |
| Penfold & Provis (1986) | Human | TEM study | Müller cells phagocyte cell debris during ontogeny |
| Mano & Puro (1990) | Human | Cell culture and TEM study | Müller cell cultures from post mortem eyes are able to phagocytose retinal fragments as well as latex beads |
| Stolzenburg et al. (1992) | Rabbit | TEM, brightfield light microscopy and fluorescence microscopy study | Müller cells show an intense phagocytosis of latex beads in vitro |
| Nishizono et al. (1993) | Rabbit | TEM study | Müller cells phagocytose egg‐lecithin‐coated silicone particles after intraocular injection |
| Egensperger et al. (1996) | Rabbit | TUNEL and lectin histochemistry | Fragmenting DNA is principally phagocytosed by microglia and Müller cells. Müller cells appear to be able to phagocytose dying cells in any retinal layer during development |
| Crafoord et al. (2000) | Albino rabbits | TEM study and immunohistochemistry | Implantation of melanin granules in the subretinal space may induce a cellular phagocytic response in macrophages and Müller cells |
| Francke et al. (2001) | Rabbit | TEM study | Retinal pigment epithelium melanin granules are phagocytosed by Müller glial cells in experimental retinal detachment |
| Ponsioen et al. (2007) | Human | Immunocytochemistry and cell culture | Müller cells in culture phagocytose collagen type II |
| Singh et al. (2014) | Human | Cell culture, phagocytosis assay, PCR analysis, measurement of intracellular metabolites, immunocytochemistry | Immortalized Müller glia can phagocytose and kill bacteria in vitro. Müller cells also produce a variety of antimicrobial molecules in response to bacterial challenge |
Müller cells in the vertebrate retina are involved in cell debris removal during embryonic development and mature tissue homeostasis, but also under experimental and pathological conditions. ILM, inner limiting membrane; ONL, outer nuclear layer; PCR, polymerase chain reaction; TEM, transmission electron microscopy; TUNEL, TdT dUTP nick‐end labelling.
This article reviews the current state of knowledge regarding the potential of Müller glia to phagocytose degenerating cells in developing, mature, pathological and injured retinas. We summarize recent findings regarding the secreted factors, signalling pathways and intrinsic factors that have been implicated in the phagocytic process and in the possible interactions between Müller glia and microglial cells. Moreover, we analyse the possible involvement of phagocytosis in other retinal events such as regeneration, degeneration and neuroprotection. This will be done through a review of the pertinent literature, as well as by using our own experience in recent studies of the phagocytosis of cell debris in retinal tissue.
Development and function of Müller glia
The retina is a laminated tissue in which the different neurons and their synaptic connections are arranged in different layers that have been remarkably conserved in vertebrate evolution (Fig. 1). Light‐sensing neurons, the photoreceptors, occupy the outermost layer, the ONL, while the retinal projection neurons, the ganglion cells, are located in the innermost neuronal layer, the ganglion cell layer (GCL). The interneurons (horizontal, bipolar and amacrine cells) reside between the ONL and GCL, in the so‐called inner nuclear layer (INL; Fig. 1). The cell bodies of Müller glia are also located in the INL (Fig. 1). These cells have a radial morphology, with two stem processes that extend in opposite directions, and span the entire retinal thickness (Fig. 1). Due to their ontogeny, morphology and distribution, these cells are considered analogous to radial glial cells of the cortex. The vitreal (inner) process of the Müller cells approaches the vitreal surface of the neural retina where it forms a so‐called endfoot abutting the basal lamina, forming the inner limiting membrane (ILM). The scleral (outer) process reaches out the subretinal space, into which it sends numerous microvilli. These apical processes of Müller cells are attached to each other and to the inner segments of the photoreceptor cells by continuous heterotypic adherens junctions that collectively form the outer limiting membrane (OLM). Furthermore, scleral processes branch around the photoreceptor nuclei, removing neurotransmitters from the extracellular space of surrounding neurons (Newman & Reichenbach, 1996; Bejarano‐Escobar et al. 2009, 2010). Therefore, these stem processes extend side branches that contact and/or ensheath different neuronal elements of the retina as well as the blood vessels in vascularized retinas.
Figure 1.
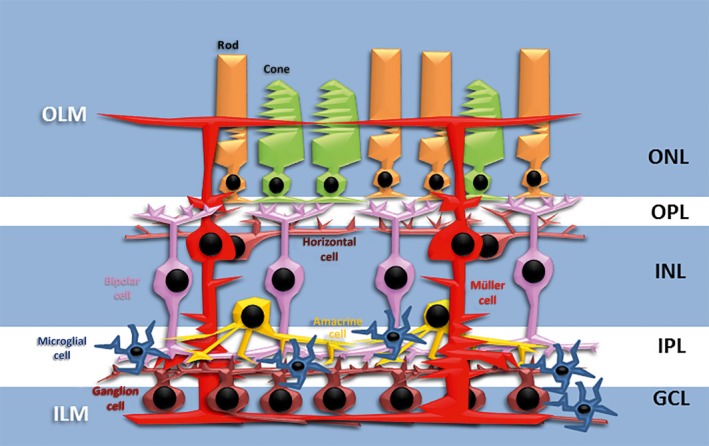
Diagram of a vertebrate retina shows its typical multi‐layered structure. The mature Müller cell is characterized by a centralized soma located in the INL, and processes extending to, and forming, the ILM and OLM. The Müller cell processes contain many branches that surround photoreceptor somata and neuronal processes. GCL, ganglion cell layer; ILM, inner limiting membrane; INL, inner nuclear layer; IPL, inner plexiform layer; OLM, outer limiting membrane; ONL, outer nuclear layer; OPL, outer plexiform layer.
Müller cells could be identified and characterized in accordance with their location and morphological clues. However, the utilization of immunochemical markers facilitates the study of these glial cells in the developing and adult tissue (for a review, see Bejarano‐Escobar et al. 2014). Due to the plasticity of Müller cells, they can be characterized by glial markers, cell cycle markers and stem cell markers (Roesch et al. 2008). Among them, the enzyme glutamine synthetase (GS) has long been considered a good molecular marker for Müller glia in the developing and mature retinal tissue (Peterson et al. 2001; Bejarano‐Escobar et al. 2009, 2010, 2012a; Pavón‐Muñoz et al. 2016). These cells also express selected classes of intermediate filament proteins, such as vimentin, glial fibrillary acidic protein (GFAP) and nestin (Raymond et al. 2006; Sánchez‐Farías & Candal, 2016). Müller glia also express some genes involved in phagocytosis, suggesting that this cell type can phagocytose in vivo. Thus, lysosomal cathepsin B and D are highly expressed in specialized phagocytes such as macrophages and microglial precursors in the retina of mammals (Fig. 2A–C; Bejarano‐Escobar et al. 2011). Abundant punctate cytosolic immunolabelling for cathepsin D is restricted to radially oriented cells in the fish retina (Fig. 2D,E). Phagocytic Müller cells could also be labelled by using deoxynucleotidyl transferase (TdT) dUTP nick‐end labelling (TUNEL) histochemistry (see below).
Figure 2.
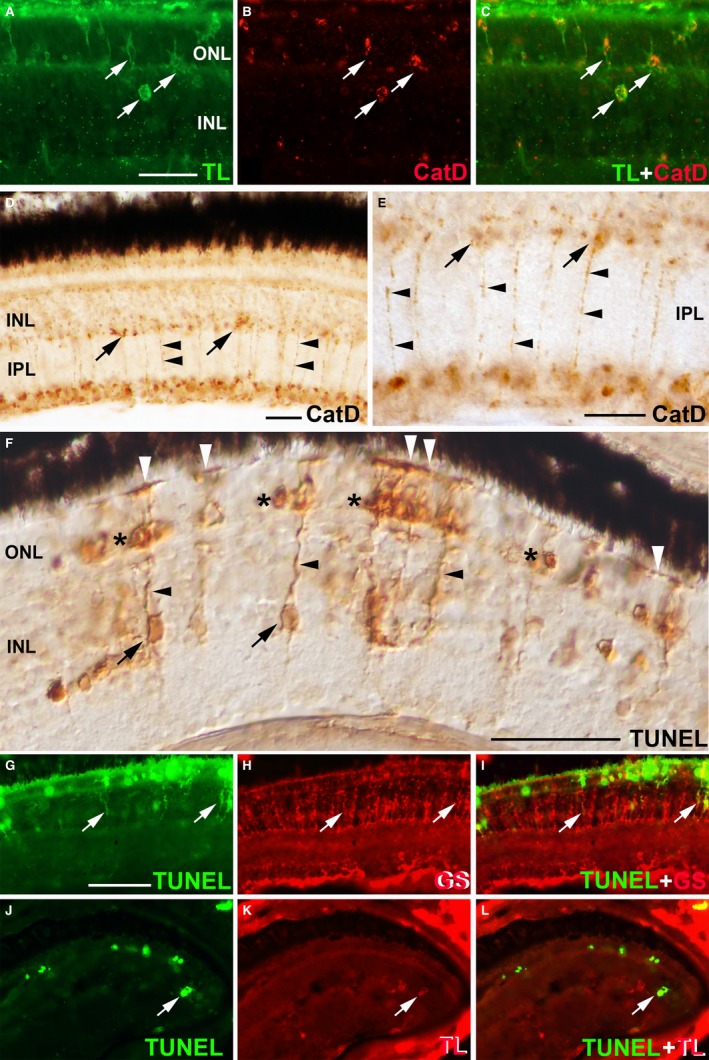
Müller glia and phagocytosis. (A–C) Microglial cells can be detected with tomato lectin (TL) histochemistry in the developing mouse retina (arrows in A,C). These phagocytic cells also express high levels of cathepsin D (Cat D; arrows in B,C). (D,E) (E) is a magnification of (D). Strong immunoreactivity against cathepsin D is detected in the cell somata (arrows) and processes (arrowheads) of a sub‐population of Müller cells in the teleost retina. (F) TdT dUTP nick‐end labelling (TUNEL) histochemistry shows retinal cell death in the photoreceptor layer (asterisks) in the light‐damaged larval teleost retina. The cell somata (arrows) and processes (black arrowheads) of a sub‐population of Müller cells are also stained with TUNEL histochemistry. The OLM occasionally appeared labelled (white arrowheads). (G–I) TUNEL‐positive Müller cells are also labelled with antibodies against glutamine synthetase (GS) (arrows) in the light‐damaged larval teleost retina. (J–L) TUNEL analysis also reveals cytoplasmic signal in TL‐positive microglial cells in the light‐damaged larval teleost retina (arrows). Scale bars: 25 μm (A–C); 50 μm (D–F; in G–L). INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer.
The vertebrate retina is mainly composed of cells of neuroectodermal origin. Thus, Müller cells and retinal neurons are derived from a retinal progenitor cell that is multipotent at all stages of retinal histogenesis (Xiang, 2013). During retinogenesis, neurogenesis precedes gliogenesis, as elsewhere in the vertebrate CNS. Therefore, Müller cells are considered the last cell type generated during vertebrate retinogenesis (Prada et al. 1991; Rapaport et al. 2004; Francisco‐Morcillo et al. 2006; Bejarano‐Escobar et al. 2009, 2010, 2012a). These data disagree with the results described in the developing shark retina by Harahush et al. (2009). Their ultrastructural analysis revealed that Müller cells are present throughout the retina from very early stages of development, simultaneously with ganglion cell differentiation. However, these authors did not characterize these early differentiated Müller cells neurochemically. Morphological changes in the transition between young and mature Müller cells could be observed in the teleost retina (Bejarano‐Escobar et al. 2009, 2010; Pavón‐Muñoz et al. 2016). By using antibodies against GS, HNK‐1 epitope and carbonic anhydrases, Peterson et al. (2001) defined immunohistochemically different stages of maturation for Müller cells in the developing zebrafish retina. They also recognized the first immature Müller cells very early in development, coinciding with the presence of the first newborn ganglion cells. More recently, an immunohistochemical analysis performed by Sánchez‐Farías & Candal (2016) described a different multi‐step Müller glia differentiation process in the developing shark retina. Thus, coinciding with early neurogenesis, neuroepithelial cells differentiate into early radial glial cells. With the subsequent increase of GFAP immunoreactivity, these authors were then able to define these cells as late radial glial cells, which, coinciding with the appearance of GS immunoreactivity, acquire the typical morphology of young Müller cells with quite thick cell somata and processes. Finally, as cell maturation proceeds, Müller cells become long and slender and are identifiable as mature Müller cells. Therefore, the analysis of the processes underlying the transition from neuroepithelial cells to radial cells, and from radial cells to Müller cells, could shed some light on Müller glia differentiation.
Müller cells are noted for their wide range of roles in neural development and function. They exhibit many of the functions observed for radial glia, astrocytes and ependymal cells in other areas of the CNS. Excellent reviews on the role of Müller glia in homeostasis, retinal innate immunity, retinal diseases and regeneration of the visual system have been published in the last decade (Bringmann et al. 2006; de Melo Reis et al. 2008; Jadhav et al. 2009; Bringmann & Wiedemann, 2012; Wohl et al. 2012; Kumar et al. 2013; Reichenbach & Bringmann, 2013; Seitz et al. 2013; Gallina et al. 2014; Goldman, 2014; Gorsuch & Hyde, 2014; Lenkowski & Raymond, 2014; Hamon et al. 2016). In addition to all the functions described by these authors, Müller glia are known to phagocytose cell debris. The regulation of the phagocytic activity of retinal glial cells is poorly understood even though phagocytosis may play a role in retinal homeostasis, pathobiology, degeneration and regeneration. Recent findings indicate the presence of highly coordinated dynamic interactions between Müller cells and microglia that regulate the phagocytosis of cell debris. Furthermore, the proliferative response of Müller glia during photoreceptor degeneration could be induced by their phagocytic activity, which would suggest the possible involvement of phagocytosis in cell proliferation and tissue regeneration.
Müller glia and phagocytosis
Müller cell phagocytic activity
Müller cells are capable of phagocytosing debris from dead cells, pigment epithelial cells and diverse foreign bodies under physiological, pathological and experimental conditions (Table 1). The first evidence of phagocytic activity by Müller cells was reported by Friedenwald & Chan (1932). Melanin granules injected intravitreally in albino rabbits were engulfed by these glial cells. These results were confirmed many years later by different techniques, including transmission electron microscopy (TEM). Thus, following the intravitreal injection of different foreign bodies, these particles appeared inside radial processes of Müller cells in TEM sections (Rosenthal & Appleton, 1975; Nishizono et al. 1993). They also engulfed intravitreally injected erythrocytes (Miller et al. 1986). Under these experimental conditions, the inner limiting lamina was thinned, interrupted or had disappeared, allowing Müller cell processes to make contact with the foreign bodies. Furthermore, they engulf melanin granules injected into the subretinal space (Crafoord et al. 2000) and erythrocyte debris from subretinal or vitreous haemorrhages (Koshibu, 1978; Miller et al. 1986). Surprisingly, fish Müller cells were found to be able to phagocytose latex beads in vitro, but not in vivo (Wagner & Raymond, 1991). Cultured human (Mano & Puro, 1990; Ponsioen et al. 2007) and rabbit (Stolzenburg et al. 1992) Müller cells are capable of phagocytosing latex beads. More recently, in vitro studies using immortalized human retinal Müller glia showed that they can phagocytose and kill bacteria in a time‐dependent manner (Singh et al. 2014). Additionally to the engulfment of external substances, Müller cells have also been reported to be active in the phagocytosis of cellular debris during the permanent renewal of photoreceptor outer segments in the mammalian retina (Long et al. 1986). They also phagocytose melanin granules derived from retinal pigment epithelial cells in models of experimental retinal detachment, where pigment epithelium is occasionally detached together with the neural retina (Francke et al. 2001). Recent evidence suggests that this phagocytic clearance following injury is more than simple tidying‐up, but instead plays a fundamental role in facilitating the reorganization of neuronal circuits and triggering repair.
The phagocytic activity of Müller cells becomes more relevant with the clearance of cell debris during development and retinal injury. TEM examination revealed that apoptotic neurons are removed by Müller cells during human (Penfold & Provis, 1986), rat (Kuwabara & Weidman, 1974), chick (Hughes & McLoon, 1979) and quail (Marín‐Teva et al. 1999c) retinal development. Egensperger et al. (1996) studied the spatiotemporal patterns of cell death and phagocytic cells in the developing retina of several mammals. They used the TUNEL technique that has been designed to detect apoptotic cells that undergo extensive DNA degradation during the late stages of apoptosis (Gavrieli et al. 1992). The method is based on the ability of TdT to label blunt ends of double‐stranded DNA breaks independent of a template, allowing the detection of fragmenting chromatin in degenerating nuclei. The technique showed intense labelling in the nuclei of degenerating cells, in cell fragments containing condensed chromatin, and in intracellular chromatin fragments (micronuclei). Surprisingly, there was also diffuse TUNEL labelling within the cytoplasm of radially oriented cells. Similar results have been found by our group in the developing retina of fish (Bejarano‐Escobar et al. 2013), reptiles (Francisco‐Morcillo et al. 2004) and birds (Francisco‐Morcillo et al. 2014). Furthermore, cytoplasmic TUNEL labelling is also found in cells with the same morphology in the teleost retina when photoreceptor degeneration is induced by treatment with constant intense light (Fig. 2F; Thummel et al. 2008; Bailey et al. 2010; Bejarano‐Escobar et al. 2012b) and in a transgenic model of rod degeneration in zebrafish (Morris et al. 2005). Radially oriented TUNEL‐positive cells have a morphology typical of Müller cells, and labelled cells also express GS, a typical Müller cell marker (Fig. 2G–I; Bejarano‐Escobar et al. 2012b). Some authors suggest that this TUNEL labelling is specific of cell death and therefore identifies degenerating Müller cells (Thummel et al. 2008). However, various morphological changes occur in apoptotic cells. Thus, during early stages of apoptosis, when cell shrinkage occurs, cells show a smaller size, which means that the cytoplasm is dense and the organelles are more tightly packed. Furthermore, extensive plasma membrane blebbing occurs, followed by destructive fragmentation of the nucleus and separation of cell fragments into apoptotic bodies during a process called ‘budding’ (Kerr et al. 1972). These morphological changes of apoptotic cells have been observed in the degenerating photoreceptors located in the ONL (Morris et al. 2005; Thummel et al. 2008; Bailey et al. 2010; Bejarano‐Escobar et al. 2012b). The apparent intact healthy morphology of radially oriented TUNEL‐positive cells and the absence of apoptotic nuclei in the INL suggest that cytoplasmic TUNEL labelling results from the dispersion of photoreceptor DNA into the cytoplasm of Müller cells, which engulfed cell debris that originated during the cell death process (Egensperger et al. 1996; Francisco‐Morcillo et al. 2004; Morris et al. 2005; Bailey et al. 2010; Bejarano‐Escobar et al. 2012b). Phagocytosing microglia may occasionally also be labelled with the TUNEL technique (Fig. 2J–L). Therefore, TUNEL staining may generally be regarded as a method for the detection of DNA fragmentation (DNA damage) and, under the appropriate circumstances, more specifically as a method for identifying apoptotic cells. Bailey et al. (2010) also detected weak cytoplasmic TUNEL labelling in a subset of Müller cells that engulf apoptotic photoreceptor bodies in the light‐damaged zebrafish retina, and that all proliferating Müller cells co‐labelled with TUNEL, suggesting that phagocytosis could be involved in the Müller glial proliferative response after injury. Photoreceptor degeneration induces the activation of microglia, contributing to the de‐differentiation and proliferation of the Müller cells (Fischer et al. 2014). By using O‐phospho‐L‐serine (L‐SOP), a molecule that blocks microglial phagocytosis of cell debris, Bailey et al. (2010) found a reduction in the number of proliferating Müller glia, suggesting that the mechanism disrupted by L‐SOP is required to activate Müller glia proliferation in the injured retina. Several hypotheses have been advanced to explain the mechanisms of L‐SOP‐mediated suppression of Müller glia proliferation. One of these hypotheses is that L‐SOP‐suppressed microglial phagocytosis of degenerating photoreceptors could affect the paracrine activation of the Müller cell proliferation by microglial cells. Therefore, Müller glial proliferation processes and phagocytosis could be linked, with both being regulated by microglia.
Coordinated phagocytic activity between Müller cells and microglia
Retinal neurons, microglia and macroglia exchange functionally significant signals under both uninjured and pathological conditions (for a review, see Vecino et al. 2016). In the healthy retina, inhibitory and excitatory neurotransmission modulates ATP release from Müller glia, regulating microglial motility (Uckermann et al. 2006; Fontainhas et al. 2011). When retinal neurons are damaged, Müller cells and microglia undergo a quite substantial functional and structural phenotype change. Thus, microglial cells are activated – from a ‘resting’ to an ‘activated’ phenotype – during retinal inflammation, injury or disease (Roque et al. 1996; Zeiss & Johnson, 2004; Bailey et al. 2010; Santos et al. 2010; Bejarano‐Escobar et al. 2012b). Moreover, under these pathological conditions, Müller cells also demonstrate some degree of activation and reactive gliosis (Bringmann et al. 2006). The activation of both types of cells promotes functional interactions between them, regulating photoreceptor cell survival (Harada et al. 2002). In other cases, photoreceptor degeneration attracts and recruits microglia into the ONL. Activated microglial cells phagocytose cell debris and increase the secretion of inflammatory cytokines, either acting directly on rod photoreceptors (Scuderi et al. 2015), or indirectly via the pro‐inflammatory activation of Müller cells that induce photoreceptor degeneration (Liu et al. 2015). In the case of glaucoma, the change of both cell types to a reactive phenotype initiates signalling cascades that may serve a neuroprotective role, but may also proceed to promote damaging effects on retinal neurons, especially in the ganglion cells (Seitz et al. 2013). It has also been described that activated Müller cells upregulate inflammatory factors, including monocyte chemoattractant protein‐1, recruiting microglial cells to the injured area in a positive‐feedback manner (Nakazawa et al. 2006; Hollborn et al. 2008). In vitro studies have shown that Müller cells change shape, and decrease the expression of gliosis markers when they are co‐cultured with activated microglia (Wang et al. 2011). They also present reduced proliferative activity and express higher protein and mRNA levels of trophic factors such as glial cell‐derived neurotrophic factor and leukaemia inhibitory factor, and increase the protein expression of pro‐inflammatory factors capable of inducing microglia activation. Moreover, co‐cultured Müller cells also show increased expression of chemotactic cytokines and cell‐adhesion molecules, allowing microglial cell processes to adhere closely with Müller cell processes forming fascicles that may serve as a scaffold for the radial migration of microglia (Wang et al. 2011), as occurs during embryonic development (Marín‐Teva et al. 1998; Sánchez‐López et al. 2004). These results indicate that, in situations of pathology or injury, Müller cells and microglia can perform mutual and reciprocal signalling that amplifies local inflammation, adaptive neuroprotection and physical interaction (Wang et al. 2011).
With respect to phagocytosis, although Müller glia participate in the clearance of cell debris, amoeboid microglia seem to be the principal phagocytic cell for retinal cells that die during development and under experimental conditions (Hume et al. 1983; Ashwell et al. 1989; Cuadros et al. 1991a; Egensperger et al. 1996; Moujahid et al. 1996; Thanos et al. 1996; Thanos, 1999; Rodríguez‐Gallardo et al. 2005; Santos et al. 2008; Bailey et al. 2010; Bejarano‐Escobar et al. 2011, 2012a). The phagocytic role of Müller glia becomes relevant in degenerative regions where microglial cells are absent. Different phases of PCD have been described during retinal development (for reviews, see Valenciano et al. 2009; Francisco‐Morcillo et al. 2014). During these phases, there has been observed occasional spatiotemporal coincidence of dying cells with macrophages and microglial precursors in the developing avian retina (Cuadros et al. 1991), and in both the embryonic and postnatal mammalian retina (Hume et al. 1983; Ashwell et al. 1989; Egensperger et al. 1996; Rodríguez‐Gallardo et al. 2005; Santos et al. 2008; Bejarano‐Escobar et al. 2011), suggesting that cell death is the stimulus attracting specialized phagocytes migrating into the developing visual system. However, other researchers have reported that, in some stages of retinal development, there is no chronotopographical coincidence between specialized phagocytes and PCD. Thus, in the developing quail retina, microglial precursors arrive to the INL only after PCD has ceased in this layer (Marín‐Teva et al. 1999c). Furthermore, no evident correlation is found between the chronotopographical distribution patterns of TUNEL‐positive bodies and of macrophages/microglial cells during early and late stages of visual system development in sharks (Bejarano‐Escobar et al. 2013). During ontogeny in these species, increased phagocytic activity is observed in such non‐specialized phagocytes as neuroepithelial cells and Müller glia. Thanos (1999) observed staining of vital and dying ganglion cells in the developing chick retina following the intraocular injection of carbocyanine dyes. Phagocytic cells that remove the fluorescent debris become fluorescently labelled themselves. Radial Müller glia are the only class of cells to become phagocytic between embryonic day 9 (E9) and E16. They are replaced exclusively with microglial cells from E17 onwards, suggesting an interaction between Müller cells and immigrating microglia that regulates the phagocytic process. Similar results were obtained in our laboratory in the teleost retina following light‐induced photoreceptor degeneration. Microglial cells are not present in the mature ONL of vertebrates (Santos et al. 2008; Bejarano‐Escobar et al. 2013). Therefore, if photoreceptor degeneration occurs, microglial cells invade this layer from inner regions of the retina (Harada et al. 2002; Bailey et al. 2010; Santos et al. 2010; Bejarano‐Escobar et al. 2012b). Microglial cells, located mainly in the inner plexiform layer (IPL), become activated and undergo a program of morphological and molecular changes, becoming deramified and developing an enlarged cell body with several short, thickened processes. Later, microglial cells develop an amoeboid appearance and become phagocytic. Depending on the species, this morphological transformation may occur within days or even hours of the initial activation. Our own laboratory's studies in the teleost retina show that the first amoeboid microglial cells appear in the ONL 55 h after the initiation of intense light exposure (Bejarano‐Escobar et al. 2012b). During this period, abundant TUNEL‐positive Müller cells are observed (Fig. 3A), while microglial cells, mainly located in the IPL, clearly show an activated morphology in response to photoreceptor degeneration (Fig. 3B). The number of TUNEL‐positive Müller cells decreases (Fig. 3C), coinciding with the arrival of microglial cells to the ONL (Fig. 3D). Phagocytic Müller cells disappear after 96 h of bright light exposure (Fig. 3), while abundant microglial cells are located in the ONL, coinciding chronotopographically with abundant TUNEL‐positive nuclei (Fig. 3,F). Our results thus clearly show that cell debris that originates during the first hours of constant light treatment is phagocytosed by Müller cells, coinciding with the activation of microglial cells. As activated microglial cells invade the photoreceptor layer, the phagocytic activity of Müller glia progressively decreases (Bejarano‐Escobar et al. 2012b). However, Santos et al. (2010) show the mouse ONL to be invaded by microglial cells immediately after the light exposure. This difference in the timing of microglial activation and migration may be attributable to inter‐specific differences and/or attenuation of the microglial activation under hypothermic conditions. Hypothermia has been described as reducing microglial activation in a temperature‐dependent manner both in vivo and in vitro (Seo et al. 2012). Indeed, those workers demonstrate that hypothermia below 29 °C has major inhibiting effects on microglia. Fish are ectotherms, meaning that they rely on the environment to control their temperature. The water temperature in our experimental study was 25 °C, perhaps low enough to attenuate microglial activation and migration in the damaged teleost retina.
Figure 3.
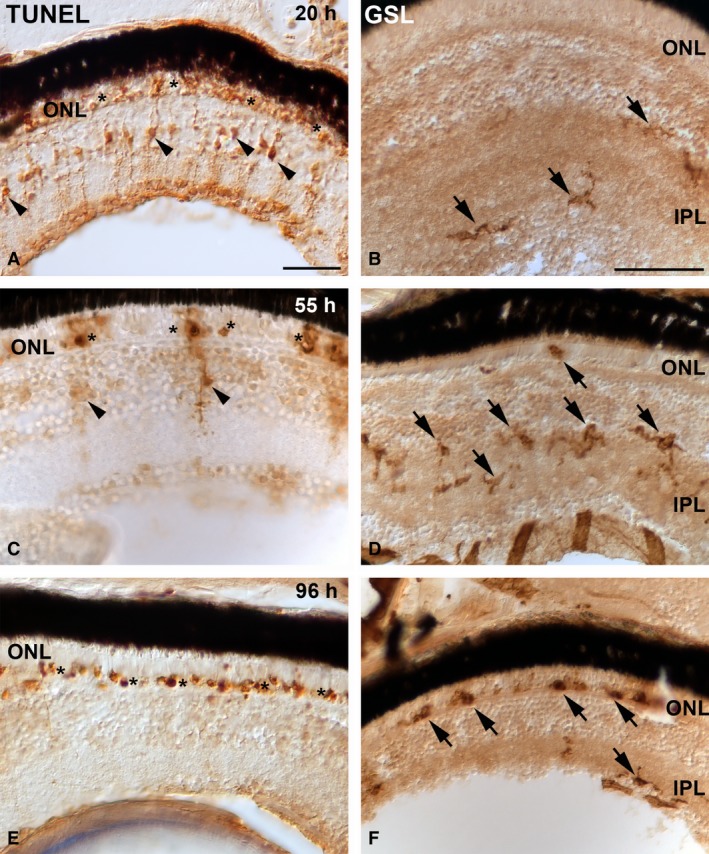
TdT dUTP nick‐end labelling (TUNEL) histochemistry (A,C,E) and Griffonia simplicifolia lectin (GSL) histochemistry (B, D, F) showing the progression of retinal cell death in the photoreceptor layer and changes in distribution pattern and microglial morphology, respectively, in larval teleost after 20 h (A, B), 55 h (C, D) and 96 h (E, F) of light exposure. TUNEL labelling shows that abundant photoreceptors are dying during light treatment (A, C, E) (asterisks). In the first hours of constant light treatment, the number of TUNEL‐positive Müller cells is high (arrowheads in A). However, during the experimental period, cytoplasmic TUNEL staining in the INL became progressively restricted to fewer cells (arrowheads in C, E). In the first hours of constant light treatment, microglial cells are mainly located in the inner border of the INL and in the IPL (arrows in B). As the treatment advances, they progressively become larger and show thicker processes after activation in response to photoreceptor degeneration, and they migrate towards the ONL (arrows in D). Microglial cells invade the photoreceptor layer phagocytosing cell debris (arrows in F). Scale bars: 25 μm (A); 50 μm (B–F). IPL, inner plexiform layer; ONL, outer nuclear layer.
We therefore hypothesized that there are bidirectional feedback signals between microglia and Müller cells that may constitute a coordinated phagocytic response following the induction of retinal damage (Fig. 4).
Figure 4.
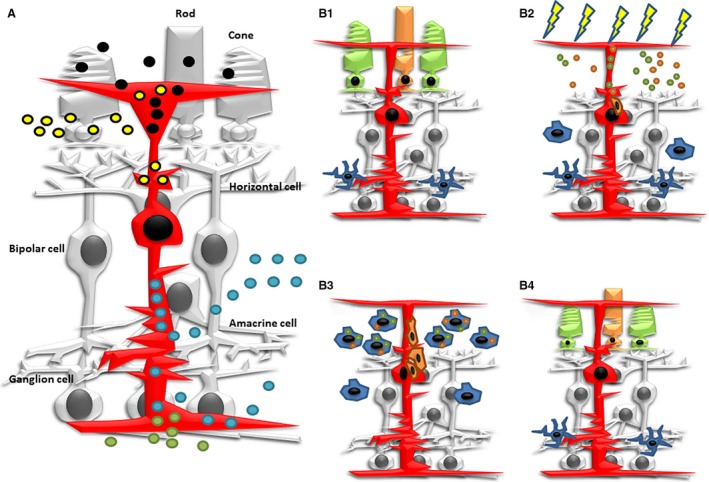
Schematic diagrams showing several aspects of phagocytosis in Müller glia. (A) Müller cells (in red) phagocytose melanin and outer segment discs (black circles) under physiological conditions. They also engulf photoreceptor cell debris (yellow circles) under pathological and experimental conditions. They participate in the removal of cell debris during development of the retina (blue circles). They also engulf foreign molecules that are injected into the eye (green circles). (B1) Microglial cells in the fish retina are mainly observed in the IPL. (B2) During the first hours of constant intense‐light treatment, cell debris from photoreceptor degeneration is phagocytosed by Müller cells. Signalling molecules activate proliferation activity in phagocytic Müller cells. These signals also activate microglia activation and migration to the photoreceptor layer. (B3) As microglial cells invaded the photoreceptor layer, they become highly phagocytic and participate in the removal of cell debris. However, phagocytic activity of Müller cells progressively decreased. (B4) Migrating precursors from Müller cells proliferate and differentiate into rod and cone photoreceptors, regenerating the missing neurons.
Conclusions
Müller cells are involved in the phagocytosis of neuronal debris under both physiological and pathological conditions. Phagocytosis has been shown to play a key role in retinal regeneration. In particular, disruption of phagocytic activity significantly reduces both the proliferation of Müller cells in response to injury and the regeneration of photoreceptors. New evidence suggests that upregulation of Müller cell/microglial cell cross‐talk occurs during phagocytosis in response to photoreceptor degeneration in the teleost retina. These bidirectional interactions between macroglial and microglial cells may mediate adaptive responses within the retina following injury, and photoreceptor degeneration could induce phagocytic activity in Müller cells and the activation of microglial cells located in more internal regions. Furthermore, activated Müller cells release soluble factors that play a crucial role in driving microglial activation and migration. Müller cells may also form an adhesive cellular scaffold that guides the migration of microglia through the layers of the retina. In sum, therefore, phagocytic activity of Müller cells could be involved in processes of degeneration, proliferation and regeneration in the retina of vertebrates. Understanding the cellular mechanisms regulating retinal cell degeneration and regeneration is crucial for the development of treatments for neurodegenerative diseases. In other regions of the CNS, insufficient clearance by microglia, prevalent in several neurodegenerative diseases and increasing with ageing, is associated with an inadequate regenerative response (Neumann et al. 2009). Thus, gaining a clearer understanding of the mechanism behind and the functional significance of Müller cell/microglial‐mediated clearance of retinal cell debris following injury may help to open up exciting new approaches to therapy.
Author contributions
R.B.E., H.S.C. and J.O.A. performed immunohistochemical and histochemical studies. G.M.P. and J.F.M conceived and designed the experiments and constructed. J.F.M. wrote the paper.
Acknowledgements
The authors express their gratitude to M.S. Holguín‐Arévalo for her excellent technical assistance. R.B.E. was a recipient of a PhD studentship from the Junta de Extremadura. This work was supported by grants from the Spanish Ministerio de Ciencia y Tecnología (BFU2007‐67540) and Junta de Extremadura (PRI06A195, GR10152).
References
- Algvere P, Kock E (1983) Experimental epiretinal membranes induced by intravitreal carbon particles. Am J Ophthalmol 96, 345–353. [DOI] [PubMed] [Google Scholar]
- Ashwell KW, Hollander H, Streit W, et al. (1989) The appearance and distribution of microglia in the developing retina of the rat. Vis Neurosci 2, 437–448. [DOI] [PubMed] [Google Scholar]
- Bailey TJ, Fossum SL, Fimbel SM, et al. (2010) The inhibitor of phagocytosis, O‐phospho‐L‐serine, suppresses Müller glia proliferation and cone cell regeneration in the light‐damaged zebrafish retina. Exp Eye Res 91, 601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejarano‐Escobar R, Blasco M, DeGrip WJ, et al. (2009) Cell differentiation in the retina of an epibenthonic teleost, the Tench (Tinca tinca, Linneo 1758). Exp Eye Res 89, 398–415. [DOI] [PubMed] [Google Scholar]
- Bejarano‐Escobar R, Blasco M, Degrip WJ, et al. (2010) Eye development and retinal differentiation in an altricial fish species, the Senegalese sole (Solea senegalensis, Kaup 1858). J Exp Zool B Mol Dev Evol 314, 580–605. [DOI] [PubMed] [Google Scholar]
- Bejarano‐Escobar R, Holguín‐Arévalo MS, Montero JA, et al. (2011) Macrophage and microglia ontogeny in the mouse visual system can be traced by the expression of Cathepsins B and D. Dev Dyn 240, 1841–1855. [DOI] [PubMed] [Google Scholar]
- Bejarano‐Escobar R, Blasco M, Durán AC, et al. (2012a) Retinal histogenesis and cell differentiation in an elasmobranch species, the small‐spotted catshark Scyliorhinus canicula . J Anat 220, 318–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejarano‐Escobar R, Blasco M, Martín‐Partido G, et al. (2012b) Light‐induced degeneration and microglial response in the retina of an epibenthonic pigmented teleost: age‐dependent photoreceptor susceptibility to cell death. J Exp Biol 215, 3799–3812. [DOI] [PubMed] [Google Scholar]
- Bejarano‐Escobar R, Blasco M, Durán AC, et al. (2013) Chronotopographical distribution patterns of cell death and of lectin‐positive macrophages/microglial cells during the visual system ontogeny of the small‐spotted catshark Scyliorhinus canicula . J Anat 223, 171–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejarano‐Escobar R, Blasco M, Martín‐Partido G, et al. (2014) Molecular characterization of cell types in the developing, mature, and regenerating fish retina. Rev Fish Biol Fisheries 24, 127–158. [Google Scholar]
- Blanks JC, Mullen RJ, LaVail MM (1982) Retinal degeneration in the pcd cerebellar mutant mouse. II. Electron microscopic analysis. J Comp Neurol 212, 231–246. [DOI] [PubMed] [Google Scholar]
- Bringmann A, Pannicke T, Grosche J, et al. (2006) Muller cells in the healthy and diseased retina. Prog Retin Eye Res 25, 397–424. [DOI] [PubMed] [Google Scholar]
- Bringmann A, Wiedemann P (2012) Müller glial cells in retinal disease. Ophthalmologica 227, 1–19. [DOI] [PubMed] [Google Scholar]
- Caley DW, Johnson C, Liebelt RA (1972) The postnatal development of the retina in the normal and rodless CBA mouse: a light and electron microscopic study. Am J Anat 133, 179–212. [DOI] [PubMed] [Google Scholar]
- Crafoord S, Dafgard Kopp E, Seregard S, et al. (2000) Cellular migration into neural retina following implantation of melanin granules in the subretinal space. Graefes Arch Clin Exp Ophthalmol 238, 682–689. [DOI] [PubMed] [Google Scholar]
- Cuadros MA, García‐Martín M, Martin C, et al. (1991) Haemopoietic phagocytes in the early differentiating avian retina. J Anat 177, 145–158. [PMC free article] [PubMed] [Google Scholar]
- De Melo Reis RA, Marques Ventura AL, Sampaio Schitine C, et al. (2008) Müller glía as an active compartment modulating nervous activity in the vertebrate retina: neurotransmitters and trophic factors. Neurochem Res 33, 1466–1474. [DOI] [PubMed] [Google Scholar]
- Egensperger R, Maslim J, Bisti S, et al. (1996) Fate of DNA from retinal cells dying during development: uptake by microglia and macroglia (Müller cells). Brain Res Dev Brain Res 97, 1–8. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Zelinka C, Gallina D, et al. (2014) Reactive microglia and macrophage facilitate the formation of Müller glia‐derived retinal progenitors. Glia 62, 1608–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontainhas AM, Wang M, Liang KJ, et al. (2011) Microglial morphology and dynamic behavior is regulated by ionotropic glutamatergic and GABAergic neurotransmission. PLoS One 6(1), e15973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco‐Morcillo J, Hidalgo‐Sánchez M, Martín‐Partido G (2004) Spatial and temporal patterns of apoptosis during differentiation of the retina in the turtle. Anat Embryol (Berl) 208, 289–299. [DOI] [PubMed] [Google Scholar]
- Francisco‐Morcillo J, Hidalgo‐Sánchez M, Martín‐Partido G (2006) Spatial and temporal patterns of proliferation and differentiation in the developing turtle eye. Brain Res 1103, 32–48. [DOI] [PubMed] [Google Scholar]
- Francisco‐Morcillo J, Bejarano‐Escobar R, Rodríguez‐León J, et al. (2014) Ontogenetic cell death and phagocytosis in the visual system of vertebrates. Dev Dyn 243, 1203–1225. [DOI] [PubMed] [Google Scholar]
- Francke M, Makarov F, Kacza J, et al. (2001) Retinal pigment epithelium melanin granules are phagocytozed by Müller glial cells in experimental retinal detachment. J Neurocytol 30, 131–136. [DOI] [PubMed] [Google Scholar]
- Friedenwald JS, Chan E (1932) Pathogenesis of retinitis pigmentosa with a note on the phagocytic activity of Meller's fibers. Arch of Opthamol 8, 173. [Google Scholar]
- Gallina D, Todd L, Fischer AJ (2014) A comparative analysis of Müller glia‐mediated regeneration in the vertebrate retina. Exp Eye Res 123, 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Porrero JA, Ojeda JL (1979) Cell death and phagocytosis in the neuroepitelium of the developing retina. A TEM and SEM study. Experientia 35, 375–376. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben‐Sasson SA (1992) Identification of programmed cell death insitu via specific labeling of nuclear DNA fragmentation. J Cell Biol 119, 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D (2014) Muller glial cell reprogramming and retina regeneration. Nat Rev Neurosci 15, 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorsuch RA, Hyde DR (2014) Regulation of Muller glial dependent neuronal regeneration in the damaged adult zebrafish retina. Exp Eye Res 123, 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon A, Roger JE, Yang XJ, et al. (2016) Muller glial cell‐dependent regeneration of the neural retina: an overview across vertebrate model systems. Dev Dyn 245, 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, Harada C, Kohsaka S, et al. (2002) Microglia‐Müller glia cell interactions control neurotrophic factor production during light‐induced retinal degeneration. J Neurosci 22, 9228–9236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harahush BK, Hart NS, Green K, et al. (2009) Retinal neurogenesis and ontogenetic changes in the visual system of the brown banded bamboo shark, Chiloscyllium punctatum (Hemiscyllidae, Elasmobranchii). J Comp Neurol 513, 83–97. [DOI] [PubMed] [Google Scholar]
- Hollborn M, Francke M, Iandiev I, et al. (2008) Early activation of inflammation‐ and immune response‐related genes after experimental detachment of the porcine retina. Invest Ophthalmol Vis Sci 49, 1262–1273. [DOI] [PubMed] [Google Scholar]
- Hughes WF, Lavelle A (1975) The effects of early tectal lesions on development in the retina ganglion cell layer of chick embryos. J Comp Neurol 163, 265–284. [DOI] [PubMed] [Google Scholar]
- Hughes WF, McLoon SC (1979) Ganglion cell death during normal retinal development in the chick: comparisons with cell death induced by early target field destructions. Exp Neurol 66, 587–601. [DOI] [PubMed] [Google Scholar]
- Hume DA, Perry VH, Gordon S (1983) Immunohistochemical localization of a macrophage‐specific antigen in developing mouse retina: phagocytosis of dying neurons and differentiation of microglial cells to form a regular array in the plexiform layers. J Cell Biol 97, 253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav AP, Roesch K, Cepko CL (2009) Development and neurogenic potential of Muller glial cells in the vertebrate retina. Prog Retin Eye Res 28, 249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JFR, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide‐ranging implications in tissue kinetic. British J Cancer 26, 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knabe W, Suss M, Kuhn HJ (2000) The patterns of cell death and of macrophages in the developing forebrain of the tree shrew Tupaia belangeri . Anat Embryol (Berl) 201, 157–168. [DOI] [PubMed] [Google Scholar]
- Koshibu A (1978) Ultrastructural studies on absorption of experimentally produced subretinal hemorrhage. 2. Autolysis of macrophages and disappearance of erythrocytes from the subretinal space at the late stage (author's transl). Nippon Ganka Gakkai Zasshi 82, 471–479. [PubMed] [Google Scholar]
- Kumar A, Pandey RK, Miller LJ, et al. (2013) Muller glia in retinal innate immunity: a perspective on their roles in endophthalmitis. Crit Rev Immunol 33, 119–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara T, Weidman TA (1974) Development of the prenatal rat retina. Invest Ophthalmol 13, 725–739. [PubMed] [Google Scholar]
- Lenkowski JR, Raymond PA (2014) Muller glia: stem cells for generation and regeneration of retinal neurons in teleost fish. Prog Retin Eye Res 40, 94–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ye F, Xiong H, et al. (2015) IL‐1beta induces IL‐6 production in retinal Müller cells predominantly through the activation of p38 MAPK/NF‐kappaB signaling pathway. Exp Cell Res 331, 223–231. [DOI] [PubMed] [Google Scholar]
- Long KO, Fisher SK, Fariss RN, et al. (1986) Disc shedding and autophagy in the cone‐dominant ground squirrel retina. Exp Eye Res 43, 193–205. [DOI] [PubMed] [Google Scholar]
- Mano T, Puro DG (1990) Phagocytosis by human retinal glial cells in culture. Invest Ophthalmol Vis Sci 31, 1047–1055. [PubMed] [Google Scholar]
- Marín‐Teva JL, Almendros A, Calvente R, et al. (1998) Tangential migration of ameboid microglia in the developing quail retina: mechanism of migration and migratory behavior. Glia 22, 31–52. [DOI] [PubMed] [Google Scholar]
- Marín‐Teva JL, Almendros A, Calvente R, et al. (1999a) Proliferation of actively migrating ameboid microglia in the developing quail retina. Anat Embryol (Berl) 200, 289–300. [DOI] [PubMed] [Google Scholar]
- Marín‐Teva JL, Calvente R, Cuadros MA, et al. (1999b) Circumferential migration of ameboid microglia in the margin of the developing quail retina. Glia 27, 226–238. [DOI] [PubMed] [Google Scholar]
- Marín‐Teva JL, Cuadros MA, Calvente R, et al. (1999c) Naturally occurring cell death and migration of microglial precursors in the quail retina during normal development. J Comp Neurol 412, 255–275. [PubMed] [Google Scholar]
- Martín‐Partido G, Rodríguez‐Gallardo L, Álvarez IS, et al. (1988) Cell death in the ventral region of the neural retina during the early development of the chick embryo eye. Anat Record 222, 272–281. [DOI] [PubMed] [Google Scholar]
- Mellén MA, de la Rosa EJ, Boya P (2008) The autophagic machinery is necessary for removal of cell corpses from the developing retinal neuroepithelium. Cell Death Differ 15, 1279–1290. [DOI] [PubMed] [Google Scholar]
- Miller B, Miller H, Ryan SJ (1986) Experimental epiretinal proliferation induced by intravitreal red blood cells. Am J Ophthalmol 102, 188–195. [DOI] [PubMed] [Google Scholar]
- Morris AC, Schroeter EH, Bilotta J, et al. (2005) Cone survival despite rod degeneration in XOPS‐mCFP transgenic zebrafish. Invest Ophthalmol Vis Sci 46, 4762–4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa T, Matsubara A, Noda K, et al. (2006) Characterization of cytokine responses to retinal detachment in rats. Mol Vis 12, 867–878. [PubMed] [Google Scholar]
- Navascués J, Moujahid A, Almendros A, et al. (1995) Origin of microglia in the quail retina: central‐to‐peripheral and vitreal‐to‐scleral migration of microglial precursors during development. J Comp Neurol 354, 209–228. [DOI] [PubMed] [Google Scholar]
- Neumann H, Kotter MR, Franklin RJ (2009) Debris clearance by microglia: an essential link between degeneration and regeneration. Brain 132, 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E, Reichenbach A (1996) The Müller cell: a functional element of the retina. Trends Neurosci 19, 307–312. [DOI] [PubMed] [Google Scholar]
- Nishizono H, Murata Y, Tanaka M, et al. (1993) Evidence that Müller cells can phagocytize egg‐lecithin‐coated silicone particles. Tissue Cell 25, 305–310. [DOI] [PubMed] [Google Scholar]
- Pavón‐Muñoz T, Bejarano‐Escobar R, Blasco M, et al. (2016) Retinal development in the gilthead seabream Sparus aurata . J Fish Biol 88, 492–507. [DOI] [PubMed] [Google Scholar]
- Penfold PL, Provis JM (1986) Cell death in the development of the human retina: phagocytosis of pyknotic and apoptotic bodies by retinal cells. Graefes Arch Clin Exp Ophthalmol 224, 549–553. [DOI] [PubMed] [Google Scholar]
- Peterson RE, Fadool JM, McClintock J, et al. (2001) Müller cell differentiation in the zebrafish neural retina: evidence of distinct early and late stages in cell maturation. J Comp Neurol 429, 530–540. [DOI] [PubMed] [Google Scholar]
- Ponsioen TL, van Luyn MJ, van der Worp RJ, Los LI et al. (2007) In vitro phagocytosis of collagens by immortalised human retinal Müller cells. Graefes Arch Clin Exp Ophthalmol 245, 82–92. [DOI] [PubMed] [Google Scholar]
- Prada P, Puga J, Pérez‐Méndez L, et al. (1991) Spatial and temporal patterns of neurogenesis in the chick retina. Eur J Neurosci 3, 559–569. [DOI] [PubMed] [Google Scholar]
- Rapaport DH, Wong LL, Wood ED, et al. (2004) Timing and topography of cell genesis in the rat retina. J Comp Neurol 474, 304–324. [DOI] [PubMed] [Google Scholar]
- Ravichandran KS (2011) Beginnings of a good apoptotic meal: the find‐me and eat‐me signaling pathways. Immunity 35, 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK, Bernardos RL, et al. (2006) Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev Biol 6, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach A, Bringmann A (2013) New functions of Müller cells. Glia 61, 651–678. [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Gallardo L, Lineros‐Domínguez MC, Francisco‐Morcillo J, et al. (2005) Macrophages during retina and optic nerve development in the mouse embryo: relationship to cell death and optic fibres. Anat Embryol (Berl) 210, 303–316. [DOI] [PubMed] [Google Scholar]
- Roesch K, Jadhav AP, Trimarchi JM, et al. (2008) The transcriptome of retinal Müller glial cells. J Comp Neurol 509(2), 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque RS, Imperial CJ, Caldwell RB (1996) Microglial cells invade the outer retina as photoreceptors degenerate in Royal College of Surgeons rats. Inv Ophthal Visual Sci 37, 196–203. [PubMed] [Google Scholar]
- Rosenthal AR, Appleton B (1975) Histochemical localization of intraocular copper foreign bodies. Am J Ophthalmol 79, 613–625. [DOI] [PubMed] [Google Scholar]
- Sánchez‐Farías N, Candal E (2016) Identification of radial glia progenitors in the developing and adult retina of sharks. Front Neuroanat 10, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez‐López A, Cuadros MA, Calvente R, et al. (2004) Radial migration of developing microglial cells in quail retina: a confocal microscopy study. Glia 46, 261–273. [DOI] [PubMed] [Google Scholar]
- Santos AM, Calvente R, Tassi M, et al. (2008) Embryonic and postnatal development of microglial cells in the mouse retina. J Comp Neurol 506, 224–239. [DOI] [PubMed] [Google Scholar]
- Santos AM, Martín‐Oliva D, Ferrer‐Martín RM, et al. (2010) Microglial response to light‐induced photoreceptor degeneration in the mouse retina. J Comp Neurol 518, 477–492. [DOI] [PubMed] [Google Scholar]
- Scuderi S, D'Amico AG, Federico C, et al. (2015) Different retinal expression patterns of IL‐1alpha, IL‐1beta, and their receptors in a rat model of type 1 STZ‐induced diabetes. J Mol Neurosci 56, 431–439. [DOI] [PubMed] [Google Scholar]
- Seitz R, Ohlmann A, Tamm ER (2013) The role of Müller glia and microglia in glaucoma. Cell Tissue Res 353, 339–345. [DOI] [PubMed] [Google Scholar]
- Seo JW, Kim JH, Kim JH, et al. (2012) Time‐dependent effects of hypothermia on microglial activation and migration. J Neuroinflammation 9, 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PK, Shiha MJ, Kumar A (2014) Antibacterial responses of retinal Müller glia: production of antimicrobial peptides, oxidative burst and phagocytosis. J Neuroinflammation 11, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzenburg JU, Haas J, Hartig W, et al. (1992) Phagocytosis of latex beads by rabbit retinal Müller (glial) cells in vitro . J Hirnforsch 33, 557–564. [PubMed] [Google Scholar]
- Thanos S, Moore S, Hong YM (1996) Retinal microglia. Prog Retin Eye Res 15, 331–361. [Google Scholar]
- Thanos S (1999) Genesis, neurotrophin responsiveness, and apoptosis of a pronounced direct connection between the two eyes of the chick embryo: a natural error or a meaningful developmental event? J Neurosci 19, 3900–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel R, Kassen SC, Montgomery JE, et al. (2008) Inhibition of Müller glial cell division blocks regeneration of the light‐damaged zebrafish retina. Dev Neurobiol 68, 392–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckermann O, Wolf A, Kutzera F, et al. (2006) Glutamate release by neurons evokes a purinergic inhibitory mechanism of osmotic glial cell swelling in the rat retina: activation by neuropeptide Y. J Neurosci Res 83, 538–550. [DOI] [PubMed] [Google Scholar]
- Valenciano AI, Boya P, de la Rosa EJ (2009) Early neural cell death: numbers and cues from the developing neuroretina. Int J Dev Biol 53, 1515–1528. [DOI] [PubMed] [Google Scholar]
- Vecino E, Rodríguez FD, Ruzafa N, et al. (2016) Glia‐neuron interactions in the mammalian retina. Prog Retin Eye Res 51, 1–40. [DOI] [PubMed] [Google Scholar]
- Wagner EC, Raymond PA (1991) Müller glial cells of the goldfish retina are phagocytic in vitro but not in vivo . Exp Eye Res 53, 583–589. [DOI] [PubMed] [Google Scholar]
- Wang M, Ma W, Zhao L, et al. (2011) Adaptive Müller cell responses to microglial activation mediate neuroprotection and coordinate inflammation in the retina. J Neuroinflammation 8, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohl SG, Schmeer CW, Isenmann S (2012) Neurogenic potential of stem/progenitor‐like cells in the adult mammalian eye. Prog Retin Eye Res 31, 213–242. [DOI] [PubMed] [Google Scholar]
- Xiang M (2013) Intrinsic control of mammalian retinogenesis. Cell Mol Life Sci 70, 2519–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiss CJ, Johnson EA (2004) Proliferation of microglia, but not photoreceptors, in the outer nuclear layer of the rd‐1 mouse. Invest Ophthalmol Vis Sci 45, 971–976. [DOI] [PubMed] [Google Scholar]


