Abstract
Early-life stress (ELS) increases the risk for psychopathology. Immune and endocrine changes have been reported in adults and are associated with maladaptation of stress-responsive systems. Here we investigated the effects of ELS on endocrine and immune pathways in adolescents without psychopathology. Thirty adolescents with a history of childhood maltreatment and 27 adolescents without ELS history were recruited. Blood and hair samples were obtained from all participants. Lymphocytes were isolated and stimulated in vitro. Flow cytometry was used to evaluate lymphocyte subsets, Th1/Th2/Th17 cytokines, mitogen-activated protein kinase (MAPK), and nuclear factor kappa B (NF-κB) signaling pathways, as well as lymphocyte sensitivity to dexamethasone. Brain-derived neurotrophic factor (BDNF) and hair cortisol were assessed with enzyme-linked immunosorbent assays (ELISAs). Adolescents with a history of ELS had increased percentages of T-cell activation markers (CD3+CD4+CD25+ and CD3+CD69+) and senescent T cells (CD8+CD28− and CD4+CD28−), as well as decreased percentages of NK (CD3-CD56+) and NK T cells (CD3+CD56+). Following stimulation, lymphocytes of ELS+ adolescents produced significantly more IL-2, IL-4, IFN-γ, and IL-17 and engaged more MAPK ERK and NF-κB signaling. ELS was associated with increased hair cortisol levels in parallel with increased lymphocyte resistance to dexamethasone and low plasma BDNF levels. These data provide the first indication of the presence of immune activation and pro-inflammatory profiles in healthy adolescents exposed to ELS, which could contribute to increased vulnerability of trauma-related psychopathology later in life. The underlying mechanisms of this impairment may include the enhanced activation of both MAPK and NF-κB signaling in parallel to partial resistance to glucocorticoids.
Introduction
Childhood maltreatment has been associated with early-life reprogramming of stress-responsive systems, including immune responses and hypothalamic–pituitary–adrenal (HPA) axis signaling in adult life (Danese and McEwen, 2012; McGowan et al, 2009). Early-life stress (ELS) increases the risk for psychopathology, including major depression (MD), posttraumatic stress disorder (PTSD), drug abuse, and schizophrenia in adulthood (Levandowski et al, 2014; McCrory et al, 2011; Pace et al, 2012). However, very little is known about the biological alterations related to ELS independent of mental disorders.
Abnormalities in HPA function following child maltreatment include children exhibiting elevated basal cortisol (Tarullo and Gunnar, 2006) and a blunted stress response (Ouellet-Morin et al, 2011), whereas maltreated adults show contradictory results regarding cortisol or adrenocorticotropic hormone levels when short- or long-term assessment approaches are used (Carpenter et al, 2007; Schalinski et al, 2015). In this sense, it is thought that lack of appropriate neuroendocrine control related to ELS would disrupt immune responses (Coelho et al, 2014).
Indeed, data from human and animal studies indicates enhanced cell-mediated immune markers, including expansion of activated CD4+ and CD8+ T cells, natural killer (NK) cells, and increased CD4/CD8 T-cell ratios in adults with ELS history (Bielas et al, 2012; Vanbesien-Mailliot et al, 2007). Furthermore, higher plasma levels of pro-inflammatory cytokines, including interleukin 6 (IL-6), interleukin-1β (IL-1β), and serum tumor necrosis factor-alpha (TNF-α), have been described in healthy adults exposed to ELS (Carpenter et al, 2010), as well as in adults with any psychiatric disorder and reporting ELS (Coelho et al, 2014). This immunological imbalance could be explained by the differential expression of intracellular signaling cascades involved with cell activation, proliferation, and inflammation. In this context, NF-κB has an important role in a number of cellular functions, including immune signaling, inflammation, and cell survival (Hayden and Ghosh, 2004). Activation of NF-κB occurs in response to a variety of stimuli, which include bacterial and viral peptides, inflammatory cytokines, growth factors, and stress. Previous findings have reported increased NF-κB signaling in healthy individuals following acute psychosocial stress (Bierhaus et al, 2003). Also, Pace et al (2012) found increased NF-κB activity in women with PTSD as compared with controls (Pace et al, 2012). In the same vein, engagement of MAPKs consists of additional stress-responsive pathways that convert extracellular stimuli into cellular responses, including cell proliferation, apoptosis, and inflammation (Wieck et al, 2013). Although the activation of lymphocyte MAPK was associated with pro-inflammatory profiles in patients with mood disorders (do Prado et al, 2013; Wieck et al, 2013), the potential involvement of the MAPK system in ELS is unknown.
ELS has been shown to affect the expression of both CNS and circulating levels of brain-derived neurotrophic factor (BDNF; Grassi-Oliveira et al, 2008). BDNF regulates diverse biological processes during development, including neuronal survival, growth, differentiation, and neuroplasticity. Despite the crosstalk between peripheral and central nervous systems, it is believed that most circulating BDNF is derived from endothelium, immune cells, and platelets, being upregulated in activated T and B cells, as well as during inflammatory processes, which suggests immunomodulatory actions (Kerschensteiner et al, 1999). Specifically, a previous study reported decreased BDNF plasma levels in adult women with MD reporting ELS (Grassi-Oliveira et al, 2008). Furthermore, Bucker et al (2015) found increased BDNF levels in the serum of children exposed to ELS without mental disorders when compared with control children (Bucker et al, 2015). It is thought that glucocorticoid (GC)–BDNF crosstalk is essential for the early-life programming of the HPA axis and neurotrophin signaling (Daskalakis et al, 2015).
Here we investigated a comprehensive set of neuroendocrine and immune parameters of adolescents reporting ELS without current psychopathology. In particular, we evaluated the lymphocyte subsets involved with immune cell activation/regulatory profiles, the Th1/Th2/Th17 cytokines in cell supernatants, and the intracellular expression of MAPKs p38 (p-p38), ERK (p-ERK), and NF-κB. We also investigated whether tonic cortisol levels are associated with neuroimmunological parameters. Therefore, we investigated hair cortisol levels, lymphocyte sensitivity to GCs, and plasma BDNF levels.
Materials and methods
Participants
Adolescents aged between 13 and 17 years with a history of childhood maltreatment (CM) were recruited by telephone from a previous study (primary database) that assessed CM in schools of Porto Alegre (Brazil) using the Portuguese version of Childhood Trauma Questionnaire (CTQ) (Grassi-Oliveira et al, 2014). CTQ is a retrospective 28-item self-report instrument that assesses exposure to sexual, physical, and emotional abuse and physical and emotional neglect. As there is a dose-dependent association of exposure to multiple types of trauma with poor health outcomes (Elton et al, 2014), and because the CTQ subscales load onto a higher-order factor, a CTQ total score can be calculated by the sum of scores from each abuse and/or neglect subtype. Total CTQ scores ranged from 25 to 125 and took the severity of multiple forms of abuse and neglect into account (Grassi-Oliveira et al, 2014).
A total of 243 adolescents that took part in a previous study and answered the CTQ were contacted and invited to voluntarily participate in the present study, attending two interviews in our laboratory. Forty-three students declined to take part in our study. We identified 41 adolescents with CTQ scores above the 60th percentile in normative data who self-reported no history of mental disorders to be included in the ELS group. However, 11 dropped out before concluding all of the assessments (n=30). The participants who did not reach the primary end point had higher CTQ scores in comparison to included cases. Normative data considered gender and age in order to generate percentiles. The forms of ELS reported were sexual abuse (25%), physical abuse (53.1%), emotional abuse (56.3%), physical neglect (56.3%), and emotional neglect (37.5%). The Stressful Life Events Schedule—Adolescents (SLES-A) was used in our study to investigate current stressors taking place in the 12 months preceding inclusion in the study. The interview instrument consists of a self-reported scale containing 79 items that serve to examine the perceived impact of events in the interviewee’s life (‘how did this affect you? Not at all, a little, somewhat, and a lot’), assessing both the nature (dependent/independent) and the intensity of distress generated by these events (high/low impact). High-impact SLEs were defined as those rated as ‘somewhat’ and ‘a lot’ in the self-reported data (Williamson et al, 2003).
Thirty-three adolescents without history of abuse or neglect and self-reporting no history of mental disorders or substance use were randomly selected from the primary CTQ database and included as the control group. Five dropped out and one was excluded before all the assessments were concluded (n=27). To exclude current mental disorders, major axis I disorders (ie, psychotic disorders, mood disorders, anxiety disorders, and trauma-related disorders) were assessed using the Schedule for Affective Disorders and Schizophrenia for School Aged Children—Present and Lifetime Version (Brasil and Bordin, 2010; Kaufman et al, 1997). In addition, estimated IQ was assessed by the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). Childhood Depressive Inventory was used to evaluate the severity of depressive symptoms (Kovacs, 1985). Exclusion criteria for both groups were (a) adolescents taking any type of medications at the time of participation, (b) presence of major axis I disorder, (c) intellectual disability, (d) presence of acute (eg, infections) or chronic (eg, neoplasia, diabetes) illnesses, (e) neurological disorders, and (f) use of controlled substances. We did not measure sleep quality in our study. This study was approved by the Ethical Committee of PUCRS, and all participants and their parents or guardians provided their written informed consent prior to inclusion in the study.
Blood Collection and Cell Isolation
Peripheral blood (10 ml) was collected by venipuncture in EDTA-containing tubes. Plasma was isolated and stored at −80 °C prior to analysis. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll (Ge Healthcare Life Sciences—Marlborough, MA, USA) density gradient centrifugation (30 min at 900 g). Cells were counted using a microscope (100 ×) and viability always exceeded 95%, as judged on ability to exclude Trypan Blue (Sigma-Aldrich—St Louis, MO, USA). PBMCs were resuspended in complete culture medium (RPMI-1640, supplemented with 0.5% gentamycin, 1% glutamine, 1% HEPES, 0.1% fungizone, and 10% fetal calf serum (FCS); all from Sigma-Aldrich) and adjusted to 1 × 105 cells/well.
Immunophenotyping
A comprehensive panel of lymphocyte subsets was identified by multicolor flow cytometry. Briefly, PBMCs were washed in flow cytometry buffer (PBS containing 1% FCS and 0.01% sodium azide) and treated with Fc Block solution for 20 min. Cells were stained for 30 min with combinations of the following monoclonal antibodies: anti-CD3 FITC, anti-CD3 PECy5, anti-CD4 PE, anti-CD8 PE, anti-CD8 PECy5, anti-CD19 PE, anti-CD56 FITC, anti-CD28 FITC, and anti-CD69 FITC (all from BD Biosciences—San Jose, CA, USA). Immediately after staining, cells were washed, resuspended, and analyzed by flow cytometry. At least 20 000 lymphocytes were identified by size (FSC) and granularity (SSC) and acquired using a FACS Canto II flow cytometer (BD Biosciences). The instrument has been checked for sensitivity and overall acquisition. Data were analyzed using the Flowjo 7.2.5 software (Tree Star, Ashland, OR, USA).
Analysis of Secreted Cytokines In Vitro
To determine cytokine production, PBMCs were cultured (1 × 105 cells) in RPMI medium with 10% FCS (Sigma-Aldrich) and 1% phytohemagglutinin (PHA, Invitrogen—Carlsbad, CA, USA), for 72 h at 37 °C and in a 5% CO2 atmosphere. The supernatants were collected and stored at −80 °C for subsequent analysis. The samples were thawed on the same day and processed together. Multiple soluble cytokines (IL-2, IL-10, IL-4, IL-6, IFN-γ, TNF-α, and IL-17) were quantified simultaneously with flow cytometry using the Cytometric Bead Array (CBA) Human Th1/Th2/Th17 Kit (BD Biosciences). Quantitative results were generated using the FCAP Array v1.0.1 software (Soft Flow—Pecs, Hungary). The minimal detection limits for these assays ranged from 2.4 to 4.9 pg/ml (IL-2, IL-10, IL-4, IL-6, IFN-γ, TNF-α) and 18.9 pg/ml for IL-17. The intra-assay and interassay coefficients of variation were <10%.
Analysis of Intracellular Activated MAPKs and NF-κB in Lymphocytes
Intracellular phospho-p38 and phospho-ERK expression was determined by flow cytometry in CD3+CD4+ and CD3+CD8+ cells (Human T Cell Activation Kit, BD Biosciences). PBMCs were cultured in RPMI medium with 10% FCS and stimulated with 40 nM phorbol 12-myristate 13-acetate (PMA) and 1 μM ionomycin (IONO, all from Sigma-Aldrich) for 15 min at 37 °C and in 5% CO2 atmosphere. Cells were harvested, immediately fixed, and stored (−80 °C) in Cytofix solution (BD Biosciences) for later analysis. All samples were thawed on the same day and processed together to reduce variation. Cells were permeabilized on ice for 30 min with Phosflow Perm Buffer III (BD Biosciences) according to the manufacturer’s instructions. Next, cells were washed (600 g, 6 min), stained for 60 min at room temperature with appropriate antibodies, washed once more (600 g, 6 min), and resuspended at a final concentration of 4.5 × 105 cells/200 μl in Pharmingen Staining Buffer (BD Biosciences). The staining procedure was performed with the following monoclonal antibodies (all from BD Biosciences): anti-CD3 PerCP (clone SK7), anti-CD4 FITC (clone SK3), and anti-CD8 PE (clone SK1) with anti-phospho ERK1/2 Alexa Fluor 647 (clone 20A) or anti-phospho p38 Alexa Fluor 647 (clone 36/p38). Lyophilized human control cells (BD Biosciences) were used as positive and negative controls.
NF-κB activation was assessed in T cells by flow cytometry through the assessment of the intracellular phospho-p65 (NF-κB) subunit. Cells were stimulated with 10 μg/ml LPS (NF-κB assay; Sigma-Aldrich) for 45 min, harvested and immediately fixed and stored (−80 °C) in Cytofix solution (BD Biosciences) according to the manufacturer’s instructions.
Analysis of Hair Cortisol
The majority of previous studies assessing HPA axis functionality investigated cortisol in the serum/plasma, saliva, or urine samples—which reflect phasic responses but not tonic levels. Conversely, hair cortisol is a long-term measure, reflecting cumulative cortisol levels (Stalder et al, 2012). Considering that hair grows at a median rate of 1 cm/month, each centimeter distant from the scalp indicates 30 days of cumulative cortisol exposure. Hair cortisol analysis was made as described previously (Grassi-Oliveira et al, 2012). Briefly, 20 mg of 3 cm hair strands with a 3 mm diameter were cut from the vertex posterior of participants, properly identified, and stored at room temperature for further analysis. One cm hair sections were cut and minced into pieces no larger than 1 mm separately with clean, fine-tipped surgical scissors. For cortisol extraction, 10 mg of powdered hair per 1 cm section was weighed, separated into individual glass vials, and 1 ml of 50 °C methanol added, sonicated for 30 min, and incubated overnight (52 °C for approximately 16 h). Following overnight incubation, 0.75 ml of methanol supernatant was removed, placed into new glass tubes, and evaporated. Residues were then dissolved in 250 μl PBS (pH 8.0) and vortexed for 1 min. Hair cortisol levels were quantified by ELISA (Salimetrics—State College, PA, USA) following the manufacturer’s instructions. Dyed hair color have no effects on cortisol concentrations.
Cellular Activation and Sensitivity to GCs
Cellular activation profiles and GC sensitivity were analyzed by flow cytometry by staining cells for CD3 and CD25 markers. The lymphocyte GC sensitivity was estimated using functional assays developed to measure the ability of steroids to suppress T-cell activation in vitro (Knijff et al, 2006). Briefly, PBMCs were cultured (1 × 105 cells) in RPMI medium with 10% FCS (Sigma-Aldrich), stimulated with 1% PHA (Invitrogen) and treated with 10−6 nM dexamethasone (DEX) (Sigma-Aldrich) for 72 h at 37 ° C and in a 5% CO2 atmosphere. Activation profile was described as the percentage of CD3+CD25+ stimulated cells minus unstimulated cells. Cellular sensitivity data are represented by basal cell activation, with 100% (basal) corresponding to 1% PHA stimulation without DEX.
Plasma BDNF Levels
Plasma BDNF levels were measured by ELISA (Quantikine Human Free BDNF ELISA, R&D Systems, Minneapolis, USA) following the manufacturer’s guidelines. The sensitivity of these assays was 20 pg/ml. The intra-assay and interassay coefficients of variation were <12%.
Statistical Analyses
All variables were tested for normality of distribution by Shapiro–Wilk tests. Clinical and socio-demographic characteristics were compared by Student’s t-test and Chi-square test, according to the outcome/distribution. As we found non-parametric distribution for many variables, Generalized Linear Modeling or Generalized Estimating Equations (GEE) were used to compare differences between groups adjusting for potential confounders (age, BMI, and sex). Linear or gamma distribution was selected based on the distribution outcome and robust estimation with unstructured working correlation matrix was set. Hair cortisol data and sensitivity to GC were analyzed by GEE to investigate between group effects (ELS × Controls) and within time (30, 60, and 90 days) or treatment (Unstimulated, PHA, PHA+DEX) effects and possible interactions (Group × Time or Treatment). Multiple comparisons were adjusted with Bonferroni’s correction. Effect sizes are reported as Cohen’s d. Conventionally, Cohen’s d values of 0.2, 0.5, and 0.8 are considered small-, medium- and large-effect sizes, respectively. Statistical analyses were performed using the Statistical Package for Social Sciences, SPSS Statistics 17.0 software (SPSS—Chicago, IL, USA).
Results
Demographic and clinical characteristics of the study population are described in Table 1. Age, BMI, sex, and parental income were homogeneous between groups. As expected, CTQ scores were statistically different between the two groups (F(1,54)=11.19, p=0.002, η2=0.22). There were no significant differences between control and ELS groups in current stressors taking place in the past 12 months according with SLES-A (t51=−0.71, p=0.47), and no current or prior maltreatment within the past 12 months were reported.
Table 1. Characteristics of the Studied Populations.
| Controls (n=27) | ELS (n=30) | Statistics | p-Value | Effect size (Cohen’s d) | |
|---|---|---|---|---|---|
| Age, years (mean±SD) | 14.19±1.57 | 16.47±1.25 | t55=−0.75 | 0.45 | 0.20 |
| Sex | χ2=1.11 | 0.29 | |||
| Female | 18 | 15 | |||
| Male | 9 | 15 | |||
| BMI (mean±SD) | 25.16±3.44 | 24.07±2.12 | t56=1.47 | 0.14 | 0.39 |
| CDI (mean±SD) | 35.25±5.25 | 34.20±4.80 | t55=0.79 | 0.43 | 0.21 |
| Parental income monthly (US$) | 50.1±9.99 | 52.8±10.80 | t44=0.46 | 0.38 | 0.14 |
| ELS history (mean±SD) | |||||
| Emotional neglect | 6.79±2.02 | 10.83±3.83 | t53=−4.78 | <0.0001 | 1.31 |
| Physical neglect | 5.37±0.57 | 6.45±1.78 | t53=−2.83 | 0.006 | 0.77 |
| Sexual abuse | 5.04±0.20 | 6.09±3.50 | t53=1.46 | <0.0001 | 0.40 |
| Physical abuse | 5.29±0.55 | 7.03±2.89 | t53=−2.90 | 0.005 | 0.79 |
| Emotional abuse | 6.41±2.14 | 10.03±3.95 | t53=−4.03 | <0.0001 | 1.10 |
| CTQ | 28.45±3.52 | 42.72±10.57 | F1,53=10.03 | 0.003 | |
| SLES-A | 6.30±4.86 | 7.27±5.11 | t51=−0.71 | 0.47 | 0.20 |
Abbreviations: BMI, body mass index; CDI, Childhood Depression Inventory; CTQ, Childhood Trauma Questionnaire; SLES-A, Stressful Life Events Schedule—Adolescents.
Data are shown as mean±SD. Statistically significant differences are highlighted in bold.
Healthy Adolescents with ELS Had Lymphocyte Profiles Associated with Cell Activation, Regulation, and Early Senescence
We investigated different peripheral lymphocyte subpopulations associated with activation, regulation, and immunosenescence profiles (Table 2). Percentages of CD3+CD4+CD25+ and CD3+CD69+ activated T cells were higher in ELS as compared with controls (p<0.001 and p<0.001, respectively, Table 2). Furthermore, the ELS group had higher levels of CD8+CD28− and CD4+CD28− regulatory T cells (p=0.002 and p<0.001, respectively). Reduced percentages of NK, NK T cells, as well as CD4+CD28+ activated T cells, were observed in ELS as compared with controls (p<0.001; p=0.001 and p=0.035, respectively). No significant differences were found between groups for the remaining lymphocyte subsets.
Table 2. Immunophenotyping of Lymphocyte Subsets.
| Markers | Cell type | Controls (%) | ELS (%) | Statistics | p-Value | Effect size (Cohen’s d) |
|---|---|---|---|---|---|---|
| CD3+CD4+ | Th | 38.46±6.34 | 38.18 ±8.01 | χ2 (1)=0.59 | 0.43 | 0.12 |
| CD3+CD8+ | Tc | 24.63±6.97 | 26.41±5.76 | χ2 (1)=1.04 | 0.30 | 0.30 |
| CD4/CD8 | Ratio | 1.69±0.59 | 1.57±0.56 | χ2 (1)=0.14 | 0.71 | 0.21 |
| CD3−CD19+ | B | 12.67±4.35 | 12.86±5.46 | χ2 (1)=0.08 | 0.77 | 0.03 |
| CD3−CD56+ | NK | 18.03±7.57 | 11.57±6.38 | χ2 (1)=13.68 | <0.0001 | 0.99 |
| CD3+CD56+ | NK T | 12.54±8.97 | 4.97±4.63 | χ2 (1)=13.91 | <0.0001 | 1.28 |
| CD3+CD4+CD25+ | Activated T cell | 1.50±1.99 | 5.91±6.06 | χ2 (1)=26.54 | <0.0001 | 0.92 |
| CD3+CD69+ | Activated T cell | 0.48±0.62 | 1.49±2.50 | χ2 (1)=15.11 | 0.002 | 0.50 |
| CD8+CD28+ | Activated T cell | 13.15±7.92 | 12.71±8.27 | χ2 (1)=0.12 | 0.72 | 0.002 |
| CD8+CD28− | Senescent or regulatory T cell | 12.17±9.61 | 20.53 ±10.67 | χ2 (1)=9.29 | 0.005 | 0.86 |
| CD4+CD28+ | Activated T cell | 32.36±10.23 | 22.37±13.26 | χ2 (1)=4.45 | 0.009 | 0.88 |
| CD4+CD28− | Senescence T cell | 6.95±7.61 | 17.93±17.27 | χ2 (1)=13.30 | 0.008 | 0.87 |
Abbreviations: Th, helper T cell; Tc, cytotoxic T cell.
Data shown as mean±SD. Statistically significant differences are highlighted in bold.
Lymphocytes from Adolescents with ELS Produced More Pro-inflammatory Cytokines
Next, we analyzed multiple in vitro Th1/Th2/Th17 cytokines (IL-2, IL-4, IL-6, IL-10, IL-17, IFN-γ, and TNF-α) implicated with inflammation, proliferation, and cellular differentiation (Table 3). Increased IL-2, IL-4, IFN-γ, and IL-17 levels were found in ELS compared with the control group (p<0.001; p<0.001; p<0.001, and p<0.001, respectively). No significant differences were observed for IL-6, TNF-α, and IL-10.
Table 3. In Vitro Production of Th1/Th2/Th17 Cytokines by PBMCs.
| Controls (pg/ml) | ELS (pg/ml) | Fold change | Statistics | p-Value | Effect size (Cohen’s d) | |
|---|---|---|---|---|---|---|
| IL-2 | 4.43±15.28 | 54.29±100.38 | 12 × | Wald-χ2 (1)=27.95 | <0.0001 | 0.56 |
| IL-4 | 1.17±2.88 | 3.96±4.56 | 3.4 × | Wald-χ2 (1)=30.49 | <0.0001 | 0.47 |
| IL-6 | 113.13±9.11 | 90.98±40.64 | 0.8 × | Wald-χ21)=0.09 | 0.34 | 0.65 |
| IL-17 | 8.94±11.01 | 41.50±47.95 | 4.6 × | Wald-χ2 (1)=14.04 | <0.0001 | 0.81 |
| IL-10 | 1334.27±838.21 | 1359.8±936.85 | 1 × | Wald-χ2 (1)=0.70 | 0.40 | 0.03 |
| TNF-α | 20.10±16.09 | 14.39±18.94 | 0.7 × | Wald-χ2 (1)=0.48 | 0.48 | 0.30 |
| IFN-γ | 9113.97±3409.83 | 51 678.6±71 314.9 | 5.6 × | Wald-χ2 (1)=34.39 | <0.0001 | 0.71 |
Peripheral blood mononuclear cells (PBMCs) were isolated and stimulated with PHA for 72 h. Supernatants were collected and cytokines measured by CBA (Cytometric Bead Array, BD). Data are shown as mean (pg/ml)±SD. Statistically significant differences are highlighted in bold.
Lymphocytes from Adolescents with ELS had Activated MAPK and NF-κB
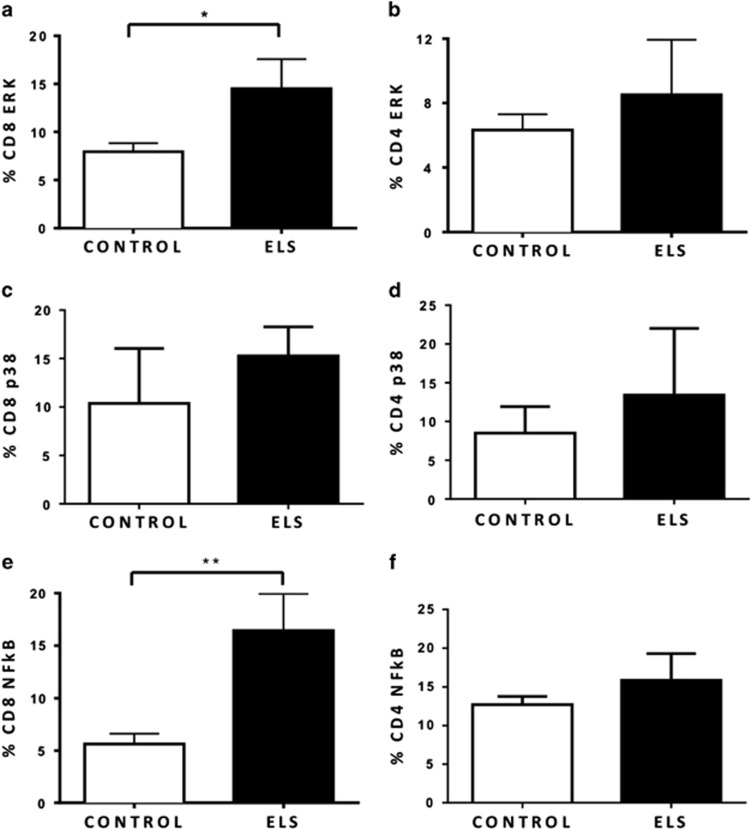
To better understand the molecular mechanisms underlying the pro-inflammatory and lymphocyte activation profiles observed in ELS, we explored downstream intracellular signaling pathways involved with cell activation, proliferation, and inflammation. As depicted in Figure 1, the percentages of p-ERK+CD8+ and p-NF-κB+CD8+ cells were found to be increased in ELS compared with controls (Wald-χ2 (1)=4.035, p=0.045, Cohen’s d=0.57 and Wald-χ2 (1)=12.498, p<0.001, Cohen’s d=0.83, respectively). The same was not observed for the percentages of p-ERK+CD4+, p-p38+CD8, p-38+CD4, and p-NF-κB+CD4 between the two groups.
Figure 1.
ELS was associated with increased activation of intracellular signaling pathways involved with cell activation, proliferation, and inflammation. Phosphorylated MAPKs and NF-κB were evaluated in peripheral T-cell subsets. Data show the expression of MAPK profiles following stimulation with 40 nM PMA and 1 μM ionomycin (IONO) for 15 min and the expression of NF-κB in T cells after stimulation with 10 μg/ml LPS for 45 min. (a and b) Percentage of T cells expressing phospho-ERK with increased percentage of p-ERK CD8+ cells in ELS. (c and d) Percentage of T cells expressing phospho-p38. (e and f) Percentage of T cells expressing phospho-NF-κβ with increased percentage of pNF-κB in ELS. Data are shown as mean±SE. Statistically significant differences are indicated *p<0.05 and **p<0.001. n=27 controls and 30 ELS.
ELS was Associated with Activation of the HPA Axis
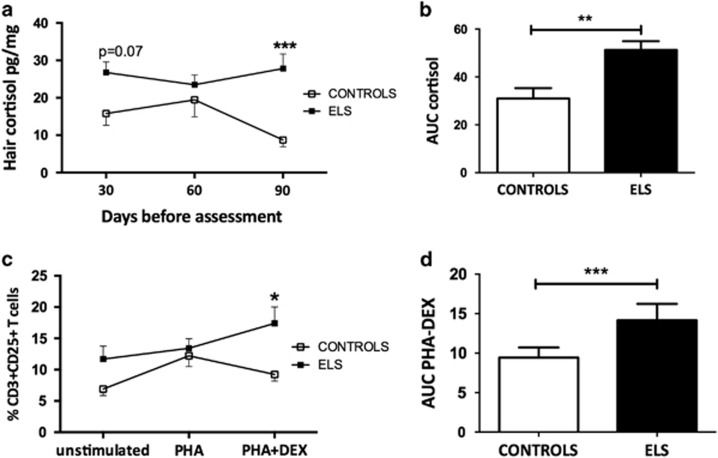
Hair cortisol levels were analyzed to identify the long-term endocrine consequences of ELS. We observed significant Group effects for hair cortisol (Wald-χ2 (1)=20.75; p<0.0001), driven by increased hair cortisol levels in the ELS group (Figure 2a). No Time effects were observed (Wald-χ2 (2)=2.65; p=0.26). A significant Group × Time interaction was observed (Wald-χ2 (2)=6.57; p=0.01), indicating increased hair cortisol levels in ELS adolescents 30 days before assessment (p=0.07, approaching significance) and 90 days before assessment (p<0.0001). As a consequence, the adolescents exposed to childhood maltreatment had higher cumulative hair cortisol levels, as estimated by the area under the curve (AUC), as compared with controls (Wald-χ2 (1)=8.58, p=0.003; Figure 2b).
Figure 2.
Healthy adolescents with ELS had activation of the HPA axis and reduced lymphocyte sensitivity to GC. Following extraction, hair cortisol levels were determined using a high-sensitivity salivary cortisol ELISA test. Lymphocyte sensitivity to GC was estimated by measuring the ability of steroids to suppress T-cell activation in vitro. (a) Increased levels of hair cortisol in the 3 months prior to collection (3 cm) in ELS. (b) The area under curve of hair cortisol levels. Increased AUC indicates higher integrated levels of cortisol. (c) Following treatment with DEX, cells from ELS group were more resistant to GC effects. (d) Increased AUC PHA-DEX indicates higher CD3+CD25+ T cells following GC treatment. Data are shown as mean±SE. Statistically significant differences are indicated *p<0.05, **p<0.01 and ***p<0.001. n=27 controls and 30 ELS.
T-Cell Activation was Related to Partial GC Resistance in ELS
Additionally, we investigated whether increased cortisol levels were related to impaired GC signaling to immune functions. Lymphocyte sensitivity to GC was estimated by determining the ability of DEX to suppress T-cell activation in vitro. Significant group effects were found, with lower suppression observed in cells of ELS adolescents (Wald-χ2 (1)=7.70; p=0.006). PHA or DEX treatments produced similar effects (Wald-χ2 (2)=12.381; p<0.002). In addition, there was a significant interaction between Group × Treatment (Wald-χ2 (2)=6.54 p<0.05), as demonstrated by increased proportions of CD3+CD25+ activated T cells in the ELS group after in vitro DEX treatment (p<0.05; Figure 2c). Integrated CD3+CD25+ cell percentages were estimated by the trapezoidal rule to calculate the AUC for both groups. Increased AUC was observed for ELS, further indicating higher CD3+CD25+ T cells following GC treatment or lower GC sensitivity (Wald-χ2 (1)=19.82; p<0.0001; Figure 2d).
Adolescents with ELS Had Lower BDNF Levels
It is known that GC may regulate BDNF expression and ELS could disrupt peripheral BDNF levels. As we observed high cortisol levels in ELS participants, we decided to investigate the levels of BDNF in both groups. Adolescents with ELS had significantly lower BDNF levels compared with controls (200.07±97.48 vs 347.96±224.11 pg/ml, respectively, Wald-χ2 (1)=14.37; p<0.001).
Discussion
Exposure to ELS may lead to long-lasting neuroimmunoendocrine alterations involved in increased vulnerability to psychiatric disorders later in life, a process known as developmental programming (Danese and McEwen, 2012). However, little is known about the biological alterations related to ELS before psychiatric disorders are already established or in resilient individuals, which adapt well to trauma exposure without developing mental illness. It follows that individual characteristics and protective factors have a profound impact on vulnerability to psychiatric outcomes or resilience associated with ELS (McCrory et al, 2011). Protective mechanisms include genetic and epigenetic factors, different forms of environmental enrichment (eg social support or school environment), physical activity, and psychosocial interventions. All of which could lead to a reorganization of brain structure during the early years and prevent the onset of clinical symptoms in adolescence or adulthood (Danese and Lewis, 2017; McCrory et al, 2011).
Here we found that ELS was associated with increased hair cortisol levels and impaired GC signaling, as shown by in vitro resistance to DEX effects. Hyperactivity of the HPA axis is generally observed following acute or chronic stressful events (Horowitz and Zunszain, 2015). However, exposure to stressful events during sensitive periods of development may lead to alterations in the HPA axis and stress reactivity later in life (Danese and McEwen, 2012; McCrory et al, 2011). We observed increased hair cortisol levels in the ELS group, suggesting persistent HPA axis activation during adolescence as a result of childhood maltreatment exposure.
GCs are responsible for modulating both the HPA axis and immune system through binding of GC receptors (GRs). Once activated, the GR translocates into the nucleus and upregulates the expression of anti-inflammatory genes and represses the activation of signaling pathways. Despite its modulatory effects, continuous exposure to high cortisol levels may lead to insensitivity to the immunosuppressive effects of steroids (Heim et al, 2008). In fact, some studies have reported increased cortisol concentrations but decreased responsiveness to GCs in patients with MD after oral administration of DEX (Pariante and Miller, 2001). In the absence of adequate inhibitory control of GC, increased immune signaling can be observed as increased levels of activated cells and pro-inflammatory cytokines (Cohen et al, 2012).
Adolescents exposed to childhood maltreatment had more circulating lymphocyte subsets associated with cell activation and early immunosenescence. We observed an increased percentage of activated T cells (CD3+CD4+CD25+ and CD3+CD69+) in ELS participants. These data are in accordance with previous work that reported increased levels of activated HLA-DR helper T cells and cytotoxic T cells in children exposed to ELS (Bielas et al, 2012). It is worth considering that the immune system is also activated during a normal stress response (Danese and McEwen, 2012). Together with HPA axis activation, stress-induced immune activation occurs in order to restore homeostasis, in a process called allostasis (Danese and McEwen, 2012; McEwen, 2001). However, constant immune system activation, as suggested by our data, may lead to detrimental consequences (ie, allostatic load) when maintaining homeostasis becomes a physiological burden (McEwen, 2001). Thus we found increased T-cell senescence (CD8+CD28− and CD4+CD28−) in the ELS group. CD28 is a co-stimulatory molecule involved in T-cell activation and the expansion of CD28− subsets is a biomarker of early immunosenescence (Bauer et al, 2015). Interestingly, expansion of CD4+CD28− T cells has been associated with inflammatory process, due to their increased production of pro-inflammatory cytokines, such as IL-2, IFN-γ, and TNF-α, as well as by induction of cytotoxicity through cytolytic enzymes (ie, perforin and granzyme B) (Bauer et al, 2015). The early T-cell senescence found in the ELS group may occur as part of the allostatic load produced by persistent immune activation. These findings are particularly exciting considering emerging work suggesting a robust and perhaps dose-dependent relationship with cellular aging and ELS (Levandowski et al, 2016; Tyrka et al, 2016).
On the other hand, we observed decreased NK and NKT cell percentages in ELS participants. Reduced NK cells have been reported in healthy individuals following psychosocial stress (Bellingrath et al, 2010), while others found increased levels of NK cells in individuals with depression and ELS (Pace et al, 2006). Interestingly, exposure to increased levels of GC results in reduced NK cytotoxic activity due to epigenetic modifications of these cells (Eddy et al, 2014). Considering the crucial role of NK and NKT cells in immunity against viral infections and in inflammation, we hypothesize that reduction in the numbers or function of these cells could lead to increased susceptibility to infectious disease, which is often observed in ELS populations (Miller et al, 2011).
We found that lymphocytes from adolescents with no mental disorders who were exposed to ELS produced more pro-inflammatory cytokines in comparison to controls. There is evidence relating peripheral plasma/serum cytokines and exposure to childhood maltreatment (Coelho et al, 2014); however, we demonstrated the in vitro effects for the first time. Inflammation following immune activation is part of the normal stress response; however, increased expression of pro-inflammatory cytokines has been described in psychiatric disorders, including MD (Lopes et al, 2012), bipolar disorder (Brietzke et al, 2009; do Prado et al, 2013; Redlich et al, 2015; Wieck et al, 2013), and cocaine addiction (Levandowski et al, 2016). Curiously, ELS is an important risk factor for all of these conditions (Teicher and Samson, 2013). Despite the absence of psychiatric disorders in our sample, we observed higher levels of IL-2, IFN-γ, IL-17, and IL-4 within ELS participants. IL-2 is a key cytokine involved with T-cell activation and proliferation. Together with impaired GC sensitivity, increased IL-2 levels in ELS participants might be an important change contributing to the observed immune activation. IFN-γ is regularly produced by activated Th1 cells (pro-inflammatory). In a recent study, Redlich et al (2015) demonstrated an interaction between IFN-γ genotype (rs1861494) and ELS affecting amygdala reactivity to emotional stimuli and emotion processing (Redlich et al, 2015). IL-17 is a pro-inflammatory cytokine produced by Th17 cells, as well as other innate immune cell types (ie, NK cells, NKT cells, and macrophages). Previous research has shown effects of IL-17 on the induction of inflammation by activating many signaling pathways (ie, NF-κB and MAPKs) and stimulating release of other pro-inflammatory cytokines, including IL-6, TNF-α, and IL-1β (Zhu and Qian, 2012). Increased IL-17 levels have been described in mood disorders and autoimmune diseases (do Prado et al, 2013). It should be noted that immune system influences on neurocircuitry largely depend on developmental stage. Appropriate levels of cytokines are necessary for healthy neurodevelopment, as explained by the highest levels of cytokines being observed in the neonatal brain (Bilbo and Schwarz, 2012). Therefore, disrupted levels of pro-inflammatory cytokines during adolescence may have a deleterious effect on brain development, particularly prefrontal and cognitive circuits (Grassi-Oliveira et al, 2016). Overall, we hypothesize that the exacerbated cellular inflammatory processes are a consequence of ELS and may contribute to increased vulnerability to mental disorders.
IL-4 is an anti-inflammatory cytokine that induces the differentiation of naive T cells into Th2 cells (anti-inflammatory), by limiting the production of IFN-γ from Th1 cells and macrophages. The increase in inflammatory and anti-inflammatory cytokines observed here is consistent with previous studies (do Prado et al, 2013) and indicates regulatory loops induced by robust cellular stimulation. In the same vein, it is expected to have higher levels of soluble TNF receptors (not measured here) following stimulation, as another example of regulation of the inflammatory response. Therefore, the increase of IL-4 in adolescents exposed to ELS may be a compensatory mechanism in response to the increased pro-inflammatory cytokines observed. Indeed, we found no difference between the ELS and control groups in regard to IL-6 and TNF-α levels during adolescence. If we consider that higher levels of IL-6 during preadolescence of 4500 individuals in a prospective general population birth cohort study was associated with an increased risk of developing depression and psychosis in young adulthood (Khandaker et al, 2014), then the compensatory mechanism described in our sample could be understood as a protective factor. Cytokines are subject to protein degradation in vitro after reaching peak levels, which could explain the lower TNF and IL-6 levels observed here. We have previously found that 72 h is the optimal time point to detect high levels of most assayed cytokines using this CBA Kit. Degradation is certainly an issue raised by multiplex kits. Therefore, future studies shall investigate the production of inflammatory cytokines at early time points. In contrast, a recent meta-analysis found that individuals exposed to childhood trauma had significantly elevated baseline plasma IL-6 and TNF-α levels (Baumeister et al, 2016), therefore we cannot conclude that this is true when PBMCs are stimulated in vitro. It should be noted that in vitro data from stimulated PBMCs cannot be compared with those obtained from plasma samples. When stimulated in vitro, the pro-inflammatory cytokines (TNF-α and IL-6) peak at earlier time points than Th2 cytokines (Sullivan et al, 2000).
To develop insight into the molecular mechanisms underlying the pro-inflammatory and lymphocyte activation profiles observed in ELS, we explored two major intracellular signaling pathways (MAPK and NF-κB) involved with cell activation, proliferation, and inflammation. In particular, we observed activated ERK and NF-κB signaling in the CD8+ subset but not in the CD4+ population. Given the crucial role of ERK1/2 and NF-κB pathways in immune responses such as proliferation and cell survival, increased activation and phosphorylation of these pathways could in part explain the increased senescent CD8+CD28− T-cell and pro-inflammatory cytokines observed in ELS. Furthermore, expansion of CD4+ lymphocytes, as shown by increased CD3+CD4+CD25+ activated T cells and senescent CD4+CD28− T cells in ELS participants, may occur through the activation of different signaling pathways, including the JAK (janus kinase) and STAT (signal transducers and activators of transcription). These data also support the hypothesis that ELS is related to increased cell activation and accelerated cell aging (Tyrka et al, 2016).
BDNF is known to promote neuroplasticity, regulate cell survival, and increase cellular excitability in the peripheral and central nervous system. In the brain, BDNF exerts its roles by activating the MAPK ERK1/2 signaling pathway via its receptor Trkb. Furthermore, the extent of activation is important for many cellular activities, including synaptic plasticity. It is known that chronic exposure to GC inhibits ERK1/2 activation via BDNF (Numakawa et al, 2013). In addition, GC modulates BDNF expression, as observed by the inhibitory effects of DEX on BDNF mRNA. Considering that cells from ELS adolescents were less sensitive to GC signaling, it is possible that reduced BDNF levels might be acting as a counterbalance mechanism to modulate the imbalanced MAPK ERK1/2 activation observed in CD8+ cells of ELS participants. Furthermore, reduced BDNF expression has previously been associated with inflammation (Guan and Fang, 2006; Lapchak et al, 1993). For example, Guan and Fang (2006) reported low BDNF levels in the brain of rats following immune activation induced by LPS (Guan and Fang, 2006). Similarly, administration of pro-inflammatory cytokines reduced BDNF levels in the brain (Lapchak et al, 1993). The immunomodulatory role of BDNF is not yet clear. BDNF is peripherally produced by activated T and B cells, as well as platelets (Kerschensteiner et al, 1999). Therefore, reduced BDNF levels as observed here might be an additional explanation for the increased inflammatory markers and cell-mediated immune activation observed after ELS exposure. Future studies shall explore the functional effects of BDNF on peripheral immune cells.
This study has some limitations and particularities. First, owing to the difficulty in recruiting adolescents exposed to ELS without psychiatric disorders, the sample size is relatively small and type II error should not be underestimated. This was the main reason that the specific effects of different types of ELS have not been investigated. Therefore, this study should be replicated with larger sample sizes. Second, despite the cessation of abuse/maltreatment for all adolescents, the CTQ scale did not provide information regarding onset and end dates of maltreatment. Thus we cannot address the impact of ELS exposure duration and time latency since the end of ELS exposure. However, CTQ answers are based on the frequency of abuse and/or neglect experiences occurring when the individual ‘was growing up’, thus the higher the CTQ score, the more frequent the exposure to maltreatment events in childhood. In addition, based on SLES information collected, we can be assured that all maltreatment ended at least 12 months prior to the beginning of our study. Only one study has associated immune markers with time latency of childhood maltreatment (Cicchetti et al, 2015). Cicchetti et al (2015) reported that maltreatment children (mean age 9 years) with recent onset maltreatment and AT/AA C reactive protein (CRP) genotype had higher levels of CRP. Third, adolescents who declined to participate in the study had higher CTQs scores than those who actually agreed. Our data may have a selection bias as adolescents who could still be living in violent environments, under ongoing abuse, or having more severe histories of childhood maltreatment were not included. Such bias is inexorably associated with this type of study, despite very few studies having addressed it. The fourth limitation is the absence of longitudinal measures to evaluate whether observed changes in neuroimmunoendocrine biomarkers will persist over time or to determine the vulnerability or resilience to the development of mental disorders in our sample. Importantly, we reported for the first time that ELS induces significant endocrine and immunological changes in adolescents with no psychiatric disorders. Fifth, accumulating evidence demonstrates a critical role of sex hormones in neurotropic modulation, including BDNF expression, and HPA axis, which could contribute to sexual differentiation of the brain and neuroplasticity (Chan and Ye, 2017). This is important because during pubertal development, the HPA axis matures, and there are dramatic changes in reactivity to stressors, which is a critical stage of brain and behavior development (Blaustein and Ismail, 2013). Sixth, in this study we used PHA, which is a polyclonal mitogen widely used in immunological studies (Careaga et al, 2017; Nagarajan et al, 2017; van Zuiden et al, 2015) to induce T-cell activation, cytokine production (Th1/Th2/Th17 profiles) and proliferation in vitro. It should be noted that other stimuli can be used to stimulate T cells in vitro, eg, anti-CD3/CD28 (Motamedi et al, 2016) or PMA/ionomycin (Rao et al, 2017). It has been shown that PMA/ionomycin only induces Th1 cytokines, whereas PHA stimulates both Th1/Th2 cytokines (Baran et al, 2001), which was our goal. Therefore, one should consider such information in order to compare our results to others using different stimuli. Moreover, considering that some studies have found interesting differences in cytokines not evaluated here (eg, IL-1β and IL-8), future studies should explore these cytokines in stimulated PBMCs, as well as differing stimuli and culture times to replicate the findings of this study.
Taken together, our data provide further evidence that immune dysregulation during pubertal stages associated with ELS is due to greater stress-induced neuroendocrine activation. In addition, the molecular mechanisms may include the enhanced activation of both MAPK and NF-κB signaling in parallel with partial resistance to GCs. The biological alterations associated with ELS could increase the risk of psychiatric disorders later in life. Further verification is needed to replicate these findings and provide proof of evidence for this hypothesis.
Funding and disclosure
The authors declare no conflict of interest.
This work was supported by grants from CNPq (RGO, 476468/2012-4, RGO, 305141/2011-2; MEB, 304528/2013-7), FAPERGS, and CAPES. The funding institutions had no further roles in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The authors declare no conflict of interest.
References
- Baran J, Kowalczyk D, Ozog M, Zembala M (2001). Three-color flow cytometry detection of intracellular cytokines in peripheral blood mononuclear cells: comparative analysis of phorbol myristate acetate-ionomycin and phytohemagglutinin stimulation. Clin Diagn Lab Immunol 8: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer ME, Wieck A, Petersen LE, Baptista TS (2015). Neuroendocrine and viral correlates of premature immunosenescence. Ann NY Acad Sci 1351: 11–21. [DOI] [PubMed] [Google Scholar]
- Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V (2016). Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-alpha. Mol Psychiatry 21: 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingrath S, Rohleder N, Kudielka BM (2010). Healthy working school teachers with high effort-reward-imbalance and overcommitment show increased pro-inflammatory immune activity and a dampened innate immune defence. Brain Behav Immun 24: 1332–1339. [DOI] [PubMed] [Google Scholar]
- Bielas H, Jud A, Lips U, Reichenbach J, Landolt MA (2012). Increased number of activated T cells in lymphocyte subsets of maltreated children: data from a pilot study. J Psychosom Res 73: 313–318. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D et al (2003). A mechanism converting psychosocial stress into mononuclear cell activation. P Natl Acad Sci USA 100: 1920–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM (2012). The immune system and developmental programming of brain and behavior. Front Neuroendocrinol 33: 267–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein JD, Ismail N. Enduring influence of pubertal stressors on behavioral response to hormones in female mice. Horm Behav 2013; 64:390–398.. [DOI] [PMC free article] [PubMed]
- Brasil HH, Bordin IA (2010). Convergent validity of K-SADS-PL by comparison with CBCL in a Portuguese speaking outpatient population. BMC Psychiatry 10: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brietzke E, Stertz L, Fernandes BS, Kauer-Sant'anna M, Mascarenhas M, Escosteguy Vargas A et al (2009). Comparison of cytokine levels in depressed, manic and euthymic patients with bipolar disorder. J Affect Disord 116: 214–217. [DOI] [PubMed] [Google Scholar]
- Bucker J, Fries GR, Kapczinski F, Post RM, Yatham LN, Vianna P et al (2015). Brain-derived neurotrophic factor and inflammatory markers in school-aged children with early trauma. Acta Psychiat Scand 131: 360–368. [DOI] [PubMed] [Google Scholar]
- Careaga M, Rogers S, Hansen RL, Amaral DG, Van de Water J, Ashwood P (2017). Immune endophenotypes in children with autism spectrum disorder. Biol Psychiatry 81: 434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF et al (2007). Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry 62: 1080–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH (2010). Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology 35: 2617–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CB, Ye K (2017). Sex differences in brain-derived neurotrophic factor signaling and functions. J Neurosci Res 95: 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Handley ED, Rogosch FA (2015). Child maltreatment, inflammation, and internalizing symptoms: investigating the roles of C-reactive protein, gene variation, and neuroendocrine regulation. Dev Psychopathol 27: 553–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho R, Viola TW, Walss-Bass C, Brietzke E, Grassi-Oliveira R (2014). Childhood maltreatment and inflammatory markers: a systematic review. Acta Psychiatr Scand 129: 180–192. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS et al (2012). Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci USA 109: 5995–5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, McEwen BS (2012). Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav 106: 29–39. [DOI] [PubMed] [Google Scholar]
- Danese A, Lewis SJ (2017). Psychoneuroimmunology of early-life stress: the hidden wounds of childhood trauma? Neuropsychopharmacology 42: 99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis NP, De Kloet ER, Yehuda R, Malaspina D, Kranz TM (2015). Early life stress effects on glucocorticoid-BDNF interplay in the hippocampus. Front Mol Neurosci 8: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Prado CH, Rizzo LB, Wieck A, Lopes RP, Teixeira AL, Grassi-Oliveira R et al (2013). Reduced regulatory T cells are associated with higher levels of Th1/TH17 cytokines and activated MAPK in type 1 bipolar disorder. Psychoneuroendocrinology 38: 667–676. [DOI] [PubMed] [Google Scholar]
- Eddy JL, Krukowski K, Janusek L, Mathews HL (2014). Glucocorticoids regulate natural killer cell function epigenetically. Cell Immunol 290: 120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton A, Tripathi SP, Mletzko T, Young J, Cisler JM, James GA et al (2014). Childhood maltreatment is associated with a sex-dependent functional reorganization of a brain inhibitory control network. Hum Brain Mapp 35: 1654–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi-Oliveira R, Cogo-Moreira H, Salum GA, Brietzke E, Viola TW, Manfro GG et al (2014). Childhood Trauma Questionnaire (CTQ) in Brazilian samples of different age groups: findings from confirmatory factor analysis. PLoS ONE 9: e87118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi-Oliveira R, Honeycutt JA, Holland FH, Ganguly P, Brenhouse HC (2016). Cognitive impairment effects of early life stress in adolescents can be predicted with early biomarkers: impacts of sex, experience, and cytokines. Psychoneuroendocrinology 71: 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi-Oliveira R, Pezzi JC, Daruy-Filho L, Viola TW, Francke ID, Leite CE et al (2012). Hair cortisol and stressful life events retrospective assessment in crack cocaine users. Am J Drug Alcohol Abuse 38: 535–538. [DOI] [PubMed] [Google Scholar]
- Grassi-Oliveira R, Stein LM, Lopes RP, Teixeira AL, Bauer ME (2008). Low plasma brain-derived neurotrophic factor and childhood physical neglect are associated with verbal memory impairment in major depression—a preliminary report. Biol Psychiatry 64: 281–285. [DOI] [PubMed] [Google Scholar]
- Guan Z, Fang J (2006). Peripheral immune activation by lipopolysaccharide decreases neurotrophins in the cortex and hippocampus in rats. Brain Behav Immun 20: 64–71. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S (2004). Signaling to NF-kappaB. Genes Dev 18: 2195–2224. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB (2008). The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology 33: 693–710. [DOI] [PubMed] [Google Scholar]
- Horowitz MA, Zunszain PA (2015). Neuroimmune and neuroendocrine abnormalities in depression: two sides of the same coin. Ann NY Acad Sci 1351: 68–79. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P et al (1997). Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36: 980–988. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE et al (1999). Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med 189: 865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB (2014). Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry 71: 1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knijff EM, Breunis MN, van Geest MC, Kupka RW, Ruwhof C, de Wit HJ et al (2006). A relative resistance of T cells to dexamethasone in bipolar disorder. Bipolar Disord 8: 740–750. [DOI] [PubMed] [Google Scholar]
- Kovacs M (1985). The Children's Depression Inventory (CDI). Psychopharmacol Bull 21: 995–998. [PubMed] [Google Scholar]
- Lapchak PA, Araujo DM, Hefti F (1993). Systemic interleukin-1 beta decreases brain-derived neurotrophic factor messenger RNA expression in the rat hippocampal formation. Neuroscience 53: 297–301. [DOI] [PubMed] [Google Scholar]
- Levandowski ML, Tractenberg SG, de Azeredo LA, De Nardi T, Rovaris DL, Bau CH et al (2016). Crack cocaine addiction, early life stress and accelerated cellular aging among women. Prog Neuropsychopharmacol Biol Psychiatry 71: 83–89. [DOI] [PubMed] [Google Scholar]
- Levandowski ML, Viola TW, Wearick-Silva LE, Wieck A, Tractenberg SG, Brietzke E et al (2014). Early life stress and tumor necrosis factor superfamily in crack cocaine withdrawal. J Psychiatr Res 53: 180–186. [DOI] [PubMed] [Google Scholar]
- Lopes RP, Grassi-Oliveira R, de Almeida LR, Stein LM, Luz C, Teixeira AL et al (2012). Neuroimmunoendocrine interactions in patients with recurrent major depression, increased early life stress and long-standing posttraumatic stress disorder symptoms. Neuroimmunomodulation 19: 33–42. [DOI] [PubMed] [Google Scholar]
- McCrory E, De Brito SA, Viding E (2011). The impact of childhood maltreatment: a review of neurobiological and genetic factors. Front Psychiatry 2: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (2001). Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann NY Acad Sci 933: 265–277. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonte B, Szyf M et al (2009). Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci 12: 342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ (2011). psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull 137: 959–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motamedi M, Xu L, Elahi S (2016). Correlation of transferrin receptor (CD71) with Ki67 expression on stimulated human and mouse T cells: the kinetics of expression of T cell activation markers. J Immunol Methods 437: 43–52. [DOI] [PubMed] [Google Scholar]
- Nagarajan V, Hernandez AV, Cauthen CA, Starling RC, Tang WH (2017). Usefulness of cell-mediated immune function in risk stratification for patients with advanced heart failure. Am Heart J 183: 35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numakawa T, Adachi N, Richards M, Chiba S, Kunugi H (2013). Brain-derived neurotrophic factor and glucocorticoids: reciprocal influence on the central nervous system. Neuroscience 239: 157–172. [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I, Odgers CL, Danese A, Bowes L, Shakoor S, Papadopoulos AS et al (2011). Blunted cortisol responses to stress signal social and behavioral problems among maltreated/bullied 12-year-old children. Biol Psychiatry 70: 1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH et al (2006). Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry 163: 1630–1633. [DOI] [PubMed] [Google Scholar]
- Pace TW, Wingenfeld K, Schmidt I, Meinlschmidt G, Hellhammer DH, Heim CM (2012). Increased peripheral NF-kappaB pathway activity in women with childhood abuse-related posttraumatic stress disorder. Brain Behav Immun 26: 13–17. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Miller AH (2001). Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry 49: 391–404. [DOI] [PubMed] [Google Scholar]
- Rao DA, Gurish MF, Marshall JL, Slowikowski K, Fonseka CY, Liu Y et al (2017). Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 542: 110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redlich R, Stacey D, Opel N, Grotegerd D, Dohm K, Kugel H et al (2015). Evidence of an IFN-gamma by early life stress interaction in the regulation of amygdala reactivity to emotional stimuli. Psychoneuroendocrinology 62: 166–173. [DOI] [PubMed] [Google Scholar]
- Schalinski I, Elbert T, Steudte-Schmiedgen S, Kirschbaum C (2015). The cortisol paradox of trauma-related disorders: lower phasic responses but higher tonic levels of cortisol are associated with sexual abuse in childhood. PLoS ONE 10: e0136921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder T, Steudte S, Miller R, Skoluda N, Dettenborn L, Kirschbaum C (2012). Intraindividual stability of hair cortisol concentrations. Psychoneuroendocrinology 37: 602–610. [DOI] [PubMed] [Google Scholar]
- Sullivan KE, Cutilli J, Piliero LM, Ghavimi-Alagha D, Starr SE, Campbell DE et al (2000). Measurement of cytokine secretion, intracellular protein expression, and mRNA in resting and stimulated peripheral blood mononuclear cells. Clin Diagn Lab Immunol 7: 920–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarullo AR, Gunnar MR (2006). Child maltreatment and the developing HPA axis. Horm Behav 50: 632–639. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Samson JA (2013). Childhood maltreatment and psychopathology: a case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am J Psychiatry 170: 1114–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Parade SH, Price LH, Kao HT, Porton B, Philip NS et al (2016). Alterations of mitochondrial DNA copy number and telomere length with early adversity and psychopathology. Biol Psychiatry 79: 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zuiden M, Kavelaars A, Vermetten E, Olff M, Geuze E, Heijnen C (2015). Pre-deployment differences in glucocorticoid sensitivity of leukocytes in soldiers developing symptoms of PTSD, depression or fatigue persist after return from military deployment. Psychoneuroendocrinology 51: 513–524. [DOI] [PubMed] [Google Scholar]
- Vanbesien-Mailliot CCA, Wolowczuk I, Mairesse J, Viltart O, Delacre M, Khalife J et al (2007). Prenatal stress has pro-inflammatory consequences on the immune system in adult rats. Psychoneuroendocrino 32: 114–124. [DOI] [PubMed] [Google Scholar]
- Wechsler DWechsler Abbreviated Scale of Intelligence. The Psychological Corporation: Harcourt Brace & Company. New York, NY, 1999.
- Wieck A, Grassi-Oliveira R, do Prado CH, Rizzo LB, de Oliveira AS, Kommers-Molina J et al (2013). Differential neuroendocrine and immune responses to acute psychosocial stress in women with type 1 bipolar disorder. Brain Behav Immun 34: 47–55. [DOI] [PubMed] [Google Scholar]
- Williamson DE, Birmaher B, Ryan ND, Shiffrin TP, Lusky JA, Protopapa J et al (2003). The stressful life events schedule for children and adolescents: development and validation. Psychiatry Res 119: 225–241. [DOI] [PubMed] [Google Scholar]
- Zhu S, Qian Y (2012). IL-17/IL-17 receptor system in autoimmune disease: mechanisms and therapeutic potential. Clin Sci (Lond) 122: 487–511. [DOI] [PubMed] [Google Scholar]