Abstract
Cross-sectional studies of the effects of cannabis on cognition in schizophrenia have produced mixed results. Heavy and persistent cannabis use in schizophrenia is a common clinical problem, and effects of controlled abstinence from cannabis in these patients have not been carefully evaluated. The present study sought to determine the effects of cannabis abstinence on cognition in patients with schizophrenia and co-occurring cannabis dependence. We utilized a 28-day cannabis abstinence paradigm to investigate the state-dependent effects of cannabis on select cognitive outcomes in cannabis-dependent patients with schizophrenia and non-psychiatric controls. Nineteen patients and 20 non-psychiatric male cannabis-dependent participants underwent 28 days of cannabis abstinence. Cognition was assessed on day 0, 14, and 28 using a comprehensive neuropsychological battery. Clinical symptoms were assessed weekly. Abstinence was facilitated by contingency reinforcement confirmed by twice weekly urinalysis. Forty-two percent of patients and 55% of controls achieved end-point abstinence (p=0.53), which was biochemically-verified (day 28 urinary THC-COOH <20 ng/ml). In this preliminary study, schizophrenia-abstainers demonstrated improvements in Hopkins Verbal Learning Test-Revised (HVLT-R) performance over time [F(2,14)=4.73, p<0.03] (d=1.07). Lesser improvements on HVLT-R were observed in non-psychiatric control abstainers (d=0.66), and with abstinence on other cognitive test measures, in both patients and controls. Verbal memory and learning may improve in schizophrenia and control subjects with cannabis abstinence, but larger more definitive studies are needed. Our findings underscore the importance of developing effective interventions for cannabis use disorders in schizophrenia.
Introduction
Cannabis may be a risk factor for the development of psychotic disorders such as schizophrenia (Moore et al, 2007). Importantly, rates of cannabis use disorders (CUD) are higher among patients with schizophrenia (~25%) compared to the general population (~2.9% Hasin et al, 2015; Koskinen et al, 2010). Cannabis users with schizophrenia are more likely to be male, have an earlier onset of the illness and experience a more severe course of the disorder compared to non-cannabis using patients (Koskinen et al, 2010; Large et al, 2011; Zammit et al, 2008). Clinical evidence demonstrates that cannabis is associated with increased rates of psychotic relapse, decreased treatment adherence, and poorer psychosocial functioning in patients with schizophrenia (Manrique-Garcia et al, 2014; Patel et al, 2016; Zammit et al, 2008). Accordingly, one might predict that cannabis compromises core cognitive processes in these patients. However, findings remain controversial (Coulston et al, 2007b; Rabin et al, 2011; Yucel et al, 2012), and warrant further investigation.
Surprisingly, several studies report that cannabis-using patients with schizophrenia have superior cognition compared to their non-using counterparts, and two recent meta-analyses examining this relationship support this notion (Rabin et al, 2011; Yucel et al, 2012). One theory proposed to explain this paradox is that patients with co-morbid CUDs represent a higher functioning subgroup with inherently superior cognition and prognosis (Dixon et al, 1991; Leeson et al, 2011; Rodriguez-Sanchez et al, 2010). These individuals are thought to have better premorbid adjustment, social skills, and lower levels of negative symptoms that enable them to navigate and socialize within drug scenes to maintain their habit (Arndt et al, 1992; Potvin et al, 2005). Other investigators have posited that these individuals possess a lower vulnerability for developing psychosis compared to patients without a history of cannabis use (Schnell et al, 2009; Yucel et al, 2012). Cannabis may interact with underlying neurobiological vulnerability factors to trigger the transition to psychosis that may not have occurred in the absence of cannabis (Caspi et al, 2005). Together, these theories suggest that cannabis is associated with better cognition, rather than cannabis itself leading to improved cognitive function. Thus, better cognition may reflect trait characteristics of this subgroup, a phenomenon not seen among non-psychiatric cannabis users (Broyd et al, 2016). Unlike studies in patient populations, there are no reported observations that cannabis using non-psychiatric controls perform better than non-users (Broyd et al, 2016). Thus, it is conceivable that cannabis may differentially affect cognitive function in schizophrenia compared to controls.
Evidence that cannabis has a deleterious effect on cognition is well documented (Lev-Ran et al, 2012; Ringen et al, 2010). Acute administration of tetrahydrocannabinol (THC) worsens, rather than enhances, cognition in patients with schizophrenia specifically in domains of verbal memory and attention (D'Souza et al, 2005). Further, these patients were more vulnerable to THC’s effects compared to non-psychiatric controls. Results from a previous study from our group demonstrated that cumulative cannabis use dose-dependently impaired cognitive performance in tasks mediated by the prefrontal cortex and the hippocampus in current, but not former cannabis-dependent patients who were abstinent for at least 6 months (Rabin et al, 2013). Taken together, these findings suggest that cannabis exerts a state-dependent negative effect on neuropsychological performance facilitated by brain regions rich in cannabinoid type-1 receptors (CB1Rs; Mackie, 2005). Moreover, these cannabis-induced impairments may be reversible with sustained periods of abstinence.
Explanations for conflicting results on effects of cannabis on cognition in schizophrenia may be due to methodological limitations in published studies. For example, many studies have failed to control for the period of time elapsed between the last use of cannabis and neuropsychological testing. Depending on this interval, studies may be assessing the impact of acute cannabis intoxication (D'Souza et al, 2005), withdrawal effects (Coulston et al, 2007a), or the residual or longer lasting consequences of cumulative cannabis exposure (Jockers-Scherubl et al, 2007; Schnell et al, 2009). Moreover, all research studies examining the relationship between cannabis and cognition in schizophrenia have employed cross-sectional designs, making it difficult to draw firm conclusions about direct effects of cannabis on cognitive function. In fact, studies may be simultaneously assessing both the state- and trait-dependent effects of cannabis. This is problematic given the premise that they may be associated with opposite effects on cognitive function. A more reliable and robust method is to employ a longitudinal design to examine within-subject differences related to cannabis abstinence and reinstatement of use.
In the present study, we employed a 28-day cannabis abstinence paradigm to more appropriately assess the long-term state-dependent effects of cannabis on key cognitive outcomes, within regions of high CB1R density, in cannabis-dependent patients with schizophrenia and non-psychiatric controls. More specifically, our main outcome of interest was verbal learning and memory given its high sensitivity to cannabis (D'Souza et al, 2005; Rabin et al, 2013) and documented impairment in schizophrenia (Heinrichs and Zakzanis, 1998). Secondary aims of the study examined other areas of moderate to high cannabinoid density, such as the prefrontal cortex and the cerebellum (Herkenham, 1991).
Therefore, we hypothesized that cognitive performance as assessed by the HVLT would improve over time in patients with schizophrenia who successfully remained abstinent from cannabis for the 28-day abstinent period. In addition, we expected greater magnitude of cognitive change in abstaining patients compared to abstaining controls given that patients with schizophrenia demonstrate enhanced sensitivity to the cognitive-impairing effects of cannabis (D'Souza et al, 2005). The 28-day abstinence period was based on the unique pharmacokinetic profile of THC (Nahas, 2001). This duration reflects the time needed for full cannabinoid elimination (ie, including residual cannabinoids) in order to achieve biochemically confirmed abstinence at a standardized cut-off of 20 ng/ml (Ellis et al, 1985; Smith-Kielland et al, 1999).
Materials and methods
Participants
Patients with schizophrenia were recruited through the Centre for Addiction and Mental Health (CAMH) using flyers posted around the hospital and through referrals made by outpatient clinicians. Non-psychiatric cannabis users were recruited from the community by posted ads. Study eligibility was assessed by an initial telephone screening, followed by an in-person comprehensive interview. Recruitment began in April 2012 and ended by December 2015.
Male participants between the ages of 18 and 55 were recruited for the study. All participants met criteria for current cannabis dependence based on DSM-IV-TR (APA, 2000). A positive urine test for THC-COOH (MEDTOX; Wilmington, NC) was required to confirm current cannabis use. To control for the effects of tobacco on cognition, all participants were daily cigarette smokers (⩾5 cigarettes per day, CPD). Moreover, all participants had to achieve Full Scale Intelligent Quotient (FSIQ) scores ⩾80, using the Weschsler Adult Reading Test (WTAR; Wechsler, 2001). Psychiatric participants met DSM-IV-TR criteria for schizophrenia or schizoaffective disorder; we excluded subjects with schizophreniform disorder and psychosis NOS. Patients were psychiatrically stable at the time of the interview with a total score <70 on the Positive and Negative Syndrome Scale for Schizophrenia (PANSS; Kay et al, 1987) and no hospitalizations in the 3 months prior to enrollment. In addition, patients had to be maintained on a stable dose of antipsychotic medication with no changes for at least 1 month. Non-psychiatric controls were excluded if they met criteria for a current or past DSM-IV Axis I diagnosis (except for major depression in remission >1 year) or if they were taking psychotropic medications. Individuals with a current substance use disorder (SUD) or past (remission <6 months) SUD (other than cannabis, nicotine, and caffeine) or those testing positive on urine toxicology for illicit drugs other than cannabis (ie, cocaine, opiates, amphetamine, phencyclidine, and barbiturates) were not eligible for study participation. In addition, participants were excluded if they were actively seeking treatment for cannabis. Head injury with loss of consciousness for >30 min requiring hospitalization, or evidence of intracranial injury or neurological/medical conditions affecting cognition was also exclusionary. Written informed consent was obtained from all participants, as approved by the Research Ethics Board at CAMH.
Measures
Substance use measures
Current cannabis dependence, past alcohol and other SUD were diagnosed using DSM-IV-TR criteria. Cumulative cannabis exposure was indexed as joint-years, where one joint-year is the equivalent of using one joint per day for 1 year (Rabin et al, 2013). The Timeline Follow-Back (Sobell et al, 1988; TLFB) is a self-report of substance use frequency and was collected for cannabis in grams, tobacco cigarettes, alcoholic beverages and caffeine in the seven previous days. Level of nicotine dependence was measured using the Fagerstrom Test of Nicotine Dependence (FTND) (Heatherton et al, 1991) and the Alcohol Use Disorders Identification Test (AUDIT; Saunders et al, 1993) assessed problematic drinking behaviors. Lastly, cannabis withdrawal was assessed using the Marijuana Withdrawal Checklist (MWC; Budney et al, 2003).
Neuropsychological test battery
All participants completed a comprehensive neuropsychological test battery. Neuropsychological test measures included those with demonstrated sensitivity in patients with schizophrenia and/or cannabis users. Cognitive sessions took an average of 2.5 h to complete, and were administered by R.A.R. The Test of Memory Malingering (TOMM) (Tombaugh, 1997), a measure of effort and motivation (Sharland and Gfeller, 2007) was only administered at the neuropsychological training session to ensure credibility of performance across other measures. Similarly, the Wisconsin Card Sorting Test (WCST; Heaton et al, 1993) and Iowa Gambling Test (IGT; Bechara et al, 1994), measures of executive function and decision-making respectively were also only administered at the training session. Other tests, less sensitive to repeated testing, were administered at Days 0, 14, and 28 and included: Spatial Delayed Response task (SDR; Hershey et al, 1998), a measure of visuospatial working memory; Digit Span Forwards and Backwards (Wechsler, 1997), a measure of working memory and executive function respectively; Continuous Performance Test II (CPT-II; Conners, 2000), an assessment of sustained attention; Trail Making Test A and B (TMT; Lezak et al, 2004) to evaluate psychomotor speed and executive function respectively; Grooved Pegboard (Lafayette Instrument Company, 1989) to measure manual dexterity and fine motor movement; Balloon Analog Risk Task (BART; Lejuez et al, 2002) a computerized measure of risk taking behavior; and lastly, Kirby Delayed Discounting Test (KDDT; Kirby et al, 1999) to assess impulsive choices. We utilized the Hopkins Verbal Learning Test Revised (HVLT-R; Brandt and Benedict, 2001) to assess verbal memory and learning. Six alternate word lists from this test were administered in a counterbalanced order.
Clinical Measures
In patients with schizophrenia, positive and negative symptoms were assessed using the PANSS (Kay et al, 1987) and extrapyramidal symptoms were measured with the Simpson Angus Rating Scale (SARS) (Simpson and Angus, 1970), the Abnormal Involuntary Movement Scale (AIMS; Guy and Cleary, 1976), and the Barnes Akathisia Rating Scale (BARS; Barnes, 1989). The Calgary Depression Scale for Schizophrenia (CDSS; Addington et al, 1993) assessed depression exclusively in patients and the Hamilton Rating Scale for Depression (HAM-D; Hamilton, 1967) assessed mood symptoms in both patients and controls.
Laboratory Procedures
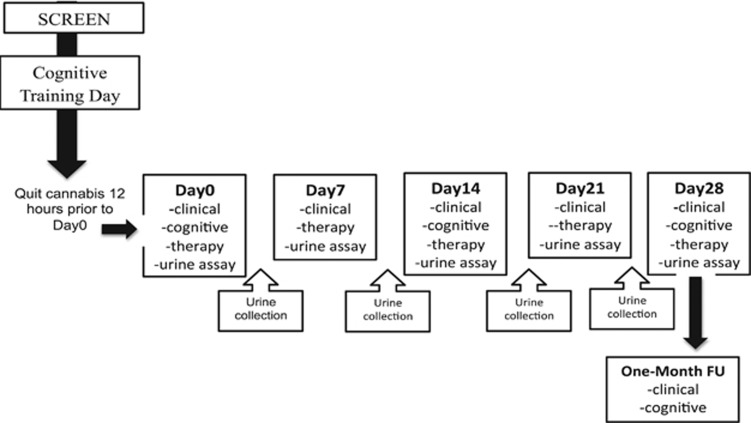
Once eligibility was confirmed, participants were invited to the lab for a neuropsychological training visit to familiarize participants with test measure procedures so as to minimize practice effects associated with repeated testing (Sacco et al, 2006). Participants were instructed to abstain from cannabis for 12 h prior to this visit to minimize the possibility of cannabis intoxication as well as withdrawal (Budney et al, 2003). Given that acute periods of abstinence cannot be biochemically confirmed, this was done through clinical observation (ie, conjunctival injection, euphoria, giggling, sedation, and lethargy (APA, 1994)). Participants were then scheduled to quit cannabis the night before coming into the lab for the day 0 (baseline) visit for a full 28-day period. Participants attended weekly study visits. Clinical measures assessing psychiatric, depressive, and withdrawal symptoms were assessed weekly, while cognitive function was evaluated biweekly, on days 0, 14, and 28. Urine was collected twice weekly and then stored in a −80 °C freezer for future gas chromatography mass spectrometry (GC–MS) analysis. To encourage cannabis abstinence individual supportive therapy was given weekly (20–30 min) by trained clinical staff in the Schizophrenia Division at CAMH. Sessions included a combination of motivational interviewing, psycho-education, and coping skills. Contingency management was used as the primary reinforcer: participants who successfully abstained from cannabis for the full 28 days (MEDTOX; THC-COOH <50 ng/ml) were rewarded with a $300 bonus. Four weeks later (day 56), participants attended a follow-up study visit, where both clinical and cognitive outcomes were evaluated. See Figure 1.
Figure 1.
Study Design. Outline of the study design including screening and training visit, weekly assessments and twice weekly urine collections.
Abstinence Verification
Abstinence verification was based on meeting all three of the following criteria:
No self-reported cannabis use for 28 days based on TLFB.
Biochemical confirmation that no new cannabis was introduced over the abstinence period. GC–MS analysis was conducted to calculate THC-COOH/creatinine ratios on 9 urine samples collected twice weekly (baseline+2 samples per week, for 4 weeks). Urine samples were collected at the weekly study visit and the other sample was collected 3 days later. Each ratio was divided by the previously collected sample quotient (urine2/urine1). A prediction model was applied to these quotients to biochemically determine whether new cannabis use was introduced during the 28-day abstinence period (Schwilke et al, 2011).
No THC-COOH was present in urines at Day 28. A sensitivity cut-off of <20 ng/ml at day28 was employed (Ellis et al, 1985).
Data Analysis
Independent t-tests and χ2 tests were used to analyze demographic variables between cannabis-dependent patients with schizophrenia and cannabis-dependent non-psychiatric controls. Between group differences on baseline neuropsychological measures were assessed using ANCOVAs controlling for education level and IQ. Bonferroni corrections were performed to account for multiple comparisons of cross-sectional data comparisons (α/41, p=0.001).
2 × 2 × 3 repeated measures of analysis of co-variance (RM-ANCOVA) were used to assess change over time for cognitive and clinical variables in patients with schizophrenia and controls. Time was the within-subject factor, and diagnosis and abstinence status were employed as the between-subject factors. This analytic approach was adopted in order to capture time × abstinence status interactions and time × diagnosis × abstinent status interaction. Time × abstinence status evaluated potential differences in recovery in the sample as a whole and time × diagnosis × abstinence status evaluated. Whether abstaining and non-abstaining patients cognitively behave in a differential manner compared to non-psychiatric controls. Separate models were estimated for each cognitive and clinical outcome. When significant differences were detected, post-hoc univariate ANOVAs were conducted. Main effects of time were followed up with LSD post hoc tests to detect between which time-point significant change occurred. Because of group differences in education level and IQ, these were controlled for in separate analyses presented in the Supplementary Material (see Table 1). Effect sizes (Cohen’s d) were computed to determine the magnitude of change between baseline and day 28 in patient-abstainers (see Supplementary Material, Table 3). Difference scores in cognitive outcomes between baseline and day 28 were computed. MANCOVAs were conducted with the cognitive difference score as the dependent variable and diagnostic group and abstinence status as fixed factors to see if cognitive change differed by diagnostic group and/or abstinence status (Supplementary Material). Finally, we also conducted Linear Regression models in patients and controls separately to determine whether duration of abstinence (ie, days of sustained abstinence) predicted change in cognitive performance between baseline (day 0) and day 28. These results are now included in the Supplementary Material (Table 2).
Table 1. Demographic, Clinical and Substance-using Characteristics.
| SCZ (n=19) | CTL (n=20) | p-value | |
|---|---|---|---|
| Demographic variables: | |||
| Age (years) | 31.58±9.1 | 30.80±8.1 | 0.78 |
| Race (C/A/O)a | 8/8/3 | 15/3/3 | 0.10 |
| FSIQ | 91.21±8.6 | 101.60±9.2 | <0.01* |
| Education (years) | 10.97±1.8 | 13.35±2.4 | <0.01* |
| Clinical variables: | |||
| CPZ equivalents | 352.42±194.0 | NA | NA |
| Atypical/typical antipsychotics | 16/2 | NA | NA |
| PANSS positive | 13.84±3.9 | NA | NA |
| PANSS negative | 13.1 1±4.0 | NA | NA |
| PANSS General | 25.8±44.3 | NA | NA |
| PANSS total | 52.84±9.9 | NA | NA |
| CDSS | 2.6 3±2.8 | NA | NA |
| HAM-D | 4.37±2.9 | 2.75±3.1 | 0.06 |
| Substance-using variables: | |||
| Joint-years | 10.10±7.3 | 9.76±6.6 | 0.88 |
| GPD | 1.22±0.8 | 1.63±1.2 | 0.21 |
| Baseline THC-COOH:Cr | 49.00±47.7 | 100.26±104.2 | 0.12 |
| ($)/Week on cannabis | 43.95±36.2 | 64.75±58.6 | 0.19 |
| Age of first use of cannabis | 15.00±2.5 | 15.05±2.9 | 0.95 |
| Age of onset of regular (weekly) cannabis use | 17.05±3.6 | 18.25±5.2 | 0.41 |
| MWC | 10.58±6.8 | 7.55±7.2 | 0.19 |
| CPD | 12.56±7.3 | 11.19±10.6 | 0.64 |
| CO level | 19.42±6.9 | 17.05±10.7 | 0.42 |
| FTND | 4.95±2.1 | 3.30±2.8 | <0.05* |
| AUDIT | 5.70±4.4 | 6.10±3.7 | 0.78 |
| Alcoholic drinks/week | 0.50±0.6 | 0.50±0.6 | 0.81 |
| Caffeinated beverages/week | 2.05±2.9 | 2.69 2.2 | 0.44 |
Abbreviations: A, African; AUDIT, alcohol use identification test; C, Caucasian; CDSS, Calgary Depression Scale for Schizophrenia; CO, carbon monoxide; CPD, cigarettes per day; CPZ equivalents, chlorpromazine equivalents, FTND, Fagerstrom test of nicotine dependence; GPD, average grams of cannabis per day; HAM-D, Hamilton Depression Rating Scale; O, other race; MWC, marijuana withdrawal checklist; PANSS, Positive and Negative Syndrome Scale; THC-COOH:Cr, carboxy-tetrahydrocannabinol (THC) normalized to creatinine. Values given in mean±SD; a, values are in numbers; *p<0.05.
Table 2. Baseline Relationships Between Cannabis and Cognition.
| Cognitive measure | Subtest | SCZ (n=19) | CTL (n=20) | p-value |
|---|---|---|---|---|
| TOMM | Trial 2 | 49.52±1.2 | 49.70±0.8 | 0.97 |
| WCST | # Trial | 106.16±20.3 | 100.15±23.3 | 0.38 |
| # Correct | 73.21±8.6 | 74.15±12.4 | 0.58 | |
| % Error | 28.68±14.5 | 23.40±14.4 | 0.61 | |
| % Perseverative response | 17.2 1±10.4 | 12.45±9.1 | 0.77 | |
| % Perseverative error | 15.36±8.4 | 11.6±7.4 | 0.83 | |
| % Non perseverative error | 13.53±7.4 | 11.9±9.4 | 0.30 | |
| Categories completed | 4.89±1.7 | 5.15±1.5 | 0.29 | |
| Conceptual response | 63.68±21.1 | 69.75±19.7 | 0.56 | |
| IGT | Net total | −1.89±24.0 | 5.90±28.2 | 0.89 |
| Total money | −1250.53±1108.3 | −670.75±1301.7 | 0.47 | |
| CPT | % Hits | 98.70±1.7 | 99.45±0.7 | 0.11 |
| % Commission | 42.39±24.7 | 30.92±20.4 | 0.21 | |
| Hit rate | 392.19±63.2 | 382.87± 57.9 | 0.45 | |
| Variability | 14.96±14.4 | 11.30±12.8 | 0.41 | |
| Attentiveness | 0.65±0.49 | 0.93±0.4 | 0.15 | |
| HVLT | Trial 1 | 4.68±2.1 | 6.915±1.6 | 0.16 |
| Trial 2 | 7.21±1.6 | 8.63±1.9 | 0.21 | |
| Trial 3 | 8.4±1.9 | 9.94±.1.6 | 0.30 | |
| Sum of trial1-3 | 20.16±5.4 | 24.74±4.5 | 0.16 | |
| Delayed recall | 5.57±2.6 | 8.79±2.1 | 0.01 | |
| Repetitions | 1.74±2.6 | 1.42±1.6 | 0.89 | |
| Intrusions | 1.57±1.8 | 0.89±1.4 | 0.56 | |
| True positives | 10.63±1.4 | 11.31±1.2 | 0.31 | |
| False positives | 1.47±1.8 | 0.47±0.9 | 0.08 | |
| % Retention | 63.78±20.8 | 85.11±17.8 | 0.01 | |
| Discrimination index | 9.15±2.1 | 10.84±1.9 | 0.07 | |
| Digit span | Forwards | 9.89±2.3 | 11.50±2.9 | 0.71 |
| Backwards | 5.63±1.9 | 8.00±2.8 | 0.18 | |
| Total | 15.53±3.5 | 19.50±4.8 | 0.53 | |
| TMT | A (seconds) | 31.71±11.3 | 26.10±10.2 | 0.50 |
| B (seconds) | 91.97±49.3 | 56.38±20.6 | 0.10 | |
| B minus A (seconds) | 60.25±42.2 | 27.65±12.6 | 0.07 | |
| SDR | 5-s delay | 17.6 3±4.8 | 18.26±5.6 | 0.33 |
| 15-s delay | 25.32±8.7 | 24.21±10.2 | 0.66 | |
| 30-s delay | 30.52±10.2 | 30.79±16.0 | 0.79 | |
| KDDT | Geomean | 0.04±0.03 | 0.05±0.06 | 0.63 |
| BART | Avg. adjusted pumps | 39.68±17.5 | 47.78±21.8 | 0.48 |
| Grooved pegboard | Total time | 170.52±46.4 | 142.48±22.8 | 0.06 |
| Total pegs dropped | 1.84±2.0 | 0.350±0.59 | 0.05 |
Abbreviations: BART, Balloon Analog Risk Task; CPT, Continuous Performance Test; HVLT, Hopkins Verbal Learning Test; KDDT, Kirby Delay Discounting; SDR, Spatial Delay Response; TOMM, Test of Memory Malingering; TMT, Trail Making Test; WCST, Wisconsin Card Sorting Test. Values given in mean±SD; a, values are in numbers; analyses controlled for IQ and education; p-value was set at <0.001.
Data were analyzed using the Statistical Program for Social Sciences (SPSS) version 24.0 (SPSS Inc., Chicago, IL). All tests were two-tailed and the level of significance was set at p<0.05. Our a priori hypothesis based on previous studies (Rabin et al, 2013) was that verbal memory and learning as measured by the Hopkins Verbal Learning Test (HVLT) would be altered by cannabis abstinence. Therefore, Bonferroni corrections were applied to account for multiple time-point comparisons (α/3, p=0.0167).
Results
Sample Demographics
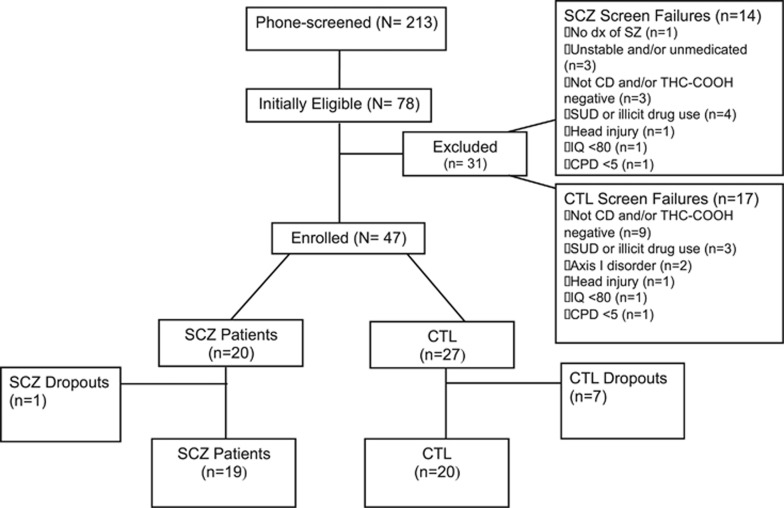
Nineteen cannabis-dependent patients with schizophrenia and 20 cannabis-dependent controls completed the study. A CONSORT diagram to outline subject disposition is provided in Figure 2. The completion rate of the 4-week abstinence period was 83%. The difference in attrition rates between patients and controls did not statistically differ χ2=3.50 (df=1), p>0.06. The patient group had a 5% attrition rate, while the controls had a 25% attrition rate. Notably, the majority of controls (n=5) dropped out after the screening visit, before cannabis abstinence was initiated.
Figure 2.
CONSORT Subject Flow Diagram. Details of recruitment, screening, drop-outs, and completion rates of participants for this study.
Thus, it was not cannabis abstinence itself that led to attrition. After the baseline visit (post-quit) only one patient and two controls dropped-out.
Demographic and clinical variables are presented in Table 1. According to DSM-IV, 14 patients met criteria for schizophrenia and five patients met criteria for schizoaffective disorder. (collectively designated ‘schizophrenia’ patients). We included patients with schizophrenia with past Axis 1 disorders [anxiety (n=1) and MDD (n=1)] as well as those with past SUD in remission for at least 6 months (other than cannabis). In the same respect, we included controls that had an SUD in remission for at least 6 months, and included controls that met for MDD in full remission (n=2). Because of our within-subjects design, these past diagnoses should not have influenced cognitive outcome trajectories.
Patients and the non-psychiatric control group did not differ significantly on age and race. Group differences emerged on IQ [t(37)=3.64, p<0.01] and years of education [t(37)=3.45, p<0.01], with controls demonstrating higher IQ and greater number of years of education. On the CDSS at baseline, patients with schizophrenia had mean scores of 2.6±2.8.
The majority of patients were taking atypical antipsychotics [clozapine (n=1), risperidone (n=7), olanzapine (n=4), quetiapine (n=2), and paliperidone (n=3)]. One patient was taking a typical antipsychotic (flupenthixol) and one patient was on a combination of an atypical and typical medication (quetiapine+fluphenazine). Chlorpromazine equivalents are listed in Table 1. Patients were also prescribed medication for their mood [citalopram (n=2), escitalopram (n=1) and valproate (n=1)], and one patient was prescribed a benzodiazepine (lorazepam). Patients were taking medication for hypertension (n=2), Type 2 diabetes (n=3) and hypercholesterolemia (n=3). Among controls, only one participant was taking medication, and this was for hypercholesterolemia (rosuvastatin).
Substance Use Characteristics
Substance use characteristics are listed in Table 1. Groups were matched on cannabis-using variables such as cumulative use (joint-years), grams of cannabis used in the previous week (GPD), money spent on cannabis in the prior week and baseline THC-COOH/creatinine levels. Age of onset of weekly cannabis use was also similar between groups, as were the mean baseline scores for MWC. On FTND, patients demonstrated higher levels of nicotine dependence compared to controls [t(37)=1.98, p=0.04]. Interestingly, the average number of cigarettes per day did not differ between groups. Problematic alcohol use and alcoholic beverages consumed over the previous week did not differ between groups. Similarly, caffeine use was comparable between patients and controls.
Abstinence Classification
Participant abstinence rates were not significantly different between patients with schizophrenia and non-psychiatric control groups: 42.1% of patients (8/19) and 55% of controls (11/20) successfully achieved abstinence verification criteria [χ2=0.65 (df=1), p=0.53]. Notably, the non-abstaining group collectively included individuals with varying abstaining/relapse trajectories.
These included: (1) individuals who quit, but whose day 28 THC-COOH levels did not drop below 20 ng/ml (SCZ, n=2; CTL, n=3); (2) individuals who lapsed even just once (SCZ, n=3; CTL, n=2); (3) individuals who did not quit but who nevertheless cut down on their current use (SCZ, n=1; CTL, n=1); (4) Participants who relapsed after minimal cessation and then continued to use cannabis throughout the study period (SCZ, n=5; CTL, n=3).
Baseline Neurocognitive Performance
The TOMM was completed at the neuropsychological test training session to examine effort exerted and motivation. All participants scored⩾45 on trial 2, suggesting high motivation (Rees et al, 1998). In addition, no difference was observed between patients and controls; [t(37)=0.53, p=0.60]. Comparing baseline cognitive function between patients with schizophrenia and controls, no group differences emerged on WCST, IGT, CPT, TMT, SDR, KDDT, or the BART. Groups differed on the HVLT and Grooved Pegboard, with controls demonstrating better performance than patients (p<0.05). However, when we corrected for multiple comparisons, these were no longer significant. All significant and non-significant relationships are presented in Table 2.
Effects of Cannabis Abstinence on Clinical Symptoms
PANSS scores remained constant across the abstinence period in both abstaining and non-abstaining patients. Among patient abstainers and non-abstainers, there was no change on SARS, BARS, or AIMS scores between baseline and day 28. However, for CDSS, there was a significant reduction in the total depression score over time in schizophrenia abstainers and non-abstainers [F(4, 68)=4.44, p<0.01]. The interaction [F(4, 68)=0.337, p=0.88] was not significant. In patients and control abstainers and non-abstainers, there was no significant change in the total MWC severity score over time [SCZ: F(4, 68)=1.61, p=0.18] [CTL: F(4, 72)=2.17, p=0.08].
Trajectory of Cognitive Symptoms with Abstinence
Hippocampus-mediated tasks
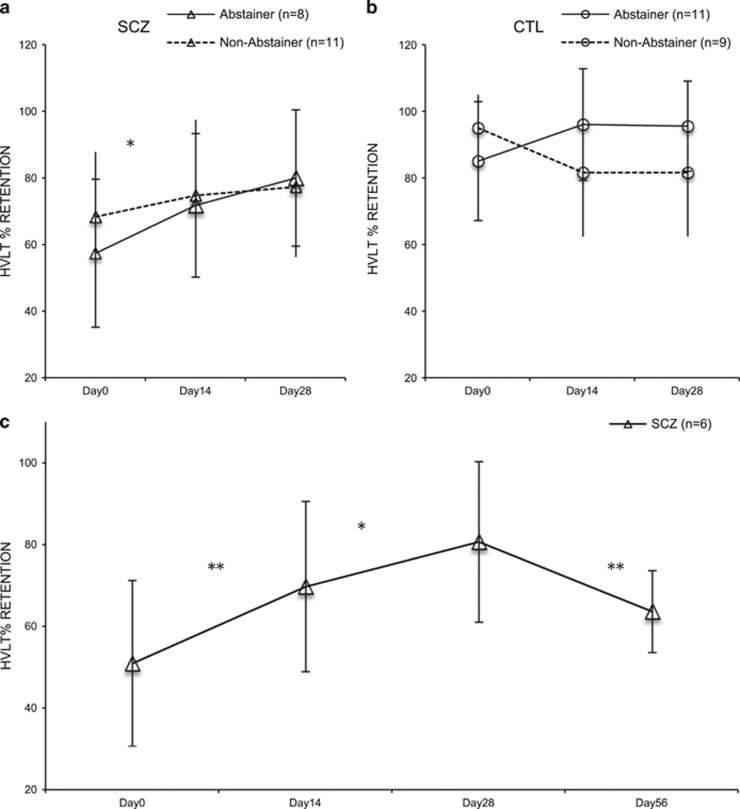
(i) Verbal Memory and Learning: using a 2 × 2 × 3 ANOVA, there was a main effect of time [F(2, 68)=5.03, p<0.01] on HVLT percent retention. The interaction between time and abstinence status was also significant [F(2, 34)=3.86, p<0.03]. The interactions between time × psychiatric diagnosis, and the three-way interaction term (time × abstinence status × psychiatric diagnosis) were not significant. Planned contrasts, controlling for multiple pairwise comparisons (with α=0.0167), revealed improvement in patient abstainers [F(2, 14) =4.73, p=0.02] over time. The magnitude of this improvement was 39.3%. (d=1.07), suggesting a clinically significant change in cognitive performance (Cohen, 1988); Figure 3a. Non-abstainers had no significant change in performance over time [F(2, 20)=2.19, p=0.14]. Similarly, change over time was non-significant in controls; [F(2, 34)=0.65, p=0.53] the magnitude of this improvement was 12.0% (d=0.67); Figure 3b. Observed power for key analyses were as follows: main effects of time, 80%, F(2, 68)=5.034, p<0.01; time × abstinence status, 68.1%, F(2, 68)=3.86, p=0.026; time × diagnosis × abstinence status, 25.4%, F(2, 68)=1.20, p=0.308.
Figure 3.
Trajectory of HVLT % Retention over Time. Schizophrenia abstainers demonstrated improved performance on the HVLT % retention over time, between days 0 and 28 (a). Controls showed no improvements over time in HVLT performance (b). Among patients, significant improvements in HVLT % retention performance occurred with abstinence, while cannabis relapse led to reversal of this abstinence-related cognitive change. In contrast, there was no significant change in HVLT % retention performance with abstinence, or cannabis relapse assessed at day 56 in control participants (c). Error bars reflect SD. *p<0.05; **p<0.01.
In exploratory analyses, since HVLT % retention improved in patients with schizophrenia with abstinence, we determined the effects of 28 days of cannabis reinstatement. Six (of 8) abstaining patients completed the follow-up, and cannabis reinstatement occurred immediately after the abstinence period ended (day 28). Thus, we examined HVLT performance across the 56-day period using four time-points (days 0, 14, 28, and 56). RM-ANOVAs revealed a significant change in HVLT % retention performance over time [F(3, 15)=5.026, p<0.02]. LSD post-hoc analyses revealed that improvement occurred with cannabis abstinence between days 0 and 28 [p<0.04], and between days 28 and 56, when cannabis was reintroduced, there was a decline in HVLT performance [p=0.03]. Among controls (N=4; of 11), the re-introduction of cannabis had little effect on HVLT performance, similar to cannabis abstinence (data not shown). RM-ANOVAs revealed no significant change in HVLT % retention performance over time; [F(3, 9)=1.820, p=0.21]. Other HVLT outcomes showed no significant change over time either in patients or controls.
Prefrontal tasks
Attention: using a 2 × 2 × 3 ANOVA demonstrated there was no significant change in CPT [% Hits F(1.5, 52.8)= 1.04, p=0.85; Hit Rate F(2, 70)= 1.55, p=0.22] or TMT-A [F(2, 70)= 0.45, p=0.64] outcomes over time.
Executive function: using a 2x2x3 ANOVA showed no change on the Digit Span Backwards [F(2, 70)= 0.72, p=0.49] or the TMT-B [F(1.5, 53.86)= 3.87, p=0.04].
Working memory: there was a non-significant effect of time on the Digit Span Forward [F(2, 70)= 2.61, p=0.08]. Performance on the SDR 5 [F(2, 68)= 1.59, p=0.21], 15 [F(2, 70)= 0.74, p=0.48] and 30 [F(2, 70)= 1.67, p=0.20] second delay also demonstrated no change over time.
Impulsivity: there was no significant change on measures that assess impulsivity such as the KDDT [F(2, 70)= 0.42, p=0.66] and BART [F(2, 50)= 0.36, p=0.70].
Cerebellar task
Motor function: RM-ANOVA yielded a significant main effect of time on the Pegboard task [F(1.7, 59.4)=5.61, p<0.01]. The interaction effect between time and diagnostic group [F(2, 70)=0.31, p=0.73] and time and abstinence status were non-significant [F(2, 70)=0.76, p=0.47].
Discussion
This is the first prospective investigation of the effects of extended cannabis abstinence on cognition in cannabis-dependent patients with schizophrenia and non-psychiatric controls. We implemented a novel cannabis abstinence paradigm to isolate the state-dependent effects of cannabis on cognitive function. At study end point, 42% of patients (8/19) and 55% of non-psychiatric controls (11/20) remained cannabis free for the full 28-day period. Moreover, study retention rate was high in patients (95%) and controls (25%).
Chronic cannabis use in non-psychiatric controls generates deficits that resemble the cognitive profile of patients with schizophrenia (Solowij et al, 2002). However, it has been proposed that cannabis-using patients with schizophrenia may belong to a subgroup of patients who are higher functioning, have better pre-morbid adjustment and have better cognition (eg, Schnell et al, 2009; Yucel et al, 2012). Therefore, the expected difference between cannabis-using controls and schizophrenia may be minimized when compared to this higher functioning patient subgroup. We posit that the true magnitude of impairment in cognitive performance may only become evident when cannabis is ceased.
However, over time, patient abstainers demonstrated improvements in verbal memory and learning. The magnitude of cognitive change (while not significant when correcting for multiple comparisons) may be of clinical importance, with scores improving by approximately 40% from pre- to post-abstinence (d=1.07). Despite the modest sample sizes, cognitive improvement with cannabis abstinence may have been preferential for verbal memory and learning, as performance on other cognitive tests were less pronounced (see Supplementary Table 3). These results suggest that chronic cannabis use may be associated with poorer cognitive function in select cognitive domains (ie, verbal memory) in patients with schizophrenia. Importantly, these deficits may improve with 28 days of abstinence. Conversely, deficits in other cognitive domains may be insensitive to change within this abstinence timeframe.
This finding was further substantiated by exploratory data from a subset of patient abstainers. Preliminary data were collected at a 4-week follow-up visit (day 56). Six out of the eight patients (two patients were lost to follow-up) who successfully abstained from cannabis until day 28 relapsed immediately after the bonus was paid. We observed that while cannabis abstinence led to significant improvements in verbal memory and learning, performance worsened when cannabis was reintroduced; suggesting a reversal of abstinence-related improvements in schizophrenia. Further study and replication of this finding in larger samples is needed.
While some studies have attributed greater cognitive capacities of cannabis-using patients to cannabis itself (Coulston et al, 2007a,b), others have proposed that cannabis users belong to a subgroup of higher functioning patients (Schnell et al, 2009; Yucel et al, 2012). Our data do not support the former hypothesis, however our findings work in concert with the latter. Thus, we speculate that cannabis exerts a deleterious effect on cognition (state-effect) that is superimposed upon a higher functioning subgroup of patients (trait-effect). However, given that we did not have non-using comparison groups, this could not be empirically confirmed. Findings are in line with our previous study that reported associations between increasing cumulative cannabis use and progressive cognitive impairment in current, but not former (>6 months abstinent) cannabis-dependent patients with schizophrenia (Rabin et al, 2013). Thus, while continued cannabis use results in deterioration of cognitive performance, when compared to non-users cannabis-users appear to have relatively higher cognitive function.
Although selective deficits may improve with 28 days of abstinence, other cognitive domains may do so at differential rates. Thus, continued abstinence beyond 28 days may be warranted for full cognitive recovery. It is possible that specific brain regions are more vulnerable and/or resilient to cannabis compared to others. Therefore, recovery of one cognitive domain does not necessarily predict recovery in others. In other words, while verbal learning and memory appears to improve with 28 days of abstinence, more enduring deficits may occur within other cognitive domains.
Interestingly, verbal learning and memory have been the most consistently impaired cognitive functions in studies of cannabis use (Ranganathan and D’Souza, 2006). Moreover, this domain is also the area in which patients with schizophrenia demonstrate the most significant deficits (Heinrichs and Zakzanis, 1998). A laboratory study by D’Souza et al (2005) showed that of all tests administered the HVLT was most sensitive to THC administration in both patients and controls (eg, verbal fluency, distractibility, and vigilance; D'Souza et al, 2005). Other studies are in agreement that heavy cannabis use selectively impairs verbal learning and memory tasks in patients (Nunez et al, 2016). Thus, the ability and/or time to cognitively recover may correlate with CB1R density in the brain region responsible for mediating that specific cognitive task. That is, improved cognitive function (with 28 days of abstinence) may first occur in tasks facilitated by areas with high CB1R concentrations, such as the hippocampus (Herkenham, 1991). Although chronic cannabis exposure may induce CB1R down-regulation and desensitization, conceivably abstinence may reverse these processes and increase CB1R availability Notably, the magnitude of rate of down-regulation and desensitization are region-dependent (Ceccarini et al, 2015; Hirvonen et al, 2012), thus adaptations do not occur uniformly across the brain. Accordingly, tasks mediated by brain regions with lower CB1R concentrations may require longer abstinence periods for recovery of performance. This proposed underlying mechanism supports findings from the current study in that patients with schizophrenia demonstrated improvements in the HVLT, a verbal memory and learning task predominantly mediated by the hippocampus (Squire, 1992).
It is important to note that the non-abstaining group collectively included individuals with varying abstaining/relapse trajectories. The heterogeneity of these abstaining/relapsing trajectories may help to explain the upward trend of improvement in HVLT scores in non-abstaining patients with schizophrenia. It should also be emphasized that improvement in performance is unlikely related to practice effects. First, alternate HVLT forms were administered on different days. Second, at the one-month follow-up, upon the fifth administration of the HVLT, there was a decrement in performance in patients with schizophrenia who re-introduced cannabis following abstinence.
In contrast to patients, cognitive change was not observed with abstinence in our non-psychiatric controls. It has been suggested that compared to controls, patients with schizophrenia possess an enhanced sensitivity to the cognitive-impairing effects of cannabis (D'Souza et al, 2005) and hence may also be more susceptible for recovery of cognitive function. A dysfunctional endocannabinoid system, including reduced CB1R availability (Ranganathan and D’Souza, 2006), has been implicated in the pathophysiology of schizophrenia and may help to explain this observation (Muller-Vahl and Emrich, 2008). Thus, if hippocampus-mediated tasks are most susceptible to cognitive impairment, and patients with schizophrenia have increased susceptibility to these effects, then it follows that this brain region may also be most vulnerable to the reversal of deficits in patients. However, given our study design we cannot fully exclude the possibility that cannabis exposure might not have impaired cognition in the control group.
Importantly, cannabis cessation in patients with schizophrenia did not lead to any adverse effects. According to the self-medication hypothesis (Khantzian, 1985), cannabis may alleviate symptoms associated with the disorder. Our results do not support this theory. Psychiatric symptoms and extrapyramidal symptoms did not worsen with abstinence. Other studies have found that cannabis use is not associated with beneficial effects in patients with schizophrenia (Henquet et al, 2010; Ringen et al, 2013). The only adverse outcomes experienced with abstinence were cannabis withdrawal symptoms, which dissipated within the first 14 days of cessation (Rabin et al, 2017).
Recovery of cannabis-induced cognitive impairment in controls may not be as rapid as in patients. Our finding that cognitive impairment does not fully recover following 28 days of cannabis cessation is consistent with some (Bolla et al, 2002; Medina et al, 2007), but not all, prior studies (Pope et al, 2001). One possible explanation for this finding is that cannabis may produce irreversible neurotoxic effects (Meier et al, 2012; Solowij and Battisti, 2008). Results are consistent with the speculation that cannabis use during adolescence, when the brain is undergoing critical neurodevelopment processes, may produce neurobiological dysfunction (Bossong and Niesink, 2010). This has been proposed as a mechanism for the development of schizophrenia in individuals with pre-existing susceptibility factors (Caspi et al, 2005). However, for individuals who use cannabis during adolescence and do not develop a psychotic illness, the resulting consequences may be enduring cognitive impairment irrespective of whether or not cannabis use is sustained. In addition, given that the mean age of onset in controls was 15 and cumulative years of use was reported to be almost 10 years, this supports the idea that permanent cognitive deficits may result. While cumulative cannabis use was comparable between groups, indexed as joint-years, baseline THC-COOH was twice as high in controls vs schizophrenia patients, perhaps suggesting heavier use, or the use of higher potency cannabis strains. This may make the control group more vulnerable to permanent, non-reversible deficits relative to our schizophrenia group (Tait et al, 2011). Moreover, given that schizophrenia patients have been shown to be more sensitive to the cognitive effects of cannabis (D'Souza et al, 2005), this may explain why improvement more readily occurred in the patients group vs the control group.
Our study results must be interpreted in the context of several limitations. First, we did not include non-cannabis using control groups (eg, non-cannabis-using non-psychiatric controls or non-cannabis using patients), or cannabis-using patients and controls receiving non-contingent (yoked) control interventions. The lack of these comparison groups makes it difficult to characterize the magnitude of baseline cognitive deficits, and prospective changes in cognitive outcomes in cannabis non-using and using subjects. Future studies should include these comparative controls. Second, ceiling effects on specific cognitive tests (eg, HVLT) at baseline may have prevented the detection of cognitive change in cannabis abstaining control participants. For example, HVLT-R may not provide be complex enough (eg, high cognitive task load) to elicit sufficient variability in scores and error types in healthy controls (Lacritz and Cullum, 1998). Thus, we were unable to detect a large enough magnitude of change in memory retention. It should be noted that mean HVLT % retention for our control group at baseline was 85.11±17.8, and by week 4 scores improved to 95.61±13.5. As mentioned, this improvement did not achieve statistical significance (p=0.27). HVLT % retention normative data for this age group is 91.15+13.07 (from the HVLT Professional manual) (Brandt and Benedict, 2001). In addition, repeated testing (four times in total by day 28) may have induced some practice effects. There is evidence to suggest that controls may experience greater practice effects form repeated testing compared to patients with schizophrenia (Szoke et al, 2008). Third, we set a biochemical cut-off level to distinguish abstainers from non-abstainers. This may have inadvertently posed a bias towards classifying heavier cannabis users who abstained as non-abstainers, as they may have not been able to eliminate cannabinoids to levels below 20 ng/ml within 28 days (Ellis et al, 1985; Goodwin et al, 2008). Thus, it follows that if cognitive function is most likely to show the greatest magnitude of change in heavier users then lengthier abstinence periods are warranted. However, we believe that there may be a trade-off as a longer duration of abstinence may be related to subject attrition. Perhaps with the implementation of extended duration contingency management and adequate incentives, increasing the length of abstinence might be feasible. Finally, this study exclusively studied males. Future studies should assess whether findings extend to female cannabis users. This is especially important because despite the lower rates of cannabis use among women, females are more susceptible to the development of CUDs, have more severe withdrawal symptoms, and are more likely to relapse compared to men (Cooper and Haney, 2014; Craft et al, 2013; Fergusson et al, 2006). These limitations should be addressed in future studies.
Conclusions
Our findings provide additional justification to support clinical efforts to encourage patients with schizophrenia to abstain from cannabis. While it may be thought of as a benign drug with low addiction potential, we provide evidence that cannabis might possess cognitive-impairing properties in schizophrenia. Intact cognitive function is especially critical in schizophrenia given that cognition is one of the most reliable predictors of functional outcomes in these patients (Green et al, 2000). Accordingly, remedying cannabis-related cognitive dysfunction may provide a critical element in the successful rehabilitation of patients with schizophrenia. Future studies should explore the cognitive effects of longer abstinence periods and functional neuroimaging techniques should be incorporated into these paradigms to monitor accompanying changes in brain activity. Understanding the degree to which the human brain may recover as a function of extended cannabis abstinence has important implications for the treatment of SUDs as well as the neurobiology of co-morbid psychiatric disorders.
Funding and disclosure
TPG reported that in the past 12 months he has had investigated initiated and industry sponsored grants from Pfizer, and served as a member of a Data Monitoring Committee for Novartis. SJK receives research funding from Jazz Pharmaceuticals and U.S. NIH NIDA for studies not related to this investigation. GR has received research support from Novartis and consultant or speaker fees from Neurocrine Biosciences, Novartis, and Synchroneuron. The remaining authors declare no conflict of interest.
Acknowledgments
This work was supported in part by an operating grant from the Canadian Institutes of Health Research (CIHR; MOP#115145 to Dr George), a CIHR Doctoral Fellowship (to Ms. Rabin), a Brain and Behavior Research Foundation/NARSAD Young Investigator Award (to Dr Barr), and the Chair in Addiction Psychiatry at the University of Toronto (to Dr George). We would like to thank Emily Simpkin, R.N. for her assistance with the study. We also want to extend our gratitude to all of our study participants.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Addington D, Addington J, Maticka-Tyndale E (1993). Assessing depression in schizophrenia: the Calgary Depression Scale. Br J Psychiatry Suppl 22: 39–44. [PubMed] [Google Scholar]
- APADiagnostic and Statistical Manual Of Mental Disorders. 4th edn. American Psychiatric Association: Washington, DC, USA, 1994.
- APADiagnostic and Statistical Manual Of Mental Disorders (Revised 4th ed.). American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- Arndt S, Tyrrell G, Flaum M, Andreasen NC (1992). Comorbidity of substance abuse and schizophrenia: the role of pre-morbid adjustment. Psychol Med 22: 379–388. [DOI] [PubMed] [Google Scholar]
- Barnes TR (1989). A rating scale for drug-induced akathisia. Br J Psychiatry 154: 672–676. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW (1994). Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50: 7–15. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL (2002). Dose-related neurocognitive effects of marijuana use. Neurology 59: 1337–1343. [DOI] [PubMed] [Google Scholar]
- Bossong MG, Niesink RJ (2010). Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Prog Neurobiol 92: 370–385. [DOI] [PubMed] [Google Scholar]
- Brandt J, Benedict R (2001) Hopkins Verbal Learning Test-Revised. In: Professional Manual. PAR, Inc.: Lutz, FL.
- Broyd SJ, van Hell HH, Beale C, Yucel M, Solowij N (2016). Acute and chronic effects of cannabinoids on human cognition-A systematic review. Biol Psychiatry 79: 557–567. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Vandrey RG, Hughes JR (2003). The time course and significance of cannabis withdrawal. J Abnorm Psychol 112: 393–402. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H et al (2005). Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry 57: 1117–1127. [DOI] [PubMed] [Google Scholar]
- Ceccarini J, Kuepper R, Kemels D, van Os J, Henquet C, Van Laere K (2015). [18F]MK-9470 PET measurement of cannabinoid CB1 receptor availability in chronic cannabis users. Addict Biol 20: 357–367. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Erlbaum Associates, Inc.: Hillsdale, New Jersey, 1988..
- Conners CK (2000) Conners’ Continuous Performance Test II: Computer Program for Windows Technical Guide and Software Manual. Multi-Health Systems: North Tonawanda, NY, USA. [Google Scholar]
- Cooper ZD, Haney M (2014). Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug Alcohol Depend 136: 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulston CM, Perdices M, Tennant CC (2007. a). The neuropsychological correlates of cannabis use in schizophrenia: lifetime abuse/dependence, frequency of use, and recency of use. Schizophr Res 96: 169–184. [DOI] [PubMed] [Google Scholar]
- Coulston CM, Perdices M, Tennant CC (2007. b). The neuropsychology of cannabis and other substance use in schizophrenia: review of the literature and critical evaluation of methodological issues. Aust N Z J Psychiatry 41: 869–884. [DOI] [PubMed] [Google Scholar]
- Craft RM, Kandasamy R, Davis SM (2013). Sex differences in anti-allodynic, anti-hyperalgesic and anti-edema effects of Delta(9)-tetrahydrocannabinol in the rat. Pain 154: 1709–1717. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Abi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G et al (2005). Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry 57: 594–608. [DOI] [PubMed] [Google Scholar]
- Dixon L, Haas G, Weiden PJ, Sweeney J, Frances AJ (1991). Drug abuse in schizophrenic patients: clinical correlates and reasons for use. Am J Psychiatry 148: 224–230. [DOI] [PubMed] [Google Scholar]
- Ellis GM Jr, Mann MA, Judson BA, Schramm NT, Tashchian A (1985). Excretion patterns of cannabinoid metabolites after last use in a group of chronic users. Clin Pharmacol Ther 38: 572–578. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM, Horwood LJ (2006). Cannabis use and other illicit drug use: testing the cannabis gateway hypothesis. Addiction 101: 556–569. [DOI] [PubMed] [Google Scholar]
- Goodwin RS, Darwin WD, Chiang CN, Shih M, Li SH, Huestis MA (2008). Urinary elimination of 11-nor-9-carboxy-delta9-tetrahydrocannnabinol in cannabis users during continuously monitored abstinence. J Anal Toxicol 32: 562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J (2000). Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the "right stuff"? Schizophr Bull 26: 119–136. [DOI] [PubMed] [Google Scholar]
- Guy W, Cleary PA (1976). Pretreatment status and its relationship to the length of drying-out period. Psychopharmacol Bull 12: 20–22. [PubMed] [Google Scholar]
- Hamilton M (1967). Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 6: 278–296. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H et al (2015). Prevalence of Marijuana use disorders in the United States between 2001-2002 and 2012-2013. JAMA Psychiatry 72: 1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991). The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 86: 1119–1127. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL (1993) Wisconsin Card Sorting Test Manual. Psychological Assessment Resources: Odessa, FL, USA. [Google Scholar]
- Heinrichs RW, Zakzanis KK (1998). Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 12: 426–445. [DOI] [PubMed] [Google Scholar]
- Henquet C, van Os J, Kuepper R, Delespaul P, Smits M, Campo JA et al (2010). Psychosis reactivity to cannabis use in daily life: an experience sampling study. Br J Psychiatry 196: 447–453. [DOI] [PubMed] [Google Scholar]
- Herkenham M (1991). Characterization and localization of cannabinoid receptors in brain: an in vitro technique using slide-mounted tissue sections. NIDA Res Monogr 112: 129–145. [PubMed] [Google Scholar]
- Hershey T, Craft S, Glauser TA, Hale S (1998). Short-term and long-term memory in early temporal lobe dysfunction. Neuropsychology 12: 52–64. [DOI] [PubMed] [Google Scholar]
- Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C et al (2012). Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry 17: 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockers-Scherubl MC, Wolf T, Radzei N, Schlattmann P, Rentzsch J, Gomez-Carrillo de Castro A et al (2007). Cannabis induces different cognitive changes in schizophrenic patients and in healthy controls. Prog Neuropsychopharmacol Biol Psychiatry 31: 1054–1063. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13: 261–276. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ (1985). The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatry 142: 1259–1264. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK (1999). Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen 128: 78–87. [DOI] [PubMed] [Google Scholar]
- Koskinen J, Lohonen J, Koponen H, Isohanni M, Miettunen J (2010). Rate of cannabis use disorders in clinical samples of patients with schizophrenia: a meta-analysis. Schizophr Bull 36: 1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacritz LH, Cullum CM (1998). The Hopkins Verbal Learning Test and CVLT: a preliminary comparison. Arch Clin Neuropsychol 13: 623–628. [PubMed] [Google Scholar]
- Lafayette Instrument Company (1989) Instruction Manual for the 32025 Grooved Pegboard Test. Lafayette Instrument Company: Lafayette, Indiana. [Google Scholar]
- Large M, Sharma S, Compton MT, Slade T, Nielssen O (2011). Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Arch Gen Psychiatry 68: 555–561. [DOI] [PubMed] [Google Scholar]
- Leeson VC, Harrison I, Ron MA, Barnes TR, Joyce EM (2011). The effect of cannabis use and cognitive reserve on age at onset and psychosis outcomes in first-episode schizophrenia. Schizophr Bull 38: 873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL et al (2002). Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART). J Exp Psychol Appl 8: 75–84. [DOI] [PubMed] [Google Scholar]
- Lev-Ran S, Segev A, Braw Y, Levkovitz Y (2012). Neurocognitive functions of heavy cannabis using schizophrenia patients. Eur Psychiatry 27: 365–368. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DWNeuropsychological Assessment. 4th edn. Oxford University Press: New York, 2004. [Google Scholar]
- Mackie K (2005). Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol 168: 299–325. [DOI] [PubMed] [Google Scholar]
- Manrique-Garcia E, Zammit S, Dalman C, Hemmingsson T, Andreasson S, Allebeck P (2014). Prognosis of schizophrenia in persons with and without a history of cannabis use. Psychol Med 44: 2513–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF (2007). Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. J Int Neuropsychol Soc 13: 807–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS et al (2012). Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci USA 109: E2657–E2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M et al (2007). Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet 370: 319–328. [DOI] [PubMed] [Google Scholar]
- Muller-Vahl KR, Emrich HM (2008). Cannabis and schizophrenia: towards a cannabinoid hypothesis of schizophrenia. Exp Rev Neurother 8: 1037–1048. [DOI] [PubMed] [Google Scholar]
- Nahas G (2001). The Pharmacokinetics of the THC in fat and brain: resulting functional responses to marihuana smoking: G. Nahas, Volume 16 Issue 3, April 2001, pages 247-255. Hum Psychopharmacol 16: 549. [DOI] [PubMed] [Google Scholar]
- Nunez C, Ochoa S, Huerta-Ramos E, Banos I, Barajas A, Dolz M et al (2016). Cannabis use and cognitive function in first episode psychosis: differential effect of heavy use. Psychopharmacology 233: 809–821. [DOI] [PubMed] [Google Scholar]
- Patel R, Wilson R, Jackson R, Ball M, Shetty H, Broadbent M et al (2016). Association of cannabis use with hospital admission and antipsychotic treatment failure in first episode psychosis: an observational study. BMJ Open 6: e009888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D (2001). Neuropsychological performance in long-term cannabisusers. Arch Gen Psychiatry 58: 909–915. [DOI] [PubMed] [Google Scholar]
- Potvin S, Briand C, Prouteau A, Bouchard RH, Lipp O, Lalonde P et al (2005). CANTAB explicit memory is less impaired in addicted schizophrenia patients. Brain Cogn 59: 38–42. [DOI] [PubMed] [Google Scholar]
- Rabin R, Zakzanis K, George T (2011). The effects of cannabis use on neurocognition in schizophrenia: a meta-analysis. Schizophr Res 128: 111–116. [DOI] [PubMed] [Google Scholar]
- Rabin RA, Kozak K, Zakzanis KK, Remington G, George TP (2017). A method to achieve 28 days of cannabis abstinence in cannabis dependent patients with schizophrenia and non-psychiatric controls. Schizophr Res (e-pub ahead of print). [DOI] [PubMed]
- Rabin RA, Zakzanis KK, Daskalakis ZJ, George TP (2013). Effects of cannabis use status on cognitive function, in males with schizophrenia. Psychiatry Res 206: 158–165. [DOI] [PubMed] [Google Scholar]
- Ranganathan M, D’Souza DC (2006). The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology 188: 425–444. [DOI] [PubMed] [Google Scholar]
- Rees LM, Tombaugh TN, Gansler DA, Moczynski NP (1998). Five validation experiments of the test of memory malingering(TOMM). Psychol Assess 10: 10–20. [Google Scholar]
- Ringen PA, Melle I, Berg AO, Agartz I, Spigset O, Simonsen C et al (2013). Cannabis use and premorbid functioning as predictors of poorer neurocognition in schizophrenia spectrum disorder. Schizophr Res 143: 84–89. [DOI] [PubMed] [Google Scholar]
- Ringen PA, Vaskinn A, Sundet K, Engh JA, Jonsdottir H, Simonsen C et al (2010). Opposite relationships between cannabis use and neurocognitive functioning in bipolar disorder and schizophrenia. Psychol Med 40: 1337–1347. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sanchez JM, Ayesa-Arriola R, Mata I, Moreno-Calle T, Perez-Iglesias R, Gonzalez-Blanch C et al (2010). Cannabis use and cognitive functioning in first-episode schizophrenia patients. Schizophr Res 124: 142–151. [DOI] [PubMed] [Google Scholar]
- Sacco KA, Termine A, Dudas MM, Seyal AA, Allen TM, Vessicchio JC et al (2006). Neuropsychological deficits in nonsmokers with schizophrenia: effects of a nicotinic antagonist. Schizophr Res 85: 213–221. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M (1993). Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—II. Addiction 88: 791–804. [DOI] [PubMed] [Google Scholar]
- Schnell T, Koethe D, Daumann J, Gouzoulis-Mayfrank E (2009). The role of cannabis in cognitive functioning of patients with schizophrenia. Psychopharmacology 205: 45–52. [DOI] [PubMed] [Google Scholar]
- Schwilke EW, Gullberg RG, Darwin WD, Chiang CN, Cadet JL, Gorelick DA et al (2011). Differentiating new cannabis use from residual urinary cannabinoid excretion in chronic, daily cannabis users. Addiction 106: 499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharland MJ, Gfeller JD (2007). A survey of neuropsychologists' beliefs and practices with respect to the assessment of effort. Arch Clin Neuropsychol 22: 213–223. [DOI] [PubMed] [Google Scholar]
- Simpson GM, Angus JW (1970). A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 212: 11–19. [DOI] [PubMed] [Google Scholar]
- Smith-Kielland A, Skuterud B, Morland J (1999). Urinary excretion of 11-nor-9-carboxy-delta9-tetrahydrocannabinol and cannabinoids in frequent and infrequent drug users. J Anal Toxicol 23: 323–332. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Leo GI, Cancilla A (1988). Reliability of a timeline method: assessing normal drinkers' reports of recent drinking and a comparative evaluation across several populations. Br J Addict 83: 393–402. [DOI] [PubMed] [Google Scholar]
- Solowij N, Battisti R (2008). The chronic effects of cannabis on memory in humans: a review. Curr Drug Abuse Rev 1: 81–98. [DOI] [PubMed] [Google Scholar]
- Solowij N, Stephens R, Roffman RA, Babor T (2002). Does marijuana use cause long-term cognitive deficits? J Am Med Assoc 287: 2653–2654 author reply 2654. [PubMed] [Google Scholar]
- Squire LR (1992). Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev 99: 195–231. [DOI] [PubMed] [Google Scholar]
- Szoke A, Trandafir A, Dupont ME, Meary A, Schurhoff F, Leboyer M (2008). Longitudinal studies of cognition in schizophrenia: meta-analysis. Br J Psychiatry 192: 248–257. [DOI] [PubMed] [Google Scholar]
- Tait RJ, Mackinnon A, Christensen H (2011). Cannabis use and cognitive function: 8-year trajectory in a young adult cohort. Addiction 106: 2195–2203. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN (1997). The Test of Memory Malingering (TOMM): normative data from cognitively intact and cognitively impaired individuals. Psychol Assess 9: 260–268. [Google Scholar]
- Wechsler D (2001) Wechsler Test of Adult Reading. Psychological Corporation: San Antonio. [Google Scholar]
- Wechsler DA (1997) Wechsler Adult Intelligence Scale. 3rd (edn). The Psychological Corporation.: San Antonio, TX, USA. [Google Scholar]
- Yucel M, Bora E, Lubman DI, Solowij N, Brewer WJ, Cotton SM et al (2012). The impact of cannabis use on cognitive functioning in patients with schizophrenia: a meta-analysis of existing findings and new data in a first-episode sample. Schizophr Bull 38: 316–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit S, Moore TH, Lingford-Hughes A, Barnes TR, Jones PB, Burke M et al (2008). Effects of cannabis use on outcomes of psychotic disorders: systematic review. Br J Psychiatry 193: 357–363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.