Abstract
Background and purpose
Charcot-Marie-Tooth disease (CMT) type 1A is characterized by uniformly reduced nerve conduction velocity (NCV) that is fully penetrant since the first years of life, remains fairly stable through the life and does not correlate with disability whereas compound muscular action potential (CMAP) amplitude does. The aim of the present study was to analyze the large amount of electrophysiological data collected in the ascorbic acid trial in Italy and the UK (CMT-TRIAAL/CMT-TRAUK) and to use these data to gain insights into the pathophysiology of NCV in CMT1A.
Methods
Baseline electrophysiological data from 271 patients were analysed. Electrophysiological recordings were taken from the motor ulnar, median and peroneal nerves and the sensory ulnar nerve. Distal motor latency (DML), motor (MNCV) and sensory (SNCV) nerve conduction velocity, and amplitudes of CMAPs and sensory action potentials were assessed. Electrophysiological findings were correlated with age of patients at examination and the Charcot–Marie–Tooth Examination Score (CMTES).
Results
NCV was markedly and uniformly reduced. CMAP amplitudes were overall reduced but more severely in lower limbs. DML decreased and MNCV and SNCV increased with age of the patients, whereas CMAP amplitudes worsened with age and also correlated with CMTES.
Conclusions
This is the largest sample of electrophysiological data obtained so far from CMT1A patients. Axonal degeneration as assessed by means of CMAP amplitude reflected clinical impairment and was consistent with a slowly progressive length-dependent neuropathy. All patients typically had markedly slowed NCV that did, however, slightly increase with age of the patients. The improvement of NCV might depend on myelin thickness remodelling that occurs during the adult life of CMT1A patients.
Keywords: Charcot Marie Tooth disease, CMT1A, CMT-TRIAAL/CMT-TRAUK, hereditary neuropathies, nerve conduction velocities
Introduction
Charcot–Marie–Tooth (CMT) disease is the most frequent neurological hereditary disorder and is characterized by clinical and genetic heterogeneity. Motor nerve conduction velocity (MNCV) enables separation of dysmyelinating (CMT1) from axonal (CMT2) CMT on the basis of a 38 m/s cut-off value in upper limb nerves [1]. CMT1A is the most common form of CMT and is characterized by low MNCV, the electrophysiological surrogate marker for myelin integrity. In CMT1A, MNCV is uniformly reduced in all nerves and values <38 m/s are highly diagnostic [2, 3]. This electrophysiological hallmark, fully penetrant since the first years of life [4–6], remains fairly stable through life [7–9] and does not correlate with disability, whereas compound motor action potential (CMAP) amplitude that is a surrogate marker of axonal degeneration does [3].
Recently, the ascorbic acid trial in Italy and the UK allowed us to collect and analyse electrophysiological data from a large cohort of CMT1A patients [10]. The aim of the present study was to further analyse the large amount of data collected in the trial and to use these data to gain insights into the pathophysiology of nerve conduction velocity (NCV) in CMT1A.
Patients and methods
Data were obtained from the baseline electrophysiological evaluation of 271 patients recruited in the CMT-TRIAAL (CMT Trial with Ascorbic Acid Long Term, EudraCT 2006-000032-27)/CMT-TRAUK (CMT Trial with Ascorbic Acid UK, ISRCTN61074476) [10]: 108 males and 163 females, mean age 41.8 ± 13.2 years, range 18–70 years.
Electrophysiological recordings were taken from the motor ulnar, median and peroneal nerves (non-dominant side) and the sensory ulnar nerve (non-dominant side and antidromic technique). Distal motor latency (DML), MNCV and sensory nerve conduction velocity (SNCV), and amplitudes (baseline to negative peak) of CMAPs and sensory action potentials (SAPs) were assessed.
Compound motor action potential was recorded, by using a pair of surface electrodes with belly-tendon arrangement, from abductor digiti minimi muscle for the ulnar nerve, abductor pollicis brevis muscle for the median nerve, and extensor digitorum brevis muscle for the peroneal nerve. Standardized distal distances for DML were used: ulnar and median nerves 7 cm and peroneal nerve 9 cm. The following nerve segments were used for calculating MNCV: below elbow to wrist for the ulnar nerve, elbow to wrist for the median nerve, and fibular head to ankle for the peroneal nerve.
For SAP recording ring electrodes were placed around the proximal (cathode) and distal (anode) interphalangeal joint of the fifth finger. SNCV was measured along the ulnar nerve from the wrist to fifth finger. Foot and hand skin temperature was checked and kept at 32–34°C.
To ensure supra-threshold stimulation, when no distal CMAP was recordable or when the amplitude of proximal CMAP was <50% compared to distal CMAP amplitude, the intensity of electrical stimulus was increased to 100 mA and if necessary the duration of electrical stimulus was increased to 0.5 ms.
Electrophysiological procedures were established by a neurophysiology committee and, to ensure the quality of nerve conduction studies performed in multiple institutions, all neurophysiological recordings were revised by the centre of Naples (by LS, FM and CP).
Standard protocol approvals, registrations and patient consents
The protocol was approved by the institutional review board at every site, and patients gave written informed consent before any study-related procedures. This study is registered, numbers ISRCTN61074476 (CMT-TRAUK) and EudraCT 2006-000032-27 (CMTTRIAAL). CMT-TRIAAL and CMT-TRAUK shared a common protocol but have different names and registration numbers because funding was separate in Italy and the UK.
Statistical analysis
Statistical analysis was performed using STATA 12.1 for Windows (StataCorp LP, College Station, TX, USA).
The Pearson or the Spearman coefficient with Bonferroni-adjusted significance level was used, for parametric or non-parametric data respectively, to investigate the correlations between electrophysiological (i.e. DML, MNCV, SNCV, CMAP and SAP) and clinical [age and Charcot–Marie–Tooth Examination Score (CMTES)] variables. A multiple regression model was applied to assess the independent associations between electrophysiological variables and CMTES; mean MNCV and CMAP amplitude summations of the three explored nerves (ulnar, median and peroneal) were the independent variables and CMTES was the dependent variable.
Moreover, in order to evaluate MNCV changes over 2 years the longitudinal data of CMT-TRIAAL/CMT-TRAUK were examined. The anova model was applied for repeated measures with time (five levels: baseline, 6, 12, 18 and 24 months) as within-subjects factor and group of treatment (two levels: placebo and ascorbic acid) as between-subjects factor. A P value of <0.05 was considered significant.
Results
Electrophysiological data from 271 CMT1A patients are reported in Table 1. DML, MNCV and SNCV were abnormal in all patients. NCV slowing ranged from 4 to 38 m/s. The mean value of both MNCV and SNCV was around 20 m/s.
Table 1.
Correlation between electrophysiological and clinical data
| Variables | Mean ± SD (range) | Normal values | Correlation with age r (P value) | Correlation with CMTES ρ (P value) |
|---|---|---|---|---|
| 1. CMTES, Charcot–Marie–Tooth Examination Score; dCMAP, distal compound motor action potential; DML, distal motor latency; MNCV/SNCV, motor/sensory nerve conduction velocity; NS, not significant; SAP, sensory action potential; significant values are reported in bold. | ||||
| DML (ms) | ||||
| Ulnar nerve (n = 261) | 7.6 ± 1.7 (4–15.8) | ≤3.3 ms (×7 cm) | −0.2374 (<0.001) | 0.0840 (NS) |
| Median nerve (n = 255) | 10.0 ± 2.1 (5.6–19.9) | ≤4.1 ms (×7 cm) | −0.0571 (NS) | 0.2203 (<0.01) |
| Peroneal nerve (n = 70) | 10.9 ± 3.1 (6.3–20.7) | ≤5 ms (×9 cm) | −0.3657 (0.01) | 0.0816 (NS) |
| MNCV (m/s) | ||||
| Ulnar nerve (n = 255) | 19.8 ± 4.8 (4–33.6) | ≥50 m/s | 0.2779 (<0.0001) | −0.1967 (0.01) |
| Median nerve (n = 249) | 21.0 ± 5.0 (5.2–38) | ≥50 m/s | 0.2531 (<0.001) | −0.1560 (NS) |
| Peroneal nerve (n = 70) | 19.8 ± 4.9 (12–32.3) | ≥41 m/s | 0.1852 (NS) | −0.1452 (NS) |
| Mean MNCV | 20.3 ± 4.5 (5.5–33.4) | 0.2975 (<0.0001) | −0.1552 (NS) | |
| dCMAP (mV) | ||||
| Ulnar nerve (n = 268) | 3.3 ± 2.0 (0–9.5) | ≥5 mV | −0.1655 (0.03) | −0.4250 (<0.0001) |
| Median nerve (n = 268) | 3.2 ± 2.2 (0–11.6) | ≥5 mV | −0.3149 (<0.0001) | −0.3590 (<0.0001) |
| Peroneal nerve (n = 264) | 0.2 ± 0.6 (0–5.2) | ≥3 mV | −0.1569 (NS) | −0.4297 (<0.0001) |
| dCMAP summation | 6.7 ± 4.0 (0–19.2) | −0.2701 (<0.0001) | −0.4339 (<0.0001) | |
| SAP (μV) | ||||
| Ulnar nerve (n = 267) | 0.9 ± 2.3 (0–16) | ≥6 μV | −0.0316 (NS) | −0.1733 (0.01) |
| SNCV (m/s) | ||||
| Ulnar nerve (n = 45) | 20.2 ± 4.9 (10.9–37.1) | ≥50 m/s | 0.3896 (0.02) | −0.2747 (NS) |
Motor nerve conduction velocity slowing was concordant amongst the three explored nerves (ulnar/median, r = 0.7519, P < 0.0001; ulnar/peroneal, r = 0.6412, P < 0.0001; median/peroneal, r = 0.6405, P < 0.0001). Moreover, MNCV slowing was concordant between distal and proximal nerve segments (DML/MNCV in ulnar nerve, r = −0.5045, P < 0.0001; DML/MNCV in median nerve, r = −0.3667, P < 0.0001; DML/MNCV in peroneal nerve, r = −0.2950, P = 0.01).
Temporal dispersion was not reported. A reduction of proximal CMAP amplitude >50% compared with distal CMAP amplitude (consistent with partial conduction block) was occasionally observed (26 out of 574 evaluable nerves, 4.5%).
Distal CMAP amplitudes were overall reduced but more severely in lower limbs. No distal CMAP was recordable in 7/268 (2.6%) patients in the ulnar nerve, in 13/268 (5.9%) patients in the median nerve and in 194/264 (73.4%) patients in the peroneal nerve.
No SAP was recordable in 215/267 (80.5%) patients in the ulnar nerve.
Correlations between electrophysiological and clinical data
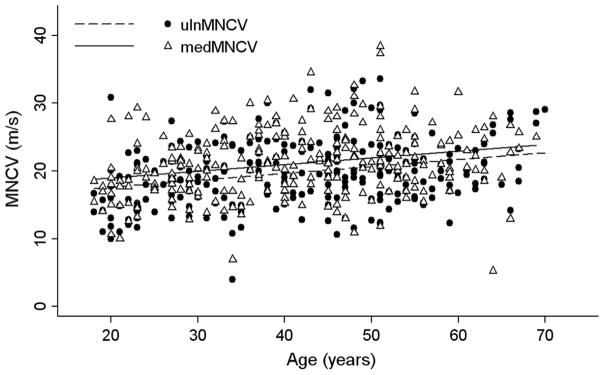
Correlations and their significance are reported in Table 1. Briefly, DML decreased with age in ulnar and peroneal nerves whilst in median nerve DML did not change with age. MNCV increased with age in ulnar and median nerves (Fig. 1) and SNCV increased with age in ulnar nerve. MNCV did not change over 2 years of follow-up (F = 2.098; P = 0.079). Treatment did not influence MNCV over 2 years of follow-up (F = 0.791; P = 0.531). Distal CMAP amplitude decreased with age in ulnar and median nerves whilst in peroneal nerve CMAP amplitude showed only a tendency (P = 0.06) to decrease with age.
FIGURE 1.
Scatterplot with correlation analysis between age and motor nerve conduction velocity (MNCV) of the ulnar and median nerves
Clinical disability, as assessed by CMTES, worsened with age (ρ = 0.2760, P < 0.0001). Moreover, CMTES correlated inversely with CMAP and SAP amplitudes and only partially with MNCV. However, multiple regression analysis showed that only CMAP amplitude correlated with CMTES (coefficient = −0.37; 95% confidence interval = −0.48 to 0.27; P < 0.001).
Overall, NCV increased and DML decreased with age, whereas CMAP amplitudes, a surrogate marker of axonal degeneration, showed a slight decline with age and correlated with clinical disability as well.
Discussion
In this study, the largest sample of electrophysiological data obtained so far from CMT1A patients was analysed.
All patients had marked and uniform NCV slowing. Moreover, axonal degeneration as assessed by means of CMAP amplitude reflected clinical impairment and was consistent with a slowly progressive length-dependent neuropathy. Distal CMAP amplitude deteriorated with age in the ulnar and the median nerves but not in the peroneal nerve. The latter finding has probably been influenced by the high prevalence of unrecordable CMAPs in the peroneal nerve that may have resulted in a floor effect.
Somewhat unexpectedly, it was found that NCV increased with the age of CMT1A patients. Consistently, DML decreased with age, even though only in the ulnar and peroneal nerves and not in the median nerve. The possible coexistence of a carpal tunnel syndrome, which is the most common entrapment neuropathy in the general population, may have influenced the lack of correlation for the median nerve.
It is of interest that, although these findings have already been noted in previous reports [2, 11], the current notion is that NCV does not change significantly through the life of CMT1A patients [7–9]. Birouk and colleagues found in their large sample of CMT1A patients that NCV increased with age and they interpreted the more evident lower NCV in younger patients as an expression of early diagnosis in younger CMT1A patients due to their more severe symptoms [2]. However, the deterioration of clinical disability with age makes it unlikely in our population that a more severe clinical impairment may have influenced the results of slower NCV in young patients. Therefore, it is believed that our NCV findings are rather an expression of patients’ age per se.
It is well known that NCV slowing in CMT1A reflects myelin abnormalities. The NCV that is typically uniformly reduced and fully penetrant from the first years of life [4–6] supports the view that CMT1A may be mainly a dysmyelinating neuropathy rather than a demyelinating neuropathy. The latter is expected to cause temporal dispersion and conduction block, in addition to non-uniform and variable NCV slowing.
In keeping with such electrophysiological features, pathological evidence supports that a developmental abnormality in internode formation resulting in uniformly shortened internodes may have a major role in NCV slowing in CMT1A [12, 13]. Abnormalities of the nodal–paranodal regions might play an additional role in NCV slowing but this remains to be determined [12, 13].
In addition, altered myelin thickness provides a further explanation for NCV slowing in dysmyelinating neuropathies [14–18]. Experimental models showed that reduced expression of neuregulin 1 (Nrg1), a master regulator of myelin thickness, causes a reduction of myelin thickness resulting in reduced NCV [16, 17]. On the other hand, Fledrich and colleagues have observed that the overexpression of Nrg1 in CMT1A rats does not restore myelin sheath thickness and accordingly does not modify the impaired NCV [18].
Interestingly, an increased myelin sheath thickness (expressed as reduced g ratio) has been demonstrated in sural nerve biopsies from young CMT1A patients [19–21]. At the same time, neuropathological data demonstrated that this myelin thickness tends to decrease (g ratio increases) with age of patients [21, 22].
All these data taken together might provide a possible explanation for the correlation between NCV and the age of patients. The reduction of over-thick myelin that results in an improvement of the g ratio (toward its optimal value for conduction velocity) [23] could lead to an increase of NCV.
However, NCV remains always markedly reduced in CMT1A patients, and this fits well with the shortened internodal lengths that persist stably through adult life [12, 13].
Over-thick myelin is also a possible trigger for demyelination that might further influence NCV slowing. However, the increase of NCV and the lack of temporal dispersion suggest that de-remyelinating phenomena may be less relevant than other myelin abnormalities in the pathophysiology of NCV slowing in CMT1A. This also puts in question repetitive de-remyelination as the cause of the onion bulbs that are a prominent pathological feature of CMT1A. Indeed, although onion bulbs occur in diseases that exhibit repeated de-remyelination (e.g. chronic inflammatory demyelinating neuropathy), our NCV findings are rather in keeping with the assumption that onion bulbs in CMT1A are an expression of an altered Schwann cell differentiation that results in an onion-bulb-like arrangement of Schwann cells around the axon [24].
In conclusion, the strength of this study is the large sample of patients, although much of the data presented especially with regard to changes with aging is cross-sectional. A prospective study would certainly be more appropriate, but in practice difficult to do, for evaluating the changes of NCV over the life of patients. Longitudinal analysis of data from CMT-TRIAAL/CMT-TRAUK demonstrates that 2 years of follow-up are too short a time to detect changes of NCV in both the treated and placebo groups. However, in the ascorbic acid trial in a paediatric CMT1A cohort, although there was not a statistical significance, a marked improvement of MNCV in the median nerve was observed in some patients treated with ascorbic acid [25]. This finding might indicate partial restoration of peripheral nerve myelin (e.g. myelin thickness) suggesting that NCV could represent, at least in children, a useful outcome in the clinical trial in CMT1A.
Therefore, it is believed that our observation offers additional insights into the pathophysiology of NCV slowing in CMT1A. The observed improvement of NCV, which anyway remains markedly reduced, highlights the role of myelin thickness remodelling in NCV changes. On the other hand, the reduced NCV, that represents the electrophysiological hallmark of CMT1A, may primarily reflect the abnormally developed shorter internodes.
Acknowledgments
F.M. is grateful to Professor Dario Bruzzese (Department of Public Health, University Federico II of Naples, Naples 80131, Italy) for assistance in statistical analysis and M.M.R. and M.L. are grateful to the Medical Research Council.
The study was supported by Telethon (GUP04002 and GUP05007)-UILDM and AIFA (Italian Medicines Agency) for the CMT-TRIAAL and Muscular Dystrophy UK (grant number RA3/736/1) for the CMT-TRAUK. Part of this work in the UK was undertaken at University College London Hospitals/University College London, which received a proportion of funding from the Department of Health's National Institute for Health Research Biomedical Research Centres funding scheme.
Additional members of the CMT-TRIAAL and CMT-TRAUK Group: Maria Nolano and Rosa Iodice (Department of Neurosciences, Reproductive Sciences and Odontostomatology, University Federico II of Naples, Naples, Italy); Marina Grandis (Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal Infantile Sciences, University of Genoa, Genoa, Italy); Ilaria Cabrini and Laura Bertolasi (Department of Neuroscience, Biomedicine and Movement Sciences, University of Verona, Verona, Italy); Anna Mazzeo and Giovanni Majorana (Department of Clinical and Experimental Medicine, School of Neurosciences, University of Messina, Messina, Italy); Francesca Vitetta (Department of Neurosciences, University of Parma, Parma, Italy); Vidmer Scaioli, Claudia Ciano and Daniela Calabrese (Carlo Besta Neurological Institute, IRCCS Foundation, Milan, Italy); Aldo Quattrone (Neurology Clinic, Neuroimaging Research Unit, National Research Council, Magna Graecia University, Catanzaro, Italy); Julian Blake (MRC Centre for Neuromuscular Diseases, Institute of Neurology, University College London, London, UK, and Department of Clinical Neurophysiology, Norfolk and Norwich University Hospital, Norwich, UK).
Footnotes
Disclosure of conflicts of interest
Dr Solari reports grants from the Foundation of the Italian National Multiple Sclerosis Society, was a board member for Biogen Idec, Novartis, and has received speaker honoraria from Biogen Idec, Novartis, Excemed, Genzyme, Merck Serono and Teva. All other authors declare that they have no conflicts of interest.
References
- 1.Shy M, Lupski JR, Chance PF, Klein CJ, Dyck PJ. Hereditary motor and sensory neuropathies: an overview of clinical, genetic, electrophysiologic and pathologic features. In: Ravi A, editor. Peripheral Neuropathy. 4. Philadelphia: Elsevier Saunders; 2005. pp. 1623–1658. [Google Scholar]
- 2.Birouk N, Gouider R, Le Guern E, et al. Charcot–Marie–Tooth disease type 1A with 17p11.2 duplication. Clinical and electrophysiological phenotype study and factors influencing disease severity in 119 cases. Brain. 1997;120:813–823. doi: 10.1093/brain/120.5.813. [DOI] [PubMed] [Google Scholar]
- 3.Krajewski KM, Lewis RA, Fuerst DR, et al. Neurological dysfunction and axonal degeneration in Charcot–Marie–Tooth disease type 1A. Brain. 2000;123:1516–1527. doi: 10.1093/brain/123.7.1516. [DOI] [PubMed] [Google Scholar]
- 4.García A, Combarros O, Calleja J, Berciano J. Charcot–Marie–Tooth disease type 1A with 17p duplication in infancy and early childhood: a longitudinal clinical and electrophysiologic study. Neurology. 1998;50:1061–1067. doi: 10.1212/wnl.50.4.1061. [DOI] [PubMed] [Google Scholar]
- 5.Berciano J, García A, Calleja J, Combarros O. Clinico-electrophysiological correlation of extensor digitorum brevis muscle atrophy in children with Charcot–Marie–Tooth disease 1A duplication. Neuromuscul Disord. 2000;10:419–424. doi: 10.1016/s0960-8966(99)00114-5. [DOI] [PubMed] [Google Scholar]
- 6.Yiu EM, Burns J, Ryan MM, Ouvrier RA. Neurophysiologic abnormalities in children with Charcot–Marie–Tooth disease type 1A. J Peripher Nerv Syst. 2008;13:236–241. doi: 10.1111/j.1529-8027.2008.00182.x. [DOI] [PubMed] [Google Scholar]
- 7.Killian JM, Tiwari PS, Jacobson S, Jackson RD, Lupski JR. Longitudinal studies of the duplication form of Charcot–Marie–Tooth polyneuropathy. Muscle Nerve. 1996;19:74–78. doi: 10.1002/(SICI)1097-4598(199601)19:1<74::AID-MUS10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 8.Shy ME, Chen L, Swan ER, et al. Neuropathy progression in Charcot–Marie–Tooth disease type 1A. Neurology. 2008;70:378–383. doi: 10.1212/01.wnl.0000297553.36441.ce. [DOI] [PubMed] [Google Scholar]
- 9.Verhamme C, van Schaik IN, Koelman JH, de Haan RJ, de Visser M. The natural history of Charcot–Marie–Tooth type 1A in adults: a 5-year follow-up study. Brain. 2009;132:3252–3262. doi: 10.1093/brain/awp251. [DOI] [PubMed] [Google Scholar]
- 10.Pareyson D, Reilly MM, Schenone A, et al. Ascorbic acid in Charcot–Marie–Tooth disease type 1A (CMT-TRIAAL and CMT-TRAUK): a double-blind randomised trial. Lancet Neurol. 2011;10:320–328. doi: 10.1016/S1474-4422(11)70025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang LW, Lin KP, Chang MH, et al. Electrophysiological characterization of Charcot–Marie–Tooth disease type 1A in Taiwan. J Chin Med Assoc. 2012;75:197–202. doi: 10.1016/j.jcma.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Saporta MA, Katona I, Lewis RA, Masse S, Shy ME, Li J. Shortened internodal length of dermal myelinated nerve fibres in Charcot–Marie–Tooth disease type 1A. Brain. 2009;132:3263–3273. doi: 10.1093/brain/awp274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manganelli F, Nolano M, Pisciotta C, et al. Charcot–Marie–Tooth disease: new insights from skin biopsy. Neurology. 2015;85:1202–1208. doi: 10.1212/WNL.0000000000001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J. Molecular regulators of nerve conduction – lessons from inherited neuropathies and rodent genetic models. Exp Neurol. 2015;267:209–218. doi: 10.1016/j.expneurol.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verhamme C, King RH, ten Asbroek AL, et al. Myelin and axon pathology in a long-term study of PMP22-overexpressing mice. J Neuropathol Exp Neurol. 2011;70:386–398. doi: 10.1097/NEN.0b013e318217eba0. [DOI] [PubMed] [Google Scholar]
- 16.Michailov GV, Sereda MW, Brinkmann BG, et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- 17.Taveggia C, Zanazzi G, Petrylak A, et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fledrich R, Stassart RM, Klink A, et al. Soluble neuregulin-1 modulates disease pathogenesis in rodent models of Charcot–Marie–Tooth disease 1A. Nat Med. 2014;20:1055–1061. doi: 10.1038/nm.3664. [DOI] [PubMed] [Google Scholar]
- 19.Gabreëls-Festen AA, Bolhuis PA, Hoogendijk JE, Valentijn LJ, Eshuis EJ, Gabreëls FJ. Charcot–Marie–Tooth disease type 1A: morphological phenotype of the 17p duplication versus PMP22 point mutations. Acta Neuropathol. 1995;90:645–649. doi: 10.1007/BF00318579. [DOI] [PubMed] [Google Scholar]
- 20.Sander S, Nicholson GA, Ouvrier RA, McLeod JG, Pollard JD. Charcot–Marie–Tooth disease: histopathological features of the peripheral myelin protein (PMP22) duplication (CMT1A) and connexin32 mutations (CMTX1) Muscle Nerve. 1998;21:217–225. doi: 10.1002/(sici)1097-4598(199802)21:2<217::aid-mus9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 21.Fabrizi GM, Simonati A, Morbin M, et al. Clinical and pathological correlations in Charcot–Marie–Tooth neuropathy type 1A with the 17p11. 2p12 duplication: a cross-sectional morphometric and immunohistochemical study in twenty cases. Muscle Nerve. 1998;21:869–877. doi: 10.1002/(sici)1097-4598(199807)21:7<869::aid-mus4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Thomas PK, Marques W, Jr, Davis MB, et al. The phenotypic manifestations of chromosome 17p11. 2 duplication. Brain. 1997;120:465–478. doi: 10.1093/brain/120.3.465. [DOI] [PubMed] [Google Scholar]
- 23.Smith RS, Koles ZJ. Myelinated nerve fibers: computed effect of myelin thickness on conduction velocity. Am J Physiol. 1970;219:1256–1258. doi: 10.1152/ajplegacy.1970.219.5.1256. [DOI] [PubMed] [Google Scholar]
- 24.Hanemann CO, Gabreëls-Festen AA, Stoll G, Müller HW. Schwann cell differentiation in Charcot–Marie–Tooth disease type 1A (CMT1A): normal number of myelinating Schwann cells in young CMT1A patients and neural cell adhesion molecule expression in onion bulbs. Acta Neuropathol. 1997;94:310–315. doi: 10.1007/s004010050712. [DOI] [PubMed] [Google Scholar]
- 25.Burns J, Ouvrier RA, Yiu EM, et al. Ascorbic acid for Charcot–Marie–Tooth disease type 1A in children: a randomised, double-blind, placebo-controlled, safety and efficacy trial. Lancet Neurol. 2009;8:537–544. doi: 10.1016/S1474-4422(09)70108-5. [DOI] [PubMed] [Google Scholar]