Abstract
Converging lines of behavioral, electrophysiological, and biochemical evidence suggest that 5-HT2C receptor signaling may bidirectionally influence reward-related behavior through an interaction with the mesolimbic dopamine (DA) system. Here we directly test this hypothesis by examining how modulating 5-HT2C receptor activity affects DA-dependent behaviors and relate these effects to changes in nucleus accumbens (NAc) DA release. In C57BL/6 mice, locomotor activity and responding for a conditioned reinforcer (CRf), a measure of incentive motivation, were examined following treatment with three 5-HT2C receptor ligands: the agonist CP809101 (0.25–3 mg/kg), the antagonist SB242084 (0.25–1 mg/kg), or the antagonist/inverse agonist SB206553 (1–5 mg/kg). We further tested whether doses of these compounds that changed locomotor activity and responding for a CRf (1 mg/kg CP809101, 0.5 mg/kg SB242084, or 2.5 mg/kg SB206553) also altered NAc DA release using in vivo microdialysis in anesthetized mice. CP809101 reduced locomotor activity, responding for a CRf, and NAc DA release. In contrast, both SB242084 and SB206553 enhanced locomotor activity, responding for a CRf, and NAc DA release, although higher doses of SB206553 produced opposite behavioral effects. Pretreatment with the non-selective DA receptor antagonist α-flupenthixol prevented SB242084 from enhancing responding for a CRf. Thus blocking tonic 5-HT2C receptor signaling can release serotonergic inhibition of mesolimbic DA activity and enhance reward-related behavior. The observed bidirectional effects of 5-HT2C receptor ligands may have important implications when considering the 5-HT2C receptor as a therapeutic target for psychiatric disorders, particularly those presenting with motivational dysfunctions.
Introduction
The ascending serotonin (5-hydroxytryptamine (5-HT)) system innervates virtually the entire brain and modulates many behavioral processes. The specific behavioral and physiological effects of 5-HT depend on signaling at multiple receptor subtypes (Barnes and Sharp, 1999). Several lines of evidence suggest that the 5-HT2C receptor in particular has an important role in modulating behaviors directed toward obtaining rewarding stimuli. 5-HT2C receptor agonists, including Ro60–1075, CP809101, WAY161503, and lorcaserin, reduce food intake (Clifton et al, 2000; Fletcher et al, 2009) and operant responding for food reinforcement (Grottick et al, 2000; Wolff and Leander, 2000). 5-HT2C receptor agonists also reduce the efficacy of brain stimulation reward (Hayes et al, 2009; Zeeb et al, 2015) and the reinforcing efficacy of self-administration of several abused drugs, including cocaine (Grottick et al, 2000; Manvich et al, 2012b), nicotine (Higgins et al, 2012), oxycodone (Neelakantan et al, 2017), and methamphetamine (Gerak et al, 2016). In mice, a conditioned place preference produced by cocaine, nicotine, or THC is also blocked by 5-HT2C agonists (Craige and Unterwald, 2013; Ji et al, 2006). Further, 5-HT2C receptor agonists reduce appetitive behaviors elicited by stimuli associated with rewards such as food and drugs of abuse (Guy and Fletcher, 2014; Higgins et al, 2012; Neisewander and Acosta, 2007). Taken together, these studies suggest that 5-HT2C receptor activation produces a general reduction in reward-related behavior.
Recently, we found that elevating endogenous extracellular 5-HT levels through pharmacological or genetic disruption of the 5-HT transporter reduced multiple operant measures of motivation (Browne and Fletcher, 2016). One of these measured motivation elicited by a reward-associated stimulus serving as a conditioned reinforcer (CRf). Reductions in responding for a CRf produced by increasing 5-HT activity could be prevented by a low dose of the selective 5-HT2C receptor antagonist SB242084 (Bromidge et al, 1997; Kennett et al, 1997), further illustrating the importance of this receptor in reward-related behavior. In the course of this work, we also noted that higher doses of SB242084, when administered alone, tended to increase responding for a CRf. This observation is in keeping with findings that SB242084 enhances some reward-related behaviors supported by cocaine (Capriles et al, 2012; Fletcher et al, 2002). However, relatively few studies have examined the effects of 5-HT2C receptor antagonists on non-drug reward-related behavior.
A recent study found that SB242084 increased instrumental responding for food in several tasks in mice (Bailey et al, 2016). However, other groups report no effect of SB242084 on responding for food on a progressive ratio schedule of reinforcement in mice (Fletcher et al, 2010), while others attribute an increase in responding to changes in motor processes (Bezzina et al, 2015). These mixed results regarding the effects of 5-HT2C receptor antagonists indicate that a more systematic investigation of 5-HT2C receptor antagonists on reward and motivation is required.
One plausible mechanism by which 5-HT2C receptor signaling influences reward-related behavior is through an interaction with the mesolimbic dopamine (DA) system—a critical neural substrate for promoting reward and reinforcement (Salamone and Correa, 2002; Schultz, 1998). Consistent with their behavioral effects, 5-HT2C receptor agonists inhibit the firing rate of DA neurons in the ventral tegmental area (VTA; Di Matteo et al, 2000), leading to a reduction in DA release in the nucleus accumbens (NAc; De Deurwaerdère et al, 2004; Di Matteo et al, 2000). In contrast, the antagonist SB242084 increases the firing rate of VTA DA neurons producing a modest increase in NAc DA release (De Deurwaerdère et al, 2004; Di Matteo et al, 1999). This potentiation of mesolimbic DA system function may explain the ability of SB242084 to enhance some reward-related behavior. Another compound commonly used as a 5-HT2C receptor antagonist, SB206553, exhibits inverse agonist properties at 5-HT2C receptors in vitro (Aloyo et al, 2009; Kennett et al, 1996). Consistent with this idea, SB206553 also increases NAc DA release to a much greater extent than does SB242084 (De Deurwaerdère et al, 2004). However, the effects of SB206553 on reward-related behavior have not been well studied. Based on its ability to potentiate mesolimbic DA activity, we hypothesize that SB206553 should enhance DA-dependent, reward-related behavior to a greater extent than SB242084.
The present experiments addressed several issues related to 5-HT2C receptor ligands and reward-related behavior. The first objective of this work was to systematically compare the effects of the selective 5-HT2C receptor agonist CP809101 (Siuciak et al, 2007), the selective 5-HT2C receptor antagonist SB242084, and the putative 5-HT2C receptor inverse agonist SB206553 on locomotor activity and responding for a CRf in mice. These behaviors were chosen because both are sensitive to bidirectional changes in mesolimbic DA activity; increasing DA enhances locomotor activity and responding for a CRf (Kelly et al, 1975; Taylor and Robbins, 1984), while decreasing DA reduces both behaviors (Fletcher and Higgins, 1997). The second objective was to examine whether doses of 5-HT2C receptor ligands that altered locomotor activity and responding for a CRf also produced parallel changes in NAc DA release. Third, having found that 5-HT2C receptor blockade increased both responding for a CRf and NAc DA release, we sought to establish a causal relationship between these two effects. Thus we examined whether the DA receptor antagonist α-flupenthixol could prevent SB242084-mediated increases in responding for a CRf.
Materials and methods
Animals
In these studies, 150 male C57BL/6N mice (Charles River, QC) were used. Mice were pair-housed in temperature and humidity controlled rooms on a 12-h light–dark cycle with lights on at 0700 hours. In tests of responding for a CRf, all mice received restricted access to water, with 2 h of daily water access. Food was available ad libitum. This work adhered to the Canadian Council on Animal Care guidelines and was approved by the Centre for Addiction and Mental Health Animal Care Committee.
Behavioral Testing Apparatus
Locomotor activity was measured using a custom-built system of 16 clear polycarbonate chambers measuring 25 × 45 × 20 cm3. The long axis of each box had an array of 11 externally mounted infrared photodetectors spaced 4 cm apart and 2 cm above the cage floor. Photocell interruptions were recorded as locomotor activity counts on a DELL desktop computer.
Responding for a CRf was assessed using 12 operant conditioning boxes (Med Associates, St Albans, VT) measuring 22 × 18 × 13 cm3. The front wall of the chamber housed a horizontally centered reinforcer magazine that contained an infrared photodetector and a roof-mounted light. A motor-driven dipper could be raised to deliver 0.02 ml of liquid through a hole in the floor of the reinforcer magazine. The wall also contained two retractable levers flanking the reinforcer magazine. Positioned above each lever was a yellow stimulus light. Each operant conditioning box was illuminated by a houselight and was enclosed in a sound-attenuating chamber equipped with a ventilation fan.
Procedure for Assessing Locomotor Activity
Mice were first habituated to testing chambers for three 1-h sessions. In subsequent tests, locomotor activity was examined for 1 h following drug treatment. All testing was carried out under red lighting.
Procedure for Assessing Responding for a CRf
The conditioned reinforcement paradigm was conducted as previously described (Browne et al, 2014).
Pavlovian conditioning
Mice received 14 daily 40-min sessions in which a conditioned stimulus (CS) was presented just prior to the delivery of 0.02 ml 0.2% saccharin 30 times on a RT 60-s schedule. The compound CS consisted of houselights off, both stimulus lights on for 5 s, and the sound of the mechanical dipper being elevated at the end of the 5-s period. The saccharin reinforcer was presented for 8 s during which time both stimulus lights remained on and the houselight remained off (13 s total CS duration). At the end of this period, stimulus lights were extinguished, the houselight was reilluminated, and the dipper descended. The main dependent variables measured were head entries into the reward magazine during the 5-s CS period (prior to saccharin delivery) and a control measure of head entries during a 5-s period just prior to CS onset (PreCS period).
Operant conditioning
Following Pavlovian conditioning, two response levers were introduced to the operant chambers: an active lever and an inactive lever. Responding on the active lever produced a shortened version of the CS from the Pavlovian conditioning phase (5-s period with the houselight off and both stimulus lights on and elevation of the empty dipper during the last 2 s), now referred to as a CRf, according to a random ratio 2 schedule of reinforcement. Responding on the inactive lever had no programmed consequences. Mice were tested in 40-min sessions. We have previously shown that, in mice, this procedure produces stable responding and does not require additional Pavlovian conditioning sessions (Browne et al, 2014).
Surgical Procedures for Microdialysis
Mice maintained under inhaled isoflurane anesthesia received stereotaxic surgery to implant a microdialysis guide cannula above the left or right NAc (counterbalanced; targeting A/P +1.54, M/L ±0.70, D/V −2.0, according to Franklin and Paxinos, 2007), which was fixed to the skull with dental cement (RelyX unicem, 3M, Minnesota, USA). Mice received ketoprofen analgesia (5 mg/kg, subcutaneously) prior to surgery and once daily for 2 days following surgery. Dialysis procedures commenced 1 week following surgery.
Microdialysis and High-Performance Liquid Chromatography (HPLC)
Microdialysis sampling was conducted in mice maintained under 2% inhaled isoflurane anesthesia. Throughout the procedure, body temperature was maintained at 37 °C with an electric heating pad (CWE, Ardmore, PA). A dialysis probe (2 mm cuprophane membrane; Scientific Products, Toronto, Canada) was lowered through the guide cannula. This probe length was used to ensure sampling of DA levels throughout the entire dorsal–ventral axis of the NAc, which is approximately 1.5 mm. However, in some cases a small portion of the dialysis probe may have extended to the caudate (dorsal) or the olfactory tubercle (ventral). Probes were continuously perfused with artificial cerebrospinal fluid at 0.5 μl/min using a 1.0 ml gastight syringe and a syringe pump (CMA Microdialysis, Holliston, USA). Sampling began 1 h following probe insertion. Samples were collected every 20 min, and baseline DA concentration was considered stable when three consecutive samples varied <10% from the previous sample (Baseline; three samples required on average).
Measurement of DA levels in dialysate samples was conducted on an analytical system consisting of an Antec Leyden LC110 Alexys HPLC system coupled to a Decade-II electrochemical detection cell and an ALF 105 50 × 1 mm column with C-18 3 μm packing material (ATS Scientific, Burlington, Canada). DA detection potential was 400 mV against an Ag/AgCl electrode. The mobile phase consisted of 50 mM phosphoric acid, 8 mM NaCl, 0.1 mM EDTA, 500 mg/l OSA, and 12.5% methanol in purified distilled water. The pH was adjusted to 6.0 and the solution was filtered through a 0.22 μm nylon filter. The flow rate was 65 μl/min. Chromatograms were interpreted using the Clarity Chromatography software. The average in vitro probe recovery was 9.4%.
Drugs
CP809101 (Tocris, Bristol, UK) was dissolved in a 0.9% saline solution containing 5% tween; SB242084 (Tocris) and SB206553 (Tocris) were dissolved in a 0.9% saline solution containing 8% β-cyclodextrin (Sigma-Aldrich, Oakville, Canada); α-flupenthixol (Tocris) was dissolved in 0.9% saline. All drugs were administered intraperitoneally in a volume of 10 ml/kg. CP809101 was injected 20 min before testing, SB242084 was injected 40 min before testing, and α-flupenthixol was injected 60 min before testing. SB206553 was injected 5 min prior to testing, which was determined from published work (Gleason et al, 2001) and pilot studies conducted in our laboratory. Doses of all drugs are expressed in terms of the free base. For all behavioral experiments, drug treatments followed a within-subjects design with dose order determined from a Latin square and with testing sessions separated by 72 h.
Experimental Procedures
Experiment 1: Effects of 5-HT2C receptor ligands on locomotor activity
Locomotor activity was measured for 1 h following treatment with either CP809101 (0.3, 1, 3 mg/kg, or vehicle; n=16), SB242084 (0.25, 0.5, 1 mg/kg, or vehicle; n=16), or SB206553 (1, 2.5, 5 mg/kg, or vehicle; n=16).
Experiment 2: Effects of 5-HT2C receptor ligands on responding for a CRf
Following Pavlovian conditioning, baseline responding for a CRf was first measured over three test sessions. Subsequently, mice were randomly separated into three groups and tested following drug treatment. One group of mice (n=12) was tested following treatment with CP809101 (0.25, 0.5, 1 mg/kg, or vehicle). This group was also retested with 1 and 3 mg/kg doses to extend the dose–response curve for CP809101. The second group (n=24) was tested following treatment with SB242084 (0.25, 0.5, 1 mg/kg, or vehicle). The third group (n=24) was tested following treatment with SB206553 (1, 2.5, 5 mg/kg, or vehicle).
Experiment 3: Effects of 5-HT2C receptor ligands on NAc DA release
In this experiment, we measured changes in extracellular levels of DA in the NAc following treatment with 1 mg/kg CP809101, 0.5 mg/kg SB242084, or 2.5 mg/kg SB206553. These doses were chosen based on their ability to alter behavior in Experiments 1 and 2.
Once baseline DA measurements were stable, mice received an injection of vehicle, and three samples were collected (60 min). Subsequently, mice were injected with CP809101 (1 mg/kg; n=6), SB242084 (0.5 mg/kg; n=6), or SB206553 (2.5 mg/kg; n=6), and 9 more samples were collected (180 min). Extracellular DA levels following vehicle and drug treatment were expressed as a percentage of change from baseline DA concentration. In one mouse from the group tested with CP80901, DA levels in the last four samples could not be analyzed owing to technological complications. The percentage of change from baseline DA concentration for these missing values was estimated using the group average.
Immediately following collection of the last sample, mice were killed via cervical dislocation, and the brains were extracted and flash-frozen on dry ice. Forty-μm sections throughout the NAc were collected on a cryostat, and dialysis probe location was verified with cresyl violet staining.
Experiment 4: Effects of α-flupenthixol on SB242084-potentiated responding for a CRf
The results of Experiments 2 and 3 suggested that blocking the 5-HT2C receptor might increase responding for a CRf by enhancing mesolimbic DA activity. To test this hypothesis, we examined whether the broad-spectrum DA receptor antagonist α-flupenthixol could prevent the effects of SB242084 on responding for a CRf. SB242084 was chosen over SB206553 due to its consistent effect on responding irrespective of dose.
An experimentally naive group of mice (n=24) underwent Pavlovian conditioning followed by three baseline sessions of responding for a CRf (data not shown). Subsequently, mice were tested after treatment with 0.4 mg/kg α-flupenthixol or its vehicle followed by 0.5 mg/kg SB242084 or its vehicle. As this dose of α-flupenthixol produced a minor but non-significant reduction in responding on its own, we repeated the experiment with a lower dose. Thus responding for a CRf was again tested in these mice after treatment with 0.25 mg/kg α-flupenthixol or its vehicle followed by 0.5 mg/kg SB242084 or its vehicle.
Statistical analysis
Data were analyzed using Statistica (Statsoft, Tulsa, OK). All data were analyzed using repeated-measures ANOVAs. Within-subjects factors included: dose of drug used (Dose), session time (Time), magazine entries period (CS vs PreCS), test session number (Session), active or inactive lever responses (Lever), dialysate sample number (Sample), SB242084 condition, and α-flupenthixol condition. In Experiment 3, dialysis samples following both vehicle and drug treatments were included in the overall ANOVA. Post hoc pairwise comparisons were performed using Tukey’s HSD tests.
Results
Experiment 1: Effects of 5-HT2C Receptor Ligands on Locomotor Activity
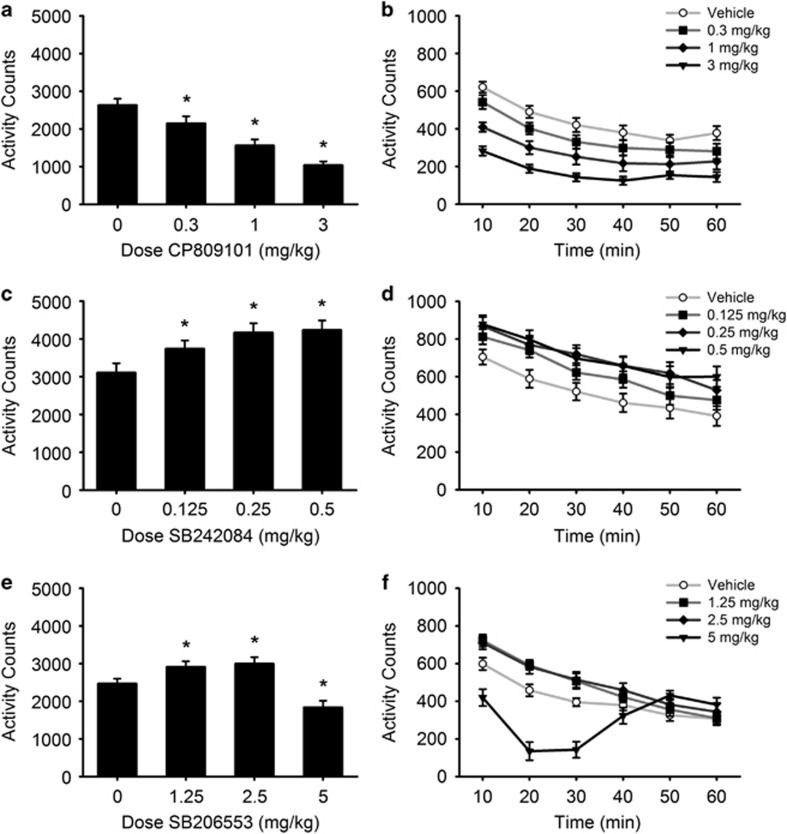
CP809101 significantly reduced total activity counts (Figure 1a; Dose: F(3,63)=44.43, p<0.001) at all doses compared with vehicle (all p<0.05). Analysis of activity counts across time (Figure 1b) found a main effect of Dose (F(3,45)=35.11, p<0.001), a main effect of Time (F(5,75)=62.47, p<0.001), and a Dose × Time interaction (F(15,225)=1.75, p=0.042). In contrast, SB242084 significantly increased total activity counts (Figure 1c; Dose: F(3,63)=16.14, p<0.001) at all doses compared with vehicle (all p<0.01). Analysis of activity counts across time (Figure 1d) found a main effect of Dose (F(3,45)=19.19, p<0.001) and a main effect of Time (F(5,75)=80.06, p<0.001) but no Dose × Time interaction (F(15,225)=0.94, NS). SB206553 significantly increased total activity counts (Figure 1e; Dose: F(3,63)=23.32, p<0.001) at 1 and 2.5 mg/kg doses compared with vehicle (both p<0.05), while 5 mg/kg reduced activity counts (p<0.01). Analysis of activity counts across time (Figure 1f) revealed a main effect of Dose (F(3,45)=23.32, p<0.001), a main effect of Time (F(5,75)=45.08, p<0.001), and a Dose × Time interaction (F(15,225)=19.85, p<0.001). This reflects the fact that, within the first 30 min of testing, SB206553 increases activity at 1.25 and 2.5 mg/kg doses (both p<0.05) but decreases activity at the 5 mg/kg dose (p<0.05).
Figure 1.
Modulating 5-HT2C receptor activity produces bidirectional changes in locomotor activity. Data are expressed as mean activity counts±SEM. Left panels show total activity counts following treatment with CP809101 (a; n=16), SB242084 (c; n=16), and SB206553 (e; n=16), respectively. Right panels (b, d, and f) show activity counts within the session in 10-min time bins. *p<0.05 vs vehicle.
Experiment 2: Effects of 5-HT2C Receptor Ligands on Responding for a CRf
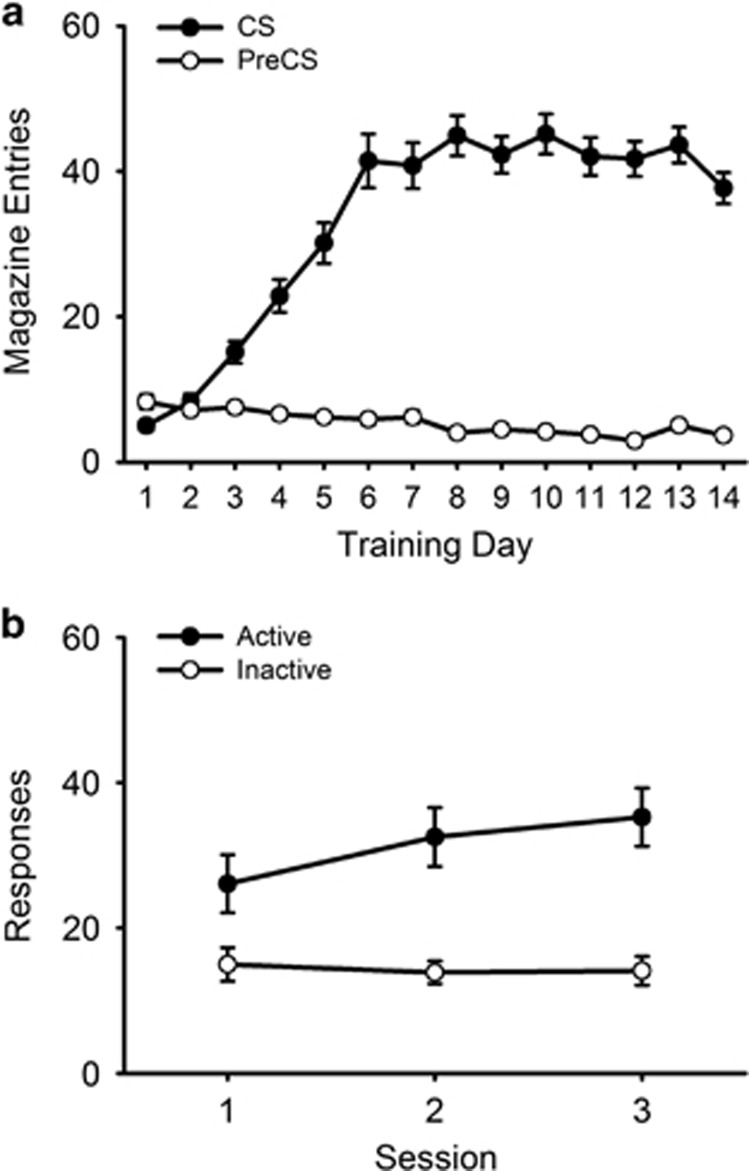
In the Pavlovian conditioning phase, mice learned to approach the reward magazine when the CS was presented (Figure 2a; CS vs PreCS × Session: F(13,767)=57.41, p<0.001). In the operant conditioning phase, all mice made significantly more responses on the active lever delivering the CRf compared with an inactive lever across three baseline sessions (Figure 2b; Lever: F(1,59)=34.72, p<0.001).
Figure 2.
Acquisition of responding for a conditioned reinforcer (N=60). Panel (a) shows reward magazine entries made during the 5-s conditioned stimulus (CS) presentation prior to saccharin delivery (CS; filled symbols) or a 5-s period just before CS onset (PreCS; open symbols) across 14 sessions of Pavlovian conditioning. Panel (b) shows responding on a lever delivering the CS, now serving as a conditioned reinforcer (active lever; filled bars), or an inactive lever (open bars) over three baseline testing sessions. Data are expressed as mean±SEM.
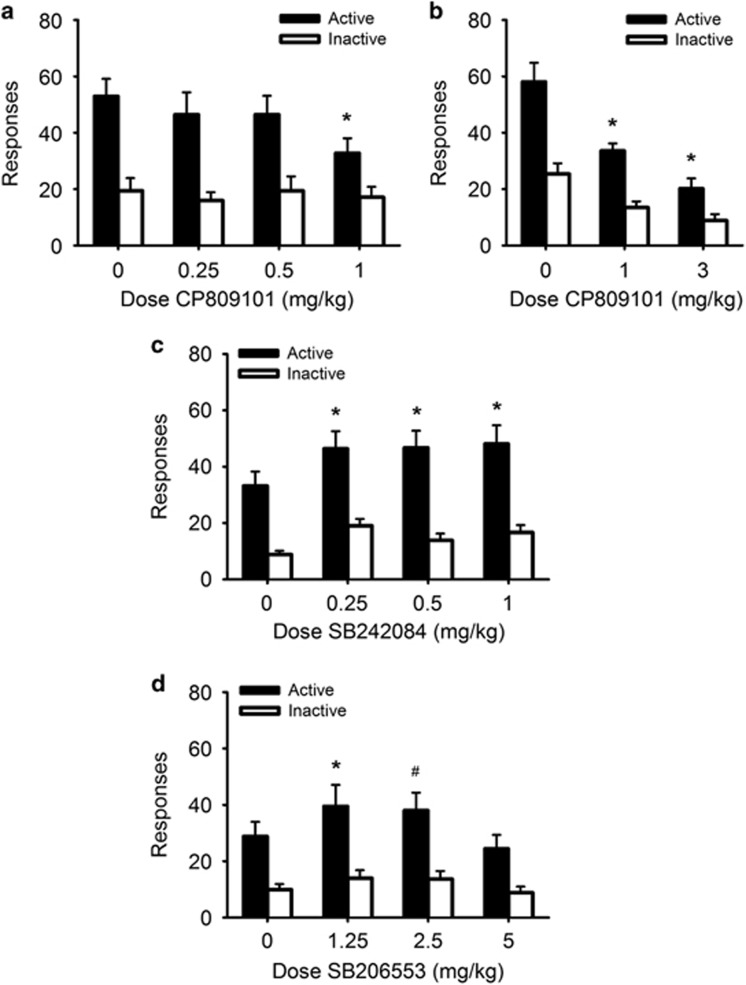
CP809101 significantly reduced responding for the CRf (Figure 3a; Dose × Lever: F(3,33)=4.36, p<0.05; Figure 3b; Dose × Lever: F(2,22)=9.30, p<0.01) at 1 and 3 mg/kg doses compared with vehicle (p<0.001). SB242084 significantly increased responding (Figure 3c; Dose: F(3,69)=7.65, p<0.001), although no Dose × Lever interaction was observed (F(3,69)=1.26, p=0.28). However, post hoc tests found that, compared with vehicle treatment, responding on the active lever was significantly increased at all doses tested (all p<0.01), while responding on the inactive lever was not significantly different across doses (all p>0.08). SB206553 generally increased responding (Figure 3d; Dose: F(3,69)=5.20, p<0.01), although the Dose × Lever interaction was not significant (F(3,69)=2.36, p=0.08). However, post hoc tests found that SB206553 increased responding on the active lever at the 1.25 mg/kg dose at p<0.05 but the increase for the 2.5 mg/kg dose was significant at p=0.06. The increase in response magnitude produced by the 1.25 and 2.5 mg/kg doses was similar (39.5 and 38.1, respectively), prompting further analysis. A paired-samples t-test found that the increase in active lever responding produced by 2.5 mg/kg SB206553 was significantly different from vehicle (t(23)=2.42, p<0.05). Post hoc tests from the overall ANOVA also found that, compared with vehicle treatment, the 5 mg/kg dose produced no change in responding (p>0.05) and that inactive lever responding was unchanged across all doses tested (all p>0.05).
Figure 3.
Modulating 5-HT2C receptor activity produces bidirectional changes in responding for a conditioned reinforcer. Graphs show the number of responses made on the active lever delivering the conditioned reinforcer (filled bars) and the inactive lever (open bars) following treatment with CP809101 or its vehicle (a and b; n=12), SB242084 or its vehicle (c; n=24), and SB206553 or its vehicle (d; n=24). Data are expressed as mean responses±SEM. *p<0.05 vs vehicle; #p=0.06 vs vehicle from post hoc tests.
Experiment 3: Effects of 5-HT2C Receptor Ligands on NAc DA Release
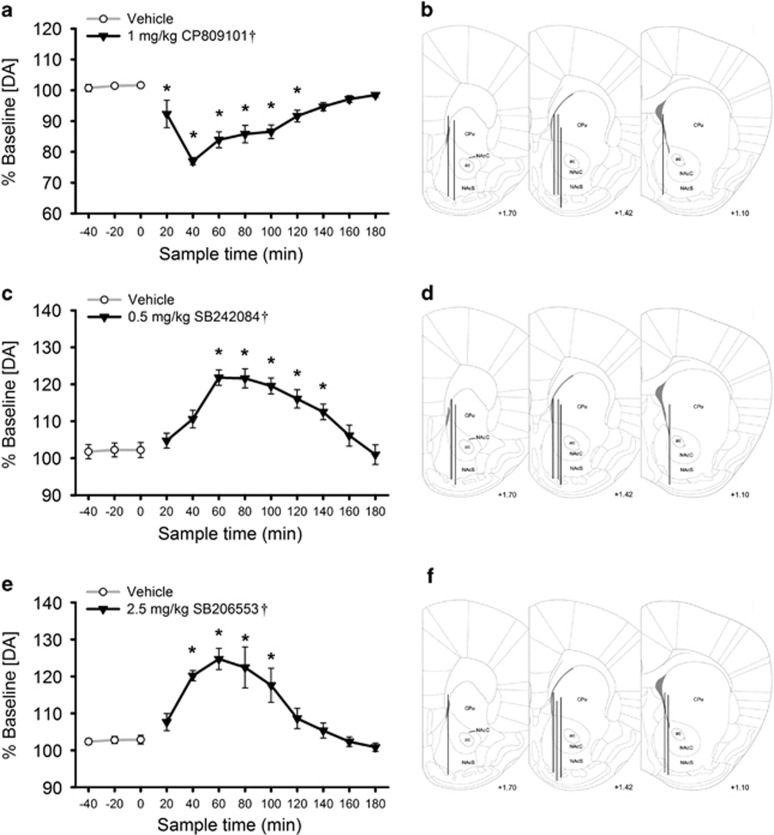
Relative to baseline, extracellular DA concentration in the NAc was significantly reduced by 1 mg/kg CP809101 (Figure 4a and b; 22.6% largest decrease; F(11,55)=22.04, p<0.001). In contrast, extracellular DA was increased by 0.5 mg/kg SB242084 (Figure 4c and d; 21.8% peak increase; F(11,55)=14.09, p<0.001) and 2.5 mg/kg SB206553 (Figure 4e and f; 24.7% peak increase; F(11,55)=14.13, p<0.001). Post hoc tests found that the change in extracellular DA returned to baseline within 120 min for CP809101, 140 min for SB242084, and 100 min for SB206553. Histological verification confirmed that dialysis probes covered the entire dorso-ventral aspect of the NAc (Figure 4b, d, and f).
Figure 4.
Modulating 5-HT2C receptor activity using doses of 5-HT2C receptor ligands that alter locomotor activity and responding for a conditioned reinforcer produces bidirectional changes in mesolimbic DA release. Left panels (a, c, and e) show extracellular dopamine concentration, expressed as a mean percentage of baseline (±SEM), measured in the nucleus accumbens following treatment with CP809101 or its vehicle (a; n=6), SB242084 or its vehicle (b; n=6), and SB206553 or its vehicle (c; n=6). Concentration was not corrected for probe recovery. Right panels (b, d, and f) depict microdialysis probe placements for mice treated with CP809101 (b), SB242084 (d), or SB206553 (f) transposed onto right hemispheres for simplicity. ac, anterior commissure; CPu, caudate/putamen; NAcC, nucleus accumbens core; NAcS, nucleus accumbens shell. †p<0.05 main effect of drug treatment. *p<0.05 vs third vehicle sample.
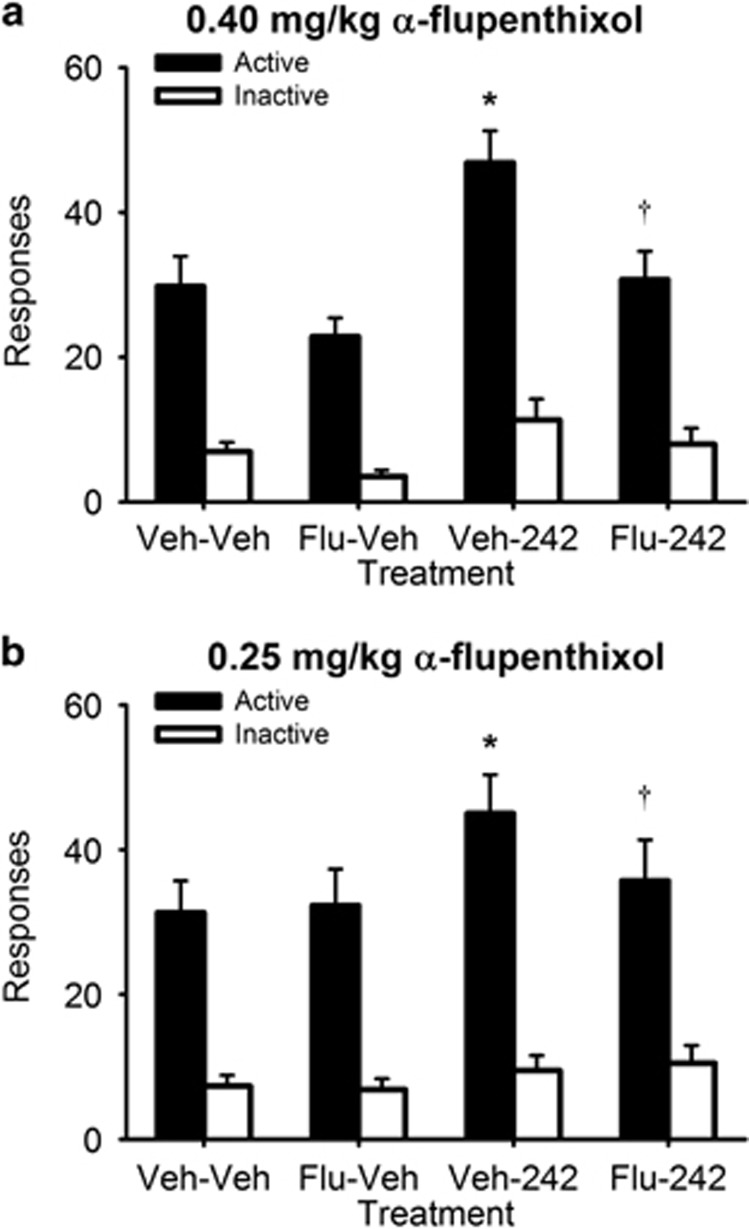
Experiment 4: Effects of α-Flupenthixol on SB242084-Potentiated Responding for a CRf
Figure 5 demonstrates that α-flupenthixol attenuated the response-enhancing effects of SB242084. In the experiment using 0.4 mg/kg α-flupenthixol (Figure 5a), the three-way interaction was not significant (F(1,22)=1.8, NS). This may have been due to variability produced by a moderate but non-significant reduction in active lever responding in the α-flupenthixol alone condition (p=0.07). However, compared with vehicle conditions, SB242084 enhanced active lever responding (p<0.001), and combining α-flupenthixol and SB242084 treatment attenuated the effect of SB242084 (p<0.05), resulting in a similar level of responding to vehicle conditions (p>0.05). In the experiment using 0.25 mg/kg α-flupenthixol (Figure 5b), a significant three-way interaction was observed (F(1,22) 4.63=p<0.05). To identify the source of this interaction, separate two-way ANOVAs were conducted for responses made on active and inactive levers. These analyses found an interaction between α-flupenthixol and SB242084 for responses made on the active lever (F(1,22)=5.07, p<0.05) but not on the inactive lever (F(1,22)=0.22, ns). Post hoc tests found that, compared with vehicle conditions, responding on the active lever was unchanged by 0.25 mg/kg α-flupenthixol alone (p>0.05) and enhanced by SB242084 (p<0.01). Combining α-flupenthixol and SB242084 treatment attenuated the effect of SB242084 (p<0.05), resulting in a similar level of responding to vehicle conditions (p>0.05).
Figure 5.
Blocking DA receptors with α-flupenthixol prevents the ability of the 5-HT2C antagonist SB242084 to enhance responding for a CRf (n=24). Data are expressed as the mean (±SEM) number of responses made on the active lever delivering the CRf (filled bars) or inactive lever (open bars). Responding was examined across four conditions in which animals were treated with α-flupenthixol or its vehicle followed by SB242084 or its vehicle. Panel (a) shows results from these experiments using 0.4 mg/kg α-flupenthixol and 0.5 mg/kg SB242084, while panel (b) shows results using 0.25 mg/kg α-flupenthixol and 0.5 mg/kg SB242084. *p<0.05 vs vehicle-vehicle condition; †p<0.05 vs vehicle-SB242084 condition.
Discussion
The present experiments examined the effects of 5-HT2C receptor ligands on two DA-dependent behaviors, locomotor activity and responding for a CRf, and related these effects to changes in mesolimbic DA system activity. The 5-HT2C receptor agonist CP809101 reduced locomotor activity and responding for a CRf, whereas the 5-HT2C receptor antagonist SB242084 and the antagonist/inverse agonist SB206553 enhanced these behaviors. These 5-HT2C receptor ligands also produced changes in mesolimbic DA activity consistent with their behavioral effects: CP809101 reduced NAc DA release, whereas SB242084 and SB206553 enhanced NAc DA release. Finally, pretreatment with the DA receptor antagonist α-flupenthixol prevented the ability of SB242084 to potentiate responding for a CRf.
The effects of CP809101 observed here in mice support and extend findings that 5-HT2C receptor agonists reduce locomotor activity and responding for a CRf in rats (Guy and Fletcher, 2014; Kennett et al, 2000). These behaviors are highly dependent on mesolimbic DA system function, which is inhibited by 5-HT2C receptor agonists (De Deurwaerdère et al, 2004; Di Matteo et al, 2000). The present results also demonstrate a direct correlation between DA activity and the behavioral effects of 5-HT2C receptor activation, in that a dose of CP809101 (1 mg/kg) that reduces DA-dependent behavior also reduces NAc DA release. Taken together, these findings support the hypothesis that 5-HT2C receptor activation may suppress reward-related behavior by inhibiting mesolimbic DA system function.
The 5-HT2C receptor antagonist SB242084 and antagonist/inverse agonist SB206553 both enhanced locomotor activity and responding for a CRf. These effects occurred at all doses of SB242084, while the effects of SB206553 were lost at the highest dose tested (5 mg/kg). We also showed that doses of SB242084 (0.5 mg/kg) and SB206553 (2.5 mg/kg), which enhanced locomotor activity and responding for a CRf, also enhanced NAc DA release. These neurochemical effects are consistent with previous reports in rats and monkeys (De Deurwaerdère et al, 2004; Manvich et al, 2012b). In the final Experiment, the broad-spectrum DA receptor antagonist α-flupenthixol blocked the ability of SB2424084 to enhance responding for a CRf. Collectively, these results provide strong evidence that 5-HT2C receptor antagonists can increase reward-related behaviors by enhancing mesolimbic DA system function. These findings have two important implications for understanding the interaction between 5-HT and DA systems in regulating reward-related behavior. First, they show that 5-HT suppresses behavior and DA release by tonic signaling at the 5-HT2C receptor, suggesting that this receptor mediates an inhibitory interaction between 5-HT and DA systems. Second, these results provide an indirect demonstration that agonist action at the 5-HT2C receptor, either by 5-HT itself or by compounds such as CP809101, reduces reward-related behavior by inhibiting mesolimbic DA system function.
SB206553 has been characterized as having inverse agonist properties in vitro (Aloyo et al, 2009; Kennett et al, 1996) and has been shown to enhance NAc DA release to a greater degree than does SB242084 (De Deurwaerdère et al, 2004). This latter finding has been used to suggest an in vivo inverse agonist effect of SB206533. Based on this evidence, we hypothesized that SB206553 would perhaps increase DA-dependent behavior to a greater magnitude than SB242084. However, we observed no differences in the ability of SB206553 and SB242084 to enhance locomotor activity or responding for a CRf. In fact, 5 mg/kg of SB206553, which was previously shown to enhance mesolimbic DA to a greater extent than any dose of SB242084 in rats (De Deurwaerdère et al, 2004), had no effect on responding for a CRf (unlike lower doses; Figure 3d) and significantly reduced locomotor activity below control levels (Figure 1e and f). The extent to which the 2.5 mg/kg dose of SB206553 increased locomotor activity, responding for a CRf, and NAc DA release was similar to the increases induced by SB242084. Thus these studies were unable to demonstrate an inverse agonist effect of SB206553 on DA-dependent, reward-related behavior and instead suggest that SB206533 altered behavior in a manner consistent with 5-HT2C receptor antagonist action.
At 5 mg/kg, SB206553 suppressed locomotor activity and failed to increase responding for CRf. This complements other reports that high doses of SB206553 exert inconsistent effects on behavior. For example, SB206553 potentiated the psychostimulant effects of cocaine at 1 and 2 mg/kg doses, an effect also seen with SB242084, but this effect was lost at a dose of 4 mg/kg (McCreary and Cunningham, 1999). Another study reported that SB206553, unlike SB242084, reduced cue-induced reinstatement of methamphetamine seeking (Graves and Napier, 2012). However, it seems unlikely that this effect of SB206553 is mediated by 5-HT2C receptors as it was not blocked by SB242084. Overall, the behavioral change produced by higher doses of SB206553 and the 5-HT2C receptor-independent reduction in methamphetamine seeking suggest that SB206553 loses selectivity for 5-HT2C receptors as dose increases. Activity at other targets such as 5-HT2B receptors (Kennett et al, 1996), 5-HT1A receptors (Shumsky et al, 2005), or nicotinic acetylcholine a7 receptors (Möller-Acuña et al, 2015) may change or mask the expression of 5-HT2C receptor-mediated effects of SB206533.
The 5-HT2C receptor has recently gained attention as a therapeutic target for psychiatric disorders that present with motivational dysfunctions. For example, the 5-HT2C receptor agonist lorcaserin, which is FDA approved for treating obesity, has shown positive results in a clinical trial for treating nicotine dependence (Shanahan et al, 2016). Further, preclinical studies find that lorcaserin reduces self-administration of cocaine (Harvey-Lewis et al, 2016) and opiates (Neelakantan et al, 2017), suggesting a general utility in treating substance abuse. In the present studies, we show that 5-HT2C receptor ligands can bidirectionally influence responding for a CRf—a measure of incentive motivation elicited by a reward-associated stimulus. The ability of CP809101 to decrease responding for a CRf supports a role for 5-HT2C receptor agonists in blunting motivation, whereas the ability of SB242084 and SB206553 to enhance responding for a CRf suggests that 5-HT2C receptor antagonists may potentiate motivation. This idea is consistent with a recent report showing that SB242084 can enhance food-motivated behavior (Bailey et al, 2016). Thus the 5-HT2C receptor can be targeted to bidirectionally modulate motivational processes, which may be useful in developing treatment strategies for psychiatric disorders such as substance abuse, as well as schizophrenia and depression (De Deurwaerdère and Di Giovanni, 2017; Meltzer, 1999; Rocha et al, 2002).
One potential barrier to using 5-HT2C antagonists in the clinic are findings that SB242084 substitutes for cocaine in a self-administration paradigm in monkeys, which suggests an abuse liability of such compounds (Manvich et al, 2012a). However, the present results observe only mild behavioral and neurochemical enhancements following SB242084 treatment. Further, the effects of SB242084 appear to have an upper limit; escalating doses of SB242084 do not produce dose-dependent increases in behavior (present results) or NAc DA release (De Deurwaerdère et al, 2004). These findings contrast with the ability of typical drugs of abuse, which produce much larger, dose-dependent increases in mesolimbic DA release and DA-dependent behavior. Thus it is possible that 5-HT2C antagonists may potentiate motivation to a minor extent without escalation of intake, but further investigation of the abuse-like properties of these compounds is certainly warranted.
In conclusion, the present results show that modulating 5-HT2C receptor function can produce a bidirectional shift in reward-related behaviors through an interaction with the mesolimbic DA system. These findings provide strong support for further exploring the 5-HT2C receptor as a therapeutic target in treating psychiatric disorders, particularly those with motivational dysfunctions.
Funding and disclosure
This research was supported by a Canadian Institutes of Health Research operating grant to PJF (Grant number MOP-13628). CJB was supported by a Doctoral award from the Natural Sciences and Engineering Research Council of Canada. The authors declare no conflict of interest.
Acknowledgments
We thank Dr Fiona D Zeeb, Dr Junchul Kim, and Dr Suzanne Erb for their helpful comments on some of this work.
References
- Aloyo VJ, Berg KA, Spampinato U, Clarke WP, Harvey JA (2009). Current status of inverse agonism at serotonin2A (5-HT2A) and 5-HT2C receptors. Pharmacol Ther 121: 160–173. [DOI] [PubMed] [Google Scholar]
- Bailey MR, Williamson C, Mezias C, Winiger V, Silver R, Balsam PD et al (2016). The effects of pharmacological modulation of the serotonin 2C receptor on goal-directed behavior in mice. Psychopharmacology (Berl) 233: 615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes NM, Sharp T (1999). A review of central 5-HT receptors and their function. Neuropharmacology 38: 1083–1152. [DOI] [PubMed] [Google Scholar]
- Bezzina G, Body S, Cheung THC, Hampson CL, Bradshaw CM, Glennon JC et al (2015). Evidence for a role of 5-HT2C receptors in the motor aspects of performance, but not the efficacy of food reinforcers, in a progressive ratio schedule. Psychopharmacology (Berl) 232: 699–711. [DOI] [PubMed] [Google Scholar]
- Bromidge SM, Duckworth M, Forbes IT, Ham P, King FD, Thewlis KM et al (1997). 6-Chloro-5-methyl-1-[[2-[(2-methyl-3-pyridyl)oxy]-5-pyridyl]carbamoyl]- indoline (SB-242084): the first selective and brain penetrant 5-HT2C receptor antagonist. J Med Chem 40: 3494–3496. [DOI] [PubMed] [Google Scholar]
- Browne CJ, Fletcher PJ (2016). Decreased incentive motivation following knockout or acute blockade of the serotonin transporter: role of the 5-HT2C receptor. Neuropsychopharmacology 41: 2566–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne JDC, Soko AD, Fletcher PJ (2014). Responding for conditioned reinforcement in C57BL/6 and CD-1 mice, and Sprague-Dawley rats: effects of methylphenidate and amphetamine. Psychopharmacology (Berl) 231: 4503–4516. [DOI] [PubMed] [Google Scholar]
- Capriles N, Watson S, Akil H (2012). Individual differences in the improvement of cocaine-induced place preference response by the 5-HT2C receptor antagonist SB242084 in rats. Psychopharmacology (Berl) 220: 731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton PG, Lee MD, Dourish CT (2000). Similarities in the action of Ro 60-0175, a 5-HT2C receptor agonist and d-fenfluramine on feeding patterns in the rat. Psychopharmacology (Berl) 152: 256–267. [DOI] [PubMed] [Google Scholar]
- Craige CP, Unterwald EM (2013). Serotonin (2C) receptor regulation of cocaine-induced conditioned place preference and locomotor sensitization. Behav Brain Res 238: 206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Deurwaerdère P, Di Giovanni G (2017). Serotonergic modulation of the activity of mesencephalic dopaminergic systems: therapeutic implications. Prog Neurobiol 151: 175–236. [DOI] [PubMed] [Google Scholar]
- De Deurwaerdère P, Navailles S, Berg KA, Clarke WP, Spampinato U (2004). Constitutive activity of the serotonin2C receptor inhibits in vivo dopamine release in the rat striatum and nucleus accumbens. J Neurosci 24: 3235–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E (1999). SB 242084, a selective serotonin2C receptor antagonist, increases dopaminergic transmission in the mesolimbic system. Neuropharmacology 38: 1195–1205. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E (2000). Biochemical and electrophysiological evidence that RO 60-0175 inhibits mesolimbic dopaminergic function through serotonin(2C) receptors. Brain Res 865: 85–90. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Grottick AJ, Higgins GA (2002). Differential effects of the 5-HT(2A) receptor antagonist M100907 and the 5-HT(2C) receptor antagonist SB242084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology 27: 576–586. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Higgins GA (1997). Differential effects of ondansetron and alpha-flupenthixol on responding for conditioned reward. Psychopharmacologia 134: 64–72. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Sinyard J, Higgins GA (2010). Genetic and pharmacological evidence that 5-HT2C receptor activation, but not inhibition, affects motivation to feed under a progressive ratio schedule of reinforcement. Pharmacol Biochem Behav 97: 170–178. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tampakeras M, Sinyard J, Slassi A, Isaac M, Higgins GA (2009). Characterizing the effects of 5-HT(2C) receptor ligands on motor activity and feeding behaviour in 5-HT(2C) receptor knockout mice. Neuropharmacology 57: 259–267. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. 3rd edn. Academic Press: San Diego, 2007..
- Gerak LR, Collins GT, France CP (2016). Effects of lorcaserin on cocaine and methamphetamine self-administration and reinstatement of responding previously maintained by cocaine in rhesus monkeys. J Pharmacol Exp Ther 359: 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason SD, Lucaites VL, Shannon HE, Nelson DL, Leander JD (2001). m-CPP hypolocomotion is selectively antagonized by compounds with high affinity for 5-HT(2C) receptors but not 5-HT(2A) or 5-HT(2B) receptors. Behav Pharmacol 12: 613–620. [DOI] [PubMed] [Google Scholar]
- Graves SM, Napier TC (2012). SB 206553, a putative 5-HT2C inverse agonist, attenuates methamphetamine-seeking in rats. BMC Neurosci 13: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grottick AJ, Fletcher PJ, Higgins GA (2000). Studies to investigate the role of 5-HT(2C) receptors on cocaine- and food-maintained behavior. J Pharmacol Exp Ther 295: 1183–1191. [PubMed] [Google Scholar]
- Guy EG, Fletcher PJ (2014). Responding for a conditioned reinforcer, and its enhancement by nicotine, is blocked by dopamine receptor antagonists and a 5-HT2C receptor agonist but not by a 5-HT2A receptor antagonist. Pharmacol Biochem Behav 125: 40–47. [DOI] [PubMed] [Google Scholar]
- Harvey-Lewis C, Li Z, Higgins GA, Fletcher PJ (2016). The 5-HT(2C) receptor agonist lorcaserin reduces cocaine self-administration, reinstatement of cocaine-seeking and cocaine induced locomotor activity. Neuropharmacology 101: 237–245. [DOI] [PubMed] [Google Scholar]
- Hayes DJ, Clements R, Greenshaw AJ (2009). Effects of systemic and intra-nucleus accumbens 5-HT2C receptor compounds on ventral tegmental area self-stimulation thresholds in rats. Psychopharmacology (Berl) 203: 579–588. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Silenieks LB, Rossmann A, Rizos Z, Noble K, Soko AD et al (2012). The 5-HT2C receptor agonist lorcaserin reduces nicotine self-administration, discrimination, and reinstatement: relationship to feeding behavior and impulse control. Neuropsychopharmacology 37: 1177–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji S-P, Zhang Y, Van Cleemput J, Jiang W, Liao M, Li L et al (2006). Disruption of PTEN coupling with 5-HT2C receptors suppresses behavioral responses induced by drugs of abuse. Nat Med 12: 324–329. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Seviour PW, Iversen SD (1975). Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res 94: 507–522. [DOI] [PubMed] [Google Scholar]
- Kennett G, Lightowler S, Trail B, Bright F, Bromidge S (2000). Effects of RO 60 0175, a 5-HT(2C) receptor agonist, in three animal models of anxiety. Eur J Pharmacol 387: 197–204. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Wood MD, Bright F, Cilia J, Piper DC, Gager T et al (1996). In vitro and in vivo profile of SB 206553, a potent 5-HT2C/5-HT2B receptor antagonist with anxiolytic-like properties. Br J Pharmacol 117: 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett GA, Wood MD, Bright F, Trail B, Riley G, Holland V et al (1997). SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology 36: 609–620. [DOI] [PubMed] [Google Scholar]
- Manvich DF, Kimmel HL, Cooper DA, Howell LL (2012. a). The serotonin 2C receptor antagonist SB 242084 exhibits abuse-related effects typical of stimulants in squirrel monkeys. J Pharmacol Exp Ther 342: 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manvich DF, Kimmel HL, Howell LL (2012. b). Effects of serotonin 2C receptor agonists on the behavioral and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther 341: 424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreary AC, Cunningham KA (1999). Effects of the 5-HT2C/2B antagonist SB 206553 on hyperactivity induced by cocaine. Neuropsychopharmacology 20: 556–564. [DOI] [PubMed] [Google Scholar]
- Meltzer HY (1999). The role of serotonin in antipsychotic drug action. Neuropsychopharmacology 21: 106S–115S. [DOI] [PubMed] [Google Scholar]
- Möller-Acuña P, Contreras-Riquelme JS, Rojas-Fuentes C, Nuñez-Vivanco G, Alzate-Morales J, Iturriaga-Vásquez P et al (2015). Similarities between the binding sites of SB-206553 at serotonin type 2 and alpha7 acetylcholine nicotinic receptors: rationale for its polypharmacological profile. PLoS ONE 10: e0134444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelakantan H, Holliday ED, Fox RG, Stutz SJ, Comer SD, Haney M et al (2017). Lorcaserin suppresses oxycodone self-administration and relapse vulnerability in rats. ACS Chem Neurosci (doi:10.1021/acschemneuro.6b00413; e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- Neisewander JL, Acosta JI (2007). Stimulation of 5-HT2C receptors attenuates cue and cocaine-primed reinstatement of cocaine-seeking behavior in rats. Behav Pharmacol 18: 791–800. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Goulding EH, O'Dell LE, Mead AN, Coufal NG, Parsons LH et al (2002). Enhanced locomotor, reinforcing, and neurochemical effects of cocaine in serotonin 5-hydroxytryptamine 2C receptor mutant mice. J Neurosci 22: 10039–10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M (2002). Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res 137: 3–25. [DOI] [PubMed] [Google Scholar]
- Schultz W (1998). Predictive reward signal of dopamine neurons. J Neurophysiol 80: 1–27. [DOI] [PubMed] [Google Scholar]
- Shanahan WR, Rose JE, Glicklich A, Stubbe S, Sanchez-Kam M (2016). Lorcaserin for smoking cessation and associated weight gain: a randomized 12-week clinical trial. Nicotine Tob (doi:10.1093/ntr/ntw301; e-pub ahead of print). [DOI] [PubMed]
- Shumsky JS, Kao T, Amato N, Simansky K, Murray M, Moxon KA (2005). Partial 5-HT(1A) receptor agonist activity by the 5-HT(2C) receptor antagonist SB 206,553 is revealed in rats spinalized as neonates. Exp Neurol 191: 361–365. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, Chapin DS, McCarthy SA, Guanowsky V, Brown J, Chiang P et al (2007). CP-809,101, a selective 5-HT2C agonist, shows activity in animal models of antipsychotic activity. Neuropharmacology 52: 279–290. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Robbins TW (1984). Enhanced behavioural control by conditioned reinforcers following microinjections of d-amphetamine into the nucleus accumbens. Psychopharmacology (Berl) 84: 405–412. [DOI] [PubMed] [Google Scholar]
- Wolff MC, Leander JD (2000). A comparison of the behavioural effects of 5-HT2A and 5-HT2C receptor agonists in the pigeon. Behav Pharmacol 11: 355–364. [DOI] [PubMed] [Google Scholar]
- Zeeb FD, Higgins GA, Fletcher PJ (2015). The serotonin 2C receptor agonist lorcaserin attenuates intracranial self-stimulation and blocks the reward-enhancing effects of nicotine. ACS Chem Neurosci 6: 1231–1240. [DOI] [PubMed] [Google Scholar]